Abstract
Rationale & Objective
Evidence is limited on how estimated glomerular filtration rate (eGFR) and urine albumin-creatinine-ratio (UACR) relate to dementia at different ages. We evaluated eGFR and UACR in midlife and older age as risk factors for dementia. Additionally, we assessed whether the association between eGFR and dementia was altered when cystatin-C and beta-2-microglobulin (B2M) were used for GFR estimation.
Study Design
Prospective Cohort Study.
Setting & Participants
Two baselines from the Atherosclerosis Risk in Communities (ARIC) Study were used: Visit 4 (1996–1998) including 9967 participants, 54–74 years old, and visit 5 (2011–2013) including 4626 participants, 70–90 years old. Participants were followed until 2017.
Predictors
Log-UACR and eGFR based on creatinine, cystatin-C, creatinine and cystatin-C, and B2M.
Outcome
Incident dementia.
Analytical Approach
Multivariable Cox proportional hazards regression models fit separately for each of the 5 predictors and based on a change in the predictor equivalent to the interquartile range (IQR) for that predictor at visit 4. eGFR models were adjusted for log-UACR and log-UACR models were adjusted for eGFR cystatin-C.
Results
We observed 1821 dementia cases after visit 4 and 438 cases after visit 5. Dementia risk increased with higher albuminuria levels (HR [95%-CI] per Visit 4 IQR [equivalent to a 4.2 fold increase in the log albuminuria], 1.15 [1.09–1.21] after Visit 4; and 1.27 [1.13–1.42] after Visit 5). An association with lower eGFR was only seen for cystatin-C (HR per Visit 4 IQR [equivalent to a 24.3 m/min decrease], 1.12 [1.04–1.21] Visit 4; and 1.30 [1.12–1.52] after Visit 5) and B2M (HR per Visit 4 IQR [equivalent to 18.3 ml/min decrease], 1.15 [1.07–1.23] after Visit 4; and 1.34 [1.17–1.55] after Visit 5). Differences between these associations in midlife and older age were not statistically significant.
Limitations
Changes of potentially time-varying covariates were not measured. Dementia was not sub-classified by etiology.
Conclusions
Albuminuria was consistently associated with dementia incidence. Lower eGFR based on cystatin-C or B2M, but not creatinine, was also associated with dementia. Risk associations were similar when CKD markers were measured at midlife and older age.
Keywords: Albuminuria, Atherosclerosis Risk in Communities (ARIC) Study, beta-2-microglobulin (B2M), chronic kidney disease (CKD), creatinine, cystatin-C, albuminuria, estimated glomerular filtration rate (eGFR), dementia, midlife, older age, urine albumin-creatinine-ratio (UACR)
PLAIN LANGUAGE SUMMARY:
To understand chronic kidney disease as a risk factor for dementia in different age groups, we measured glomerular filtration rate (GFR) using different biomarkers as well as albuminuria in participants of the Atherosclerosis Risk in Communities (ARIC) Study at two different time points. The participants, who were aged 54–74 years at the first and aged 70–90 years at the second time point, were then observed over several years. We demonstrated that elevated urinary albumin was similarly associated with an increased risk of dementia in both of these age groups. In addition, estimates of kidney function based on beta-2-microglobulin and cystatin-C may also be associated with dementia while more traditional estimates of kidney function based on serum creatinine were not.
INTRODUCTION
Dementia and cognitive decline are a growing public health problem in older adults leading to reduced quality of life, loss of independence, heavier caregiver burden and premature mortality.1 Besides the major personal toll, healthcare costs of dementia are estimated to be more than $150 billion annually in the US, a financial burden comparable to heart disease or cancer.2 Therefore, the identification of high-risk patient groups and possible risk factors is vital to achieve better surveillance, earlier diagnosis, and eventually improved prevention and treatment strategies.3
Patients with chronic kidney disease (CKD) have been shown to have an increased risk of dementia and cognitive impairment, with more advanced CKD stages exhibiting more severe cognitive decline.4, 5 More specifically, recent longitudinal studies have focused on the association of incident dementia with reduced estimated glomerular filtration rate (eGFR) as well as increased urine albumin-creatinine-ratio (UACR), which combined are used in the definition of CKD; these studies reported a sizable relation between UACR and dementia while the association between eGFR and dementia was weaker or non-existent.6–10 In previous studies, the equation used for GFR estimation was generally based on creatinine, which is widely used in routine clinical practice but can potentially be influenced by non-GFR determinants of creatinine, such as unusual muscle mass, diet with high meat content or dietary supplements containing creatine.11 Especially reduced muscle mass is a concern in elderly patients, which may lead to an overestimation of GFR and, by not accurately determining low GFR readings, obscure a potential association with dementia.12 Recently, novel biomarkers, such as cystatin-C and beta-2-microglobulin (B2M) have been introduced, and GFR estimating equations based on these markers have been validated.13 Additionally, there is limited evidence on the strength of association between CKD markers and dementia when those markers are measured at different age groups. Certain vascular risk factors, such as hypertension, abnormal body mass index (BMI) and elevated blood glucose in midlife, but not in older age, have been reported to increase the risk of dementia and neuro-degenerative brain damage.14–16 However, the age association for risk factors may not be seen for biomarkers of disease, and a similar age-comparison with CKD measures has not been performed to date.
In this study, we compared the relationship of eGFR and UACR as risk factors for dementia when they are measured at two different time points 15 years apart in the Atherosclerosis Risk in Communities (ARIC) Study cohort. We examined whether these CKD measures are more strongly associated with dementia when measured at midlife as opposed to older age, in a similar fashion as has been found previously for vascular risk factors.14–17 Since GFR estimation based on creatinine can potentially be influenced by non-GFR determinants, we also assessed whether the association between eGFR and dementia is altered when novel markers like cystatin-C and B2M are used instead.
METHODS
Study Population
In 1987–1989, the prospective ARIC cohort study enrolled 15792 participants aged 44–66 years from 4 US communities (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs; Minnesota).18 ARIC participants attended 3 study visits between 1986 and 1995 followed by visit 4 (1996–1998), which was the first visit with simultaneous assessment of eGFR and UACR, and therefore was a baseline for the present analysis. As part of the ARIC Neurocognitive Study (NCS), eGFR and UACR were measured again at visit 5 (2011–2013), considered baseline 2 in this analysis.
At visit 4 (11656 participants), all participants with end-stage renal disease (ESRD), stroke, prevalent dementia, missing information about education level, missing measures of chronic kidney disease (CKD) as well as non-white and non-African-American participants were excluded. Therefore, the baseline 1 sample included 9967 participants (Figure S1). At visit 5 (6538 participants), the same exclusion criteria led to 4626 participants in the baseline 2 sample (Figure S2).
This study was approved by the institutional review boards at the study sites and all participants gave written informed consent.
Exposures: Measures of Chronic Kidney Disease
The exposures of interest were UACR and eGFR at ARIC visits 4 and 5. Urine albumin was measured from spot urine samples, frozen and stored at −70°C by a nephelometric method with either the Dade Behring BN-100 (Dade Behring / Siemens Healthcare Diagnostics, Tarrytown, NY, USA) or the Beckman Nephelometer (Beckman Coulter, Brea, CA, USA). Creatinine was measured in plasma specimens using a modified kinetic Jaffé method, calibrated to the Cleveland Clinic laboratory measurements and standardized to an isotope-dilution mass spectrometry–traceable method.19, 20 Cystatin-C and B2M were measured in plasma specimens using a particle-enhanced immuno-nephelometric assay (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) and also calibrated and standardized.21 The estimation of GFR was based on the CKD Epidemiology Collaboration (CKD-EPI) equations using creatinine 22, cystatin-C, both alone and in combination with creatinine 23, and B2M.24
Outcome: Dementia Incidence
Dementia incidence was classified according to 3 levels:25 Level 1 is based on data from longitudinal cognitive evaluations performed at ARIC visits 2 (1990–1992), 4 (1996–1998), 5 (2011–2013), and 6 (2016–2017), a complete neuro-psychological battery at visits 5 and 6 and informant interviews with expert classification of cognitive status. Standardized definitions of dementia were computer-generated based on these data elements and confirmed by the expert panel.25 Level 2 includes all level 1 cases with additional diagnoses based on Telephone Interviews for Cognitive Status-Modified (TICSm) of participants not attending visits 5 or 6. For participants unable to be interviewed over the phone, informant interviews were used when dementia was suspected or could not be ruled out 25.
Level 3, which is the outcome of interest in this analysis, includes levels 1 and 2 as well as dementia ascertainment using surveillance based on a prior discharge hospitalization International Classification of Diseases-9 (ICD-9) or death certificate code for dementia through the date of last participant contact, up to completion of the ARIC visit 6 period.17, 26 The number of incident events reported via the different levels of dementia events are summarized in Table S1.
Covariates
The covariates included in statistical models were known dementia risk factors.27 Sex, race, date of birth, education level (less than high school, high school graduate or general equivalency diploma, beyond high school) and smoking status (current smoker, former smoker, never smoker) were self-reported. Additional adjustment variables were apolipoprotein E (APOE) ε4 genotype (0, 1 or 2 alleles; genotyping was performed using the TaqMan assay (Applied Biosystems, Foster City, CA, USA)), BMI (kg/m2), diabetes (fasting glucose level ≥126 mg/dl with serum glucose assessed by the hexokinase method, self-reported physician-diagnosed diabetes or use of diabetes medication), hypertension (systolic blood pressure >140 mm-Hg, diastolic blood pressure >90 mm-Hg or use of anti-hypertensive medication) and C-reactive protein (CRP, measured in plasma frozen and stored at –70°C with the immuno-turbidimetric assay using the Siemens BN-II analyzer (Siemens, Deerfield, Il, USA)). For non-time varying covariates (sex, race, date of birth, education level, APOE genotype), the information was obtained at ARIC visit 1. For all other covariates, the measurements were taken at the two baseline index visits used for this statistical analysis (ARIC visits 4 and 5).
Statistical Analysis
All statistical analyses were performed using Stata Version 15.1 (StataCorp LLC, College Station, TX, USA). The primary outcome was time to onset of dementia according to the level 3 classification of the ARIC study 26 after midlife (study visit 4 as baseline 1) and older age (study visit 5 as baseline 2).
Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (95%-CI). Participants without the primary outcome were censored at the latest date among either visit 6, TICSm/informant interview (when available) or at the date of last participant contact up to December 31st, 2017. Results of the Cox proportional hazards regression analyses were presented either as HRs per a change in the predictor equivalent to the interquartile range (IQR) at visit 4 for that respective predictor, or as relative hazards comparing predictor categories. Models were adjusted for the covariates described above, but only the analyses of eGFR (B2M) were adjusted for CRP as B2M has been reported to be associated with inflammation.28–30 A p-value of <0.05 was considered statistically significant for all analyses and tests for significance were 2-sided.
To address potential bias caused by an overlap of the two observational periods, one between visit 4 and 6 and the other between visit 5 and 6, a sensitivity analysis was performed; comparing these periods with the observational period between visit 4 and 5. In an additional analysis, all models using eGFR as predictor were fitted with a linear spline knot at 60 ml/min per 1.73 m2 to address a potential non-linear association between eGFR measures and incident dementia.
RESULTS
Participant Characteristics
At ARIC study visit 4, we included 9967 participants without prevalent dementia, ESRD or previous stroke (Figure S1). The mean (standard deviation, SD) age was 62.8 (5.7) years, 5701 (57.2%) participants were female and 2029 (20.4%) participants were African-American. After visit 4, we observed 1821 cases of incident dementia over a median (interquartile range, IQR) follow-up time of 18.4 (14.4– 19.5) years, equating to 11.2 events per 1000 person-years. At ARIC study visit 5, we included 4626 participants without prevalent dementia, ESRD or previous stroke (Figure S2). The mean (SD) age was 75.5 (5.2) years, 2637 (57.0%) were female and 1003 (21.7%) were African-American. After visit 5, we observed 438 cases of incident dementia over a median (IQR) follow-up of 4.6 (4.0–5.1) years, equating to 21.4 events per 1000 person-years.
Visit 5 participants were on average 12.7 years older, more likely to have diabetes and use antihypertensive medication, showed higher levels of albuminuria and lower eGFR compared to participants of visit 4 (Table 1). Patient characteristics at visit 4 and visit 5 stratified by eGFR and UACR categories are provided in Tables S2-S5.
Table 1:
Characteristics of the study population at baseline visit 4 (1996–1998) and baseline visit 5 (2011–2013), the Atherosclerosis Risk in Communities (ARIC) Study cohort.
| Visit 4 (1996–1998) N = 9967 |
Visit 5 (2011–2013) N = 4626 |
|
|---|---|---|
| Age, years, mean (SD) | 62.8 (5.7) | 75.5 (5.2) |
| Female | 5701 (57.2) | 2637 (57.0) |
| African-American | 2029 (20.4) | 1003 (21.7) |
| Education | ||
| <High school | 1850 (18.6) | 599 (12.9) |
| High school or GED | 4243 (42.6) | 1908 (41.2) |
| >High school | 3874 (38.9) | 2119 (45.8) |
| Smoking | ||
| Current | 1413 (14.2) | 256 (5.7) |
| Former | 4312 (43.5) | 2165 (47.9) |
| Never | 4195 (42.3) | 1708 (37.8) |
| Apolipoprotein E4 status | ||
| TT = 0 Alleles | 6743 (69.9) | 3236 (72.2) |
| CT = 1 Allele | 2651 (27.5) | 1167 (26.1) |
| CC = 2 Alleles | 247 (2.6) | 80 (1.8) |
| Body mass index (BMI), kg/m2 | ||
| Underweight (<18.5) | 73 (0.7) | 45 (1.0) |
| Normal weight (18.5 – 25) | 2523 (25.3) | 1091 (23.5) |
| Overweight (25 – 30) | 3934 (39.5) | 1793 (38.8) |
| Obese (>30) | 3437 (34.5) | 1697 (36.7) |
| Diabetes | 1543 (15.5) | 1459 (32.3) |
| Systolic blood pressure, mmHg, mean (SD) | 133.9 (21.2) | 130.5 (18.1) |
| Antihypertensive medication | 4165 (41.8) | 3470 (75.1) |
| C-reactive protein (CRP), mg/l, median (IQR) | 2.4 (1.1, 5.4) | 2.0 (1.0 – 4.2) |
| Measures of kidney function | ||
|
Estimated Glomerular Filtration Rate (eGFR), ml/min per 1.73m2, median (IQR) |
||
| CKD-EPI, Creatinine | 89.2 (76.7, 96.2) | 70.9 (58.2, 83.0) |
| CKD-EPI, Cystatin-C | 85.2 (73.0, 97.3) | 60.9 (47.9, 74.7) |
| CKD-EPI, Creatinine + Cystatin-C | 87.3 (76.6, 97.1) | 66.1 (53.8, 78.3) |
| CKD-EPI, Beta-2-Microglobulin | 75.3 (66.4, 84.6) | 64.0 (54.0, 73.4) |
|
Urine albumin-to-creatinine ratio (UACR), mg/g Creatinine, median (IQR) |
3.7 (1.8, 7.5) | 10.3 (6.3, 21.7) |
Data are presented as number (%) unless otherwise specified.
Association of Kidney Disease Measures with Incident Dementia
Participants with higher levels of albuminuria at baseline had an increased risk of incident dementia with an adjusted HR of 1.15 [95%-CI, 1.09–1.21] per V4-IQR-fold higher log-urine albumin-creatinine-ratio (log-UACR) after visit 4 and 1.27 [ 1.13–1.42] after visit 5.
Lower levels of eGFR were similarly associated with incident dementia, but the association was statistically significant only when the estimation was based on either cystatin-C (HR per V4-IQR, 1.12 [1.04–1.21] after visit 4 and 1.30 [1.12–1.52] after visit 5) or B2M (HR per V4-IQR, 1.15 [1.07–1.23] after visit 4 and 1.34 [1.17–1.55] after visit 5). Estimated GFR based on creatinine in combination with cystatin-C was also associated with incident dementia but was statistically significant only after visit 5, while no such association was evident for eGFR based on creatinine alone (Table 2). Alternative models of eGFR with linear spline-term knots at 60 ml/min per 1.73 m2 did not indicate a non-linear association between eGFR measures and incident dementia (Table S6). When assessing dementia risk according to standard categories of eGFR and UACR, an increase was visible in categories of higher UACR and lower eGFR (Tables S7 and S8).
Table 2:
Adjusted hazard ratios (95%-CI) of dementia incidence after midlife (baseline visit 4, ages 54–74 years during 1996–1998) and after older age (baseline visit 5, ages 70–90 years during 2011–2013), by measures of CKD, the Atherosclerosis Risk in Communities (ARIC) Study cohort.
| Measure of CKD | Baseline visit 4, ages 54–74 years | Baseline visit 5, ages 70–90 years | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| eGFR CKD-EPI, Creatinine | 0.98 (0.92–1.04) | 0.98 (0.91–1.04) | 1.11 (0.99–1.24) | 1.06 (0.94–1.19) |
| per V4-IQR (19.4ml/min) decrease | ||||
| eGFR CKD-EPI, Cystatin-C | 1.16 (1.07–1.25)* | 1.12 (1.04–1.21)* | 1.33 (1.16–1.53)* | 1.30 (1.12–1.52)* |
| per V4-IQR (24.3ml/min) decrease | ||||
| eGFR CKD-EPI, Creatinine and Cystatin-C | 1.08 (1.01–1.16)* | 1.07 (0.99–1.14) | 1.23 (1.09–1.38)* | 1.19 (1.04–1.35)* |
| per V4-IQR (20.7ml/min) decrease | ||||
| eGFR CKD-EPI, Beta-2-Microglobulin | 1.18 (1.11–1.27)* | 1.15 (1.07–1.23)* | 1.36 (1.20–1.54)* | 1.34 (1.17–1.55)* |
| per V4-IQR (18.3ml/min) decrease | ||||
| Log Urine albumin-to-creatinine ratio | 1.19 (1.13–1.25)* | 1.15 (1.09–1.21)* | 1.32 (1.18–1.46)* | 1.27 (1.13–1.42)* |
| per V4-IQR (4.2) – fold increase | ||||
Model 1 adjusted for age, sex, race, education level and Apolipoprotein E4 level.
Model 2 adjusted for age, sex, race, education level, Apolipoprotein E4 level, smoking, body mass index, diabetes and antihypertensive medication. eGFR values adjusted for log-UACR and log-UACR values adjusted for eGFR (cystatin-C). B2M additionally adjusted for CRP.
Baseline visit 4: N = 9,967 patients, 1,821 events / 162,644 person-years.
Baseline visit 5: N = 4,626 patients, 438 events / 20,497 person-years.
p < 0.05
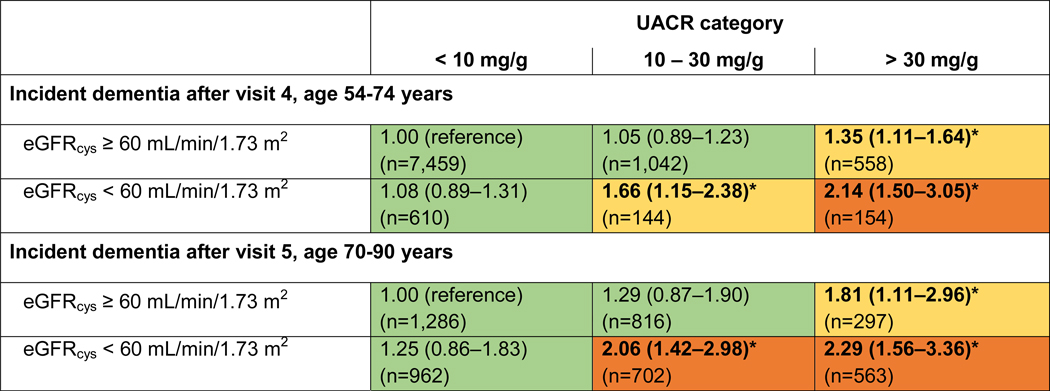
Both UACR and eGFR were included in the same statistical model demonstrating that they are both independent risk factors for dementia. Modeling the combined effects of UACR and eGFR as categorical variables showed the highest dementia risk if participants had both high levels of UACR (>30 mg/g) and low levels of eGFR (<60 ml/min per 1.73 m2). Compared to the reference group (UACR <10 mg/g; eGFR ≥60 ml/min per 1.73 m2), the HR of incident dementia was increased more than two-fold, both after visit 4 and visit 5. This result was largely consistent, whether eGFR was estimated using cystatin-C alone (Figure 1), creatinine alone, creatinine + cystatin-C or B2M (Figues S3-S5).
Figure 1:
Adjusted relative hazards (95%-CI) of dementia incidence after midlife (baseline visit 4, ages 54–74 years during 1996–1998) and after older age (baseline visit 5, ages 70–90 years during 2011–2013), by eGFR (cystatin-C) and UACR categories, the Atherosclerosis Risk in Communities (ARIC) Study cohort.
Models adjusted for age, sex, race, education level, Apolipoprotein E4 level, smoking, body mass index, diabetes and antihypertensive medication.
*p < 0.05
Dementia Risk Factors in Midlife Compared with Older Age
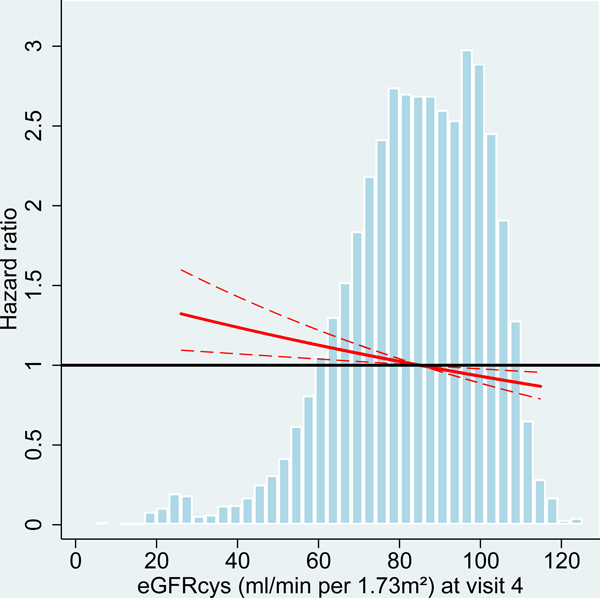
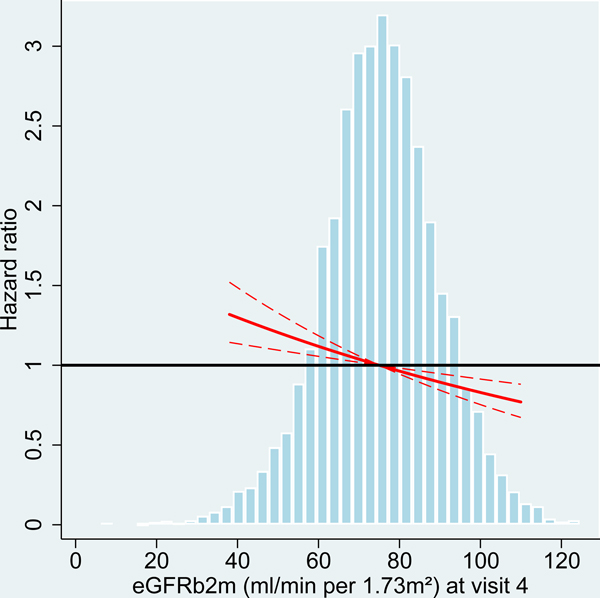
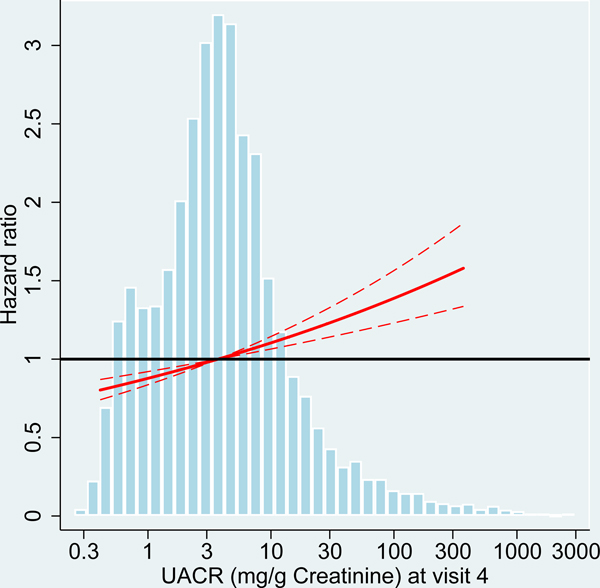
We analyzed risk factors for incident dementia at two different ARIC study visits with participants’ age ranging from 54–74 years (visit 4) and 70–90 years (visit 5). At older age (visit 5), average kidney function among the participants was reduced with lower levels of eGFR and higher levels of UACR compared to midlife (visit 4 participants; Table 1, Figure 2). Comparing the associations of kidney disease measures with incident dementia between the study visits, effect sizes were consistently larger at older age for both albuminuria and eGFR, albeit the differences were not statistically significant) (Table 2, Figure 2).
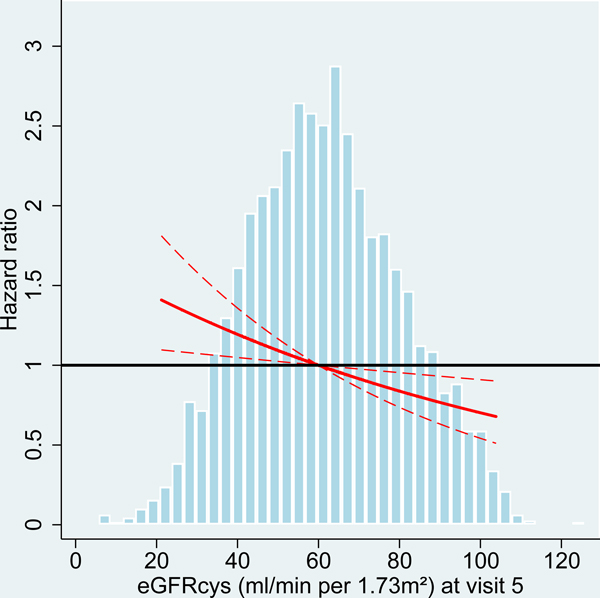
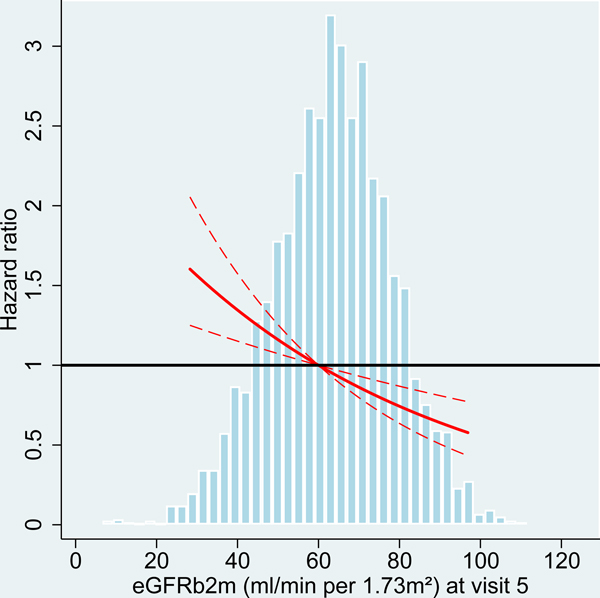
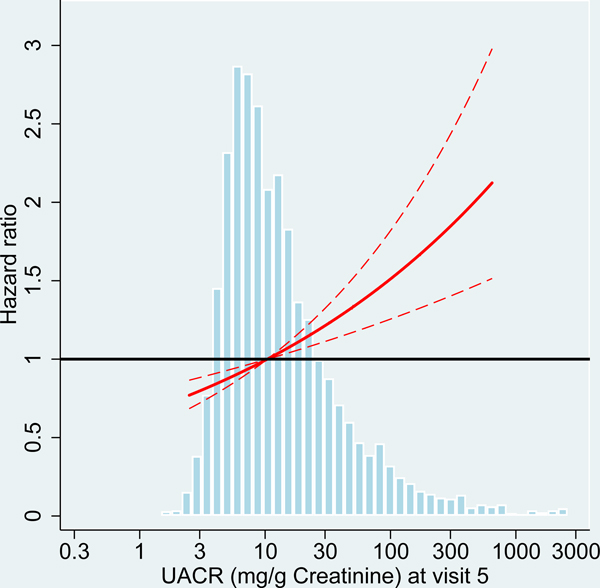
Figure 2:
Hazard ratio for dementia incidence after midlife (baseline visit 4, ages 54–74 years during 1996–1998) and after older age (baseline visit 5, ages 70–90 years during 2011–2013), by measures of kidney disease at baseline visit, the Atherosclerosis Risk in Communities (ARIC) Study cohort.
Panels A-C: HRs with 95%-CI of dementia incidence after visit 4 by measures of kidney disease at visit 4: eGFR based on cystatin-C (A), eGFR based on B2M (B) and UACR (C).
Panels D-F: HRs with 95%-CI of dementia incidence after visit 5 by measures of kidney disease at visit 5: eGFR based on cystatin-C (D), eGFR based on B2M (E) and UACR (F).
UACR log (base e) transformed. Models adjusted for age, sex, race, education level, Apolipoprotein E4 level, smoking, body mass index, diabetes and antihypertensive medication. eGFR values adjusted for log-UACR and log-UACR values adjusted for eGFR (cystatin-C). B2M additionally adjusted for CRP. Regression lines are truncated at the 1st and 99th percentile. The frequency distribution of the respective kidney disease measure at each baseline is shown in the background.
Analysis of additional risk factors showed a stronger association of lower education and diabetes with dementia at midlife compared to older age. However, the presence of one or two apolipoprotein E4 alleles or abnormally low BMI (<18.5 kg/m2) was associated with a greater HR in older age compared to midlife. Moreover, overweight and obesity at older age were associated with a reduced risk of dementia (Table S9).
Sensitivity Analyses
To rule out a potential bias due to partly overlapping observational periods after visit 4 (period between visit 4 and 6) and visit 5 (period between visit 5 and 6), we repeated the analysis after visit 4 with right censoring of all observations at visit 5, creating one observational period between visit 4 and 5. The results in this analysis are generally similar to the main analysis (Table S10).
An additional analysis stratified by race was performed to increase comparability with a previous study of mostly white participants.6 Compared with the complete ARIC cohort, the associations between CKD measures and incident dementia in white and African-American participants were similar, but with overall larger 95% confidence intervals of the HRs (Table S11).
A further analysis was performed using only incident dementia cases identified via level 1 assessment, which is based on expert classification of participants’ cognitive status and is considered to be the most accurate. Compared with the main analysis, the trends in dementia risk associated with CKD measures were similar, but also had larger confidence intervals (Table S12).
To address potential bias caused by prevalent mild cognitive impairment (MCI) at baseline visit 5 (MCI ascertainment was not available at visit 4), we excluded these participants in an additional sensitivity analysis. The association between UACR and dementia risk was even stronger and the associations between measures of eGFR and dementia risk were similar, compared to the main analysis (Table S13).
DISCUSSION
This study of incident dementia in a general population cohort shows an increased risk of dementia among participants with higher levels of UACR and lower levels of GFR, but only when GFR estimation is based on novel kidney markers, such as cystatin-C or B2M; there is no statistically significant relationship between GFR and dementia when GFR estimation is based on creatinine or a combination of creatinine and cystatin-C. Both GFR and UACR were independently associated with dementia, which has previously been reported.31 These associations were not weaker when UACR and GFR were measured at ages 70–90 than when they were measured at younger ages. The incidence of dementia was 11.2 events per 1000 person-years at midlife and 21.4 events per 1000 person-years at older age, which is similar to the incidence rates reported in other general population studies.6, 7, 32
The association between UACR and incident dementia is largely supported by prior studies; assessing albuminuria in various general population cohorts, a cohort of women and in diabetic patients, associations of albuminuria (or proteinuria) with incident dementia 7, 9 and cognitive decline 31, 33–36 were reported. Our findings reinforce the role of UACR as an important risk factor for dementia commonly used in routine clinical practice.
Conversely, the evidence is less conclusive about the connection between eGFR and incident dementia, with some studies reporting no increased risk of dementia 6, 8, 34, 37, 38, others reporting marginal results 7, 33, and some demonstrating an increased dementia risk associated with lower eGFR.31, 39–44 Our study adds new information by comparing GFR estimates based on creatinine with other estimations, based on the novel markers cystatin-C and B2M.
Comparability was ensured by standardizing all changes of predictor variables to their respective IQRs at visit 4. We found eGFR to be associated with incident dementia only when the CKD-EPI equations used these novel markers, as opposed to creatinine. This could be explained by novel kidney markers being less affected by the non-GFR determinants of creatinine, such as unusual muscle mass, diet with a high meat content or dietary supplements containing creatine.11 Especially in elderly populations, muscle mass is often reduced, leading to low creatinine values and under-estimation of CKD severity, which could conceal associations between impaired kidney function and adverse outcomes. In fact, using the CKD-EPI equations based on cystatin-C and B2M, on average lead to lower GFR estimates and therefore more participants being diagnosed as having CKD in our cohort compared to when the CKD-EPI equation for creatinine was used.
The aforementioned studies all used creatinine to estimate kidney function, except one study performed on predominantly white participants of similar age, which also included cystatin-C and a combination of the two markers, but it did not find an association between GFR and dementia.6 A subgroup analysis stratified by race showed overall similar associations of eGFR and UACR with incident dementia in white participants as in the full cohort, so this discrepancy ultimately remains unexplained.
The potential advantages of using novel CKD markers has previously been reported. Cystatin-C has been shown to be as good or even better at estimating GFR compared to creatinine.23, 45 In addition to its application in GFR estimation, smaller studies have also found B2M to be associated with various cognitive outcomes, such as postoperative cognitive decline, general age-related cognitive decline and Alzheimer’s Disease.24, 46–48 These findings support the usefulness of novel CKD markers, such as cystatin-C and B2M, which could prove to be valuable tools for clinicians in the risk assessment of dementia and cognitive decline.
This study compares the relationship of different measures of CKD with incident dementia between different age groups in the same cohort. We found that the effect size of dementia risk associated with higher UACR and lower eGFR was on average larger when measured at older age (70–90 years) compared to midlife (54–74 years), but the difference was not statistically significant. This result differs from the age association for vascular risk factors, such as hypertension, smoking, abnormal BMI and diabetes, which prior studies have found to increase the risk of dementia and cognitive impairment at middle age, while such associations in late life were weaker or non-existent.14–17 Our findings imply that UACR and eGFR are risk factors for dementia across a wide age range. Consequently, preservation of kidney function to prevent dementia could be just as vital in the clinical care of older patients as it is for middle-aged patients.
Strengths of this study include a large population-based sample, measurement of novel CKD markers and a long observation period (median follow-up 18.4 years after visit 4 and 4.6 years after visit 5). Detailed cognitive assessments and consistent definitions were used to define incident dementia and our extensive dementia surveillance system was capable of detecting dementia in participants who missed in-person study visits thereby reducing potential bias from loss to follow-up.
However, this study has limitations as well. Although the aforementioned steps were taken to reduce missed cases, the accuracy of Level 2 and Level 3 ascertainment is not expected to be as good as for Level 1 cases, which included in-person neuro-psychological assessment with informant interviews and expert review. As a consequence, some dementia cases identified via TICSm, hospitalization codes or solely based on informant interviews may be incorrectly classified. We also considered a potential bias due to hospitalization codes being picked-up more frequently in participants with other comorbidities, such as diabetes or hypertension. However, this seems unlikely as a recent study analyzing the association of cigarette smoking with dementia did not find evidence for such a bias in a sensitivity analysis involving censoring hospitalization codes for smoking-related comorbidities, such as cerebrovascular or ischemic heart disease.49 When comparing B2M measurements, it is important to know that it can be measured by different methods, such as nephelometry, turbidimetry or immunoassay, however, there is discordance between methods due to a lack of standardization.50 This should not impact our study as all measurements were performed using the same immuno-nephelometric assay followed by calibration and standardization.21 In this study, we only analyzed incident dementia diagnoses and did not model trajectories of cognitive decline as a time-varying variable, nor did we consider changes of potentially time-varying covariates during the follow-up period. Sub-classification of dementia into certain causes, such as vascular dementia or Alzheimer’s Disease was not possible due to limited data on dementia etiology.
A principal objective of the ARIC Neurocognitive Study is to analyze different risk factors for the development of dementia and cognitive decline. This study builds upon previous reports linking measures of CKD to dementia and confirms UACR and eGFR as risk factors with a particular emphasis on novel biomarkers of GFR, that are less prone to bias than creatinine. These findings were consistent both in midlife and older age, underlining the importance of preserving kidney function throughout adulthood to reduce the risk of incident dementia.
Future studies need to further characterize the etiology of dementia to allow for conclusions about potential pathomechanisms.
Supplementary Material
Table S1: Number of diagnosed incident dementia events after midlife and after older age by ascertainment levels.
Table S2: Characteristics of the study population at baseline visit 4 stratified by eGFR (cystatin-C) categories.
Table S3: Characteristics of the study population at baseline visit 4 stratified by UACR categories.
Table S4: Characteristics of the study population at baseline visit 5 stratified by eGFR (cystatin-C) categories.
Table S5: Characteristics of the study population at baseline visit 5 stratified by UACR categories.
Table S6: Adjusted hazard ratios of dementia incidence (95%-CI) after midlife and after older age for linear splines of eGFR measures with a knot at 60 ml/min.
Table S7: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (cystatin-C) categories.
Table S8: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by UACR categories.
Table S9: Adjusted hazard ratios of dementia incidence (95%-CI) after midlife and after older age by eGFR (creatinine) and significant covariates.
Table S10: Adjusted hazard ratios (HRs, 95%-CI) of dementia incidence after visit 4 by measures of CKD. Comparison of HRs with observational period after visit 4 and restricted observational period between visit 4 and visit 5 with right censoring at visit 5.
Table S11: Adjusted hazard ratios (95%-CI) of dementia incidence after visit 4 and after visit 5 by measures of CKD. Subgroup analysis stratified by ethnicity.
Table S12: Adjusted hazard ratios (95%-CI) of dementia incidence (according to ascertainment Level 1) after midlife and after older age by measures of CKD.
Table S13: Adjusted hazard ratios (95%-CI) of dementia incidence after older age by measures of CKD. Patients with prevalent mild cognitive impairment (MCI) at visit 5 were excluded.
Figure S1: Study population at baseline Visit 4.
Figure S2: Study population at baseline Visit 5.
Figure S3: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (creatinine) and UACR categories.
Figure S4: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (creatinine + cystatin-C) and UACR categories.
Figure S5: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (B2M) and UACR categories.
Acknowledgements:
We thank the staff and participants of the Atherosclerosis Risk in Communities (ARIC) Study for their important contributions.
Support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I and HHSN268201700004I). Neurocognitive data were collected with support by grants U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the National Institute of Health (National Heart, Lung, and Blood Institute, with support from the National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders) and with previous brain MRI examinations funded by R01-HL70825 from the National Heart, Lung, and Blood Institute. The funding sources had no role in the design and conduct of the study, collection, management, analysis, reporting, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Financial Disclosure: MEG: Received travel support from DCI in May 2019. DSK: Serves on a Data Safety Monitoring Board for the DIAN study; is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California; receives research support from the NIH. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fiest KM, Jette N, Roberts JI, et al. The Prevalence and Incidence of Dementia: a Systematic Review and Meta-analysis. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2016;43 Suppl 1: S3–s50. [DOI] [PubMed] [Google Scholar]
- 2.Hurd MD, Martorell P, Langa KM. Monetary costs of dementia in the United States. The New England journal of medicine. 2013;369(5): 489–490. [DOI] [PubMed] [Google Scholar]
- 3.Fratiglioni L, Qiu C. Prevention of cognitive decline in ageing: dementia as the target, delayed onset as the goal. The Lancet. Neurology. 2011;10(9): 778–779. [DOI] [PubMed] [Google Scholar]
- 4.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(8): 2446–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madan P, Kalra OP, Agarwal S, Tandon OP. Cognitive impairment in chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22(2): 440–444. [DOI] [PubMed] [Google Scholar]
- 6.Koop-Nieuwelink C, Sedaghat S, Mutlu U, et al. Kidney Function and the Risk of Stroke and Dementia: The Rotterdam Study. Journal of Alzheimer’s disease : JAD. 2019;67(3): 821–826. [DOI] [PubMed] [Google Scholar]
- 7.Takae K, Hata J, Ohara T, et al. Albuminuria Increases the Risks for Both Alzheimer Disease and Vascular Dementia in Community-Dwelling Japanese Elderly: The Hisayama Study. Journal of the American Heart Association. 2018;7(2): e006693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hare AM, Walker R, Haneuse S, et al. Relationship between longitudinal measures of renal function and onset of dementia in a community cohort of older adults. Journal of the American Geriatrics Society. 2012;60(12): 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higuchi M, Chen R, Abbott RD, et al. Mid-life proteinuria and late-life cognitive function and dementia in elderly men: the Honolulu-Asia Aging Study. Alzheimer disease and associated disorders. 2015;29(3): 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgakis MK, Dimitriou NG, Karalexi MA, et al. Albuminuria in Association with Cognitive Function and Dementia: A Systematic Review and Meta-Analysis. Journal of the American Geriatrics Society. 2017;65(6): 1190–1198. [DOI] [PubMed] [Google Scholar]
- 11.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of internal medicine. 2013;158(11): 825–830. [DOI] [PubMed] [Google Scholar]
- 12.Zaman T, Filipowicz R, Beddhu S. Implications and importance of skeletal muscle mass in estimating glomerular filtration rate at dialysis initiation. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2013;23(3): 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karger AB, Inker LA, Coresh J, Levey AS, Eckfeldt JH. Novel Filtration Markers for GFR Estimation. Ejifcc. 2017;28(4): 277–288. [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA neurology. 2014;71(10): 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12(5): e426–437. [DOI] [PubMed] [Google Scholar]
- 16.Power MC, Tingle JV, Reid RI, et al. Midlife and Late-Life Vascular Risk Factors and White Matter Microstructural Integrity: The Atherosclerosis Risk in Communities Neurocognitive Study. Journal of the American Heart Association. 2017;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso A, Mosley TH Jr., Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. Journal of neurology, neurosurgery, and psychiatry. 2009;80(11): 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129(4): 687–702. [PubMed] [Google Scholar]
- 19.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002;39(5): 920–929. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145(4): 247–254. [DOI] [PubMed] [Google Scholar]
- 21.Parrinello CM, Grams ME, Couper D, et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clinical chemistry. 2015;61(7): 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9): 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. New England Journal of Medicine. 2012;367(1): 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker LA, Tighiouart H, Coresh J, et al. GFR Estimation Using beta-Trace Protein and beta2-Microglobulin in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67(1): 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimer’s & dementia (Amsterdam, Netherlands). 2016;2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider AL, Gottesman RF, Mosley T, et al. Cognition and incident dementia hospitalization: results from the atherosclerosis risk in communities study. Neuroepidemiology. 2013;40(2): 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2009;5(3): 207–214. [DOI] [PubMed] [Google Scholar]
- 28.Forman DT. Beta-2 microglobulin--an immunogenetic marker of inflammatory and malignant origin. Annals of clinical and laboratory science. 1982;12(6): 447–452. [PubMed] [Google Scholar]
- 29.Topciu-Shufta V, Miftari R, Haxhibeqiri V, Haxhibeqiri S. Association of Beta-2 Microglobulin with Inflammation and Dislipidemia in High-Flux Membrane Hemodialysis Patients. Medical archives (Sarajevo, Bosnia and Herzegovina). 2016;70(5): 348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz B, Koklu S, Yuksel O, Arslan S. Serum beta 2-microglobulin as a biomarker in inflammatory bowel disease. World journal of gastroenterology. 2014;20(31): 10916–10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurella Tamura M, Muntner P, Wadley V, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58(5): 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmer C, Stengel B, Metzger M, et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology. 2011;77(23): 2043–2051. [DOI] [PubMed] [Google Scholar]
- 33.Barzilay JI, Lovato JF, Murray AM, et al. Albuminuria and cognitive decline in people with diabetes and normal renal function. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(11): 1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sajjad I, Grodstein F, Kang JH, Curhan GC, Lin J. Kidney dysfunction and cognitive decline in women. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(3): 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barzilay JI, Gao P, O’Donnell M, et al. Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Archives of internal medicine. 2011;171(2): 142–150. [DOI] [PubMed] [Google Scholar]
- 36.Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. American journal of epidemiology. 2010;171(3): 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seliger SL, Wendell CR, Waldstein SR, Ferrucci L, Zonderman AB. Renal function and long-term decline in cognitive function: the Baltimore Longitudinal Study of Aging. American journal of nephrology. 2015;41(4–5): 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng L, Yap KB, Yeoh LY, Ng TP. Kidney function and cognitive and functional decline in elderly adults: findings from the Singapore longitudinal aging study. Journal of the American Geriatrics Society. 2012;60(7): 1208–1214. [DOI] [PubMed] [Google Scholar]
- 39.Miwa K, Tanaka M, Okazaki S, et al. Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology. 2014;82(12): 1051–1057. [DOI] [PubMed] [Google Scholar]
- 40.Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(7): 1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Zhang L, Liu L, Wang H. Level of kidney function correlates with cognitive decline. American journal of nephrology. 2010;32(2): 117–121. [DOI] [PubMed] [Google Scholar]
- 42.Khatri M, Nickolas T, Moon YP, et al. CKD associates with cognitive decline. Journal of the American Society of Nephrology : JASN. 2009;20(11): 2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etgen T, Sander D, Chonchol M, et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(10): 3144–3150. [DOI] [PubMed] [Google Scholar]
- 44.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. Journal of the American Society of Nephrology : JASN. 2004;15(7): 1904–1911. [DOI] [PubMed] [Google Scholar]
- 45.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51(3): 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominici R, Finazzi D, Polito L, et al. Comparison of beta2-microglobulin serum level between Alzheimer’s patients, cognitive healthy and mild cognitive impaired individuals. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2018;23(6): 603–608. [DOI] [PubMed] [Google Scholar]
- 47.Yang R, Fu S, Zhao L, et al. Quantitation of circulating GDF-11 and beta2-MG in aged patients with age-related impairment in cognitive function. Clinical science (London, England : 1979). 2017;131(15): 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaleska-Kociecka M, Jezierski P, Grabowski M, et al. Role of beta2-microglobulin in postoperative cognitive decline. Biomarkers in medicine. 2017;11(3): 245–253. [DOI] [PubMed] [Google Scholar]
- 49.Deal JA, Power MC, Palta P, et al. Relationship of Cigarette Smoking and Time of Quitting with Incident Dementia and Cognitive Decline. Journal of the American Geriatrics Society. 2020;68(2): 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fedele PL, Choy KW, Doery JC, Grigoriadis G, Shortt J, Lu ZX. Inter-laboratory discordance of beta-2 microglobulin results: impact on the validity of the international staging system for multiple myeloma. British journal of haematology. 2014;166(6): 951–953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Number of diagnosed incident dementia events after midlife and after older age by ascertainment levels.
Table S2: Characteristics of the study population at baseline visit 4 stratified by eGFR (cystatin-C) categories.
Table S3: Characteristics of the study population at baseline visit 4 stratified by UACR categories.
Table S4: Characteristics of the study population at baseline visit 5 stratified by eGFR (cystatin-C) categories.
Table S5: Characteristics of the study population at baseline visit 5 stratified by UACR categories.
Table S6: Adjusted hazard ratios of dementia incidence (95%-CI) after midlife and after older age for linear splines of eGFR measures with a knot at 60 ml/min.
Table S7: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (cystatin-C) categories.
Table S8: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by UACR categories.
Table S9: Adjusted hazard ratios of dementia incidence (95%-CI) after midlife and after older age by eGFR (creatinine) and significant covariates.
Table S10: Adjusted hazard ratios (HRs, 95%-CI) of dementia incidence after visit 4 by measures of CKD. Comparison of HRs with observational period after visit 4 and restricted observational period between visit 4 and visit 5 with right censoring at visit 5.
Table S11: Adjusted hazard ratios (95%-CI) of dementia incidence after visit 4 and after visit 5 by measures of CKD. Subgroup analysis stratified by ethnicity.
Table S12: Adjusted hazard ratios (95%-CI) of dementia incidence (according to ascertainment Level 1) after midlife and after older age by measures of CKD.
Table S13: Adjusted hazard ratios (95%-CI) of dementia incidence after older age by measures of CKD. Patients with prevalent mild cognitive impairment (MCI) at visit 5 were excluded.
Figure S1: Study population at baseline Visit 4.
Figure S2: Study population at baseline Visit 5.
Figure S3: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (creatinine) and UACR categories.
Figure S4: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (creatinine + cystatin-C) and UACR categories.
Figure S5: Adjusted relative hazards (95%-CI) of dementia incidence after midlife and after older age by eGFR (B2M) and UACR categories.