Abstract
Animals engage in motivated behaviors, such as feeding and mating behaviors, to ensure their own survival and the survival of their species. However, the neural circuits mediating the generation and persistence of these motivational drives remain poorly understood. Here we review recent studies on the circuit mechanisms underlying motivational states in Drosophila, with a focus on feeding, courtship, and aggression. These studies shed light on the molecular and cellular mechanisms by which key drive neurons receive relevant input signals, integrate information, and decide on a specific behavioral output. We also discuss conceptual models for integrating these circuit mechanisms, distinguishing between those for homeostatically- vs non-homeostatically-regulated motivated behaviors. We suggest that the ability to trigger persistence of a motivated behavior may be a feature of integrator or apex/command neurons.
Introduction:
Animals engage in goal-directed behaviors for their own survival and for the propagation of their species. Motivation is considered the force that initiates and directs these behaviors, but how is this motivation generated? At a conceptual level, psychologists have proposed different theories to address this question, of which the “drive” and “instinct” theories are most relevant for innate behaviors [1]. The “drive theory” of motivation posits that maintenance of organismal homeostasis is the key factor underlying motivation [2,3]. For example, the need for nutrients generates hunger, which serves as the motivational drive for eating. However, not all innate behaviors are clearly homeostatically regulated; social interactions such as fighting and mating can be triggered by sensory cues, and are not classically considered to be under the control of homeostatic drive [4**]. Instead, as predicted by “instinct” theory, animals engage in inborn behavior which may promote fitness or reproductive success.
The fruit fly Drosophila melanogaster is a powerful system to study the neural circuits underlying motivational states. Flies exhibit a wide range of innate and learned behaviors and have a complex yet compact nervous system [5]. The genetic toolkit for this system is highly sophisticated, and numerous driver lines exist that readily allow manipulation of small subsets of neurons [6]. Moreover, large-scale electron microscopy projects are delineating the fly brain connectome [7]. In this review, we will discuss recent studies in Drosophila describing central neural circuits mediating motivated behaviors, with a focus on feeding, reproductive behaviors, and aggression. We will then attempt to synthesize these findings in a broader conceptual framework and discuss how persistence of motivated behaviors may be a characteristic feature of critical neural circuits involved in motivated behaviors.
Feeding and hunger circuits:
Feeding is an exemplar homeostatically-regulated behavior. A number of studies over the past several years has identified molecular and circuit mechanisms by which general and specific hungers are controlled. Neuropeptides are key neuromodulators that encode information about the external environment or internal state [8], and several neuropeptides have been shown to regulate feeding in fruit flies [9–20]. Min et al. found that either silencing myoinhibitory peptide (MIP)-expressing neurons or genetic knockout of mip resulted in obese flies and increased consumption of a yeast/sugar mix, suggesting that this circuit normally acts to promote satiety [17]. Martelli et al. studied 4 neurons expressing SIFamide [16] and found that thermogenetic activation of these neurons led to an increase in feeding and food-seeking behaviors. Interestingly, the SIFamide neurons receive input from other neuropeptides, including MIP and hugin-PK, which is considered a hunger-inducing signal. Activation of hugin-PK neurons increases intracellular Ca2+ in SIFamide neurons, while activation of MIP neurons does the opposite, suggesting that SIFamide neurons integrate satiety (MIP) and hunger (hugin-PK) signals to drive feeding behavior. Another set of neurons shown to encode hunger drive are “taotie” neurons, which are in the pars intercerebralis (PI), a region consisting of neurosecretory cells that function similarly to the mammalian hypothalamus [20]. Activation of these taotie neurons leads to increased food consumption, while silencing suppresses feeding in starved flies. Chronic activation results in obese flies, and interestingly, transient activation of these neurons leads to persistent hunger state. The taotie neurons likely promote feeding by inhibiting insulin-producing cells of the PI, given that insulin functions as a satiety factor in mammals [21].
Hunger is not a monolithic state, but rather can exist for specific nutrients, such as sugar or protein [22]. The Suh lab identified a subset of ellipsoid body (EB) ring neurons required for sugar hunger [23]. These neurons are labeled by a gene SLC5A11 (a.k.a., cupcake), which encodes a sodium/solute cotransporter-like protein required for taste-independent sugar preference. Increasing or decreasing SLC5A11-expressing neuron activity enhances or reduces sugar consumption, respectively. Unexpectedly, these neurons do not appear to directly flux glucose, but instead inhibit a specific K+ channel (dKCNQ), to increase excitability of these neurons following starvation [24*]. Animals must balance their food intake with water intake. Jourjine et al. identified 4 neurons (named ISNs for interoceptive subesophageal neurons) that regulate both sugar and water consumption [25**]. Strikingly, these neurons are activated by either starvation or decreased extracellular osmolality (suggestive of water abundance). They directly receive signals indicating these states; these neurons are stimulated by Adipokinetic hormone (AKH), the fly analog of glucagon, via the AKH receptor, as well as low extracellular osmolality, detected by Nanchung (an osmosensitive TRPV channel) in the ISNs. When activated, the ISNs promote sugar intake but suppress water consumption, to guide the animal’s choice between two distinct behaviors.
Recently, a neural circuit encoding protein hunger was identified [26**]. This circuit comprises a subset of dopaminergic (DA) neurons, named DA-WED neurons. These 4 neurons are both necessary and sufficient for protein hunger, and their activity is significantly increased with protein starvation. The DA-WED neurons have 2 distinct projections: one to FB-LAL neurons (which promote protein feeding) and another to PLP neurons (which promote sugar feeding). Following protein starvation, the DA-WED neurons simultaneously enhance the activity of FB-LAL neurons and suppress the activity of PLP neurons, thus triggering a behavioral switch from consuming sugar to protein. Remarkably, the DA-WED neurons drive persistent intake of protein, but only transient inhibition of sugar feeding, via branch-specific plasticity of the projection to the FB-LAL neurons.
Courtship and copulation circuits:
Although reproductive behavior is sometimes considered a pre-programmed “instinct”, it is clear that these behaviors are flexible and modulated by past experience and internal states. Among innate social behaviors, the circuit mechanisms mediating reproductive behavior in Drosophila are arguably among the best understood [4**,27]. Reproductive behavior is exhibited by both females and males, but here we review recent work on two aspects of male reproductive behavior: courtship and copulation.
Male flies undergo a stereotyped courtship ritual that includes following the female, tapping her, playing a courtship song with extended wing, licking genitalia, and finally mounting [28]. The discovery of the sex-determining transcription factor Fruitless enabled the discovery of a particularly important set of neurons for courtship, named P1 neurons [29–31] (Figure 1). These neurons comprise a cluster of ~20 male-specific Fruitless (FruM)-positive neurons per hemibrain that integrate multiple sensory inputs from females [32–42] and sexual experience [43]. Importantly, ectopic activation of these neurons can trigger courtship singing behavior [34,44,45]. Recent studies show that P1 neuronal activity is regulated by neuromodulatory signals such as dopamine [43,46*,47*] and neuropeptides (e.g., NPF [48], Dsk [49]). DA neurons in the anterior superior medial protocerebrum (SMPa) have been shown to encode mating drive [43]. These DA SMPa neurons promote courtship initiation by desensitizing P1 neuron inhibition derived from the GABAergic mAL neurons [46*]. Moreover, the persistence of courtship behavior is likely sustained by a recurrent excitation loop, involving neuropeptide F (NPF) and pCd neurons. The output of this recurrent excitation loop increases mating drive in the DA SMPa neurons, which then act on the P1 neurons [46*,47*]. Interestingly, this courtship circuit mechanism appears to be under homeostatic control, as copulation-reporting neurons (CRNs) in the abdominal ganglia suppresses the NPF signaling acting on the DA SMPa neurons, in order to reduce mating drive. Satiety resulting from mating is driven by CREB2-dependent transcription of specific K+ channels (TASK7) in the NPF-pCd recurrent loop, which leads to prolonged inhibition of loop dynamics and thus persistent satiety.
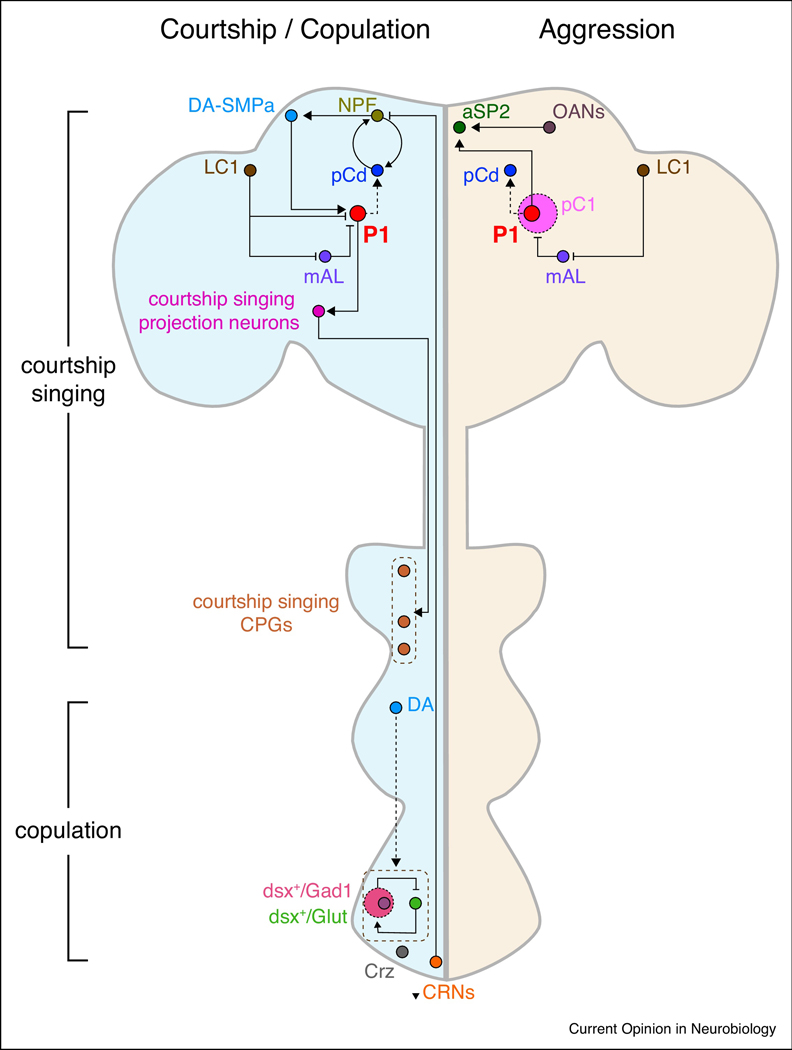
Figure 1:
Neural circuit pathways mediating mating and aggression behaviors in male Drosophila.
The schematic illustrates neurons regulating male courtship, aggression, and copulation discussed in this review. For clarity, sensory inputs to P1 neurons are not illustrated. Male courtship song is generated by a circuit consisting of fru+ neuronal clusters: P1 neurons, descending interneurons (P2b/plP10), and central pattern generators (“CPG”, dPR1/vPR6/vMS11). GABAergic LC1 neurons, via indirect or direct actions on P1 or pC1, shift behavior towards aggression. DA-SMPa neurons encode mating drive and desensitize P1 neurons to GABAergic inhibition from mAL neurons. A positive recurrent NPF-pCd circuit sustains motivation for courtship, which is inhibited by copulation reporting neurons (CRNs). An indirect action of P1 neurons on pCd neurons also promotes persistent courtship singing. For copulation, dsx+ glutamatergic motor neurons promote genital coupling, and this is suppressed by dsx+ GABAergic neurons. A small subset of these dsx+ GABAergic cells terminates copulation, while dopamine tone in the ventral nerve cord conversely maintains copulation. A cell-autonomous molecular timer (CaMKII) in corazonin (Crz)-releasing neurons also sustains copulation duration. Signals from P1 and octopaminergic (OA) neurons converge in aSP2 to promote aggression. Similar to courtship singing, an indirect action of P1 neurons on pCd neurons plays an important role for persistence of aggression. Data sources: aSP2 [56], CPGs [40], CRNs [47], Crz [52,53], DA [51], DA-SMPa [43,46,47], dsx+/Gad1 [50,51], dsx+/Glut [50], LC1 [55], mAL [55], NPF [47], OANs [56], P1 [34,43,45–47,54–56,58], P2b [34], pC1 [55], pCd [47,58], plP10 [40].
The ultimate goal of courtship behavior is copulation and successful mating. The act of copulation itself is composed of different components, including genital coupling, sperm transfer, and copulation duration [50]. A recent study used genetic intersectional approaches to identify neurons important for genital coupling (Figure 1). doublesex (dsx), like fru, is an important sex-determination gene. The authors labelled and manipulated subsets of dsx neurons and found that a glutamatergic subset (~80 neurons) in the abdominal ganglion is important for genital coupling [50]. In addition, a GABAergic subset (~150 neurons) in the abdominal ganglion likely inhibits the glutamatergic dsx neurons to terminate genital coupling.
Crickmore and Vosshall had previously shown that the duration of copulation is regulated by DA neurons in the VNC (which increase duration) and ~8 GABAergic neurons in the abdominal ganglion (which decrease duration) [51]. It is worth mentioning that the phenotypes seen by silencing of these 8 dsx+ GABAergic neurons vs the 150 dsx+ GABAergic neurons described above are distinct. The former reduces motivation to continue copulation, while the latter inhibits genital uncoupling, resulting in a “stuck” phenotype. It is possible that the 150 dsx+ GABAergic cell cluster comprises distinct sub-groups, including the 8 dsx+ GABAergic neurons. Thornquist et al. have further explored the molecular and cellular mechanisms underlying copulation duration [52*]. Although male flies will mate for >20 mins, they are particularly motivated in the first ~6 mins (the time it takes to transfer sperm) and will resist terminating copulation even when facing a threatening stimulus. Their data suggest that Ca2+/calmodulin-dependent protein kinase II (CaMKII) acts as a molecular timer for this 6 min period of high motivation, as the activity of CaMKII parallels this ~ 6 min timeframe. A small group of Fru+ corazonin (Crz)-expressing neurons was previously shown to be important for sperm transfer and terminating mating [53], and expression of a constitutively active CaMKII in these neurons leads to a profound increase in copulation duration.
Aggression Circuits:
When encountering a conspecific, male flies may engage in one of two mutually exclusive behaviors— courtship or aggression. Remarkably, growing evidence suggests that the neural circuits underlying courtship are also involved in aggression [45,54*, 55–56] (Figure 1). Hoopfer et al. found that optogenetic stimulation of Fru+ P1 neurons at 10–20 Hz promotes aggression, particularly after the offset of photostimulation, whereas high frequency stimulation (30–50 Hz) induces wing extension during photostimulation [54*]. Thermogenetic activation of a small subset of Fru+ P1 neurons triggered aggression alone, but not courtship behavior. Interestingly, the aggression induced by optogenetic activation of P1 neurons could persist for minutes. It remains unclear whether the same P1 neurons, or different subsets of P1 neurons, mediate aggression vs courtship behaviors.
The P1 neuron group (classically defined by FruM expression) is a subset of a larger neuron cluster called pC1 (defined by Dsx expression). Koganezawa et al. found that Fru−/Dsx+ pC1 neurons promotes aggression, while Fru+/Dsx+ pC1 neurons promotes courtship [55]. The decision of whether to court or fight is regulated by a two-level inhibitory network. Fru+ mAL GABAergic neurons inhibit both the Fru−/Dsx+ and Fru+/Dsx+ pC1 sub-clusters, while upstream Fru+ LC1 GABAergic neurons inhibit mAL neurons and also the Fru+/Dsx+ pC1 neurons. Thus, the net effect of the LC1 neurons is to promote aggression. Having this multi-layered regulatory network may allow for more inputs to modulate this behavioral choice. It remains to be determined how to reconcile the precise identities of the aggression-promoting neurons in pC1 identified by Koganezawa et al. vs Hoopfer et al., and whether aggression and courtship can be induced by the same or distinct subsets of P1 neurons. Although we have focused our discussion on male-male aggression, it is worth noting that female-female aggression has also been observed in Drosophila, and that a subset of pC1 neurons appears to be important for regulating this latter type of fighting [57].
Recent work has also delineated circuit mechanisms that act downstream of P1 neurons to regulate aggression. Watanabe et al. reported that Fru+ aSP2 neurons receive inputs from P1 neurons as well as neurons expressing octopamine (OA, the insect analog of norepinephrine) [56]. The octopaminergic input appears to potentiate P1 signaling onto aSP2 neurons to enhance fighting between male flies. It has been previously shown that activation of P1 neurons can lead to persistent aggressiveness [54*] and courtship singing [32,45]. How is this persistence generated? The Anderson lab recently determined that the activity of pCd neurons, an indirect downstream target of P1 neurons, is required for the perdurance of aggression and courtship behaviors generated by P1 neurons [58**]. However, pCd neurons are not sufficient for generating these behaviors. Interestingly, these pCd neurons are the same neurons previously implicated as participating in a recurrent excitation loop with NPF neurons for the persistence of courtship behavior [47*].
Concluding Remarks:
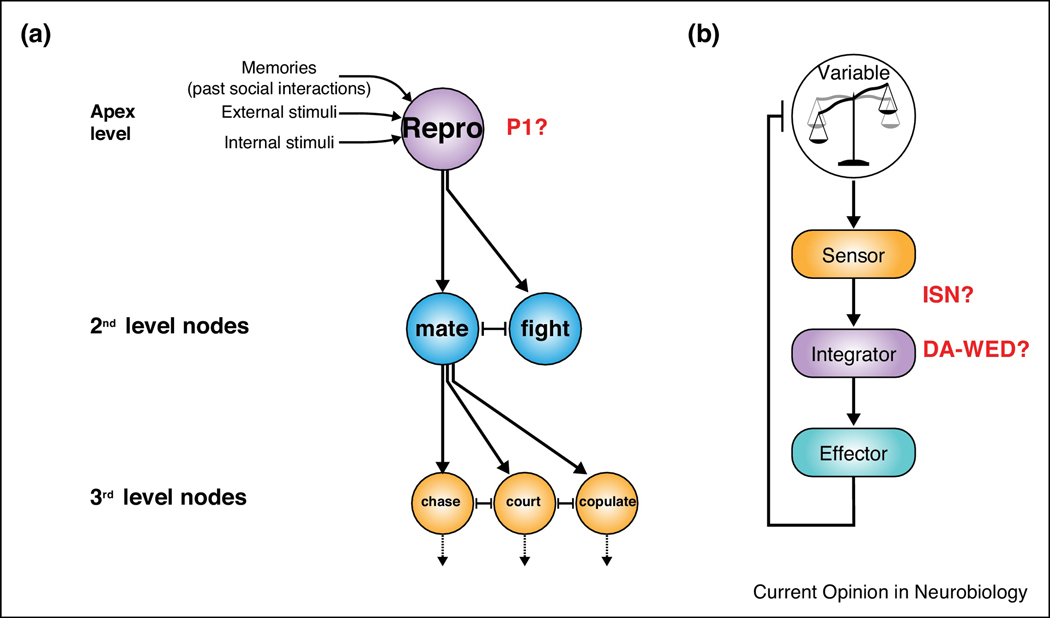
Motivation is broadly considered to have 3 key features: activation, intensity, and persistence [1]. The process of activation requires a decision to be made, which Tinbergen proposed occurs by integration of intrinsic and extrinsic inputs to an apex node of a hierarchical feedforward structure [59] (Figure 2a). This structure consists of circuit nodes that individually promote distinct behaviors and has been proposed to serve as a circuit scheme for motivated behaviors not traditionally considered to be under homeostatic control, such as courtship and aggression [60]. It is worth noting, however, that the distinction between “homeostatic” and “non-homeostatic” behaviors is not always clear-cut; for example, Zhang et al. find that mating drive is under homeostatic control [47]. The P1 neurons serve as a good example of an “apex” node in this model. These neurons receive multiple relevant sensory inputs [32–42], are sufficient to generate multiple downstream behaviors [34,44,45,54*], and likely choose between these different behaviors [40,54*,55]. Interestingly, the P1 neurons can also trigger persistent courtship and aggression [45,54*,58], and this persistence may be an important characteristic of drive neurons in general, as discussed below.
Figure 2:
Circuit schemes for feedforward and homeostatically-regulated motivated behaviors.
(a) Tinbergen’s hierarchical model for behavioral decisions, modified from [59]. The apex neurons (“Reproductive” program, e.g. P1 neurons) receive input from the environment, internal states, and prior social interactions. Based on this information, the apex neurons choose between different 2nd level downstream behavioral outputs, which tend to inhibit one another. These 2nd level nodes can further trigger specific aspects of the behavior. (b) The homeostatic controller system model, modified from [61]. The sensor detects the state variable and sends this information to the integrator component that computes the difference between the state variable and a setpoint. This difference generates drive to activate the effectors, which then provide feedback control on the state variable. The ISNs may act as sensorintegrators, and the DA-WED neurons may serve as an integrator component. Persistence of protein hunger is encoded by cell-autonomous plastic changes in the DA-WED cells.
For homeostatic motivated behaviors, such as feeding, the circuit scheme can be visualized as a simple homeostatic controller system (consisting of a “sensor”, an “integrator”, and an “effector”) [61] (Figure 2b). Sensors detect the state variable and send this information to an integrator that computes the difference between the state variable and an internal setpoint. This difference is the “need”, which results in “drive” in the integrator component, leading to activation of the effector. Integrator circuits are of particular interest, but may be difficult to empirically identify. We suggest two properties related to their computational nature that may help distinguish such circuits: the ability to 1) arbitrate between qualitatively different behavioral outcomes, and 2) to generate persistent behavior following transient activation. Using these criteria, the ISNs [25**] and the DA-WED neurons [26**], of the hunger circuits described above, are most likely to function as integrator circuits. The ISNs may serve as “sensor-integrators”, as they directly respond to two different types of inputs and then integrate this information to coordinate feeding vs drinking. DA-WED neurons also arbitrate between different behavioral outcomes and moreover can generate persistent protein feeding. The downstream FB-LAL and PLP neurons likely represent effector circuits for protein and sugar feeding, respectively.
A number of neural circuits described above are capable of generating persistent states [20,26**,46,47,51,52,54*,58]. Whether by cell-autonomous mechanisms [26**,52] or by recurrent excitation loops [46,47], the ability to generate persistence may point towards a critical role in motivational drive. Why would persistence be a characteristic of an integrator or apex circuit? Persistence of a behavior is crucial to satisfying a need, but needs to be flexible in response to changes in the environment or internal states [51], which implies a computational function. Thus, we suggest this feature, which is easily experimentally tested, may help identify “apex” or “integrator” neurons going forward and may be relevant for mammals as well. For example, agouti-related protein (AgRP) neurons, which are critical hunger neurons in mammals [62,63], fit criteria for “integrator” neurons, as they receive input from hunger-related signals [64,65], undergo plastic changes with increased hunger [66,67], and can drive persistent feeding behavior following brief optogenetic stimulation [68,69]. AgRP neurons project to and regulate multiple core feeding nodes [70–74], suggesting that these neurons represent “apex” or “integrator” neurons for hunger. Taken together, the studies above in Drosophila have substantially advanced our understanding of how motivational drive is generated and regulated, but many questions remain. The comprehensive networks underlying hierarchical or homeostatic circuit mechanisms are still unclear, but addressing this gap should be greatly facilitated by the recent release of the first hemi-brain connectome [7]. In addition, future studies exploiting the powerful genetic tools in Drosophila should reveal the precise molecular pathways by which “apex” or “integrator” neurons are able to consider input from internal states and the environment, make a decision on which behavior to promote, and maintain persistence of this behavior.
Highlights:
Motivated behaviors can be under homeostatic or non-homeostatic control
Related circuits can be modeled as homeostatic control or feedforward systems
Circuits that drive behavioral persistence may comprise integrator or apex neurons
Acknowledgements:
We thank the reviewers for helpful feedback and apologize to investigators in this field, whose work we were unable to cover due to space limitations. This work was supported by NIH grant R01NS100792 (M.N.W.)
Footnotes
Conflict of Interest Statement Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hockenbury DH, Hockenbury SE: Psychology, 5th ed New York, NY, US: Worth Publishers; 2010. [Google Scholar]
- 2.Cannon WB: The wisdom of the body. New York, NY, US: W W Norton & Co; 1932. [Google Scholar]
- 3.Hull CL: Principles of behavior: an introduction to behavior theory. Oxford, England: Appleton-Century; 1943. [Google Scholar]
- 4.Anderson DJ: Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci 2016, 17:692–704.**In this review article, the author argues persuasively that circuit mechanisms for 2 innate social behaviors (courtship and aggression) are shared and that this organization is an ancient evolutionarily conserved circuit motif.
- 5.Sokolowski MB: Drosophila: genetics meets behaviour. Nat Rev Genet 2001, 2:879–890. [DOI] [PubMed] [Google Scholar]
- 6.Caygill EE, Brand AH: The GAL4 System: A Versatile System for the Manipulation and Analysis of Gene Expression. Methods Mol Biol 2016, 1478:33–52. [DOI] [PubMed] [Google Scholar]
- 7.Xu CS, Januszewski M, Lu Z, Takemura S-y, Hayworth KJ, Huang G, Shinomiya K, Maitin-Shepard J, Ackerman D, Berg S, et al. : A connectome of the adult Drosophila central brain. bioRxiv 2020:2020.2001.2021.911859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nassel DR, Zandawala M: Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog Neurobiol 2019, 179:101607. [DOI] [PubMed] [Google Scholar]
- 9.Al-Anzi B, Armand E, Nagamei P, Olszewski M, Sapin V, Waters C, Zinn K, Wyman RJ, Benzer S: The leucokinin pathway and its neurons regulate meal size in Drosophila. Curr Biol 2010, 20:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beshel J, Dubnau J, Zhong Y: A leptin analog locally produced in the brain acts via a conserved neural circuit to modulate obesity-linked behaviors in Drosophila. Cell Metab 2017, 25:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung BY, Ro J, Hutter SA, Miller KM, Guduguntla LS, Kondo S, Pletcher SD: Drosophila neuropeptide F signaling independently regulates feeding and sleep-wake behavior. Cell Rep 2017, 19:2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hentze JL, Carlsson MA, Kondo S, Nassel DR, Rewitz KF: The neuropeptide allatostatin A regulates metabolism and feeding decisions in Drosophila. Sci Rep 2015, 5:11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hergarden AC, Tayler TD, Anderson DJ: Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci U S A 2012, 109:3967–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DH, Shin M, Jung SH, Kim YJ, Jones WD: A fat-derived metabolite regulates a peptidergic feeding circuit in Drosophila. PLoS Biol 2017, 15:e2000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KS, You KH, Choo JK, Han YM, Yu K: Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem 2004, 279:50781–50789. [DOI] [PubMed] [Google Scholar]
- 16.Martelli C, Pech U, Kobbenbring S, Pauls D, Bahl B, Sommer MV, Pooryasin A, Barth J, Arias CWP, Vassiliou C, et al. : SIFamide translates hunger signals into appetitive and feeding behavior in Drosophila. Cell Rep 2017, 20:464–478. [DOI] [PubMed] [Google Scholar]
- 17.Min S, Chae HS, Jang YH, Choi S, Lee S, Jeong YT, Jones WD, Moon SJ, Kim YJ, Chung J: Identification of a peptidergic pathway critical to satiety responses in Drosophila. Curr Biol 2016, 26:814–820. [DOI] [PubMed] [Google Scholar]
- 18.Ren GR, Hauser F, Rewitz KF, Kondo S, Engelbrecht AF, Didriksen AK, Schjott SR, Sembach FE, Li S, Sogaard KC, et al. : CCHamide-2 Is an orexigenic brain-gut peptide in Drosophila. PLoS One 2015, 10:e0133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Liu C, Bai X, Li X, Li J, Zhang Z, Zhang Y, Guo J, Li Y: Drosophila FIT is a protein-specific satiety hormone essential for feeding control. Nat Commun 2017, 8:14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan YP, Liu L, Zhu Y: Taotie neurons regulate appetite in Drosophila. Nat Commun 2016, 7:13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Q, Horvath TL: Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 2007, 30:367–398. [DOI] [PubMed] [Google Scholar]
- 22.Simpson SJ, Raubenheimer D: The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity: Princeton University Press; 2012. [Google Scholar]
- 23.Dus M, Ai M, Suh GS: Taste-independent nutrient selection is mediated by a brain-specific Na+ /solute co-transporter in Drosophila. Nat Neurosci 2013, 16:526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JY, Dus M, Kim S, Abu F, Kanai MI, Rudy B, Suh GSB: Drosophila SLC5A11 mediates hunger by regulating K(+) channel activity. Curr Biol 2016, 26:2550.*This paper, together with [23], describes a novel molecule, cupcake, that labels a set of hunger-encoding EB ring neurons. Cupcake is upregulated by starvation and inhibits a specific K+ channel to increase the activity of these neurons.
- 25.Jourjine N, Mullaney BC, Mann K, Scott K: Coupled sensing of hunger and thirst signals balances sugar and water consumption. Cell 2016, 166:855–866.**How hunger and thirst drives interact is poorly understood. This paper beautifully describes molecular and circuit mechanisms by which these signals are integrated to drive a behavioral output (increasing sugar consumption and decrease water consumption).
- 26.Liu Q, Tabuchi M, Liu S, Kodama L, Horiuchi W, Daniels J, Chiu L, Baldoni D, Wu MN: Branchspecific plasticity of a bifunctional dopamine circuit encodes protein hunger. Science 2017, 356:534539.**This paper characterizes a neural circuit that promotes protein feeding, while simultaneously restricting sugar consumption, and demonstrates that branch-specific plasticity of this circuit underlies the selective persistence of protein hunger. This circuit mechanism also serves as a good example of integrator neurons signaling to distinct effector neurons to generate behavior.
- 27.Ellendersen BE, von Philipsborn AC: Neuronal modulation of D. melanogaster sexual behaviour. Curr Opin Insect Sci 2017, 24:21–28. [DOI] [PubMed] [Google Scholar]
- 28.Greenspan RJ, Ferveur JF: Courtship in Drosophila. Annu Rev Genet 2000, 34:205–232. [DOI] [PubMed] [Google Scholar]
- 29.Demir E, Dickson BJ: fruitless splicing specifies male courtship behavior in Drosophila. Cell 2005, 121:785–794. [DOI] [PubMed] [Google Scholar]
- 30.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS: Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 2005, 436:395–400. [DOI] [PubMed] [Google Scholar]
- 31.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ: fruitless regulates aggression and dominance in Drosophila. Nat Neurosci 2006, 9:1469–1471. [DOI] [PubMed] [Google Scholar]
- 32.Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V: Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 2015, 87:1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallman BR, Kim H, Scott K: Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. Elife 2015, 4:e11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohatsu S, Koganezawa M, Yamamoto D: Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 2011, 69:498–508. [DOI] [PubMed] [Google Scholar]
- 35.Kohatsu S, Yamamoto D: Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nat Commun 2015, 6:6457. [DOI] [PubMed] [Google Scholar]
- 36.Kohl J, Ostrovsky AD, Frechter S, Jefferis GS: A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell 2013, 155:1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan Y, Meissner GW, Baker BS: Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A 2012, 109:10065–10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R: A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 2010, 468:686–690. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan AG, Zhou C, Manoli DS, Baker BS: Neural pathways for the detection and discrimination of conspecific song in D. melanogaster. Curr Biol 2014, 24:1039–1049. [DOI] [PubMed] [Google Scholar]
- 40.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ: Neuronal control of Drosophila courtship song. Neuron 2011, 69:509–522. [DOI] [PubMed] [Google Scholar]
- 41.Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ: Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol 2010, 20:1602–1614. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C, Franconville R, Vaughan AG, Robinett CC, Jayaraman V, Baker BS: Central neural circuitry mediating courtship song perception in male Drosophila. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang SX, Rogulja D, Crickmore MA: Dopaminergic circuitry underlying mating drive. Neuron 2016, 91:168–181. [DOI] [PubMed] [Google Scholar]
- 44.Bath DE, Stowers JR, Hormann D, Poehlmann A, Dickson BJ, Straw AD: FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat Methods 2014, 11:756–762. [DOI] [PubMed] [Google Scholar]
- 45.Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson DJ: Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods 2014, 11:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang SX, Miner LE, Boutros CL, Rogulja D, Crickmore MA: Motivation, perception, and chance converge to make a binary decision. Neuron 2018, 99:376–388 e376.*This study demonstrates that dopamine tone in the male brain promotes the initiation of courtship, and sustains high motivation through potentiation of a P1 recurrent excitation loop.
- 47.Zhang SX, Rogulja D, Crickmore MA: Recurrent circuitry sustains Drosophila courtship drive while priming itself for satiety. Curr Biol 2019, 29:3216–3228 e3219.*The mechanisms underlying motivational persistence are largely unknown. The authors identify a recurrent excitation loop for persistent courtship drive in male Drosophila. The mating-dependent satiety state is maintained in this loop circuitry through CREB2-dependent expression of specific K+ channels.
- 48.Liu W, Ganguly A, Huang J, Wang Y, Ni JD, Gurav AS, Aguilar MA, Montell C: Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu S, Guo C, Zhao H, Sun M, Chen J, Han C, Peng Q, Qiao H, Peng P, Liu Y, et al. : Drosulfakinin signaling in fruitless circuitry antagonizes P1 neurons to regulate sexual arousal in Drosophila. Nat Commun 2019, 10:4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavlou HJ, Lin AC, Neville MC, Nojima T, Diao F, Chen BE, White BH, Goodwin SF: Neural circuitry coordinating male copulation. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crickmore MA, Vosshall LB: Opposing dopaminergic and GABAergic neurons control the duration and persistence of copulation in Drosophila. Cell 2013, 155:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thornquist SC, Langer K, Zhang SX, Rogulja D, Crickmore MA: CaMKII measures the passage of time to coordinate behavior and motivational state. Neuron 2020, 105:334–345 e339.*This study presents an elegant molecular mechanism for an interval timer (slow decay of CaMKII activity) that determines persistence of copulation.
- 53.Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ: A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc Natl Acad Sci U S A 2012, 109:20697–20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ: P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. Elife 2015, 4.*The authors present data suggesting that a subset of P1 interneurons can promote either courtship or aggression, depending on the stimulation paradigm. In addition, activation of these neurons can trigger a persistent aggressive state.
- 55.Koganezawa M, Kimura K, Yamamoto D: The neural circuitry that functions as a switch for courtship versus aggression in Drosophila males. Curr Biol 2016, 26:1395–1403. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe K, Chiu H, Pfeiffer BD, Wong AM, Hoopfer ED, Rubin GM, Anderson DJ: A circuit node that integrates convergent input from neuromodulatory and social behavior-promoting neurons to control aggression in Drosophila. Neuron 2017, 95:1112–1128 e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palavicino-Maggio CB, Chan YB, McKellar C, Kravitz EA: A small number of cholinergic neurons mediate hyperaggression in female Drosophila. Proc Natl Acad Sci U S A 2019, 116:17029–17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung Y, Kennedy A, Chiu H, Mohammad F, Claridge-Chang A, Anderson DJ: Neurons that function within an integrator to promote a persistent behavioral state in Drosophila. Neuron 2020, 105:322–333 e325.**The authors identify an indirect downstream target of P1 neurons, pCD neurons, that promotes persistence of courtship or aggression, depending on sensory cues. Notably, the pCd activity does not initiate these behaviors, but rather prolongs them following their initiation by P1 neurons.
- 59.Tinbergen N: The Study of Instinct; 1951. [Google Scholar]
- 60.Anderson DJ, Adolphs R: A framework for studying emotions across species. Cell 2014, 157:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berridge KC: Motivation concepts in behavioral neuroscience. Physiol Behav 2004, 81:179–209. [DOI] [PubMed] [Google Scholar]
- 62.Aponte Y, Atasoy D, Sternson SM: AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 2011, 14:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB: Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 2011, 121:1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. : An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 2014, 507:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Atasoy D, Su HH, Sternson SM: Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 2011, 146:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong D, Dagon Y, Campbell JN, Guo Y, Yang Z, Yi X, Aryal P, Wellenstein K, Kahn BB, Sabatini BL, et al. : A Postsynaptic AMPK-->p21-Activated Kinase Pathway Drives Fasting-Induced Synaptic Plasticity in AgRP Neurons. Neuron 2016, 91:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB: Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 2012, 73:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y, Essner RA, Kosar S, Miller OH, Lin YC, Mesgarzadeh S, Knight ZA: Sustained NPY signaling enables AgRP neurons to drive feeding. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Lin YC, Zimmerman CA, Essner RA, Knight ZA: Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atasoy D, Betley JN, Su HH, Sternson SM: Deconstruction of a neural circuit for hunger. Nature 2012, 488:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Betley JN, Cao ZF, Ritola KD, Sternson SM: Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 2013, 155:1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, MandelblatCerf Y, Livneh Y, et al. : A rapidly acting glutamatergic ARC-->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat Neurosci 2017, 20:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. : A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 2015, 18:863871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. : Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 2017, 546:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]