Abstract
Background:
Bipolar radiofrequency (RF) clamps have been shown to be capable of reproducibly creating transmural lesions with a single ablation in animal models. Unfortunately, in clinical experience, the bipolar clamps have not been as effective, and often require multiple ablations to create conduction block. This study created a new experimental model using fresh, cardioplegically-arrested human hearts turned down for transplantation to evaluate the performance of a non-irrigated bipolar RF clamp.
Methods:
Nine human hearts turned down for transplantation were harvested and the Cox-Maze IV lesion set was performed with a non-irrigated bipolar RF clamp. In the first seven hearts, a single ablation was performed for each lesion. In the last two hearts, a set of two successive ablations without unclamping were performed. The heart tissue was stained with 2,3,5-triphenyl-tetrazolium chloride. Each ablation lesion was cross-sectioned to assess lesion depth and transmurality.
Results:
A single ablation with the bipolar RF clamp resulted in 89% (469/529) of the histologic sections and 65% (42/65) of the lesions being transmural. Of the non-transmural sections, 92% occurred in areas with epicardial fat. Performing two successive ablations without unclamping resulted in 100% of the cross-sections (201/201) and lesions (25/25) being transmural.
Conclusions:
A single ablation failed to create a transmural lesion 35% of the time, and this was associated with the presence of epicardial fat. Two successive ablations without unclamping resulted in 100% lesion transmurality using the bipolar RF clamp.
Keywords: atrial fibrillation, surgical ablation, bipolar radiofrequency ablation, transmurality
The Cox-Maze procedure (CMP) was introduced in 1987 and was the first successful interventional treatment for atrial fibrillation (AF). The original operation employed a pattern of biatrial incisions designed to create lines of conduction block.1,2 Though it achieved excellent results, the CMP III was not widely adopted due to its complexity.3 Advances in ablation technology have helped to replace the “cut-and-sew” technique with energy sources capable of producing transmural lesions and conduction block. In particular, bipolar radiofrequency (RF) and cryoablation technology have been shown to reliably create transmural lesions in a less-invasive manner. This led to the development of the Cox-Maze IV procedure (CMP-IV).4,5 The most commonly used ablation devices in the world today are the bipolar radiofrequency clamps.
Bipolar RF technology works by delivering high-density current between closely approximated electrodes embedded in the jaws of a clamp. The size of the lesion created by thermal energy is determined by the tissue-electrode contact area, interface temperature, power, and duration of energy delivery.6 The electrical resistance encountered by the current as it passes through the myocardium produces heat that kills the myocardial cells. Focal coagulation necrosis occurs at temperatures greater than 50°C.6 The clamps shield the electrodes from the circulating blood pool, which improves lesion creation, reduces collateral injury, and allows for faster ablations.4
This ablation technology tries to predict lesion transmurality in real-time by measuring the conductance between the electrodes. The technology measures tissue conductance and impedance throughout the ablation cycle, and controls the application of energy to the tissue. The maximal energy delivery occurs when the constant power (CP) zone is reached as defined by the manufacturer, which occurs when tissue impedance is 30–70 ohms, or when tissue conductance reaches 15–30 millisiemens (mS). During the ablation cycle, energy is delivered to the tissue until the tissue conductance reaches a stable minimum and impedance reaches a maximum threshold, at which point energy delivery ceases. A signal is given once the stable low conductance level is reached as a sign that an effective lesion has been created, with the knowledge that transmurality itself can only be confirmed with histologic analysis.
Our laboratory has performed numerous experimental studies which have shown that bipolar RF ablation can safely create reliable transmural lesions on a beating heart in animal models, and that this conductance algorithm was effective at predicting transmurality.7–10 Early iterations of the disposable non-irrigated bipolar RF clamp (Isolator™, AtriCure Inc, Cincinnati, OH) were successful in producing 100% transmurality after a single ablation in both acute and chronic animal studies.7–9 The present device on the market, the dual-electrode bipolar non-irrigated radiofrequency ablation device (Synergy™, AtriCure Inc, Cincinnati, OH) also was capable of creating transmural lesions of the modified CMP-IV procedure in a porcine beating heart model with a single ablation.10
Unfortunately, in clinical experience, bipolar clamps were not effective with a single ablation. Multiple ablations were often necessary to achieve conduction block, resulting in a large variation in clinical practice, with different cardiac surgeons advocating between 3–20 ablations. This practice puts patients at risk for both under- and over-ablation. We hypothesized that the ineffective lesions occurred due to low conductance between the electrodes during the entire ablation, which we occasionally observed clinically by the absence of conduction block after an ablation. This often occurred in areas with excessive epicardial fat. However, other factors including fat, char, air, foreign bodies, or folded tissue were also possible factors promoting inadequate lesion formation. Unfortunately, this phenomenon, which we observed in the operating room, could not be replicated in any of our animal models. Solving this conundrum was extremely important, since the success of surgical ablation depends on creating conduction block and transmural lesions. Our laboratory and others have shown that even small gaps in an ablation resulted in ineffective conduction block and could lead to procedural failure.14 Thus, we sought to develop a more clinically relevant experimental model, and settled on using ex-vivo human hearts that were turned down for transplantation. The purpose of this study was to determine the efficacy of the bipolar RF clamp in performing the Cox-Maze IV procedure in this novel model.
Material and Methods
Nine donor human hearts from adults 18 years or older that were rejected for transplantation were used in this study. The hearts were obtained from Mid-America Transplant in St. Louis, Missouri by a trained transplant harvest team. Each heart was arrested using cold cardioplegia solution and the tissue was harvested without excessive dissection. The hearts were immediately transported on ice to the laboratory and studied within one-two hours of harvest. A thermocouple was placed in the atrial septum to perform serial temperature recordings. Myocardial temperature was maintained between 10–20°C in order to simulate clinical ablation conditions. Baseline donor characteristics and gross cardiac tissue appearance were recorded (Table 1).
Table 1.
Baseline characteristics of human heart donors. Hearts rejected for transplantation.
| Human Heart Donor | Age | Gender | BMI | Past Medical History | Cause of Death |
|---|---|---|---|---|---|
| #1 | 62 | Male | 32 | Mitral valve prolapse s/p mitral valve repair, HTN | CVA |
| #2 | 60 | Male | 40 | HTN, HLD, COPD, CAD, schizophrenia | Cardiac arrest |
| #3 | 67 | Female | 27 | HTN, DM, Asthma, mitral valve prolapse | CVA |
| #4 | 46 | Female | 23 | Drug abuse | CVA |
| #5 | 60 | Female | 21 | HTN,COPD | CVA |
| #6 | 34 | Female | 30 | Drug abuse, autoimmune hepatitis, sarcoidosis | CVA |
| #7 | 60 | Male | 47 | HTN | Cardiac arrest |
| #8 | 69 | Female | 25 | Mitral valve prolapse, endocarditis | CVA |
| #9 | 55 | Male | 34 | HTN, HLD, COPD, CAD | CVA |
Abbreviations: HTN, hypertension; HLD, hyperlipidemia; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; CHF, chronic heart failure
The device used in this study was the Isolator Synergy™ Ablation System (AtriCure, Inc., Cincinnati, OH). This non-irrigated, impedance-controlled clamp contained jaws 65 mm in length with dual-electrode pairs embedded in each jaw (Figure 1). Impedance and conductance measurements were analyzed to determine if the ablations reached the CP zone as defined by the manufacturer. In this zone, maximal CP is delivered to the tissue, as the conductance is optimal for safe energy delivery. An ablation reached the CP zone when the measured tissue impedance was 30–70 ohms, or when tissue conductance reached 15–30 millisiemens (mS). The ablation algorithm delivers current specific to the tissue conditions until the conductance reaches a stable minimum level. This has been associated with lesion transmurality in multiple chronic animal studies.7–9,15 The Cox-Maze IV procedure was performed on each heart as anatomy allowed, and included the standard biatrial lesion set (Figure 2). Cryoablation was not performed in this study, and therefore was not used to connect the mitral and tricuspid ablation lines to the valvular annuli or to complete the left atrial isthmus ablation line. The mitral isthmus and both tricuspid annuli ablation lines were performed using the bipolar clamp, with care taken to terminate the tip of the clamp approximately 1–2 cm from the valve annuli, replicating our clinical practice. In the first seven hearts, the lesions were completed by performing a single ablation at each location. The ablation lines in the last two hearts were created by performing two successive ablations without unclamping. The extent of each ablation line was marked with sutures. The electrodes were cleaned between each set of ablations to remove any char or debris. Ablation characteristics were recorded by the Ablation Sensing Unit™ (ASU) (AtriCure Inc., Cincinnati, OH), and the raw data for conductance, impedance, power, and ablation duration were transferred to a specially designed software program for further analysis.
Figure 1.
AtriCure Isolator Synergy bipolar radiofrequency clamp. Courtesy of AtriCure, Inc., used with permission.
Figure 2.
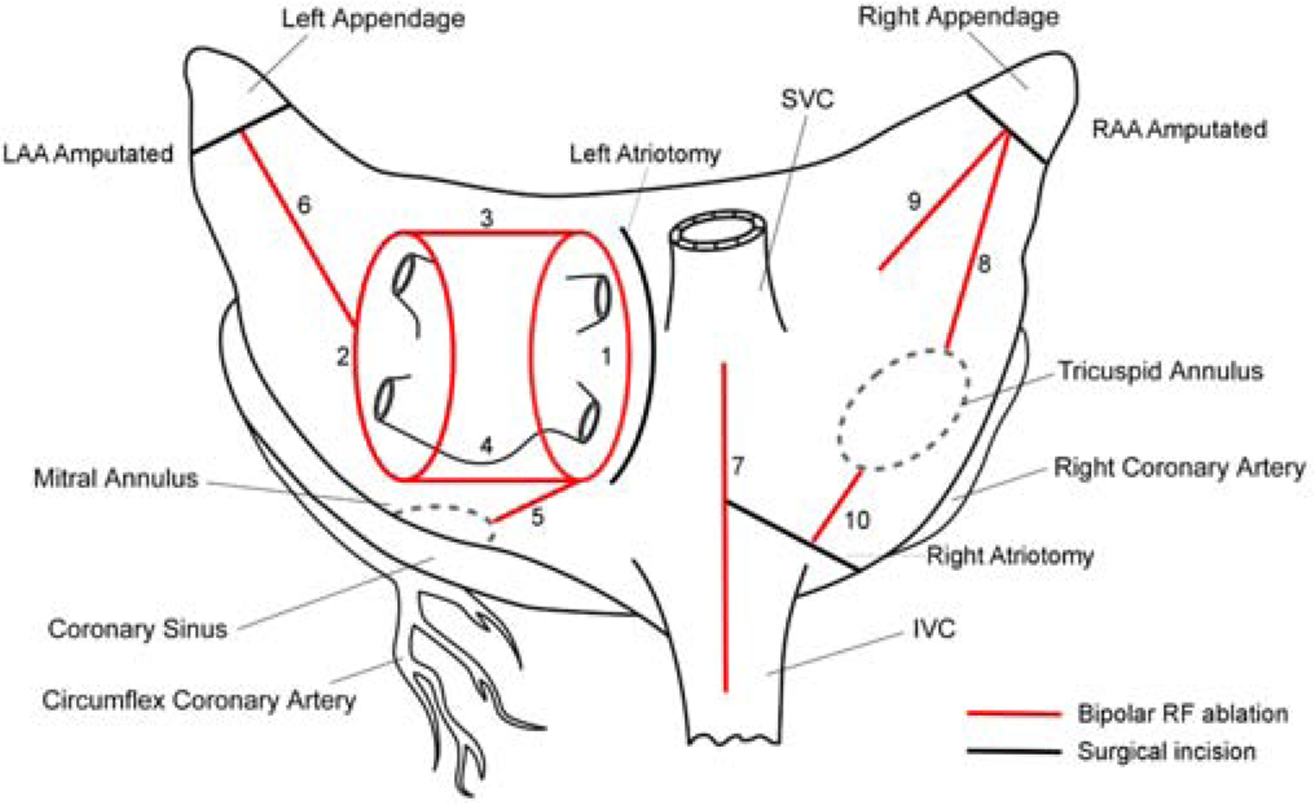
Lesions used for the study. Red line denoting bipolar RF clamp ablation lines. Black line denoting incision sites. The ablation lines are ordered numerically in the order by which they were performed.
After completion of the lesion set, each ablation line was excised and immersed in 1% triphenyl tetrazolium chloride (TTC) for 24 hours. Cross-sections were made as described in previous studies from our laboratory.11,12 Each lesion was sectioned for histology approximately every 5 mm along its length. Each section was photographed using a high-resolution digital camera with a ruler for calibration. The ablation depth and tissue thickness were measured for each section using ImageJ (National Institutes of Health, Bethesda, Maryland, USA).13 A transmural ablation was defined as a continuous area of necrosis (unstained by TTC) from endocardium to epicardium (Figure 3). We examined the transmurality of each histological section, and also for the entire lesion, which included all of the histological sections from a single ablation of the bipolar clamp (usually 7–8 sections). The epicardial fat thickness was measured in the same fashion. The thickness of the unclamped atrial tissue was measured, and defined as the atrial tissue immediately adjacent to the clamped tissue. Descriptive statistics were performed using commercial software (IBM SPSS Statistics, Version 25). Data were expressed as mean ± standard deviation unless otherwise noted. Comparisons were performed using a two-tailed, Student’s t test for normally distributed, continuous variables.
Figure 3.
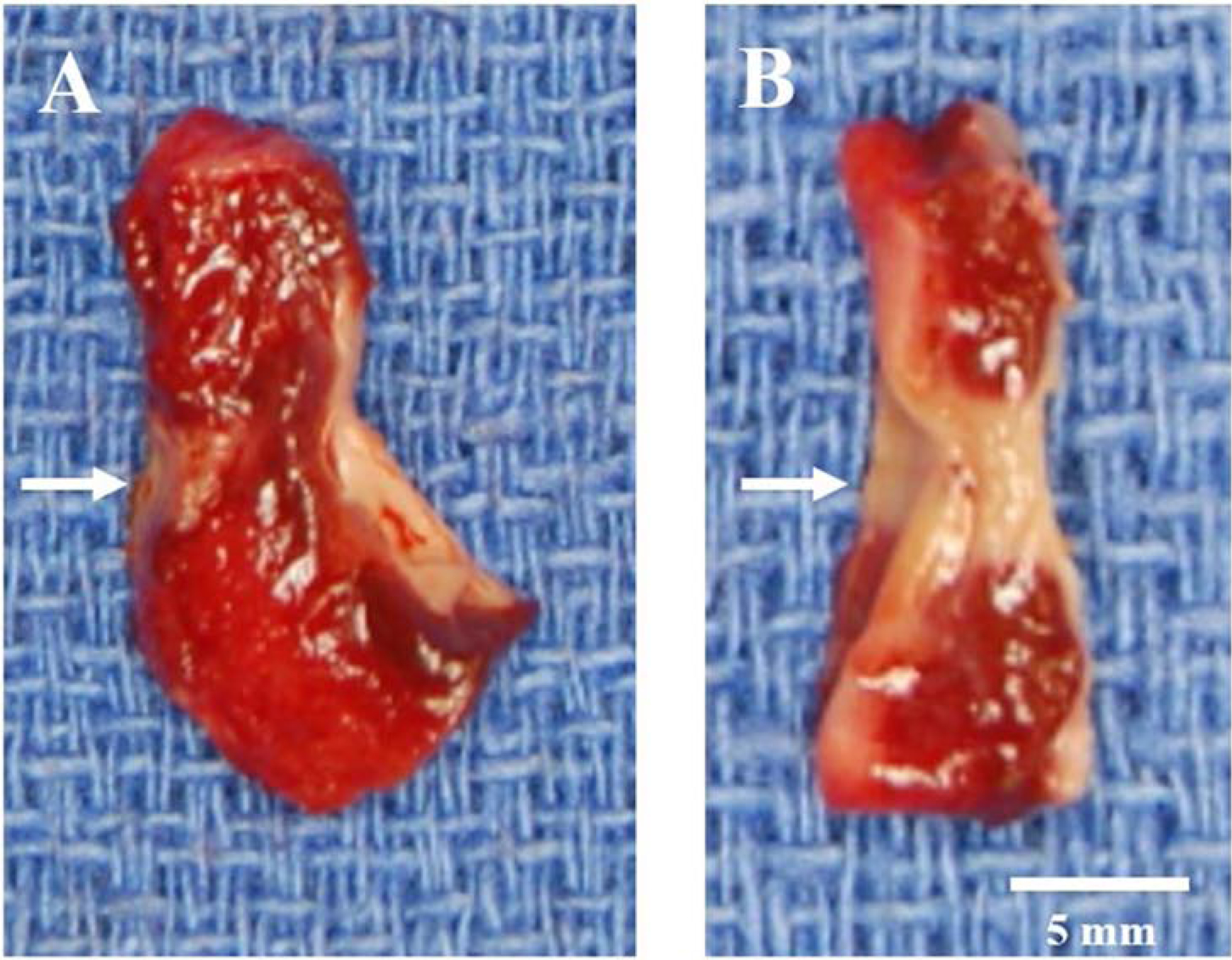
Cross-section samples of non-transmural (A) and transmural (B) ablations after TTC staining. The ablation line is indicated by arrows. Devitalized tissue does not take up TTC, thus the ablations appear white or pale. The non-transmural ablated tissue is indicated by a red color. The endocardial surface is on the right side of the tissue samples.
Results
Baseline Characteristics
The average age of the human donors was 57.0±10.9 years. The majority of the donors were female (56%) and the average BMI was 31.0±8.4 kg/m2. Two human donors had known coronary artery disease, one of whom had previously underwent coronary stent placement. One donor had a previous mitral valve intervention (Table 1).
Lesion Characteristics
A total of 90 lesions and 730 histologic cross-sections were examined from the nine human donor hearts. Two successive ablations without unclamping between each ablation produced a total of 25 lesions and 201 histologic cross-sections. Gross examination revealed all lesions were discrete and identifiable. No tissue disruption, endocardial clot, char, or excess debris were observed for any ablation.
Analysis of the TTC-stained gross photographs for all nine hearts revealed a mean lesion tissue thickness of 1.67±0.77 mm and a lesion depth of 1.47±0.59 mm. The mean lesion width was 3.33±0.98 mm. The average unclamped thickness of the atrial tissue was 2.62±0.93 mm and the mean epicardial fat thickness was 1.21±1.53 mm. When comparing the characteristics of the first seven hearts to the final two hearts, the atrial wall thickness was not significantly different (2.55±0.90 vs 2.82±1.01 mm, P=0.29), and there was no significant difference in epicardial fat thickness (1.31±1.02 vs 0.90±0.76 mm, P=0.14).
Section and Lesion Transmurality
Overall, 670 of the 730 histologic sections (92%) were transmural, and 67/90 lesions (74%) were transmural at every section along their length (Figure 4). In the first seven hearts, during which a single ablation was performed, 466/528 sections (88%) were transmural. Sixty-five percent (42/65) of lesions were transmural at every section along their length. Of the non-transmural ablations, the majority (14/23, 61%) occurred near the AV groove, which included the mitral valve connecting, right atrial appendage to tricuspid valve, and right atriotomy to tricuspid valve lesions. The non-transmural lesions in the first seven hearts, when compared to the transmural lesions, were found to have a greater atrial wall thickness (3.05±0.90 vs 2.25±0.60 mm, P=0.001), greater amount of epicardial fat (2.26±3.63 vs 0.75±1.68 mm, P=0.002), and smaller lesion width (2.47±1.04 vs 3.73±0.72 mm, P<0.001). Representative photographs of the tissue cross-sections found to have both transmural and non-transmural ablations are depicted in Figure 3.
Figure 4.
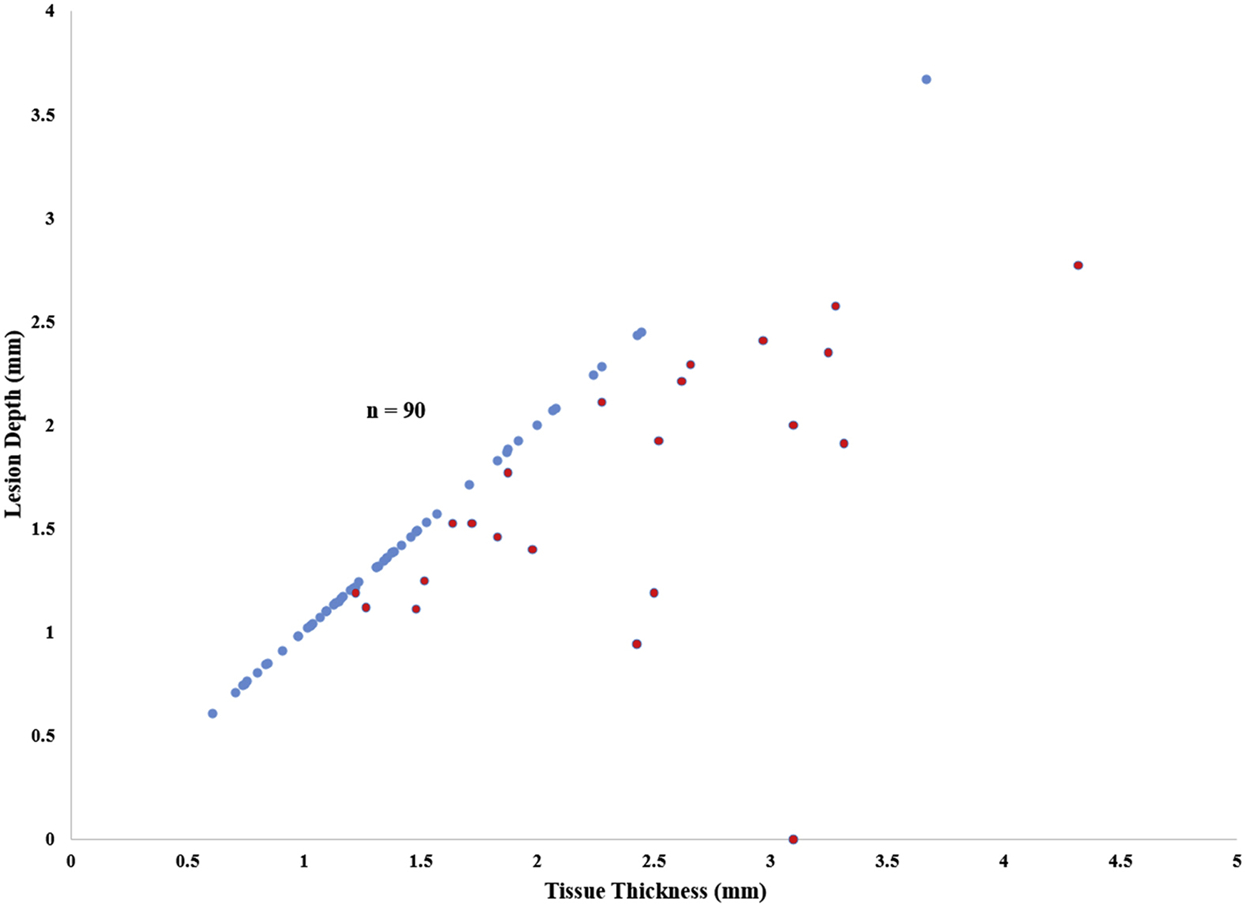
Lesion depth versus tissue thickness by TTC for all lesions performed. Transmural lesions (blue dots) fall along a line of unity; non-transmural lesions (red dots) lie below this line of unity.
In the last two hearts, for which two successive ablations were performed without unclamping, 201 of 201 sections (100%) were transmural, and 25/25 lesions (100%) were transmural at every section along their length (Figure 5). When compared to performing a single ablation using the same device, two successive ablations without unclamping the device significantly improved lesion transmurality (100% vs 65%, P=0.013).
Figure 5.
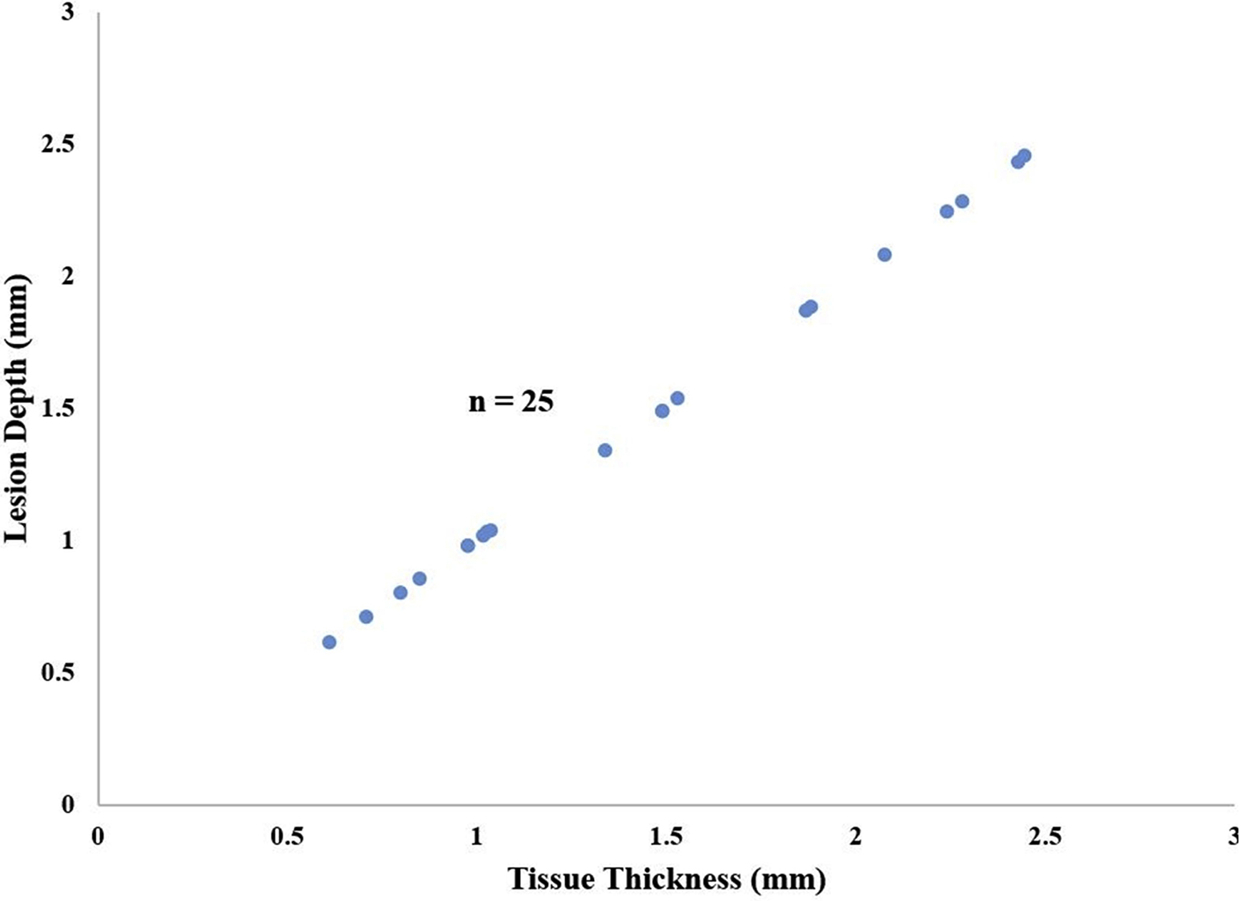
Lesion depth versus tissue thickness by TTC for Heart #8 and 9. Ablations were performed as two successive applications of energy without unclamping the device. Transmural lesions (blue dots) fall along a line of unity; non-transmural lesions (red dots) lie below this line of unity. There were no non-transmural lesions.
Overall, 182 of the 195 (93%) histologic sections from the pulmonary vein lesions were transmural, and 10/14 lesions (71%) were transmural at every section along their length. In the first seven hearts, during which a single ablation was performed, 118/131 sections (90%) were transmural. Sixty percent (6/10) of the lesions were transmural at every section along their length. In the last two hearts, for which two successive ablations were performed without unclamping, 64 of 64 sections (100%) were transmural, and 4/4 lesions (100%) were transmural at every section along their length.
Ablation Characteristics
The ablation durations were not significantly different between transmural and non-transmural lesions (47.4±43.7 vs 32.9±26.9 sec, P=0.12) (Table 2). However, there was a trend towards a greater amount of energy delivered to the tissue in the transmural lesions (539.7±322.4 vs 404.4±373.5 J, P=0.08), though this was not statistically significant. The maximum conductance measured was lower in the non-transmural lesions (18.9±11.3 vs 30.9±9.67 mS, P<0.001).
Table 2.
Ablation characteristics between transmural and non-transmural lesions.
| Transmural Lesions (n = 67) | Non-transmural Lesions (n = 23) | P value | |
|---|---|---|---|
| Tissue Thickness (mm) | 1.39±0.55 | 2.40±0.80 | <0.001 |
| Lesion Depth (mm) | 1.39±0.55 | 1.68±0.65 | 0.07 |
| Lesion Width (mm) | 3.59±0.88 | 2.47±1.02 | <0.001 |
| Ablation Time (s) | 47.4±43.7 | 32.9±26.9 | 0.12 |
| Maximum Power (W) | 22.6±3.19 | 25.9±3.58 | 0.001 |
| Maximum Conductance (mS) | 30.9±9.67 | 18.9±11.3 | <0.001 |
Impedance and conductance measurements for each ablation were analyzed to determine if the ablations reached the CP zone as defined by the manufacturer. In this zone, maximal CP is delivered to the tissue, as the conductance is optimal for safe energy delivery. Of the 42 transmural lesions produced from a single ablation in the first seven hearts, 100% of the ablations reached the CP zone. For non-transmural ablations, only 7/23 (30%) reached the CP zone. While reaching the CP zone did not guarantee transmurality, all lesions (16/16) that did not reach the CP zone were found to be non-transmural. In the lesions in which two successive ablations were performed without unclamping between ablations, only 13/25 (52%) ablations reached the CP zone, despite 100% transmurality in those lesions. Representative conductance graphs from single ablations that reached and did not reach the CP zone are demonstrated in Figure 6.
Figure 6.
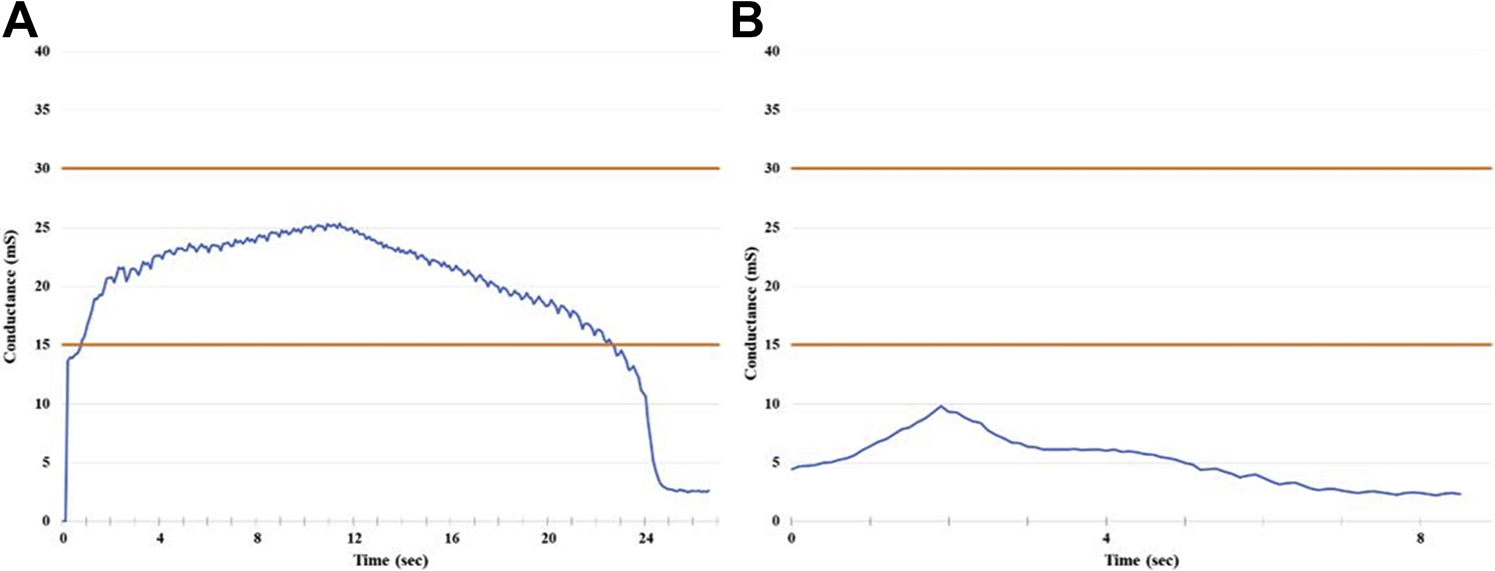
Representative conductance graphs from ablations reaching (A) and not reaching (B) the Constant Power (CP) zone. CP zone (15–30mS) is depicted between the orange lines.
Comment
The surgical treatment of AF has evolved and expanded over the past two decades due to improvements in ablation technology and surgical techniques. Ablation devices have been used to replace surgical incisions, and have both simplified and shortened surgical therapy.5 This has greatly increased the number of surgical ablations performed each year. The ability to consistently create transmural lesions is critical to prevent AF recurrence.14 Our laboratory has previously shown that bipolar radiofrequency clamps were always able to reliably create transmural lesions in animal models.7–9,15 Ablations performed in chronic ovine and canine studies using an early bipolar radiofrequency clamp design produced 100% transmural sections and lesions with conduction block with a single ablation.7–9 A similar experiment using the device tested in this study also produced uniformly transmural lesions and achieved complete electrical isolation with a single ablation.15 Unfortunately, in our clinical experience, the bipolar clamps were not as effective and often required multiple ablations to create conduction block. This resulted in a large variation in ablation practices across the world. We hypothesized that ablations with low initial conductance between the electrodes were associated with ineffective lesion formation, due to inadequate energy delivery to the tissue. In a search for a more relevant experimental model to evaluate this problem, we developed a new and unique model, using donor human hearts rejected for transplantation.
This study investigated the ability of the Isolator Synergy Clamp (AtriCure, Inc., Cincinnati, OH) to reliably create transmural lesions in human atrial tissue. Our results showed that performing a single ablation with the bipolar radiofrequency device resulted in only 88% of histologic sections being transmural, as opposed to the 100% efficacy in animal models. While this was still quite good, it is necessary for every section to be transmural across the entire line of ablation, in order to effectively create conduction block. Examined this way, only 65% of lesions were transmural throughout their entire length. This would result in a failure of more than a third of the lesions to be transmural and would be expected to increase the failure rate of surgical ablation procedures to terminate AF. Factors contributing to non-transmurality in this study included regions of thicker atrial tissue and the presence of more epicardial fat. As we had hypothesized, non-transmural lesions had a significantly lower conductance than transmural ones (18.9±11.3 vs 30.9±9.67 mS, P<0.001) and they were often in areas with excess epicardial fat.
Fat is known to have a high resistance and impedes RF lesion formation.16 This resulted in inadequate energy delivery to the tissue and non-transmural ablations. Variable thickness of tissue may also have resulted in poor approximation of the jaws of the clamp, impeding conduction in those regions, as seen by a smaller lesion width (2.47±1.04 vs 3.74±0.72 mm, P<0.001) in non-transmural compared to transmural sections.
In order to overcome the shortcomings of bipolar clamps in areas of thick and fatty tissue, we tested a simple strategy of performing two successive ablations without unclamping the device. The hypothesis was that the second ablation would selectively drive the energy into the remaining viable tissue which would have a lower conductance than the previously ablated nonviable tissue. An alternative possibility was that there would be improved thermal conductive heat transfer during the second ablation.
Our results showed that by performing two successive ablations with the bipolar clamp, 100% of both the histologic sections and ablation lesions were transmural. This technique was successful in reliably creating a transmural ablation regardless of the thickness of the atrial tissue, presence of epicardial fat, or location of the ablation line. Performing two successive ablations without unclamping the device did not result in any adverse events, such as atrial perforation or excessive char formation.
It should be noted that early research into RF ablation of cardiac tissue highlighted that the heterogeneous nature of the cardiac tissue required wide variation in the amount of energy required to reach transmurality.17 The ablation algorithm associated with this clamp limits the power delivery to minimize the risk of over-delivery of energy, and therefore excess char formation and perforation. While this has been effective in preventing complications, we hypothesized that in certain applications where the maximum conductance seen during the ablation is limited by epicardial fat or other factors, the device may not deliver enough energy. However, we found that repeating the ablation cycle without unclamping the device was able to overcome this limitation.
Analysis of the impedance and conductance data for each ablation revealed that in transmural lesions produced by single ablation, 100% of the ablations reached the CP zone. However, reaching the CP zone did not guarantee transmurality, as 30% of non-transmural ablations reached the required conductance threshold for optimal power delivery. Interestingly, the technique of performing two successive ablations without unclamping resulted in 100% transmurality, regardless of whether the CP zone was reached. Thus, it is not necessary to focus on the conductance during ablation if this technique is employed.
In summary, a single ablation with the bipolar RF clamp did not result in consistent transmural ablation, with over one third of lesions not being transmural. Performing two successive ablations without unclamping the device produced transmural lesions in 100% of histologic sections in ex-vivo human hearts. The technique reliably produced transmural lesions in this ex-vivo human heart model regardless of tissue characteristics (i.e. presence of epicardial fat, atrial wall thickness) or ablation location. Based on these findings, surgeons performing AF ablation with non-irrigated bipolar RF clamps should consider employing a double ablation technique to increase the likelihood of achieving conduction block. This ablation strategy should improve clinical outcomes with surgical ablation. Further clinical data are needed to verify these experimental observations in a limited number of explanted hearts.
Limitations
This study had several limitations. The experiment was performed on explanted human hearts procured using rigorous protocols. While very similar, these conditions were not exactly identical to those experienced clinically during surgical ablation. However, this study was unique in that we utilized explanted human tissue, and did our best to simulate the temperature and environment of a cardioplegically-arrested heart. Additionally, a detailed cardiac history was not able to be obtained for some of the human heart donors, and therefore differences in underlying cardiac pathologies between specimens may have existed. The pathologic human atrial wall has been shown to differ significantly from healthy atrial myocardium in thickness, degree of fibrosis, and amount of epicardial fat, all of which could impact energy delivery and lesion transmurality.18 Another limitation was the relatively small number of hearts testing the double ablation algorithm. This was due to the limited availability of these hearts. However, we did examine over 200 histologic sections and there was a significant difference in lesion efficacy when compared to a single ablation. Despite these shortcomings, these results have mirrored our clinical experience, and this model is more clinically relevant than an animal model. Finally, the present study was an acute study, and it is possible that transmurality could be different in a chronic model. However, this would not be possible using this human tissue model. Previous studies from our laboratory have not identified any differences in acute and chronic transmurality when using a bipolar device.19
Acknowledgement:
The authors acknowledge the technical assistance of Dr. Michael K. Pasque and the staff of Mid-America Transplant in the procurement of the donated human heart specimens.
This study was sponsored by AtriCure, Inc., the manufacturer of the device. The authors attest that they have examined all data and had full freedom of investigation and freedom to publish the results regardless of the outcome of the study. Expenses for Washington University staff were paid through a research grant to Dr. Ralph J. Damiano, Jr. from AtriCure Inc. Dr. Ali Khiabani and Dr. Robert MacGregor were supported by NIH grant T32-HL007776. Dr. Ralph J. Damiano Jr. and Dr. Richard B. Schuessler were supported by NIH grant R01-HL032257 and the Barnes Jewish Hospital Foundation. Dr. Richard B. Schuessler was also supported by a research grant from Atricure, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox JL, Schuessler RB, D’agostino HJ, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101(4):569–83. [PubMed] [Google Scholar]
- 2.Cox JL, Schuessler RB, Boineau JP. The development of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12(1):2–14. [DOI] [PubMed] [Google Scholar]
- 3.Prasad S, Maniar H, Camillo C, et al. The Cox Maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126(6):1822–8. [DOI] [PubMed] [Google Scholar]
- 4.Melby SJ, Schuessler RB, Damiano RJ. Ablation technology for the surgical treatment of atrial fibrillation. ASAIO J. 2013;59(5):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ, Jr. The Cox-maze IV procedure in its second decade: still the gold standard? European Journal of Cardiothoracic Surgery. 2018;53(suppl_1):i19–i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melby SJ, Damiano RJ Jr.. Chapter 58. Surgery for Atrial Fibrillation In: Cohn LH. eds. Cardiac Surgery in the Adult, 4e New York, NY: McGraw-Hill;2012. [Google Scholar]
- 7.Prasad SM, Maniar HS, Schuessler RB, Damiano RJ Jr. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg. 2002; 124:708–713. [DOI] [PubMed] [Google Scholar]
- 8.Prasad SM, Maniar HS, Diodato MD, Schuessler RB, Damiano RJ Jr. Physiological consequences of bipolar radiofrequency energy on the atria and pulmonary veins: A chronic animal study. Ann Thorac Surg. 2003; 76:836–841. discussion 841. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor SL, Ishii Y, Diodato MD, et al. Successful performance of Cox-Maze procedure on beating heart using bipolar radiofrequency ablation: A feasibility study in animals. Ann Thorac Surg. 2004;78:1671–77. [DOI] [PubMed] [Google Scholar]
- 10.Voeller RK, Zierer A, Schuessler RB, Damiano RJ. Performance of a novel dual-electrode bipolar radiofrequency ablation device: a chronic porcine study. Innov Phila Pa. 2011;6:17–22. [DOI] [PubMed] [Google Scholar]
- 11.Lee AM, Aziz A, Sakamoto S, et al. Epicardial ablation on the beating heart: Limited efficacy of a novel, cooled radiofrequency ablation device. Innovations. 2009; 4:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schill M, Melby S, Speltz M, et al. Evaluation of a Novel Cryoprobe for Atrial Ablation in a Chronic Ovine Model. Ann Thorac Surg. 2017;104(3):1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. 2012. Nature methods;9(7):671–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melby SJ, Lee AM, Zierer A, et al. Atrial fibrillation propagates through gaps in ablation lines: Implications for ablative treatment of atrial fibrillation. Heart Rhythm. 2008; 5:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voeller RK, Zierer A, Schuessler RB, Damiano RJ. Performance of a novel dual-electrode bipolar radiofrequency ablation device: a chronic porcine study. Innov Phila Pa. 2011;6:17–22. [DOI] [PubMed] [Google Scholar]
- 16.Berjano E, Hornero F. Thermal-Electrical Modeling for Epicardial Atrial Radiofrequency Ablation. IEEE Transactions on Biomedical Engineering. 2004;51(8):1348–57. [DOI] [PubMed] [Google Scholar]
- 17.Melby SJ, Zierer A, Voeller RK, et al. Wide variations in energy delivery using an impedance-controlled algorithm in bipolar radiofrequency ablation: evidence against fixed time ablation. Innov Phila Pa. 2007;2(2):67–72. [DOI] [PubMed] [Google Scholar]
- 18.Platonov PG, Ivanov V, Ho SY, et al. Left atrial posterior wall thickness in patients with and without atrial fibrillation: Data from 298 consecutive autopsies. Journal of cardiovascular electrophysiology. 2008; 19:689–692. [DOI] [PubMed] [Google Scholar]
- 19.Lee A, Aziz A, Clark K, et al. Chronic Performance of a Novel Radiofrequency Ablation Device on the Beating Heart: Limitations of Conduction Delay to Assess Transmurality. J Thorac Cardiovasc Surg. 2012;144(4):859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


