Abstract
Purpose:
The extracellular matrix (ECM) is an intriguing yet understudied component of therapy resistance. Here we investigated the role of ECM remodeling by the collagenase MT1-MMP in conferring resistance of BRAF-mutant melanoma to BRAF inhibitor therapy.
Experimental Design:
Publicly available RNA sequencing (RNAseq) data and reverse-phase-protein-array (RPPA) were used to determine the relevance of MT1-MMP up-regulation in BRAFi-resistant melanoma in patients, PDX and cell line derived tumors. shRNA-mediated knockdown of MT1-MMP; inhibition via the selective MT1-MMP/MMP2 inhibitor, ND322; or overexpression of MT1-MMP were used to assess the role of MT1-MMP in mediating resistance to BRAFi.
Results:
MT1-MMP was consistently up-regulated in post-treatment tumor samples derived from patients upon disease progression and melanoma xenografts and cell lines that acquired resistance to BRAFi. shRNA or ND322 mediated inhibition of MT1-MMP synergized with BRAFi leading to re-sensitization of resistant cells and tumors to BRAFi. The resistant phenotype depends on the ability of cells to cleave the ECM. Resistant cells seeded in MT1-MMP un-cleavable matrixes were re-sensitized to BRAFi similarly to MT1-MMP inhibition. This is due to the inability of cells to activate integrinβ1/FAK signaling, as restoration of integrinβ1 activity is sufficient to maintain resistance to BRAFi in the context of MT1-MMP inhibition. Finally, the increase in MT1-MMP in BRAFi-resistant cells is TGFβ-dependent, as inhibition of TGFβ receptors I/II dampens MT1-MMP overexpression and restores sensitivity to BRAF inhibition.
Conclusions:
BRAF inhibition results in a selective pressure towards higher expression of MT1-MMP. MT1-MMP is pivotal to an ECM-based signaling pathway that confers resistance to BRAFi therapy.
Introduction
Melanoma is the most deadly form of skin cancer, with an estimated death toll of 6,850 patients in 2020 in the United States and costing approximately 3 billion USD in treatment. Metastatic melanoma is one of the most aggressive forms of cancer, with a five-year survival at 16–20% for distant metastasis. Despite recent advances in melanoma treatment, its incidence is increasing annually. These economic and demographic trends underscore the necessity for developing new therapies to complement current treatment methods, particularly those that target metastasis (1).
In approximately 50% of all melanomas, a mutation in the v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) has been found (2). This results in the constitutive activation of BRAF, resulting in the over-activation of the mitogen-activated protein kinase (MAPK) growth pathway and melanoma proliferation. Inhibitors specific to mutant BRAF have impressive response rates of 80% initially; however, after six months most patients relapse with BRAFi resistant melanoma, even in combination with a MEK inhibitor (3–6).
Several mechanisms of resistance have been identified mostly consisting of the rewiring in tumor cells of several survival pathways independent of BRAF (4, 7–10). A potential role of the tumor microenvironment, specifically of the extracellular matrix (ECM), in the resistant phenotype has also been suggested. In the work by Fedorenko et al (11), fibronectin was found increased in BRAF resistant cells and partly responsible of BRAFi resistance through the activation of integrin α5β1/AKT signaling. Also, paradoxical activation of melanoma-associated fibroblasts by PLX4720 has been linked to the promotion of matrix production and induction of integrinβ1/FAK/Src signaling in melanoma cells, providing a mechanism of resistance (12).
While activation of integrins may provide survival cues that can promote resistance, the mechanisms linking ECM production and integrin activation to BRAFi resistance remains undescribed. Here we provide evidence that BRAFi resistant cells and tumors selectively up-regulate the metalloproteinase MT1-MMP. MT1-MMP is a major collagenase essential for the cleavage and activation of collagen I, II and III as well as other substrates such as EGF, CD44, Notch1, fibronectin, and the invasion promoting MMP2 (13–20). MT1-MMP has been shown to lead to the activation of integrinβ1 via collagen processing (21). We have previously shown that MT1-MMP is highly expressed in melanoma where it drives invasion and metastases (22, 23); and that inhibition of MT1-MMP by either RNAi or the selective catalytic inhibitor ND322 significantly impairs metastatic dissemination in a melanoma orthotopic model (22, 24). Here we demonstrate that BRAFi resistant cells selectively up-regulate ECM components such as collagen and fibronectin, as well as MT1-MMP, their major processing enzyme, via up-regulation of TGFβ signaling. Inhibition of MT1-MMP via RNAi or ND322 restores sensitivity to BRAFi in previously resistant cells and tumors. MT1-MMP dependent resistance to BRAFi is mediated by its ability to remodel the ECM and activate integrinβ1 signaling. Thus severing the interaction of melanoma cells with the supporting ECM by inhibiting MT1-MMP function is an effective means to simultaneously inhibit melanoma growth, metastasis and treatment resistance.
Methods
Chemicals
PLX4720 was provided by LC Laboratories and dissolved in DMSO or incorporated into animal chow at a concentration of 200 ppm (25) (OpenSource Diets). ND322 was synthesized as described (26). ND322 was solubilized in DMSO in 10mM stocks and used at 0.32μM for in vitro work. For in vivo work, ND322 was solubilized with 25% DMSO, 45% propylene glycol and 30% H2O for mouse subcutaneous injections at a dose of 25mg/Kg once daily. The TGF-receptor type I/II dual inhibitors LY21109761 and LY364947 were purchased from Selleck Chemicals, dissolved in DMSO and used at 10μM concentration in cell cultures.
Viral plasmids and transductions
shRNAs against MT1-MMP (TRCN0000050855, TRCN0000050856), MMP2 (TRCN00000051526, TRCN00000051527) and MMP9 (TRCN0000373008) were purchased from Sigma. shMT1-MMP and shMMP2 were previously described (24). MT1-MMP over expression plasmids were previously described (22). ITGB1 over expression plasmids were a generous gift from Dr. Valerie Weaver (University of California, San Francisco, CA) and were previously described (21). Plasmids were transfected in HEK-293T (from ATCC) using XtremeGene-9 (Sigma) to produce viral particles. Supernatant was collected and viral transduction onto primary melanoma cells using 8μg/mL polybrene was done. Cells were selected using 2μg/mL puromycin.
Cell lines
The human melanoma cell lines A375, K457, V2387, WM266–4, WM115, 1205Lu, WM9 acquired resistance to PLX4720 after the chronic treatment with PLX4720 at 5μM for 1–2 months until no cell death was observed as described in (27). WM793 and WM164 pairs of parental/resistant cells were a gift from Dr. Keiran Smalley (Moffitt Cancer Institute, Tampa, FL). Resistant cells were designated A375R, WM793R, K457R, V237R, 1205luR, WM9R and WM164R and WM164RR (dual resistance to BRAFi/MEKi). Cells were routinely tested for mycoplasma once a month. Cells were used within a month from thawing.
Reverse-Phase protein array
Biopsies were collected from xenograft melanoma tumors derived from A375 and 1205Lu cells and from PDX lines WM4007 and WM3929, before and after in vivo treatment with PLX4720 or PLX4720+PD0325901. Samples were prepared as described in (25) and submitted to UT MD Anderson Center RPPA core facility (Houston, Tx) as described in (28) and data reported as normalized log2. MT1-MMP expression levels as well as levels of phospho-tyrosine-397-FAK, phospho-serine-473-AKT, and p42/44-MAPK were analyzed.
Western blotting
Cell seeding, collection of protein and western blot methods were previously described (22, 23). Membranes were probed with the following antibodies: anti-MT1-MMP (Millipore, MAB3328), anti-GAPDH (Santa Cruz Biotechnology, C65), anti-FAK-(pY397) (BD Biosciences, 611722), anti-FAK (Cell Signaling, D2R2E), anti-ITGB1 (Cell Signaling, D2E5), anti-Cleaved-PARP (Cell Signaling, D64E10), anti-SMAD3-(pS423/S425) and anti-SMAD3 (EP568Y) (Abcam).
Real-Time PCR
cDNA synthesis, and PCR amplification were done as described in (24). Primers were selected from the Harvard Primer Bank as described here (29).
Cell viability
Promega’s Cell Titer-Glo viability assay was used to determine relative [ATP]. Cells were seeded at 5×103 density in 96 well plates in triplicate in 100uL. One day after seeding, 100uL of lysis reagent was added to the time 0 (T0) plate and baseline luminescence was detected. Media was changed in other plates and drug was added. 3 days after treatment cells were again lysed and luminesce was detected based on total [ATP]. Time points were normalized to the T0 reading.
Cooperativity Index
CI was calculated based on viability assay values using Compusyn as described (30). Three doses of PLX4720 were used in combination with shMT1-MMP (for WM266–4) or full-length and ΔCAT (for WM115) MT1-MMP constructs, in triplicate. Three days after treatment, viability was measured via Promega Cell Titer-Glo.
Survival assay
2×104 cells were seeded in 24 well plates. One day after seeding cells were treated with drug and a time zero was collected. Detached cells present in the media were combined with trypsinzed cells, spun down and suspended in 100uL of media:Trypan blue at a 1:1 ratio. Counting was performed via the T20 Biorad cell counter.
Apoptosis assay
Promega’s RealTime-Glo Annexin V Apoptosis assay was used to determine relative annexinV levels. 5×103 cells were seeded on 96 well plates in triplicate. One day after seeding cells were treated with PLX4720 at 5μM in DMSO. Three days after treatment the kit reagents were added and luminescence was detected. Relative annexinV was normalized to DMSO shGFP controls.
qGEL 3D matrix assay
Cell suspension of 106 cells/100uL was combined with 400uL of HEPES with qGEL lyophilized powder (Formulation IDs: NSC4QA432R and NSC4EN562R) (qGelBio, Lausanne, Switzerland) (31). The mixture was incubated at 37°C, 5% CO2 for 30m until a solid matrix was formed with cells embedded inside. 2 mL media was then added and survival assay was performed as above.
In vivo tumor growth
Female Nude mice were provided by Charles River Laboratories and cared for by the division of animal resources (DVR) at the University of Miami. All experimental models were IACUC approved. 2×106 cells were injected in the dorsal flanks of each mouse, totaling 5 mice and 10 tumors per group. When tumor volumes reached 100mm3, tumors and body weight were measured every other day. Tumor volume was calculated using ((W2 × L) × 0.5). Mice were treated with chow containing 200ppm PLX4720 and/or dailiy s.c. 25mg/Kg doses of ND322. Once tumors reached approximately 1000mm3 mice were euthanized and tumors collected.
Immunohistochemistry
FFPE tumor sections derived from 1205Lu (both parental and resistant to BRAFi) were rehydrated and antigen retrieval performed using a citric acid based antigen unmasking solution (Vector Laboratories) as per manufacturer’s instructions. Primary anti MT1-MMP (clone EP1264Y, Abcam) was used at 1:250 dilution overnight at 4C. HRP conjugated secondary antibody was incubated at RT as per manufacturer’s instructions (ImmPRESS anti rabbit, Vector laboratories) and was followed by ImmPACT DAB substrate (Vector Laboratories).
Statistics
Statistical significance was determined using the Student’s t test with a significant difference being p<0.05. Significance of correlation was detected using the Pearson correlation calculated via Graph pad Prism with a correlation significant when p was at least <0.05. All experiments were repeated at least three times.
Results
MT1-MMP increases after BRAFi treatment
We have previously shown that MT1-MMP expression correlates with reduced outcome for patients with melanoma (22, 23). Further, we have shown that MT1-MMP plays a key role in melanoma invasion and metastasis, in part through the activation of pro-MMP2 (22, 24). It has been shown that BRAFi-resistant melanoma becomes more aggressive and metastatic. Since MT1-MMP is a key player in cell invasion and migration, we sought to investigate whether MT1-MMP may play a role in resistance to BRAFi (32).
By analyzing two data sets with RNAseq data available for patients’ pre- and post-treatment tumor specimens, we found that MT1-MMP is significantly increased at the mRNA level in post-treatment tumor biopsies that progressed on BRAFi treatment compared to pre-treatment biopsies (Fig. 1A) (33, 34). To further support these findings we created BRAFi-resistant cell lines by chronically treating them with PLX4720 at 5uM (35). The expression of MT1-MMP at both mRNA (Fig. 1B) and protein (Fig. 1C–D) levels were consistently increased in all seven cell lines that acquired resistance to PLX4720 compared to their treatment-naïve parental counterparts. Interestingly, the analysis of RNAseq data derived from four patients’ longitudinal tumor specimens, who were treated at Massachusetts General Hospital with sequential targeted therapies and cancer immunotherapies and then progressed on all, demonstrated an increase in MT1-MMP mRNA (Suppl. Fig. 1).
Figure 1: MT1-MMP is associated with a worse prognosis and increases after BRAFi treatment.

A, Analysis of RNAseq geodata-set GSE50509 (left panel) and GSE99898 (right panel) for MT1-MMP mRNA expression. Tumor biopsies were separated into two groups: Pre-treatment biopsy and Progressed on treatment with BRAFi (dabrafenib or vemurafenib). B, MT1-MMP mRNA levels in resistant cells normalized to each parental counterpart. MT1-MMP is significantly up-regulated in all lines with respect to parental controls (p<0.001). C, MT1-MMP protein levels in parental (P) or PLX4720 resistant (R) cell line pairs. GAPDH was used as loading control. D, densitometry for MT1-MMP parental vs resistant obtained with ImageJ. Values were normalized to GAPDH for each sample. E, MT1-MMP protein expression in 1205Lu derived tumors before BRAFi treatment (PAR) and after resistance to BRAFi treatment occurred (RES).
To further support patients’ clinical data, 1205Lu xenografted tumors that progressed on PLX4720 were immunostained with an anti MT1-MMP antibody. All resistant tumors showed increased expression of MT1-MMP at the protein level compared to parental tumors (Fig. 1E). Similarly, the analysis of reverse phase protein array (RPPA) data of pre- and post-treatment A375 derived tumors as well as two Patient Derived Xenografts (WM4007 and WM3929) (35), showed increased MT1-MMP protein upon acquiring resistance to PLX4720 (Suppl. Fig. 2). Even short-term treatment with combination BRAFi (PLX4720) and MEKi (PD0325901) demonstrated higher MT1-MMP expression after treatment.
We also found other pathways that were consistently upregulated in resistant tumors, including AKT and ERK signaling, as well as an increase in phosphorylation of FAK at tyrosine Y397, a marker of integrin activation (Suppl. Fig. 3). Together these data indicate MT1-MMP expression increases in BRAFi resistant tumors and cell lines making it a potential novel target to reverse resistance.
MT1-MMP correlates to Vemurafenib response in BRAFV600E mutant melanoma cell lines
In order to better assess the relationship of MT1-MMP with the sensitivity of BRAF-mutant melanoma cells to PLX4720, 5 melanoma cell lines expressing various relative levels of MT1-MMP (Fig. 2A) were treated with increasing doses of PLX4720 and cell viability determined (Fig. 2B). The IC50 for each line was calculated by the curve fit method (GraphPad). By comparing each IC50 with the relative amount of MT1-MMP expressed by each line, we found that the higher the amount of MT1-MMP the higher the IC50 (Fig. 2C), suggesting MT1-MMP is inversely associated to the response of melanoma cells to the BRAFi.
Figure 2: MT1-MMP correlates to Vemurafenib response in BRAFV600E mutant melanoma cell lines.

A, MT1-MMP expression levels in several melanoma cell lines. B, viability of the cells in A after 72 hours of treatment with different doses of PLX4720. IC50 values were determine by the curve fit method. Values were normalized to DMSO treated control. C), correlation between IC50s of the cells in A,B and the relative MT1-MMP expression levels determined by densitometry after normalization to GAPDH. D-E, WM266–4 cells transfected with lentivirus containing shRNA targeting MT1-MMP (shMT1) or GFP (shGFP) and treated with PLX4720 at three different doses. Cell growth was measured 72 hours after treatment and normalized to DMSO control. F-G, WM115 cells transfected with either PLM (empty vector), full length MT1-MMP (FL) or dead mutant MT1-MMP (ΔCAT) and treated with PLX4720 as in D-E. Viability was measured 72 hours after treatment. H, cooperativity indexes of the cells in D and F.
To determine if MT1-MMP is actively playing a role in the resistance to BRAFi, we next knocked down (Fig. 2D, E) or over-expressed (Fig. 2F, G) MT1-MMP in a pair of syngeneic lines that have either high or low relative MT1-MMP expression, respectively (22). Knockdown of MT1-MMP in WM266–4, which has high endogenous MT1-MMP levels, resulted in further decrease in cell growth when coupled with PLX4720 compared to control cells expressing shGFP. On the other hand, over-expression of full-length (FL) MT1-MMP in WM115, which has a lower endogenous MT1-MMP expression, increased growth. A catalytically inactive mutant MT1-MMP (ΔCAT) (22) however, failed to promote cell growth, suggesting MT1-MMP may protect cells from BRAFi via its proteolytic activity. By using Compusyn to determine the cooperativity of PLX4720 and MT1-MMP expression, we found that PLX4720 synergizes with MT1-MMP inhibition at all doses tested (Fig. 2H, top panel) in WM266–4 cells, whereas in WM115, the overexpression of full length MT1-MMP exert antagonistic effects (Fig. 2H, bottom panel) (30, 36). Synergy between PLX4720 and shMT1-MMP combination was observed for additional cell lines (Suppl. Fig. 4). These data support a role of MT1-MMP in modulating susceptibility to BRAFi treatment, highlighting MT1-MMP as a potential target for combination therapy and further study.
MT1-MMP knockdown sensitizes cells and tumors to Vemurafenib (PLX4720)
Given the link between the expression levels of MT1-MMP and sensitivity of BRAF-mutant melanoma cells to BRAFi, we next sought to determine if cells that acquired resistance could be re-sensitized to PLX4720 via the knockdown of MT1-MMP. We first examined cell survival of BRAFi-sensitive and -resistant cells treated with PLX4720 and expressing either a control shRNA (shGFP) or shMT1-MMP. In both PLX4720 sensitive K457 (Fig. 3A) and WM793 (Fig. 3B) cells, we observed PLX4720 alone significantly decreased cell survival but did not significantly affect resistant cells. However, the combination of MT1-MMP knockdown with PLX4720 restored sensitivity of resistant cells to the levels of parental cells treated with PLX4720 alone or even lower. Similar results were observed on an additional pair of parental and resistant cell lines (Suppl. Fig. 5A). Inhibition of MT1-MMP also reduced cell growth in several melanoma cell lines, particularly when combined with PLX4720. Importantly, in resistant cells, in which the BRAFi did not significantly affect cell growth, depletion of MT1-MMP restored the drug inhibitory effects (Suppl. Fig. 5B).
Figure 3: MT1-MMP knockdown sensitizes cells and tumors to Vemurafenib.

A-B, Survival of K457 (A) and WM793 (B) parental and PLX4720 resistant cells treated with the BRAFi at 5uM for three day and expressing either shGFP or shMT1-MMP. Cell survival was measured by trypan blue exclusion assay. C-D, Levels of annexinV of the cells in A and B, respectively. E and F, Western blot showing cleaved PARP (c-PARP) and MT1-MMP levels of the cells in A and B. G, Growth in nude mice of K457 parental (PAR) and resistant (RES) cells expressing either shGFP or shMT1-MMP and fed with control or 200ppm PLX4720 chow. Treatment with the BRAFi started when tumors in all groups reach an average 100mm3 in volume. Tumor volumes were measured every other day.
Finally, to address potential off-target effects, a second shRNA against MT1-MMP we have employed previously (22–24) was used. Inhibition of MT1-MMP by this shRNA also led to reduced cell growth and increased cell death when combined with PLX4720, restoring sensitivity to the drug in resistant cells (Suppl. Fig. 6).
To further define the mechanism of action of BRAFi resistance mediated by MT1-MMP, apoptosis was assessed. The combination of the knockdown of MT1-MMP and PLX4720 increased apoptosis, as measured by the percentage of Annexin V positive cells (Fig. 3C, D) (37); and led to higher levels of cleaved-PARP (Fig. 3E, F). Taken together, our results indicate the depletion of MT1-MMP in combination with PLX4720 increases apoptosis and cell death. Likewise, cells resistant to both BRAFi and MEKi, are re-sensitized to the inhibitors by the depletion of MT1-MMP (Suppl. Fig. 7B, C).
Having established a strong link between the knockdown of MT1-MMP and the sensitivity to PLX4720, we next sought to verify the effect of the knockdown of MT1-MMP in a mouse xenograft model to investigate its potential as a therapeutic target. K457 parental and resistant cells were transduced with either shMT1-MMP or shGFP and grafted onto mice. Mice were then treated with PLX4720 ad libitum and tumor growth was measured over time (Fig. 3G). Tumors derived from parental cells in which MT1-MMP was depleted displayed the least tumor growth when treated with the BRAFi. The treatment of tumors derived from resistant cells expressing shGFP had little effect on growth as expected; however, tumors derived from resistant cells expressing shMT1-MMP showed a significant reduction in tumor growth. These results indicate that MT1-MMP confers resistance to BRAFi and the depletion of MT1-MMP can overcome resistance.
MT1-MMP mediates resistance to Vemurafenib via processing of the ECM
MT1-MMP is one of the most important invasion promoting, pro-tumorigenic MMPs that control progression of cancer cells (35). Active MT1-MMP is able to process a wide variety of ECM proteins, adhesion and signaling receptors, cytokines and growth factors including EGF, CD44, and Notch1 (13–20). MT1-MMP also activates pro-migratory/invasive MMPs such as MMP2 and MMP13, promoting tumorigenesis (38). Although MT1-MMP is known to signal independently of its catalytic function (39–43), data in figure 2F suggest the response of BRAF-mutant cells to BRAFi is dependent on the catalytic activity of MT1-MMP as a catalytically inactive MT1-MMP construct (ΔCAT) did not promote cell growth upon BRAFi treatment. To further confirm these data, WM115 cells transduced with either the full length catalytically proficient or the ΔCAT MT1-MMP constructs (Suppl. Fig. 8A) were treated with 5uM PLX4720 and survival assessed. Only the catalytically proficient MT1-MMP provided a survival benefit to cells treated with PLX4720 (Suppl. Fig. 8B), indicating MT1-MMP acts through the enzymatic processing of a substrate(s) to mediate resistance to BRAFi.
MMP2 is a main accessory soluble metalloproteinase acting downstream of MT1-MMP in several types of cancers. MMP2 is directly activated by MT1-MMP (44, 45) and we have previously shown MMP2 mediates the migration and invasion of melanoma downstream of MT1-MMP (22). We therefore sought to determine whether MMP2 mediates MT1-MMP dependent BRAFi resistance. MMP2 was depleted by two specific shRNAs (shMMP2–1 and shMMP2–2) in both parental and resistant WM793 cells (Suppl. Fig. 9A). The survival was then measured after three days of treatment with PLX4720 at 5uM (Suppl. Fig. 9B). We found that the depletion of MMP2 did not sensitize cells to BRAFi, supporting a specific role of MT1-MMP in this phenomenon.
We therefore asked whether MT1-MMP confers resistance through its ability of directly processing the ECM (46). To answer this question, parental and resistant K457 cells were embedded in a synthetic (ethylene glycol) hydrogel that incorporates MT1-MMP cleavable or non-cleavable collagen sequences (21, 31). Cells were then treated with PLX4720 and the survival was measured by trypan blue staining (Fig. 4A). The survival of resistant cells encapsulated in a non-MT1-MMP cleavable gel was significantly decreased compared to cleavable matrix when treated with PLX4720 (Suppl. Fig. 10). This demonstrates that the role of MT1-MMP in ECM cleavage is important for resistance to BRAFi and that inhibiting the catalytic function of MT1-MMP is beneficial to restoring sensitivity to BRAFi.
Figure 4: MT1-MMP mediates resistance to Vemurafenib via processing of the ECM.

A, Survival of parental and resistant K457 cells encapsulated in a hydrogel containing either MT1-MMP degradable or not degradable collagen sites and treated with PLX4720 (5uM) for 72 hours. Survival was measured via trypan blue exclusion assay. *p<0.01. B, Collagen I secretion in parental and resistant melanoma pairs. ELISA for collagen I was conducted from serum deprived conditioned media from 106 cells. *p<<0.05. C, Fibronectin expression in conditioned media and MT1-MMP from cell lysates of the cells in B. D, MT1-MMP, FAK and Phospho-FAKY397 in K457 cells transfected with shGFP or shMT1-MMP. GAPDH was used as loading control. E, integrinβ1 (ITGB1), MT1-MMP, FAK and phospho-FAKY397 expression levels in K457 cells transfected with a combination of shMT1-MMP or shGFP and a constitutively active integrinβ1 over expression vector (pLV-VN-ITGB1). F, Survival of the cells in E, treated with 5uM PLX4720 for 72 hours. *p<0.01.
MT1-MMP mediates BRAFi resistance by engaging integrinβ1/FAK signaling
Given the requirement of ECM cleavage in MT1-MMP dependent BRAFi resistance, we next asked whether ECM components cleaved by MT1-MMP were also upregulated in resistant cells, as a potential mechanism of protection. Indeed, an increase in collagen I secretion as well as fibronectin, was found in resistant cells (Fig. 4B, C).
These data suggest resistant cells selectively acquire a mesenchymal-like phenotype by up-regulating a repertoire of ECM factors as well as MT1-MMP, a major processing enzyme of both collagen and fibronectin.
Integrinβ1 (ITGB1) is a main cell surface receptor of ECM collagen and fibronectin (47, 48). Binding of collagen promotes integrin clustering and activation, which is a key step in allowing ECM-integrin mediated outside-in signaling (49). The intracellular domain of integrinβ1 can bind several effectors. A main signaling factor activated by active integrinβ1 is FAK, which autophosphorylates at tyrosine 397 (50). MT1-MMP has been shown to activate ITGB1 and drive osteogenic versus adipogenic differentiation through processing of ECM components and activation of FAK at tyrosine 397 (21). Importantly, ITGB1 has been suggested to play a role in the resistance to BRAFi (11, 12). In the work by Hirata et al (12), treatment with PLX4720 has been shown to activate stromal fibroblasts, which in turn, secrete ECM components leading to ITGB1/Src activation in melanoma cells. Thus, we sought to determine whether in our system, in which resistant melanoma cells themselves secrete more ECM factors and at the same time increase MT1-MMP expression, MT1-MMP might confer resistance to BRAFi via ECM processing and activation of ITGB1. FAK phosphorylation of tyrosine 397 was used as a read out of ITGB1 activity since, as mentioned above, this tyrosine is specifically phosphorylated upon ECM mediated integrin activation (21). Inhibition of MT1-MMP in both parental and resistant K457 cells resulted in a reduction in FAK phosphorylation, indicating reduced ITGB1 activation (Fig. 4D). Thus, to test whether ITGB1 indeed mediates resistance to BRAFi downstream of MT1-MMP, parental and resistant cells expressing shMT1-MMP were co-transduced with a construct expressing a self-clustering ITGB1 mutant (ITGB1-VN) (21). This construct causes high ITGB1 clustering at the focal adhesions, which results in its constitutive downstream signaling such as higher FAK phosphorylation (Fig. 4E, suppl. Figs. 7A and 11A). We found that resistant cells that were re-sensitized to PLX4720 by the knockdown of MT1-MMP regained their resistance when activated ITGB1 was introduced (Fig. 4F and Suppl. Fig. 11B). This suggests MT1-MMP acts as a mediator between the ECM and integrinβ1 signaling to promote resistance to BRAFi. Of note, data in Suppl. Fig. 3, show phospho-FAKY397 is upregulated in tumors that progressed on PLX4720, further supporting a link between MT1-MMP, ECM remodeling and the activation of integrin/FAK signaling.
Inhibition of MT1-MMP activity by ND322 restores sensitivity to Vemurafenib in vivo
Our data shows that MT1-MMP confers resistance to BRAFi through the processing of the ECM and consequent activation of ITGB1 signaling. Thus, we reasoned that specifically targeting the catalytic activity of MT1-MMP would restore responses of resistant cells to BRAFi. To test this, we made use of ND322. ND322 is a selective, slow-binding inhibitor with inhibition constants of 0.02uM, 0.24uM and 0.87uM, for MMP2, MT1-MMP, and MMP9 respectively (51). ND322 however, is a very poor inhibitor, with short residence time and low affinity, of several other MMPs, as we have shown previously (24, 26, 51). In an earlier study, we have shown that ND322 counteracts melanoma growth and delays metastases in a melanoma orthotopic mouse model while displaying no side effects in vivo (24). At a concentration of 0.32uM, 2.7 times below the Ki for MMP9, ND322 was able to restore sensitivity of resistant cells to PLX4720 as shown by a reduction in survival compared to cells treated with either agent alone (Fig. 5A–B).
Figure 5: Inhibition of MT1-MMP activity by ND322 restores sensitivity to Vemurafenib.

A-B, Survival of parental and resistant K457 (A) and WM793 (B) cells treated with 5uM PLX4720 and 0.32uM ND322 either alone or in combination for 72 hours. *p<0.01. C-D, Growth of K457 parental and resistant cells in mice treated with daily doses of 25mg/Kg ND322 (s.c) and fed with 200ppm PLx4720 in chow. Treatment started when tumors in all groups reach an average 100mm3 in volume. Tumor volumes were measured every other day. Statistically significant difference between the combination and the individual treatment groups is shown.
We excluded the effects of ND322 on resistance to BRAFi are dependent on MMP2 inhibition since we have shown that MMP2 knock down does not sensitize resistant cells to PLX4720 (suppl. Fig. 9). However, since ND322 is also an MMP9 inhibitor, albeit at higher concentrations required to inhibit MT1-MMP, we determined whether MMP9 could play a role in resistance to BRAFi. Knockdown of MMP9 in parental and resistant cells (Suppl. Fig 12) did not have an impact on cell survival when cells were treated with PLX4720, indicating MMP9 does not play an important role in conferring resistance to BRAFi and that ND322 sensitizes cells to BRAFi mainly through MT1-MMP inhibition.
Next, ND322 was tested in vivo to determine whether it could restore responses to PLX4720. BRAF-mutant K457 parental and resistant cells were inoculated subcutaneously in nude mice. Mice were then fed PLX4720 or control chow as previously described [(25) and figure 3]. Treatment with PLX4720 and/or ND322 at 25mg/Kg s.c. daily was started when tumors in all groups reached an average volume of 100mm3. The combination of ND322 and PLX4720 demonstrated a significant reduction in tumor growth compared to either agent alone in mice inoculated with BRAFi-sensitive cells, while stable disease was observed in the combination setting in mice inoculated with resistant cells (Fig. 5C–D). Overall, these data indicate the specific targeting of the catalytic activity of MT1-MMP can restore sensitivity of resistant tumors to BRAFi.
MT1-MMP upregulation in resistant cells is mediated by TGFβ signaling.
It has been previously shown that resistance to BRAFi causes an increase in TGFβ release, which in turn, induces the secretion of ECM components, such as fibronectin, from the surrounding fibroblasts (52). In our experimental system we find that melanoma cells themselves secrete ECM components and even more so when they acquire resistance to BRAFi. Also, MT1-MMP has been shown to be controlled by TGFβ signaling (53, 54). Hence we posited that increased MT1-MMP in BRAFi-resistant cells could be a function of TGFβ signaling. To test this possibility, we used the TGF-receptor I/II dual inhibitors LY2109761 and LY364947. Both inhibitors effectively blunt the signaling downstream of TGFβ as indicated by a reduction of SMAD3 phosphorylation (Fig 6A). Treatment of both parental and resistant K457 cells with LY2109761 lead to a decrease in MT1-MMP expression in resistant cells, likely because the upregulation of MT1-MMP in these cells is TGFβ dependent; and was accompanied by an increase in cleaved PARP, suggesting inhibition of MT1-MMP by blockade of TGFβ signaling may lead to cell death. To definitively assess this possibility, cells were treated with PLX4720 and LY2109761 either alone or in combination, and then they were stimulated by active recombinant MT1-MMP, to determine whether the latter could rescue cell survival. Indeed, the addition of active MT1-MMP reduced the amount of cleaved PARP in both parental and resistant cells, and importantly, rescued cell survival (Fig. 6C, D). Similar results were obtained with the inhibitor LY364947 (Suppl. Fig. 13). Hence, these data indicate TGFβ signaling in BRAFi-resistant cells may leads to an increase in MT1-MMP resulting in cell protection.
Figure 6. MT1-MMP up-regulation is dependent on TGFβ signaling.
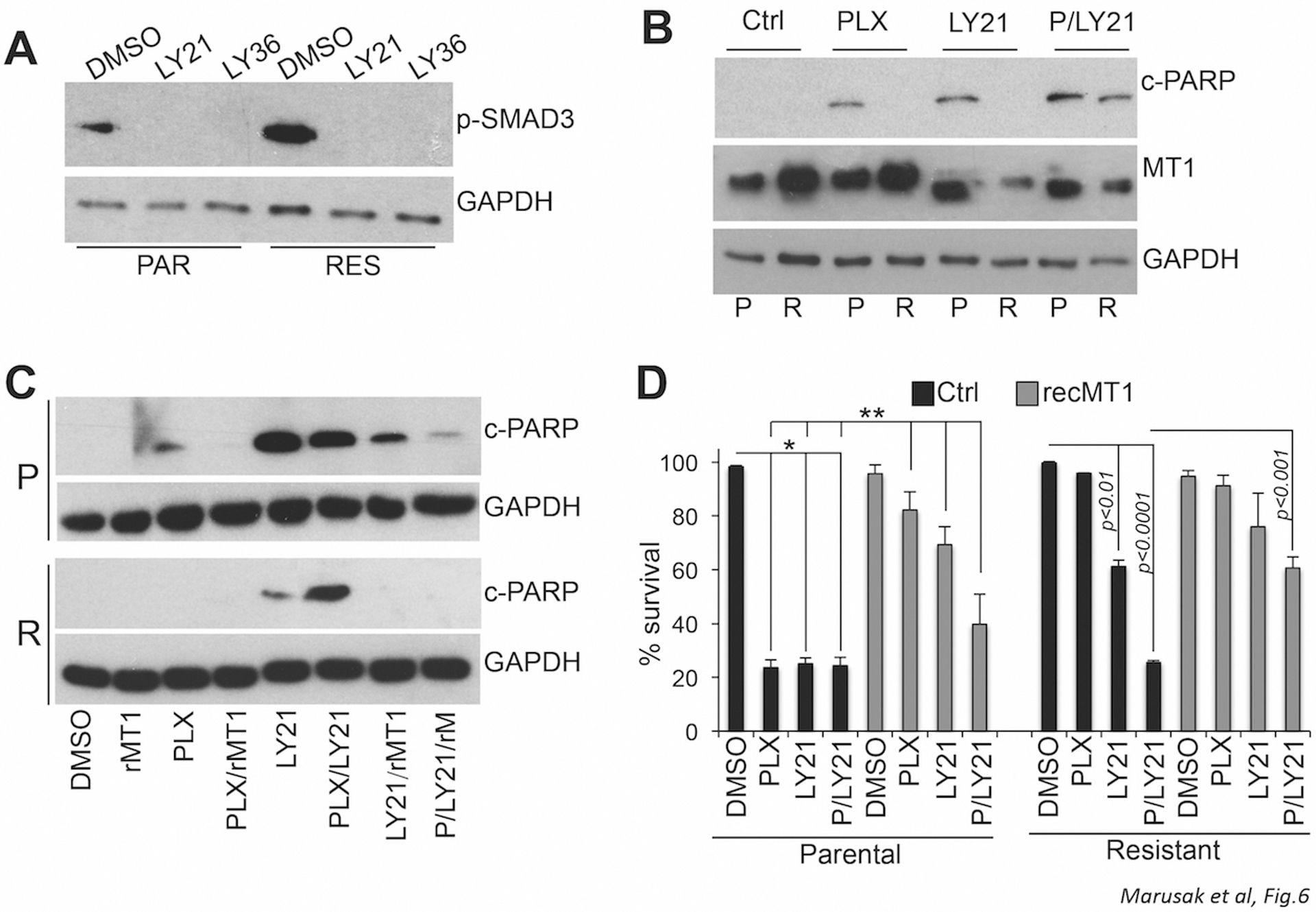
A, levels of phohpo-SMAD3S423/S425 in parental and resistant K457 cells treated with 10uM of the TGF-receptor I/II (TRI/II) dual inhibitors LY21109761 and LY364947 overnight. B, Cleaved PARP and MT1-MMP expression levels in parental and resistant K457 cells treated with 5uM PLX4720 and 10uM LY21109761 for 72 hours. C, cleaved PARP in parental and resistant K457 treated as in B but with the addition of 20ng/ml active recombinant MT1-MMP (rMT1) where indicated. D, survival of the cells in C. *p<0.001.
Discussion
In this study, we show that MT1-MMP dependent remodeling of the ECM is a novel mechanism of resistance to BRAF inhibitors. A selective advantage exists in BRAFi resistant melanomas to overexpress MT1-MMP, as well as components of the ECM (i.e. collagen and fibronectin), likely as a mechanism of protection against BRAFi mediated cytotoxicity. Indeed, we show that MT1-MMP activates an outside-in survival signaling pathway through the activation of ITGB1; and that genetic or pharmacological inhibition of MT1-MMP synergizes with BRAF inhibition restoring sensitivity to BRAFis both in vitro and in vivo. This indicates MT1-MMP is playing a role beyond its canonical migratory and invasive functions and that MT1-MMP inhibition can not only counteract tumor progression by inhibiting metastases, as we have previously shown (22–24); but importantly, can be combined with BRAFis to increase their efficacy and prevent or revert resistance.
It has been previously shown that matrix deposition by tumor associated fibroblasts following BRAF inhibitor treatment can induce an integrinβ1 dependent survival pathway in melanoma cells (11, 12). Here we show that melanoma cells under the selective pressure of BRAF inhibition consistently activate an autogenous mechanism of protection by producing their own ECM, such as collagen I and fibronectin, as well as MT1-MMP, the rate-limiting enzyme in ECM remodeling and of paramount importance in the activation of integrinβ1 and its downstream survival effects.
Inhibition of integrins has been investigated as anticancer target, however, targeting them specifically has proved difficult because of high homology between the different integrins and because of the important role of integrins in general cell homeostasis (55). Instead, targeting of MT1-MMP is a feasible alternative in view of its role in linking the ECM to integrinβ1 signaling and the availability of novel selective MMP inhibitors.
Indeed, earlier trials with pan-MMP inhibitors failed mostly due to severe side effects such as musculoskeletal pain and inflammation, accompanied by negligible anti-cancer effects (56). This is because several MMPs play important roles in inflammation and immune responses and some, such as MMP8, possess anti-cancer and pro-immune surveillance properties; as well as the fact that these inhibitors were tested on late-stage melanoma patients when pan MMP inhibition might be more useful as an adjuvant (56).
However, research into the individual roles of the MMPs in cancer has revealed MT1-MMP as a major driver of melanomagenesis. MT1-MMP increases in melanoma as it progresses; it inversely correlates to BRAF treatment responses as demonstrated here; it is a poor prognosis marker, and it is critical for metastasis (22–24, 38). Selective targeting of MT1-MMP would, therefore, provide effective anti-tumor responses while reducing deleterious side effects. In fact, while daily treatment with the selective MT1-MMP/MMP2 inhibitor ND322 either alone or in combination with PLX4720 revealed significant anti-cancer activity, it did not show any evident morbidity such as changes in body weight, hunched posture, reduced motility, highlighting its potential safety and efficacy even in a combination setting. Similar safety profiles were previously observed in animal models of brain ischemia and wound healing (57–59).
That said, it is worth mentioning that MT1-MMP knockout mice are the only MMP knockout mice that cannot fully develop, with systemic growth defects across the body that eventually lead to mortality (60). These defects are likely due to the inability to properly deposit collagen early on in development. This may therefore potentially limits the use of MT1-MMP inhibitors to non-pediatric cancers (61). Still, MT1-MMPs established roles in metastasis combined with our data demonstrating its importance in cell survival and BRAFi resistance make it an attractive target for a wide range of aggressive cancers, in addition to melanoma.
In summary, our findings highlight a previously unidentified role of MT1-MMP in mediating BRAFi resistance and demonstrate that MT1-MMP inhibition via the selective inhibitor ND322, when combined with BRAFi in BRAFV600E mutant melanoma, has a profound synergistic inhibitory response, reverting resistant tumors to responsive ones. Overall, blockade of MT1-MMP provides an effective means to simultaneously inhibit melanoma growth, metastasis and treatment resistance by severing the interaction of melanoma cells with the supporting ECM.
Supplementary Material
Translational Relevance:
While BRAFi therapies have dramatically changed the outlook for many patients with BRAF-mutant metastatic melanoma, the vast majority of patients inevitably develop resistance. We have previously shown that MT1-MMP inhibition significantly reduces the ability of melanomas to metastasize. Here we highlight a novel mechanism of resistance to BRAFi involving MT1-MMP. By using BRAFi-resistant xenografts we demonstrate that targeting of MT1-MMP by a selective MT1-MMP catalytic inhibitor (ND322) with high selectivity towards the target, and low toxicity, can effectively re-sensitize tumors to BRAFi treatment. MT1-MMP inhibition disrupts the activation of integrinβ1/FAK signaling by blunting the cleavage of collagen, a major ligand of integrinβ1. This result suggests that targeted inhibition of MT1-MMP by ND322 may be used in the clinic in combination with BRAFi, to prevent both metastasis and treatment resistance.
Acknowledgments
This work was in part supported by NIH grant R01CA177652, and by start up funds from Sylvester Comprehensive Cancer Center and the Frankel Family Division Research Fund . C.M. Marusak, V. Thakur, Y. Li, J.T. Freitas, P. M. Zmina, were supported by NIH grant R01CA177652 and by start up funds from Sylvester Comprehensive Cancer Center and the Frankel Family Division Research Fund.
References
- 1.American Cancer society: Cancer Facts & Figures 2020. Atlanta: American Cancer Society, Ga. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Testa U, Castelli G, Pelosi E. Melanoma: Genetic Abnormalities, Tumor Progression, Clonal Evolution and Tumor Initiating Cells. Med Sci (Basel) 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol 2011; 82: 201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010; 468: 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perna D, Karreth FA, Rust AG, Perez-Mancera PA, Rashid M, Iorio F, Alifrangis C, Arends MJ, Bosenberg MW, Bollag G, Tuveson DA, Adams DJ. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proc Natl Acad Sci U S A 2015; 112: E536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irvine M, Stewart A, Pedersen B, Boyd S, Kefford R, Rizos H. Oncogenic PI3K/AKT promotes the step-wise evolution of combination BRAF/MEK inhibitor resistance in melanoma. Oncogenesis 2018; 7: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abel EV, Basile KJ, Kugel CH 3rd, Witkiewicz AK, Le K, Amaravadi RK, Karakousis GC, Xu X, Xu W, Schuchter LM, Lee JB, Ertel A, Fortina P, Aplin AE. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest 2013; 123: 2155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D’Andrea K, Pushparajan A, Hayden JE, Brown KD, Laquerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010; 18: 683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010; 468: 968–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, Ribas A, Palma MD, Nathanson KL, Koomen JM, Messina JL, Smalley KS. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 2011; 71: 2750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedorenko IV, Abel EV, Koomen JM, Fang B, Wood ER, Chen YA, Fisher KJ, Iyengar S, Dahlman KB, Wargo JA, Flaherty KT, Sosman JA, Sondak VK, Messina JL, Gibney GT, Smalley KS. Fibronectin induction abrogates the BRAF inhibitor response of BRAF V600E/PTEN-null melanoma cells. Oncogene 2016; 35: 1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell 2015; 27: 574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci 2009; 122: 3015–24. [DOI] [PubMed] [Google Scholar]
- 14.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol 2001; 153: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin G, Zhang F, Chan KM, Xavier Wong HL, Liu B, Cheah KS, Liu X, Mauch C, Liu D, Zhou Z. MT1-MMP cleaves Dll1 to negatively regulate Notch signalling to maintain normal B-cell development. EMBO J 2011; 30: 2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshikawa N, Mizushima H, Minegishi T, Iwamoto R, Mekada E, Seiki M. Membrane type 1-matrix metalloproteinase cleaves off the NH2-terminal portion of heparin-binding epidermal growth factor and converts it into a heparin-independent growth factor. Cancer Res 2010; 70: 6093–103. [DOI] [PubMed] [Google Scholar]
- 17.Sabbota AL, Kim HR, Zhe X, Fridman R, Bonfil RD, Cher ML. Shedding of RANKL by tumor-associated MT1-MMP activates Src-dependent prostate cancer cell migration. Cancer Res 2010; 70: 5558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golubkov VS, Aleshin AE, Strongin AY. Potential relation of aberrant proteolysis of human protein tyrosine kinase 7 (PTK7) chuzhoi by membrane type 1 matrix metalloproteinase (MT1-MMP) to congenital defects. J Biol Chem 2011; 286: 20970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golubkov VS, Chekanov AV, Cieplak P, Aleshin AE, Chernov AV, Zhu W, Radichev IA, Zhang D, Dong PD, Strongin AY. The Wnt/planar cell polarity protein-tyrosine kinase-7 (PTK7) is a highly efficient proteolytic target of membrane type-1 matrix metalloproteinase: implications in cancer and embryogenesis. J Biol Chem 2010; 285: 35740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Tang X, Wong P, Jacobs B, Borden EC, Bedogni B. Noncanonical Activation of Notch1 Protein by Membrane Type 1 Matrix Metalloproteinase (MT1-MMP) Controls Melanoma Cell Proliferation. J Biol Chem 2014; 289: 8442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Rowe RG, Botvinick EL, Kurup A, Putnam AJ, Seiki M, Weaver VM, Keller ET, Goldstein S, Dai J, Begun D, Saunders T, Weiss SJ. MT1-MMP-dependent control of skeletal stem cell commitment via a beta1-integrin/YAP/TAZ signaling axis. Dev Cell 2013; 25: 402–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaverdashvili K, Wong P, Ma J, Zhang K, Osman I, Bedogni B. MT1-MMP modulates melanoma cell dissemination and metastasis through activation of MMP2 and RAC1. Pigment Cell Melanoma Res 2014; 27: 287–96. [DOI] [PubMed] [Google Scholar]
- 23.Shaverdashvili K, Zhang K, Osman I, Honda K, Jobava R, Bedogni B. MT1-MMP dependent repression of the tumor suppressor SPRY4 contributes to MT1-MMP driven melanoma cell motility. Oncotarget 2015; 6: 33512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marusak C, Bayles I, Ma J, Gooyit M, Gao M, Chang M, Bedogni B. The thiirane-based selective MT1-MMP/MMP2 inhibitor ND-322 reduces melanoma tumor growth and delays metastatic dissemination. Pharmacol Res 2016; 113: 515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krepler C, Xiao M, Sproesser K, Brafford PA, Shannan B, Beqiri M, Liu Q, Xu W, Garman B, Nathanson KL, Xu X, Karakousis GC, Mills GB, Lu Y, Ahmed TA, Poulikakos PI, Caponigro G, Boehm M, Peters M, Schuchter LM, Weeraratna AT, Herlyn M. Personalized Preclinical Trials in BRAF Inhibitor-Resistant Patient-Derived Xenograft Models Identify Second-Line Combination Therapies. Clin Cancer Res 2016; 22: 1592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gooyit M, Lee M, Schroeder VA, Ikejiri M, Suckow MA, Mobashery S, Chang M. Selective water-soluble gelatinase inhibitor prodrugs. J Med Chem 2011; 54: 6676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesi G, Philippidou D, Kozar I, Kim YJ, Bernardin F, Van Niel G, Wienecke-Baldacchino A, Felten P, Letellier E, Dengler S, Nashan D, Haan C, Kreis S. A new ALK isoform transported by extracellular vesicles confers drug resistance to melanoma cells. Mol Cancer 2018; 17: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther 2006; 5: 2512–21. [DOI] [PubMed] [Google Scholar]
- 29.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 2010; 38: D792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou TC MN. CompuSyn for drug combinations: PC Software and User’s Guide: a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values. Paramus (NJ): Combosyn 2005. [Google Scholar]
- 31.Thakur V, Zhang K, Savadelis A, Zmina P, Aguila B, Welford SM, Abdul-Karim F, Bonk KW, Keri RA, Bedogni B. The membrane tethered matrix metalloproteinase MT1-MMP triggers an outside-in DNA damage response that impacts chemo- and radiotherapy responses of breast cancer. Cancer Lett 2019; 443: 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, McQuade JL, Panka DJ, Hudgens CW, Amin-Mansour A, Mu XJ, Bahl S, Jane-Valbuena J, Wani KM, Reuben A, Creasy CA, Jiang H, Cooper ZA, Roszik J, Bassett RL Jr., Joon AY, Simpson LM, Mouton RD, Glitza IC, Patel SP, Hwu WJ, Amaria RN, Diab A, Hwu P, Lazar AJ, Wargo JA, Garraway LA, Tetzlaff MT, Sullivan RJ, Kim KB, Davies MA. Clinical, Molecular, and Immune Analysis of Dabrafenib-Trametinib Combination Treatment for BRAF Inhibitor-Refractory Metastatic Melanoma: A Phase 2 Clinical Trial. JAMA Oncol 2016; 2: 1056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiesner T, Kutzner H, Cerroni L, Mihm MC Jr., Busam KJ, Murali R. Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy. Pathology 2016; 48: 113–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakavand H, Rawson RV, Pupo GM, Yang JYH, Menzies AM, Carlino MS, Kefford RF, Howle JR, Saw RPM, Thompson JF, Wilmott JS, Long GV, Scolyer RA, Rizos H. PD-L1 Expression and Immune Escape in Melanoma Resistance to MAPK Inhibitors. Clin Cancer Res 2017; 23: 6054–61. [DOI] [PubMed] [Google Scholar]
- 35.Song C, Piva M, Sun L, Hong A, Moriceau G, Kong X, Zhang H, Lomeli S, Qian J, Yu CC, Damoiseaux R, Kelley MC, Dahlman KB, Scumpia PO, Sosman JA, Johnson DB, Ribas A, Hugo W, Lo RS. Recurrent Tumor Cell-Intrinsic and -Extrinsic Alterations during MAPKi-Induced Melanoma Regression and Early Adaptation. Cancer Discov 2017; 7: 1248–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, Bosenberg MW, McMahon M, Cantley LC, Zheng B. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A 2013; 110: 18226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 2010; 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thakur V, Bedogni B. The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis. Pharmacol Res 2016; 111: 17–22. [DOI] [PubMed] [Google Scholar]
- 39.Turunen SP, Tatti-Bugaeva O, Lehti K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim Biophys Acta Mol Cell Res 2017; 1864: 1974–88. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Kasberg WC, Celo A, Liang Z, Quispe K, Stack MS. Post-translational modification of the membrane type 1 matrix metalloproteinase (MT1-MMP) cytoplasmic tail impacts ovarian cancer multicellular aggregate dynamics. J Biol Chem 2017; 292: 13111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cepeda MA, Pelling JJ, Evered CL, Leong HS, Damjanovski S. The cytoplasmic domain of MT1-MMP is dispensable for migration augmentation but necessary to mediate viability of MCF-7 breast cancer cells. Exp Cell Res 2017; 350: 169–83. [DOI] [PubMed] [Google Scholar]
- 42.Pratt J, Iddir M, Bourgault S, Annabi B. Evidence of MTCBP-1 interaction with the cytoplasmic domain of MT1-MMP: Implications in the autophagy cell index of high-grade glioblastoma. Mol Carcinog 2016; 55: 148–60. [DOI] [PubMed] [Google Scholar]
- 43.Terawaki S, Kitano K, Aoyama M, Hakoshima T. Crystallographic characterization of the radixin FERM domain bound to the cytoplasmic tail of membrane-type 1 matrix metalloproteinase (MT1-MMP). Acta Crystallogr Sect F Struct Biol Cryst Commun 2008; 64: 911–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 1995; 270: 5331–8. [DOI] [PubMed] [Google Scholar]
- 45.Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, Seiki M. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem 1998; 273: 16098–103. [DOI] [PubMed] [Google Scholar]
- 46.Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: a tethered collagenase. J Cell Physiol 2004; 200: 11–9. [DOI] [PubMed] [Google Scholar]
- 47.Zeltz C, Gullberg D. The integrin-collagen connection - a glue for tissue repair? J Cell Sci 2016; 129: 1284. [DOI] [PubMed] [Google Scholar]
- 48.Nakayamada S, Okada Y, Saito K, Tamura M, Tanaka Y. Beta1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor kappaB ligand on osteoblasts and osteoclast maturation. J Biol Chem 2003; 278: 45368–74. [DOI] [PubMed] [Google Scholar]
- 49.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 2010; 11: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guan JL. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol 1997; 29: 1085–96. [DOI] [PubMed] [Google Scholar]
- 51.Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang M, Fridman R, Mobashery S. Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem 2005; 280: 33992–4002. [DOI] [PubMed] [Google Scholar]
- 52.Fedorenko IV, Wargo JA, Flaherty KT, Messina JL, Smalley KSM. BRAF Inhibition Generates a Host-Tumor Niche that Mediates Therapeutic Escape. J Invest Dermatol 2015; 135: 3115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 2007; 109: 4055–63. [DOI] [PubMed] [Google Scholar]
- 54.Kuo YC, Su CH, Liu CY, Chen TH, Chen CP, Wang HS. Transforming growth factor-beta induces CD44 cleavage that promotes migration of MDA-MB-435s cells through the up-regulation of membrane type 1-matrix metalloproteinase. Int J Cancer 2009; 124: 2568–76. [DOI] [PubMed] [Google Scholar]
- 55.Tolomelli A, Galletti P, Baiula M, Giacomini D. Can Integrin Agonists Have Cards to Play against Cancer? A Literature Survey of Small Molecules Integrin Activators. Cancers (Basel) 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer 2006; 94: 941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R, Hadass O, Lehmidi T, Blair GJ, Lee M, Chang M, Mobashery S, Sun GY, Gu Z. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener 2012; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song W, Peng Z, Gooyit M, Suckow MA, Schroeder VA, Wolter WR, Lee M, Ikejiri M, Cui J, Gu Z, Chang M. Water-Soluble MMP-9 Inhibitor Prodrug Generates Active Metabolites That Cross the Blood-Brain Barrier. ACS Chem Neurosci 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao M, Nguyen TT, Suckow MA, Wolter WR, Gooyit M, Mobashery S, Chang M. Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc Natl Acad Sci U S A 2015; 112: 15226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999; 99: 81–92. [DOI] [PubMed] [Google Scholar]
- 61.Mori H, Bhat R, Bruni-Cardoso A, Chen EI, Jorgens DM, Coutinho K, Louie K, Bowen BB, Inman JL, Tecca V, Lee SJ, Becker-Weimann S, Northen T, Seiki M, Borowsky AD, Auer M, Bissell MJ. New insight into the role of MMP14 in metabolic balance. PeerJ 2016; 4: e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


