Abstract
Significant attrition limits drug discovery. The available chemical entities present with drug-like features contribute to this limitation. Using specific examples of promiscuous receptor-ligand interactions, we make a case for expanding the chemical space for drug-like molecules. These ligand-receptor interactions are poor candidates for the drug discovery process. However, we provide specific examples of ligand-receptor or transcription factor interactions, namely, the pregnane X receptor (PXR) and the aryl hydrocarbon receptor (AhR), and its interactions with microbial metabolites. We show discrete examples of microbial metabolite mimicry to yield more potent and non-toxic therapeutic leads for pathophysiological conditions regulated by PXR and AhR. These examples underscore our opinion that microbial metabolite mimicry of promiscuous ligand-receptor interactions is warranted and will likely expand the existing chemical space of drugs.
Keywords: biomimicry, metabolites, receptors, drugs, chemical space, disease
Expanding the Chemical Space of Drugs via Microbial Metabolite Mimicry of Promiscuous Metabolite-Receptor Interactions.
Promiscuous receptor-ligand interactions, while on the one hand problematic for necessary science investigations into receptor biology, maybe a treasure trove for investigating the bounds of chemical space available for that receptor (1). If the receptor per se influences a biological phenomenon, then there is theoretical potential to develop more potent small molecules mimicking that chemical space (2, 3). To mine this concept, we start with orphan or adopted orphan nuclear receptors as it is well established that these sets of receptors and related ligand-activated transcription factors have significant ligand promiscuity (4).
Orphan nuclear receptors and transcription factors as attractive targets for host modulation of physiology and pathophysiology.
The absence of a well-defined endogenous ligand defines orphan nuclear receptors. Once the endogenous ligand is discovered, the terms, de-orphanization or adopted orphan receptors are used. The most studied sub-groups of the orphan nuclear receptors comprise xenobiotic receptors and metabolic and energy sensors. Unlike nuclear hormone receptors, orphan nuclear receptors have a large ligand-binding domain, and they are promiscuous in binding a wide array of structurally unrelated compounds. Also, ligands of orphan nuclear receptors usually have lower affinity and potency than nuclear hormone receptors. Therefore, orphan nuclear receptors (we elaborate salient receptors below) are sometimes referred to as high-capacity and low-specificity (affinity) receptors. The discovery of the first xenosensor, the aryl hydrocarbon receptor (AhR), a ligand activated transcription factor, dates back to the early 1970s´. The AhR is activated by several xenobiotics, including environmental pollutants, plant polyphenolics, alkaloids, synthetic compounds, and drugs. The examples of endogenous AhR ligands are eicosanoids, indirubin, bilirubin, or 6-formylindolo[3,2-b]carbazole (FICZ) (5). Whereas the AhR was considered for a long time as a transcriptional mediator of xenoprotective and drug-metabolizing genes, there is a substantial body of evidence for the involvement of the AhR in many physiological processes, such as hematopoiesis, restorative neurogenesis, organ development, embryogenesis and immunity (6). Moreover, the AhR is a pivotal actor in the pathogenesis of cancer (7), intestinal inflammation (8), hepatic steatosis (9), and atopic dermatitis (10). Given the broad and essential roles of the AhR in human physiology and pathophysiology, the attempts for therapeutic targeting of the AhR have emerged. Multiple reports proposed the AhR signaling pathway as a target for anti-cancer therapy, including a strategy of repurposing existing drugs (7, 11). Clinically approved AhR active drugs lansoprazole, omeprazole, raloxifene, flutamide, sulindac, tranilast, leflunomide, nimodipine, or mexeletine were suggested as off-target chemotherapeutics for the treatment of breast cancers (12). Off-targeting the AhR with tranilast was used in the treatment of atopic dermatitis (13). Topical application of coal tar induced skin barrier repair in atopic dermatitis via the AhR pathway (14). A topical cream containing tapinarof, a bacterial stilbenoid, and an AhR agonist, was examined, as the agents for atopic dermatitis (10) and plaque psoriasis (15). The roles of the AhR in the pathogenesis of inflammatory bowel diseases are widely known(16), and the insufficient or inappropriate activation of the AhR is associated with inflammatory conditions (8). Whereas the activation of the intestinal AhR by various xenobiotics was reported to trigger and to attenuate colitis, therapeutic targeting of the intestinal AhR was not applied to date. Interestingly, very few AhR antagonists described, and more recently, natural vitamins, folate, and B12 seem to function as endogenous AhR antagonists (17).
The pregnane X receptor (PXR) was initially described as an orphan nuclear receptor activated by natural and synthetic steroids. In this context, it is important to recognize that the mouse and human receptors respond to distinct ligands (e.g., mouse receptor is activated by pregnenolone carbonitrile; the human receptor by rifampicin) and therefore, displays species-specificity. In this context, with respect to intestinal microbial metabolites, indolyl-3-propionate (IPA), is a mouse PXR ligand but not an efficient human PXR ligand. However, combinatorial indole metabolite mixtures can activate the human PXR receptor. These metabolites are produced in both rodent and human intestines. Thus, there is specificity in terms of the types of ligands found as microbial metabolites and their ligand activation of PXR. For translation to human conditions, human PXR ligands also necessitate the use of humanized mice and these mice have been created by several groups and companies (e.g., Taconic). The PXR is activated by xenobiotics that induce drug-metabolizing enzymes, which causes pharmacokinetic drug interactions, and the activation of which is undesired (18). Xenobiotic ligands of the PXR are structurally unrelated compounds, including drugs (e.g., rifampicin), natural compounds (e.g., hyperforin, solomonsterols) and environmental pollutants (e.g., phthalates, bisphenols) (19, 20). Nowadays, the PXR is “de-orphanized," and endogenous ligands such as bile acids, estrogens, or vitamin K2 were identified. Besides the central role in the regulation of xenobiotic-metabolizing enzymes, the PXR controls the intermediary metabolism of lipids, glucose and bile acids, and it is involved in the etiology of various diseases such as diabetes, metabolic syndrome, cardiovascular pathologies, inflammatory bowel disease, acute kidney injury, neurological pathologies and cancer (20). Hence, the therapeutic targeting of the PXR with both agonists and antagonists is of particular interest (19, 20).
The constitutive androstane receptor (CAR) is a “sister” receptor of the PXR (21) because they share and overlap ligands and activators, transcriptional partners, specific response elements in DNA, a set of target genes, and in general biological functions (22). In contrast to other nuclear receptors, the CAR constitutively activates gene transcription. Initially, it was observed that the steroids androstanol and androstenol inhibit the constitutive activity of the CAR (inverse agonist effects) by the mechanism involving the promotion of co-activator release from the ligand-binding domain. Therefore, an unanticipated steroidal signaling pathway that functions in a manner opposite to that of the conventional nuclear receptor pathways was defined (21). Targeting the CAR in the therapy of metabolic diseases, cancer, diabetes, inflammatory, and liver diseases were proposed (23).
The liver X receptor (LXR) exists in two forms, i.e., LXRα and LXRβ, which have tissue-specific expression. The endogenous ligands of the LXRs are oxysterols and intermediates of the mevalonate metabolic pathway. The example of the xenobiotic activator is synthetic compound GW3965. Physiological roles for the LXRs are the regulation of glucose, fatty acids, and cholesterol metabolism. Therefore, the LXRs are promising targets for the therapy of obesity, diabetes, and atherosclerosis (24). Therapeutic targeting of the LXRs, using dual or LXRα/LXRβ-selective ligands, is also considered in cancer (25) and inflammatory and neurodegenerative diseases (26). Oltipraz, an antagonist of the LXRα, reduced the liver fat content in patients with nonalcoholic fatty liver disease (NAFLD) in phase II clinical trial (27). The LXRβ-selective agonist BMS-852927 increased reverse cholesterol transport pathways, but adverse effects, such as elevated LDL cholesterol and triglycerides, were reported (28). An improvement in barrier differentiation and lipids was observed in patients with mild to moderate atopic dermatitis, topically administered with LXRβ-selective ligand VTP-38543 (29).
The farnesoid X receptor (FXR; NR1H4) was named after its first identified ligand farnesol. Endogenous ligands of the FXR are bile acids; hence the FXR is sometimes referred to as bile acid receptor. The physiological function of the FXR is the regulation of the metabolism of bile acids, fatty acids, triglycerides, and glucose. Consistently, FXR is an attractive target for the treatment of metabolic and hepatic diseases (24, 30). Several clinical trials were carried out with highly potent FXR synthetic agonist obeticholic acid, including those against type II diabetes and NAFLD (31), non-cirrhotic nonalcoholic steatohepatitis (32), primary sclerosing cholangitis (33) and primary biliary cholangitis (34). For the latter, FDA-approved drug Ocaliva® is available. The examples of other FXR agonists currently under clinical investigations are Cilofexor (Gilead Sciences) and PX-102 (Phenex Pharmaceuticals).
The peroxisome proliferator-activated receptors (PPARs) regulate glucose and lipid homeostasis, inflammation, proliferation, and differentiation (35). Fatty acids activate the PPARs and their derivatives, the examples of endogenous ligands for PPARα and PPARγ are oleoylethanolamide and 15-deoxy-prostaglandin J2, respectively. Xenobiotic ligands for the PPARs are various endocrine-disrupting chemicals than cause obesogenic effects. The therapeutic target for hypolipidemic drugs of fibrates class is PPARα, whereas anti-diabetes type-II thiazolidinediones target PPARγ. Targeting PPARs in cancer therapy (35) and diabetic microvascular damage (36) was proposed.
Microbial metabolites as modulators of AhR, PXR, FXR, LXRs, and PPARs receptors.
Human gut microbiota produces a broad spectrum of metabolic products that interact with the host organism and mediate beneficial health effects and pathogenicity. The microbial metabolites are structurally diverse compounds, including short and medium-chain fatty acids, polysaccharides, polyamines, formyl-peptides, bile acids, tryptophan metabolites, acyl-homoserine lactones, gaseous substances (hydrogen sulfide, methane), toxins and others (37). At cellular levels, the gut microbial metabolites interact mostly with G protein-coupled receptors and nuclear receptors, through which they mediate their biological effects both onsite and distantly.
The following examples illustrate how multiple microbial metabolites can serve as weak but promiscuous ligands for a given nuclear receptor and across ligand-activated transcription factor systems. In this context we only highlight PXR and AhR as the other receptors mentioned, LXR, FXR, and PPAR are not per se promiscuous receptors. Metabolites of tryptophan, produced by human intestinal microbiota, were extensively studied as ligands of the AhR and mediators of intestinal health and disease. To date, numerous tryptophan catabolites were identified as low-affinity AhR ligands (agonist and antagonist), including indole, skatole, tryptamine a series of indolyl-3-(lactate, pyruvate, acrylate, propionate, acetate, aldehyde, acetamide, ethanol) (38, 39). Ligand-dependent transcriptional activation of the AhR was synergistically enhanced by short-chain fatty acids (SCFAs) (40). Other examples of intestinal-microbiota derived AhR ligands comprise 2,8-dihydroxy-quinoline, urolithin A (formed from ellagitannins), and 1,4-dihydroxy-2-naphthoic acid (41).
The most studied microbial PXR ligands were the indole products of tryptophan catabolism, including skatole (42), indolyl-3-acetate (IAA), and indolyl-3-propionate (IPA); the latter being reported to diminish intestinal inflammation through PXR pathway (43). Intestinal pro-inflammatory responses in the early stages of experimental necrotizing entero-colitis were attenuated by lithocholic acid through targeting the PXR (44). Indirect effects of various xenobiotics such as statins or green tea polyphenols through PXR were shown to result in dysregulation of intestinal SCFAs and bile acids composition. Whereas the involvement of CAR in the intestinal mucosal response to injury was demonstrated, direct proof of CAR targeting with microbial metabolites was not provided (45).
The antioxidant tempol displayed anti-obesity activity by reducing intestinal genus Lactobacillus, leading to the accumulation of tauro-β-muricholic acid, which is the FXR antagonist (46). Similarly, theabrownin suppressed gut microbes with bile-salt hydrolase activity, which led to the increased levels of taurochendoxycholic, tauroursodeoxycholic, glycochenodeoxycholic and glycoursodeoxycholic acids that exhibited hypolipidemic effects through inhibition of the intestinal FXR (47). Hypoglycemic activity of metformin was partly explained by shaping gut microbiota when Bacteroides fragilis diminished, and the levels of glycoursodeoxycholic acid, an FXR antagonist, increased (48). Recently, a new class of bile acids with FXR agonist activity, produced by human gut microbiota was identified. These acids are conjugated with atypical amino acids, yielding novel phenylalanocholic, tyrosocholic and leucocholic acids (49). Overall, gut bacterial bile acids are the agonist and antagonists of the FXR that display their biological effects partly through this receptor; however, the activation of the PXR cannot be excluded. Interestingly, there are no available data about the interactions between LXR and gut microbial metabolites.
Several studies reported the activation of the intestinal PPARγ by SCFAs, mainly by propionate and butyrate (50). Depletion of butyrate-producing microbes by antibiotics reduced epithelial signaling through PPARγ (51). Probiotic bacteria producing conjugated linoleic acids locally in the gut were shown to suppress colitis through targeting PPARγ (52).
Indole and its microbial metabolites as promiscuous ligands for a given receptor and across receptor systems.
L-tryptophan catabolism, specifically via microbes in the intestine, is essential for host-microbe symbiosis (53, 54) (Figure 1). Indole per se has been extensively studied concerning AhR. Indole is a very weak PXR ligand [manuscript submitted] (43). However, indoles (~ 1 mM) can also inhibit voltage-gated K (+) channels leading to acute stimulation of GLP-1 secretion from intestinal L cells; however, a more chronic effect of indoles reduces GLP-1 via inhibition of NADH dehydrogenases and a slow loss of ATP (55). Indole also has effects on bacteria such that it causes spatial segregation of bacteria in the gut that resist invaders but helps recruit beneficial organisms (56). Additionally, indole can attenuate the IFN1 pathway and limit graft-versus-host disease in mice that have undergone allogeneic bone marrow transplantation (57). Indolyl-3-propionate (IPA) is a mouse PXR agonist, and for efficient activation of the human receptor requires indole (43). However, IPA is not an efficient AhR ligand (39). Indole metabolite, skatole (3-methylindole) is an efficient ligand for AhR but also demonstrates AhR-independent activation of p38 in intestinal epithelial cells (58). Tryptamine mediates ionic flux in the intestines directly via a 5-HT4 receptor (5-HT4R), a G-protein-coupled receptor (GPCR)(59). Indoleacrylic acid (IAC) and indole 3-pyruvic acid (IPY), suppresses inflammation via actions on Nrf2 and AhR, respectively (60, 61). These examples serve to illustrate the complexity of host receptor interactions within just one amino acid microbial catabolite family. Efficient metabolite-receptor interactions are usually in the micromolar range. The combinatorial effects of metabolites also have different outcomes – some together act as agonists, while other agonists, in combination, could result in antagonism of the receptor(s)(62). Using the indole metabolite family as an example, we will explore how quantitative structure-activity relationships (QSAR) focused on PXR and AhR yield potential lead small molecules. We can show that these lead molecules are relatively safe, compared with known xenobiotic ligands for AhR and PXR, for in vivo applications. Since PXR and AhR have potent anti-inflammatory properties, there is a potential for developing the leads into therapeutic drugs for conditions such as inflammatory bowel disease.
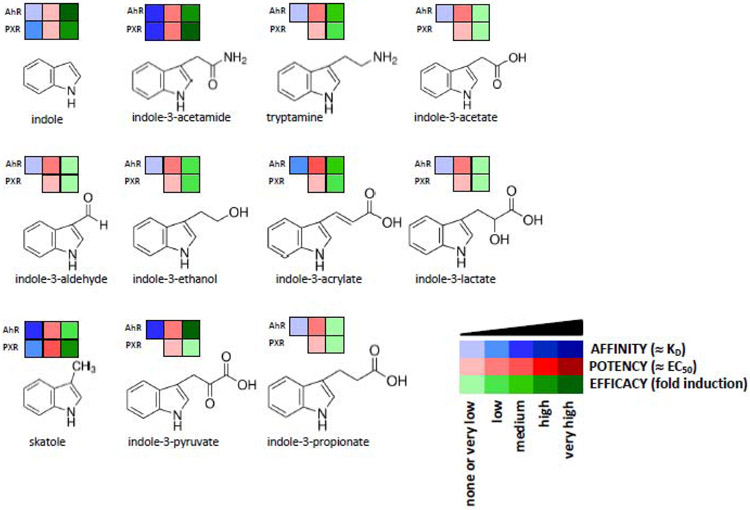
Figure 1. Tryptophan Intestinal Microbial Catabolites as Ligands and Agonists of Xenosensors, AhR and PXR.
A chart summarizes molecular effects of known human intestinal microbial metabolites of tryptophan at AhR and PXR receptors. Heat-map shows a semi-quantitative profile of interactions: (i) AFFINITY, is a measure of compound binding at the receptor ligand binding domain (dissociation constant ≈ KD); (ii) POTENCY, refers to the concentration of the compound to produce 50% of maximal effect (half-maximal effective concentration ≈ EC50); (iii) EFFICACY is the maximum response that can be reached by the compound.
Quantitative Structure-Activity Relationships between Indole(s) and receptors, PXR, and AhR.
The correlation between a structure of xenobiotic and microbial indoles and their capability to activate PXR and AhR receptors in vitro or in vivo is not straightforward, because there is mutual regulatory cross-talk between the AhR and the PXR. Also, numerous compounds are dual agonists for both receptors (Figure 1). Whereas indole is a very low potency (EC50 ≈ 1000 μM) ligand of the PXR, its mono-methylation at any position significantly increased its potency (EC50 ≈ 25-75 μM) (42). The substitution of indole at position 3 with acetamide moiety led to medium-potency derivative, whereas the introduction of 2-hydroxyethyl, 2-aminoethyl, or acryloyl moiety maintained feeble agonist effects. However, 3-substitutions with acetate, propionate, lactate, pyruvate, or aldehyde resulted rather in loss of even weak indole activity (manuscript submitted). The presence of indole-N-acetamide moiety was identified as a PXR-active pharmacophore of viral polymerase inhibitors (63). Interestingly, the combination of IPA and indole yielded much stronger intestinal anti-inflammatory effects via the PXR, as compared with the individual metabolites (43). We designed highly potent (EC50 ≈ 1 μM) and efficacious PXR-selective agonists by exploiting indoles-based hybrid structure scaffolds. The key PXR-active pharmacophore was 1-(phenylsulfonyl)-indol-2-yl backbone. The new introduction of the indol-2-yl group led to the acquisition of the AhR activity and the AhR/PXR-dual agonists; the removal of phenyl sulfonyl group resulted in AhR supra-agonist compounds (64). Eleven metabolites formed from dietary tryptophan by human intestinal microbiota, all being 3-substituted indoles, were identified as the ligands and activators of the AhR, substantially differing in their pharmacology parameters (39). Highly potent (EC50 ≈ 1 nM) AhR-agonists, are based on 3-mono-substituted indoles [6-(indole-3-carbonyl)picolinonitrile and (indol-3-yl)(6-(trifluoromethyl)pyridin-2-yl)methanone] (65).
Examples of mimicry of AhR and PXR ligand metabolites derived from microbes as therapeutic leads.
As discussed previously, we had demonstrated that the combination of indole, together with its metabolite indolyl-3-propionate (IPA), activates the human pregnane X receptor (PXR). PXR agonism regulates intestinal inflammation via the TLR4-NFκB pathway (43). Subsequently, based on the binding pharmacophore of indole and IPA on the PXR ligand-binding domain (LBD), we tested a series of indole analogs arising for reaction intermediates and final products in PXR and AhR reporter assays (64). In these studies, we found several indole analog synthetic intermediates (FKK1-10) that were effective PXR agonists; however, only two of these, FFK2 and FKK9, had potent AhR agonist effects. In picking for selective PXR agonists, we focused on FKK5 and FKK6 based on the most favorable biophysical and biochemical activity profile. As a first example, FKK6 was further evaluated in human cell lines and intestinal organoid model systems of inflammation, and finally, in a mouse model of chemical colitis. The data suggest that FKK6 significantly abrogates indices of pro-inflammatory activity (IL8, lipocalin) in all model systems studied.
Furthermore, experiments in primary human intestinal biopsy tissues obtained from patients with inflammatory bowel disease (IBD) ex vivo are planned (personal communication, Drs. Hao Li, Albert Einstein College of Medicine, Bronx, NY & Efi Kokkotou, Beth Israel Deaconess Medical Center, Boston MA). A more focused approach is seen with AhR. Indole is an AhR agonist (EC50~ 3μM to 1.5 mM in luciferase reporter assays) (66, 67). However, at Harvard, investigators screened a chemical library of indoles and indazoles, which resulted in developing a potent hit, PY109. This small molecule is a potent AhR agonist (EC50 ~1.2 nM) and is a more potent and drug-like indole mimic that has demonstrated anti-inflammatory properties in mice (68). PY109, when orally administered to mice, potently induced IL-22 and expanded tissue expand ILC3 and γδ T cell subpopulations. The investigators found several other hits like PY108, all capable of similar actions as PY109. These design and chemical synthesis principles may be extended to the microbial synthesis of potent alkaloids that serve as AhR ligands (69, 70).
Potential Application and Pitfalls of PXR and AhR Activating Microbial Metabolite Mimics in human diseases.
It is tempting to dive into classical drug development, but it is prudent to understand the effects of chronically perturbing PXR/AhR signaling homeostasis. In the case of both receptors, PXR and AhR, hyperstimulation of either receptor has tissue-specific effects, some that will not be beneficial for drug discovery. For example, hyperstimulation of AhR systemically can lead to anemia, fatty livers, and developmentally, cleft palate (17, 71). In this context, the aim of an AhR targeting treatment might be to restore the normal activation of the receptor and not to overstimulate it. Similarly, systemic activation of PXR can have untoward effects with regards to unanticipated drug interactions, liver steatosis, and the potential to accelerate de novo malignancy (72).
Furthermore, as with any receptor involved in homeostasis, tachyphylaxis of response is also possible. Hence, applications of principles of clinical pharmacology then become salient in the drug development process for agents targeting these types of receptors. For example, in the case of FKK6 and PXR, it is imperative to drive PXR activation, perhaps in an intermittent manner and with local confinement. So, if we target inflammatory bowel disease, the ideal setting is distal colitis, in which FKK6 formulations could only be delivered as enemas for local effect. Alternatively, intermittent rectal bolus could be applied to avoid consistent hyperstimulation of PXR. Thus, a combination of patient and disease selection, coupled with some regional approaches for drug delivery, might be the best option to move these potent agents forward.
Concluding Remarks and Future Perspectives.
This opinion article covers the feasibility of developing potential therapeutic leads using the concept of chemical biomimicry. The bio-mimics are of "weak," but host targeted microbial metabolites, where the exact relationship between the ligand and its cognate therapeutic target(s) are known. We posit some pre-requisites for this to happen. First, the chemistry of deriving mimics should be simple and well defined. There should be a resolution of enantiomers, as many of these receptors have enantiomer-specific interactions. Second, the relationship between the host target and the microbial metabolite should be well defined, preferably by direct binding studies. Third, there should be a firm ability to perform classical biochemistry studies with the target. The analogs will need to be tested in terms of its affinity for binding to the receptor. If possible, the crystal structures of the ligand-bound receptor should be ascertained. Fifth, the association, in terms of cause and effect between the host target and the physiology (or pathophysiology) under therapy, should be well characterized. However, even with these pre-requisites, broader questions are looming (see Outstanding Questions). For example, it is unknown as to what the true extent of weak (and promiscuous) metabolite-receptor pairings is in humans, specifically those that modify host physiology or pathophysiology. If each is mined, how many can genuinely be subjected to the pre-requisites mentioned above to further drug discovery? Drug discovery is already replete with ideas that expand chemical space simply based on the limits of chemistry per se (73, 74). Hence, an outstanding question is whether this could help our concept develop further or if our concept of directed metabolite mimicry enlarges the chemical space. There is much to be learned from mining microbial metabolite - host receptor interactions, and there is a ray of hope that future work will exploit these interactions favorably.
Outstanding Questions.
To what extent are host receptor-metabolite pairings promiscuous and weak (affinity in the micro to millimolar range) ?
How many of these interactions actually influence host physiology or pathophysiology ?
How many receptor-metabolite pairs that influence host physiology are actually amenable to chemical analog investigations ? A major pre-requisite for this would be that the ligand in question must be amenable to chemistry that is simple and dramatically changes receptor interactions.
Highlights.
The lack of chemical diversity in drug like molecules is one important reason for drug attrition
Microbial metabolite mimicry of promiscuous ligand-receptor interactions is warranted and will likely expand the existing chemical space of drugs
Prototypical host receptors that exhibit weak ligand interactions include the xenobiotic nuclear receptors like PXR and AhR
Using PXR and AhR as exemplar xenobiotic receptors, we show discrete examples of microbial metabolite mimicry that yields more potent and non-toxic therapeutic leads for pathophysiological conditions regulated by these receptors
ACKNOWLEDGEMENTS
Supported in part by The Peer Reviewed Medical Research Program – Investigator Initiated Research Award under Award No. W81XWH-17-1-0479; NIH grants (ES030197; CA 222469); Czech Health Research Council, grant number [NV19-05-00220] (to Z.D.).
Footnotes
DISCLAIMER STATEMENT
We (SM, ZD) have filed a patent application US 2019/0367475 A1 on PXR agonists and uses thereof for gut barrier dysfunction and treatment prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keah HH, Hearn MT. A molecular recognition paradigm: promiscuity associated with the ligand-receptor interactions of the activin members of the TGF-beta superfamily. J Mol Recognit. 2005;18(5):385–403. Epub 2005/06/11. doi: 10.1002/jmr.715. PubMed PMID: 15948132. [DOI] [PubMed] [Google Scholar]
- 2.Ekins S, Kortagere S, Iyer M, Reschly EJ, Lill MA, Redinbo MR, Krasowski MD. Challenges Predicting Ligand-Receptor Interactions of Promiscuous Proteins: The Nuclear Receptor PXR. PLOS Computational Biology. 2009;5(12):e1000594. doi: 10.1371/journal.pcbi.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giani Tagliabue S, Faber SC, Motta S, Denison MS, Bonati L. Modeling the binding of diverse ligands within the Ah receptor ligand binding domain. Scientific Reports. 2019;9(1):10693. doi: 10.1038/s41598-019-47138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sladek FM. What are nuclear receptor ligands? Mol Cell Endocrinol. 2011;334(1-2):3–13. Epub 2010/07/10. doi: 10.1016/j.mce.2010.06.018. PubMed PMID: 20615454; PMCID: PMC3010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annual review of pharmacology and toxicology. 2003;43:309–34. doi: 10.1146/annurev.pharmtox.43.100901.135828. PubMed PMID: 12540743. [DOI] [PubMed] [Google Scholar]
- 6.Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nature reviews Immunology. 2019;19(3):184–97. doi: 10.1038/s41577-019-0125-8. PubMed PMID: 30718831. [DOI] [PubMed] [Google Scholar]
- 7.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nature reviews Cancer. 2014;14(12):801–14. doi: 10.1038/nrc3846. PubMed PMID: 25568920; PMCID: 4401080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metidji A, Omenetti S, Crotta S, Li Y, Nye E, Ross E, Li V, Maradana MR, Schiering C, Stockinger B. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity. 2018;49(2):353–62 e5. doi: 10.1016/j.immuni.2018.07.010. PubMed PMID: 30119997; PMCID: 6104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Wada T, Febbraio M, He J, Matsubara T, Lee MJ, Gonzalez FJ, Xie W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010;139(2):653–63. doi: 10.1053/j.gastro.2010.03.033. PubMed PMID: 20303349; PMCID: 2910786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppers J, Paller AS, Maeda-Chubachi T, Wu S, Robbins K, Gallagher K, Kraus JE. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. Journal of the American Academy of Dermatology. 2019;80(1):89–98 e3. doi: 10.1016/j.jaad.2018.06.047. PubMed PMID: 30554600. [DOI] [PubMed] [Google Scholar]
- 11.Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities. Trends in pharmacological sciences. 2018;39(3):307–25. doi: 10.1016/j.tips.2017.11.007. PubMed PMID: 29254698. [DOI] [PubMed] [Google Scholar]
- 12.Baker JR, Sakoff JA, McCluskey A. The aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Medicinal research reviews. 2020;40(3):972–1001. doi: 10.1002/med.21645. PubMed PMID: 31721255. [DOI] [PubMed] [Google Scholar]
- 13.Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharmacological research. 2015;91:15–28. doi: 10.1016/j.phrs.2014.10.009. PubMed PMID: 25447595. [DOI] [PubMed] [Google Scholar]
- 14.van den Bogaard EH, Bergboer JG, Vonk-Bergers M, van Vlijmen-Willems IM, Hato SV, van der Valk PG, Schroder JM, Joosten I, Zeeuwen PL, Schalkwijk J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. The Journal of clinical investigation. 2013;123(2):917–27. doi: 10.1172/JCI65642. PubMed PMID: 23348739; PMCID: 3561798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins K, Bissonnette R, Maeda-Chubachi T, Ye L, Peppers J, Gallagher K, Kraus JE. Phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. Journal of the American Academy of Dermatology. 2019;80(3):714–21. doi: 10.1016/j.jaad.2018.10.037. PubMed PMID: 30612986. [DOI] [PubMed] [Google Scholar]
- 16.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nature medicine. 2016;22(6):598–605. Epub 2016/05/10. doi: 10.1038/nm.4102. PubMed PMID: 27158904; PMCID: PMC5087285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DJ, Venkataraman A, Jain PC, Wiesler EP, DeBlasio M, Klein J, Tu SS, Lee S, Medzhitov R, Iwasaki A. Vitamin B12 and folic acid alleviate symptoms of nutritional deficiency by antagonizing aryl hydrocarbon receptor. Proceedings of the National Academy of Sciences of the United States of America. 2020. Epub 2020/06/24. doi: 10.1073/pnas.2006949117. PubMed PMID: 32571957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. The Journal of clinical investigation. 1998;102(5):1016–23. doi: 10.1172/JCI3703. PubMed PMID: 9727070; PMCID: 508967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai SC, Lin W, Li Y, Chen T. Drug discovery technologies to identify and characterize modulators of the pregnane X receptor and the constitutive androstane receptor. Drug discovery today. 2019;24(3):906–15. doi: 10.1016/j.drudis.2019.01.021. PubMed PMID: 30731240; PMCID: 6421094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai SC, Wright WC, Chen T. Strategies for developing pregnane X receptor antagonists: Implications from metabolism to cancer. Medicinal research reviews. 2020;40(3):1061–83. doi: 10.1002/med.21648. PubMed PMID: 31782213; PMCID: 7166136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395(6702):612–5. doi: 10.1038/26996. PubMed PMID: 9783588. [DOI] [PubMed] [Google Scholar]
- 22.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nature reviews Drug discovery. 2002;1(4):259–66. doi: 10.1038/nrd753. PubMed PMID: 12120277. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee M, Robbins D, Chen T. Targeting xenobiotic receptors PXR and CAR in human diseases. Drug discovery today. 2015;20(5):618–28. doi: 10.1016/j.drudis.2014.11.011. PubMed PMID: 25463033; PMCID: 4433851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nature reviews Molecular cell biology. 2012;13(4):213–24. doi: 10.1038/nrm3312. PubMed PMID: 22414897; PMCID: 3597092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CY, Gustafsson JA. Targeting liver X receptors in cancer therapeutics. Nature reviews Cancer. 2015;15(4):216–24. doi: 10.1038/nrc3912. PubMed PMID: 25786697. [DOI] [PubMed] [Google Scholar]
- 26.Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nature reviews Drug discovery. 2014;13(6):433–44. doi: 10.1038/nrd4280. PubMed PMID: 24833295. [DOI] [PubMed] [Google Scholar]
- 27.Kim W, Kim BG, Lee JS, Lee CK, Yeon JE, Chang MS, Kim JH, Kim H, Yi S, Lee J, Cho JY, Kim SG, Lee JH, Kim YJ. Randomised clinical trial: the efficacy and safety of oltipraz, a liver X receptor alpha-inhibitory dithiolethione in patients with non-alcoholic fatty liver disease. Alimentary pharmacology & therapeutics. 2017;45(8):1073–83. doi: 10.1111/apt.13981. PubMed PMID: 28225186. [DOI] [PubMed] [Google Scholar]
- 28.Kirchgessner TG, Sleph P, Ostrowski J, Lupisella J, Ryan CS, Liu X, Fernando G, Grimm D, Shipkova P, Zhang R, Garcia R, Zhu J, He A, Malone H, Martin R, Behnia K, Wang Z, Barrett YC, Garmise RJ, Yuan L, Zhang J, Gandhi MD, Wastall P, Li T, Du S, Salvador L, Mohan R, Cantor GH, Kick E, Lee J, Frost RJ. Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell metabolism. 2016;24(2):223–33. doi: 10.1016/j.cmet.2016.07.016. PubMed PMID: 27508871. [DOI] [PubMed] [Google Scholar]
- 29.Czarnowicki T, Dohlman AB, Malik K, Antonini D, Bissonnette R, Chan TC, Zhou L, Wen HC, Estrada Y, Xu H, Bryson C, Shen J, Lala D, Ma'ayan A, McGeehan G, Gregg R, Guttman-Yassky E. Effect of short-term liver X receptor activation on epidermal barrier features in mild to moderate atopic dermatitis: A randomized controlled trial. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2018;120(6):631–40 e11. doi: 10.1016/j.anai.2018.03.013. PubMed PMID: 29567358. [DOI] [PubMed] [Google Scholar]
- 30.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nature reviews Gastroenterology & hepatology. 2014;11(1):55–67. doi: 10.1038/nrgastro.2013.151. PubMed PMID: 23982684. [DOI] [PubMed] [Google Scholar]
- 31.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–82 e1. doi: 10.1053/j.gastro.2013.05.042. PubMed PMID: 23727264. [DOI] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E, Network NCR. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4. PubMed PMID: 25468160; PMCID: 4447192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, Shrestha R, Trotter J, Goldberg D, Rushbrook S, Hirschfield GM, Schiano T, Jin Y, Pencek R, MacConell L, Shapiro D, Bowlus CL, Investigators AS. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. Journal of hepatology. 2020. doi: 10.1016/j.jhep.2020.02.033. PubMed PMID: 32165251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D, Group PS. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. The New England journal of medicine. 2016;375(7):631–43. doi: 10.1056/NEJMoa1509840. PubMed PMID: 27532829. [DOI] [PubMed] [Google Scholar]
- 35.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nature reviews Cancer. 2012;12(3):181–95. doi: 10.1038/nrc3214. PubMed PMID: 22318237; PMCID: 3322353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiukka A, Maranghi M, Matikainen N, Taskinen MR. PPARalpha: an emerging therapeutic target in diabetic microvascular damage. Nature reviews Endocrinology. 2010;6(8):454–63. doi: 10.1038/nrendo.2010.89. PubMed PMID: 20567246. [DOI] [PubMed] [Google Scholar]
- 37.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nature reviews Immunology. 2016;16(6):341–52. doi: 10.1038/nri.2016.42. PubMed PMID: 27231050; PMCID: 5541232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. Epub 2018/08/19. doi: 10.1038/s41467-018-05470-4. PubMed PMID: 30120222; PMCID: PMC6098093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vyhlidalova B, Krasulova K, Pecinkova P, Marcalikova A, Vrzal R, Zemankova L, Vanco J, Travnicek Z, Vondracek J, Karasova M, Mani S, Dvorak Z. Gut Microbial Catabolites of Tryptophan Are Ligands and Agonists of the Aryl Hydrocarbon Receptor: A Detailed Characterization. International journal of molecular sciences. 2020;21(7). doi: 10.3390/ijms21072614. PubMed PMID: 32283770; PMCID: 7177849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin UH, Cheng Y, Park H, Davidson LA, Callaway ES, Chapkin RS, Jayaraman A, Asante A, Allred C, Weaver EA, Safe S. Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci Rep. 2017;7(1):10163. doi: 10.1038/s41598-017-10824-x. PubMed PMID: 28860561; PMCID: 5579248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pernomian L, Duarte-Silva M, de Barros Cardoso CR. The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor. Clinical reviews in allergy & immunology. 2020. doi: 10.1007/s12016-020-08789-3. PubMed PMID: 32279195. [DOI] [PubMed] [Google Scholar]
- 42.Vyhlidalova B, Bartonkova I, Jiskrova E, Li H, Mani S, Dvorak Z. Differential activation of human pregnane X receptor PXR by isomeric mono-methylated indoles in intestinal and hepatic in vitro models. Toxicology letters. 2020;324:104–10. doi: 10.1016/j.toxlet.2020.02.010. PubMed PMID: 32092453. [DOI] [PubMed] [Google Scholar]
- 43.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41(2):296–310. Epub 2014/07/30. doi: 10.1016/j.immuni.2014.06.014. PubMed PMID: 25065623; PMCID: Pmc4142105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang K, Mukherjee S, DesMarais V, Albanese JM, Rafti E, Draghi Ii A, Maher LA, Khanna KM, Mani S, Matson AP. Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatric research. 2018;83(5):1031–40. doi: 10.1038/pr.2018.14. PubMed PMID: 29360809; PMCID: 5959752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudson GM, Flannigan KL, Erickson SL, Vicentini FA, Zamponi A, Hirota CL, Alston L, Altier C, Ghosh S, Rioux KP, Mani S, Chang TK, Hirota SA. Constitutive androstane receptor regulates the intestinal mucosal response to injury. British journal of pharmacology. 2017;174(12):1857–71. doi: 10.1111/bph.13787. PubMed PMID: 28320072; PMCID: 5446585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. PubMed PMID: 24064762; PMCID: 6595219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang F, Zheng X, Ma X, Jiang R, Zhou W, Zhou S, Zhang Y, Lei S, Wang S, Kuang J, Han X, Wei M, You Y, Li M, Li Y, Liang D, Liu J, Chen T, Yan C, Wei R, Rajani C, Shen C, Xie G, Bian Z, Li H, Zhao A, Jia W. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat Commun. 2019;10(1):4971. doi: 10.1038/s41467-019-12896-x. PubMed PMID: 31672964; PMCID: 6823360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, Zhang S, Yun C, Lian G, Zhang X, Zhang H, Bisson WH, Shi J, Gao X, Ge P, Liu C, Krausz KW, Nichols RG, Cai J, Rimal B, Patterson AD, Wang X, Gonzalez FJ, Jiang C. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nature medicine. 2018;24(12):1919–29. doi: 10.1038/s41591-018-0222-4. PubMed PMID: 30397356; PMCID: 6479226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn RA, Melnik AV, Vrbanac A, Fu T, Patras KA, Christy MP, Bodai Z, Belda-Ferre P, Tripathi A, Chung LK, Downes M, Welch RD, Quinn M, Humphrey G, Panitchpakdi M, Weldon KC, Aksenov A, da Silva R, Avila-Pacheco J, Clish C, Bae S, Mallick H, Franzosa EA, Lloyd-Price J, Bussell R, Thron T, Nelson AT, Wang M, Leszczynski E, Vargas F, Gauglitz JM, Meehan MJ, Gentry E, Arthur TD, Komor AC, Poulsen O, Boland BS, Chang JT, Sandborn WJ, Lim M, Garg N, Lumeng JC, Xavier RJ, Kazmierczak BI, Jain R, Egan M, Rhee KE, Ferguson D, Raffatellu M, Vlamakis H, Haddad GG, Siegel D, Huttenhower C, Mazmanian SK, Evans RM, Nizet V, Knight R, Dorrestein PC. Global chemical effects of the microbiome include new bile-acid conjugations. Nature. 2020;579(7797):123–9. Epub 2020/02/28. doi: 10.1038/s41586-020-2047-9. PubMed PMID: 32103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nepelska M, de Wouters T, Jacouton E, Beguet-Crespel F, Lapaque N, Dore J, Arulampalam V, Blottiere HM. Commensal gut bacteria modulate phosphorylation-dependent PPARgamma transcriptional activity in human intestinal epithelial cells. Sci Rep. 2017;7:43199. doi: 10.1038/srep43199. PubMed PMID: 28266623; PMCID: 5339702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Baumler AJ. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357(6351):570–5. doi: 10.1126/science.aam9949. PubMed PMID: 28798125; PMCID: 5642957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Carbo A, Shaykhutdinov R, Jobin C, Arthur JC, Corl BA, Vogel H, Storr M, Hontecillas R. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR gamma to suppress colitis. PloS one. 2012;7(2):e31238. doi: 10.1371/journal.pone.0031238. PubMed PMID: 22363592; PMCID: 3283634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borghi M, Puccetti M, Pariano M, Renga G, Stincardini C, Ricci M, Giovagnoli S, Costantini C, Romani L. Tryptophan as a Central Hub for Host/Microbial Symbiosis. Int J Tryptophan Res. 2020;13:1178646920919755. Epub 2020/05/22. doi: 10.1177/1178646920919755. PubMed PMID: 32435131; PMCID: PMC7225782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716–24. Epub 2018/06/15. doi: 10.1016/j.chom.2018.05.003. PubMed PMID: 29902437. [DOI] [PubMed] [Google Scholar]
- 55.Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell reports. 2014;9(4):1202–8. Epub 2014/12/03. doi: 10.1016/j.celrep.2014.10.032. PubMed PMID: 25456122; PMCID: PMC4308618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Chawla R, Rhee KY, Gupta R, Manson MD, Jayaraman A, Lele PP. Biphasic chemotaxis of Escherichia coli to the microbiota metabolite indole. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(11):6114–20. Epub 2020/03/04. doi: 10.1073/pnas.1916974117. PubMed PMID: 32123098; PMCID: PMC7084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swimm A, Giver CR, DeFilipp Z, Rangaraju S, Sharma A, Ulezko Antonova A, Sonowal R, Capaldo C, Powell D, Qayed M, Kalman D, Waller EK. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood. 2018;132(23):2506–19. Epub 2018/09/28. doi: 10.1182/blood-2018-03-838193. PubMed PMID: 30257880; PMCID: PMC6284212 applied for a patent on “Methods of Managing Graft Versus Host Disease (GVHD) Using Indole Carboxyaldehydes or Derivatives Thereof.” The remaining authors declare no competing financial interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurata K, Kawahara H, Nishimura K, Jisaka M, Yokota K, Shimizu H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochemical and Biophysical Research Communications. 2019;510(4):649–55. doi: 10.1016/j.bbrc.2019.01.122. [DOI] [PubMed] [Google Scholar]
- 59.Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, Kaunitz JD, Sonnenburg JL, Fischbach MA, Farrugia G, Kashyap PC. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe. 2018;23(6):775–85.e5. Epub 2018/06/15. doi: 10.1016/j.chom.2018.05.004. PubMed PMID: 29902441; PMCID: PMC6055526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wlodarska M, Luo C, Kolde R, d'Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O'Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe. 2017;22(1):25–37.e6. Epub 2017/07/14. doi: 10.1016/j.chom.2017.06.007. PubMed PMID: 28704649; PMCID: PMC5672633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y. Indole-3-Pyruvic Acid, an Aryl Hydrocarbon Receptor Activator, Suppresses Experimental Colitis in Mice. J Immunol. 2018;201(12):3683–93. Epub 2018/11/16. doi: 10.4049/jimmunol.1701734. PubMed PMID: 30429284. [DOI] [PubMed] [Google Scholar]
- 62.Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85(5):777–88. Epub 2014/02/25. doi: 10.1124/mol.113.091165. PubMed PMID: 24563545; PMCID: PMC3990014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harper S, Avolio S, Pacini B, Di Filippo M, Altamura S, Tomei L, Paonessa G, Di Marco S, Carfi A, Giuliano C, Padron J, Bonelli F, Migliaccio G, De Francesco R, Laufer R, Rowley M, Narjes F. Potent inhibitors of subgenomic hepatitis C virus RNA replication through optimization of indole-N-acetamide allosteric inhibitors of the viral NS5B polymerase. Journal of medicinal chemistry. 2005;48(14):4547–57. doi: 10.1021/jm050056+. PubMed PMID: 15999993. [DOI] [PubMed] [Google Scholar]
- 64.Dvorak Z, Kopp F, Costello CM, Kemp JS, Li H, Vrzalova A, Stepankova M, Bartonkova I, Jiskrova E, Poulikova K, Vyhlidalova B, Nordstroem LU, Karunaratne CV, Ranhotra HS, Mun KS, Naren AP, Murray IA, Perdew GH, Brtko J, Toporova L, Schon A, Wallace WG, Walton WG, Redinbo MR, Sun K, Beck A, Kortagere S, Neary MC, Chandran A, Vishveshwara S, Cavalluzzi MM, Lentini G, Cui JY, Gu H, March JC, Chatterjee S, Matson A, Wright D, Flannigan KL, Hirota SA, Sartor RB, Mani S. Targeting the pregnane X receptor using microbial metabolite mimicry. EMBO molecular medicine. 2020;12(4):e11621. doi: 10.15252/emmm.201911621. PubMed PMID: 32153125; PMCID: 7136958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Haller CA, Jernigan FE, Koerner SK, Wong DJ, Wang Y, Cheong JE, Kosaraju R, Kwan J, Park DD, Thomas B, Bhasin S, De La Rosa RC, Premji AM, Liu L, Park E, Moss AC, Emili A, Bhasin M, Sun L, Chaikof EL. Modulation of lymphocyte-mediated tissue repair by rational design of heterocyclic aryl hydrocarbon receptor agonists. Science advances. 2020;6(3):eaay8230. doi: 10.1126/sciadv.aay8230. PubMed PMID: 31998845; PMCID: 6962035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43(10):1522–35. Epub 2015/06/05. doi: 10.1124/dmd.115.064246. PubMed PMID: 26041783; PMCID: PMC4576673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vyhlídalová B, Krasulová K, Pečinková P, Marcalíková A, Vrzal R, Zemánková L, Vančo J, Trávníček Z, Vondráček J, Karasová M, Mani S, Dvořák Z. Gut Microbial Catabolites of Tryptophan Are Ligands and Agonists of the Aryl Hydrocarbon Receptor: A Detailed Characterization. International journal of molecular sciences. 2020;21(7). Epub 2020/04/15. doi: 10.3390/ijms21072614. PubMed PMID: 32283770; PMCID: PMC7177849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Haller CA, Jernigan FE, Koerner SK, Wong DJ, Wang Y, Cheong JE, Kosaraju R, Kwan J, Park DD, Thomas B, Bhasin S, De La Rosa RC, Premji AM, Liu L, Park E, Moss AC, Emili A, Bhasin M, Sun L, Chaikof EL. Modulation of lymphocyte-mediated tissue repair by rational design of heterocyclic aryl hydrocarbon receptor agonists. Science Advances. 2020;6(3):eaay8230. doi: 10.1126/sciadv.aay8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mexia N, Koutrakis S, He G, Skaltsounis AL, Denison MS, Magiatis P. A Biomimetic, One-Step Transformation of Simple Indolic Compounds to Malassezia-Related Alkaloids with High AhR Potency and Efficacy. Chem Res Toxicol. 2019;32(11):2238–49. Epub 2019/10/28. doi: 10.1021/acs.chemrestox.9b00270. PubMed PMID: 31647221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J, Luo Y, Zhu Z, Zhou Y, Sun L, Gao J, Sun J, Wang G, Yao X, Li W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J Allergy Clin Immunol. 2019;143(6):2108–19.e12. Epub 2018/12/24. doi: 10.1016/j.jaci.2018.11.036. PubMed PMID: 30578876. [DOI] [PubMed] [Google Scholar]
- 71.Turski WA, Wnorowski A, Turski GN, Turski CA, Turski L. AhR and IDO1 in pathogenesis of Covid-19 and the "Systemic AhR Activation Syndrome" Translational review and therapeutic perspectives. Restor Neurol Neurosci. 2020. doi: 10.3233/rnn-201042. PubMed PMID: 32597823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biswas A, Mani S, Redinbo MR, Krasowski MD, Li H, Ekins S. Elucidating the 'Jekyll and Hyde' nature of PXR: the case for discovering antagonists or allosteric antagonists. Pharm Res. 2009;26(8):1807–15. Epub 2009/05/06. doi: 10.1007/s11095-009-9901-7. PubMed PMID: 19415465; PMCID: PMC2846309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naveja JJ, Medina-Franco JL. Finding Constellations in Chemical Space Through Core Analysis. Frontiers in Chemistry. 2019;7(510). doi: 10.3389/fchem.2019.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Llanos EJ, Leal W, Luu DH, Jost J, Stadler PF, Restrepo G. Exploration of the chemical space and its three historical regimes. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(26):12660–5. Epub 2019/06/13. doi: 10.1073/pnas.1816039116. PubMed PMID: 31186353; PMCID: PMC6600933. [DOI] [PMC free article] [PubMed] [Google Scholar]