Abstract
Localized translation is a proposed biological event that allows mRNA to be translated on site, providing an additional level of protein regulation within a cell. Examples of localized translation have been found or proposed in a variety of cellular contexts from neurons to cancer cells and implicated in both normal development and disease for over a half century. For example, mRNA translation on the mitotic apparatus (MA) was initially hypothesized in the 1950–60s. However, its proof of existence, biological significance and mechanistic details have remained sparse and it is still unclear how well conserved this mechanism may be among different cell types or organisms. In this review, we provide a brief historic summary of translation on the MA and discuss how current and future work may help us understand this biological process that provides a subcellular level of regulation in protein synthesis within a cell.
1. History of translation on the mitotic apparatus
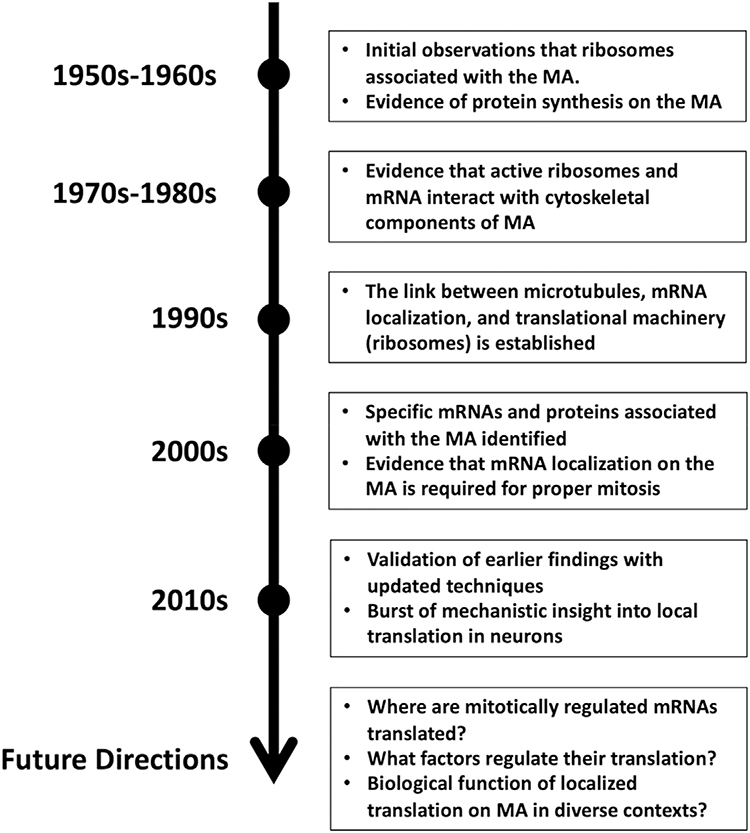
The initial concept of translation on the MA emerged in the field in the late 1950s and early 1960s (Fig. 1). A wealth of observations on the subcellular structure and morphology of a wide variety of cells had shown that ribosomes frequently appeared associated with the cytoskeleton and, particularly, with the microtubules of the mitotic apparatus (Gross et al., 1958; Kane, 1962; Roth and Daniels, 1962). Furthermore, several studies from mammalian cells and embryos of the sea urchin suggested that protein synthesis continues throughout the cell cycle including M-phase (though to varying degrees in different systems) (Gross and Fry, 1966; Konrad, 1963; Prescott and Bender, 1962).
Fig. 1.
Timeline highlighting the main advances in our understanding of localized translation on the mitotic apparatus and questions still to be answered.
The question of whether protein synthesis occurs on the MA was then addressed using radiolabeled amino acids and electron microscopy that visualizes newly synthesized proteins in the cells. In these studies, researchers found that a high concentration of radioactive labelling is consistently present over the MA in sea urchin blastomeres (Gross and Cousineau, 1963; Mangan et al., 1965; Stafford and Iverson, 1964). Based on these labeling experiments it appeared that newly synthesized proteins associated with the microtubules of the MA, similar to the ribosomal localization. Connecting these major observations, researchers hypothesized that protein synthesis occurs from ribosomes that are localized on the MA, and that this localized synthesis may be important for the structure and function of the MA. Around the same time, however, some reports suggested that rates of protein synthesis decrease during mitosis in mammalian cell culture (Fan and Penman, 1970; Konrad, 1963). Therefore, the question of whether the increase in radioactive labelling on the MA was due to active translation there or accumulation of new proteins made prior to mitosis remained.
Over the following decades, evidence supporting the hypothesis of localized translation on the MA continued to arise. The development of techniques that allowed researchers to isolate intact cytoskeletal frameworks of cells suitable for electron microscopy were particularly helpful. Despite the harsh conditions required to remove cellular membranes, it was found that ribosomes and RNA remained associated with cytoskeletal structures (Lenk et al., 1977). Researchers also found that cytoskeletal-associated ribosomes were predominantly polyribosome (considered actively translating ribosomes) and that the interaction required the presence of mRNA (Cervera et al., 1981; Fulton et al., 1980; Lenk et al., 1977; Moon et al., 1983; Suprenant et al., 1989). However, many of these studies were carried out in interphase cells and could not directly support the idea of localized translation within the MA.
By the late 1980s–1990s, the idea that the cytoskeleton is critical for trafficking of mRNAs and translational machinery became accepted in the field. In various organisms such as Drosophila, Xenopus, and mouse, it was reported that many of the mRNA distribution patterns necessary for embryonic and cellular polarity were largely dependent on trafficking of mRNAs along microtubules (Ainger et al., 1993; Pokrywka and Stephenson, 1991; Raff et al., 1990; Yisraeli et al., 1990). Further, mature mRNAs that coded for only a subset of proteins were found enriched in the microtubule fraction of sea urchin embryonic lysate (Hamill et al., 1994). These results suggested that specific mRNAs interact with microtubules perhaps in a regulated fashion as opposed to random trapping of mRNAs by microtubules. Despite the growing evidence that the cytoskeleton (particularly the microtubule component) could act as a scaffold for mRNA translation, little direct evidence was available that proves the presence of active translation on the microtubule fraction of the MA itself. Furthermore, questions remained as to whether translation is differentially regulated at a sub-cellular level within the cell or whether translation remains constant throughout the cell while the products vary based on mRNA localization.
By the 2000s, more evidence of MA translation began to emerge. Groisman et al. used Xenopus embryos to show in vivo that at least two proteins involved in regulating translation of specific mRNAs (Maskin and CPEB) colocalize with their target mRNAs (Xbub3 and cyclin B1) on the mitotic spindle. They further showed that localization of cyclinB1 mRNA on the MA is required for proper localization of its corresponding protein and proper cell divisions in the embryo (Groisman et al., 2000). This study provided valuable evidence that mRNAs translated on the mitotic spindle are involved in the process of mitosis. Further, this study provides an example of a sequence motif involved in localizing specific mRNAs to the mitotic spindle.
Proteomic and genomic analyses provided additional support that active translation occurs on the MA. Through the use of mass spectrometry to characterize the microtubule-associated proteome of Xenopus eggs, Liska et al. revealed that many proteins involved in translation interact with microtubules, including ribosomal proteins, elongation factors, and aminoacyl-tRNA synthetases (Liska et al., 2004). It is important to note that the structures they were analyzing were meiotic spindles, which differ from mitotic spindles in how they form and in microtubule organization (Severson et al., 2016). A similar approach was used to identify MA-associated proteins in human cancer cells (HeLa), which revealed an association of ~200 proteins involved in translation with the MA (Sauer et al., 2005). This list includes many of the homologous proteins found associated with the Xenopus meiotic spindle, suggesting that local translation may be a shared feature of these two structures. As a complement to this proteomic data, Blower, et al. used microarray analysis to identify mRNAs associated with the meiotic spindle of Xenopus eggs and the mitotic spindle of human cancer cells (Blower et al., 2007). In both Xenopus eggs and human cancer cells, mRNAs coding for proteins involved in DNA metabolism (DNA repair, synthesis, etc.) and mitosis were found enriched on the spindle microtubules. Furthermore, Blower et al. showed that translation could actively occur on the meiotic spindle in vitro using Xenopus egg lysate. These findings supported for the hypothesis that localized translation on the MA is present, and furthermore, may be important for cellular functions such as proper cell divisions and controlled distribution of products into daughter cells. However, direct in vivo evidence that proves active translation within the MA and its biological function in developing cells and organisms remained speculative.
Research on this topic over the last decade has further supported the hypothesis in more model systems and with updated techniques. Many of these studies validate earlier results with updated technologies, i.e. reanalysis of the mitotic spindle proteome in hamster ovary (CHO) cells (Bonner et al., 2011), and provided additional correlative evidence, i.e. immunofluorescence showing dynamic patterns of translation regulators on the meiotic spindle of the mouse oocyte (Romasko et al., 2013). Other studies have provided additional trans-acting factors that may regulate the translation of localized mRNAs. For example, the translational regulator Vasa was found to localize to the MA in early sea urchin embryos and to directly interact with mRNAs such as cyclinB1 (Yajima and Wessel, 2011, 2015). Recently, Hassine et al. identified a population of RNAs that are enriched on the mitotic spindle of HCT116 cells (colorectal cancer) and non-transformed hTERT-RPEI cells (immortalized retinal pigment epithelial cells) and show that many of these RNAs depend on the RNA-binding protein Staufen1 for their localization (Hassine et al., 2020). Additionally, this study also shows that OP-puromycin labeling co-localizes with microtubules in mitotic cells, suggesting that mitotic translational activity is enriched in the area of the mitotic spindle. However, whether these mRNAs are translated on the mitotic spindle or have physiological significance has not been directly tested.
2. Lessons on local translation from elsewhere in the cell
A context in which localized translation has been well studied is in the translation of proteins destined for cellular membranes or secretion. Secretory pathway proteins are targeted to the endoplasmic reticulum during their translation; a process termed co-translational targeting. As these proteins are translated in the cytoplasm, the first portion of the protein to exit the ribosome contains a signal sequence that is recognized by a protein known as Signal Recognition Particle (SRP). SRP binds to the signal sequence and then to SRP receptors on the endoplasmic reticulum (ER), thereby bringing the nascent protein and associated translation complex to the ER. Once associated with the ER, the growing polypeptide will be translocated into the lumen of the ER and processed in a variety of ways depending on the ultimate destination. One could imagine a scenario in which mitotic proteins begin their translation in the cytoplasm but are quickly localized to and completed on the MA due to a similar mechanism. Interestingly, multiple recent studies suggest that co-translational targeting of mRNAs may be a more widely used mechanism within the cell than just to target mRNAs to the ER (Cioni et al., 2019; Sepulveda et al., 2018).
One particularly relevant example is a study from 2018 in which researchers used zebrafish embryos and human cell culture (HeLa and RPE-1 cells) to show co-translational targeting of pericentrin (PCNT) to the centrosome, a region of the MA (Sepulveda et al., 2018). In this study, they show that PCNT mRNA and nascent PCNT protein are highly localized to the centrosome of mitotic cells in a translation-, microtubule-, and dynein-dependent manner. While they did not directly test how this co-translational targeting is regulated, they note that there is a dynein-interaction domain near the N-terminus of PCNT and propose a model in which dynein carries partially translated PCNT along with attached polysomes and mRNA to the centrosome. Translation is completed at or near the centrosome, allowing for efficient accumulation of PCNT into the pericentriolar material and ultimately proper maturation of the structure, however a functional assay that directly proves this point is still awaited. This study also provides evidence that this mechanism may also apply to another centrosomal protein, suggesting that this could be a widely used mechanism that cells use to ensure proteins are synthesized with spatiotemporal precision.
Mechanisms of localized translation have also been vigorously studied in neuronal cells. Since comprehensive reviews are already available in this field of research, we will discuss it only briefly (Batista and Hengst, 2016; Biever et al., 2019; Cioni et al., 2018; Farias et al., 2019; Holt et al., 2019). Neuronal cells are known for their distinct and compartmentalized morphology, having a cell body as well as dendrites, an axon, and synapses. Recent studies incorporating advanced imaging and sequencing technologies such as transcriptome and translatome analyses suggest that protein synthesis occurs in specific regions such as the presynaptic terminal and the axon of the neuronal cell. Researchers have also identified some of the pathways involved in this process of local translation (Cagnetta et al., 2018, 2019; Hafner et al., 2019; Shigeoka et al., 2016; Wong et al., 2017). Many of the functions and mechanisms of local translation reported so far remain largely specific to neuronal cells. However, these findings still provide a good foundation for one to speculate that this biological event may be used in many different cells and/or biological contexts. Recent technological advancements in the field of neuroscience may be applied to other cells and biological contexts to address fundamental questions left in the field: if and how localized translation occurs on the MA and how that contributes to cellular functions and ultimately organismal development
3. Future directions
Part of the reason little attention has been given to localized translation on the MA is that global translation has been found to be repressed during mitosis (Fan and Penman, 1970; Konrad, 1963). However, more recent work is beginning to show that while translation may be generally depressed, a population of mRNAs still needs to be actively translated during mitosis in various cell types (Park et al., 2016; Shuda et al., 2015; Stumpf et al., 2013; Tanenbaum et al., 2015). These findings reinforce the need for further studies on mechanisms of translation during mitosis. Some of the main questions still to be addressed include: 1) Where in the MA are mitotically regulated mRNAs translated? 2) What factors regulate localization and/or translation?3) What is the biological function of local translation on the MA?
Question 1 relates to the need for better resolution of where within the MA translation of specific mRNAs occurs? The MA is a complex region composed of the centrosomes, kinetochore, the mitotic spindle, which itself is composed of different types of microtubules. Does translation occur in all of these locations and are there specific mRNAs that are translated in one region over another? The example of PCNT indicates that at least some mRNAs are translated specifically where the protein product is needed. Parsing apart which specific components of the MA are involved in the translation of a set of targets will help us understand how the process is regulated and if it is related to specific biological function. It will also be important to distinguish between mRNAs that are translated on the MA from mRNAs that associate with the MA as a cargo for proper segregation into daughter cells. Examples of the latter process have been well documented in a wide range of species including yeast and spiralians (Chartrand et al., 2002; Kingsley et al., 2007; Rabinowitz and Lambert, 2010). Live imaging of translation in dividing cells will help distinguish between these two fates of MA-localizaed mRNAs.
Question 2 refers to the need for a better understanding of the molecules involved in regulating local translation on the MA. As we have seen, microtubules clearly play a crucial role, but we have also seen that additional proteins such as RNA-binding proteins and other mitotic regulators are involved as well. On the other side of the equation, there are likely also specific sequences within MA-translated mRNAs that help determine their localization and ultimate translation. For example, work done in Xenopus eggs and embryos has shown that the Cytoplasmic Polyadenylation Element (CPE), found in the 3’ UTR of some mRNAs is important for the spindle localization of mRNAs for proteins such as CyclinB1, Xkid, and others (Blower et al., 2007; Eliscovich et al., 2008). Identifying the cis- and trans-acting factors that regulate local translation will be critical for understanding the molecular mechanism governing this process.
Question 3 seeks to understand why cells would need mechanisms for local translation during mitosis rather than relying on cytoplasmic translation and subsequent accumulation of proteins on the MA? Again, the PCNT example starts to provide a potential explanation. The authors of this study propose that for a protein as large as PCNT (>3k amino acids) local translation at the centrosome allows for more efficient incorporation of PCNT during early mitosis. By localizing polysome-laden PCNT mRNA directly to the centrosome, larger numbers of PCNT proteins may be incorporated into the centrosome than if PCNT proteins had to be individually trafficked to the centrosome from elsewhere in the cell. This efficiency may be important for centrosome maturation, which occurs in ~30 min in a human cell. One could imagine that efficient incorporation of mitotic proteins would be even more important in rapidly cycling cells such as those in early embryos. As we gain a more complete understanding of the types of mRNAs that are translated within the MA, as well as when and how they are regulated we will be able to better address this hypothesis.
Moving forward, in vivo and live imaging studies will provide insight into where mitotically-associated mRNAs are translated subcellularly and if/how that contributes to specific cellular activities and/or fate specifications. Although it is not yet clear how extensively localized translation may be used in adult mitotic cells, growing evidence suggests that embryonic cells appear to take advantage of this biological process across organisms. The relatively large size and rapid cell cycling and differentiation of embryonic cells may increase the demand for more precise control of protein synthesis. Given that observational evidence of localized translation on the MA is already available in several models such as early embryos of Xenopus and sea urchin, revisiting these models with recently developed technologies will likely advance the field. Future work should address the biological significance and the molecular mechanisms of localized translation on the MA in developing cells and organisms, which has been proposed yet unanswered for over a half century.
Funding
This work was supported by the American Heart Association Scientist Development Grant (14SDG18350021) and by NIH 1R01GM126043-01 to MY.
References
- Ainger K, Avossa D, Morgan F, Hill SJ, Barry C, Barbarese E, Carson JH, 1993. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J. Cell Biol 123, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AFR, Hengst U, 2016. Intra-axonal protein synthesis in development and beyond. Int. J. Dev. Neurosci 55, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biever A, Donlin-Asp PG, Schuman EM, 2019. Local translation in neuronal processes. Curr. Opin. Neurobiol 57, 141–148. [DOI] [PubMed] [Google Scholar]
- Blower MD, Feric E, Weis K, Heald R, 2007. Genome-wide analysis demonstrates conserved localization of messenger RNAs to mitotic microtubules. J. Cell Biol 179, 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MK, Poole DS, Xu T, Sarkeshik A, Yates JR, Skop AR, 2011. Mitotic spindle proteomics in Chinese hamster ovary cells. PloS One 6, e20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnetta R, Frese CK, Shigeoka T, Krijgsveld J, Holt CE, 2018. Rapid cue-specific remodeling of the nascent axonal proteome. Neuron 99, 29–460000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnetta R, Wong HH-W, Frese CK, Mallucci GR, Krijgsveld J, Holt CE, 2019. Noncanonical modulation of the eIF2 pathway controls an increase in local translation during neural wiring. Mol. Cell 73, 474–489 e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M, Dreyfuss G, Penman S, 1981. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell 23, 113–120. [DOI] [PubMed] [Google Scholar]
- Chartrand P, Meng X, Huttelmaier S, Donato D, Singer RH, 2002. Asymmetric sorting of Ash1p in yeast results from inhibition of translation by localization elements in the mRNA. Mol. Cell 10. [DOI] [PubMed] [Google Scholar]
- Cioni J-M, Koppers M, Holt CE, 2018. Molecular control of local translation in axon development and maintenance. Curr. Opin. Neurobiol 51, 86–94. [DOI] [PubMed] [Google Scholar]
- Cioni J-M, Lin JQ, Holtermann AV, Koppers M, Jakobs MAH, Azizi A, Turner-Bridger B, Shigeoka T, Franze K, Harris WA, et al. , 2019. Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell 176, 56–72 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliscovich C, Peset I, Vernos I, Méndez R, 2008. Spindle-localized CPE-mediated translation controls meiotic chromosome segregation. Nat. Cell Biol 10, 858–865. [DOI] [PubMed] [Google Scholar]
- Fan H, Penman S, 1970. Regulation of protein synthesis in mammalian cells. J. Mol. Biol 50, 655–670. [DOI] [PubMed] [Google Scholar]
- Farias J, Sotelo JR, Sotelo-Silveira J, 2019. Toward axonal system biology: genome wide views of local mRNA translation. Proteomics 19, 1900054. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Wan KM, Penman S, 1980. The spatial distribution of polyribosomes in 3T3 cells and the associated assembly of proteins into the skeletal framework. Cell 20, 849–857. [DOI] [PubMed] [Google Scholar]
- Groisman I, Huang Y-S, Mendez R, Cao Q, Theurkauf W, Richter JD, 2000. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus. Cell 103, 435–447. [DOI] [PubMed] [Google Scholar]
- Gross PR, Cousineau GH, 1963. Synthesis OF spindle-associated proteins IN early cleavage. J. Cell Biol 19, 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross PR, Fry BJ, 1966. Continuity of protein synthesis through cleavage metaphase. Science 153, 749–751. [DOI] [PubMed] [Google Scholar]
- Gross PR, Philpott DE, Nass S, 1958. The fine-structure of the mitotic spindle in sea urchin eggs. J. Ultra. Mol. Struct. Res 2, 55–72. [DOI] [PubMed] [Google Scholar]
- Hafner A-S, Donlin-Asp PG, Leitch B, Herzog E, Schuman EM, 2019. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science 364, eaau3644. [DOI] [PubMed] [Google Scholar]
- Hamill D, Davis J, Drawbridge J, Suprenant KA, 1994. Polyribosome targeting to microtubules: enrichment of specific mRNAs in a reconstituted microtubule preparation from sea urchin embryos. J. Cell Biol 127, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassine S, Bonnet-Magnaval F, Bouvrette LPB, Doran B, Ghram M, Bouthillette M, Lecuyer E, DesGroseillers L, 2020. Staufen1 localizes to the mitotic spindle and controls the localization of RNA populations to the spindle. J. Cell Sci 133 (jcs), 247155. [DOI] [PubMed] [Google Scholar]
- Holt CE, Martin KC, Schuman EM, 2019. Local translation in neurons: visualization and function. Nat. Struct. Mol. Biol 26, 557–566. [DOI] [PubMed] [Google Scholar]
- Kane RE, 1962. The mitotic apparatus. J. Cell Biol 15, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley EP, Chan XY, Duan Y, Lambert JD, 2007. Widespread RNA segregation in a spiralian embryo: RNA segregation in Ilyanassa. Evol. Dev 9, 527–539. [DOI] [PubMed] [Google Scholar]
- Konrad CG, 1963. Protein synthesis and RNA synthesis during mitosis IN animal cells. J. Cell Biol 19, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R, Ransom L, Kaufmann Y, Penman S, 1977. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell 10, 67–78. [DOI] [PubMed] [Google Scholar]
- Liska AJ, Popov AV, Sunyaev S, Coughlin P, Habermann B, Shevchenko A, Bork P, Karsenti E, Shevchenko A, 2004. Homology-based functional proteomics by mass spectrometry: application to the Xenopus microtubule-associated proteome. Proteomics 4, 2707–2721. [DOI] [PubMed] [Google Scholar]
- Mangan J, Miki-Noumura T, Gross PR, 1965. Protein synthesis and the mitotic apparatus. Science 147, 1575–1578. [DOI] [PubMed] [Google Scholar]
- Moon RT, Nicosia RF, Olsen C, Hille MB, Jeffery WR, 1983. The cytoskeletal framework of sea urchin eggs and embryos: developmental changes in the association of messenger RNA. Dev. Biol 95, 447–458. [DOI] [PubMed] [Google Scholar]
- Park J-E, Yi H, Kim Y, Chang H, Kim NV, 2016. Regulation of poly(A) tail and translation during the somatic cell cycle. Mol. Cell 62, 462–471. [DOI] [PubMed] [Google Scholar]
- Pokrywka NJ, Stephenson EC, 1991. Microtubules mediate the localization of bicoid RNA during Drosophila oogenesis. Dev. Camb. Engl 113, 55–66. [DOI] [PubMed] [Google Scholar]
- Prescott DM, Bender MA, 1962. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp. Cell Res 26, 260–268. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JS, Lambert JD, 2010. Spiralian quartet developmental potential is regulated by specific localization elements that mediate asymmetric RNA segregation. Development 137, 4039–4049. [DOI] [PubMed] [Google Scholar]
- Raff JW, Whitfield WGF, Glover DM, 1990. Two Distinct Mechanisms Localise Cyclin B Transcripts in Syncytial Drosophila Embryos. Development. [DOI] [PubMed] [Google Scholar]
- Romasko EJ, Amarnath D, Midic U, Latham KE, 2013. Association of maternal mRNA and phosphorylated EIF4EBP1 variants with the spindle in mouse oocytes: localized translational control supporting female meiosis in mammals. Genetics 195, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth LE, Daniels EW, 1962. Electron microscopic studies OF mitosis IN amebae. J. Cell Biol 12, 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G, Körner R, Hanisch A, Ries A, Nigg EA, Silljé HHW, 2005. Proteome analysis of the human mitotic spindle. Mol. Cell. Proteomics 4, 35–43. [DOI] [PubMed] [Google Scholar]
- Sepulveda G, Antkowiak M, Brust-Mascher I, Mahe K, Ou T, Castro NM, Christensen LN, Cheung L, Jiang X, Yoon D, et al. , 2018. Co-translational protein targeting facilitates centrosomal recruitment of PCNT during centrosome maturation in vertebrates. Elife 7, e34959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson AF, von Dassow G, Bowerman B, 2016. Chapter five - oocyte meiotic spindle assembly and function In: Wassarman PM (Ed.), Curr Top Dev Biol Academic Press, pp. 65–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka T, Jung H, Jung J, Turner-Bridger B, Ohk J, Lin J, Amieux PS, Holt CE, 2016. Dynamic axonal translation in developing and mature visual circuits. Cell 166, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuda M, Velásquez C, Cheng E, Cordek DG, Kwun HJ, Chang Y, Moore PS, 2015. CDK1 substitutes for mTOR kinase to activate mitotic cap-dependent protein translation. Proc. Natl. Acad. Sci. Unit. States Am 112, 5875–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford DW, Iverson RM, 1964. Radioautographic evidence for the incorporation of leucine-carbon-14 into the mitotic apparatus. Science 143, 580–581. [DOI] [PubMed] [Google Scholar]
- Stumpf CR, Moreno MV, Olshen AB, Taylor BS, Ruggero D, 2013. The translational landscape of the mammalian cell cycle. Mol. Cell 52, 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprenant KA, Tempero LB, Hammer LE, 1989. Association of ribosomes with in vitro assembled microtubules. Cell Motil. Cytoskelet 14, 401–415. [DOI] [PubMed] [Google Scholar]
- Tanenbaum ME, Stern-Ginossar N, Weissman JS, Vale RD, 2015. Regulation of mRNA translation during mitosis. Elife 4, e07957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HH-W, Lin JQ, Ströhl F, Roque CG, Cioni J-M, Cagnetta R, Turner-Bridger B, Laine RF, Harris WA, Kaminski CF, et al. , 2017. RNA docking and local translation regulate site-specific axon remodeling in vivo. Neuron 95, 852–868 e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM, 2011. The DEAD-box RNA helicase Vasa functions in embryonic mitotic progression in the sea urchin. Dev. Camb. Engl 138, 2217–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM, 2015. Essential elements for translation: the germline factor Vasa functions broadly in somatic cells. Development 142, 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisraeli JK, Sokol S, Melton DA, 1990. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Dev. Camb. Engl 108, 289–298. [DOI] [PubMed] [Google Scholar]