Abstract
The older listener’s ability to understand speech in challenging environments may be affected by impaired temporal processing. This review summarizes objective evidence of degraded temporal processing from studies that have used the auditory brainstem response, auditory steady-state response, the envelope- or frequency-following response, cortical auditory-evoked potentials, and neural tracking of continuous speech. Studies have revealed delayed latencies and reduced amplitudes/phase locking in subcortical responses in older vs. younger listeners, in contrast to enhanced amplitudes of cortical responses in older listeners. Reconstruction accuracy of responses to continuous speech (e.g., cortical envelope tracking) shows over-representation in older listeners. Hearing loss is a factor in many of these studies, even though the listeners would be considered to have clinically normal hearing thresholds. Overall, the ability to draw definitive conclusions regarding these studies is limited by the use of multiple stimulus conditions, small sample sizes, and lack of replication. Nevertheless, these objective measures suggest a need to incorporate new clinical measures to provide a more comprehensive assessment of the listener’s speech understanding ability, but more work is needed to determine the most efficacious measure for clinical use.
Keywords: Temporal processing, Aging, Auditory brainstem response, Auditory steady-state response, Frequency-following response, Cortical auditory-evoked potential, Magnetoencephalography, Envelope tracking, Hearing impairment
Introduction
Older adults often struggle to understand speech in situations where their younger counterparts can hear with comparative ease. Three main factors are suggested to contribute to age-related declines in speech perception, namely peripheral hearing loss, central auditory processing deficits, and decreased cognitive function (CHABA, 1988). The role of central auditory processing in the deterioration of speech understanding has not been firmly established, however, because hearing loss and reduced cognition may confound performance on measures of speech understanding assessed in adverse listening environments (Humes et al., 2012). Objective measures that tap into the auditory nervous system’s ability to accurately represent the speech signal may provide a means of evaluating central auditory processing without requiring active cooperation. Electrophysiologic (EEG) and magnetoencephalographic (MEG) recordings are objective measures that have been used to investigate the processing deficits that may contribute to speech perception impairments in older listeners.
Most studies that evaluate age-related impairments in neural speech processing recruit younger and older participants who have audiometrically normal hearing to reduce the confound of hearing loss. Generally, decreased temporal resolution is associated with aging (Gordon-Salant and Fitzgibbons, 1993; Gordon-Salant et al., 2007; Pichora-Fuller et al., 2007), whereas decreased frequency resolution is associated with hearing loss (Florentine et al., 1980; Phillips et al., 2000). It is difficult, however, to completely eliminate the influence of peripheral deficits, as almost all older individuals have decreased hearing in the extended high-frequency region above 8000 Hz (Davis et al., 1992; Matthews et al., 1997). There is evidence that elevated thresholds in this high-frequency region may contribute to speech-in-noise difficulties (Motlagh Zadeh et al., 2019; Yeend et al., 2019). Furthermore, behavioral and neural findings may be affected by other deficits that are not evident on the audiogram, including a subclinical loss of outer hair cells (Abdala and Dhar, 2012; Fabijańska et al., 2012; Hoben et al., 2017; Uchida et al., 2008), strial dysfunction (Ohlemiller, 2009; Schmiedt et al., 1990; Syka, 2010), broadened auditory filters (Hopkins and Moore, 2011; Patterson et al., 1982), cochlear synaptopathy and decreased numbers of auditory nerve fibers (Makary et al., 2011; Schmiedt et al., 1996; Sergeyenko et al., 2013; Viana et al., 2015; Wu et al., 2019), and loss of neural synchrony (Harris and Dubno, 2017; Marmel et al., 2013).
Many previous studies have revealed age-related temporal processing deficits when using compressed or reverberant speech signals (Fujihira et al., 2017; Gordon-Salant and Fitzgibbons, 1993; Gordon-Salant and Fitzgibbons, 2001; Gordon-Salant et al., 2007; Grose et al., 2009; Helfer and Wilber, 1990; Humes et al., 2007; Jenstad and Souza, 2007; Peelle and Wingfield, 2005; Wingfield et al., 2006), or when speech signals are presented in noise (Decruy et al., 2019; Dubno et al., 1984; Füllgrabe et al., 2015; Goossens et al., 2017; Helfer and Freyman, 2014; Presacco et al., 2016b). This review will consider the evidence from studies that have used EEG and MEG recordings to investigate age-related temporal processing deficits and will examine the possible roles of peripheral deficits in these studies. The sections are divided into five main types of measures: auditory brainstem response (ABR), envelope-following response (EFR)/auditory steady-state response (ASSR), frequency-following response (FFR), cortical auditory-evoked potential (CAEP), and cortical envelope tracking. Within each section, we separate effects of aging and hearing loss. For the purposes of this review, peripheral disorders will encompass cochlear hair cell loss, cochlear synaptopathy, and auditory nerve dysfunction, and central disorders will encompass deterioration beyond the auditory nerve. The final section will consider how this information might influence management recommendations for older adults who have difficulty understanding speech in noise.
Auditory brainstem response (ABR)
Aging
The ABR is dependent on synchronous firing of populations of neurons that is sufficient to be detected with scalp electrodes. For this reason, disorders that result in disrupted neural synchrony, such as auditory neuropathy spectrum disorder, result in absent or highly degraded ABRs (Kraus et al., 2000; Starr et al., 1996; White-Schwoch et al., 2019). Latency differences on the order of fractions of milliseconds can be clinically significant in the detection of retrocochlear pathology (Chandrasekhar et al., 1995). The peaks of the ABR have sources from the auditory nerve through the inferior colliculus (IC); furthermore, the later peaks have multiple sources because of crossed auditory pathways (Kaga et al., 1997; Kuokkanen et al., 2018; Land et al., 2016; Møller and Jannetta, 1983). Aging may result in a loss of auditory nerve fibers, cochlear synaptopathy (Garrett and Verhulst, 2019; Makary et al., 2011; Schmiedt et al., 1996; Sergeyenko et al., 2013; Viana et al., 2015), and changes in neural coding in the auditory brainstem (Brecht et al., 2017; Parthasarathy et al., 2014; Schatteman et al., 2008; Williamson et al., 2015). Because ABR peak latencies are delayed and amplitudes are reduced by neuronal loss or by changes in synchronous firing patterns, the ABR may provide a sensitive measure of temporal processing in older individuals.
Large cross-sectional studies have shown that ABR latencies increase with age. In a study of 98 normal-hearing listeners ranging in age from 25 to 55 years, the click-evoked Wave V latency increased ~ 0.2 ms and amplitude decreased ~ 0.05 µV (Jerger and Hall, 1980). Similarly, a study with 586 normal-hearing subjects ranging in age from infancy to older adulthood revealed that click-evoked Wave V latencies begin to change at the age of ~ 30 years, increasing from ~ 5.7 to 6.0 ms at > 60 years (Skoe et al., 2015). A similar increase in Wave V latencies was also found in recordings to a 40-ms [da] syllable in the Skoe et al. study.
Aging effects on ABR latency and amplitude may differ depending on stimulus parameters and protocols. Using recordings to a high-level click stimulus of 115 dB peSPL and a tympanic-membrane electrode to maximize detection of Wave I in 11 ONH and YNH listeners, Burkard and Sims (2001) found that ONH listeners had slightly longer Wave I latencies than YNH listeners across stimulation rates that varied from 11 to 75 Hz (obtained using conventional ABR) and from 100 to 500 Hz (obtained using a maximum length sequence procedure). Progressively longer latencies were noted with increasing stimulation rates, with equivalent effects between age groups, suggesting that increasing stimulation rate does not tax the auditory system to a greater degree in ONH than in YNH listeners, at least at this high intensity presentation level. The authors reported smaller Wave I amplitudes and somewhat smaller Wave V amplitudes in ONH compared to YNH listeners. The authors did not provide standard deviations for the amplitude means, however, and ABR amplitudes are highly variable because of differences in baseline noise levels in awake listeners. Therefore, the amplitude effects do not appear to be as robust as the effect for Wave I latency.
In a follow-up study using the same participants, Burkard and Sims (2002) recorded ABRs to the same click stimulus presented at a constant rate of 25 Hz and compared effects of broad-band noise between YNH and ONH listeners. They found similar age-related increases in Wave I latency as in their 2001 rate study, and they also found that noise had equivalent effects on ABRs in older and younger listeners. Assuming that noise and/or rate desynchronize neural firing to stimulus onsets, as suggested by Don et al. (1977), the results of the Burkard studies suggest that ONH listeners are not affected by neural desynchronization to a greater extent than YNH listeners. Note that the sample sizes in both Burkard studies (2001, 2002) were relatively small (n=11, each group); therefore, the studies may have been underpowered for revealing amplitude differences that tend to be highly variable.
Konrad-Martin et al. (2012) found similar results in a study that recorded ABRs to a 100-µsec broadband click presented at 110 dB peSPL at three stimulation rates (11, 51, and 71 Hz) from a sample of 131 predominantly male veterans (ages 26 to 71). Using regression modeling, they demonstrated that aging decreased amplitudes of all ABR wave peaks (I, III, and V) and increased latencies for peaks I and III. They also found that aging did not exacerbate the effects of rate; in contrast, the effect of increased rate on amplitude was somewhat attenuated in the older participants due to their smaller baseline amplitudes at lower rates.
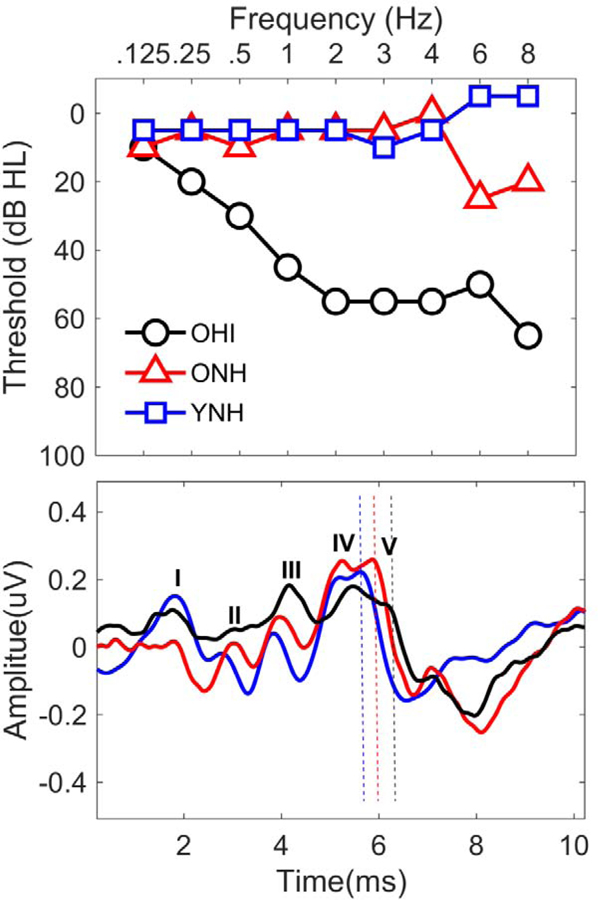
Despite the presence of audiometrically normal thresholds in the older listeners in these studies, their thresholds, particularly in the high frequencies, were elevated compared to those of the younger listeners. The click-ABR is dominated by the high-frequency components of the stimulus. This high-frequency emphasis arises due to temporal dispersion of the low frequency components on the basilar membrane and due to responses of low-frequency tails of higher frequency nerve fibers (Dau, 2003). Therefore, even small elevations in high-frequency hearing thresholds may lead to delayed latencies (Bauch and Olsen, 1986), although recruitment may minimize latency delays at high intensity levels (Kavanaugh and Beardsley, 1979). An example of the effects of slight high-frequency hearing loss is displayed in Figure 1. In the ONH listener with “normal hearing” (thresholds of 25 and 20 dB HL at 6000 Hz and 8000 Hz, respectively), Wave V latency is delayed compared to the YNH listener and is earlier compared to the OHI listener.
Fig. 1.
Individual audiograms (top panel) and auditory brainstem response waveforms (bottom panel) are displayed for representative young normal-hearing (YNH, blue squares), older normal-hearing (ONH, red triangles), and older hearing-impaired (OHI, black circles) listeners. Even slight high-frequency loss above 4000 Hz is associated with Wave V latency delays.
The Burkard and Konrad-Martin studies mentioned above controlled for the effects of hearing through recruitment of individuals who had normal hearing thresholds and through further subdividing the older adults into a group with “better hearing” (Burkard & Sims, 2001, 2002: all thresholds ≤20 dB; Konrad-Martin et al. (2012): pure-tone average of 2000, 3000, and 4000 Hz < 17.5 dB HL). Although these studies controlled for the effects of hearing threshold, threshold elevations above 4000 Hz exist even for the better-hearing older participants compared to the YNH participants. For example, there is a mean difference of 9.83 dB HL between the YNH participants and the better hearing older participants in Burkard and Sims (2001; Table 1, p.2 and Table 2, p. 3). Furthermore, given that the majority of participants in the Konrad-Martin study were men, sex may have played a role in evaluation of age vs. hearing loss effects. Jerger and Johnson (1988) evaluated interactions of age, sex, and hearing level on the ABR latencies and found that hearing loss effects were more pronounced in their female participants. Therefore, effects of elevated thresholds cannot be ruled out in these human studies.
Hearing loss
The effects of sensorineural hearing loss on the ABR are well documented, including delayed latencies and decreased amplitudes with increases in hearing thresholds; however, Wave V latencies may be normal at high presentation levels due to recruitment in cases of mild-to-moderate hearing loss (Stapells et al., 1985). Slope metrics, calculated from latency or amplitude changes with intensity, may have potential for differentiating between outer hair cell loss and cochlear synaptopathy or auditory nerve degradation (Vasilkov and Verhulst, 2019; Verhulst et al., 2016). However, these metrics are highly variable in individuals with hearing loss, and more work needs to be done before it is possible to tease apart effects that might be related to aging vs. outer hair cell loss (Vasilkov and Verhulst, 2019).
Animal studies
Because environmental causes of hearing loss can be controlled, lab-based animal studies may reduce the confounds of hearing loss in aging studies. Many studies have compared auditory function in the “normal-hearing” CBA/CaJ mouse strain to the C57 strain which exhibits significant progressive age-related sensorineural hearing loss. However, even the CBA mice gradually lose hearing at a rate similar to humans but within a compressed time frame (Williamson et al., 2015). ABR recordings in CBA/CaJ mice show no changes with aging; however, hearing loss in the C57 mice results in prolonged ABR latencies and reduced amplitudes, particularly in the early waves (Hunter and Willott, 1987). Studies in other species have shown that peripheral function contributes to ABR abnormalities. Older rhesus monkeys with reduced outer hair cell function (lower distortion-product otoacoustic emissions) have delayed ABR latencies (Torre and Fowler, 2000) and older rats with a greater loss of cochlear ribbon synapses have lower amplitudes, especially for Wave I (Cai et al., 2018). In contrast to these studies showing only peripheral effects on the ABR, a study comparing ABRs in younger and older Mongolian gerbils found an age-related decrease in ABR amplitudes, even when limiting the comparison to older gerbils who had lower ABR thresholds (Boettcher et al., 1993). In addition, the slopes of amplitude-intensity functions were shallower in the older compared to the younger gerbils and there was no relationship between slopes and hearing thresholds. The results should be viewed with some caution, however, as the thresholds of the better hearing older gerbils were slightly elevated compared to the young gerbils (Boettcher et al., 1993; Fig. 2).
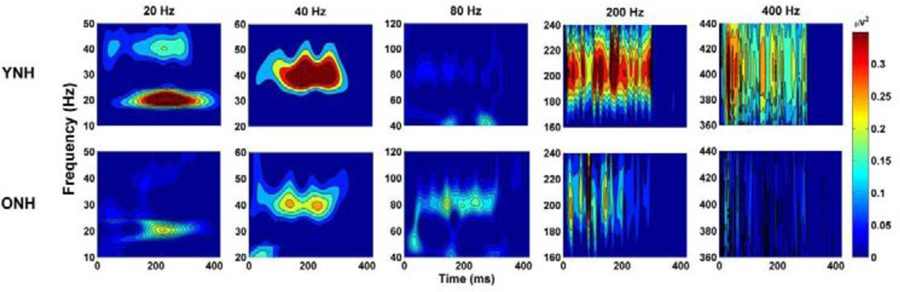
Fig. 2.
Spectral energy corresponding to pulse-train presentation rates shows that responses in younger normal-hearing listeners (YNH, top panel) have higher frequency energy than in older normal-hearing listeners (ONH, bottom panel) that is significant for the 400-Hz rate. Furthermore, a decrease in spectral energy with higher rates is only significant in the ONH listeners. Used with permission from Gaskins et al. (2019).
Summary
Across studies, the “aging” effects of decreased amplitude and delayed latency appear to be confounded to some extent by hearing loss, especially at frequencies above 4000 Hz. The normal-hearing requirement in aging studies usually includes frequencies up to 4000 Hz, and almost all older individuals have some degree of threshold elevation in the extended high frequency range. Animal studies have shown that these group differences are most pronounced for the earlier ABR components which are more likely to be affected by peripheral dysfunction. At this time, it is not possible to disentangle aging effects from hearing loss, but perhaps new metrics can be developed that can differentiate these effects.
Envelope-following response (EFR) and auditory steady-state response (ASSR)
To obtain a better understanding of aging effects on auditory processing, studies have employed transient and amplitude-modulated stimuli, as aging may selectively target neurons that respond to amplitude-modulated stimuli rather than neurons that encode onsets (Palombi and Caspary, 1996; Rabang et al., 2012; Walton et al., 2002). In an aging rat model, Parthasarathy et al. (2014) compared aging effects for ABRs and EFRs and found that the relationships between these measures changed with age, such that correlations that were found between ABR and EFR amplitudes in the younger rodents were not found in the older rodents. These findings suggest that the ABR and EFR provide complimentary information regarding neurophysiological changes with aging, and the authors suggest including both assessments when evaluating age-related changes in auditory function.
EFR/FFR/ASSR studies have employed different approaches to investigate temporal processing deficits in older adults. One approach is to challenge the auditory system by varying stimulus parameters, such as modulation depth, stimulation rates, and stimulus trajectory, or by degrading the signal. Another approach is to use complex stimuli that allow examination of neural representation to specific regions of the stimulus (e.g., consonant transition vs. sustained vowel). These approaches may help to clarify the specific nature of temporal processing deficits and may lead to management strategies that target these specific deficits.
Aging
By varying stimulus parameters, an aging effect may be revealed that is limited to a specific stimulus presentation rate or modulation depth. Caution should be employed with this approach, however, to ensure that these parametric studies are guided by prior hypotheses and are corrected for multiple comparisons.
Presentation rate can be manipulated to better understand the locus of the deficit (Gaskins et al., 2019; Herdman et al., 2002) or to approximate the modulations that occur in natural speech (Goossens et al., 2016; Schoof and Rosen, 2016). For example, Leigh-Paffenroth and Fowler (2006) recorded ASSRs to AM tones with carrier frequencies of 500 and 2000 Hz and modulation rates of 20, 40, and 90 Hz and measured numbers of phase-locked responses in YNH and ONH listeners. They found a significant age × rate interaction, such that the ONH listeners had smaller numbers of phase-locked responses than the YNH listeners to the 90-Hz rate but not to the 20- or 40-Hz rates. Given that responses to the 20-Hz and 40-Hz rates are dominated by cortical generators and responses to the 90-Hz rate by brainstem generators (Herdman et al., 2002), these results suggest that brainstem, but not cortical phase locking, is affected by aging. Because there were hearing threshold differences between the groups, it is possible that there were peripheral hearing loss effects on the brainstem responses that were then compensated in cortex.
Grose et al. (2009) investigated aging effects on ASSR amplitudes at low and high modulation frequencies (32 and 128 Hz, respectively) and at low and high carrier frequencies (500 and 2000 Hz, respectively). These frequencies were chosen to resolve discrepancies in the literature regarding aging effects on temporal processing that showed no aging effects using relatively low modulation rates of 40 Hz (Boettcher et al., 2001) or 30–50 Hz (Purcell et al., 2004), but the existence of aging effects for a relatively higher rate of 90 Hz (Leigh-Paffenroth and Fowler, 2006). Grose et al. (2009) demonstrated an age-related reduction in amplitude for the 128-Hz modulation frequency at both carrier frequencies, but no aging effects were noted for the 32-Hz modulation frequency. They noted that these results were consistent with psychophysical data showing age-related deficits for high but not low modulation rates (He et al., 2008; Purcell et al., 2004).
Based on previous studies, Gaskins et al. (2019) hypothesized that aging effects would be specific to high frequencies (age × rate interaction) in a study that recorded ASSRs to 300-ms bandlimited pulse trains (1000 Hz bandwidth centered around 4000 Hz) presented at five rates (20, 40, 80, 200, and 400 Hz). In this study, the ASSRs showed lower signal-to-noise ratios (SNRs) in the ONH than in the YNH listeners, but only for the highest rate of 400 Hz (after correcting for multiple comparisons). In addition, ASSR energy decreased with increasing rate, but only in the ONH listeners (Figure 2). Given previous results from the Leigh-Paffenroth and Fowler (2006) and Grose et al. (2009) studies, aging effects might have been expected for the 80-Hz and 200-Hz rates as well. The Gaskins study tested 15 participants in each age group, whereas the Grose et al study tested 10 participants in each group and the Leigh-Paffenroth and Fowler tested 11 participants in each group. Therefore, it is possible that true age differences exist only for the highest rates (> 200 Hz), and it would be beneficial to perform a follow-up study with a larger number of participants to obtain a more accurate assessment of aging effects for high-rate modulation stimuli.
Studies that have examined effects of modulation depth have not found aging differences. McClaskey et al. (2019) examined the effects of aging and modulation depth on ASSRs to AM tones with a carrier frequency of 3000 Hz, a modulation frequency of 80 Hz, and modulation depths of 0, −4, and −8 dB (full, moderate, and shallow modulation, respectively). Based on the results of previous studies (Boettcher et al., 2001; Purcell et al., 2004), they did not expect to find aging differences for the relatively low modulation rate of 80 Hz, but instead they focused on effects of AM depth. Although both younger (n=22) and older (n=35) groups were defined as having normal hearing, there were significant group differences in pure tone thresholds, and the authors controlled for thresholds (average of 2000, 3000, and 4000 Hz) in their statistical analysis. They found that shallower AM depths reduced phase locking and amplitude in both listener groups, and there were no age group differences or interactions between age group and AM depth. The authors suggested that the use of transposed tones rather than sinusoidal AM tones may have elicited higher phase locking and reduced differences between the younger and older listeners. They also suggested that deficits at more peripheral levels may be partially compensated at midbrain and cortical levels, thus reducing or eliminating age differences.
Hearing loss
Effects of hearing loss have also been demonstrated with EFR recordings that vary in rate and/or modulation depth. In recordings of EFRs to white noise modulated at 25% depth and at rates that swept from 20 to 600 Hz, older listeners (n=13) with mild ARHL demonstrated detectable EFRs at a lower maximum frequency (mean = 294 Hz) compared to the maximum frequency (mean = 494 Hz) in YNH listeners (n=25) (Purcell et al., 2004). Dimitrijevic et al. (2016) recorded EFRs in YNH listeners and two groups of middle-aged and older listeners with hearing loss (O1, 41–62 yrs, pure-tone average (PTA) = 30 dB HL; O2, 67–82 yrs, PTA = 49 dB HL) in two conditions, with 12 listeners in each group. In the first condition, EFRs were recorded to a wideband noise carrier modulated at a rate of 41 Hz. The AM depth was continuously varied from 2% to 100% at a rate of 5%/sec. In the second condition, ASSRs (41 Hz) were recorded to fixed AM depths (100, 75, 50, and 25%). As expected, given the relatively low modulation rate of 41 Hz, the overall response amplitude was equivalent between groups. However, the YNH and O1 listeners exhibited a relatively linear increase in amplitude with greater AM depth, whereas the O2 listeners exhibited a narrower dynamic range with saturation at higher AM depths. In addition, EFR phase was relatively constant in the YNH listeners but decreased as a function of AM depth in the older listeners. The authors performed linear regression modeling to partial out effects of age and hearing loss and determined that the 4000-Hz threshold was the significant predictor of response amplitude. It should be noted that both older groups of participants had some degree of hearing loss, so it is difficult to disentangle the effects of aging from hearing loss in the results.
The best approach to differentiating effects of aging and hearing loss would be to match participants on these factors; however, the etiologies of hearing loss differ between younger and older individuals, and these etiologies may contribute to observed group differences. Nevertheless, when feasible, it is informative to compare temporal processing in groups that have been matched on both age and hearing loss, as was done in Goossens et al. (2019). In this study, the authors recruited normal hearing (NH) and hearing impaired (HI) young (20–30 yrs; 20 NH, 10 HI), middle-aged (50–60 yrs; 20 NH, 14 HI), and older (70–80 yrs; 14 NH, 13 HI) individuals. They recorded ASSRs to octave bands of white noise that were modulated at rates of 4, 20, 40, and 80 Hz to represent cortical, thalamus, thalamus/upper brainstem, and brainstem, respectively (Herdman et al., 2002). Based on previous animal models, they hypothesized that hearing loss would be associated with envelope enhancement, especially when stimuli have been adjusted for audibility, and that this association may differ between age groups. They presented the stimuli to both NH and HI groups at 70 dB SPL, rated as comfortably loud by the NH listeners, and they also presented the stimuli at levels that were individually rated as comfortably loud by the HI listeners. Overall, the young and middle-aged HI listeners’ responses showed enhanced encoding of envelope modulations in both subcortical and cortical regions compared to their normal-hearing counterparts, whereas the OHI listeners’ responses did not show this enhancement. Further, the enhancement to the cortical rates (4 to 40 Hz) disappeared when stimuli were presented at 70 dB SPL to the HI and the NH listeners, but the enhancement remained for the 80-Hz (brainstem) rate. Overall, it appears that hearing loss leads to exaggerated responses to the envelope, but this effect may be reduced with advanced age (over 70).
The Goossens et al. (2019) study included age as a factor in the analysis, but they did explicitly report the effects of age, and it would have been helpful to compare effects of age vs. effects of hearing loss. As noted, the sample sizes of each group were relatively small, due to the difficulty in recruiting and matching NH and HI subjects by age. There were also a large number of conditions, and therefore, the positive findings of the study may have resulted from random statistical variation. The authors have nevertheless demonstrated that a comparison of age vs. hearing loss effects is feasible, and it would be important to determine if these results are replicable in another study.
Animal studies
Various animal models have been used to clarify the nature of age-related degradation in AM encoding. Homeostatic changes that occur secondary to a loss of afferent input lead to decreased levels of inhibitory neurotransmission, including lower glycine levels and altered glycine receptors in the cochlear nucleus (Banay-Schwartz et al., 1989; Milbrandt and Caspary, 1995; Willott et al., 1997) and lower Gamma aminobutyric acid (GABA) levels and decreased numbers of GABA receptors in the IC and cortex (Caspary et al., 2013; Gutiérrez et al., 1994; Ling et al., 2005; Milbrandt et al., 1996). Given the role of GABA in shaping IC responses to AM stimuli (Caspary et al., 2002), these changes may underlie age-related deficits in AM encoding.
As noted above, these changes are presumed to result from a loss of input from the periphery, and may therefore reflect a central consequence from hearing loss rather than from aging, per se. To separate the effects of aging and hearing loss, Lai et al. (2017) compared EFRs in younger and older Fischer-344 rats using stimulus levels that were equalized for peripheral and central activation between the two groups. Using this approach, the authors aimed to account for potential synaptopathy and threshold elevations that are expected to occur with aging. To accomplish peripheral equalization, the mean Wave I amplitude obtained in the older animals with a stimulus intensity of 85 dB SPL served as a reference, and the stimulus level needed to obtain an equivalent Wave I amplitude was used in ABR and EFR recordings in the younger rats. To accomplish central equalization, the mean EFR amplitude (at 45-, 128-, and 256-Hz modulation rates) obtained in the older animals with a stimulus intensity of 85 dB SPL served as a reference, and the stimulus level needed to obtain an equivalent EFR amplitude was used in EFR recordings to the same modulation rates at several modulation depths in the younger animals. Using matched ABR I amplitudes, the authors found no age differences for Wave V amplitude; however, EFRs were enhanced at 100% but not at 25% modulation depths. Using matched EFR amplitudes, they found no age differences for either AM depth or AM frequency. Overall, these results demonstrate that apparent age-related differences in ABR amplitude are likely due to peripheral degradation, either in the outer hair cells, cochlear synapses, or auditory nerve fibers. However, the EFR results show that aging may affect enhancement of sustained modulations (EFR) to a greater extent than the effects on onset responses (ABR).
The nature of the peripheral deficit in temporal processing was further explored in a gerbil model (Heeringa et al., 2020). The authors found that coding of the temporal envelope and fine structure in the auditory nerve was equivalent between younger and older gerbils when measured at comparable sensation levels. Auditory nerve spontaneous firing rate, however, was decreased in older vs. younger gerbils, and this decrease was related to the extent of hearing threshold elevation and the decrease in auditory nerve fibers. Based on these findings, the authors concluded that age-related temporal coding deficits arise from deficits central to the auditory nerve associated with age-related decreases in inhibition (Canlon et al., 2010; Caspary et al., 1995), but that these deficits may be a consequence of decreased numbers of auditory nerve fibers.
Summary
Overall, these studies appear to show that 1) aging affects temporal processing of higher-rate modulation frequencies, and 2) aging does not affect processing of AM depth at relatively lower modulation frequencies. Aging effects have also been observed in studies that used very low-rate stimuli (e.g., < 10 Hz) (Decruy et al., 2019; Goossens et al., 2016; Henry et al., 2017; Tlumak et al., 2015), suggesting a possible cortical-cognitive role in aging deficits. For example, Henry et al. (2017) found a greater attention-related enhancement to a 2.8-Hz modulation rate (approximating speech rhythms) in YNH than in ONH listeners. The enhanced neural synchronization in young and middle-aged HI listeners noted in Goossens et al. (2019) is consistent with the results of animal studies (Henry et al., 2014; Parthasarathy et al., 2018; Qiu et al., 2000; Zhong et al., 2014). Enhanced synchronization likely reflects homeostatic mechanisms that compensate for reduced afferent input.
Conclusions regarding these studies are not definitive, however, due to the many stimulus conditions and small numbers of subjects that increase the possibility of a Type I error. Confidence in these results would be increased by studies that replicate these findings. It should also be noted that all ONH listeners across the studies were affected by age-related hearing loss (ARHL) to some degree, at least in the higher frequencies and therefore peripheral hearing loss may be a factor in any potential age-related differences.
Frequency-following response (FFR)
Aging
Older adults’ poorer speech perception may reflect temporal jitter or neural noise in the auditory system (Pichora-Fuller et al., 2007; Wang et al., 2011). Temporal jitter may result from loss of neural synchrony (Agmon, 2012; Luo et al., 2018) that would interfere with the auditory system’s ability to generate the synchronous firing necessary to produce a precise representation of the auditory stimulus (Plack et al., 2014). A study that evaluated effects of temporal jitter on word-in-noise identification in YNH listeners found that jitter reduced their performance to levels previously found in ONH listeners for the same task using non-jittered words (Pichora-Fuller et al., 2007). Mamo et al. (2016) tested this same idea by recording the FFR to a synthesized speech syllable [da] presented in clean and jittered conditions to 22 YNH and 22 ONH listeners. They found a main effect of age, in that ONH listeners had significantly reduced magnitudes of the fundamental frequency (F0) and its principle harmonics compared to YNH listeners in response to the clean syllable. However, jitter decreased spectral magnitudes only in the YNH listeners, not in the ONH listeners. They concluded that reduced synchrony in the ONH listeners mimicked neural jitter and therefore the introduction of stimulus jitter did not further decrease response magnitude.
Another approach to evaluating aging effects on temporal processing is to determine the specific components of the speech syllable or word that are affected by aging. Anderson et al. (2012) recorded FFRs in 17 YNH and 17 ONH listeners to a 170-ms synthesized [da] syllable presented in quiet and performed several analyses on the consonant-transition (20–60 ms) and steady-state vowel (60–170 ms) regions. They found that the ONH listeners had prolonged latencies compared to YNH listeners, but only for the onset and transition peaks, and not for the steady-state peaks. The other measures, phase locking, response consistency, and response amplitudes, showed aging deficits for both the transition and steady-state regions. The authors suggested that the reduced inhibitory neurotransmission may interfere with the ONH listeners’ ability to phase lock to the rapidly changing formants in the consonant transition.
Two alternate hypotheses for the Anderson et al. (2012) findings would be that ONH listeners experience a general delay in consistent phase locking that is not specific to changing formants or that high-frequency threshold differences between the two groups impact latencies for the high-frequency components of the stop consonant. Presacco et al. (2015) performed a follow-up study to test these hypotheses and contrasted aging effects on responses to [da] and [a]. Although they found age-related latency delays for [da] and not for [a], they noted that within-group differences between the stimuli were only found in the 15 YNH and not in the 15 ONH listeners. In the YNH listeners, the latencies to [da] were earlier than to [a], as would be expected given the higher-frequency energy content of the [da] that would be encoded earlier on the basilar membrane. These same latency differences between the [da] and [a] were not found in the ONH listeners, presumably because a slight loss of audibility impaired encoding of the high-frequency transients, even though their hearing thresholds were within the normal clinical range. A better approach in the future would be to record responses to syllables that begin with the same formants but vary in the length of the transition prior to the vowel, such as the syllables [ba] and [wa].
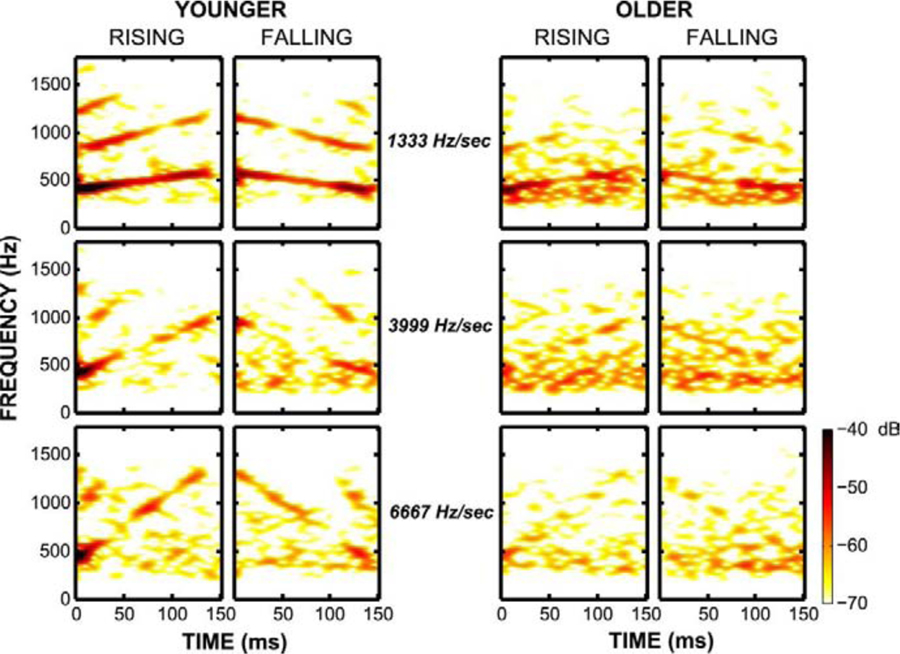
Clinard and Cotter (2015) adopted a different approach to determine if reduced inhibitory neurotransmission affects encoding of rapidly changing formants. Instead of presenting speech syllables, they recorded FFRs to tones that linearly increased or decreased in frequencies that mimicked formant changes in speech. The starting or ending frequency was 400 Hz for rising or falling tones, respectively, and the frequency changed at rates of 1333, 3999, and 6667 Hz/sec. They used stimulus-to-response (STR) correlations to assess the fidelity of frequency representation and found lower correlations and spectral energy in 9 ONH compared to 10 YNH listeners (Fig. 3). Of particular significance is the fact that they found aging effects even in the lower frequencies (~400 Hz), in contrast to a previous study that showed aging effects only for the relatively higher frequencies (~1000 Hz) and not for the lower frequencies (~500 Hz) when using static stimuli (Clinard et al., 2010). At this low frequency, it is less likely that peripheral hearing loss plays a role in the age effects. The authors suggested that an age-related reduction in inhibitory neurotransmission may lead to a reduction in phase locking to dynamic frequency sweeps, given the apparent role of GABA and glycinergic inhibition in neural processing of frequency modulations (Covey and Casseday, 1999).
Fig. 3.
FFR spectral amplitudes to dynamic frequency changes are displayed for a representative young normal-hearing listener (left panel) and a representative older normal-hearing listener (right panel). The responses in the younger listener show more robust tracking of dynamic frequency than the responses of the older listener across rates of change. Used with permission from Clinard et al. (2015).
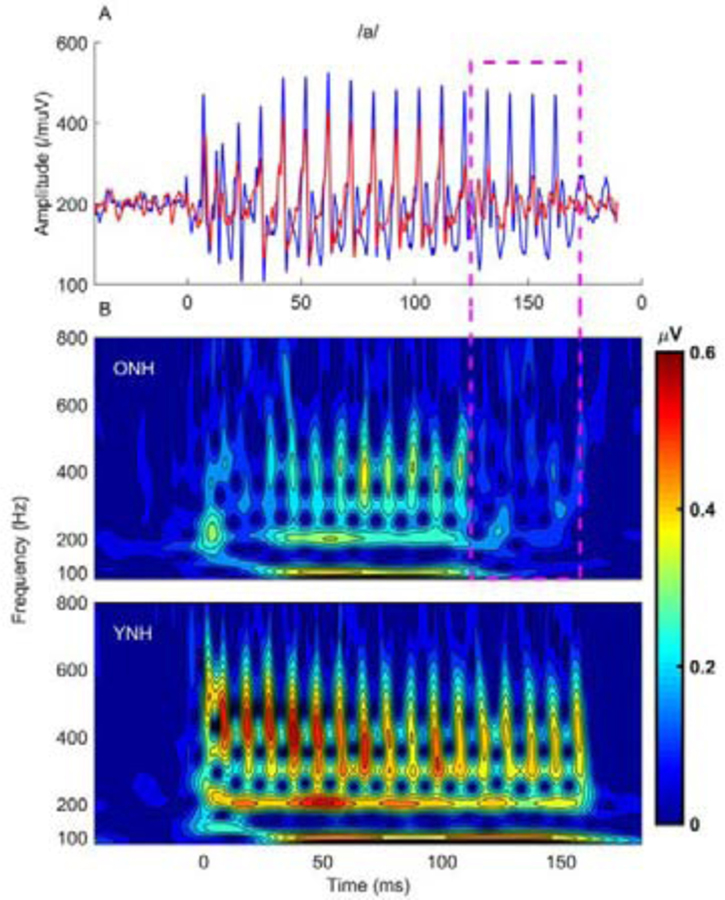
Although the Clinard et al. (2010) study did not find aging effects in FFRs to low-frequency 500-Hz static tones, later studies found reduced phase locking/neural synchrony when using stimuli that contain sustained vowel regions (Anderson et al., 2012; Bidelman et al., 2014; Presacco et al., 2016b; Presacco et al., 2015). In particular, one finding that could not be easily explained by peripheral hearing loss was the abrupt drop in synchrony at ~110 ms in ONH listeners’ responses to a sustained 170-ms [a] stimulus (Presacco et al., 2015) (Fig. 4). The authors suggested that an age-related reduction in auditory nerve fibers may limit the auditory system’s ability to sustain neural firing, similar to the phenomenon of excessive stapedial reflex decay that is associated with the presence of vestibular schwannomas on the VIII nerve (Olsen et al., 1975). An alternate explanation for these results would be prolonged neural recovery and fatigue in the older listeners, as suggested by the findings of Walton et al. (1998) who observed that neurons in the IC had delayed recovery times in older compared to younger mice.
Fig. 4.
An abrupt loss of synchrony in the later response regions to the vowel [a] is noted in older normal-hearing (ONH) listeners’ responses in panels A and B. This drop was not seen in the younger normal-hearing (YNH) listeners. The dashed pink rectangle delineates this later region where a dramatic decrease in response amplitude (A) and frequency energy (B) can be seen in the ONH responses. Modified with permission from Presacco et al. (2015).
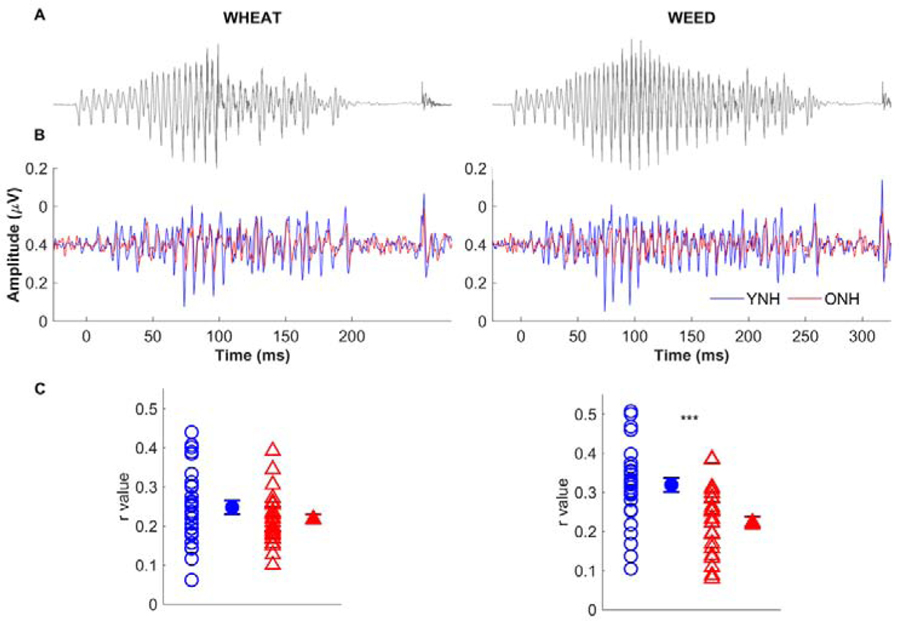
Previous behavioral studies have demonstrated an age-related perceptual deficit in the ability to use duration cues, such as vowel duration to cue final voicing or silence duration to cue an affricate vs. fricative (Gordon-Salant et al., 2008; Gordon-Salant et al., 2006). Roque et al. (2019 a,b) investigated the potential neural basis of these deficits by recording FFRs to word contrasts that were identical except for a silence duration cue (DISH-DITCH; 2019b) in 15 YNH, 15 ONH, and 15 OHI listeners and a vowel duration cue (WHEAT-WEED; 2019a) in 30 YNH and 30 ONH listeners. They found that STR correlations were lower in the ONH and OHI listeners for the words with longer duration cues, DITCH and WEED, compared to the YNH listeners. These same group differences were not noted for DISH or WHEAT and they surmised that the older listeners had degraded neural representation of the longer duration cues in DITCH and WEED. The lower STR correlation in DITCH but not DISH may arise from a reduction in the aging auditory system’s ability to detect short gaps or silent durations that naturally occur in speech (Walton et al., 1998). The lower STR correlation in WEED but not WHEAT (Fig. 5) may arise from an age-related reduction in the ability to sustain neural firing to the longer vowel, associated with a loss of auditory nerve fibers (Schmiedt et al., 1996) or delayed neural recovery (Walton et al., 1998). Upon close examination of the waveforms, one can see a breakdown in neural synchrony in the latter part of the vowel region in the ONH listeners (Fig. 5). The drop in synchrony was not as abrupt as it was in the Presacco et al. (2015) study, perhaps because the F0 and formants were not as “steady” for the naturally-produced WEED as they were for the synthesized vowel [a]. We note that few studies have investigated aging deficits in the subcortical representation of naturally-produced words, and it would be important to determine if these results can be replicated in another study.
Fig. 5.
Stimulus-to-response correlation ‘r’ values are lower in ONH than in YNH listeners for the word WEED but not for WHEAT. Panel A shows stimulus waveforms that differ only in vowel duration. Panel B shows response waveforms in young normal-hearing (YNH, blue) and older normal-hearing (ONH, red) listeners. A drop in neural synchrony is apparent in the ONH listeners at approximately 185 ms. Panel C displays individual (open symbols) and mean (closed symbols) for YNH (blue circles) and ONH (red triangles) listeners. Error bars: ± 1 standard error. ***p < 0.001. Modified with permission from Roque et al. (2019a).
Hearing loss
While the above-mentioned studies recruited listeners with clinically-normal hearing thresholds, the role of hearing loss cannot be ruled out given the inevitable elevation of high-frequency thresholds in the older listeners. For example, Vander Werff and Burns (2011) found that 18 ONH listeners had reduced amplitudes and delayed latencies to a 40-ms [da] syllable compared to 19 YNH listeners, but after covarying for high-frequency hearing thresholds, aging effects were limited to the onset amplitude and offset latency. Similar combined contributions of age and hearing were found in a study that recorded responses to a 40-ms [da] syllable in NH participants in whom age was a continuous variable (22–77) (Clinard and Tremblay, 2013). Therefore, it is important to consider hearing loss effects on the FFR when evaluating aging effects in participants with audiometrically-normal hearing thresholds.
Based on animal models demonstrating that hearing loss results in enhanced envelope encoding in the auditory nerve/midbrain (Dong et al., 2010; Kale and Heinz, 2010; Zhong et al., 2014), it might be expected that the FFR would also be enhanced with age-related hearing loss. To our knowledge, however, only one study showed larger F0 magnitudes in 15 OHI listeners compared to 15 age-matched ONH listeners in recordings of responses to a 40-ms [da] syllable (Anderson et al., 2013c). This finding has not been replicated in any subsequent studies. Most studies that have compared FFRs in ONH, OHI, and YNH listeners have either found no differences in response magnitude/phase locking between ONH and OHI listeners (Presacco et al., 2019; Roque et al., 2019b) or reduced magnitudes in OHI listeners (Ananthakrishnan et al., 2016; Hao et al., 2018). The Anderson et al. findings may have been the result of false discovery, but it is also possible that the subsequent studies did not replicate the findings because the ages of the groups were not matched, and it is expected that age would reduce amplitudes. Another explanation for differing findings is that different etiologies of sensorineural hearing loss may produce different responses at suprathreshold levels. For example, an individual’s hearing loss may be dominated by cochlear hair cell loss and cochlear synaptopathy to different degrees (Verhulst et al., 2018; Verhulst et al., 2016). Cochlear hair cell loss is associated with broader tuning curve and steeper rate-level functions, two factors that contributed to enhanced envelope coding in chinchillas with moderate-to-severe noise-induced hearing loss (Kale and Heinz, 2010). In contrast, age-related cochlear synapatopathy in CBA mice, resulting in a progressive loss of synapses with intact outer hair cells, is associated with reduced EFR amplitudes (Parthasarathy and Kujawa, 2018). We speculate that a measure that could differentiate between these sources of hearing loss may further the understanding of the effects of hearing loss on temporal processing.
Animal studies
As reviewed above, most animal studies have recorded EFRs to AM tones to evaluate aging effects. Recently, however, Parthasarathy et al. (2019) recorded single-unit and multi-unit responses to a 260-ms speech syllable [ba] in younger and older Fischer-344 rats to better understand the mechanisms contributing to age-related decreases in speech processing in humans. Although ABR Wave I amplitude was reduced for the older vs. younger rats, there was an age-related increase in synchronization to the temporal envelope measured in multi-unit neural activity from the IC. Furthermore, despite reduced synaptic input to IC neurons in local field potential recordings, the population response of the IC showed over-representation of the envelope in older vs. younger animals, suggesting a compensatory central gain mechanism along the auditory pathway. This enhancement of the envelope may be associated with changes in the balance of neural representation of the envelope vs. the temporal fine structure and subsequent reduction in decreased speech discrimination (Guo et al., 2017).
Summary
Aging may reduce synchronization to sustained stimulus components and may degrade processing of duration components of stimuli. Latency delays to the onset and transition regions of the speech stimulus appear to be most affected by subtle degrees of hearing loss. Synchronization can be enhanced, however, given a sufficient degree of cochlear hearing loss, consistent with a compensatory gain mechanism suggested by Parthasarathy et al. (2019).
Cortical auditory-evoked potentials (CAEP)
Aging
The EFR, FFR and ASSR studies reveal evidence of substantial degradation of the neural signal in brainstem and midbrain in individuals with ARHL. Yet, ONH listeners generally understand speech well in quiet or easy listening situations. The cortex has a remarkable ability to at least partially compensate for a degraded signal, as demonstrated by a gradual restoration of tone-evoked neural firing rates in IC and cortex and near-normal tone detection in mice after ouabain-induced cochlear denervation (Chambers et al., 2016). In this study, however, the compensatory plasticity necessary to generate these responses was not sufficient to restore the timing precision required for encoding speech stimuli. An examination of cortical responses may therefore lead to a better understanding of the limits of compensatory plasticity for understanding speech.
As mentioned previously, the ability to detect short gaps appears to be important for speech understanding, especially in noise. Furthermore, lesion studies in rats have demonstrated that the auditory cortex is necessary for perceptual identification of gaps (Ison et al., 1991; Syka et al., 2002). The gap termination response, a burst of neural firing in auditory cortex at the end of a gap, enables the listener to perceive the gap (Weible et al., 2014). Therefore, cortical responses to gaps in noise may reveal neural mechanisms underlying poor gap detection ability in older listeners. Cortical recordings to gaps embedded in noise reveal the traditional onset response (P1-N1-P2 peaks) to the onset of the noise stimulus and a second smaller onset response to onset of the gap. Lister et al. (2011) recorded CAEPs from 24 ONH listeners to silent gaps embedded between two segments of narrow-band noise. They compared these results to previous data collected in 12 YNH listeners (Lister et al., 2007) and found that the ONH listeners had delayed P2 latencies compared to YNH listeners (significant after correcting for multiple comparisons). They suggested that altered auditory inhibition and slower neural conduction times accounted for the aging differences.
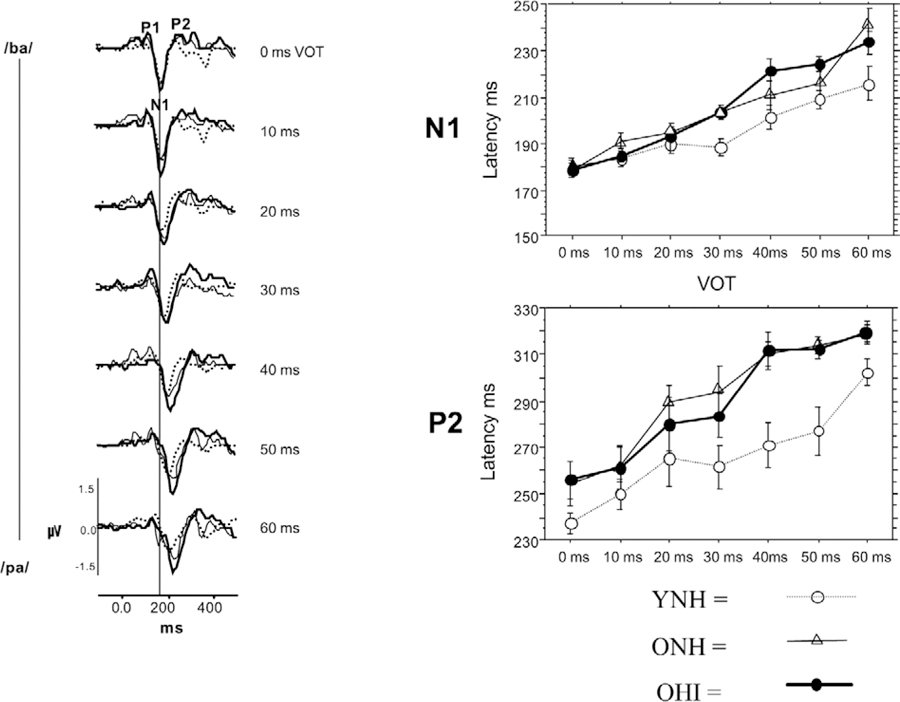
Age-related decreases in processing of gaps in noise may extend to speech stimuli. Tremblay et al. (2003) investigated perceptual and cortical representation of voice-onset-time (VOT), a temporal contrast that enables one to distinguish between voiced and unvoiced consonants in YNH, ONH, and OHI listeners (n=10 per group). They used a synthesized [pa] to [ba] continuum that gradually decreased the VOT in 10-ms steps. They found that the ONH and OHI listeners had more difficulty discriminating the 10-ms VOT contrasts than the YNH listeners. They also recorded CAEPs to the 7-step VOT continuum and found that 1) latency of the N1 and P2 peaks increased with increasing VOT, 2) the effect of VOT on latency was greater in the ONH and OHI listeners than in the YNH listeners, and 3) the latencies were equivalent between the ONH and OHI listeners (Fig. 6). The authors suggested a number of possible neural mechanisms for the peak delays observed in the older listeners, including delayed neural refraction, decreased neural synchrony, and a broader distribution of individual neural firing to the onset of the syllable. They also observed that restoration of audibility through the use of hearing aids may not entirely ameliorate central processing deficits that contribute to decreased perception in older listeners. However, extended use of amplification may improve some aspects of impaired temporal processing that have resulted from a lack of afferent input (Karawani et al., 2018a; Karawani et al., 2018b; Rao et al., 2017).
Fig. 6.
Left panel: Group average waveforms show that increasing voice-onset-time (VOT) results in increased latencies of P1, N1, and P2 peaks of the cortical auditory-evoked response. Right panels: N1 and P2 peaks show greater delays with increasing VOTs in older normal-hearing (ONH, filled circles) and older hearing-impaired (OHI, open triangles) listeners compared to young normal-hearing (YNH, open circles) listeners. Error bars: 1 standard error. Used with permission from Tremblay et al. (2003).
The Roque et al. (2019a,b) studies cited in the FFR section also recorded CAEPs to words that differed in silent duration (DISH vs. DITCH) and vowel duration (WHEAT vs. WEED). The effects on cortical peaks differed between the studies. In the vowel duration study (Roque et al., 2019a), which included YNH and ONH listeners, the aging effects were limited to the P1 peak-decreased latency and increased amplitude for WEED and decreased latency for WHEAT in ONH vs YNH listeners. In the silence duration study (Roque et al., 2019b), which included YNH, ONH, and OHI listeners, the only group effect on amplitude was a large N1 amplitude in the OHI listeners compared to either the ONH or YNH listeners. In addition, P2 latency was delayed in the ONH and OHI listeners relative to the YNH listeners. These differing aging effects between the studies may be explained by stimulus and subject differences. The WHEAT/WEED stimuli begin with a glide and the DISH/DITCH stimuli begin with stop consonant. The transient in the stop consonant may generate more synchronous firing than the glide consonant and may be less sensitive to age differences that would be present for the P1 component. Furthermore, the hearing levels and ages were higher in the DISH/DITCH study than in the WHEAT/WEED study. The silence duration study recruited older subjects ≥ 60 yrs of age with hearing levels ≤ 25 dB HL from 125–4000 Hz and no hearing limit at 6000 and 8000 Hz. In an effort to reduce the effects of aging, the subsequent vowel duration study recruited older subjects who were younger than in the previous study (≥ 55 yrs of age) and had better hearing thresholds (≤ 20 dB HL from 125–4000 Hz and ≤ 30 dB HL at 6000 and 8000 Hz). Therefore, the P2 latency age effects in the silence duration study may be due to either age and/or hearing loss effects. The P2 peak is a putative marker of object identification (Näätänen and Winkler, 1999; Ross et al., 2013), and an imprecise stimulus representation associated with age or hearing loss may lead to delays in processing this component. The N1 amplitude effect in the silence duration study was only seen in the OHI listeners, and this group was not recruited in the vowel duration study. Another possible reason for the discrepancies between the two studies is a random statistical variation rather than a true aging difference; this possibility is especially likely given the large range of normal values for cortical peak latencies and amplitudes (Zhang et al., 2009).
Hearing loss
Effects of hearing loss are best evaluated in age-matched groups, such as in the study conducted by Campbell and Sharma (2013). They recorded CAEPs to nonsense syllables in middle-aged listeners (37–68 yrs) using a 128-channel electrode cap. They found that P2 latency was smaller in amplitude and delayed in latency in nine HI listeners compared to eight age-matched NH listeners. The levels of hearing loss in this study were relatively mild; therefore, aging effects on the P2 effect in previous studies (Lister et al., 2011; Roque et al., 2019b) may reflect small but significant differences in high-frequency thresholds. In another study that employed linear mixed-effects modeling, no hearing loss effects were noted for N1 and P2 (Koerner and Zhang, 2018) in 18 listeners that varied in age from 40–71 and had hearing levels from normal to moderate; rather aging effects were noted for N1 and P2. Small sample sizes in these and other studies may limit interpretation and replicability of findings (Billings and Madsen, 2018). Another important factor that may limit reproducibility between studies is the stimulus presentation rate; age-related latency changes in amplitude and latency for CAEP peaks may be present for faster but not for slower rates (Tremblay et al., 2004).
Although the above-mentioned studies found age-related decreases in the representation of the temporal components in speech, even when presented in quiet, the most frequent complaint voiced by most older adults is difficulty understanding speech in background noise. To address this complaint, studies have investigated aging effects on neural processing of speech in noise. For example, Billings et al. (2015) investigated the effects of SNR and level and compared CAEPs to the syllable [ba] presented at 50, 60, 70, and 80 dB SPL and SNRs of −5, 15, 25, and 35 dB in 15 ONH and 15 OHI listeners and compared these data to previously published data in 15 YNH listeners (Billings et al., 2013). They found robust effects of SNR on almost all peak latencies and amplitudes, but effects of level were limited and specific to the OHI listeners. Age-related latency delays across SNR conditions were most pronounced for the latency of N1, P2, and N2 peaks. Similar to the Tremblay et al. (2003) study, they did not find latency differences between the ONH and OHI listeners, suggesting that an age-related decrease in neural synchrony may be the dominant factor for P2 latency in this study. The authors’ initial hypotheses concerning hearing loss and aging effects were not specific to any particular peak amplitude or latency, and therefore the results were exploratory in nature. Given the relatively small sample sizes, and the large number of statistical comparisons, it would be important to confirm these results in another study.
Animal Studies
As noted above, age-related changes in the balance of neural inhibition and excitation may partially account for a reduction in temporal precision (Caspary et al., 2008). These changes extend from dorsal cochlear nucleus (Caspary et al., 2005; Schatteman et al., 2008) to cortex (Cisneros-Franco et al., 2018; Hughes et al., 2010; Overton and Recanzone, 2016; Turner et al., 2005). Recanzone (2018) reviewed the evidence for three potential types of temporal processing deficits associated with decreased inhibition in cortex: 1) a decrease in the number of neurons responsive to temporally modulated stimuli, 2) a change in the neural code that transmits temporal information, and 3) a decrease in the temporal fidelity of the neural response. In rhesus macaques, the number of neurons tuned to temporally modulated stimuli does not differ between younger and older macaques; however, the coding strategy of the aged neurons shifts such that there is an overall increase in firing rate which reduces the dynamic range necessary for encoding the envelope (Overton and Recanzone, 2016; Yin et al., 2011). These studies also demonstrated an age-related reduction in the vector strength of cortical neurons, resulting in decreased response fidelity.
Summary
Combined results from humans and animals suggest that central compensation, associated with changes in the balance of neural excitation and inhibition, may result in increased neural firing and exaggerated cortical amplitudes, even in listeners with clinically normal audiometric thresholds. However, latency delays and decreased temporal precision reduce the fidelity of signal representation, likely contributing to perceptual deficits. We note inconsistency across studies in the effects of age and hearing loss on cortical peak latencies and amplitudes; given the large normal variability in these peak measures, we suggest that investigators conduct studies with larger numbers of subjects based on power analyses that accurately include numbers of conditions and variables.
Cortical envelope tracking
In recent years, there has been increased interest in the use of continuous speech samples to obtain a more ecologically valid measure of neural speech processing (Decruy et al., 2019; Ding and Simon, 2012; Etard et al., 2019; O’Sullivan et al., 2015; Presacco et al., 2016a; Presacco et al., 2016b; Presacco et al., 2019; Vanthornhout et al., 2018). These studies typically present selections from recorded audiobooks in quiet or in the presence of background noise, such as a single competing talker. The recorded signals are analyzed by using temporal response functions (TRFs) to facilitate mapping of speech features onto the EEG/MEG signal (Brodbeck et al., 2018; Wong et al., 2018) or by reconstructing the speech envelope from the neural signal (Ding et al., 2014; Presacco et al., 2016b; Vanthornhout et al., 2018). The TRF analysis produces a cortical waveform with peaks that mirror those of the CAEP (O’Sullivan et al., 2015), while reconstruction accuracy is assessed by linearly correlating the cortical response envelope with the speech envelope to obtain an ‘r’ value. A higher ‘r’ value would indicate higher reconstruction accuracy.
Aging
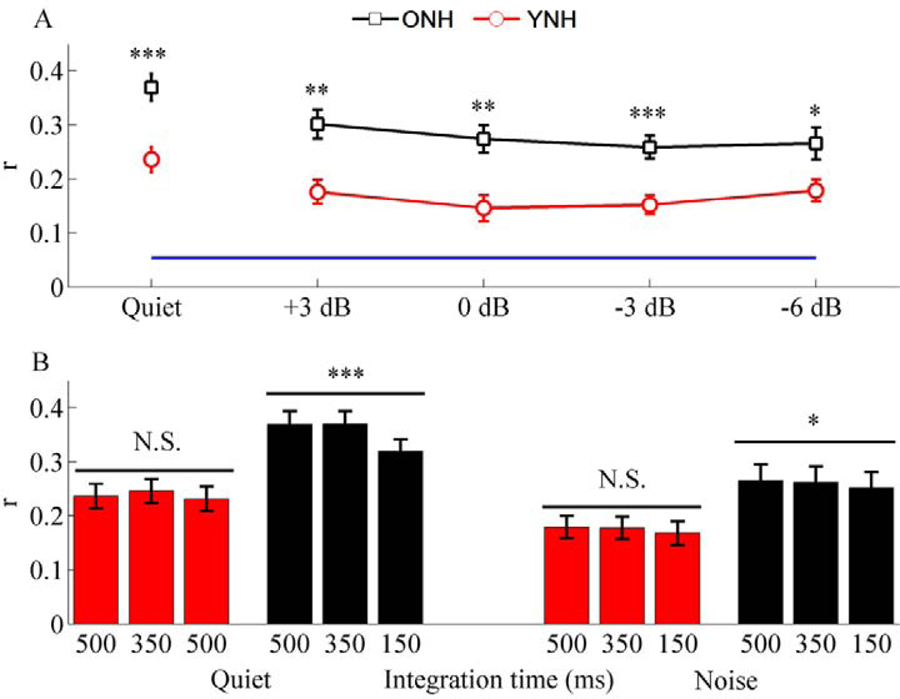
Using MEG recordings, Presacco et al. (2016b) discovered that ONH listeners (n=15) had higher reconstruction accuracy ‘r’ values than YNH listeners (n=17). The participants listened to audiobook recordings and were instructed to attend to the male talker and ignore the female talker. The male speaker was presented at ~70 dB SPL while the female speaker’s level was varied to produce SNRs of +3, 0, −3, and −6 dB SNR. An aging effect of higher reconstruction values was found across SNR conditions. The authors had hypothesized that higher fidelity (higher reconstruction accuracy) would relate to better speech-in-noise understanding, and they did not find any relationships between behavioral and neural measures. This over-representation is consistent with previous studies that found higher amplitudes to early components (P1m and N1m) in older vs. younger listeners’ MEG responses to rapidly occurring speech sounds (Sörös et al., 2009) and to the EEG equivalents of these responses (P1 and N1) (Roque et al., 2019a; Snyder and Alain, 2005; Tremblay et al., 2003). Presacco et al. (2016b) also found that reconstruction accuracy decreased when the temporal integration window was reduced from 500 to 150 ms in the ONH listeners but not in the YNH listeners, consistent with a temporal processing deficit (Fig. 7). Interestingly, FFRs to the speech syllable [da] in these same subjects showed lower response amplitudes in the ONH than in the YNH listeners. The authors suggested that the exaggerated amplitudes seen in cortex may arise from a similar central compensatory mechanism to that described in Chambers et al. (2016). In the Chambers study, the central compensation was not complete in IC, but was exaggerated in cortex. The FFRs to the [da] may contain cortical contributions but are likely dominated by responses from IC (Bidelman, 2018; Coffey et al., 2016; Smith et al., 1975; Tichko and Skoe, 2017; White-Schwoch et al., 2019). Therefore, the combined responses from FFR and MEG suggest that degradation evident in midbrain is over-compensated in cortex.
Fig. 7.
Panel A: Older listeners’ cortical responses (ONH; black squares) show higher reconstruction accuracies (r values) in quiet and across SNR conditions compared to younger responses (YNH; red circles). Responses of both groups are above the noise floor (blue line). Panel B: ONH responses show a reduction in reconstruction accuracy as the integration time window is narrowed from 500 ms to 150 ms in both quiet and noise conditions, but the YNH responses do not show a similar decrease in reconstruction accuracy. Error bars: ± 1 standard error. *p < 0.05, **p < 0.01, ***p < 0.001, N.S. = not significant. Modified with permission from Presacco et al. (2016a).
Although the Presacco et al. (2016) study had hypothesized that the older listeners’ responses would show higher reconstruction accuracy, they were nevertheless surprised regarding the magnitude of the effect which was seen across listening conditions. Therefore, it would be important to replicate this finding in another study. Decruy et al. (2019) also demonstrated this over-compensation by recording EEG to continuous speech stimuli at subject-specific SNRs. They included a middle-aged group of NH listeners so that they had a continuous distribution of age from 17 to 82 years (n=54). They replicated the finding of an increase in envelope tracking (reconstruction accuracy) with aging, and they noted that for both envelope tracking and speech-in-noise performance there was an accelerated trajectory in the ONH listeners. Three explanations may be posited for over-representation of the envelope in ONH listeners: 1) ONH listeners may rely more on the low modulation frequencies that are important for speech understanding to compensate for perceived speech understanding difficulties, leading to enhanced envelope tracking, 2) Given the negative correlation between cognitive measures of response inhibition and reconstruction accuracy found in both the Presacco et al. (2016b) and Decruy et al. (2019) studies, an inefficient use of cognitive resources may be a factor in over-representation of the speech envelope in older listeners, and 3) Reduced neural inhibition noted in animal models (Cisneros-Franco et al., 2018; de Villers-Sidani et al., 2010; Hughes et al., 2010; Syka, 2002) or redundant local processing and reduced connectivity (Peelle et al., 2010) may lead to exaggerated responses. The field would benefit from future studies that can elucidate potential sources of age-related over-representation, perhaps in cross-species models.
Hearing Loss
To test whether audibility was a key factor in the exaggerated cortical responses, Presacco et al. (2019) repeated the experiment in a group of 14 OHI listeners. They found that the responses in the ONH and OHI listeners did not differ and reasoned that the exaggerated amplitudes resulted mainly from aging rather than hearing loss effects. Nevertheless, it was not possible to clearly distinguish the effects of aging and hearing loss in this study, especially as the OHI listeners were older than the ONH listeners, and the stimulus levels were not equated for audibility between the two groups.
To address audibility concerns, Petersen et al. (2017) fit their older listeners (n=27), whose hearing ability ranged from normal to severe hearing loss, with hearing aids that used quasi-linear amplification to limit distorting effects of compression. Audiobook stories were presented through the hearing aids via direct audio input, and envelope tracking was recorded with a 103-electrode EEG system. They found that the OHI listeners had robust envelope tracking to the attended speech signal, but they also had reduced attentional modulation (higher similarities between the attended and unattended speech signals) and benefitted to a lesser extent from higher SNRs than the ONH listeners. Two limitations in the Peterson et al. study were that there was no matching of age between the ONH and OHI listeners and that the quasi-linear amplification would have distorted the signal to some extent and consequently affecting envelope tracking. Decruy et al. (2020) addressed these issues by matching 14 NH and 14 HI listeners on age (ages ranged from 21–82) and by using a linear amplification algorithm to equate for audibility. Using 64-channel EEG recordings to Matrix sentences and to two- to three-minute recordings of an audiobook, they found enhanced envelope tracking to the target talker in HI compared to NH listeners. They suggested that the results reflect enhanced envelope sensitivity in individuals with hearing loss (Moore et al., 1996) or compensatory effort in separating the target from the background speech signal.
The Decruy et al. (2020) study included a range of ages and had relatively small sample sizes to investigate envelope tracking across many conditions (20 total for the HI listeners). Therefore, even though the participants were matched on age, it would be important to examine these effects in a larger sample to ensure that the findings can be replicated. Fuglsang et al. (2020) investigated envelope tracking to simultaneous, spatially separated attended and unattended speech streams in 22 NH and 22 HI listeners whose ages ranged from 51 to 76 years. The study’s purpose was to evaluate the effects of hearing loss on cortical synchronization to the attended and unattended speech streams, and to determine if hearing loss affected attention decoding accuracy. Similar to Decruy et al. (2019), they found enhanced reconstruction accuracy in the HI compared to NH listeners for the attended talker, but there were no group differences for the unattended talker. They evaluated attention decoding accuracy by comparing reconstruction accuracy for the attended vs. unattended speech stream. They did not find an effect of hearing loss on attention decoding accuracy, and it was above chance level in both groups. These results have implications for the future of “neuro-steered” hearing aids. If the object of the listener’s attention can be identified with single-trial EEG decoding, the hearing aid algorithm may then selectively amplify that particular signal.
Animal Studies
To our knowledge, no studies have yet been published that investigated aging effects on cortical envelope tracking to continuous speech samples in an animal model. However, results from a study that investigated age-related changes in cortical processing of rapidly-presented sound sequences in rhesus macaques are consistent with the above-listed findings in older humans (Ng and Recanzone, 2018). In this study, a sparse, selective strategy was observed in the auditory cortex of young monkeys; in contrast, increased neural excitability and reduced temporal selectivity was observed in older monkeys. The authors surmised that a loss of cortical inhibition may underlie a change from sparse to more diffuse encoding with increased age.
Summary
A consistent age-related increase in envelope tracking has been revealed across studies. At this time, it is unclear how much of this increase is due to aging or hearing loss. The studies that have investigated hearing loss effects on envelope tracking have differed, likely due to the difficulty in finding a sufficient number of participants who differ in hearing thresholds but are matched in age. However, Fuglsang et al. (2020) found increased envelope tracking in a relatively large number of HI participants compared to age-matched NH participants. Cascading effects of reduced afferent input with aging that is compounded with hearing loss may lead to decreased inhibition and increased neural excitability (Ng and Recanzone, 2018).
Summary and Clinical Implications
Temporal processing deficits have been identified in older listeners across a wide range of neurophysiological measures that assess the auditory system from the auditory nerve to cortex. However, the relative contributions of aging and hearing loss are difficult to tease apart, as high-frequency hearing loss is an inevitable consequence of aging across most species. Therefore, it is not possible to state conclusively that central processing deficits, as observed in objective measures, result from aging effects that are independent of decreased afferent input from the cochlea and the auditory nerve.
Despite the difficulty of separating contributions of hearing vs. aging factors, objective measures of temporal processing may be useful components of the audiological assessment. These measures may reveal impaired neural representation that can be compensated on tests of clinical speech understanding, through increased effort or engagement of cognitive resources (Kuchinsky et al., 2013; Zekveld et al., 2018). The effort needed to perform well on a clinical measure would not be sustainable in everyday settings without incurring fatigue; therefore, behavioral clinical measures may fall short in estimating functional hearing ability. Even though there may be a need for an objective clinical measure, the most efficacious measures for this purpose has not yet been identified. The three main requirements for a clinical assessment, reliability, sensitivity, and specificity (Roeser et al., 2007), have not yet been achieved in any of the measures discussed in this review. For example, the ABR has high reliability, at least for latency, but its sensitivity for identifying speech understanding deficits has not yet been demonstrated. The FFR also has high reliability and FFR deficits appear to relate to speech perception deficits, especially in noise, but most studies that have investigated the role of the FFR in speech understanding in older listeners have been correlative in nature (Anderson et al., 2011; Hao et al., 2018; McClaskey et al., 2019; Roque et al., 2019b), and it would be important to demonstrate its sensitivity/specificity in specific clinical populations. Finally, there has been a great deal of interest in the clinical utility of envelope tracking, especially as ecologically-valid speech samples are used in the measurement. However, more work needs to be done with this measure to improve the ability to detect the response above neural noise, and to ensure that the measure reflects speech understanding itself, rather than the brain’s locking onto specific acoustical attributes of the stimulus.
Although implementation of objective measures into clinical test protocols is not yet routine, these findings have implications for patient counseling regarding management of hearing difficulties (Davis et al., 2016). Patients often complain about having difficulty understanding people who speak rapidly or in a noisy background, and they may wonder why their audiogram shows normal thresholds. They may benefit from communication strategies to improve successful communication, such as maximizing the use of visual cues, positioning oneself to reduce background noise, or asking the speaker to speak more slowly and clearly (Johnson et al., 2018).
For patients with hearing loss, the primary form of management is the provision of devices to improve audibility through the use of hearing aids, or in the case of severe to profound hearing loss, cochlear implants. But these studies demonstrate that audibility alone is not sufficient to achieve speech-in-noise performance comparable to that of younger listeners. For this reason, studies have been conducted to determine the potential benefits of auditory training, but the results have been mixed, especially for commercial programs (Henshaw and Ferguson, 2013). Some studies have demonstrated improvement in speech understanding in both normal-hearing and hearing-impaired listeners (Anderson et al., 2013a; Anderson et al., 2013b; Burk and Humes, 2007; Ferguson and Henshaw, 2015), but generalization to untrained stimuli is generally limited (Karawani et al., 2015). And, a recent randomized-control trial in a large group of veterans failed to show any additional benefit of training over the use of amplification alone (Saunders et al., 2016). More work is needed to determine the most efficacious means of treatment, and it would be important to identify the specific processing deficits that could be targeted for remediation.
An understanding of the specific nature of temporal processing deficits is hindered by inconsistent or conflicting results across studies. These inconsistencies may arise from a number of issues in aging EEG research. It is difficult to recruit older listeners with normal hearing, and for this reason, the study sample sizes are typically small. Furthermore, many of these studies are exploratory, rather than hypothesis-driven, assessing aging effects across a wide range of stimulus conditions or EEG measures, increasing the possibility of a significant finding that is actually a random statistical variation. The field would benefit from rigorous studies with a theoretical framework based on animal models, human perception, and previous human EEG studies.
Conclusion
Neural degradation at early levels of processing and overcompensation for this degradation in cortex may contribute to speech understanding difficulties experienced by older listeners. More work is needed to tease apart degradation associated with cochlear dysfunction versus more central pathologies. New clinically-feasible measures are needed to provide a comprehensive assessment of the listener’s speech understanding abilities.
Highlights.
Older normal-hearing listeners experience neural degradation at early levels of the auditory system
Neural degradation in brainstem/midbrain is over-compensated in auditory cortex
Hearing loss leads to exaggeration of neural responses; but this exaggeration may be diminished with advanced aging
Acknowledgments
The authors of this review were supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health (NIH) under Award number R21DC015843 (Anderson). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ABR
Auditory brainstem response
- ASSR
Auditory steady-state response
- EEG
Electroencephalographic
- FFR
Frequency-following response
- EFR
Envelope-following response
- CAEP
Cortical auditory-evoked potential
- MEG
Magnetoencephalographic
- YNH
Young normal-hearing
- ONH
Older normal-hearing
- OHI
Older hearing-impaired
- peSPL
Peak-equivalent sound pressure level
- VOT
Voice-onset-time
- PTA
Pure-tone-average
- F0
Fundamental frequency
- ARHL
Age-related hearing loss
- SNR
Signal-to-noise ratio
- IC
Inferior Colliculus
- STR
Stimulus-to-response
- TRF
Temporal response function
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: There are no conflicts of interest to report.
References
- Abdala C, Dhar S 2012. Maturation and aging of the human cochlea: A view through the dpoae looking glass. Journal of the Association for Research in Otolaryngology 13, 403–421, 10.1007/s10162-012-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A 2012. A novel, jitter-based method for detecting and measuring spike synchrony and quantifying temporal firing precision. Neural Systems and Circuits 2, 5, 10.1186/2042-1001-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan S, Krishnan A, Bartlett E 2016. Human frequency following response: Neural representation of envelope and temporal fine structure in listeners with normal hearing and sensorineural hearing loss. Ear Hear. 37, e91–e103, 10.1097/AUD.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, Yi H-G, Kraus N 2011. A neural basis of speech-in-noise perception in older adults. Ear Hear. 32, 750–757, 10.1097/AUD.0b013e31822229d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N 2012. Aging affects neural precision of speech encoding. J. Neurosci. 32, 14156–14164, 10.1523/jneurosci.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Choi HJ, Kraus N 2013a. Training changes processing of speech cues in older adults with hearing loss. Front. Syst. Neurosci. 7, 1–9, 10.3389/fnsys.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N 2013b. Reversal of age-related neural timing delays with training. Proc. Natl. Acad. Sci. U S A 110, 4357–4362, 10.1073/pnas.1213555110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Drehobl S, Kraus N 2013c. Effects of hearing loss on the subcortical representation of speech cues. J. Acoust. Soc. Am. 133, 3030–3038, 10.1121/1.4799804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M 1989. Changes with aging in the levels of amino acids in rat CNS structural elements II. Taurine and small neutral amino acids. Neurochemical Research 14, 563–570, 10.1007/BF00964919. [DOI] [PubMed] [Google Scholar]
- Bauch CD, Olsen WO 1986. The effect of 2000–4000 hz hearing sensitivity on ABR results. Ear Hear. 7, 314–317, 10.1097/00003446-198610000-00004. [DOI] [PubMed] [Google Scholar]
- Bidelman GM 2018. Subcortical sources dominate the neuroelectric auditory frequency-following response to speech. Neuroimage 175, 56–69, 10.1016/j.neuroimage.2018.03.060. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Villafuerte JW, Moreno S, Alain C 2014. Age-related changes in the subcortical-cortical encoding and categorical perception of speech. Neurobiol. Aging, 10.1016/j.neurobiolaging.2014.05.006. [DOI] [PubMed]
- Billings CJ, Madsen BM 2018. A perspective on brain-behavior relationships and effects of age and hearing using speech-in-noise stimuli. Hear. Res. 369, 90–102, 10.1016/j.heares.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, McMillan GP, Penman TM, Gille SM 2013. Predicting perception in noise using cortical auditory evoked potentials. J. Assoc. Res. Otolaryngol. 14, 891–903, 10.1007/s10162-013-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, Penman TM, McMillan GP, Ellis EM 2015. Electrophysiology and perception of speech in noise in older listeners: Effects of hearing impairment and age. Ear Hear. 36, 710–22, 10.1097/aud.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL 1993. Age-related changes in auditory evoked potentials of gerbils. I. Response amplitudes. Hear. Res. 71, 137–145, 10.1016/0378-5955(93)90029-z. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Poth EA, Mills JH, Dubno JR 2001. The amplitude-modulation following response in young and aged human subjects. Hear. Res. 153, 32–42, 10.1016/s0378-5955(00)00255-0. [DOI] [PubMed] [Google Scholar]
- Brecht EJ, Barsz K, Gross B, Walton JP 2017. Increasing GABA reverses age-related alterations in excitatory receptive fields and intensity coding of auditory midbrain neurons in aged mice. Neurobiol. Aging 56, 87–99, 10.1016/j.neurobiolaging.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck C, Presacco A, Simon JZ 2018. Neural source dynamics of brain responses to continuous stimuli: Speech processing from acoustics to comprehension. Neuroimage, 10.1016/j.neuroimage.2018.01.042. [DOI] [PMC free article] [PubMed]
- Burk MH, Humes LE 2007. Effects of training on speech recognition performance in noise using lexically hard words. J. Speech Lang. Hear. Res. 50, 25, 10.1044/1092-4388(2007/003). [DOI] [PubMed] [Google Scholar]
- Burkard RF, Sims D 2001. The human auditory brainstem tesponse to high click rates: Aging effects. Am. J. Audiol. 10, 53–61, 10.1044/1059-0889(2001/008). [DOI] [PubMed] [Google Scholar]
- Burkard RF, Sims D 2002. A comparison of the effects of broadband masking noise on the Auditory Brainstem Response in young and older adults. Am. J. Audiol. 11, 13–22, 10.1044/1059-0889(2002/004). [DOI] [PubMed] [Google Scholar]
- Cai R, Montgomery SC, Graves KA, Caspary DM, Cox BC 2018. The FBN rat model of aging: investigation of ABR waveforms and ribbon synapse changes. Neurobiol. Aging 62, 53–63, 10.1016/j.neurobiolaging.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Sharma A 2013. Compensatory changes in cortical resource allocation in adults with hearing loss. Front. Syst. Neurosci. 7, 10.3389/fnsys.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canlon B, Illing RB, Walton J 2010. Cell biology and physiology of the aging central auditory pathway. Springer Handbook of Auditory Research 34, 39–74, [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH 1995. Central auditory aging: GABA changes in the inferior colliculus. Experimental Gerontology 30, 349–360, 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF 2002. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hear. Res. 168, 163–173, 10.1016/s0378-5955(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF 2005. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs. J. Neurosci. 25, 10952–10959, 10.1523/jneurosci.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Ling LL 2013. Age-related GABAA receptor changes in rat auditory cortex. Neurobiol. Aging 34, 1486–1496, 10.1016/j.neurobiolaging.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF 2008. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J. Exp. Biol. 211, 1781–1791, 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABA. 1988. Speech understanding and aging. J. Acoust. Soc. Am. 83, 859–895, 10.1121/1.395965. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB 2016. Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89, 867–79, 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar SS, Brackmann DE, Devgan KK 1995. Utility of auditory brainstem response audiometry in diagnosis of acoustic neuromas. Am. J. Otol. 16, 63–7, [PubMed] [Google Scholar]
- Cisneros-Franco JM, Ouellet L, Kamal B, de Villers-Sidani E 2018. A brain without brakes: Reduced inhibition is associated with enhanced but dysregulated plasticity in the aged rat auditory cortex. eNeuro 5, 10.1523/eneuro.0051-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard C, Tremblay K 2013. Aging degrades the neural encoding of simple and complex sounds. J. Am. Acad. Audiol. 24, 590–599, 10.3766/jaaa.24.7.7. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Cotter CM 2015. Neural representation of dynamic frequency is degraded in older adults. Hear. Res. 323, 91–8, 10.1016/j.heares.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR 2010. Aging alters the perception and physiological representation of frequency: Evidence from human frequency-following response recordings. Hear. Res. 264, 48–55, 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey EB, Herholz SC, Chepesiuk AM, Baillet S, Zatorre RJ 2016. Cortical contributions to the auditory frequency-following response revealed by MEG. Nature Communications 7, 11070, 10.1038/ncomms11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey E, Casseday JH 1999. Timing in the auditory system of the bat. Annu. Rev. Physiol. 61, 457–76, 10.1146/annurev.physiol.61.1.457. [DOI] [PubMed] [Google Scholar]
- Dau T 2003. The importance of cochlear processing for the formation of auditory brainstem and frequency following responses. J. Acoust. Soc. Am. 113, 936–50, 10.1121/1.1534833. [DOI] [PubMed] [Google Scholar]
- Davis A, Stephens D, Rayment A, Thomas K 1992. Hearing impairments in middle age: the acceptability, benefit and cost of detection (ABCD). Br. J. Audiol. 26, 1–14, 10.3109/03005369209077866. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RCS, Merzenich MM 2010. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proceedings of the National Academuy of Sciences - USA 107, 13900–13905, 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decruy L, Vanthornhout J, Francart T 2019. Evidence for enhanced neural tracking of the speech envelope underlying age-related speech-in-noise difficulties. J. Neurophysiol., 10.1152/jn.00687.2018. [DOI] [PMC free article] [PubMed]
- Decruy L, Vanthornhout J, Francart T 2020. Hearing impairment is associated with enhanced neural tracking of the speech envelope. bioRxiv, 815530, 10.1101/815530v2. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Alsamri J, John MS, Purcell D, George S, Zeng FG 2016. Human envelope following responses to amplitude modulation: Effects of aging and modulation depth. Ear Hear. 37, e322–35, 10.1097/aud.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ 2012. Emergence of neural encoding of auditory objects while listening to competing speakers. Proc. Natl. Acad. Sci. U S A 109, 11854–11859, 10.1073/pnas.1205381109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Chatterjee M, Simon J 2014. Robust cortical entrainment to the speech envelope relies on the spectro-temporal fine structure. NeuroImage 88, 41–46, 10.1016/j.neuroimage.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M, Allen A, Starr A 1977. Effect of click rate on the latency of auditory brain stem responses in humans. Ann Otol. Rhinol. Laryngol. 86, 186–195, 10.1177/000348947708600209. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WHAM, Rodger J, Woo S, Robertson D 2010. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. European Journal of Neuroscience 31, 1616–1628, 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks D, Morgan D 1984. Effects of age and mild hearing loss on speech recognition in noise. J. Acoust. Soc. Am. 76, 87–96, 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- Etard O, Kegler M, Braiman C, Forte AE, Reichenbach T 2019. Decoding of selective attention to continuous speech from the human auditory brainstem response. Neuroimage 200, 1–11, 10.1016/j.neuroimage.2019.06.029. [DOI] [PubMed] [Google Scholar]
- Fabijańska A, Smurzyński J, Hatzopoulos S, Kochanek K, Bartnik G, Raj-Koziak D, Mazzoli M, Skarżyński PH, Jędrzejczak WW, Szkiełkowska A, Skarżyński H 2012. The relationship between distortion product otoacoustic emissions and extended high-frequency audiometry in tinnitus patients. Part 1: normally hearing patients with unilateral tinnitus. Med. Sci. Monit. 18, CR765–CR770, 10.12659/msm.883606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MA, Henshaw H 2015. Auditory training can improve working memory, attention, and communication in adverse conditions for adults with hearing loss. Front. Psychol. 6, 556, 10.3389/fpsyg.2015.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentine M, Buus S, Scharf B, Zwicker E 1980. Frequency selectivity in normally-hearing and hearing-impaired observers. J. Speech Lang. Hear. Res. 23, 646–669, 10.1044/jshr.2303.646. [DOI] [PubMed] [Google Scholar]
- Fuglsang SA, Märcher-Rørsted J, Dau T, Hjortkjær J 2020. Effects of sensorineural hearing loss on cortical synchronization to competing speech during selective attention. J. Neurosci. 40, 2562–2572, 10.1523/jneurosci.1936-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihira H, Shiraishi K, Remijn GB 2017. Elderly listeners with low intelligibility scores under reverberation show degraded subcortical representation of reverberant speech. Neuroscience Letters 637, 102–107, 10.1016/j.neulet.2016.11.042. [DOI] [PubMed] [Google Scholar]
- Füllgrabe C, Moore BCJ, Stone MA 2015. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front. Aging Neurosci. 6, 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M, Verhulst S 2019. Applicability of subcortical EEG metrics of synaptopathy to older listeners with impaired audiograms. Hear. Res. 380, 150–165, 10.1016/j.heares.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Gaskins C, Jaekel BN, Gordon-Salant S, Goupell MJ, Anderson S 2019. Effects of aging on perceptual and electrophysiological responses to acoustic pulse trains as a function of rate. J. Speech Lang. Hear. Res. 62, 1087–1098, 10.1044/2018_jslhr-h-ascc7-18-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, van Wieringen A 2016. Aging affects neural synchronization to speech-related acoustic modulations. Front. Aging Neurosci. 8, 133, 10.3389/fnagi.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, van Wieringen A 2017. Masked speech perception across the adult lifespan: Impact of age and hearing impairment. Hear. Res. 344, 109–124, 10.1016/j.heares.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, van Wieringen A 2019. The association between hearing impairment and neural envelope encoding at different ages. Neurobiol. Aging 74, 202–212, 10.1016/j.neurobiolaging.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ 1993. Temporal factors and speech recognition performance in young and elderly listeners. J. Speech Hear. Res. 36, 1276–1285, 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons P 2001. Sources of age-related recognition difficulty for time-compressed speech. J. Speech Lang. Hear. Res. 44, 709–719, 10.1044/1092-4388(2001/056). [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ, Friedman SA 2007. Recognition of time-compressed and natural speech with selective temporal enhancements by young and elderly listeners. J. Speech Lang. Hear. Res. 50, 1181–1193, 10.1044/1092-4388(2007/082). [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Yeni-Komshian G, Fitzgibbons P 2008. The role of temporal cues in word identification by younger and older adults: Effects of sentence context. J. Acoust. Soc. Am. 124, 3249–3260, 10.1121/1.2982409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S, Yeni-Komshian GH, Fitzgibbons PJ, Barrett J 2006. Age-related differences in identification and discrimination of temporal cues in speech segments. J. Acoust. Soc. Am. 119, 2455–2466, 10.1121/1.2171527. [DOI] [PubMed] [Google Scholar]
- Grose JH, Mamo SK, Hall JW 3rd. 2009. Age effects in temporal envelope processing: speech unmasking and auditory steady state responses. Ear Hear. 30, 568–75, 10.1097/AUD.0b013e3181ac128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Clause AR, Barth-Maron A, Polley DB 2017. A corticothalamic circuit for dynamic switching between feature detection and discrimination. Neuron 95, 180–194.e5, 10.1016/j.neuron.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez A, Khan ZU, Morris SJ, De Blas AL 1994. Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J. Neurosci. 14, 7469–7477, 10.1523/JNEUROSCI.14-12-07469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao W, Wang Q, Li L, Qiao Y, Gao Z, Ni D, Shang Y 2018. Effects of phase-locking deficits on speech recognition in older adults with presbycusis. Front. Aging Neurosci. 10, 397, 10.3389/fnagi.2018.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Dubno JR 2017. Age-related deficits in auditory temporal processing: Unique contributions of neural dyssynchrony and slowed neuronal processing. Neurobiol. Aging 53, 150–158, 10.1016/j.neurobiolaging.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N-J, Mills JH, Ahlstrom JB, Dubno JR 2008. Age-related differences in the temporal modulation transfer function with pure-tone carriers. J. Acoust. Soc. Am. 124, 3841–3849, 10.1121/1.2998779w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa AN, Zhang L, Ashida G, Beutelmann R, Steenken F, Köppl C 2020. Temporal coding of single auditory nerve fibers is not degraded in aging gerbils. J. Neurosci. 40, 343–354, 10.1523/jneurosci.2784-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer KS, Wilber LA 1990. Hearing loss, aging, and speech perception in reverberation and noise. J. Speech Hear. Res. 33, 149–155, 10.1044/jshr.3301.149. [DOI] [PubMed] [Google Scholar]
- Helfer KS, Freyman RL 2014. Stimulus and listener factors affecting age-related changes in competing speech perception. J. Acoust. Soc. Am. 136, 748–59, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JA, Roberts LE, Caspary DM, Theodoroff SM, Salvi RJ 2014. Underlying mechanisms of tinnitus: review and clinical implications. J. Am. Acad. Audiol. 25, 5–22; quiz 126, 10.3766/jaaa.25.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MJ, Herrmann B, Kunke D, Obleser J 2017. Aging affects the balance of neural entrainment and top-down neural modulation in the listening brain. Nature Communications 8, 15801, 10.1038/ncomms15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw H, Ferguson MA 2013. Efficacy of individual computer-based auditory training for people with hearing loss: a systematic review of the evidence. PLoS ONE 8, e62836, 10.1371/journal.pone.0062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman AT, Lins O, Van Roon P, Stapells DR, Scherg M, Picton TW 2002. Intracerebral sources of human auditory steady-state responses. Brain Topogr. 15, 69–86, https://doi-org.proxy-um.researchport.umd.edu/10.1023/A:1021470822922. [DOI] [PubMed] [Google Scholar]
- Hoben R, Easow G, Pevzner S, Parker MA 2017. Outer hair cell and auditory nerve function in speech recognition in quiet and in background noise. Front. Neurosci. 11, 157, 10.3389/fnins.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins K, Moore BCJ 2011. The effects of age and cochlear hearing loss on temporal fine structure sensitivity, frequency selectivity, and speech reception in noise. J. Acoust. Soc. Am. 130, 334–349, 10.1121/1.3585848. [DOI] [PubMed] [Google Scholar]
- Hughes LF, Turner JG, Parrish JL, Caspary DM 2010. Processing of broadband stimuli across A1 layers in young and aged rats. Hear. Res. 264, 79–85, 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Burk MH, Coughlin MP, Busey TA, Strauser LE 2007. Auditory speech recognition and visual text recognition in younger and older adults: similarities and differences between modalities and the effects of presentation rate. J. Speech Lang. Hear. Res. 50, 283–303, 10.1044/1092-4388(2007/021). [DOI] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH, Wingfield A 2012. Central presbycusis: A review and evaluation of the evidence. J. Am. Acad. Audiol. 23, 635–666, 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter KP, Willott JF 1987. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hear. Res. 30, 207–18, 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Ison JR, O’Connor K, Bowen GP, Bocirnea A 1991. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav. Neurosci. 105, 33–40, 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- Jenstad LM, Souza PE 2007. Temporal envelope changes of compression and speech rate: combined effects on recognition for older adults. J. Speech Lang. Hear. Res. 50, 1123–38, 10.1044/1092-4388(2007/078). [DOI] [PubMed] [Google Scholar]
- Jerger J, Hall J 1980. Effects of age and sex on auditory brainstem response. Arch. Otolaryngol. 106, 387–391, 10.1001/archotol.106.7.387. [DOI] [PubMed] [Google Scholar]
- Jerger J, Johnson K 1988. Interactions of age, gender, and sensorineural hearing loss on ABR latency. Ear Hear. 9, 168–176, 10.1097/00003446-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Kaga K, Shinoda Y, Suzuki JI 1997. Origin of auditory brainstem responses in cats: whole brainstem mapping, and a lesion and HRP study of the inferior colliculus. Acta Otolaryngol 117, 197–201, 10.3109/00016489709117768. [DOI] [PubMed] [Google Scholar]
- Kale S, Heinz M 2010. Envelope coding in auditory nerve fibers following noise-induced hearing loss. J. Assoc. Res. Otolaryngol. 11, 657–673, 10.1007/s10162-010-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karawani H, Jenkins K, Anderson S 2018a. Restoration of sensory input may improve cognitive and neural function. Neuropsychologia 114, 203–213, 10.1016/j.neuropsychologia.2018.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karawani H, Jenkins KA, Anderson S 2018b. Neural and behavioral changes after the use of hearing aids. Clin Neurophysiol 129, 1254–1267, 10.1016/j.clinph.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karawani H, Bitan T, Attias J, Banai K 2015. Auditory Perceptual Learning in Adults with and without Age-Related Hearing Loss. Front. Psychol. 6, 2066, 10.3389/fpsyg.2015.02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh KT, Beardsley JV 1979. Brain stem auditory evoked response: Ii. Clinical application in the assessment of patients with organic hearing loss. Ann Otol. Rhinol. Laryngol. 88, 11–21, 10.1177/00034894790880S402. [DOI] [PubMed] [Google Scholar]
- Koerner TK, Zhang Y 2018. Differential effects of hearing impairment and age on electrophysiological and behavioral measures of speech in noise. Hear. Res. 370, 130–142, 10.1016/j.heares.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, Austin DF 2012. Age-related changes in the auditory brainstem response. J. Am. Acad. Audiol. 23, 18–35, 10.3766/jaaa.23.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, Bradlow MA, Cunningham CJ, King CD, Koch DB, Nicol TG, McGee TJ, Stein LK, Wright BA 2000. Consequences of neural asynchrony: A case of AN. J. Assoc. Res. Otolaryngol. 01, 33–45, 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinsky SE, Ahlstrom JB, Vaden KI Jr., Cute SL, Humes LE, Dubno JR, Eckert MA 2013. Pupil size varies with word listening and response selection difficulty in older adults with hearing loss. Psychophysiology 50, 23–34, 10.1111/j.1469-8986.2012.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen PT, Kraemer A, Kempter R, Köppl C, Carr CE 2018. Auditory brainstem response wave III is correlated with extracellular field potentials from nucleus laminaris of the barn owl. Acta Acust. United Acust. 104, 874–877, 10.3813/AAA.919236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Sommer AL, Bartlett EL 2017. Age-related changes in envelope-following responses at equalized peripheral or central activation. Neurobiol. Aging 58, 191–200, 10.1016/j.neurobiolaging.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land R, Burghard A, Kral A 2016. The contribution of inferior colliculus activity to the auditory brainstem response (ABR) in mice. Hear. Res. 341, 109–118, 10.1016/j.heares.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Leigh-Paffenroth ED, Fowler CG 2006. Amplitude-modulated auditory steady-state responses in younger and older listeners. J. Am. Acad. Audiol. 17, 582–97, 10.3766/jaaa.17.8.5. [DOI] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM 2005. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience 132, 1103–1113, 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ 2007. Cortical evoked response to gaps in noise: within-channel and across-channel conditions. Ear Hear. 28, 862–78, 10.1097/AUD.0b013e3181576cba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ, Gonzalez VB 2011. Auditory evoked response to gaps in noise: Older adults. Int. J. Audiol. 50, 211–225, 10.3109/14992027.2010.526967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Macias S, Ness TV, Einevoll GT, Zhang K, Moss CF 2018. Neural timing of stimulus events with microsecond precision. PLoS Biology 16, e2006422, 10.1371/journal.pbio.2006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary C, Shin J, Kujawa S, Liberman MC, Merchant S 2011. Age-related primary cochlear neuronal degeneration in human temporal bones. J. Assoc. Res. Otolaryngol. 12, 711–717, 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo SK, Grose JH, Buss E 2016. Speech-evoked ABR: Effects of age and simulated neural temporal jitter. Hear. Res. 333, 201–9, 10.1016/j.heares.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F, Linley D, Carlyon RP, Gockel HE, Hopkins K, Plack CJ 2013. Subcortical neural synchrony and absolute thresholds predict frequency discrimination independently. J. Assoc. Res. Otolaryngol. 14, 757–766, 10.1007/s10162-013-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LJ, Lee FS, Mills JH, Dubno JR 1997. Extended high-frequency thresholds in older adults. J. Speech Lang. Hear. Res. 40, 208–14, https://doi./org/10.1044/jslhr.4001.208. [DOI] [PubMed] [Google Scholar]
- McClaskey CM, Dias JW, Harris KC 2019. Sustained envelope periodicity representations are associated with speech-in-noise performance in difficult listening conditions for younger and older adults. J. Neurophysiol, 10.1152/jn.00845.2018. [DOI] [PMC free article] [PubMed]
- Milbrandt JC, Caspary DM 1995. Age-related reduction of [3H]strychnine binding sites in the cochlear nucleus of the fischer 344 rat. Neuroscience 67, 713–719, 10.1016/0306-4522(95)00082-T. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Turgeon SM, Caspary DM 1996. GABAA receptor binding in the aging rat inferior colliculus. Neuroscience 73, 449–58, 10.1016/0306-4522(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Møller AR, Jannetta PJ 1983. Interpretation of brainstem auditory evoked potentials: results from intracranial recordings in humans. Scandinavian audiology 12, 125–33, 10.3109/01050398309076235. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Wojtczak M, Vickers DA 1996. Effect of loudness recruitment on the perception of amplitude modulation. J. Acoust. Soc. Am. 100, 481–489, 10.1121/1.415861. [DOI] [Google Scholar]
- Motlagh Zadeh L, Silbert NH, Sternasty K, Swanepoel W, Hunter LL, Moore DR 2019. Extended high-frequency hearing enhances speech perception in noise. Proc. Natl. Acad. Sci. U S A, 10.1073/pnas.1903315116. [DOI] [PMC free article] [PubMed]
- Näätänen R, Winkler I 1999. The concept of auditory stimulus representation in cognitive neuroscience. Psychol. Bull. 125, 826–859, 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- Ng CW, Recanzone GH 2018. Age-related changes in temporal processing of rapidly-presented sound sequences in the macaque auditory cortex. Cereb. Cortex 28, 3775–3796, 10.1093/cercor/bhx240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JA, Power AJ, Mesgarani N, Rajaram S, Foxe JJ, Shinn-Cunningham BG, Slaney M, Shamma SA, Lalor EC 2015. Attentional selection in a cocktail party environment can be decoded from single-trial eeg. Cereb. Cortex 25, 1697–706, 10.1093/cercor/bht355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK 2009. Mechanisms and genes in human strial presbycusis from animal models. Brain Research 1277, 70–83, 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen WO, Noffsinger D, Kurdziel S 1975. Acoustic reflex and reflex decay: Occurrence in patients with cochlear and eighth nerve lesions. Arch. Otolaryngol. 101, 622–625, 10.1001/archotol.1975.00780390036009. [DOI] [PubMed] [Google Scholar]
- Overton JA, Recanzone GH 2016. Effects of aging on the response of single neurons to amplitude-modulated noise in primary auditory cortex of rhesus macaque. J. Neurophysiol. 115, 2911–23, 10.1152/jn.01098.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM 1996. Physiology of the aged Fischer 344 rat inferior colliculus: responses to contralateral monaural stimuli. J. Neurophysiol. 76, 3114–25, 10.1152/jn.1996.76.5.3114. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Kujawa SG 2018. Synaptopathy in the aging cochlea: Characterizing early-neural deficits in auditory temporal envelope processing. J. Neurosci. 38, 7108–7119, 10.1523/jneurosci.3240-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL, Kujawa SG 2018. Age-related changes in neural coding of envelope cues: Peripheral declines and central compensation. Neuroscience 407, 21–31, 10.1016/j.neuroscience.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Herrmann B, Bartlett EL 2019. Aging alters envelope representations of speech-like sounds in the inferior colliculus. Neurobiol. Aging 73, 30–40, 10.1016/j.neurobiolaging.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JA, Hopkins C, Bartlett EL 2014. Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. J. Assoc. Res. Otolaryngol. 15, 649–61, 10.1007/s10162-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RD, Nimmo-Smith I, Weber DL, Milroy R 1982. The deterioration of hearing with age: frequency selectivity, the critical ratio, the audiogram, and speech threshold. J. Acoust. Soc. Am. 72, 1788–803, 10.1121/1.388652. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Wingfield A 2005. Dissociations in perceptual learning revealed by adult age differences in adaptation to time-compressed speech. J. Exp. Psychol. Hum. Percept. Perform. 31, 1315, 10.1037/0096-1523.31.6.1315. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M 2010. Neural processing during older adults’ comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb. Cortex 20, 773–782, 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen EB, Wostmann M, Obleser J, Lunner T 2017. Neural tracking of attended versus ignored speech is differentially affected by hearing loss. J. Neurophysiol. 117, 18–27, 10.1152/jn.00527.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SL, Gordon-Salant S, Fitzgibbons PJ, Yeni-Komshian G 2000. Frequency and temporal resolution in elderly listeners with good and poor word recognition. J. Speech Lang. Hear. Res. 43, 217–228, 10.1044/jslhr.4301.217. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, MacDonald E, Pass HE, Brown S 2007. Temporal jitter disrupts speech intelligibility: A simulation of auditory aging. Hear. Res. 223, 114–121, 10.1016/j.heares.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Barker D, Prendergast G 2014. Perceptual consequences of “hidden” hearing loss. Trends Hear. 18, 1–11, 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S 2016a. Effect of informational content of noise on speech representation in the aging midbrain and cortex. J. Neurophysiol. 116, 2356–2367, 10.1152/jn.00373.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S 2016b. Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J. Neurophysiol. 116, 2346–2355, 10.1152/jn.00372.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S 2019. Speech-in-noise representation in the aging midbrain and cortex: Effects of hearing loss. PLoS One 14, e0213899, 10.1371/journal.pone.0213899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Jenkins K, Lieberman R, Anderson S 2015. Effects of aging on the encoding of dynamic and static components of speech. Ear Hear. 36, e352–63, 10.1097/aud.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DW, John SM, Schneider BA, Picton TW 2004. Human temporal auditory acuity as assessed by envelope following responses. J. Acoust. Soc. Am. 116, 3581–3593, 10.1121/1.1798354. [DOI] [PubMed] [Google Scholar]
- Qiu C, Salvi R, Ding D, Burkard R 2000. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear. Res. 139, 153–71, 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Rabang CF, Parthasarathy A, Venkataraman Y, Fisher ZL, Gardner SM, Bartlett EL 2012. A computational model of inferior colliculus responses to amplitude modulated sounds in young and aged rats. Front Neural Circuits 6, 77, 10.3389/fncir.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Rishiq D, Yu L, Zhang Y, Abrams H 2017. Neural correlates of selective attention with hearing aid use followed by readmyquips auditory training program. Ear Hear. 38, 28–41, 10.1097/aud.0000000000000348. [DOI] [PubMed] [Google Scholar]
- Recanzone G 2018. The effects of aging on auditory cortical function. Hear. Res. 366, 99–105, 10.1016/j.heares.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser RJ, Valente M, Hosford-Dunn H 2007. Audiology: Diagnosis Thieme.
- Roque L, Karawani H, Gordon-Salant S, Anderson S 2019a. Effects of age, cognition, and neural encoding on the perception of temporal speech cues. Front. Neurosci. 13, 749, 10.3389/fnins.2019.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque L, Gaskins C, Gordon-Salant S, Goupell MJ, Anderson S 2019b. Age effects on neural representation and perception of silence duration cues in speech. J. Speech Lang. Hear. Res. 62, 1099–1116, 10.1044/2018_jslhr-h-ascc7-18-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Jamali S, Tremblay KL 2013. Plasticity in neuromagnetic cortical responses suggests enhanced auditory object representation. BMC Neuroscience 14, 151–151, 10.1186/1471-2202-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders GH, Smith SL, Chisolm TH, Frederick MT, McArdle RA, Wilson RH 2016. A randomized control trial: Supplementing hearing aid use with Listening and Communication Enhancement (LACE) auditory training. Ear Hear, 10.1097/aud.0000000000000283. [DOI] [PubMed]
- Schatteman TA, Hughes LF, Caspary DM 2008. Aged-related loss of temporal processing: Altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience 154, 329–337, 10.1016/j.neuroscience.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Adams JC 1990. Tuning and suppression in auditory nerve fibers of aged gerbils raised in quiet or noise. Hear. Res. 45, 221–236, 10.1016/0378-5955(90)90122-6. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA 1996. Age-related loss of activity of auditory-nerve fibers. J. Neurophysiol. 76, 2799–2803, 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Schoof T, Rosen S 2016. The role of age-related declines in subcortical auditory processing in speech perception in noise. J. Assoc. Res. Otolaryngol. 17, 441–60, 10.1007/s10162-016-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG 2013. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. 33, 13686–13694, 10.1523/jneurosci.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Krizman J, Anderson S, Kraus N 2015. Stability and plasticity of auditory brainstem function across the lifespan. Cereb. Cortex 25, 1415–26, 10.1093/cercor/bht311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Marsh JT, Brown WS 1975. Far-field recorded frequency-following responses: evidence for the locus of brainstem sources. Electroencephalography and Clinical Neurophysiology 39, 465–472, 10.1016/0013-4694(75)90047-4. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Alain C 2005. Age-related changes in neural activity associated with concurrent vowel segregation. Cognitive Brain Research 24, 492–499, 10.1016/j.cogbrainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Sörös P, Teismann IK, Manemann E, Lütkenhöner B 2009. Auditory temporal processing in healthy aging: a magnetoencephalographic study. BMC Neuroscience 10, 34–34, 10.1186/1471-2202-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapells D, Picton T, Perez-Abalo M, Read D, Durieux-Smith A 1985. Frequency specificity in evoked potential audiometry. pp. 147–177.
- Starr A, Picton TW, Sininger S, Hood LJ, Berlin CI 1996. Auditory Neuropathy. Brain 119, 741–753, 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Syka J 2002. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiological Reviews 82, 601–636, 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Syka J 2010. The Fischer 344 rat as a model of presbycusis. Hear. Res. 264, 70–78, 10.1016/j.heares.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Mazelova J, Druga R 2002. Gap detection threshold in the rat before and after auditory cortex ablation. Hear. Res. 172, 151–9, 10.1016/s0378-5955(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Tichko P, Skoe E 2017. Frequency-dependent fine structure in the frequency-following response: The byproduct of multiple generators. Hear. Res. 348, 1–15, 10.1016/j.heares.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Tlumak AI, Durrant JD, Delgado RE 2015. The effect of advancing age on auditory middle- and long-latency evoked potentials using a steady-state-response approach. Am. J. Audiol, 10.1044/2015_AJA-15-0036. [DOI] [PubMed]
- Torre P 3rd, Fowler CG 2000. Age-related changes in auditory function of rhesus monkeys (Macaca mulatta). Hear. Res 142, 131–40, 10.1016/s0378-5955(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Piskosz M, Souza P 2003. Effects of age and age-related hearing loss on the neural representation of speech cues. Clinical Neurophysiology 114, 1332–1343, 10.1016/S1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Billings C, Rohila N 2004. Speech evoked cortical potentials: Effects of age and stimulus presentation rate. J. Am. Acad. Audiol 15, 226–237, 10.3766/jaaa.15.3.5. [DOI] [PubMed] [Google Scholar]
- Turner JG, Hughes LF, Caspary DM 2005. Affects of aging on receptive fields in rat primary auditory cortex layer V neurons. J. Neurophysiol 94, 2738–2747, 10.1152/jn.00362.2005. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ando F, Shimokata H, Sugiura S, Ueda H, Nakashima T 2008. The effects of aging on distortion-product otoacoustic emissions in adults with normal hearing. Ear Hear 29, 176–84, 10.1097/aud.0b013e3181634eb8. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Burns KS 2011. Brain stem responses to speech in younger and older adults. Ear Hear 32, 168–180., 10.1097/AUD.0b013e3181f534b5. [DOI] [PubMed] [Google Scholar]
- Vanthornhout J, Decruy L, Wouters J, Simon JZ, Francart T 2018. Speech intelligibility predicted from neural entrainment of the speech envelope. Journal of the Association for Research in Otolaryngology : JARO 19, 181–191, 10.1007/s10162-018-0654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilkov V, Verhulst S 2019. Towards a differential diagnosis of cochlear synaptopathy and outer-hair-cell deficits in mixed sensorineural hearing loss pathologies. medRxiv, 19008680, 10.1101/19008680. [DOI] [Google Scholar]
- Verhulst S, Altoe A, Vasilkov V 2018. Computational modeling of the human auditory periphery: Auditory-nerve responses, evoked potentials and hearing loss. Hear. Res 360, 55–75, 10.1016/j.heares.2017.12.018. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Jagadeesh A, Mauermann M, Ernst F 2016. Individual differences in auditory brainstem response wave characteristics. Trends Hear 20, 233121651667218, 10.1177/2331216516672186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, Merchant SN, Liberman LD, Liberman MC 2015. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res 327, 78–88, 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O’Neill WE 1998. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J. Neurosci 18, 2764–2776, 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD 2002. Age-related alterations in the neural coding of envelope periodicities. J. Neurophysiol 88, 565–578, 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Wang M, Wu X, Li L, Schneider BA 2011. The effects of age and interaural delay on detecting a change in interaural correlation: The role of temporal jitter. Hear. Res 275, 139–149, 10.1016/j.heares.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Weible AP, Moore AK, Liu C, DeBlander L, Wu H, Kentros C, Wehr M 2014. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr. Biol 24, 1447–55, 10.1016/j.cub.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Schwoch T, Anderson S, Krizman J, Nicol T, Kraus N 2019. Case studies in neuroscience: Subcortical origins of the frequency-following response. J. Neurophysiol, 10.1152/jn.00112.2019. [DOI] [PubMed]
- Williamson TT, Zhu X, Walton JP, Frisina RD 2015. Auditory brainstem gap responses start to decline in mice in middle age: a novel physiological biomarker for age-related hearing loss. Cell Tissue Res 361, 359–69, 10.1007/s00441-014-2003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Milbrandt JC, Bross LS, Caspary DM 1997. Glycine immunoreactivity and receptor binding in the cochlear nucleus of C57BL/6J and CBA/CaJ mice: Effects of cochlear impairment and aging. Journal of Comparative Neurology 385, 405–414, . [DOI] [PubMed] [Google Scholar]
- Wingfield A, McCoy SL, Peelle JE, Tun PA, Cox CL 2006. Effects of adult aging and hearing loss on comprehension of rapid speech varying in syntactic complexity. J. Am. Acad. Audiol 17, 487–497, 10.3766/jaaa.17.7.4. [DOI] [PubMed] [Google Scholar]
- Wong DDE, Fuglsang SA, Hjortkjær J, Ceolini E, Slaney M, de Cheveigné A 2018. A comparison of regularization methods in forward and backward models for auditory attention decoding. Front. Neurosci 12, 10.3389/fnins.2018.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC 2019. Primary neural degeneration in the human cochlea: Evidence for hidden hearing loss in the aging ear. Neuroscience 407, 8–20, 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeend I, Beach EF, Sharma M 2019. Working memory and extended high-frequency hearing in adults: Diagnostic predictors of speech-in-noise perception. Ear Hear 40, 458–467, 10.1097/aud.0000000000000640. [DOI] [PubMed] [Google Scholar]
- Yin P, Johnson JS, O’Connor KN, Sutter ML 2011. Coding of amplitude modulation in primary auditory cortex. J. Neurophysiol 105, 582–600, 10.1152/jn.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekveld AA, Koelewijn T, Kramer SE 2018. The Pupil Dilation Response to Auditory Stimuli: Current State of Knowledge. Trends Hear 22, 2331216518777174, 10.1177/2331216518777174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Eliassen J, Anderson J, Scheifele P, Brown D 2009. The time course of the amplitude and latency in the auditory late response evoked by repeated tone bursts. J. Am. Acad. Audiol 20, 239–50, 10.3766/jaaa.20.4.4. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Henry KS, Heinz MG 2014. Sensorineural hearing loss amplifies neural coding of envelope information in the central auditory system of chinchillas. Hear. Res 309, 55–62, 10.1016/j.heares.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]