Abstract
Emerging research supports that soluble epoxide hydrolase (sEH), an enzyme involved in eicosanoid metabolism, could be a promising target for obesity-associated disorders. The sEH enzyme is overexpressed in many tissues of obese animals. Genetic ablation or pharmacological inhibition of sEH attenuates the development of a wide range of obesity-induced disorders, including endoplasmic reticulum stress, metabolic syndrome, kidney diseases, insulin resistance, fatty liver, hepatic steatosis, inflammation, and endothelial dysfunction. Furthermore, our recent research showed that genetic ablation or inhibition of sEH attenuated obesity-induced intestinal barrier dysfunction and its resulted bacterial translocation, which is widely regarded to be a central mechanism for the pathogenesis of various obesity-induced disorders. Together, these results support that targeting sEH could be a promising strategy to reduce risks of obesity-induced disorders, at least in part through blocking obesity-induced leaky gut syndrome.
Keywords: Soluble epoxide hydrolase, obesity, gut microbiota, intestinal barrier function
Introduction
Obesity, which is defined by body mass index (BMI) over 30, is a serious health problem in Western countries [1, 2]. It was estimated that >35% adults and >17% children in the United States are obese [1, 2]. Obese individuals have increased risks of developing many chronic diseases, including metabolic diseases, diabetes, cardiovascular diseases, and several types of cancers [3]. For example, colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death in the United States [4], and previous studies have shown that obese individuals have 30-60% greater risk of developing CRC [5, 6]. It is of critical importance to identify novel therapeutic targets for obesity-induced disorders, in order to develop novel strategies for prevention and/or treatment.
Recent research supports that soluble epoxide hydrolase (sEH), an enzyme involved in eicosanoid metabolism, could be a promising therapeutic target for obesity-associated disorders. The expression and activity of sEH, as well as the concentrations of sEH-produced lipid metabolites, have been shown to be increased in tissues of obese animals [7-10]. Genetic ablation or pharmacological inhibition of sEH attenuates the development of various obesity-induced disorders, including endoplasmic reticulum (ER) stress, metabolic syndrome, insulin resistance, fatty liver, hepatic steatosis, inflammation, and endothelial dysfunction [11-19]. More importantly, our recent research showed that genetic ablation or inhibition of sEH abolished obesity-induced intestinal barrier dysfunction and bacterial translocation [20], which is widely regarded as a central mechanism for the pathogenesis of various obesity-induced disorders [21-23]. Together, these results suggest a novel theme that targeting sEH could attenuate risks of various obesity-induced disorders, at least in part through blocking obesity-induced leaky gut syndrome. Therefore, sEH could be an important therapeutic target for preventing and/or treating a wide range of obesity-induced chronic diseases. This is clinically important because pharmacological inhibitors of sEH have been evaluated in multiple human clinical trials targeting other disorders [24, 25], and could be explored to target obesity-induced disorders.
In this review, we will discuss the biochemistry of sEH-mediated eicosanoid metabolism and the functional roles of sEH in regulating obesity-induced disorders. Moreover, we will discuss our recent finding which suggests that targeting sEH could attenuate various obesity-induced disorders through blocking obesity-caused intestinal barrier dysfunction.
1. sEH-mediated metabolism of fatty acids
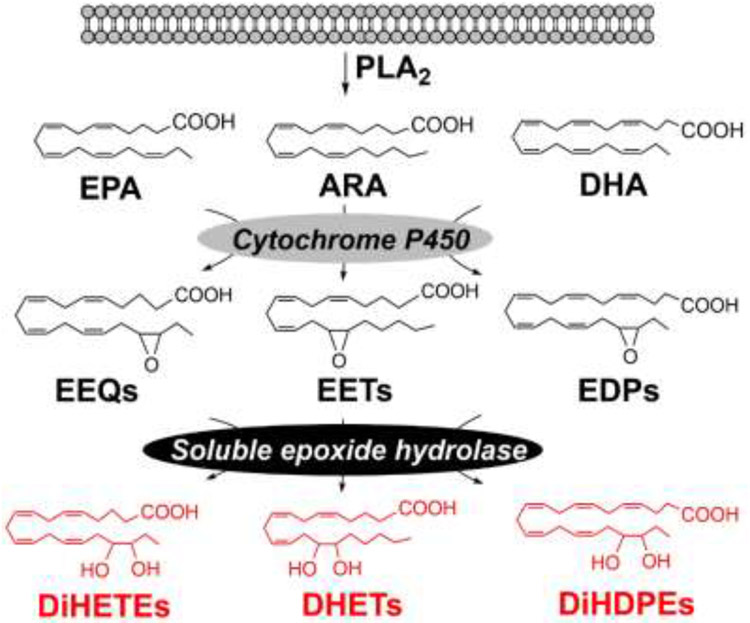
The sEH enzyme, which is encoded by Ephx2 gene, plays an important role in eicosanoid metabolism [26]. It catalyzes hydrolysis of fatty acid epoxides, which are produced from polyunsaturated fatty acids by the actions of cytochrome P450 (CYP) monooxygenases, to generate the corresponding fatty acid diols (Figure 1). The fatty acid epoxides are important lipid signaling molecules that have potent biological actions such as anti-inflammatory, cardio-protective, tissue-protective, and pro-angiogenic effects; in contrast, the fatty acid diols are usually less active or even pro-inflammatory [26-29]. Therefore, the metabolic activity of sEH leads to reduced concentrations of potentially beneficial metabolites (fatty acid epoxides) and/or increased concentrations of potentially inactive or harmful metabolites (fatty acid diols). Substantial research has shown that sEH plays critical roles in the pathogenesis of many diseases, including inflammation, hypertension, pain, and angiogenesis [26]. The pharmacological inhibitors of sEH are being evaluated in multiple human clinical trials [24, 25]. Notably, GlaxoSmithKline is conducting human clinical trials to test the effect of a sEH inhibitor and has shown that the drug candidate is safe, well-tolerated, and causes sustained inhibition of sEH in humans [24]. In addition, other classes of sEH inhibitors are being considered for human trials [25].
Figure. 1. Biochemistry of sEH-mediated metabolism of fatty acids:
sEH catalyzes hydrolysis of fatty acid epoxides to the corresponding fatty acid diols.
2. Changes of the sEH pathway in obesity
Our recent research showed that the expression of sEH and the concentrations of sEH-produced fatty acid diols are increased in the colon tissues of diet-induced obese mice [7]. We treated C57BL/6 mice with a control diet (low-fat diet) (10 kcal% from fat, catalog # D12450J from Research Diets Inc., this diet mimics a normal rodent diet which has a similar level of fat) or a high-fat diet (HFD) (60 kcal% from fat, catalog # D12492) for 8 weeks. These diets are among the most widely-used diets for obesity research (see our publications [7, 20, 30, 31] and others [32]). LC-MS/MS analysis showed that the concentrations of sEH-produced fatty acid diols, including arachidonic acid (ARA)-derived 8,9-, 11,12-, and 14,15-dihydroxyeicosatrienoic acid (DHET), eicosapentaenoic acid (EPA)-derived 17,18-dihydroxyeicosatetraenoic acid (DiHETE), and docosahexaenoic acid (DHA)-derived 7,8-, 10,11-, 16,17-, and 19,20-dihydroxydocosapentaenoic acid (DiHDPE) (see chemical structures of these metabolites in Figure 1), were significantly increased in the colon tissues of HFD-induced obese mice. Next, we analyzed expressions of proteins involved in biosynthesis of fatty acid diols, and found that the transcriptional and protein expression levels of sEH were increased in the colon tissues of HFD-induced obese mice. In contrast, other proteins, such as Pla2g4a (encoding cytosolic calcium-dependent PLA2) or Cyp monooxygenases were not changed [7]. Overall, these results showed that the sEH pathway is upregulated in the colon tissues of diet-induced obese mice.
Our result is well in agreement with previous studies which showed that the sEH pathway is upregulated in animal models of obesity. The expression and/or activity of sEH was increased in many tissues, such as adipose [33, 34], liver [8, 9, 34], and kidney [10], of HFD-induced obese mice. Furthermore, previous studies also support the hypothesis that sEH is upregulated in genetically induced obese animals. Zucker rat is a widely-used animal model of genetic obesity [35]. Zhao et. al showed that compared with lean Zucker rats, the expression of sEH in mesenteric artery is increased in obese Zucker rats [36]. There are also inconsistent results, which showed that the expression of sEH is not altered in left ventricular tissue of diet-induced obese mice [37] or renal cortical tissue of obese Zucker rats [38], suggesting that the sEH pathway could be upregulated in obesity in a tissue-specific manner.
Human studies support the hypothesis that the sEH pathway is upregulated in obese individuals, though there are inconsistent results. Theken et al. showed that compared with non-obese atherosclerotic cardiovascular disease (CAD) patients, the obese CAD patients have reduced concentrations of EETs (substrates of sEH), as well as a lower ratio of 14,15-EET (a substrate of sEH) to 14,15-DHET (a product of sEH), in the plasma [39]. This result supports that the sEH pathway is upregulated in obese individuals. We need to point out that there are inconsistent results from human studies. For example, Pickens et al. showed that compared with lean individuals, the plasma concentrations of sEH-produced fatty acid diols were decreased in the plasma of obese individuals [40]. There could be many reasons for these inconsistent results in human studies. In most human studies, only the concentrations of eicosanoids in the circulation were measured [39, 40]. Our previous studies showed that the circulating concentrations of many eicosanoids, including sEH-produced fatty acid diols, were not significantly changed in HFD-induced obese mice [31], though their concentrations were altered in the colon tissues of obese mice [7]. These findings suggest that the circulating eicosanoids could be poor predictors of metabolic changes induced by obesity.
3. Roles of sEH in mediating obesity-induced disorders
Previous studies have shown that genetic ablation or pharmacological inhibition of sEH doesn’t have a major impact on obesity (e.g. body weight), but attenuates the development of a wide range obesity-induced disorders, including endoplasmic reticulum (ER) stress, metabolic syndrome, insulin resistance, fatty liver, hepatic steatosis, inflammation, and endothelial dysfunction [11-19]. Overall, these results support the hypothesis that sEH plays an important role in the pathogenesis of various obesity-induced disorders. The details are discussed below.
3.1. Roles of sEH in obesity-induced colonic inflammation and associated disorders
Obesity is associated with enhanced colonic inflammation [41-43], which is a major risk factor of developing CRC [44]. CRC is the third most common cancer and the second leading cause of cancer-related death in the United States [4]. Every year there are ~130,000 new cases and ~50,000 fatalities from CRC in the United States [4]. It is well established that obese individuals have higher risks of developing CRC and late-stage CRC [5, 45]. Considering the obesity epidemic and the potential lethal consequence of CRC, obesity-enhanced CRC is a serious health problem in the United States. However, the mechanism by which obesity increases the risks of CRC is not well understood [6].
Our recent research shows that the sEH pathway is upregulated in the colon tissues of diet-induced obese mice; in addition, inhibition or genetic ablation of sEH abolishes obesity-induced colonic inflammation and activation of pro-tumorigenic Wnt signaling [7]. Specifically, our research shows that: (1) the expression of sEH and the concentrations of sEH-produced metabolic products (fatty acid diols) are increased in the colon tissues of diet-induced obese mice; (2) inhibition or genetic ablation of sEH attenuates obesity-induced colonic inflammation, with reduced expression of pro-inflammatory cytokines and decreased infiltration of immune cells in colon; and (3) inhibition or genetic ablation of sEH attenuates obesity-induced activation of pro-tumorigenic Wnt signaling in colon, as indicated by reduced phosphorylation of GSK3β and decreased expression of Axin2 in colon [7]. Together, these results demonstrate that sEH is important for the pathogenesis of obesity-induced colonic inflammation. Notably, we found that oral administration of two different sEH inhibitors (via drinking water), at low doses, abolished obesity-induced colonic inflammation and activation of Wnt signaling [7], supporting that the sEH inhibitors could potentially be novel agents for preventing or treating obesity-induced gut inflammation and its associated disorders. In our experiments, we performed the studies using male mice, further studies are needed to determine the extent to which genetic ablation or inhibition of sEH attenuates obesity-induced colonic inflammation in a sex-dependent manner.
Previous research suggests that it is feasible to target sEH to reduce the risks of colonic inflammation and colon cancer. Two previous studies have shown that compared with normal colon tissues, the expression of sEH is increased in human CRC samples [46, 47], supporting the notion that sEH could be a therapeutic target of CRC. Furthermore, inhibition or genetic ablation of sEH attenuates the development of dextran sodium sulfate (DSS)- or IL-10-deficiency-induced colitis and CRC [47-49]. Together with our finding [7], these results support that sEH could be a novel therapeutic target for obesity-associated CRC. A recent human study showed that obesity significantly increased the risks of developing CRC in patients with Lynch syndrome (LS, an inherited genetic condition that increases risks of developing certain cancers such as CRC), while this risk is abrogated in those taking aspirin [50]. This result supports that targeting inflammation, or more specifically eicosanoid signaling in inflammation, is a promising approach to reduce the risk of obesity-associated CRC. Further studies are needed to determine the roles of sEH-mediated eicosanoid signaling pathway in obesity-associated CRC.
3.2. Roles of sEH in obesity-induced nonalcoholic fatty liver disease (NAFLD)
NAFLD, characterized by an extra accumulation of fat in liver tissues, affects 80-100 million Americans [51]. Obesity is a major risk factor of developing NAFLD, and 30-90% of NAFLD patients are obese [52]. Animal studies support that it is feasible to target sEH to reduce the risks of obesity-induced NAFLD. Schuck et al. showed that compared with the mice fed with standard chow (14 Cal% from fat), mice treated with an atherogenic diet (40 Cal% from fat) developed NAFLD, with exaggerated systematic and hepatic inflammation, and hepatic injury. These effects were significantly attenuated in the sEH KO mice, illustrating a critical role of sEH in regulating obesity-induced NAFLD [53]. Furthermore, genetic ablation of sEH increased concentrations of EETs, as well as the EET-to-DHET ratio, in both the plasma and liver of the atherogenic diet-treated mice, supporting the involvement of sEH-mediated lipid signaling pathway [53]. Consistent with this study, other studies have also shown that genetic ablation or pharmacological inhibition of sEH, or overexpression of CYP monooxygenases, attenuates obesity-induced hepatic inflammation, ER stress and hepatic steatosis [8, 9, 34, 54, 55], demonstrating an important role of the sEH pathway in the pathogenesis of obesity-induced NAFLD.
3.3. Roles of sEH in obesity-induced Type 2 diabetes
Type 2 diabetes, which is the most common type of diabetes, is characterized by an abnormally high concentration of glucose in bloodstream [56]. Obesity is a major risk factor in developing type II diabetes [56, 57]. Recent research supports that inhibition or genetic ablation of sEH attenuates obesity-induced type 2 diabetes. Luria et al. showed that HFD treatment caused insulin resistance and glucose intolerance in mice, while such effects were significantly attenuated by genetic ablation or pharmacological inhibition of sEH, resulting in lower concentrations of plasma glucose and improved insulin tolerance in mice [58]. Interestingly, inhibition of sEH did not reduce the concentration of blood glucose in non-obese mice (fed with standard chow), while reduced blood glucose in obese mice (fed with HFD) [58]. Consistent with ith this study, other studies have also shown a beneficial effect of targeting sEH in animal models of obesity-induced type 2 diabetes [59-61].
3.4. Roles of sEH in obesity-induced kidney diseases
Obesity is also a critical risk factor for developing chronic kidney diseases [62]. Huang et al. showed that treatment with a sEH inhibitor reduced mean arterial pressure, renal vascular resistance, cumulative sodium balance, and glomerular filtration rate, while increased renal blood flow in HFD-treated rats, demonstrating that inhibition of sEH attenuates obesity-induced abnormal renal function [63]. Roche et al. showed that in HFD-induced obese mice, inhibition of sEH attenuates the development of renal dysfunction, and reduced renal inflammation with reduced activation of NF-κB pathway and decreased renal expression of MCP-1, VCAM-1 and COX-2 [64]. In agreement with these two studies, others have also shown that inhibition of sEH attenuates the development of obesity-induced kidney disorders [10, 65].
4. Intestinal barrier function plays an important role in mediating obesity-induced disorders
The exact mechanisms by which obesity increases risks of a wide range of diseases are not fully understood. Recent research supports that obesity-induced intestinal barrier dysfunction could play a central role in mediating various obesity-induced disorders [66-69]. Indeed, previous research has shown that obese subjects have compromised intestinal barrier function, and this leads to translocation of bacteria or toxic bacterial products from the gut into the bloodstream and distant organs, resulting in systemic inflammation, insulin resistance, and tissue dysfunction [66-69].
Animal studies support that obesity induces intestinal barrier dysfunction and enhances bacteria translocation. Cani et al. showed that compared with mice fed with a normal diet, the HFD-induced obese mice had impaired expression of tight-junction proteins in the colon, increased gut leakage (as assessed by a FITC-dextran permeability assay), and higher concentrations of lipopolysaccharide (LPS) in the circulation, illustrating intestinal barrier dysfunction in the diet-induced obese mice [66, 68]. Furthermore, Cani et al. showed that HFD-induced intestinal barrier dysfunction through gut microbiota-dependent mechanisms, since antibiotic cocktail-mediated suppression of gut bacteria attenuates the effects of HFD on gut leakage [68]. Besides diet-induced obesity, previous studies have also shown that genetically obese (db/db) mice had reduced expression of tight-junction protein in the colon, enhanced gut leakage (as assessed by using FITC-dextran- and Citrobacter rodentium-based permeability assays), and higher concentrations of microbial pattern recognition receptor (PRR) ligands in serum, spleen, and liver, demonstrating impaired intestinal barrier function and enhanced bacterial translocation in the db/db mice [21]. In agreement with these studies, other studies have shown that diet- or genetically induced obese mice develop intestinal barrier dysfunction [70-74].
Human studies also support the hypothesis that obese individuals have impaired intestinal barrier function, though there are inconsistent results [69]. Consistent with the results from the aforementioned animal studies [66, 68], Pendyala et al. showed that a 1-month treatment with Western-style diet (high fat diet) in healthy subjects induced a ~70% increase of the plasma concentration of LPS, while a prudent-style diet (with similar caloric density as Western-style diet but with less fat and more carbohydrates and fiber) reduced LPS by ~30% [75]. Furthermore, Erridge et al. showed that intake of HFD can induce a rapid increase of plasma concentration of LPS in human subjects: 4 hours after initiation of HFD intake, the plasma concentration of LPS was increased by ~50% [76]. Besides HFD, other human studies also support that the circulating concentration of LPS is increased in obese subjects. Basu et al. showed that the plasma concentrations of LPS in lean versus obese pregnant women were 0.5 ± 0.2 EU/mL versus 1.0 ± 0.5 EU/mL (P = 0.006), supporting that obesity induces intestinal barrier function in pregnant women [77]. A similar result was also observed in other studies [78-80].
Accumulating evidence supports the hypothesis that obesity-induced intestinal barrier dysfunction and its resulted bacterial translocation could play a central role in mediating various obesity-induced disorders [66]. The presence of gut bacteria is essentially required for the development of obesity-induced disorders. Treatment with antibiotic, which depletes bacteria and bacterial products, attenuated HFD-induced adipose inflammation and dysfunction, and insulin resistance in mice [68]. Compared with conventionally raised mice, HFD-induced obesity, insulin resistance, hepatic steatosis, and dyslipidemia were attenuated in germ-free mice [81, 82]. Furthermore, recent studies support that continuous infusion with low, non-toxic dose LPS, which mimics HFD-induced elevation of bacteria/LPS translocation, can cause disorders that are similar to those induced by obesity. Cani et al. showed that continuous infusion with 300 μg/kg/day LPS via mini-pumps increased plasma concentration of glucose and insulin, increased body weight, enlarged liver and adipose tissues, and exaggerated fat accumulation in liver tissues in non-obese mice [66]. Dapito et al. also showed that infusion with 300 μg/kg/day LPS exaggerated diethylnitrosamine (DEN)/carbon tetrachloride (CCl4)-induced hepatocellular carcinoma, with increased tumor number and tumor size [83]. Overall, these results support a potential role of the intestinal barrier dysfunction-induced bacteria/LPS translocation in promoting obesity-associated disorders.
5. Roles of sEH in regulating obesity-induced intestinal barrier dysfunction and its resulting pathologies
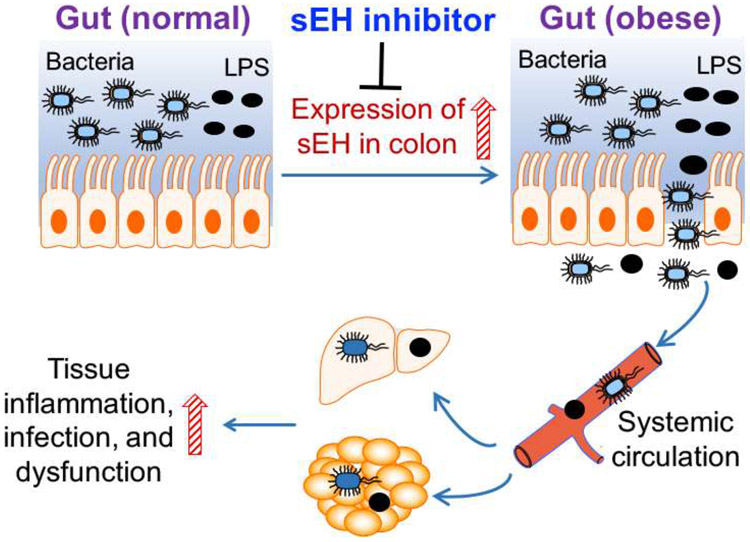
The molecular mechanisms by which obesity induces barrier dysfunction remain poorly understood, and represent a significant knowledge gap [84, 85]. Our recent research supports that sEH is a novel endogenous regulator of obesity-induced intestinal barrier dysfunction and its resulted disorders, via gut microbiota-dependent mechanisms [20]. Compared with control mice (maintained on a low-fat diet), HFD-induced obese mice had compromised intestinal barrier function (as assessed by a FITC-dextran permeability assay), a higher concentration of LPS in the plasma, and higher amount of bacterial DNA in the bloodstream and adipose tissue. These results illustrate exaggerated gut leakage and enhanced bacterial translocation in the obese mice, which are consistent with previous studies [21, 66-69, 75-77, 86]. Genetic ablation of sEH abolished such effects in the obese mice, supporting a critical role of sEH in mediating obesity-induced intestinal barrier dysfunction. Furthermore, we showed that genetic ablation of sEH altered obesity-associated gut microbiota and the detrimental actions of sEH on intestinal barrier function were abolished by antibiotic cocktail-mediated suppression of the microbiota. Indeed, without the antibiotic treatment, genetic ablation of sEH attenuated HFD-induced gut leakage and bacterial translocation; while with the antibiotic treatment, genetic ablation of sEH had no such effects [20]. This finding supports a critical role of the microbiota in mediating the activities of sEH on the barrier function. If further validated, this represents a new mechanism for the biological functions of sEH, since very little is known about the effect of sEH-mediated lipid signaling on gut microbiota and its connection to human diseases.
We found that sEH-produced lipid metabolites play critical roles in mediating the barrier-disrupting effects of sEH, further supporting sEH as a critical regulator of intestinal barrier function. Treatment with dihydroxyeicosatrienoic acids (DHETs, derived from ω-6 ARA), using mini-pumps, induced gut leakage, impaired colonic expression of a tight-junction protein Claudin-5, and enhanced colonic expression of pro-inflammatory cytokines, in mice. These results suggest that sEH-produced lipid metabolites have potent actions on inducing intestinal barrier dysfunction and colonic inflammation in vivo, further supporting a critical role of sEH in driving gut disorders [20]. This finding is interesting since most previous studies have suggested that the sEH-derived fatty acid diols are biologically inactive [26]. Previous studies showed that ω-3 versus ω-6 polyunsaturated fatty acids have opposite effects on intestinal barrier function [87], it is feasible that ω-3 versus ω-6 fatty acid diols could have different actions on barrier function and more studies are needed to determine the actions of individual sEH-produced fatty acid diols.
To date, there are few therapeutic strategies for preventing or treating intestinal barrier dysfunction and its resulting disorders. Our results support that pharmacological inhibitors of sEH could be promising agents for preventing or treating obesity-induced intestinal barrier dysfunction and its resulted disorders. Oral administration of a sEH inhibitor t-TUCB (administered via dissolving in drinking water), at a low dose (~ 1mg/kg/day), abolished HFD-induced colonic inflammation, intestinal barrier dysfunction, bacterial translocation, as well as bacterial invasion-induced adipose inflammation and dysfunction. Regarding the mechanisms by which the sEH inhibitor attenuates barrier dysfunction, we found that compared with control mice (maintained on a low-fat diet), the HFD-induced obese mice had lower expression of tight-junction proteins in the colon, while such effects were abolished by treatment with the sEH inhibitor [20]. This finding could be clinically important because the sEH inhibitors have been evaluated in multiple human clinical trials targeting other chronic disorders [24, 25].
6. Future work
Overall, previous studies from us and others support that sEH could be an important therapeutic target for preventing and/or treating a wide range of obesity-induced chronic disorders, at least in part through attenuating obesity-induced intestinal barrier dysfunction [7, 11-20]. A better understanding of the molecular mechanisms by which sEH regulates intestinal barrier function could be important for clinical applications of sEH inhibitors, as well as development of novel therapeutic targets for obesity-induced disorders. We have shown that treatment with sEH-derived metabolites, DHETs, caused colonic inflammation and gut leakage in mice, supporting a critical role of sEH metabolites in mediating the actions of sEH on barrier function [20]. Many eicosanoids act by binding to specific G-protein coupled receptors (GPCRs) [88]. Emerging research support that CYP/sEH-derived eicosanoids, such as EETs, also act via GPCR-dependent mechanisms, though the specific receptor(s) remain to be elucidated and validated [89-91]. It would also be important to discover the direct cellular targets or receptors of the sEH metabolites such as DHETs, since the identified proteins could serve as novel therapeutic targets of barrier dysfunction, and therefore have important implications in the pathogenesis of many human diseases. In addition, based on the potent actions of DHETs, the sEH-derived metabolites could be potential structural targets to develop stable antagonists that counteract their actions and serve as novel therapeutics to treat barrier dysfunction. Recent studies support that it is feasible to design synthetic mimics of CYP/sEH-derived metabolites as potential therapeutic drugs [92-95]. The elucidation of the direct cellular targets or receptors of the sEH metabolites could further help the rational design of synthetic mimics and facilitate drug discovery and development.
Besides obesity-related disorders, intestinal barrier dysfunction has also been shown as a key and direct pathogenic factor of many other human diseases, including infectious diseases [96-99], inflammatory bowel disease [100], aging [101], sepsis [102-104], and diabetes [85, 105]. It is important to determine the potential role of sEH in the development of other diseases and the roles of barrier function involved.
Figure 2.
sEH is a novel endogenous regulator of obesity-induced intestinal barrier dysfunction. Genetic ablation or inhibition of sEH could attenuate various obesity-induced disorders, through inhibiting obesity-induced intestinal barrier dysfunction.
Highlights.
Soluble epoxide hydrolase (sEH), an important enzyme involved in fatty acid metabolism, is upregulated in many tissues of obese animals.
Substantial studies have shown that genetic ablation or pharmacological inhibition of sEH attenuates the development of a wide range of obesity-induced disorders.
Our recent research has shown that genetic ablation or inhibition of sEH attenuates obesity-induced intestinal barrier dysfunction and its resulted bacterial translocation, which is widely regarded to be a central mechanism for the pathogenesis of various obesity-induced disorders.
Acknowledgement
Our research is supported by funding support from USDA NIFA 2020-67017-30844, 2019-67017-29248, and 2016-67017-24423, USDA/Hatch MAS00556, NIH/NCI R03CA237795 and R03CA218520 (to G. Zhang).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Flegal KM, Carroll MD, Kit BK, Ogden CL, Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010, JAMA, 307 (2012) 491–497. [DOI] [PubMed] [Google Scholar]
- [2].Ogden CL, Carroll MD, Kit BK, Flegal KM, Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010, JAMA, 307 (2012) 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bray GA, Medical consequences of obesity, J Clin Endocrinol Metab, 89 (2004) 2583–2589. [DOI] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA Cancer J Clin, 66 (2016) 7–30. [DOI] [PubMed] [Google Scholar]
- [5].Moghaddam AA, Woodward M, Huxley R, Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events, Cancer Epidemiol Biomarkers Prev, 16 (2007) 2533–2547. [DOI] [PubMed] [Google Scholar]
- [6].Roberts DL, Dive C, Renehan AG, Biological mechanisms linking obesity and cancer risk: new perspectives, Annu Rev Med, 61 (2010) 301–316. [DOI] [PubMed] [Google Scholar]
- [7].Wang W, Yang J, Zhang J, Wang Y, Hwang SH, Qi W, Wan D, Kim D, Sun J, Sanidad KZ, Yang H, Park Y, Liu JY, Zhao X, Zheng X, Liu Z, Hammock BD, Zhang G, Lipidomic profiling reveals soluble epoxide hydrolase as a therapeutic target of obesity-induced colonic inflammation, Proc Natl Acad Sci U S A, 115 (2018) 5283–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y, Inhibition of soluble epoxide hydrolase attenuates high-fat–dietinduced hepatic steatosis by reduced systemic inflammatory status in mice, PloS one, 7 (2012) e39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].López-Vicario C, Alcaraz-Quiles J, García-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Clària J, Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides, Proceedings of the National Academy of Sciences, 112 (2015) 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luo Y, Wu MY, Deng BQ, Huang J, Hwang SH, Li MY, Zhou CY, Zhang QY, Yu HB, Zhao DK, Zhang G, Qin L, Peng A, Hammock BD, Liu JY, Inhibition of soluble epoxide hydrolase attenuates a high-fat diet-mediated renal injury by activating PAX2 and AMPK, Proc Natl Acad Sci U S A, 116 (2019) 5154–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG, Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue, J Biol Chem, 288 (2013) 14189–14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].do Carmo JM, da Silva AA, Morgan J, Jim Wang YX, Munusamy S, Hall JE, Inhibition of soluble epoxide hydrolase reduces food intake and increases metabolic rate in obese mice, Nutr Metab Cardiovasc Dis, 22 (2012) 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Imig JD, Walsh KA, Hye Khan MA, Nagasawa T, Cherian-Shaw M, Shaw SM, Hammock BD, Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor gamma agonist improve vascular function and decrease renal injury in hypertensive obese rats, Exp Biol Med (Maywood), 237 (2012) 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, Brown L, Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats, Exp Diabetes Res, 2012 (2012) 758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y, Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice, PLoS One, 7 (2012) e39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Claria J, Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides, Proc Natl Acad Sci U S A, 112 (2015) 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roche C, Besnier M, Cassel R, Harouki N, Coquerel D, Guerrot D, Nicol L, Loizon E, Remy-Jouet I, Morisseau C, Mulder P, Ouvrard-Pascaud A, Madec AM, Richard V, Bellien J, Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice, Am J Physiol Heart Circ Physiol, 308 (2015) H1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zha W, Edin ML, Vendrov KC, Schuck RN, Lih FB, Jat JL, Bradbury JA, DeGraff LM, Hua K, Tomer KB, Falck JR, Zeldin DC, Lee CR, Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity, J Lipid Res, 55 (2014) 2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang LN, Vincelette J, Chen D, Gless RD, Anandan SK, Rubanyi GM, Webb HK, MacIntyre DE, Wang YX, Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension, Eur J Pharmacol, 654 (2011) 68–74. [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Yang J, Wang W, Sanidad KZ, Cinelli MA, Wan D, Hwang SH, Kim D, Lee KSS, Xiao H, Hammock BD, Zhang G, Soluble epoxide hydrolase is an endogenous regulator of obesity-induced intestinal barrier dysfunction and bacterial translocation, Proc Natl Acad Sci U S A, (2020) 201916189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection, Science, 359 (2018) 1376–1383. [DOI] [PubMed] [Google Scholar]
- [22].Shen J, Obin MS, Zhao L, The gut microbiota, obesity and insulin resistance, Molecular aspects of medicine, 34 (2013) 39–58. [DOI] [PubMed] [Google Scholar]
- [23].Vajro P, Paolella G, Fasano A, Microbiota and gut-liver axis: a mini-review on their influences on obesity and obesity related liver disease, Journal of pediatric gastroenterology and nutrition, 56 (2013) 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lazaar AL, Yang L, Boardley RL, Goyal NS, Robertson J, Baldwin SJ, Newby DE, Wilkinson IB, Tal-Singer R, Mayer RJ, Cheriyan J, Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor, Br J Clin Pharmacol, 81 (2016) 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McReynolds C, Schmidt WK, Wagner K, Hammock BD, Advancing Soluble Epoxide Hydrolase Inhibitors Through the Valley of Death into Phase 1 Clinical Trials for Treating Painful Diabetic Neuropathy by Utilizing University Partnerships, Collaborations, and NIH Support, The FASEB Journal, 30 (2016) 1272.1276. [Google Scholar]
- [26].Zhang G, Kodani S, Hammock BD, Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer, Prog Lipid Res, 53 (2014) 108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK, Anti-inflammatory Properties of Cytochrome P450 Epoxygenase-Derived Eicosanoids, Science, 285 (1999) 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnes CM, Mammoto A, Mammoto T, Luria A, Benny O, Chaponis DM, Dudley AC, Greene ER, Vergilio JA, Pietramaggiori G, Scherer-Pietramaggiori SS, Short SM, Seth M, Lih FB, Tomer KB, Yang J, Schwendener RA, Hammock BD, Falck JR, Manthati VL, Ingber DE, Kaipainen A, D'Amore PA, Kieran MW, Zeldin DC, Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice, J Clin Invest, 122 (2012) 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD, Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis, Proc Natl Acad Sci U S A, 110 (2013) 6530–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang W, Yang J, Qi W, Yang H, Wang C, Tan B, Hammock BD, Park Y, Kim D, Zhang G, Lipidomic profiling of high-fat diet-induced obesity in mice: Importance of cytochrome P450-derived fatty acid epoxides, Obesity (Silver Spring), 25 (2017) 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang W, Yang J, Yang H, Sanidad KZ, Hammock BD, Kim D, Zhang G, Effects of high-fat diet on plasma profiles of eicosanoid metabolites in mice, Prostaglandins Other Lipid Mediat, 127 (2016) 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hariri N, Thibault L, High-fat diet-induced obesity in animal models, Nutr Res Rev, 23 (2010) 270–299. [DOI] [PubMed] [Google Scholar]
- [33].De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, Murphy SB, Friedman DB, Hammock BB, Vaughan DE, Expression and regulation of soluble epoxide hydrolase in adipose tissue, Obesity, 18 (2010) 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG, Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue, Journal of Biological Chemistry, 288 (2013) 14189–14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oana F, Takeda H, Hayakawa K, Matsuzawa A, Akahane S, Isaji M, Akahane M, Physiological difference between obese (fa/fa) Zucker rats and lean Zucker rats concerning adiponectin, Metabolism, 54 (2005) 995–1001. [DOI] [PubMed] [Google Scholar]
- [36].Zhao X, Dey A, Romanko OP, Stepp DW, Wang M-H, Zhou Y, Jin L, Pollock JS, Webb RC, Imig JD, Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 288 (2005) R188–R196. [DOI] [PubMed] [Google Scholar]
- [37].Roche C, Besnier M, Cassel R, Harouki N, Coquerel D, Guerrot D, Nicol L, Loizon E, Remy-Jouet I, Morisseau C, Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice, American Journal of Physiology-Heart and Circulatory Physiology, 308 (2015) H1020–H1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhao X, Quigley JE, Yuan J, Wang M-H, Zhou Y, Imig JD, PPAR-α activator fenofibrate increases renal CYP-derived eicosanoid synthesis and improves endothelial dilator function in obese Zucker rats, American Journal of Physiology-Heart and Circulatory Physiology, 290 (2006) H2187–H2195. [DOI] [PubMed] [Google Scholar]
- [39].Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, Lih FB, Tomer KB, Poloyac SM, Wu MC, Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease, Atherosclerosis, 222 (2012) 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pickens CA, Sordillo LM, Zhang C, Fenton J.l., Obesity is positively associated with arachidonic acid-derived 5-and 11-hydroxyeicosatetraenoic acid (HETE), Metabolism, 70 (2017) 177–191. [DOI] [PubMed] [Google Scholar]
- [41].Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G, Denman S, Begun J, Florin TH, Perkins A, Cuív PÓ, McGuckin MA, Hasnain SZ, High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22, Scientific Reports, 6 (2016) 28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kim K-A, Gu W, Lee I-A, Joh E-H, Kim D-H, High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway, PLoS ONE, 7 (2012) e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, Greenberg AS, Mason JB, Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer, J Nutr Biochem, 23 (2012) 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Terzic J, Grivennikov S, Karin E, Karin M, Inflammation and colon cancer, Gastroenterology, 138 (2010) 2101–2114 e2105. [DOI] [PubMed] [Google Scholar]
- [45].Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H, Obesity and Risk of Colorectal Cancer: A Systematic Review of Prospective Studies, PLoS ONE, 8 (2013) e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Enayetallah AE, French RA, Grant DF, Distribution of soluble epoxide hydrolase, cytochrome P450 2C8, 2C9 and 2J2 in human malignant neoplasms, J Mol Histol, 37 (2006) 133–141. [DOI] [PubMed] [Google Scholar]
- [47].Zhang W, Li H, Dong H, Liao J, Hammock BD, Yang GY, Soluble epoxide hydrolase deficiency inhibits dextran sulfate sodium-induced colitis and carcinogenesis in mice, Anticancer Res, 33 (2013) 5261–5271. [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang W, Yang AL, Liao J, Li H, Dong H, Chung YT, Bai H, Matkowskyj KA, Hammock BD, Yang GY, Soluble epoxide hydrolase gene deficiency or inhibition attenuates chronic active inflammatory bowel disease in IL-10(−/−) mice, Dig Dis Sci, 57 (2012) 2580–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang W, Liao J, Li H, Dong H, Bai H, Yang A, Hammock BD, Yang GY, Reduction of inflammatory bowel disease-induced tumor development in IL-10 knockout mice with soluble epoxide hydrolase gene deficiency, Mol Carcinog, 52 (2013) 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Movahedi M, Bishop DT, Macrae F, Mecklin J-P, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: a prospective investigation in the CAPP2 study, Journal of Clinical Oncology, (2015). [DOI] [PubMed] [Google Scholar]
- [51].Spengler EK, Loomba R, Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis, Mayo Clin Proc, 90 (2015) 1233–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brunt EM, Wong VW-S, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME, Nonalcoholic fatty liver disease, Nature reviews Disease primers, 1 (2015) 1–22. [DOI] [PubMed] [Google Scholar]
- [53].Schuck RN, Zha W, Edin ML, Gruzdev A, Vendrov KC, Miller TM, Xu Z, Lih FB, DeGraff LM, Tomer KB, The cytochrome P450 epoxygenase pathway regulates the hepatic inflammatory response in fatty liver disease, PloS one, 9 (2014) e110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen G, Xu R, Zhang S, Wang Y, Wang P, Edin ML, Zeldin DC, Wang DW, CYP2J2 overexpression attenuates nonalcoholic fatty liver disease induced by high-fat diet in mice, American Journal of Physiology-Endocrinology and Metabolism, 308 (2015) E97–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang J, Yang J, Wang W, Yang H, Sanidad KZ, Yang S-H, Sukamtoh E, Zhang G, Pharmacological inhibition or genetic ablation of soluble epoxide hydrolase attenuates obesity-induced nonalcoholic fatty liver disease, The FASEB Journal, 32 (2018) 560.567–560.567. [Google Scholar]
- [56].Stumvoll M, Goldstein BJ, van Haeften TW, Type 2 diabetes: pathogenesis and treatment, Lancet, 371 (2008) 2153–2156. [DOI] [PubMed] [Google Scholar]
- [57].Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN, Diet-Induced Type II Diabetes in C57BL/6J Mice, Diabetes, 37 (1988) 1163. [DOI] [PubMed] [Google Scholar]
- [58].Luria A, Bettaieb A, Xi Y, Shieh G-J, Liu H-C, Inoue H, Tsai H-J, Imig JD, Haj FG, Hammock BD, Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance, Proceedings of the National Academy of Sciences, 108 (2011) 9038–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang L-N, Vincelette J, Chen D, Gless RD, Anandan S-K, Rubanyi GM, Webb HK, MacIntyre DE, Wang Y-XJ, Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension, European journal of pharmacology, 654 (2011) 68–74. [DOI] [PubMed] [Google Scholar]
- [60].Luo P, Chang H-H, Zhou Y, Zhang S, Hwang SH, Morisseau C, Wang C-Y, Inscho EW, Hammock BD, Wang M-H, Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis, Journal of Pharmacology and Experimental Therapeutics, 334 (2010) 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ramirez CE, Shuey MM, Milne GL, Gilbert K, Hui N, Yu C, Luther JM, Brown NJ, Arg287Gln variant of EPHX2 and epoxyeicosatrienoic acids are associated with insulin sensitivity in humans, Prostaglandins & other lipid mediators, 113 (2014) 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].V Mathew A, Okada S, Sharma K, Obesity related kidney disease, Current diabetes reviews, 7 (2011) 41–49. [DOI] [PubMed] [Google Scholar]
- [63].Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH, Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats, Am J Physiol Renal Physiol, 293 (2007) F342–349. [DOI] [PubMed] [Google Scholar]
- [64].Roche C, Guerrot D, Harouki N, Duflot T, Besnier M, Remy-Jouet I, Renet S, Dumesnil A, Lejeune A, Morisseau C, Richard V, Bellien J, Impact of soluble epoxide hydrolase inhibition on early kidney damage in hyperglycemic overweight mice, Prostaglandins Other Lipid Mediat, 120 (2015) 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Khan MAH, Hwang SH, Sharma A, Corbett JA, Hammock BD, Imig JD, A dual COX-2/sEH inhibitor improves the metabolic profile and reduces kidney injury in Zucker diabetic fatty rat, Prostaglandins & other lipid mediators, 125 (2016) 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Metabolic endotoxemia initiates obesity and insulin resistance, Diabetes, 56 (2007) 1761–1772. [DOI] [PubMed] [Google Scholar]
- [67].Neves AL, Coelho J, Couto L, Leite-Moreira A, Roncon-Albuquerque R Jr, Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk, J Mol Endocrinol, 51 (2013) R51–64. [DOI] [PubMed] [Google Scholar]
- [68].Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R, Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice, Diabetes, 57 (2008) 1470–1481. [DOI] [PubMed] [Google Scholar]
- [69].Boutagy NE, McMillan RP, Frisard M.l., Hulver MW, Metabolic endotoxemia with obesity: Is it real and is it relevant?, Biochimie, 124 (2016) 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang W, Xu J-H, Yu T, Chen Q-K, Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice, Biomedicine & Pharmacotherapy, 118 (2019) 109131. [DOI] [PubMed] [Google Scholar]
- [71].Brun P, Castagliuolo I, Leo VD, Buda A, Pinzani M, Palù G, Martines D, Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis, American Journal of Physiology-Gastrointestinal and Liver Physiology, 292 (2007) G518–G525. [DOI] [PubMed] [Google Scholar]
- [72].Stenman LK, Holma R, Korpela R, High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids, World journal of gastroenterology: WJG, 18 (2012) 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cremonini E, Wang Z, Bettaieb A, Adamo AM, Daveri E, Mills DA, Kalanetra KM, Haj FG, S Karakas Oteiza PI, (−)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance, Redox biology, 14 (2018) 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, G Muccioli G, Delzenne NM, Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity, Proceedings of the National Academy of Sciences, 110 (2013) 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pendyala S, Walker JM, Holt PR, A high-fat diet is associated with endotoxemia that originates from the gut, Gastroenterology, 142 (2012) 1100–1101. e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Erridge C, Attina T, Spickett CM, Webb DJ, A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation, The American journal of clinical nutrition, 86 (2007) 1286–1292. [DOI] [PubMed] [Google Scholar]
- [77].Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation, Obesity, 19 (2011) 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Radilla-Vázquez RB, Parra-Rojas I, Martínez-Hernández NE, Márquez-Sandoval YF, Illades-Aguiar B, Castro-Alarcón N, Gut microbiota and metabolic endotoxemia in young obese Mexican subjects, Obesity facts, 9 (2016) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Genser L, Aguanno D, Soula HA, Dong L, Trystram L, Assmann K, Salem JE, Vaillant JC, Oppert JM, Laugerette F, Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes, The Journal of pathology, 246 (2018) 217–230. [DOI] [PubMed] [Google Scholar]
- [80].Rainone V, Schneider L, Saulle I, Ricci C, Biasin M, Al-Daghri N, Giani E, Zuccotti G, Clerici M, Trabattoni D, Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents, International journal of obesity, 40 (2016) 1026. [DOI] [PubMed] [Google Scholar]
- [81].Bäckhed F, Manchester JK, Semenkovich CF, Gordon J.l., Mechanisms underlying the resistance to diet-induced obesity in germ-free mice, Proceedings of the National Academy of Sciences, 104 (2007) 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ, Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism, The FASEB Journal, 24 (2010) 4948–4959. [DOI] [PubMed] [Google Scholar]
- [83].Dapito DH, Mencin A, Gwak G-Y, Pradere J-P, Jang M-K, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF, Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4, Cancer cell, 21 (2012) 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM, Intestinal permeability--a new target for disease prevention and therapy, BMC Gastroenterol, 14 (2014) 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E, Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection, Science, 359 (2018) 1376–1383. [DOI] [PubMed] [Google Scholar]
- [86].Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, Forsblom C, Pörsti I, Rissanen A, Kaprio J, Mustonen J, Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation, Diabetes care, 34 (2011) 1809–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kaliannan K, Wang B, Li X-Y, Kim K-J, Kang JX, A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia, Scientific reports, 5 (2015) 11276–11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Funk CD, Prostaglandins and leukotrienes: advances in eicosanoid biology, science, 294 (2001) 1871–1875. [DOI] [PubMed] [Google Scholar]
- [89].Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB, 20-lodo-14, 15-epoxyeicosa-8 (Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14, 15-epoxyeicosatrienoic acid receptor, Biochemistry, 50 (2011) 3840–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ding Y, Frömel T, Popp R, Falck JR, Schunck W-H, Fleming I, The biological actions of 11, 12-epoxyeicosatrienoic acid in endothelial cells are specific to the R/S-enantiomer and require the Gs protein, Journal of Pharmacology and Experimental Therapeutics, 350 (2014) 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Park S-K, Herrnreiter A, Pfister SL, Gauthier KM, Falck BA, Falck JR, Campbell WB, GPR40 is a low-affinity epoxyeicosatrienoic acid receptor in vascular cells, Journal of Biological Chemistry, 293 (2018) 10675–10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck J, Campbell WB, 14, 15-Epoxyeicosa-5 (Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries, Circulation research, 90 (2002) 1028–1036. [DOI] [PubMed] [Google Scholar]
- [93].Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnés CM, Mammoto A, Mammoto T, Luria A, Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice, The Journal of clinical investigation, 122 (2012) 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids, Journal of Biological Chemistry, 285 (2010) 32720–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schunck W-H, Konkel A, Fischer R, Weylandt K-H, Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases, Pharmacology & therapeutics, 183 (2018) 177–204. [DOI] [PubMed] [Google Scholar]
- [96].Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, Schulzke JD, Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients, Gut, 58 (2009) 220–227. [DOI] [PubMed] [Google Scholar]
- [97].Dandekar S, George MD, Baumler AJ, Th17 cells, HIV and the gut mucosal barrier, Curr Opin HIV AIDS, 5 (2010) 173–178. [DOI] [PubMed] [Google Scholar]
- [98].Deitch EA, The Role of Intestinal Barrier Failure and Bacterial Translocation in the Development of Systemic Infection and Multiple Organ Failure, JAMA Surgery, 125 (1990) 403–404. [DOI] [PubMed] [Google Scholar]
- [99].McNamara BP, Koutsouris A, O'Connell CB, Nougayrede JP, Donnenberg MS, Hecht G, Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function, J Clin Invest, 107 (2001) 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Antoni L, Nuding S, Wehkamp J, Stange EF, Intestinal barrier in inflammatory bowel disease, World journal of gastroenterology, 20 (2014) 1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rera M, Clark RI, Walker DW, Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila, Proc Natl Acad Sci U S A, 109 (2012) 21528–21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH, Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock, J Infect Dis, 180 (1999) 1584–1589. [DOI] [PubMed] [Google Scholar]
- [103].Kritselis I, Tzanetakou V, Adamis G, Anthopoulos G, Antoniadou E, Bristianou M, Kotanidou A, Lignos M, Polyzos K, Retsas T, Sassopoulou P, Papaioannou AI, Sinapidis D, Sereti K, Vittoros V, Ghanas P, Gogos C, Giamarellos-Bourboulis EJ, The level of endotoxemia in sepsis varies in relation to the underlying infection: Impact on final outcome, Immunol Lett, 152 (2013) 167–172. [DOI] [PubMed] [Google Scholar]
- [104].Marshall JC, Endotoxin in the Pathogenesis of Sepsis, Contributions to Nephrology, 167 (2010) 1–13. [DOI] [PubMed] [Google Scholar]
- [105].Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V, Endotoxemia Is Associated With an Increased Risk of Incident Diabetes, Diabetes Care, 34 (2011) 392. [DOI] [PMC free article] [PubMed] [Google Scholar]