Abstract
Background:
Postoperative delirium is a common and serious problem for older adults. To better align local practices with delirium prevention consensus guidelines, we implemented a five-component intervention followed by a quality improvement (QI) project at our institution.
Methods:
This hybrid implementation-effectiveness study took place at two adult hospitals within a tertiary care academic healthcare system. We implemented a 5-component intervention: preoperative delirium risk stratification, multidisciplinary education, written memory aids, delirium prevention post-anesthesia care unit (PACU) orderset, and electronic health record enhancements between 12/1/17–6/30/18. This was followed by a department-wide QI project to increase uptake of the intervention from 7/1/18–6/30/19. We tracked process outcomes during the QI period, including frequency of preoperative delirium risk screening, percentage of “high risk” screens, and frequency of appropriate PACU orderset use. We measured practice change after the interventions using interrupted time series analysis of perioperative medication prescribing practices during baseline (12/1/16–11/30/17), intervention (12/1/17–6/30/18), and QI (7/1/18–6/30/19) periods. Participants were consecutive older patients (age 65 and over) who underwent surgery during the above timeframes and received care in the PACU, compared to a concurrent control group younger than age 65. The a priori primary outcome was a composite of perioperative American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use (Beers PIM) medications. The secondary outcome, delirium incidence, was measured in the subset of older patients who were admitted to the hospital for at least one night.
Results:
During the 12-month QI period, preoperative delirium risk stratification improved from 67% (714/1,068 patients) in month 1 to 83% in month 12 (776/931 patients). Forty percent of patients were stratified as “high-risk” over the 12-month period (4,246/10,494 patients). Appropriate PACU orderset use in high-risk patients increased from 19% in month 1 to 85% in month 12. We analyzed medication use in 7,212, 4,416, and 8,311 PACU care episodes during the baseline, intervention, and QI periods, respectively. Beers PIM administration decreased from 33% to 27% to 23% during the three time periods, with adjusted odds ratio (aOR) 0.97 ([95% CI 0.95–0.998], p=0.03] per month during the QI period in comparison to baseline. Delirium incidence was 7.5%, 9.2%, and 8.5% during the three time periods with aOR of delirium of 0.98 [(95% CI 0.91–1.05), p=0.52] per month during the QI period in comparison to baseline.
Conclusions:
A perioperative delirium prevention intervention was associated with reduced administration of Beers PIMs to older adults.
Introduction
Older surgical patients are at elevated risk of perioperative morbidity,1–4 including postoperative delirium (POD). Delirium is one of the most common postoperative complications described in older surgical patients, with a reported prevalence of up to 50%.5 It has been associated with worsened traditional and patient-centered outcomes, including prolonged length of stay, increased institutional discharge,6 impaired functional recovery,7 and cognitive decline.8 Notably, one-third to one-half of delirium cases are thought to be preventable using a multi-component intervention,9 making it an ideal target for quality improvement in the perioperative environment.
Earlier this year, the American Society for Enhanced Recovery and the Sixth Perioperative Quality Initiative (POQI-6) published a joint consensus statement on prevention of postoperative delirium.10 This document complements earlier guidelines published by other professional societies.5,11–13 Strong recommendations applying to the preoperative or intraoperative environment include implementing a multidisciplinary quality improvement process to reduce postoperative delirium, performing preoperative risk stratification and delirium assessment in high-risk patients, using multicomponent non-pharmacologic delirium prevention interventions, and minimizing use of deliriogenic medications. Medications commonly used in the perioperative period that are known to contribute to delirium in older patients include anticholinergic drugs (e.g., diphenhydramine, scopolamine), benzodiazepines, and meperidine, among others (Table 1). These are termed Potentially Inappropriate Medications (PIMs) or Beers Criteria medications by the American Geriatrics Society.14
Table 1:
Intervention recommendations for perioperative use of Beers Criteria PIMs commonly used in anesthesia*
| Medication Class | Examples | Precautions | Rationale |
|---|---|---|---|
| NSAIDs | Ketorolac Diclofenac Ibuprofen |
|
Increased risk of GI bleeding, AKI, hypertension |
| Sedative Hypnotics | Benzodiazepines | Avoid, except for specific indications such as seizure or alcohol withdrawal | Increased risk of delirium, cognitive impairment, falls, fractures |
| Gabapentin |
|
Increased risk of over-sedation | |
| Meperidine | Avoid, especially in patients with CKD | Higher risk of neurotoxicity including delirium | |
| Anticholinergics | Scopolamine Promethazine Prochlorperazine Diphenhydramine Hydroxyzine Tricyclic anti-depressants |
Avoid | Increased risk of central anti-cholinergic side effects including confusion, blurred vision, delirium, sedation |
| Other psychoactive medications | Steroids (e.g., dexamethasone) | Avoid or use cautiously | Increased risk of delirium |
| Antipsychotics |
|
Increased risk of stroke, cognitive decline, mortality in patients with dementia |
Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; GFR, glomerular filtration rate; CKD, chronic kidney disease; AKI, acute kidney injury; GI, gastrointestinal; ESRD, end-stage renal disease
Data used from AGS 2019 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults14
In 2016, the Delirium Reduction Campaign15 – a health system-wide quality improvement initiative to implement delirium screening, prevention, and treatment procedures in medical and surgical inpatients – was launched at University of California, San Francisco (UCSF) Health. In coordination with system-wide efforts, we developed a distinct multidisciplinary perioperative pathway to provide guidance on evidence-based perioperative delirium prevention (See Supplemental Document 1, which describes the UCSF Perioperative Delirium Prevention and Treatment Pathway in further detail). This was accompanied by a targeted five-component implementation intervention and quality improvement project with the goal of increasing departmental compliance with best practices recommended in the pathway. In this retrospective hybrid implementation-effectiveness study with an interrupted time series design, we report our experience with the implementation and outcomes of this perioperative initiative at UCSF Health.
Methods
Clinical Setting
UCSF Health is an urban tertiary care health system. Work described here was performed at Moffitt-Long and Mission Bay Hospitals, the health system’s two major adult inpatient hospitals with a combined 706 adult hospital beds. Between 29,000–30,000 adult anesthesia cases are performed at these two hospitals yearly. The perioperative delirium prevention intervention was implemented for all patients aged 18 years and over having surgery at one of these two hospitals. Patients receiving inpatient obstetric, electrophysiology, or cardiac catheterization procedures were not included, since they do not typically receive care in a post-anesthesia care unit (PACU) targeted by this intervention; patients who underwent endoscopy or radiology procedures were included if they received care in the PACU. UCSF Health uses the Epic electronic health record (EHR) system (Verona, WI, USA) including the Optime (periop) and Anesthesia modules. This manuscript adheres to SQUIRE 2.0 guidelines.16
IRB Approval
The Committee on Human Research at UCSF provided expedited review of the protocol for this retrospective study with minimal risk to human subjects (IRB #19–27578). The need to obtain written informed consent from study participants was waived by the committee.
Intervention Design and Implementation Strategy
The intervention was designed based on an assessment of existing organizational- and individual-level barriers to behavior change. Constructs from a framework published in the implementation science literature17 were utilized to evaluate barriers and to develop implementation interventions that would be likely to address the needs of individuals at our institution and ultimately to result in the desired behavior change. During this process, we solicited feedback and obtained buy-in from champions from the perioperative nursing department and representatives from the anesthesia resident and nurse anesthetist groups. We also collaborated with stakeholders from the hospital-wide Delirium Reduction Campaign; members of surgical, geriatrics, and neurology departments; and executive-level hospital leadership.
The final intervention (“the intervention”) consisted of five components: introduction of preoperative delirium risk screening, provision of multidisciplinary education and training, distribution of written educational materials and memory aids, launch of a delirium risk PACU orderset, and development of EHR enhancements. The intervention was implemented between December 2017 and June 2018, and was followed by a department-wide quality improvement (QI) project to improve uptake. The five-component intervention and the QI project are described in further detail below. A timeline with important implementation milestones is depicted in Figure 1, and a more granular timeline can be found in Supplemental Document 1 (Supplement Table 1).
Figure 1: Timeline of intervention.
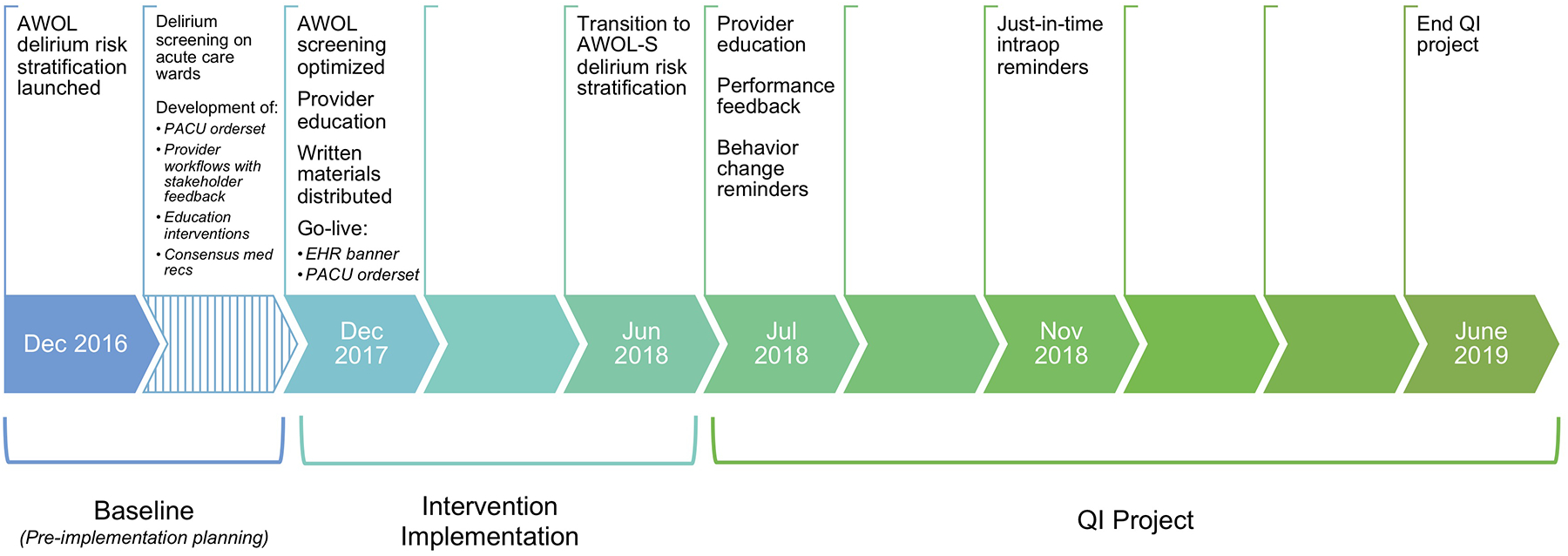
A timeline of the intervention and quality improvement project is pictured with selected implementation milestones during the baseline (12/1/16–11/30/17), intervention (12/1/17–6/30/18), and QI project (7/1/18–6/30/19) periods. (Abbreviations: PACU, post-anesthesia care unit; EHR, electronic health record; intraop, intraoperative; QI, quality improvement)
The Intervention (December 2017 – June 2018)
1. Delirium Risk Stratification:
To identify patients at highest risk of developing POD, we began risk-stratifying all adult surgical patients undergoing surgery with a planned overnight hospital stay with the AWOL tool18 in December 2017, and later the AWOL-S (surgery-specific) tool19 in June 2018. Briefly, AWOL-S is a surgery-specific delirium risk stratification tool which was modified from AWOL (Age > 80, failure to spell WORLD backward, disOrientation to place, and higher nurse-rated iLlness severity score), a simple 4-point screening tool which was used hospital-wide to screen all inpatients at the time of admission to our institution for delirium risk. AWOL-S includes terms for surgery-specific risk (based on NSQIP and local data6) and ASA physical status, while retaining the original AWOL predictors of age, inability to spell WORLD backward, and disorientation to place. AWOL-S is an EHR-based tool that generates a predicted probability of delirium for an individual patient using odds ratios derived from logistic regression. AWOL-S predicted probability of delirium ≥5% was considered “high risk” for risk stratification purposes. For more details on risk stratification procedures, refer to Supplemental Document 1.
2. Multidisciplinary Education and Training:
a. Anesthesia Department:
An initial educational session on the impact of POD and recommendations for delirium prevention was conducted at a UCSF Department of Anesthesia and Perioperative Care Grand Rounds conference by project leadership (ALD) and representatives from the Departments of Neurology (VCD) and Geriatrics (SR). Concepts presented were reinforced with an educational email to all members of the department, and email reminders were sent to anesthesia providers periodically throughout the intervention period. An introduction to the new PACU orderset was provided to all members of the anesthesia department by EHR experts (DLR) and the PACU medical director (MRB).
Actionable recommendations for anesthesia providers were primarily focused on perioperative medication management, although other important concepts contained in best practice patient care guidelines5,12–13 were also emphasized (See Supplemental Document 1 for further detail). We recommended medication management according to the Beers Criteria;14,20 specifically, we recommended avoiding administration of deliriogenic medications whenever possible (Table 1). We developed a preferred approach for management of postoperative nausea and vomiting (PONV) which incorporated guidance from institutional experts from geriatrics, neurology, and pharmacy where uncertainty existed in the literature (Table 2). Further description of the rationale for specific medication recommendations can be found in Supplemental Document 1.
Table 2:
UCSF multidisciplinary consensus recommendations for the prevention and management of postoperative nausea and vomiting in older adults
| Preferred order of anti-emetics | Avoid when possible |
|---|---|
Preventative measures:
Rescue antiemetics:
|
|
Abbreviations: mg, milligrams; IV, intravenous
b. Preoperative and PACU Nurses:
Nurses received education on the prevalence and impact of POD, effectiveness of preventative measures, and logistics of the new PACU orderset by project and PACU leadership (ALD and MRB) at staff meetings, by peer-to-peer training, and via an online educational module. They also received training by project and nursing leadership prior to launch of AWOL and AWOL-S screening through formal presentations at staff meetings, one-on-one or group peer training, email messages, and posted visual aids. For more details, refer to Supplemental Document 1.
c. Surgical Departments:
Education on postoperative delirium prevention and management was provided to surgical teams by the Delirium Reduction Campaign (SR and VCD).
3. Distribution of Written Educational Materials and Memory Aids:
Written materials, visual reminders, and memory aids were created for anesthesia providers, surgeons, and nursing staff (See Supplemental Document 2, Part 1, depicting visual aids and educational materials used for the intervention). Printed materials were placed in strategic locations around the operating rooms (ORs), including the preoperative holding area, PACU workstations, OR anesthesia carts, workspaces for surgeons, and common spaces for nursing and anesthesia providers. Certain materials were also made available online for reference in any location and were sent to providers via email.
4. Launch of a Delirium Risk PACU Orderset:
Our institution’s standard adult PACU orderset contains opt-in orders for three PIMs: meperidine, prochlorperazine, and metoclopramide. In order to minimize inadvertent exposure to PIMs, we created a novel PACU orderset for high-risk patients, termed the delirium risk PACU orderset. Modifications included removal of the three opt-in PIMs, addition of haloperidol as an alternative agent for PONV (See Supplemental Document 2, Part 2, which shows the “Other Medications” panel of the PACU orderset) and addition of a multi-component nursing care bundle (See Supplemental Document 2, Part 2, which shows the “Delirium Prevention Interventions” panel of the PACU orderset). The new PACU orderset was launched in December 2017. The rationale for specific medication changes is described further in Supplemental Document 1.
5. Implementation of EHR Enhancements:
New EHR builds were required to facilitate recommended practice changes. To aid identification of patients stratified as at high risk for delirium, a visual flag was added to the patient’s EHR banner so that providers could target the intervention to appropriate patients (See Supplemental Document 2, Part 2, which shows the anesthesia caution banner). Modifications to the PACU orderset, as described above, were launched in December 2017. As a response to initially poor compliance with appropriate orderset use, just-in-time intraoperative reminders for anesthesia providers were introduced in November 2018 (See Supplemental Document 2, Part 2 which shows intraoperative reminders for anesthesia providers). These enhancements were introduced during the QI period (described below) as an iterative part of continuous process improvement; however, we describe them here for simplicity.
Quality Improvement Project (July 2018 – June 2019)
Following implementation of the intervention, a targeted quality improvement (QI) project was conducted between July 1, 2018 and June 30, 2019. The goal of the QI project was to increase uptake of delirium prevention recommendations. The QI project most specifically targeted appropriate PACU orderset use in high-risk patients based on AWOL-S results. This process metric was selected because it was simple, measurable, and a surrogate for individual behavior change. QI activities were conducted by residents and faculty advisors on the Anesthesia Resident Quality Improvement Committee. Representatives provided additional intensive departmental education on perioperative delirium best practices at departmental conferences and via email. Committee members tracked monthly departmental compliance with appropriate PACU orderset use, provided monthly feedback on compliance to the entire anesthesia department via email and during conferences, and sent reminders to use the orderset via email and pages.
Measurement of Results
Participants analyzed retrospectively were consecutive patients age 65 and older who underwent surgery or a procedure requiring anesthesia care and received care in the PACU, excluding those who underwent electroconvulsive therapy treatments only, during 3 time periods: baseline (12/1/16–11/30/17), intervention (12/1/17–6/30/18), and quality improvement (7/1/18–6/30/19). A one-year baseline time period was selected to allow for adjustment of results for trends preceding the intervention. Patients under 65 served as a contemporaneous control group during all time periods. The primary outcome, use of one or more of seven commonly-used deliriogenic Beers Criteria PIMs (diphenhydramine, lorazepam, meperidine, midazolam, prochlorperazine, promethazine, and scopolamine) between anesthesia start and PACU end, was chosen a priori in order to measure practice change associated with implementation of the intervention. The secondary outcome measure was delirium incidence for those patients staying at least one night in the hospital. We also performed exploratory hypothesis-generating analyses on prescriber behavior for Beers Criteria antiemetics (prochlorperazine, promethazine, and scopolamine), preferred antiemetics (dexamethasone, haloperidol, and metoclopramide), and midazolam. Process measures during the QI period included frequency of preoperative delirium risk screening, frequency of high-risk delirium screen, and frequency of appropriate PACU orderset use.
Delirium rates in the first 7 postoperative days were measured in the subpopulation of adults 65 and older who received care in PACU (except electroconvulsive therapy) and were hospitalized for at least one night after surgery. This population was chosen because patients would have been eligible to receive postoperative delirium screening during their hospital stay. Delirium was identified on acute care wards using the NuDesc screen.21 Screening was performed by nurses once per shift (morning and evening), and a score of 2 or higher was defined as a positive result. Patients who were later transferred to the ICU received delirium screening with CAM-ICU.22 Compliance with delirium screens was tracked by hospital leadership and remained >90% after hospital units were on-boarded with delirium screening education, which evolved during the study period as additional hospital floors were trained over time.
Statistical Analysis
Sample size was based on the number of older patients having surgery at our institution within the specified timeframes. No statistical power calculation was conducted prior to the study because of the consecutive sample used. A two-tailed p-value less than 0.05 was considered the threshold for statistical significance, and no adjustment for multiple testing was performed.
Univariate retrospective comparisons of patient characteristics and primary (composite Beers PIM), secondary (delirium incidence), and hypothesis-generating (composite Beers PIM antiemetics, composite preferred antiemetics, midazolam) outcomes among the 3 time periods were performed with chi-square test, after checking that cell frequency assumptions were met. Statistically significant chi square test results were followed by a post-hoc examination of the Pearson residuals divided by the standard error; if the absolute value of the adjusted residual was >3, it was considered potentially contributing to the overall statistically significant test and flagged with a ‘*’ in the table to assist in interpretation. Age and hospital length of stay, which were not normally distributed, were compared with the Kruskal-Wallis test; median and interquartile range are reported.
For the primary analysis, multiple records were retained for patients with more than one encounter. Segmented regression23 was conducted using generalized estimating equations (GEE) logistic model for 39,741 patients with 54,450 observations to account for confounding and within-subject correlation (exchangeable correlation assumed). We also assessed the unstructured variance covariance matrix but this could not be fit due to the limited number of response pairs, and the simpler exchangeable structure was adequate. The outcomes were binary, so the logit link function was used in SAS proc genmod. We simultaneously estimated intercept and slopes for cases (age 65 and older) and controls (<65) for each period measured in months: baseline (12/1/2016–11/30/2017), intervention (12/1/2017–6/30/2018), and QI (7/1/2018–6/30/2019). We assessed the form of the covariate time, including quadratic, using the Z statistic (P<0.05), and we found that time as a linear effect provided the best fit.
Models were adjusted by age, gender, ASA physical status, emergency case status, type of surgery, and the Elixhauser comorbidities obesity, hypertension, diabetes, congestive heart failure, renal failure, liver failure, obstructive sleep apnea, and dementia, which were calculated from ICD-9 and ICD-10 codes available in the electronic medical record.24,25 There were no missing values for age, emergency case status, type of surgery, or Elixhauser comorbidities; for care episodes of patients at any age, 621 of the 54,450 (1.1%) were missing ASA physical status and 11/54,450 (0.02%) were missing gender, with an additional 6 (0.01%) listed as nonbinary. For those missing ASA, “ASA missing” was assigned its own categorical variable for model adjustment purposes. For those missing gender or listed as nonbinary, due to the small number, the values were imputed to all male and all female to assess sensitivity of conclusions to that missingness; we concluded that there was no impact on the findings.
Using post-hoc estimate statements in SAS, we estimated the “difference-in-difference” by first estimating the difference in the slopes of the cases and controls for each time period. We then compared the difference of those slopes between QI and baseline periods; that is, the adjusted rates of medication administration between post-intervention and pre-intervention. Adjusted odds ratios (aOR) and associated 95% confidence intervals were reported. Statistical analysis was performed with Stata 14.2 software (StataCorp LLC, College Station, TX) and SAS v9.4 (SAS Institute, Cary, NC).
Results
Patients
We analyzed medication use in 7,212 PACU care episodes for older adults during the baseline period, 4,416 during the intervention period, and 8,311 during the quality improvement period; cohort characteristics are in Table 3. Of those care episodes, 4,884, 3,051, and 5,606 respectively resulted in a hospitalization for at least 1 night after surgery, and therefore contributed to the delirium screening rates.
Table 3:
Cohort characteristics
| Baseline | Intervention | Quality Improvement | P value | |||
|---|---|---|---|---|---|---|
| All encounters resulting in care in PACUa | 20,274 | 12,161 | 22,015 | |||
| Encounters in patients 65+ who received care in PACU | 7212 | 4416 | 8311 | |||
| Age | 72 [68–77] | 72 [68–77] | 72 [68–77] | 0.14b | ||
| Female gender | 2991 (41%) | 1946 (44%) | 3566 (43%) | 0.053c | ||
| ASA physical status | 1 | 107 (1.5%) | 45 (1.0%) | 90 (1.1%) | 0.037d | |
| 2 | 3158 (44%) | 1836 (42%) | 3,489 (42%) | |||
| 3 | 3629 (51%) | 2,306 (53%) | 4,331 (53%) | |||
| 4 | 248 (3.5%) | 167 (3.8%) | 310 (3.8%) | |||
| 5 | 2 (0.03%) | 0 | 2 (0.02%) | |||
| Emergency case status | 681 (9.4%) | 468 (11%) | 808 (9.7%) | 0.12c | ||
| Type of procedure | General | 1053 (15%)* | 595 (13%) | 1055 (13%) | <0.001d | |
| Orthopedic | 1252 (17%) | 804 (18%) | 1453 (17%) | |||
| Plastic | 157 (2.2%) | 75 (1.7%) | 143 (1.7%) | |||
| Neurosurgery / Neurointer-ventional Radiology | 662 (9.2%)* | 469 (10.6%) | 914 (11%) | |||
| Cardiac / Vascular /Thoracic | 1005 (14%) | 664 (15%) | 1257 (15%) | |||
| ENT / Ophthalmic / OMFS | 546 (7.6%) | 321 (7.3%) | 555 (6.7%) | |||
| Urology / Gynecology | 1306 (18%) | 745 (17%) | 1392 (17%) | |||
| Endoscopy / Radiology | 1231 (17%) | 743 (17%) | 1542 (19%) | |||
| Elixhauser comorbidities: | ||||||
| Obesity | 450 (6.2%) | 280 (6.3%) | 580 (7.0%) | 0.14c | ||
| Hypertension | Uncomplicated | 3148 (44%) | 1840 (42%) | 3580 (43%) | <0.001d | |
| Complicated | 1016 (14%)* | 700 (16%) | 1401 (17%)* | |||
| Diabetes | Uncomplicated | 897 (12%) | 479 (11%) | 963 (12%) | 0.086c | |
| Complicated | 726 (10%) | 434 (9.8%) | 860 (10%) | |||
| Congestive heart failure | 594 (8.2%) | 386 (8.7%) | 809 (9.7%)* | 0.004d | ||
| Renal failure | 944 (13%) | 600 (14%) | 1166 (14%) | 0.23c | ||
| Liver failure | 388 (5.3%) | 240 (5.4%) | 419 (5.0%) | 0.53c | ||
| Obstructive sleep apnea | 728 (10%)* | 449 (10%) | 1063 (13%)* | <0.001d | ||
| Dementia | 169 (2.3%) | 105 (2.4%) | 207 (2.5%) | 0.83c | ||
| Depression | 566 (7.9%) | 340 (7.7%) | 722 (8.7%) | 0.072d | ||
| Encounters in patients 65+ who received care in PACU and were admitted at least 1 night | 4884 | 3051 | 5606 | |||
| Hospital LOS in those admitted overnight | 2 [1–5] | 3 [1–5] | 2 [1–5] | 0.34b | ||
Abbreviations: PACU, post-anesthetic care unit. ASA, American Society of Anesthesiologists. ENT, ear-nose-throat (otorhinolaryngology). OMFS, oral and maxillofacial surgery. LOS, length of stay.
Excluding electroconvulsive therapy encounters.
Kruskal-Wallis test
Chi square test
Chi square, with post-hoc examination of the residuals; cells marked with * are considered potentially contributing to the overall statistically significant test.
Process Measures
Preoperative Delirium Risk Stratification During QI Period
Over the QI period, frequency of preoperative delirium risk stratification with AWOL-S improved from 714/1068 (67%) in July, 2018 to 776/931 (84%) of eligible patients in June, 2019. Average risk stratification compliance in eligible patients was 79% (10,494/13,353) over the 12-month QI period and 81% (5438/6736) in the last 6 months of the QI period. Throughout the 12-month period, 4,246/10,494 (40%) of risk-stratified patients had a “high risk” result (Figure 2.A).
Figure 2: Process Measures.

Panel A shows monthly rates of preoperative delirium risk stratification with the AWOL-S tool (green line) and percentage of risk-stratified patients classified as high-risk (orange line) during the 12 months of the quality improvement (QI) period starting in July 2018. Panel B shows the frequency of appropriate post-anesthesia care unit (PACU) orderset use in high-risk individuals during the same timeframe. Note that the introduction of intraoperative electronic health record reminders to guide orderset usage was a critical implementation milestone which allowed orderset compliance to increase to approximately 80% for the remainder of the project period.
PACU Orderset Compliance During QI period
Monthly compliance with appropriate PACU orderset use (i.e., ordering the “Delirium Prevention Interventions” in the delirium reduction PACU orderset in patients stratified as high risk for delirium by AWOL-S) was tracked throughout the QI period. Compliance started at 19% (59/308 eligible patients) in July, 2018 and increased steadily to 85% (263/309 eligible patients) In June, 2019. By the end of the QI period, monthly compliance had increased to consistently above 80% in an average of 275 eligible patients per month (Figure 2.B). Compliance with orderset use increased sharply and remained high once intraoperative EHR reminders were introduced in November, 2018 (Figure 2.B).
Outcome Measures
Perioperative Medication Administration for Patients Admitted to the PACU
During the baseline period, 33% of care episodes where an older adult received anesthesia care and then went to PACU resulted in the administration of at least 1 Beers Criteria PIM. This decreased to 27% during the intervention period, and 23% during the QI period. Segmented regression analysis demonstrated an adjusted odds ratio of 0.97 ([95% CI 0.95–0.998], p=0.033) per month of the QI period compared to baseline for administration of the Beers Criteria composite medications (i.e., 3% month-on-month decrease in administration), after adjustment for patient characteristics (Table 4). Exploratory analyses of preferred antiemetics, Beers Criteria antiemetics, and midazolam suggested that this may be driven by reduced midazolam prescribing (OR 0.97 [0.94–0.997], p=0.031), although the point estimates for use of Beers Criteria and preferred antiemetics during the QI period also suggested statistically nonsignificant improvements in prescribing behavior (Table 4).
Table 4:
Outcome measures.
| Outcome | Baseline (n=7212) | Intervention (n=4416) | QI (n=8311) | Chi2 p* | aOR per montha | p |
|---|---|---|---|---|---|---|
| Beers list medicationb (primary outcome) | 2397 (33%)* | 1200 (27%) | 1937 (23%)* | <0.001 | 0.97 [0.95–0.998] | 0.033 |
| Deliriumc (secondary outcome) |
365/4884 (7.5%) | 280/3051 (9.2%) | 477/5606 (8.5%) | 0.020 | 0.98 [0.91–1.05] | 0.52 |
| Preferred antiemeticd (exploratory analysis) | 2634 (36%) | 1673 (38%) | 3214 (39%) | 0.022 | 1.01 [0.99–1.04] | 0.38 |
| Beers list antiemetice (exploratory analysis) | 268 (3.7%)* | 127 (2.9%) | 165 (2.0%)* | <0.001 | 0.96 [0.91–1.02] | 0.21 |
| Midazolam (exploratory analysis) | 2073 (29%) | 1042 (24%) | 1677 (20%) | <0.001 | 0.97 [0.94–0.997] | 0.031 |
Abbreviations: QI, quality improvement. aOR, adjusted odds ratio.
Adjusted odds ratio comparing the change in slopes between baseline and the QI period calculated via segmented regression adjusting for patient demographics and comorbidities.
Composite of any use of diphenhydramine, lorazepam, meperidine, midazolam, prochlorperazine, promethazine, or scopolamine.
Delirium incidence was calculated only in patients who were hospitalized at least 1 night; the denominator is reported in the table.
Composite of any use of dexamethasone, haloperidol, or metoclopramide.
Composite of any use of prochlorperazine, promethazine, or scopolamine.
Potentially contributing to the overall statistically significant chi squared test based on post-hoc examination of the residuals.
Delirium Incidence for Admitted Patients
Delirium incidence in older patients varied over the 3 periods, from 7.5% during the baseline period (during which delirium screening was occurring inconsistently on postoperative floors) to 9.2% during the intervention and 8.5% during the QI period. There was no statistically significant difference in adjusted delirium incidence month-by-month during the QI period compared to baseline using segmented regression (aOR 0.98 [0.91–1.05], p=0.52) (Table 4).
Discussion
We implemented a multidisciplinary perioperative quality improvement initiative designed to better align our institution’s perioperative delirium prevention practices with expert consensus recommendations. After implementation of the intervention followed by a targeted quality improvement project to increase uptake, preoperative delirium risk stratification and use of multicomponent delirium prevention interventions in the PACU occurred reliably, and perioperative administration of Beers Criteria PIMs, particularly midazolam, was reduced when controlling for patient characteristics and secular trends using an interrupted time series study design.
The practice change we demonstrated brought our institutional practice better in line with strong recommendations made in the Joint Consensus Statement from the American Society for Enhanced Recovery and Perioperative Quality Initiative (POQI-6) Workgroup, including preoperative delirium risk stratification procedures, use of multicomponent interventions in the PACU, and decreased PIM administration.10 Though most PIMs were administered to only a small percentage of older patients undergoing surgery, small differences in administration rates may still be clinically significant since each case of postoperative delirium can result in individual patient harm. Unfortunately, few specific intraoperative interventions effectively prevent POD, with recent trials failing to show a preventative effect for intraoperative use of dexmedetomidine,26 ketamine,27 and processed electroencephalography monitoring,28,29 for example. Therefore, the guidance provided by consensus statements such as that published by POQI-6,10 with which our intervention is consistent, is the current standard.
No statistical difference was observed in postoperative delirium incidence after the intervention. There are several likely explanations for this finding. First, delirium screening with NuDesc was rolled out on hospital wards at varying times during the one-year baseline period, so delirium rates were likely undercounted during that time. Data on the sensitivity of NuDesc for detecting delirium varies; it has been recommended for use in the postoperative setting,30 although it, and the CAM-ICU, have also elsewhere been criticized for low sensitivity.31 Furthermore, there were many concurrent delirium-reduction efforts occurring at a health system-wide level throughout our analysis, which further confounds the delirium data. Any changes observed would not be clearly attributed to our specific perioperative intervention. Therefore, making conclusions about the effectiveness of the intervention in this case is limited by variability in delirium detection across time periods. Since no intraoperative interventions reliably decrease delirium incidence across a broad population of surgical patients, our goal at the outset of the intervention was to bring our department’s perioperative management into better alignment with best practice care guidelines rather than to directly change delirium rates.
Effecting individual-level and organizational-level practice change is notoriously difficult.32,33 Factors crucial to our success include capitalizing on momentum from hospital-wide initiatives with high-level institutional support; early engagement of stakeholders from multiple departments (especially nursing); considering feedback about feasibility, workflow impact, and individual- and organizational-level barriers in design of the intervention; providing robust repetitive education to all stakeholder groups; and providing feedback on performance to providers. We also leveraged the EHR to improve provider practice habits through utilization of a variety of custom EHR builds, including just-in-time intraoperative reminders to increase compliance with appropriate orderset use. In fact, introduction of intraoperative reminders was the crucial step that allowed for improvement and maintenance of compliance with orderset use above our departmental goal. Various other EHR capabilities, including best practice advisories and automated order selection based on delirium risk stratification results were not used for a variety of institutional reasons including compliance with the resident QI program guidelines, but these may be good options for others to consider. We intend to continue to analyze the effectiveness of the EHR enhancements in this setting in an attempt to balance the risks of alert fatigue34–36 with the observed – and expected – effectiveness of just-in-time alerts,37 and we plan to continue support of the low-risk passive clinical decision support interventions described in Supplemental Document 2. These passive clinical decision support interventions have been non-controversial – they may not have the full effectiveness of a highly visible alert, but they also carry minimal cognitive burden and risk of distraction.
Based on our experience, we offer several recommendations to others interested in implementing similar POD prevention initiatives at their institutions. First, interprofessional collaboration is of utmost importance to the success of any large-scale initiative. Our efforts would not have been possible without collaboration from our extraordinary preoperative and PACU nursing colleagues and multiple practitioners throughout the hospital. Furthermore, delirium prevention efforts focused solely on anesthesia management are unlikely to be successful without continuation of delirium detection, prevention, and treatment throughout the patient’s entire hospital stay. Coordination with surgeons, geriatricians, consulting physicians, pharmacists, rehabilitation specialists, nurses, and other key staff on acute care wards and intensive care units and having support from hospital leadership for such coordinated efforts is crucial. Provision of constant feedback on compliance and process metrics may be helpful. In order to optimize effectiveness of interventions, involvement of experts in implementation science may also be beneficial.
Our study has several limitations in addition to those already described. The intervention was designed to be used at a single center, incorporating analysis of our institution’s organizational barriers. Our risk stratification tool and EHR builds are also institution-specific. These features limit the generalizability of the intervention; it would require adaptation to be used elsewhere. Furthermore, our intervention occurred in the context of a health system-wide delirium quality improvement intervention. In order for a more limited perioperative intervention to be successful, delirium screening and prevention practices would need to occur on a broader scale outside of the perioperative environment. Finally, sustainability of this intervention is improved by EHR enhancements but inhibited by labor-intensiveness of providing education to new groups of practitioners and ongoing performance feedback.
In conclusion, we report our experience with implementing a perioperative delirium quality improvement intervention which was associated with a statistically significant reduction in the perioperative administration of Beers Criteria PIMs to adults 65 and older. This interprofessional clinical intervention may serve as a foundation for others to build upon in their own institutions.
Supplementary Material
Key points:
Question:
Can we better align perioperative delirium prevention practices with best practice patient care guidelines?
Findings:
After a perioperative delirium prevention intervention, prevention processes occurred reliably and there was a month-over-month decrease in perioperative administration of a composite of potentially inappropriate medications for older adults.
Meaning:
A multidisciplinary quality improvement intervention targeting postoperative delirium resulted in changes to care practices which may improve perioperative outcomes for older adults at risk of delirium.
Acknowledgments:
Preoperative and PACU Nursing Delirium Champions and Nursing Leadership: Elizabeth Borczynski, RN, BSN; James Emerton, RN; Ann Gillen, RN, BSN, CPAN; Kimberly Grossweiler, RN; Sara Nedkov, RN, BSN, CPAN, Unit Director Moffitt-Long Preop/PACU; Lisa Newton, RN; Angela Yan, RN, CNIII. Department of Nursing, University of California, San Francisco, San Francisco, CA, USA. Advised the design and implementation of delirium screening procedures and patient care interventions, provided training and education to nursing staff. This initiative would not have been possible without the great dedication shown by this group.
Preoperative and PACU nursing staff: Carried out delirium screening, prevention, and treatment interventions with high compliance. Department of Nursing, University of California, San Francisco, San Francisco, CA, USA.
Nurse Anesthetist Incentive Project Leaders: Leigh-Ann Langford, MSN, CRNA; Willy Ching, CRNA, DNAP; Richard Ferguson VI, CRNA. Department of Anesthesia and Perioperative Care, University of California, San Francisco, San Francisco, CA, USA. Provided education to CRNA group.
Delirium Reduction Campaign: Jan Yeager, MDes, Service Designer, UCSF Clinical Innovation Center, University of California, San Francisco, San Francisco, CA, USA. Provided graphic design of educational materials. Brian Holt, MHA, Senior Continuous Improvement Advisor, Continuous Improvement. University of California, San Francisco, San Francisco, CA, USA. Provided project coordination and performance feedback.
Jon Spinner, Rachelle Armstrong, Adam Jacobson, Amy Hephner, and Carly Deibler. UCSF Clinical Services and Anesthesia Information Technology, University of California, San Francisco, San Francisco, CA, USA. Managed data collection and electronic health record build.
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1TR001872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Funding Statement:
Support was provided from institutional and/or departmental sources.
Anne L. Donovan received research support from the National Institute on Aging of the National Institutes of Health, under subcontract 91511 of Award Number R24AG054259 (Network for the Investigation of Delirium: Unifying Scientists).
Matthias R. Braehler has received funding support from the University of California, San Francisco Clinical and Translational Science Institute Pilot Awards Program.
Ann A. Lazar has received funding support from the National Institutes of Health UL1 TR991872 and University of California Office of the President (UCOP) CRCC Award and Awards for the HBCU Initiative.
Emily Finlayson has received research support from the National Institute on Aging (NIA R01 AG044425, NIA P30 AG04428, NIA R21AG054208).
Stephanie Rogers has received research support from Center for Disease Control (CDC-OPOIOIDS-2017-001 and CDC-STEADI-2016-001).
Vanja C. Douglas has received funding support from the Sara & Evan Williams Foundation Endowed Neurohospitalist Chair.
Elizabeth L. Whitlock has received research support from the National Institute on Aging of the National Institutes of Health (R03AG059822) and the Foundation for Anesthesia Education and Research.
Statistical support was provided by the UCSF Clinical and Translational Science Institute (CTSI), which is part of the Clinical and Translational Science Award (CTSA) program funded by the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH) (Grant Number UL1 TR991872).
The remainder of authors have no funding sources to disclose.
Glossary of Terms:
- POD
Postoperative delirium
- PIM
Potentially inappropriate medication
- UCSF
University of California, San Francisco
- EHR
Electronic health record
- PACU
Post-anesthesia care unit
- PONV
Postoperative nausea and vomiting
- QI
Quality improvement
- AWOL
“Age, WORLD backward, orientation, iLlness severity” delirium risk stratification tool
- CAM-ICU
Confusion Assessment Method for the ICU
- ICU
Intensive care unit
Footnotes
Competing interests: Emily Finlayson is a founding shareholder of Ooney, Inc. The other authors declare no competing interests.
References
- 1.Oresanya L, Zhao S, Gan S, Fries BE, Goodney PP, Covinsky KE, Conte MS, Finlayson E. Functional outcomes after lower extremity revascularization in nursing home residents: a national cohort study. JAMA Intern Med 2015;175(6):951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berian JR, Mohanty S, Ko CY, Rosenthal RA, Robinson TN. Association of Loss of Independence With Readmission and Death After Discharge in Older Patients After Surgical Procedures. JAMA Surg 2016;151(9):e161689. [DOI] [PubMed] [Google Scholar]
- 3.Mohanty S, Liu Y, Paruch JL, Kmiecik TE, Cohen ME, Ko CY, Bilimoria KY. Risk of discharge to postacute care: a patient-centered outcome for the american college of surgeons national surgical quality improvement program surgical risk calculator. JAMA Surg 2015;150(5):480–4. [DOI] [PubMed] [Google Scholar]
- 4.Finlayson E, Zhao S, Boscardin WJ, Fries BE, Landefeld CS, Dudley RA. Functional status after colon cancer surgery in elderly nursing home residents. J Am Geriatr Soc 2012;60(5):967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal Perioperative Management of the Geriatric Patient: A Best Practices Guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg 2016;222(5):930–47. [DOI] [PubMed] [Google Scholar]
- 6.Berian JR, Zhou L, Russell MM, Hornor MA, Cohen ME, Finlayson E, Ko CY, Rosenthal RA, Robinson TN. Postoperative Delirium as a Target for Surgical Quality Improvement. Ann Surg 2018;268(1):93–9. [DOI] [PubMed] [Google Scholar]
- 7.Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, Cooper Z, Rogers SO Jr, Jones RN, Marcantonio ER, Inouye SK. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg 2015;150(12):1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, Simpkins SA. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016;3:CD005563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, Gan TJ, McEvoy MD, Miller TE, Perioperative Quality Initiative (POQI) 6 Workgroup. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesth Analg 2020;130(6):1572–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger M, Schenning KJ, Brown CH 4th, Deiner SG, Whittington RA, Eckenhoff RG, Angst MS, Avramescu S, Bekker A, Brzezinski M, Crosby G, Culley DJ, Eckenhoff M, Eriksson LI, Evered L, Ibinson J, Kline RP, Kofke A, Ma D, Mathew JP, Maze M, Orser BA, Price CC, Scott DA, Silbert B, Su D, Terrando N, Wang DS, Wei H, Xie Z, Zuo Z, Perioperative Neurotoxicity Working Group. Best Practices for Postoperative Brain Health: Recommendations From the Fifth International Perioperative Neurotoxicity Working Group. Anesth Analg 2018;127(6):1406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, Cherubini A, Jones C, Kehlet H, MacLullich A, Radtke F, Riese F, Slooter AJ, Veyckemans F, Kramer S, Neuner B, Weiss B, Spies CD. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017;34(4):192–214. [DOI] [PubMed] [Google Scholar]
- 13.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015;63(1):142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.By the 2019 American Geriatrics Society Beers Criteria(R) Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019;67(4):674–94. [DOI] [PubMed] [Google Scholar]
- 15.Enhanced Recovery After Surgery. Available at: http://eras.surgery.ucsf.edu/. Accessed 1/7, 2017.
- 16.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0-Standards for Quality Improvement Reporting Excellence-Revised Publication Guidelines from a Detailed Consensus Process. J Am Coll Surg 2016;222(3):317–23. [DOI] [PubMed] [Google Scholar]
- 17.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas VC, Hessler CS, Dhaliwal G, Betjemann JP, Fukuda KA, Alameddine LR, Lucatorto R, Johnston SC, Josephson SA. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med 2013;8(9):493–9. [DOI] [PubMed] [Google Scholar]
- 19.Whitlock EL, Donovan AL, Braehler MR, Kaplan JA, Finlayson E, Rogers SE, Douglas VC. Derivation, Validation, and Sustained Performance of a Hospital-Wide Elective Surgery Delirium Risk Tool (AWOL-S). Anesthesia & Analgesia, in press DOI: 10.1213/ANE.0000000000005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015;63(11):2227–46. [DOI] [PubMed] [Google Scholar]
- 21.Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage 2005;29(4):368–75. [DOI] [PubMed] [Google Scholar]
- 22.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001;29(7):1370–9. [DOI] [PubMed] [Google Scholar]
- 23.Mascha EJ, Sessler DI. Segmented Regression and Difference-in-Difference Methods: Assessing the Impact of Systemic Changes in Health Care. Anesth Analg 2019;129(2):618–33. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control. International Classification of Diseases,Tenth Revision (ICD-10) 1990;.
- 25.Centers for Disease Control. International Classification of Diseases,Ninth Revision (ICD-9). 2015 1979;.
- 26.Deiner S, Luo X, Lin HM, Sessler DI, Saager L, Sieber FE, Lee HB, Sano M, and the Dexlirium Writing Group, Jankowski C, Bergese SD, Candiotti K, Flaherty JH, Arora H, Shander A, Rock P. Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA Surg 2017;152(8):e171505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, Veselis RA, Grocott HP, Emmert DA, Rogers EM, Downey RJ, Yulico H, Noh GJ, Lee YH, Waszynski CM, Arya VK, Pagel PS, Hudetz JA, Muench MR, Fritz BA, Waberski W, Inouye SK, Mashour GA, PODCAST Research Group. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390(10091):267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieber FE, Neufeld KJ, Gottschalk A, Bigelow GE, Oh ES, Rosenberg PB, Mears SC, Stewart KJ, Ouanes JP, Jaberi M, Hasenboehler EA, Li T, Wang NY. Effect of Depth of Sedation in Older Patients Undergoing Hip Fracture Repair on Postoperative Delirium: The STRIDE Randomized Clinical Trial. JAMA Surg 2018;153(11):987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wildes TS, Mickle AM, Ben Abdallah A, Maybrier HR, Oberhaus J, Budelier TP, Kronzer A, McKinnon SL, Park D, Torres BA, Graetz TJ, Emmert DA, Palanca BJ, Goswami S, Jordan K, Lin N, Fritz BA, Stevens TW, Jacobsohn E, Schmitt EM, Inouye SK, Stark S, Lenze EJ, Avidan MS, ENGAGES Research Group. Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. Jama 2019;321(5):473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radtke FM, Franck M, Schust S, Boehme L, Pascher A, Bail HJ, Seeling M, Luetz A, Wernecke KD, Heinz A, Spies CD. A comparison of three scores to screen for delirium on the surgical ward. World J Surg 2010;34(3):487–94. [DOI] [PubMed] [Google Scholar]
- 31.Neufeld KJ, Leoutsakos JS, Sieber FE, Joshi D, Wanamaker BL, Rios-Robles J, Needham DM. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth 2013;111(4):612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta DM, Boland RJ Jr, Aron DC. The physician’s experience of changing clinical practice: a struggle to unlearn. Implement Sci 2017;12(1):28–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards N, Saltman RB. Re-thinking barriers to organizational change in public hospitals. Isr J Health Policy Res 2017;6:8,8. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, Spurr C, Khorasani R, Tanasijevic M, Middleton B. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc 2003;10(6):523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westbrook JI, Coiera E, Dunsmuir WT, Brown BM, Kelk N, Paoloni R, Tran C. The impact of interruptions on clinical task completion. Qual Saf Health Care 2010;19(4):284–9. [DOI] [PubMed] [Google Scholar]
- 36.American Society of Anesthesiologists. Statement on Principles for Alarm Management for Anesthesia Professionals.; 2018. October 17,.
- 37.Nair BG, Newman SF, Peterson GN, Wu WY, Schwid HA. Feedback mechanisms including real-time electronic alerts to achieve near 100% timely prophylactic antibiotic administration in surgical cases. Anesth Analg 2010;111(5):1293–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


