Abstract
Aerobic exercise is believed to be an effective chronic low back pain (CLBP) intervention, although its mechanisms remain largely untested. This study evaluated whether endogenous opioid (EO) mechanisms contributed to the analgesic effects of an aerobic exercise intervention for CLBP. Individuals with CLBP were randomized to a 6-week, 18-session aerobic exercise intervention (n = 38) or usual activity control (n = 44). Before and after the intervention, participants underwent separate laboratory sessions to assess responses to evoked heat pain after receiving saline placebo or i.v. naloxone (opioid antagonist) in double-blinded, crossover fashion. Chronic pain intensity and interference were assessed before and after the intervention. EO analgesia was indexed by naloxone-placebo condition differences in evoked pain responses (blockade effects). Relative to controls, exercise participants reported significantly greater pre-post intervention decreases in chronic pain intensity and interference (p’s < .04) and larger reductions in placebo condition evoked pain responsiveness (McGill Pain Questionnaire-Short Form [MPQ]-Total). At the group level, EO analgesia (MPQ-Total blockade effects) increased significantly pre-post intervention only among female exercisers (p = .03). Dose-response effects were suggested by a significant positive association in the exercise group between exercise intensity (based on meeting heart rate targets) and EO increases (MPQ-Present Pain Intensity; p = .04). Enhanced EO analgesia (MPQ-Total) was associated with significantly greater improvement in average chronic pain intensity (p = .009). Aerobic exercise training in the absence of other interventions appears effective for CLBP management. Aerobic exercise-related enhancements in endogenous pain inhibition, in part EO-related, likely contribute to these benefits
Keywords: chronic pain, evoked pain, aerobic exercise, clinical trial, endogenous opioid, naloxone
Introduction
A recent federal report on pain management best practices recognizes exercise as a core component of chronic pain (CP) management [58]. Several reviews conclude that exercise, broadly defined, effectively reduces CP intensity and dysfunction, with small to moderate effects [14,25,53]. Aerobic exercise training is often one component of exercise programs for CP. Several studies suggest that aerobic exercise training may be effective for CP management [11,13,16,36,38,42,59]. However, this literature is relatively small, with several negative findings [12,34,40,57,60], and few studies providing a no-exercise control condition to rule out history effects.
Mechanisms contributing to pain-related benefits of aerobic exercise are not well understood, but may include alterations in pain modulatory systems. In pain-free individuals, evoked pain testing immediately before and after a single aerobic exercise session reveals exercise-related increases in pain tolerance [3,26,30] dependent on exercise intensity [43]. Some evidence suggests this acute hypoalgesia is short-lived (<60 minutes; [18]), although two studies in healthy individuals demonstrated similarly increased evoked pain tolerance following 6 weeks of aerobic training relative to pre-intervention baseline [27,33]. One study in CP patients found that 2 weeks of aerobic training significantly decreased both evoked pain responsiveness and clinical pain, although improvements were statistically similar to physical therapy controls [46].
Beyond general changes in pain responsiveness, some studies suggest that increased endogenous opioid (EO) activity specifically may contribute to hypoalgesia following acute aerobic exercise. In highly trained runners, pharmacological opioid receptor blockade with naloxone eliminates the hypoalgesic effects of running [31,47], indicating involvement of EO mechanisms. In addition, Positron Emission Tomography (PET) studies confirm that aerobic exercise releases EOs in the brain [28,49,50]. In contrast, Droste et al. [18] reported no effect of opioid blockade on aerobic exercise-induced hypoalgesia. The role of EOs in exercise-induced analgesia may be complex, involving interactions with the endocannabinoid system [15]. Whether EO mechanisms contribute to prolonged analgesic effects (i.e., outside the acute exercise context) following aerobic exercise training programs is unknown, but is an important mechanistic question regarding the benefits of exercise-based CP management.
In summary, most prior trials have not tested aerobic exercise interventions against a natural history control to permit isolation of specific aerobic training effects, and nearly all mechanistic studies have focused on individuals without CP and the effects of acute exercise rather than sustained aerobic exercise training. Finally, prior studies examining possible EO mechanisms have focused exclusively on effects of acute exercise. To address these gaps, the current randomized controlled trial examined the effects of a 6-week (18 session) supervised aerobic exercise training program on changes in clinical outcomes in CP patients, and tested whether this intervention altered evoked pain responsiveness and EO analgesia outside the acute exercise context. We hypothesized that relative to no-intervention controls, aerobic exercise would reduce pain intensity and dysfunction in CP patients, and would produce simultaneous decreases in evoked pain responsiveness and increases in EO analgesia if these mechanisms contributed to the intervention’s benefits. Given known sex differences in pain responsiveness [4], we examined possible moderation of the hypothesized effects by sex.
Method
Design
This study was part of a randomized controlled trial to evaluate the effects of a structured aerobic exercise training program on chronic low back pain and opioid analgesic responsiveness, and to assess the role of EO mechanisms in observed intervention effects (NCT02469077). The study used a parallel groups, mixed design, with study drugs (placebo, morphine, and naloxone) administered in double-blinded fashion in randomized, counterbalanced (crossover) order across 3 separate identical sessions (conducted over a 10-day period), with this lab protocol carried out both before and after either an 18-session (6 week) aerobic exercise training program or a usual activity control condition. Given the focus of the current work on the effects of the intervention on CP-related outcomes and the potential role of EO mechanisms in those effects, data for the morphine condition are not presented here. The study was conducted at two separate study locations using identical procedures in parallel and in a closely coordinated fashion. All procedures were approved by the Institutional Review Boards at the respective institutions.
Participants
Participants included 82 individuals with chronic low back pain (CLBP) who were not using opioid analgesics on a daily basis. Participants using as-needed opioid analgesics were asked to abstain from any opioid use within the 3 days prior to each laboratory session (confirmed via urine opioid screen), and all participants were instructed not to use any non-steroidal anti-inflammatory drugs or over-the-counter analgesics for at least 12 hours prior to each laboratory session. Participants were recruited through an informatics-based targeted recruiting system mining electronic medical records to identify potentially eligible patients previously indicating a willingness to participate in research studies (“My Research at Vanderbilt”), on-line advertisements on the Vanderbilt employee e-mail recruitment system, the Rush Pain Clinic, advertisements in local print media and Facebook, and posted flyers. General criteria for participation included age between 18–55; no self-reported history of liver or kidney disorders, posttraumatic stress disorder, bipolar disorder, psychotic disorder, diabetes, seizure disorder, or alcohol or drug dependence; and no daily use of opioid analgesics. To maximize potential exercise intervention effects, participants were additionally required to be low active, i.e., engaged in moderate or vigorous exercise < 2 days/week and < 60 min/week (based on responses to 6 questions assessing moderate to vigorous activity on the CDC Behavioral Risk Factor Surveillance Survey [61]). CLBP was defined as daily low back pain of at least 3 months’ duration, with an average past month severity of at least 3/10 on a 0–10 verbal numeric pain intensity scale. All participants were required to be able to provide documentation of a previous medical provider diagnosis consistent with CLBP. Individuals self-reporting CP related to malignancy or autoimmune disorders were excluded, as were individuals who were pregnant (determined by urine pregnancy screens). All participants were compensated for their time ($75 for initial screening, $100 for each lab visit, and $30 for each completed exercise session). A CONSORT flow chart is provided in Figure 1, with n=55 participants randomized to the exercise intervention and n=58 randomized to the control condition. The originally targeted sample size of n=58 per group was chosen based on an a priori power analysis to permit at least 80% power to detect group differences in exercise-related changes in EO function, assuming a two-tailed p<.05 criterion for significance. Of the randomized participants, n=38 in the Exercise group and n=44 controls completed the intervention with full pre-post intervention lab data (for EO assessment) available for analysis in the current study. All participants were recruited by the research coordinator at each site, with all study procedures carried out between 9/28/15 and 9/17/19. The trial ended when the originally targeted recruitment sample size was achieved and grant support ended.
Figure 1.
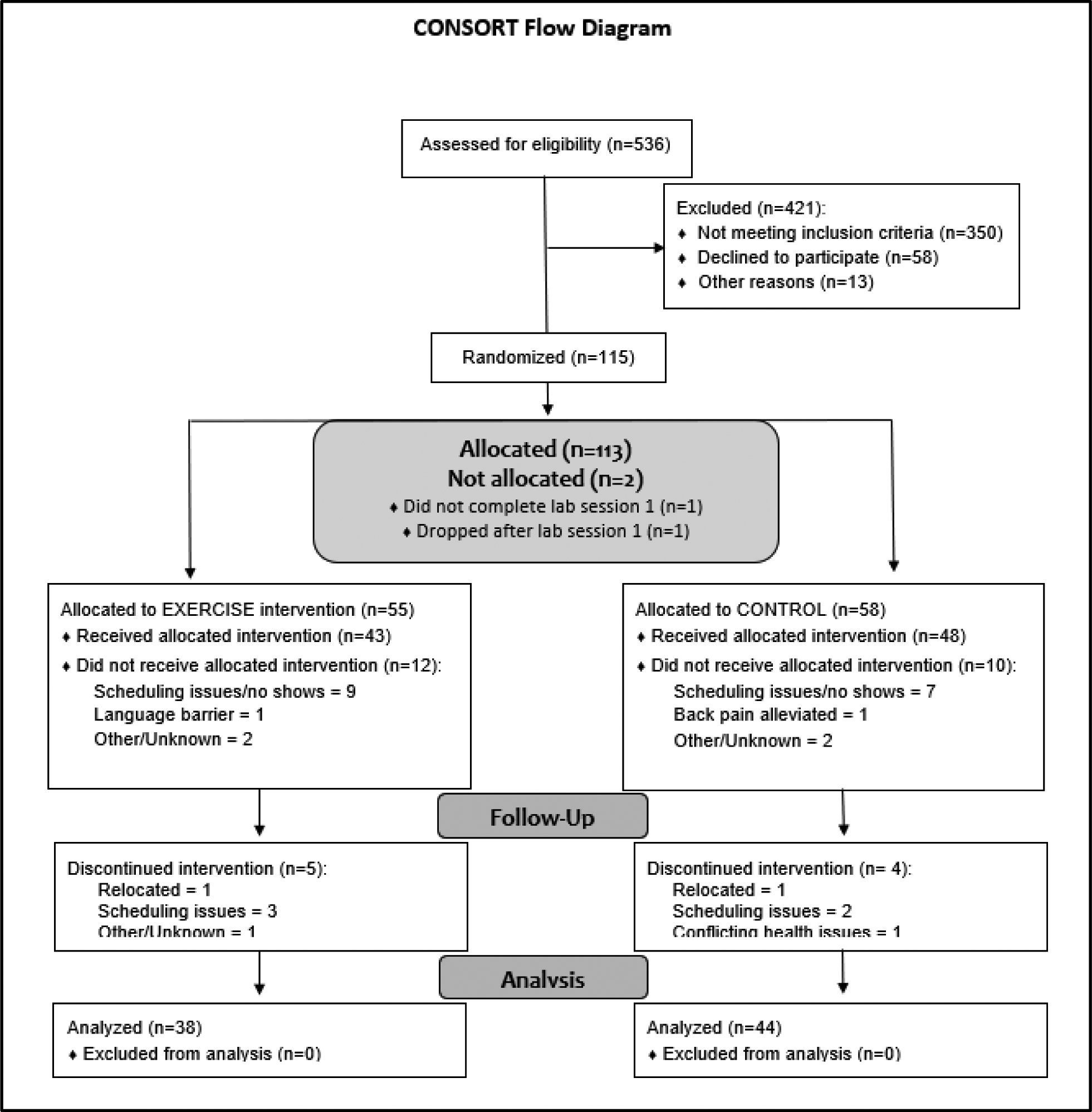
CONSORT Diagram.
Study Drugs
The opioid antagonist naloxone was administered in one laboratory session pre-intervention and one session post-intervention. This naloxone condition was included to permit derivation of a quantitative index of EO system function, specifically, the difference in evoked pain responses between naloxone and placebo conditions [7,8]. Naloxone was infused incrementally, with an initial 8mg dose administered prior to the first heat pain trial (detailed below), followed by saline placebo prior to the second heat pain trial, a 4mg maintenance dose prior to the third trial (to maintain full opioid blockade during the remaining trials), and then a final saline placebo dose before the fourth trial. Normal saline was infused in the same incremental manner across all four trials during the placebo condition. A drug order randomization schedule was prepared by an independent statistician using the Proc Plan procedure in SAS version 9.2 (SAS Institute, Cary, NC), with drug order randomized separately for pre-intervention and post-intervention lab sessions. Blinding of drug order was maintained and randomized drug assignment was carried out according to the randomization schedule by the investigational pharmacy at each site.
Measures
Clinical Chronic Pain and Functional Measures.
Numeric Rating Scale (NRS) Pain Intensity.
Consistent with IMMPACT recommendations [19], an 11-point NRS anchored with “no pain” and “worst possible pain” was used to rate average (primary clinical outcome) and worst (secondary outcome) clinical back pain over the previous 24 hours, at both pre-randomization baseline and again at the final post-intervention lab session. NRS ratings of average and worst pain are simple overall intensity measures that are sensitive to change with intervention [32].
Short Form McGill Pain Questionnaire-2.
To more specifically assess the various qualitative aspects of chronic pain, clinical back pain over the preceding week (secondary outcome) was also assessed at pre-randomization baseline and again post-intervention using the Short Form-McGill Pain Questionnaire-2 (MPQ-2) [20,21]. The MPQ-2 is a validated measure containing 22 items rated using an NRS format (0 = none and 10 = worst possible). It contains 4 subscales (Continuous, Intermittent, Neuropathic, and Affective).
Pain Interference.
The PROMIS Short Form v1.0 - Pain Interference 8a scale (PROMIS Interference) was used to assess pain-related life interference (secondary outcome) over the past week [2]. It assesses the impact of pain on daily social, emotional, physical, and recreational activities. Items are rated on a 5-point scale, ranging from “almost never” (0) to “almost always” (4). Item scores were summed and raw scores were converted into T-scores per standard scoring guidelines [48]. Higher scores indicate greater pain-related life interference. The PROMIS Interference scale has been shown to be sensitive to change with intervention [51].
Patient Global Impression of Change (PGIC).
A 7-item PGIC measure was completed at the final lab session. This secondary outcome measure used the standard format recommended by IMMPACT, with response categories ranging from “very much worse” to “very much better” [19].
Evoked Pain Intensity Measure
Perceived intensity of the thermal pain stimulus was assessed using the original MPQ-Short Form (MPQ-SF; [41]). It contains Sensory and Affective subscales (all items on a 4-point scale rated from 0 = “none” to 3 = “severe”) which are combined into a Total score (to avoid redundancy, only the MPQ-SF Total [primary evoked pain outcome] score is reported here). Use of a different version of the MPQ to assess the evoked pain stimuli versus clinical chronic pain was implemented to minimize potential participant confusion regarding the type of pain being rated (evoked vs. chronic). The MPQ-SF also includes a 6-point Present Pain Intensity (PPI) numeric scale of overall pain intensity. Ratings are made on a 0 (“no pain”) to 5 (“excruciating”) scale.
Heart Rate Measures
Heart rate (HR) was assessed in two ways. First, for all participants, resting pre-drug HR was assessed at the beginning of each lab session using an automated oscillometric blood pressure monitor (GE Dinamap Procare 400). Changes in resting HR from pre-intervention baseline to session 4 (one week following the 6 week intervention) were derived, with negative values indicating decreased HR.
To evaluate aerobic exercise intensity (in the exercise group only), the mean amount of time per exercise session (across all completed exercise sessions) in which HR was in the desired training zone was calculated (based on percentage of heart rate reserve; see Intervention description below). These data were derived from HR assessed at 5 minute intervals during all aerobic training sessions via a Polar HR monitor (Polar Electro Inc., Bethpage, NY).
Evoked Pain Stimulus
The evoked pain stimulus in the current study was a heat pain task using a computerized Medoc TSAII NeuroSensory Analyzer (Medoc US., Minneapolis, MN) as in our prior work (e.g., [7]). The thermode was placed at a slightly different location of the ventral forearm for each stimulus to avoid local sensitization effects. After administration of each incremental drug dose and a 10 minute rest to permit peak drug activity to be achieved, three trials were conducted for heat pain tolerance (with the mean value used for analyses), with the thermode starting at an adaptation temperature of 40°C and increasing at a ramp rate of 0.5°C/second until tolerance was reached. Immediately upon completion of the final heat pain tolerance trial at each drug dosage, participants were asked to rate the pain just experienced using the MPQ-SF described above. Participants underwent standardized training to familiarize them with the thermal stimulus procedures prior to undergoing the evoked pain task for the first time.
Intervention
Participants were randomly assigned to the exercise intervention or usual activity control group by the study coordinators using a 1:1 randomization schedule developed by an independent statistician prior to study initiation using the Proc Plan procedure in SAS version 9.2 (SAS Institute, Cary, NC). Experimenters were not blinded to intervention condition (but were blinded to drug order in the lab sessions). The exercise intervention was an aerobic exercise training program designed to produce a training effect for all adherent participants, but which was sensitive to the potential for initial symptom exacerbation in CLBP patients by incorporating progressive increases in workload during the first two weeks. Individuals randomly assigned to the exercise intervention participated in a supervised, individual aerobic exercise training program 3 times/week for 6 weeks. This protocol was similar to that used in prior work showing reduced evoked pain responses in healthy individuals [33] and improved endurance and function in CLBP patients [52]. To enhance and monitor adherence, all of the exercise sessions were conducted in an on-campus exercise facility and supervised by ACSM-certified personal trainers trained in study procedures.
Each exercise session consisted of a 5 minute warm-up, then 30 minutes of aerobic exercise followed by a 5 minute cool-down period. Aerobic exercise included treadmill walking/running, stepping, elliptical, or cycling exercise as preferred by the participant to minimize symptom exacerbation (i.e., acute increases in back pain) while maximizing adherence to the training program. Effort levels were standardized using HR and perceived exertion (RPE) monitoring as recommended by the American College of Sports Medicine [1]. At the beginning of the intervention, target heart rate zones were established using the Karvonen formula and heart rate reserve (HRR), with duration of exercise standardized at 30 minutes with a target exercise intensity between 70–85% HRR (RPE = 15, hard). Because of the study focus on de-conditioned individuals with CLBP, duration and intensity of exercise was progressively increased up to the target intensity during the first two weeks to avoid symptom exacerbation and minimize study drop-out. Specifically, participants began with 10–15 minutes of exercise between 40–55% HRR (RPE = 11–12, light) during the first week, 20–30 minutes of exercise between 55–70% HRR (RPE = 12–13, somewhat hard) during the second week, and then 30 minutes of exercise between 70–85% HRR (RPE= 14–16, hard) for the remainder of the study. To ensure that participants were exercising within their prescribed workload during each session, HR and RPE were assessed every 5 minutes during exercise using HR monitors (see above) and Borg’s 6–20 RPE scale [5].
Participants assigned to the usual activity Control condition (all of whom were low active per inclusion criteria) were asked to maintain their normal daily activity levels throughout the study.
Procedure
All procedures were conducted at the Vanderbilt General Clinical Research Center or a dedicated research room at the Rush University Pain Center. After providing informed consent, participants engaged in an assessment session during which they completed a packet of questionnaires, including chronic pain and functional measures and demographic information. Individuals then participated in identical experimental procedures across the drug conditions, with all sessions scheduled at the same time of day within individuals to control for variance due to circadian rhythms. The protocol for the lab sessions is summarized in Figure 2.
Figure 2.
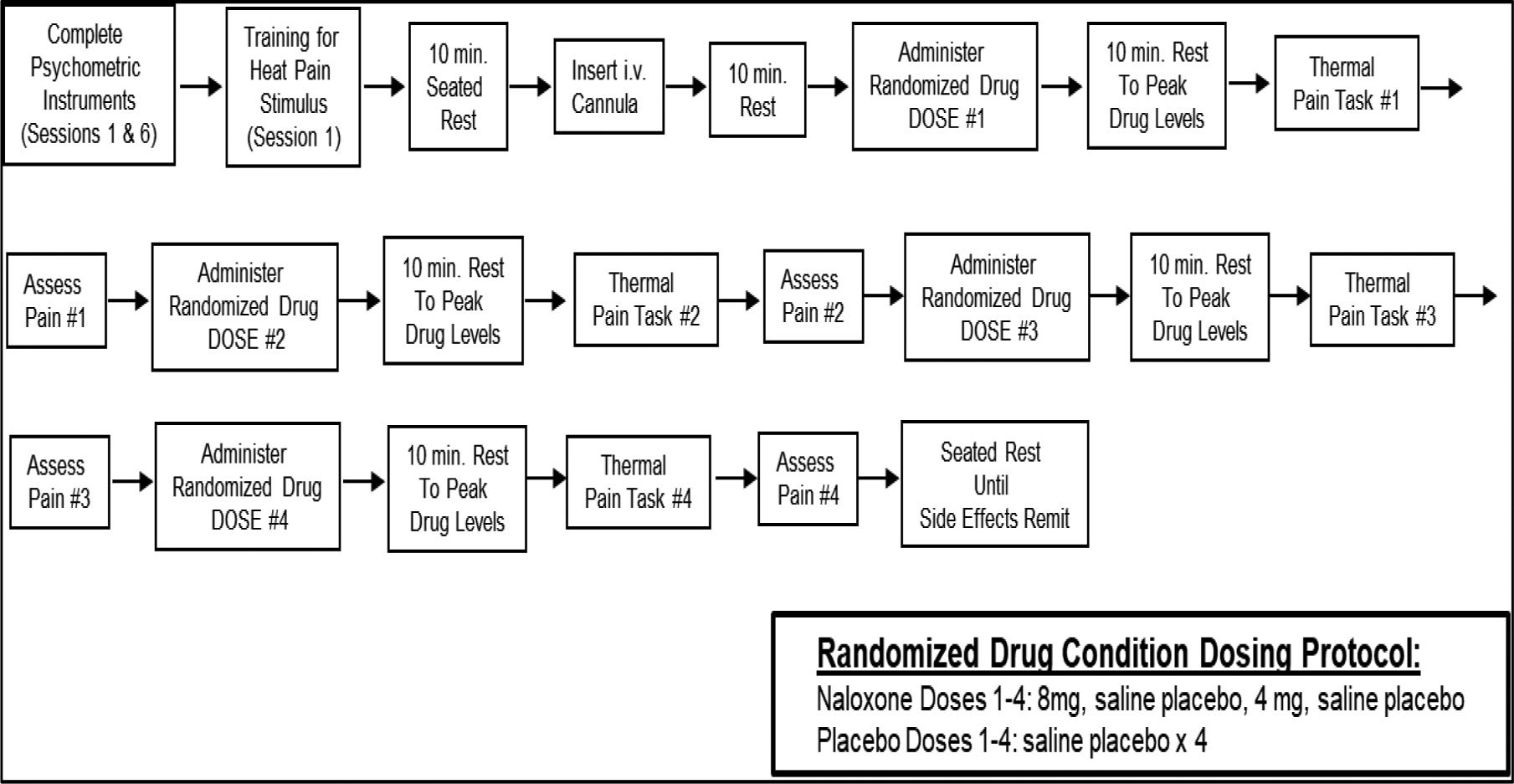
Protocol for Laboratory Sessions Pre- and Post-Intervention.
Participants remained seated upright in a chair throughout all lab procedures. The investigational pharmacy at each institution prepared and provided the study drugs in blinded fashion to the study nurses. At the beginning of each session, after a 5-min seated rest, resting HR was determined as previously described. Next, an indwelling venous cannula was inserted into the non-dominant arm by a trained research nurse under physician supervision. Participants then received (via the cannula) their first dose of the study drug as assigned per the randomization schedule. After a 10-min rest period to allow drug activity to peak, participants completed the first evoked thermal pain tasks and ratings of evoked pain. Fifteen minutes later, the second assigned drug dose was given followed by a 10-min rest and then the evoked thermal pain tasks and pain ratings, with the same procedure followed through the fourth and final drug dose. All participants remained in the lab under observation for 1 hour after the final drug dose to allow drug effects to remit, after which they were released to a responsible adult.
After completing the final pre-intervention lab session, participants then began their randomized intervention (exercise or control). The exercise group completed 18 supervised aerobic exercise sessions over the following 6 weeks (minimum per protocol was 13 sessions, but all completed at least 16 sessions). Within 10 days of the final exercise session, follow-up post-intervention lab assessment was begun, with participants again completing the lab protocol described above over a 10-day period. Post-exercise lab data indexed EO status and evoked pain responsiveness following the intervention but in the absence of any effects of recent acute exercise. This procedure was implemented specifically to determine whether exercise-related enhancements in EO analgesia could account for sustained pain relief following such interventions suggested by some prior work [27,33,46]. Post-intervention chronic pain outcomes (as well as the PGIC measure) were assessed in the last post-intervention lab session using the same questionnaires completed at the baseline assessment. All participants completing the intervention also completed all of the post-intervention laboratory sessions.
Statistical Analysis
All analyses were conducted using IBM SPSS for Windows Version 26 (SPSS Inc., Chicago, IL). In preparation for conducting analyses, change scores were calculated for all chronic pain outcomes, as well as placebo condition evoked pain responses, from pre-intervention baseline to post-treatment. Positive scores indicated improvement for all outcomes. In order to provide a quantitative index of EO analgesia, blockade effects (differences in pain response between placebo and naloxone conditions) for evoked pain tolerance and MPQ-SF ratings were derived separately for pre-intervention and post-interventions lab sessions, such that positive blockade effect values indicated greater EO modulation of evoked pain (i.e., increased evoked pain responses with naloxone administration relative to placebo). Change scores (pre- to post-intervention) were then derived from these blockade effects and were the focus of analyses [primary EO outcome: MPQ-SF Total], with positive values indicating increased EO analgesia over the course of the intervention.
Preliminary analyses used between-subject t-tests (continuous measures) or chi-square analyses (categorical measures) to test for differences between groups on baseline characteristics. Given known sex differences in chronic and evoked pain responses [4], primary analyses used analysis of covariance (ANCOVA) procedures examining main and interacting effects of intervention status (Group) and sex on outcomes, controlling for baseline values of the targeted outcome (i.e., baseline corrected change). The source of significant sex interactions was determined using simple effects analyses (ANCOVAs conducted separately in males and females). Estimated marginal means (± SE) from ANCOVAs are presented to portray the source of significant main and interaction effects, controlling for relevant covariates. Analyses were conducted on a per protocol basis given the focus on changes in EO outcomes (which were obtained at follow-up only in individuals who completed the exercise protocol). All analyses used the maximum number of available cases and a two-tailed probability value of p<.05 as the criterion for significance.
Results
Preliminary Analyses
None of the sample characteristics were significantly different between the intervention and control groups (all p’s >.10; Table 1). The majority of participants in both groups were female, white, and non-Hispanic. Chronic pain was characterized as moderate in intensity and of long duration. Less than 16% of the sample was using as-needed opioids in either group, and none had used opioids in the 3 days prior to each laboratory session (confirmed by urine screen). In terms of exercise protocol engagement, 94.7% of the exercise group participants completed the full 18 sessions of exercise, with one participants (2.6%) completing 17 sessions and one (2.6%) completing 16 sessions. The mean amount of time per session during which achieved HR was within the target training zone based on HRR was 21.1 minutes (SD = 4.65; range = 6.7 – 27.8 minutes) over the full exercise protocol, indicating that the intervention approached, but fell short of, the desired intensity for the group as a whole, but with wide variability. Two participants were able to exercise in the target HR zone only for an average of <10 minutes per session throughout the 18-session protocol. Pre- and post-intervention raw values for all outcomes are summarized by intervention group in Table 2.
Table 1.
Baseline sample characteristics by group.
| Group | ||
|---|---|---|
| Characteristic | Exercise (n=38) | Control (n=44) |
| Sex (% Female) | 55.3 | 65.9 |
| Race (%): | ||
| White | 60.5 | 58.1 |
| African-American | 26.3 | 34.9 |
| Ethnicity (% Non-Hispanic) | 94.7 | 95.1 |
| Age (Mean ± SD, years) | 40.0 ± 10.00 | 41.9 ± 9.45 |
| Body Mass Index (Mean ± SD) | 29.9 ± 5.74 | 31.9 ± 7.16 |
| Pain Duration (Median ± IQR) | 75.6 ± 112.81 | 84.8 ± 160.71 |
| Post-menopausal (%) | 19.0 | 25.9 |
| Birth Control or Hormone Replacement (%) | 19.0 | 31.0 |
| As needed opioid use (%) | 15.8 | 11.4 |
| Neuroleptic use (%) | 7.9 | 4.5 |
| Antidepressant use (%) | 23.7 | 18.2 |
Note: All group comparisons are nonsignificant (p’s > .10).
Table 2.
Clinical, placebo-condition evoked pain, and EO outcomes (Mean ± SD) pre- and post-intervention by group.
| Exercise | Control | |||
|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | |
| Past 24 Hour Average NRS Pain | 4.54 ± 2.22 | 3.22 ± 2.02 | 5.35 ± 2.26 | 4.69 ± 2.28 |
| Past 24 Hour Worst NRS Pain | 6.32 ± 2.17 | 4.27 ± 2.32 | 6.53 ± 2.04 | 5.98 ± 2.45 |
| MPQ-2 Continuous | 4.07 ± 1.87 | 2.04 ± 1.79 | 4.29 ± 2.15 | 2.91 ± 2.33 |
| MPQ-2 Intermittent | 2.94 ± 2.41 | 1.21 ± 1.48 | 3.71 ± 2.41 | 1.87 ± 2.08 |
| MPQ-2 Neuropathic | 1.27 ± 1.45 | 0.5 ± .070 | 1.80 ± 1.89 | 0.70 ± 1.11 |
| MPQ-2 Affective | 2.24 ± 2.08 | 0.60 ± 1.00 | 2.31 ± 2.35 | 1.25 ± 2.34 |
| PROMIS Interference T-score | 60.58 ± 5.86 | 55.67 ± 6.81 | 62.42 ± 7.62 | 59.51 ± 6.03 |
| Patient Global Impression of Change | ---- | 5.22 ± 0.89 | ---- | 3.98 ± 1.03 |
| Placebo Thermal Pain Tolerance | 47.64 ± 1.55 | 47.73 ± 1.72 | 47.31 ± 1.56 | 47.51 ± 1.46 |
| Placebo Thermal MPQ-TOT | 10.31 ± 9.29 | 9.91 ± 8.67 | 7.86 ± 6.18 | 9.99 ± 7.67 |
| Placebo Thermal MPQ-PPI | 2.67 ± 1.02 | 2.50 ± 0.91 | 2.45 ± 1.17 | 2.28 ± 1.21 |
| EO - Thermal Tolerance | 0.09 ± 0.76 | 0.17 ± 0.52 | −0.06 ± 0.69 | 0.08 ± 0.55 |
| EO - Thermal MPQ-TOT | −0.28 ± 4.61 | 1.61 ± 4.67 | 0.92 ± 3.81 | 0.28 ± 3.76 |
| EO - Thermal MPQ-PPI | −0.02 ± 0.52 | 0.21 ± 0.52 | −0.01 ± 0.54 | 0.10 ± 0.46 |
Note: All baseline (pre-intervention) differences between groups are nonsignificant. NRS = Numeric Rating Scale; MPQ-2 = McGill Pain Questionnaire-2; MPQ-TOT = McGill Pain Questionnaire-Short Form Total Score; MPQ-PPI = McGill Pain Questionnaire-Short Form - Present Pain Intensity; EO = Endogenous Opioid Index (naloxone minus placebo condition value for the measure of interest).
Aerobic Exercise Intervention Efficacy
Chronic Pain Outcomes.
ANCOVAs controlling for baseline values indicated that NRS ratings of past 24 hour average pain intensity declined significantly more in the exercise group than in the control group [F(1,75) = 4.74, p = .033, η2 = .059]. Parallel analyses for worst 24-hour NRS pain intensity revealed similar effects, with the exercise group again showing significantly larger improvements than controls [F(1,77) = 9.195, p = .003, η2 = .107]. The Group X Sex interaction was nonsignificant for both measures (p’s > .20). Pre-post intervention changes (estimated marginal means adjusting for baseline differences) for both average and worst 24-hour NRS pain intensity are displayed in Figure 3. A reduction of 30% in NRS pain ratings from pre-intervention baseline has been suggested as a criterion indicating at least moderate clinically important improvement in clinical trials [22]. In the exercise group, 40.5% of participants reported at least a 30% reduction in worst NRS pain from pre-intervention baseline to post-intervention, compared to only 27.3% of the control group, with comparable proportions observed for average NRS pain improvements (44.4% vs. 31.0%, respectively).
Figure 3.
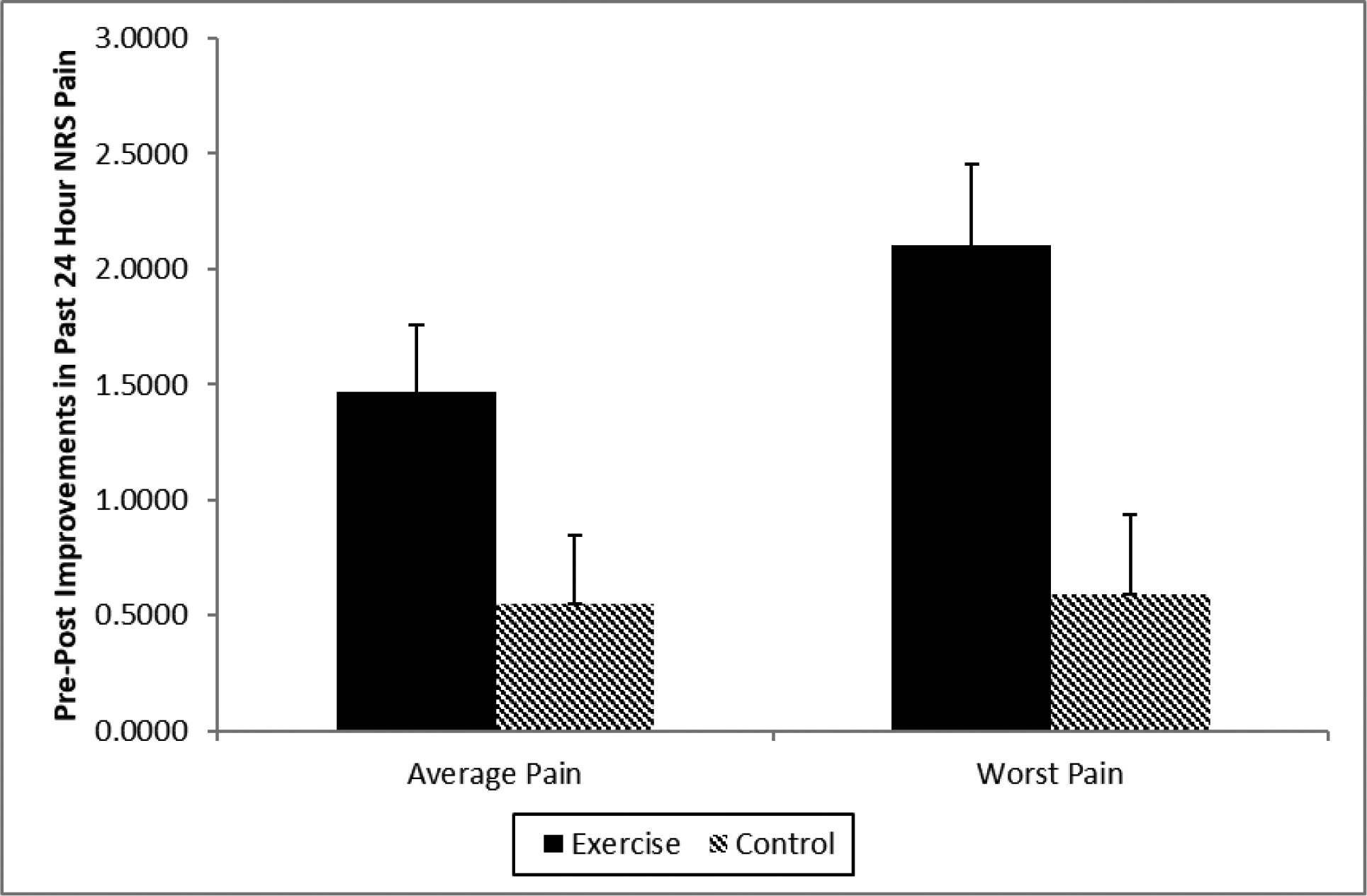
Changes in NRS ratings (0–10) of past 24 hour pain intensity by intervention group. Positive values indicate improvements in pain from pre-intervention to post-intervention. Values presented are estimated marginal means (± SE) adjusting for baseline differences in NRS ratings.
Analyses of pre-post intervention changes in specific chronic pain qualities (rated over the past week) on the MPQ-2 also revealed significant intervention effects. Pre-post intervention reductions in MPQ-2 Continuous subscale ratings were significantly greater in the exercise group than in controls [F(1,77) = 4.15, p = .045, η2 = .052; Exercise: M = 2.1, SE = 0.29; Control: M = 1.3, SE = 0.28]. A similar nonsignificant trend was also noted for the MPQ-2 Affective subscale [F(1,77) = 3.15, p = .08, η2 = .04; Exercise: M = 1.7, SE = 0.27; Control: M = 1.0, SE = 0.27]. Group X Sex interactions for both measures were nonsignificant (p’s > .46). Neither the main effect of Group nor the Group X Sex interaction were significant for the MPQ-2 Intermittent or Neuropathic subscales (p’s > .24).
Pain-Related Interference.
ANCOVA revealed significant main effects of the intervention on PROMIS Interference T-scores (baseline adjusted). Improvements in pain interference were significantly larger in the exercise group than in the control group [F(1,77) = 5.58, p = .021, η2 = .068; Exercise: M = 5.3, SE = 0.84; Control: M = 2.5, SE = 0.83]. The Group X Sex interaction was nonsignificant (p > .93). The magnitude of improvement in PROMIS Interference T-scores in the exercise intervention group equates to an effect size of 0.53 standard deviation units, a level that is considered to be clinically important for functional pain outcomes [22].
Patient Global Impression of Change (PGIC).
As suggested by IMMPACT guidelines [22], the percentage of participants in each group endorsing each response category on the PGIC measure are provided in Table 3. In the exercise group, 31.6% reported being “much improved” or “very much improved,” whereas in the control group only 9.1% met this criterion. ANCOVA revealed a significant Group X Sex interaction on PGIC ratings [F(1,78) = 4.90, p = .03, η2 = .059]. Simple effects analyses indicated that this interaction was due to male exercise participants reporting notably greater overall improvement than male controls [F(1,30) = 44.92, p < .001; Exercise: M = 5.53, SE = 0.18; Control: M = 3.73, SE = 0.20], with a smaller but nonetheless significant difference in PGIC ratings between conditions in female participants [F(1,48) = 8.16, p = .006; Exercise: M = 5.0, SE = 0.23; Control: M = 4.1, SE = 0.19].
Table 3.
Distribution (%) of Patient Global Impression of Change responses at the end of the intervention period by group.
| Group | ||
|---|---|---|
| Response Category | Exercise | Control |
| Very Much Worse | 0.0 | 0.0 |
| Much Worse | 0.0 | 4.5 |
| Minimally Worse | 2.6 | 25.0 |
| No Change | 13.2 | 50.0 |
| Minimally Improved | 52.6 | 11.4 |
| Much Improved | 23.7 | 6.8 |
| Very Much Improved | 11.0 | 3.7 |
Changes in Heart Rate Measures.
Mean baseline-adjusted changes in resting HR did not differ significantly between groups [F(1,75) = 0.45, p = 0.50, η2 = .006], although the exercise group showed a small directional reduction in group mean HR as expected (M = −0.38, SE = 1.52) while controls showed small increases in mean resting HR (M = 1.00, SE = 1.39). Within the exercise group, reductions in resting HR from pre- to post-intervention were significantly associated with improvements in chronic pain as indexed by average [r(33) = −0.52, p = .002] and worst past 24 hour NRS pain intensity [r(33) = −0.44, p = .008], as well as the MPQ-2 Continuous [r(33) = −0.36, p = .034] and Intermittent [r(33) = −0.43, p = .010] subscales. There were no comparable associations in the control group between resting HR changes over the course of the trial and chronic pain outcomes (all p’s > .42). Within the exercise group, HR changes from pre- to post-intervention were not significantly associated with mean time per exercise session spent in the target HR zone (p >.10).
Intervention Effects on Evoked Pain Outcomes
Evoked thermal pain responses at baseline did not differ significantly between groups (all p’s >.17). ANCOVA revealed a significant main effect of intervention Group on placebo-condition MPQ-SF Total ratings [F(1,77) = 5.80, p = .018, η2 = .064]. Participants in the exercise group displayed slightly improved pain responsiveness (decreased thermal pain ratings) following the 6-week intervention (M = 0.33, SE = 0.70), whereas control group participants reported an increase in thermal pain responses over time (M = −2.04, SE = 0.68). The Group X Sex interaction was not significant in this analysis (p>.63). The analysis above did not appear to be confounded by any intervention-related changes in thermal pain tolerance; inclusion of pre-post changes in pain tolerance as an additional control variable in the analysis above left the significant intervention effect on placebo-condition MPQ-TOT ratings essentially unchanged (p = 0.025). However, we note that the group-level mean reduction in evoked pain responsiveness observed in the exercise condition was not significantly different from zero [t(37) = 0.63, p = 0.54].
Similar analyses of placebo-condition MPQ-SF PPI ratings and thermal pain tolerance did not reveal any significant main effects of Group or Group X Sex interaction effects (all p’s > .40).
Intervention Effects on Endogenous Opioid (EO) Outcomes
Blockade effect values did not differ significantly at pre-intervention baseline across groups (all p’s >.11). Analyses of evoked pain MPQ-SF Total blockade effects revealed a trend approaching significance for an intervention effect moderated by participant sex [F(1,75) = 3.11, p = .082, η2 = .04]. This interaction trend was further explored given the unique nature of these data. Women in the exercise group exhibited significantly larger increases in EO function (M = 1.68, SE = 0.91) compared to women in the control group [M = −0.93, SE = 0.80; F(1,46) = 5.35, p = .025]. This intervention effect was not significant in men [F(1,28) = 0.47, p = .499; Exercise: M = 0.67, SE = 1.07; Control: M = 1.47, SE = 1.08]. Main effects of Group and Group X Sex interactions for changes in blockade effects derived for thermal pain tolerance (p’s > .15) and MPQ-SF PPI ratings (p’s > .17) were all nonsignificant, although the pattern of means for the latter measure was consistent with MPQ-SF Total results (i.e., women exercisers experienced the greatest EO function increases of any group).
We next evaluated the possibility that group-level mean intervention effects on EO function may have been obscured to some degree by individual differences in exercise dose (i.e., exercise intensity). In the exercise group, participants with a greater average number of minutes across all training sessions in the target HR zone (based on HRR), as expected, were found to exhibit significantly greater increases in EO analgesia based on the MPQ-SF PPI measure [r(35) = 0.33, p = .043]. Similar analyses for tolerance and MPQ-SF Total evoked pain blockade effects were nonsignificant (p’s > .45).
Intervention-related EO changes appeared to be clinically relevant. Greater enhancements in EO function from pre- to post-intervention in the exercise group as reflected in the MPQ-SF Total blockade effect measure were significantly associated with greater improvements in Average NRS past 24 hour pain [r(35) = 0.43, p = .009], with a similar association just failing to meet the criterion for significance for Worst NRS pain [r(35) = 0.32, p = .052]. Associations between this EO measure and MPQ-2 ratings of past week intermittent pain also approached significance [r(35) = 0.28, p = .097]. In addition, EO increases as indexed by pain tolerance blockade effects exhibited nonsignificant trends towards associations with greater clinical improvements in Worst NRS pain [r(35) = 0.28, p = .092] and the MPQ-2 Intermittent subscale [r(35) = 0.28, p = .093].
Discussion
Although it is often assumed that aerobic exercise interventions are beneficial for CLBP management, support for this is mixed [11,12,16,34,36,38,40,57,59,60]. Interpretation of existing studies is hindered by small samples [e.g., 12,29,34,43], and designs that evaluate aerobic exercise provided with other interventions [16,40,46], lack a control condition [60], or use of an active treatment control [13,42,57]. Support from well-designed randomized controlled trials (RCTs) regarding efficacy specifically of aerobic exercise for CLBP management remains sparse. This RCT evaluated the efficacy of a supervised aerobic exercise intervention for CLBP provided in the absence of other interventions compared to a usual activity control. Results indicated the intervention led to greater improvements in pain and pain-related life interference over the 8 week trial than were observed in controls. Exercise participants also reported significantly greater global improvement compared to controls. While both sexes appeared to benefit equally from the intervention in terms of pain and function, men reported greater global benefits of the intervention than women. Overall, findings for clinical outcomes support the efficacy of supervised aerobic exercise training programs in the management of CLBP.
Of more conceptual interest is the question of why aerobic exercise training may be effective for CLBP. Existing studies focusing almost exclusively on pain-free individuals suggested that aerobic exercise might enhance endogenous pain inhibitory capacity, as reflected in reduced evoked pain responsiveness, or more specifically, increased EO analgesia. This latter finding, while intriguing and consistent with the popular idea of the “runner’s high,” is to date based entirely on studies regarding effects of acute exercise in non-pain populations [15,28,31,47,49,50]. The current work sought to test for the first time whether aerobic exercise training enhances EO analgesia on a sustained basis (beyond the acute exercise setting), and whether this effect contributes to the benefits of such interventions in the daily life of CLBP patients. There was support for this EO hypothesis in the current work, although limited. Women receiving the aerobic exercise intervention exhibited significantly greater EO increases than women in the control group, although there were no intervention-related differences in men. This overall Sex X intervention interaction was only a statistical trend (p<.09). Sex differences in EO analgesic systems have previously been noted [6,23,55,62], although whether such differences account for intervention-related EO increases occurring specifically in women in the current work cannot be determined. Analyses focusing on EO dose-response effects rather than group means also support the EO hypothesis. Specifically, in the exercise group, greater time spent in the target HR zone during exercise training sessions was associated with greater EO analgesia in the lab, indicating an aerobic exercise dose-response effect. Exercise-related EO increases, in turn, were associated with greater improvements in CLBP outcomes over the same time period. Despite some support for the EO hypothesis, limited statistical power impacted adversely on our tests of this hypothesis, and prohibited an adequate test of statistical mediation. This limited power was a result of the challenges of recruiting for a study requiring 6 extended laboratory sessions and 18 exercise sessions. While the current results regarding impact of aerobic exercise on EO analgesic function must be interpreted cautiously, they do suggest the intriguing possibility that regular aerobic exercise may lead to sustained increases in EO function outside of the acute exercise setting, at least in some individuals. This finding is consistent with preclinical work showing increased EO brain levels and analgesia in animals 5 days after completing a 5-week aerobic exercise training protocol [56]. Further work in larger chronic pain samples to examine the magnitude and duration of EO function increases after aerobic training programs appear justified, and may help clarify the mechanism of action of such interventions.
Beyond intervention effects on EO function specifically, we also evaluated whether the intervention produced reductions in evoked pain responsiveness that might support a salutary intervention effect on pain modulatory systems. Reductions in evoked pain responding following aerobic exercise have been shown previously in studies of acute exercise effects [3,18,26,30,43] and studies of prolonged exercise interventions conducted in individuals without chronic pain [27,33]. Results of the current work provided only limited support for this hypothesis. Specifically, a significant main effect of the intervention on MPQ-SF Total ratings of evoked pain was noted. Although consistent with prior work, this effect was driven not only by small mean decreases in pain responding in the intervention group (not significantly different from zero), but also by increased pain responding in controls over the intervention period. Reasons for the latter changes are unclear.
Overall, this study provides some support for the hypothesis that aerobic exercise training in individuals with CLBP may exert some of its beneficial clinical effects via enhancement of pain inhibitory function, in part EO-related, that is sustained enough to be observed even outside of the acute exercise context. This is consistent with conclusions of a recent literature review regarding mechanisms underlying exercise hypoalgesia [54]. However, another review highlighted work suggesting impaired ability to elicit endogenous analgesia in chronic pain patients (e.g., [44]), hypothesizing that this may limit exercise benefits in chronic pain patients [45]. Despite possible pain modulatory impairments, our results suggest that it is possible to enhance endogenous analgesia to some degree at least in certain subgroups of CLBP patients. Factors that may moderate such effects should be considered in future studies. Our results raise the possibility that sex may impact on these training effects, and prior work suggests age [44] and negative affect [9,10] could also potentially play a role. Daily opioid analgesic use would also be expected to adversely affect ability for exercise to improve endogenous analgesia, given that it leads to reduced opioid receptor sensitivity through downregulation.
Factors beyond exercise-related EO enhancements must also be considered as potential contributors to the clinical benefits of sustained aerobic exercise training in CLBP patients. For example, isometric exercise studies suggest that increased endocannabinoid activity might contribute to exercise-related hypoalgesia [35], a finding confirmed in preclinical work [24]. Other animal studies suggest that analgesia elicited by aerobic exercise involves peripheral alpha-2 adrenergic receptors [17]. Finally, benefits of aerobic exercise training may also be related to the fact that such interventions, at least indirectly, target pain-related disuse, a factor the fear avoidance model indicates is linked to CLBP-related dysfunction [39].
Several limitations of the current work must be considered. First, intervention efficacy results generalized only to the context of progressive aerobic training supervised by certified exercise trainers. Moreover, the sample consisted of self-selected CLBP patients volunteering to complete an 18-session aerobic exercise program and multiple laboratory visits. Results in clinical patients or for self-directed aerobic exercise programs may not be comparable. Second, the control group for the exercise intervention did not incorporate a placebo to control for expectancies. In addition, assessors for trial outcomes were not blinded to intervention condition. Both factors could have contributed to greater clinical improvements in the exercise group via positive expectancies. On the other hand, laboratory measures, including EO outcomes that were the focus of this study, were assessed in a double-blinded manner, and thus were not confounded by expectancy effects. Third, the sample excluded individuals using daily opioids for safety reasons, given that planned naloxone administration would elicit withdrawal symptoms in individuals regularly using opioids. This, and the focus on individuals with low baseline activity levels, may have enhanced our ability to produce intervention-related EO changes. Finally, EO was assessed in separate naloxone and placebo laboratory sessions carried out following completion of the intervention. Due to scheduling constraints, the final laboratory session was scheduled up to nearly 3 weeks following intervention completion. While the timing of post-intervention outcome assessment matched the laboratory EO assessment schedule, exercise-related EO function enhancements may have diminished in the weeks following the intervention, particularly in individuals not continuing to exercise after study completion. Thus, our findings likely reflect an underestimate of the magnitude of EO enhancements achievable via aerobic training in CLBP patients, reducing statistical power to test EO hypotheses. In a related issue, although our sample was relatively large compared to many prior related studies, it nonetheless may have had suboptimal statistical power for testing mechanistic hypotheses. Results for EO change measures displayed a number of effects that would have been significant with even a slightly larger sample. Use of one-tailed statistical tests for EO hypotheses to enhance power given their a priori direction nature would have led to multiple additional significant EO findings.
In summary, supervised progressive aerobic exercise training, when provided in the absence of other intervention, can significantly decrease pain and interference in individuals with CLBP. There was an apparent dose response effect regarding the degree to which HR training targets were achieved, with evidence supporting enhanced pain inhibitory function, in part EO-related, as a likely contributor to intervention effects. While replication is necessary, this study for the first time suggests that aerobic training can in some chronic pain patients enhance natural ability to inhibit pain that extends outside of the acute exercise context into daily life.
Acknowledgements
This research was supported by NIH Grant R01DA037891, training grant T32GM108554, and CTSA award UL1TR002243 from the National Center for Advancing Translational Sciences. Contents of this work are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The authors report no conflicts of interest.
References
- 1.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 2014. Lippincott Williams and Wilkins; Philadephia, PA. [Google Scholar]
- 2.Amtmann DA, Cook KF, Jensen MP, Chen W-H, Choi SW, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai J-S. Development of a PROMIS item bank to measure pain interference. Pain 2010; 150: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholomew JB, Lewis BP, Linder DE, Cook DB. Post-exercise analgesia: replication and extension. J Sports Sci 1996; 14: 329–334. [DOI] [PubMed] [Google Scholar]
- 4.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013; 111: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg G Borg’s perceived Exertion and Pain Scales. 1998. Human Kinetics; Champaign, IL. [Google Scholar]
- 6.Bruehl S, al’Absi M, France CR, France J, Harju A, Burns JW, Chung OY. Anger management style and endogenous opioid function: is gender a moderator? J Behav Med. 2007; 30: 209–219. [DOI] [PubMed] [Google Scholar]
- 7.Bruehl S, Burns JW, Gupta R, Buvanendran A, Chont M, Kinner E, Schuster E, Passik S, France CR. Endogenous opioid function mediates the association between laboratory-evoked pain sensitivity and morphine analgesic responses. Pain 2013;154: 1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruehl S, Burns JW, Gupta R, Buvanendran A, Chont M, Schuster E, France CR. Endogenous opioid inhibition of chronic low back pain influences degree of back pain relief following morphine administration. Reg Anesth Pain Med 2014; 39: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns JW, Bruehl S, France CR, Schuster E, Orlowska D, Buvanendran A, Chont M, Gupta RK. Psychosocial factors predict opioid analgesia through endogenous opioid function. Pain 2017; 158: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JW, Bruehl S, France CR, Schuster E, Orlowska D, Chont M, Gupta RK, Buvanendran A. Endogenous opioid function and responses to morphine: the moderating effects of anger expressiveness. J Pain 2017; 18: 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantarero-Villanueva I, Fernández-Lao C, Fernández-de-Las-Peñas C, López-Barajas IB, Del-Moral-Ávila R, de la-Llave-Rincón AI, Arroyo-Morales M. Effectiveness of water physical therapy on pain, pressure pain sensitivity, and myofascial trigger points in breast cancer survivors: a randomized, controlled clinical trial. Pain Med 2012; 13: 1509–1519. [DOI] [PubMed] [Google Scholar]
- 12.Chan CW, Mok NW, Yeung EW. Aerobic exercise training in addition to conventional physiotherapy for chronic low back pain: a randomized controlled trial. Arch Phys Med Rehabil 2011; 92: 1681–1685. [DOI] [PubMed] [Google Scholar]
- 13.Chatzitheodorou D, Kabitsis C, Malliou P, Mougios V. A pilot study of the effects of high-intensity aerobic exercise versus passive interventions on pain, disability, psychological strain, and serum cortisol concentrations in people with chronic low back pain. Phys Ther 2007; 87: 304–312. [DOI] [PubMed] [Google Scholar]
- 14.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Cuesta-Vargas AI, Adams N, Salazar JA, Belles A, Hazañas S, Arroyo-Morales M. Deep water running and general practice in primary care for non-specific low back pain versus general practice alone: randomized controlled trial. Clin Rheumatol 2012; 31: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 15.Crombie KM, Brellenthin AG, Hillard CJ, Koltyn KF. Endocannabinoid and Opioid System Interactions in Exercise-Induced Hypoalgesia. Pain Med 2018; 19: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuesta-Vargas AI, García-Romero JC, Arroyo-Morales M, Diego-Acosta AM, Daly DJ. Exercise, manual therapy, and education with or without high-intensity deep-water running for nonspecific chronic low back pain: a pragmatic randomized controlled trial. Am J Phys Med Rehabil 2011; 90: 526–534. [DOI] [PubMed] [Google Scholar]
- 17.de Souza GG, Duarte ID, de Castro Perez A. Differential involvement of central and peripheral α2 adrenoreceptors in the antinociception induced by aerobic and resistance exercise. Anesth Analg 2013; 116: 703–711. [DOI] [PubMed] [Google Scholar]
- 18.Droste C, Greenlee MW, Schreck M, Roskamm H. Experimental pain thresholds and plasma beta-endorphin levels during exercise. Med Sci Sports Exerc 1991; 23: 334–342. [PubMed] [Google Scholar]
- 19.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, and Witter J: Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113: 9–19. [DOI] [PubMed] [Google Scholar]
- 20.Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce-Sandner S, Bhagwat D, Everton D, Burke LB, Cowan P. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009;144: 35–42. [DOI] [PubMed] [Google Scholar]
- 21.Dworkin RH, Turk DC, Trudeau JJ, Benson C, Biondi DM, Katz NP, Kim M. Validation of the Short-form McGill Pain Questionnaire-2 (SF-MPQ-2) in acute low back pain. J Pain 2015; 16: 357–366. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105–121. [DOI] [PubMed] [Google Scholar]
- 23.Frew AK, Drummond PD. Negative affect, pain and sex: the role of endogenous opioids. Pain. 2007; 132 Suppl 1:S77–85. [DOI] [PubMed] [Google Scholar]
- 24.Galdino G, Romero TR, Silva JF, Aguiar DC, de Paula AM, Cruz JS, Parrella C, Piscitelli F, Duarte ID, Di Marzo V, Perez AC. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology 2014; 77: 313–324. [DOI] [PubMed] [Google Scholar]
- 25.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev 2017; 4: CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurevich M, Kohn PM, Davis C. Exercise-induced analgesia and the role of reactivity in pain sensitivity. J Sports Sci 1994; 12: 549–559. [DOI] [PubMed] [Google Scholar]
- 27.Hakansson S, Jones MD, Ristov M, Marcos L, Clark T, Ram A, Morey R, Franklin A, McCarthy C, Carli LD, Ward R, Keech A. Intensity-dependent effects of aerobic training on pressure pain threshold in overweight men: A randomized trial. Eur J Pain 2018; 22: 1813–1823. [DOI] [PubMed] [Google Scholar]
- 28.Hiura M, Sakata M, Ishii K, Toyohara J, Oda K, Nariai T, Ishiwata K. Central μ-Opioidergic System Activation Evoked by Heavy and Severe-Intensity Cycling Exercise in Humans: a Pilot Study Using Positron Emission Tomography with 11C-Carfentanil. Int J Sports Med 2017; 38: 19–26. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman MD, Shepanski MA, Mackenzie SP, Clifford PS. Experimentally induced pain perception is acutely reduced by aerobic exercise in people with chronic low back pain. J Rehabil Res Dev 2005; 42: 183–190. [DOI] [PubMed] [Google Scholar]
- 30.Hviid JT, Thorlund JB, Vaegter HB. Walking increases pain tolerance in humans an experimental cross-over study. Scand J Pain. 2019. June 29 pii:/j/sjpain.ahead-of-print/sjpain-2019–0070/sjpain-2019–0070.xml. doi: 10.1515/sjpain-2019-0070. [DOI] [PubMed] [Google Scholar]
- 31.Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain 1984; 19: 13–25. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chonic pain intensity measures. Pain 1999; 83: 157–162. [DOI] [PubMed] [Google Scholar]
- 33.Jones MD, Booth J, Taylor JL, Barry BK. Aerobic training increases pain tolerance in healthy individuals. Med Sci Sports Exerc 2014; 46: 1640–1647. [DOI] [PubMed] [Google Scholar]
- 34.Kell RT, Asmundson GJ. A comparison of two forms of periodized exercise rehabilitation programs in the management of chronic nonspecific low-back pain. J Strength Cond Res 2009; 23: 513–523. [DOI] [PubMed] [Google Scholar]
- 35.Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain 2014; 15: 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korshøj M, Birk Jørgensen M, Lidegaard M, Mortensen OS, Krustrup P, Holtermann A, Søgaard K. Decrease in musculoskeletal pain after 4 and 12 months of an aerobic exercise intervention: a worksite RCT among cleaners. Scand J Public Health 2018; 46: 846–853. [DOI] [PubMed] [Google Scholar]
- 37.Kraegel P, Griffin J, Grusing S, Brodt ED. Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2017; 166: 493–505. [DOI] [PubMed] [Google Scholar]
- 38.Latorre PÁ, Santos MA, Heredia-Jiménez JM, Delgado-Fernández M, Soto VM, Mañas A, Carbonell-Baeza A. Effect of a 24-week physical training programme (in water and on land) on pain, functional capacity, body composition and quality of life in women with fibromyalgia. Clin Exp Rheumatol 2013; 31(6 Suppl 79):S72–80. [PubMed] [Google Scholar]
- 39.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007; 30: 77–94. [DOI] [PubMed] [Google Scholar]
- 40.Leibetseder V, Strauss-Blasche G, Marktl W, Ekmekcioglu C. Does aerobic training enhance effects of spa therapy in back pain patients? A randomized, controlled clinical trial. Forsch Komplementmed 2007; 14: 202–206. [DOI] [PubMed] [Google Scholar]
- 41.Melzack R The short form of the McGill Pain Questionnaire. Pain 1987; 30: 191–197. [DOI] [PubMed] [Google Scholar]
- 42.Murtezani A, Hundozi H, Orovcanec N, Sllamniku S, Osmani T. A comparison of high intensity aerobic exercise and passive modalities for the treatment of workers with chronic low back pain: a randomized, controlled trial. Eur J Phys Rehabil Med 2011; 47: 359–366. [PubMed] [Google Scholar]
- 43.Naugle KM, Naugle KE, Fillingim RB, Samuels B, Riley JL 3rd. Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc 2014; 46: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naugle KM, Naugle KE, Riley JL 3rd. Reduced Modulation of Pain in Older Adults After Isometric and Aerobic Exercise. J Pain 2016; 17: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 2012; 15(3 Suppl):ES205–13. [PubMed] [Google Scholar]
- 46.Öte Karaca Ş, Demirsoy N, Günendi Z. Effects of aerobic exercise on pain sensitivity, heart rate recovery, and health-related quality of life in patients with chronic musculoskeletal pain. Int J Rehabil Res 2017; 40: 164–170. [DOI] [PubMed] [Google Scholar]
- 47.Paulev PE, Thorbøll JE, Nielsen U, Kruse P, Jordal R, Bach FW, Fenger M, Pokorski M. Opioid involvement in the perception of pain due to endurance exercise in trained man. Jpn J Physiol 1989; 39: 67–74. [DOI] [PubMed] [Google Scholar]
- 48.PROMIS Pain Interference Scoring Manual. http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Pain_Interference_Scoring_Manual.pdf. Accessed on 3/9/20.
- 49.Saanijoki T, Nummenmaa L, Tuulari JJ, Tuominen L, Arponen E, Kalliokoski KK, Hirvonen J. Aerobic exercise modulates anticipatory reward processing via the μ-opioid receptor system. Hum Brain Mapp 2018; 39: 3972–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saanijoki T, Tuominen L, Tuulari JJ, Nummenmaa L, Arponen E, Kalliokoski K, Hirvonen J. Opioid Release after High-Intensity Interval Training in Healthy Human Subjects. Neuropsychopharmacology 2018; 43: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma M, Ugiliweneza B, Beswick J, Boakye M. Concurrent validity and comparative responsiveness of PROMIS-SF versus legacy measures in the cervical and lumbar spine population: Longitudinal analysis from baseline to postsurgery. World Neurosurg 2018; doi: 10.1016/j.wneu.2018.04.131. [DOI] [PubMed] [Google Scholar]
- 52.Shnayderman I, Katz-Leurer M. An aerobic walking programme versus muscle strengthening programme for chronic low back pain: a randomized controlled trial. Clin Rehabil 2013; 27: 207–214. [DOI] [PubMed] [Google Scholar]
- 53.Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, Fu R, Brodt ED, Wasson N, Winter C, Ferguson AJR. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review [Internet]. 2018. June Rockville (MD): Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- 54.Sluka KA, Frey-Law L, Hoeger Bement M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 2018; 159 Suppl 1:S91–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 2006; 26: 5777–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T Jr. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology 2011; 114: 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner JA, Clancy S, McQuade KJ, Cardenas DD. Effectiveness of behavioral therapy for chronic low back pain: a component analysis. J Consult Clin Psychol 1990; 58: 573–579. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services. Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. Retrieved from U. S. Department of Health and Human Services website: https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html. Accessed on 3/7/20.
- 59.Wewege MA, Booth J, Parmenter BJ. Aerobic vs. resistance exercise for chronic non-specific low back pain: A systematic review and meta-analysis. J Back Musculoskelet Rehabil 2018; 31: 889–899. [DOI] [PubMed] [Google Scholar]
- 60.Wormgoor ME, Indahl A, van Tulder MW, Kemper HC. The impact of aerobic fitness on functioning in chronic back pain. Eur Spine J 2008; 17: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yore MM, Ham SA, Ainsworth BE, Kruger J, Reis JP, Kohl III HW, Macera CA. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc 2007; 1267–1274. [DOI] [PubMed] [Google Scholar]
- 62.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci 2002; 22: 5100–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]


