Abstract
Field carcinogenesis describes the prevalence of tumor-related alterations in normal-appearing tissues. Here we summarize recent efforts in profiling field molecular dynamics for resolving early events in cancer evolution. We also highlight gaps in our knowledge of the molecular and cellular heterogeneity of field carcinogenesis and propose directions to tackle these voids using single cell-based approaches and unique tissue sampling models. By interrogating both the mutagenized epithelium and its microenvironment, we surmise that single cell-guided studies will help chart the spatiotemporal molecular and cellular “atlas” of field carcinogenesis, will further delineate preneoplastic initiation and progression, and will help identify cancer prevention and early intervention targets.
Keywords: Field carcinogenesis, Field cancerization, Field effects, Premalignancy, Single-cell genomics
Introduction
Molecular field carcinogenesis, also known as field cancerization, describes the inception of tumors from a preconditioned normal-appearing or premalignant ‘field’ of injured (e.g. carrying mutations) cells (1). Despite being phenotypically “silent”, the epithelial field carries molecular alterations, including transcriptomic, genomic, and epigenetic changes, that can be indicative of nearby or ensuing tumors. Field carcinogenesis has been described in various malignancies including those of the lung, head and neck, mouth, skin, breast, esophagus, colon, stomach, prostate and bladder (2). Field carcinogenesis and the generation of a cancerization field is considered to represent an initiating phase in tumor evolution (1). This cancerization field is thought to continue to accrue alterations, some of which provide a selective clonal advantage for malignant transformation and tumorigenesis (1). Since normal-appearing cancerization fields exhibit abnormal molecular pathophysiology, understanding mechanisms of field carcinogenesis may help delineate early molecular targets for early diagnosis, interception and treatment. Yet, leveraging field carcinogenesis for identification of high-potential prevention targets has been inherently limited by our inability to sensitively resolve the precise order in which “driver” alterations occur, and by our poor understanding of the roles of non-epithelial compartments (e.g. immune cells, stromal components) and their interplay with the mutagenized epithelium. Here, we briefly describe relevant hallmarks in field carcinogenesis and highlight knowledge gaps inherent to its elusive dynamic nature. We also emphasize the significance of resolving spatiotemporal timelines of field carcinogenesis at single-cell resolution to our understanding of cancer initiation, preneoplastic progression and identification of relevant targets for early cancer treatment.
Molecular field carcinogenesis
Mutagenic insults (e.g. exposure to tobacco smoke, alcohol use, ultraviolet light; UV, infections -e.g., viral- or environmental factors) or ageing-mediated DNA replication errors are thought to lead to cancerization fields in normal-appearing tissues (1,2). An expanding preneoplastic field is thought to transition through a continuum of molecular (and later histological) aberrations (1). Molecular hallmarks of preneoplastic fields include transcriptomic, genomic, and epigenetic alterations, as well as exposure- or carcinogen-specific mutational signatures in the epithelium. For instance, normal-appearing esophageal epithelium and tobacco carcinogen-exposed airways are strongly associated with a TP53-mutant field (3). Several field-associated mutations (e.g. TP53, KRAS) overlap with pan-cancer mutational drivers of clonal expansion that could precede diagnosis by decades (4), further supporting the need to prioritize clinical interrogation of histologically-normal tissues. Loss of heterozygosity at common chromosomal regions (e.g. 2q, 9p, 12p and 17p) is another field-associated genetic event and a critical step in neoplastic progression of normal bronchial, oral and esophageal mucosae (1–3), whereby many of these chromosomal losses were also ranked among the earliest events in the evolution of multiple tumors (4). Additionally, epigenetic changes were described in the respiratory, colonic and esophageal fields (1) and are often linked to environmental (e.g. dietary and lifestyle) influences (2). Cancerization fields may also comprise mutational signatures linked to known exposures. For instance, field mutational signatures in normal-appearing gastric mucosa were associated with H. pylori-infection (1). Exposures to carcinogens such as tobacco and bile acid correlate with specific mutational signatures in normal-appearing airway and esophageal fields, respectively (1,3). Interestingly, exposure-related mutational signatures were shown to follow a strong pattern of unidirectional temporal variability; signatures of tobacco smoking in lung adenocarcinoma and UV in melanoma were predominantly active in the early clonal stages of tumorigenesis, and significant changes in other mutational spectra was evident throughout the evolution of 40% of pan-tumor samples (4). These insights suggest that an in-depth interrogation of mutational processes in normal-appearing epithelium may help elucidate the role of field carcinogenesis in cancer evolution.
Despite these insights, our understanding of molecular field carcinogenesis in cancer evolution remains limited primarily due to challenges in capturing the dynamic nature of normal-appearing and premalignant fields. For instance, the rate of somatic mutations in normal cells is generally low, implying that rare alterations unique to “fit” cells are likely to be missed in an “average” sequence derived from an admixed population of cells. Recently, Martincorena and colleagues performed deep sequencing of microdissected normal esophageal epithelial tissues acquired during upper endoscopy from phenotypically healthy individuals, and found that the normal tissues frequently displayed somatic mutations, and with a high degree of positive selection for cancer-associated mutations, such as TP53, with age (5). Similar sampling of small patches from UV-exposed ageing skin revealed thousands of mutations in normal cells, including oncogenic mutations that were clonally persistent (6). Equally intriguing, and in contrast to expanded oncogenic TP53-mutant clones, normal ageing esophageal epithelium and UV-exposed skin displayed increased clonal expansion of NOTCH1–mutant clones that strongly outweighed their prevalence in tumor tissues -- suggesting that these clones may be negatively selected, perhaps in part, due to pressures of cellular competition (1,5). To fully discern the specific contribution of field alterations to the temporal evolution of tumors in situ, longitudinal tissue sampling and analysis will be crucial to optimize best practices in clinical screening timing and biomarkers (1).
Molecular and cellular field effects have been frequently described in the epithelial compartment. Yet, increasing evidence points towards a role for the microenvironment in promoting clonal expansion of mutagenized epithelial cells. More recently, the selective advantage of an epithelial phenotype induced by field carcinogenesis drivers (e.g. TP53 mutations) was shown to be the product of an interplay between mutant epithelial cells and the surrounding microenvironment (1). For instance, in the absence of inflammatory signals, clonal expansion of colonic fields does not occur even in the presence of driver TP53 mutations (1). In the healthy adult intestine, field expansion is achieved when mutagenized cells become more fit to replace their competitive neighboring wild-type crypt cells following perturbations in the crypt microenvironment, such as increased secretion of oncogenic proteins (e.g. R-spondins) that stimulate stem cell and niche compartments and drive rapid tumorigenesis (7). In addition to their direct effect on the epithelium, mutagenic insults may induce stromal atrophy, thus possibly modifying cytokine expression, signaling pathways, or microbiome-sensing metabolites in stromal cells, and promoting pro-tumor inflammatory cell recruitment, as well as adjacent epithelial proliferation and neoplastic transformation (1,2). Non-steroidal anti-inflammatory drugs reduce the progression of normal esophageal field to cancer, possibly by inducing a microenvironment that hampers clonal expansion (1,2). Microenvironment-mediated selective pressures have been linked to a number of endogenous and exogenous etiologic factors (e.g. hormone -such as estrogen or insulin- levels, mitochondrial metabolism, microbiome dysbiosis, chronic inflammation, excessive growth hormone treatment, high fat diet, alcohol intake, lifestyle) (2). Yet, our knowledge of the exact mechanisms by which these factors and their interplay predispose normal cells to a microenvironment that favors premalignancy and results in cancerized fields, remain to be investigated.
Despite novel insights into field cancerization, molecular or cellular events have been either cataloged alone or at defined points in space or time. Understanding the precise order of events, interactions between the epithelium and other cellular populations, and the contributions of different endogenous and exogenous factors will provide an “atlas” of field carcinogenesis, and thus, a scalable roadmap for developing targets for prevention.
Tracing field carcinogenesis dynamics at the single-cell level
Recent efforts to sequence normal-appearing tissues (e.g. skin, esophagus and lung) have unveiled a heterogeneous susceptibility to carcinogenesis (3,5,6). Still, it is reasonable to surmise that a wholesome map of field effects in their natural habitat can be captured by overcoming technical and computational challenges, such as by: 1) simultaneously capturing microenvironmental states, 2) enabling discovery by moving beyond targeted approaches investigating a subset of predetermined genes, and 3) stretching the narrow spectrum of accessible tissues (regions of interest ought to be small enough to contain a limited number of clonal patches, yet large enough to ensure a sufficient amount of cells and analyte). “Clonally-perceptive” sampling models were thus further refined by sequencing whole genomes from hundreds of colonies derived from normal single cells in the mutagenized field of smoking-exposed airway (3). This study revealed a striking ten-fold variation in mutational burden among cells derived from a small biopsy of normal bronchial epithelium that has been exposed to cigarette smoke (3). Similarly, interrogation of normal cells within individual colonic crypts, each deriving from a single stem cell, identified mutational processes including driver mutations and clonal dynamics of colonic stem cells, and unraveled novel and robust insights into early stages of colorectal carcinogenesis since the clones were spatially stratified and isolated prior to sequencing (8). These studies have hinted at the prospects of capturing the true single-cell landscape in premalignant fields, which could thus address key events in specific cell subsets and clones that underlie neoplastic conversion during field carcinogenesis.
Building on successful single-cell-based interrogations and recent discoveries in the context of normal development, advanced tumors, as well as premalignancy (albeit to a lesser extent), we anticipate that single-cell-guided approaches will help address emerging questions on the evolutionary dynamics of field carcinogenesis. For instance, single-cell approaches to interrogating healthy and diseased (e.g. malignant) tissues have thus far enabled a parallel investigation of mucosa and stroma, at specific points in space and time, revealing novel crosstalk between the two compartments, genomic alterations, cellular compositions and differentiation states (including novel cells types), as well as diverse immune cell phenotypes (9). In the context of preneoplastic fields, single-cell-based studies have been far and few, primarily due to the paucity of longitudinally and spatially sampled fresh tissues, and largely dictated by current standard-of-care which either does not recommend surgical resection of preneoplasias (e.g. pure ground-glass opacities in the lung) or suggests the use of preneoplastic tissue solely for diagnosis in the clinical pathological setting. In a study surveying preneoplastic lesions and early gastritis-induced gastric cancer tissues at the single-cell level, distinct cell types and states were characterized across the different lesions, and an early cancer cell cluster was identified using neoplastic and non-neoplastic biopsies from the same patient (10). Derived single-cell signatures were leveraged to characterize of precise transcriptional patterns that could help define a panel of markers for the early detection of gastric cancer (10). Along the same lines, the cellular complexities of known precursors of esophageal and colorectal cancer were demarcated at the single-cell level, whereby the latter was characterized by early cell-type specific metabolic reprogramming, possibly signifying a novel hallmark of field cancerization (9).
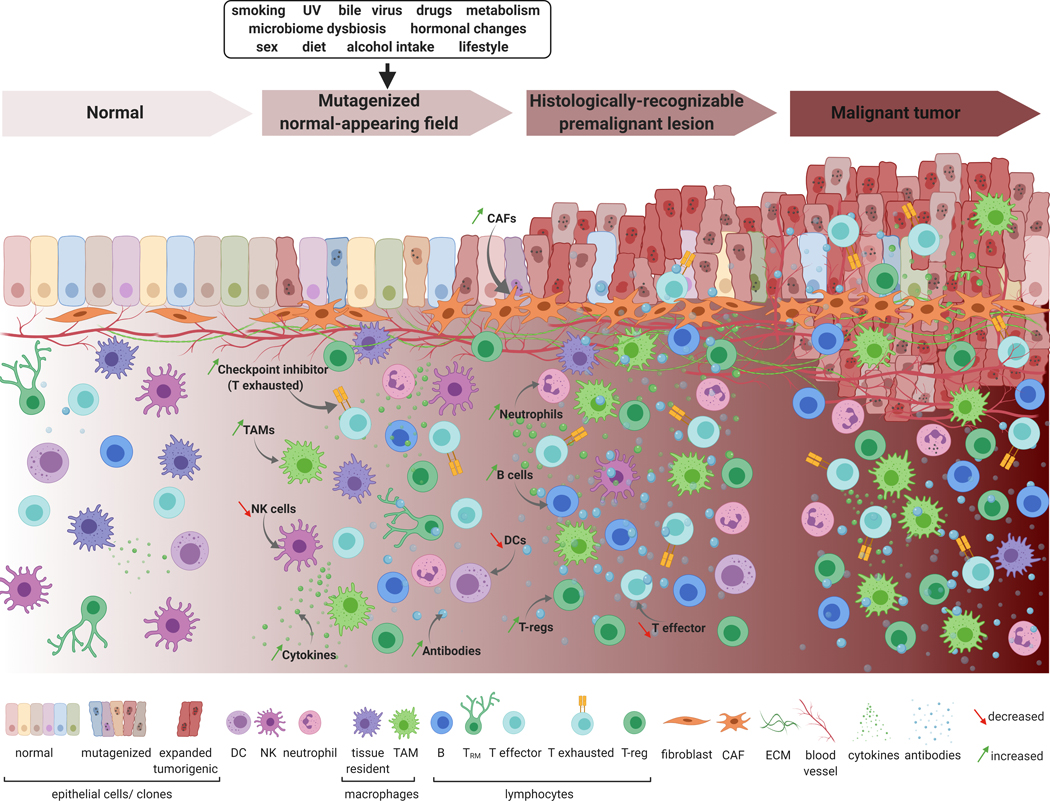
Applying single-cell-based approaches to delineate normal-appearing and premalignant field cancerization dynamics will enable the interrogation of the earliest neoplastic cells, cellular transitions, as well as potential cell-cell interactions across multiple tissues (Figure 1). Additionally, simultaneous evaluation of genomic and transcriptomic architecture of single cells in carcinogenic fields and over time, is anticipated to unravel complex dynamics in the evolution of preneoplasia. Consequently, multi-omic single-cell approaches are anticipated to address uncharted and provocative interrogations in field carcinogenesis, such as: What are the individual roles and collective contribution of cell types and states to field cancerization? Is clonal selection in the field mediated by early immune sculpting (e.g., enhanced immune evasion of epithelial clones)? Alternatively, are immune response dynamics rather driven by the mutational profile of select clones? What roles does epithelial-stromal crosstalk play in field carcinogenesis? Owing to their rapidly advancing accuracy and scale in detecting rare events in tumor evolution, single-cell approaches will serve to complement and expand the breadth of insight obtained from pathological assessment, bulk transcriptomics and genomics, molecular alterations, and cellular phenotypes (e.g. pre-malignant, malignant, non-malignant and immune). Surveying the single-cell landscape of normal and premalignant fields and their surrounding microenvironments will elucidate the roles of individual epithelial, immune, and stromal populations- defined by cell type or state -as well as interactions among them - in driving field carcinogenesis and progression (Figure 1). Valuable surrogates for studying the early events that drive field carcinogenesis include in vitro organoid-derived normal-appearing single cells, as well as animal models that recapitulate driver-specific (e.g. genetically engineered models) or carcinogen-mediated field cancerization (9). Such strategies, coupled with unique sampling of premalignant human tissue, will help identify highly specific markers to predict, and possibly intercept, the progression of premalignant lesions into cancer. Achieving these milestones heavily rests on prioritizing longitudinal sampling of fresh premalignant lesions, optimizing the processing of fixed tissues, broadening the scope of analytes attainable from the same single cell, as well as advancing and standardizing multi-omics technologies and computational analyses. By charting comprehensive atlases of the early mutagenized epithelium, its immune microenvironment, as well as stromal components in space and time, cancerized fields will convey profound implications for accelerating the development of novel strategies for early cancer interception.
Figure 1.
A panoramic model of field carcinogenesis at single-cell resolution. This model shows the inception of field carcinogenesis in normal-appearing epithelium and how this phenomenon may be influenced by or modulate the surrounding microenvironment. These field effects may be markers for the progression of normal-appearing mutagenized cells in the field into histologically-distinguishable premalignant states, and later, malignant stages. Epithelial cancerization fields may comprise different subtypes of normal epithelial cells (left) resulting in clones of mutagenized cells that carry diverse genomic alterations (rough-appearing cells with dotted nuclei). Fit mutagenized clones overcome selective pressure leading to clonal expansion and progression of the normal or premalignant field into premalignant lesions and later, malignant tumors. The model also depicts diverse cell types, populations, and components of the immune and stromal microenvironments whose dynamic interplay with field carcinogenesis in the epithelium can be best illustrated at the single-cell level. Changes in stromal compartments such as fibroblasts (e.g. increased cancer-associated fibroblasts), extracellular matrix components, as well as vasculature may be further “fuel” for field carcinogenesis or be impacted by mutagenized field epithelia. Additionally, it is speculated that co-evolution of the immune microenvironment may play important roles in field cancerization and progression. Possible early immune-response dynamics may include gradual changes to immune cell abundance and/or epithelial infiltration, which could be decreased (e.g. for natural killer, dendritic, and T cells), or potentially increased (e.g. for tumor-associated macrophages, and regulatory and exhausted T lymphocytes-such as through the increased expression of inhibitory checkpoints). This can be also accompanied by an increased expression of inhibitory immune checkpoints, or production of pro-tumor immunomodulatory molecules such as cytokines and antibodies. UV: ultraviolet; DC: dendritic cell; NK: natural killer cell; TAM: tumor-associated macrophage; TRM: tissue-resident memory T cell; T-reg: regulatory T cell; CAF: cancer-associated fibroblast; ECM: extracellular matrix. Created with BioRender.com.
Acknowledgments
Grant support: Supported by the grant R01CA205608 from the National Cancer Institute (to HK), and by start-up research funds kindly provided to LW and HK by the University of Texas MD Anderson Cancer Center.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nature reviews Cancer 2018;18:19–32 [DOI] [PubMed] [Google Scholar]
- 2.Lochhead P, Chan AT, Nishihara R, Fuchs CS, Beck AH, Giovannucci E, et al. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2015;28:14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida K, Gowers KHC, Lee-Six H, Chandrasekharan DP, Coorens T, Maughan EF, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 2020;578:266–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, et al. The evolutionary history of 2,658 cancers. Nature 2020;578:122–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science (New York, NY) 2018;362:911–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science (New York, NY) 2015;348:880–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boone PG, Rochelle LK, Ginzel JD, Lubkov V, Roberts WL, Nicholls PJ, et al. A cancer rainbow mouse for visualizing the functional genomics of oncogenic clonal expansion. Nature communications 2019;10:5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature 2019;574:532–7 [DOI] [PubMed] [Google Scholar]
- 9.Ren X, Kang B, Zhang Z. Understanding tumor ecosystems by single-cell sequencing: promises and limitations. Genome Biology 2018;19:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Yang M, Zhang Y, Xiao S, Lai X, Tan A, et al. Dissecting the Single-Cell Transcriptome Network Underlying Gastric Premalignant Lesions and Early Gastric Cancer. Cell reports 2019;27:1934–47.e5 [DOI] [PubMed] [Google Scholar]