Abstract
Background & Aims:
Non-alcoholic fatty liver disease confers increased risk for cardiovascular disease, including heart failure, for reasons that remain unclear. Possible pathways could involve an association of liver fat with cardiac structural or functional abnormalities even after accounting for body size.
Methods:
We analyzed N=2,356 Framingham Heart Study participants (age 52±12 years, 52% women) who underwent echocardiography and standardized computed tomography (CT) measures of liver fat.
Results:
In cross-sectional multivariable regression models adjusted for age, sex, cohort, and cardiovascular risk factors, liver fat was positively associated with left ventricular (LV) mass (β=1.45; 95% confidence interval (CI): 0.01,2.88), LV wall thickness (β=0.01; 95% CI: 0.00,0.02), mass volume ratio (β=0.02; 95% CI 0.01,0.03), mitral peak velocity (E) (β=0.83; 95% CI 0.31,1.36), and LV filling pressure (E/e’ ratio) (β=0.16; 95% CI 0.09,0.23); and inversely associated with global systolic longitudinal strain (β=0.20, 95% CI 0.07,0.33), diastolic annular velocity (e’) (β=−0.12; 95% CI −0.22,−0.03), and E/A ratio (β=−0.01; 95% CI-0.02,−0.00). After additional adjustment for body mass index (BMI), statistical significance was attenuated for all associations except for that of greater liver fat with increased LV filling pressure, a possible precursor to heart failure (β=0.11; 95% CI 0.03,0.18).
Conclusion:
Increased liver fat was associated with multiple subclinical cardiac dysfunction measures, with most of associations mediated by obesity. Interestingly, the association of liver fat and LV filling pressure was only partially mediated by BMI, suggesting a possible direct effect of liver fat on LV filling pressure. Further confirmatory studies are needed.
Keywords: Heart failure, Subclinical cardiovascular disease, Non-alcoholic fatty liver disease
LAY SUMMARY
It is unclear if increased liver fat is associated with subclinical cardiovascular disease after accounting for obesity. In our study, increased liver fat was linked with multiple echocardiographic markers of subclinical cardiac dysfunction, with most of associations mediated by obesity. The association of liver fat and LV filling pressure was only partially mediated by BMI, suggesting that increased liver fat may directly affect LV filling pressure, a clinical precursor to heart failure.
Non-alcoholic fatty liver disease (NAFLD) and cardiovascular diseases, particularly heart failure (HF), are obesity-related conditions1,2 that have increased in prevalence along with the obesity epidemic over the last two decades3–5. Those with NAFLD can progress to develop end-stage liver disease, though cardiovascular disease (CVD) remains the leading cause of morbidity6 and mortality7. Growing literature suggests that individuals with NAFLD manifest myocardial functional and structural changes, leading to cardiac remodeling and increased risk of HF8–13. NAFLD and HF share many common risk factors, including diabetes mellitus, obesity, hypertension, and the metabolic syndrome14–20. Mechanisms that link NAFLD to HF, beyond shared risk factors, have largely been unexplored. In NAFLD, circulating free fatty acids and triglycerides accumulate in liver and myocardial cells with resultant increased myocardial fatty acid oxidation. Fatty acid oxidation is less efficient than glucose metabolism, and this metabolic inefficiency may contribute to the development of HF21. Additionally, inflammation and oxidative stress induced by NAFLD may contribute to cardiac insulin resistance22 and cardiac fibrosis23, leading to altered cardiac structure and HF. However, obesity may contribute to HF via similar mechanisms.
Although multiple studies have observed associations with NAFLD and left ventricular (LV) dysfunction12,24–41, right ventricular (RV) dysfunction42,43 and LV hypertrophy44, some results are conflicting45,46, most include small sample sizes or narrow populations, or did not adequately account for potential confounding variables. Additionally, prior studies have not explored the possible mediation effect of general adiposity, as measured by body mass index (BMI), on the association between NAFLD and subclinical CVD.
Thus, we investigated the association between NAFLD and subclinical CVD as defined by abnormal cardiac structure and function via standard and speckle-tracked-based echocardiography in the Framingham Heart Study (FHS). We hypothesized that NAFLD is associated with subclinical CVD, even after accounting for shared risk factors, and that the association is at least partially mediated by adiposity.
Methods
The study sample was derived from the FHS Third Generation and Offspring Cohort participants, as described previously47,48. A subset of Third Generation and Offspring participants underwent multi-detector computed tomography (CT) that assessed liver fat and visceral adipose tissue (VAT) between 2002 and 200549. Third Generation participants at exam 1 (2002–2005) and Offspring cohort participants at exam 8 (2005–2008) completed a standardized medical history, laboratory evaluation and echocardiography assessment with digital image acquisition and speckle-tracking analyses. We excluded participants with excessive alcohol use (>7 drinks per week for women and >14 drinks per week for men) (n=502), missing liver fat or covariates (n=254), missing echocardiogram (n=5), and overt CVD (prevalent history of angina, acute coronary syndrome, coronary artery disease, or HF) (n=180). Informed consent was provided by all participants prior to attending the examination cycle. This study was approved by the Institutional Review Board of Boston University Medical Center and the Massachusetts General Hospital.
The CT protocol has been described previously to measure liver fat and VAT49–51. In this protocol, 25 contiguous slices of 5 mm thickness were obtained, and a phantom calibration control (Image Analysis, Lexington, KY, US) was placed under each participant. The average fat attenuation in Hounsfield Units (HU) was measured in three areas of the liver and divided by the calibration phantom (HU) to derive the liver phantom ratio (LPR). With increasing liver fat, the LPR decreases. We defined hepatic steatosis using a cut off of an LPR ≤0.33, as in prior studies50,51. The LPR was utilized as the indexed standard as the spleen was not visualized on all scans. A liver spleen ratio of 1.1 corresponds to 30% hepatic steatosis as described previously50,51.
To measure VAT volume, we identified pixels containing fat using an image display window of −195 to −45 HU and a window center of −120 HU on a dedicated workstation (Aquarius 3D Workstation, TeraRecon Inc, San Mateo, California), as described49,51. We manually traced the separation between the visceral and subcutaneous fat compartments with high intra-reader and inter-reader correlation coefficients of 0.9949.
All measurements were attained during a standardized medical history and examination session for participants in exam 1 (Third Generation) or exam 8 (Offspring). Participants were considered current smokers if they smoked at least 1 cigarette daily during the previous year. Alcohol use was recorded as drinks/week or drinks/month as reported on a clinician-administered questionnaire. Plasma glucose, triglycerides, high density lipoprotein (HDL) cholesterol and total cholesterol were measured on fasting morning samples. Diabetes was defined as fasting plasma glucose ≥126 mg/dL or treatment with insulin or hypoglycemic agent. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥ 90 mm Hg or the use of anti-hypertensive medications. BMI was calculated by dividing the weight in kilograms (kg) by height in meters squared (m2).
All participants in the study underwent routine M-mode, 2-dimensional (2D) and pulse-wave Doppler echocardiography using a Hewlett-Packard 5500 machine (Philips Healthcare, Andover, MA)52. The mean time between CT scan and echocardiogram was 9 months. All echocardiograms were evaluated by an experienced sonographer or cardiologist who was blinded to the participant’s demographic and clinical characteristics using a standardized protocol. Cardiac structure measures included: LV end-diastolic diameter and volume, LV end-systolic diameter and volume, LV wall mass and thickness, relative wall thickness (LV wall thickness/LV end-diastole diameter) and LV mass-volume ratio (LV mass/LV end-diastole volume). We calculated LV mass according to a previously validated formula53. We used previously validated formulas to calculate measures of cardiac function, including: Mitral peak (E) velocity (m/s), diastolic annular (e’) velocity (cm/s), LV filling pressure (E/e’ ratio), and the ratio of early (E) to late (A) ventricular filling velocities (E/A ratio). We used the Du Bois formula to estimate body surface area in order to calculate the LV mass index, the LV end diastolic volume index and LV end systolic volume index. We also calculated hemodynamic measures including volume-derived ejection fraction (%) and fractional shortening (%)54.
A speckle-tracking software package (2D Cardiac Performance Analysis, TomTec Imaging Systems, Unterschleissheim, Germany) was used to perform previously validated speckle-tracking-based analyses of LV myocardial deformation55. Global systolic longitudinal strain is a measure of LV mechanical function, as previously described56.
Descriptive analyses were performed to compare the baseline demographic, metabolic, and echocardiographic characteristics between participants with and without hepatic steatosis. We present descriptive summaries as means ± standard deviations for continuous variables and as proportions for categorical variables. Age-, cohort-, and sex-adjusted partial Pearson correlations were calculated to assess the correlation between LPR and multiple echocardiographic measures of cardiac structure and function. We performed multivariable linear regression analyses to examine the associations between liver fat (as a continuous or dichotomous measure) and echocardiographic measures of cardiac structure and function. Covariates in the multivariable models were selected a priori based on prior studies suggesting an association with the exposure and outcome. The initial model (base model) included adjustment for age, sex, cohort, smoking status, and alcohol intake. Additional multivariable models included additional adjustment for HF risk factors: diabetes, systolic blood pressure, anti-hypertensive medication use, lipid lowering therapy use, total cholesterol, HDL cholesterol, triglycerides, and fasting glucose. Separate models further adjusted for VAT or BMI, respectively. We evaluated for effect modification by sex. For echocardiographic measures that were associated with liver fat, we performed mediation analyses to explore BMI as a possible mediator. SAS version 9.3 was used to perform the primary analyses and R version 3.5.3 with the ‘mediation’ package was used to perform the mediation analyses57. A 2-tailed p-value <0.05 was considered statistically significant. No adjustments were made for multiple testing, since our analyses were hypothesis generating.
Results
The baseline demographic and metabolic characteristics of the 2,356 participants (mean age 52±12 years, 52% women) by hepatic steatosis presence are presented in Table 1. The prevalence of hepatic steatosis in the study sample was 16.3%. Participants with hepatic steatosis had a higher proportion of men, hypertension, diabetes, higher fasting glucose level, higher triglycerides, lower high density lipoprotein levels (HDL), and higher lipid-lowering medication use as compared to those without hepatic steatosis. Cardiac structural and functional characteristics by hepatic steatosis status are summarized in Table 2. Compared to those without hepatic steatosis, participants with hepatic steatosis had, on average, a greater LV wall mass index, greater LV wall thickness, higher regional wall thickness, and a higher mass volume ratio. Those with hepatic steatosis also had, on average, lower diastolic annular velocity (e’), higher LV filling pressure (E/e’ ratio) and worse global systolic longitudinal strain.
Table 1.
Demographic and Metabolic Characteristics of FHS Study Sample by Presence of Hepatic Steatosis
| Hepatic steatosis N=384 |
No hepatic steatosis N=1972 |
Overall N=2356 |
|
|---|---|---|---|
| Age (years) | 53 ± 12 | 52 ± 12 | 52 ± 12 |
| Women n, (%) | 177 (46.1%) | 1046 (53.0%) | 1223 (51.9%) |
| Smoking n, (%) | 42 (10.9%) | 211 (10.7%) | 253 (10.7%) |
| Drinks per Week | 3.1 ± 3.9 | 3.0 ± 3.4 | 3.0 ± 3.5 |
| Systolic Blood Pressure (mm Hg) | 127 ± 15 | 120 ± 15 | 121 ± 16 |
| Diastolic Blood Pressure (mm Hg) | 78 ± 10 | 75 ± 9 | 75 ± 9 |
| Hypertension n, (%) | 187 (48.7%) | 525 (26.6%) | 712 (30.2%) |
| Use of anti-hypertensive meds n, (%) | 136 (35.4%) | 373 (18.9%) | 509 (21.6%) |
| Use of lipid lowering meds n, (%) | 97 (25.3%) | 352 (17.8%) | 449 (19.1%) |
| Total Cholesterol (mg/dl) | 193 ± 37 | 191 ± 34 | 191 ± 35 |
| HDL Cholesterol (mg/dl) | 47 ± 14 | 56 ± 16 | 54 ± 16 |
| Triglycerides (mg/dl) | 168 ± 112 | 108 ± 62 | 117 ± 76 |
| Fasting Glucose (mg/dl) | 109 ± 29 | 98 ± 19 | 100 ± 21 |
| Diabetes n, (%) | 62 (16.1%) | 85 (4.3%) | 147 (6.2%) |
| BMI (kg/m2) | 31.6 ± 6.0 | 26.8 ± 4.9 | 27.6 ± 5.4 |
| Estimated body surface area (m2) | 2.0 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 |
| VAT volume (cm3) | 2540 ± 1008 | 1523 ± 885 | 1689 ± 981 |
HDL, high density lipoprotein; BMI, body mass index; VAT, visceral adiposity tissue
Hepatic steatosis was defined as a liver phantom ratio ≤ 0.33 on computed tomography examination
Continuous variables expressed as mean (sd), categorical variables as n, (%)
Table 2.
Echocardiographic Characteristics of FHS Study Sample by Presence of Hepatic Steatosis
| Hepatic steatosis N=384 |
No hepatic steatosis N=1972 |
Overall N=2356 |
|
|---|---|---|---|
| Cardiac Structure | |||
| LV wall mass index (g/m2) | 87 ± 17 | 85 ± 18 | 85 ± 17 |
| LV wall thickness (cm) | 2.0 ± 0.3 | 1.8 ± 0.3 | 1.9 ± 0.3 |
| LV end-diastole diameter (cm) | 4.9 ± 0.4 | 4.9 ± 0.4 | 4.9 ± 0.4 |
| LV end-systole diameter (cm) | 3.1 ± 0.4 | 3.1 ± 0.4 | 3.1 ± 0.4 |
| LV end-diastolic volume index (mL/m2) | 58 ± 10 | 61 ± 10 | 60 ± 10 |
| LV end-systolic volume index (mL/m2) | 40 ± 12 | 39 ± 11 | 39 ± 11 |
| Regional wall thickness | 0.62 (0.56–0.69) | 0.58 (0.53–0.64) | 0.58 (0.54–0.65) |
| Mass volume ratio | 1.50 (1.33–1.65) | 1.37 (1.23–1.53) | 1.38 (1.25–1.55) |
| LV Systolic Function | |||
| Global systolic longitudinal strain (%) | −19 ± 3 | −20 ± 3 | −20 ± 3 |
| Ejection Fraction (%) | 66 ± 5 | 66 ± 6 | 66 ± 6 |
| Fractional shortening (%) | 37 ± 4 | 37 ± 4 | 37 ± 4 |
| LV Diastolic Function | |||
| Mitral peak velocity (E) (cm/s) | 67 ± 13 | 66 ± 12 | 67 ± 13 |
| Diastolic annular velocity (e’) (cm/s) | 10 ± 2 | 11 ± 3 | 11 ± 3 |
| LV filling pressure (E/e’) | 7.0 (6–8) | 6.0 (5–7) | 6.0 (5–7) |
| Early-to-late ventricular filling velocity (E/A ratio) | 1.06 (0.88–1.22) | 1.16 (0.93–1.41) | 1/14 (0.91–1.38) |
LV, left ventricular
Continuous variables expressed as mean ± sd
Hepatic steatosis was defined as a liver phantom ratio ≤ 0.33 on computed tomography examination
We observed significant correlations between LPR and a majority of the echocardiographic measures of cardiac function and structure as summarized in Table 3. Results were generally consistent with the trends noted above. Increasing liver fat was weakly negatively correlated with a lower E/A ratio and reduced diastolic annular velocity (e’ velocity). We observed weak, but statistically significant, positive correlations between liver fat and a higher mitral peak velocity (E), LV wall thickness, LV end-diastolic volume index, LV end-systolic volume index, mass volume ratio, regional wall thickness, global systolic longitudinal strain and LV filling pressure (E/e’ ratio).
Table 3.
Age-, Sex-, Cohort-Adjusted Pearson’s Correlations Between Liver Phantom Ratio and Markers of Subclinical Myocardial Dysfunction
| More Liver Fat | P-value | |
|---|---|---|
| Cardiac Structure | ||
| LV wall mass index | −0.01 | 0.66 |
| LV wall thickness | 0.16 | <0.0001 |
| LV diameter at end-diastole | 0.00 | 0.85 |
| LV diameter at end-systole | 0.01 | 0.74 |
| LV end-diastolic volume index | 0.13 | <0.001 |
| LV end-systolic volume index | 0.08 | <0.001 |
| Regional wall thickness | 0.11 | <0.0001 |
| Mass volume ratio | 0.16 | <0.0001 |
| LV Systolic Function | ||
| Global systolic longitudinal strain | 0.13 | <0.0001 |
| Fractional shortening | −0.01 | 0.77 |
| Ejection fraction | 0.00 | 0.83 |
| LV Diastolic Function | ||
| Mitral peak velocity (E) | 0.07 | <0.01 |
| Diastolic annular velocity (e’) | −0.12 | <0.0001 |
| LV filling pressure (E/e’) | 0.15 | <0.0001 |
| E/A ratio | −0.10 | <0.0001 |
LV, left ventricular; LPR, liver phantom ratio
For each additional standard deviation of liver fat (lower LPR), we observed increases in mean LV wall mass (β=1.45; 95% confidence interval (CI) 0.01,2.88), LV wall thickness (β=0.01; 95% CI 0.00,0.02), and mass volume ratio (β=0.02; 95% CI 0.01,0.03) in multivariable models adjusted for demographic and HF risk factors (Table 4). After additionally adjusting for BMI or VAT, mean end-diastolic volume and diameter were negatively associated with liver fat, though the association with LV wall mass, LV wall thickness, and the mass volume ratio were no longer statistically significant (Table 4).
Table 4.
Multivariable-Adjusted Linear Regression Analysis for the Association of Liver Fat with Markers of Subclinical Myocardial Dysfunction
| Outcome | Model 1: Base Model* | Model 2: Base + HF Risk Factors** | Model 3: Model 2 + BMI | Model 4: Model 2 + VAT | ||||
|---|---|---|---|---|---|---|---|---|
| β (CI) | P value | β (CI) | P value | β (CI) | P value | β (CI) | P value | |
| Cardiac Structure | ||||||||
| LV wall mass | 4.17 (2.77,5.56) | <0.001 | 1.45 (0.01,2.88) | 0.048 | −1.19 (−2.57,0.19) | 0.09 | −1.36 (−2.82,0.11) | 0.07 |
| LV wall thickness | 0.03 (0.03,0.04) | <0.001 | 0.01 (0.00,0.02) | 0.003 | −0.00 (−0.01,0.01) | 0.89 | −0.00 (−0.01,0.01) | 0.67 |
| LV at end-diastole diameter | 0.00 (−0.01,0.02) | 0.86 | −0.00 (−0.02,0.01) | 0.58 | −0.02 (−0.04,0.01) | 0.003 | −0.02 (−0.04,0.01) | 0.005 |
| LV at end-systole diameter | 0.00 (−0.01,0.02) | 0.73 | −0.00 (−0.01,0.02) | 0.85 | −0.01 (−0.02,0.00) | 0.16 | −0.01 (−0.03,0.00) | 0.09 |
| LV end diastolic volume | 0.07 (−0.75,0.89) | 0.87 | −0.26 (−1.13,0.61) | 0.56 | −1.30 (−2.17,0.44) | 0.003 | −1.31 (−2.21,0.40) | 0.005 |
| LV end systolic volume | 0.05 (−0.36,0.47) | 0.80 | 0.02 (−0.41,0.46) | 0.92 | −0.33 (−0.77,0.12) | 0.15 | −0.39 (−0.85,0.06) | 0.09 |
| Regional wall thickness | 0.01 (0.01,0.02) | <0.001 | 0.00 (−0.00,0.01) | 0.06 | 0.00 (−0.00,0.01) | 0.40 | 0.00 (−0.00,0.01) | 0.32 |
| Mass volume ratio | 0.04 (0.03,0.05) | <0.001 | 0.02 (0.01,0.03) | 0.001 | 0.01 (−0.00,0.02) | 0.23 | 0.01 (−0.00,0.02) | 0.29 |
| LV Systolic Function | ||||||||
| Global systolic longitudinal strain | 0.39 (0.27,0.51) | <0.001 | 0.20 (0.07,0.33) | 0.002 | 0.13 (−0.00,0.26) | 0.05 | 0.09 (−0.04,0.23) | 0.17 |
| Ejection fraction | −0.03 (−0.20,0.14) | 0.75 | −0.09 (−0.27,0.10) | 0.36 | −0.10 (−0.28,0.09) | 0.30 | −0.05 (−0.24,0.14) | 0.62 |
| Fractional shortening | 0.03 (−0.20,0.14) | 0.75 | −0.09 (0.27,0.10) | 0.36 | −0.10 (−0.28,0.09) | 0.29 | −0.05 (−0.24,0.14) | 0.62 |
| LV Diastolic Function | ||||||||
| Mitral peak velocity (E) | 0.81 (0.32,1.31) | 0.001 | 0.83 (0.31, 1.36) | 0.002 | 0.60 (0.07,1.13) | 0.03 | 0.75 (0.20,1.30) | 0.008 |
| Diastolic annular velocity (e’) | −0.27 (−0.36,−0.18) | <0.001 | −0.12(−0.22,−0.03) | 0.009 | −0.07 (−0.16,0.03) | 0.17 | −0.00 (−0.10,0.09) | 0.93 |
| LV filling pressure (E/e’) | 0.26 (0.19,0.33) | <0.001 | 0.16 (0.09,0.23) | <0.001 | 0.11 (0.03,0.18) | 0.004 | 0.08 (0.01,0.16) | 0.03 |
| E/A ratio | −0.03 (−0.04,−0.02) | <0.001 | −0.01 (−0.02,−0.00) | 0.035 | −0.00 (−0.02,0.01) | 0.46 | 0.00 (−0.01,0.01) | 0.79 |
LV, left ventricular; HF, heart failure; BMI, body mass index
MV model adjusts for age, sex, cohort, smoking, alcohol use; β expressed per SD increase in liver fat
Heart failure (HF) risk factors: diabetes, systolic blood pressure, antihypertensive med use, lipid lowering med use, total cholesterol, HDL, triglycerides, and fasting glucose
With regard to cardiac function, we observed a negative association between liver fat and global systolic longitudinal strain (β=0.20; 95% CI 0.07,0.33) in multivariable adjusted models. Increases in mean liver fat was associated with greater mean mitral peak (E) velocity (β=0.83; 95% CI 0.31,1.36), lower diastolic annular velocity (e’ velocity) (β=−0.12; 95% CI −0.22,−0.03), decreased mean E/A ratio (β=−0.01; 95% CI-0.02,−0.00), and increased mean (E/e’ ratio) (β=0.16; 95% CI 0.09,0.23). After additional adjustment for BMI or VAT, most associations between liver fat and measures of cardiac dysfunction were no longer significant. However, liver fat remained positively associated with mitral peak velocity and LV filling pressure in the adjusted BMI model.
Results were generally similar for models where liver fat was defined dichotomously based on LPR ≤ 0.33 (Supplemental Table 1) Results presented in terms of standardizing ed beta coefficients are presented in Supplemental Table 2.
Results of the mediation analyses demonstrate that BMI was a significant mediator in the associations between liver fat and subclinical myocardial dysfunction (Figure 1). BMI fully mediated the associations between liver fat and the myocardial structural measures of LV wall mass, LV wall thickness, and mass volume ratio and the myocardial functional measures of diastolic annular velocity and E/A ratio. BMI partially mediated the relationships between liver fat and mitral peak velocity (percent mediation 28%), global systolic longitudinal strain (percent mediation 36%) and LV filling pressure (percent mediation 33%), respectively.
Figure 1.
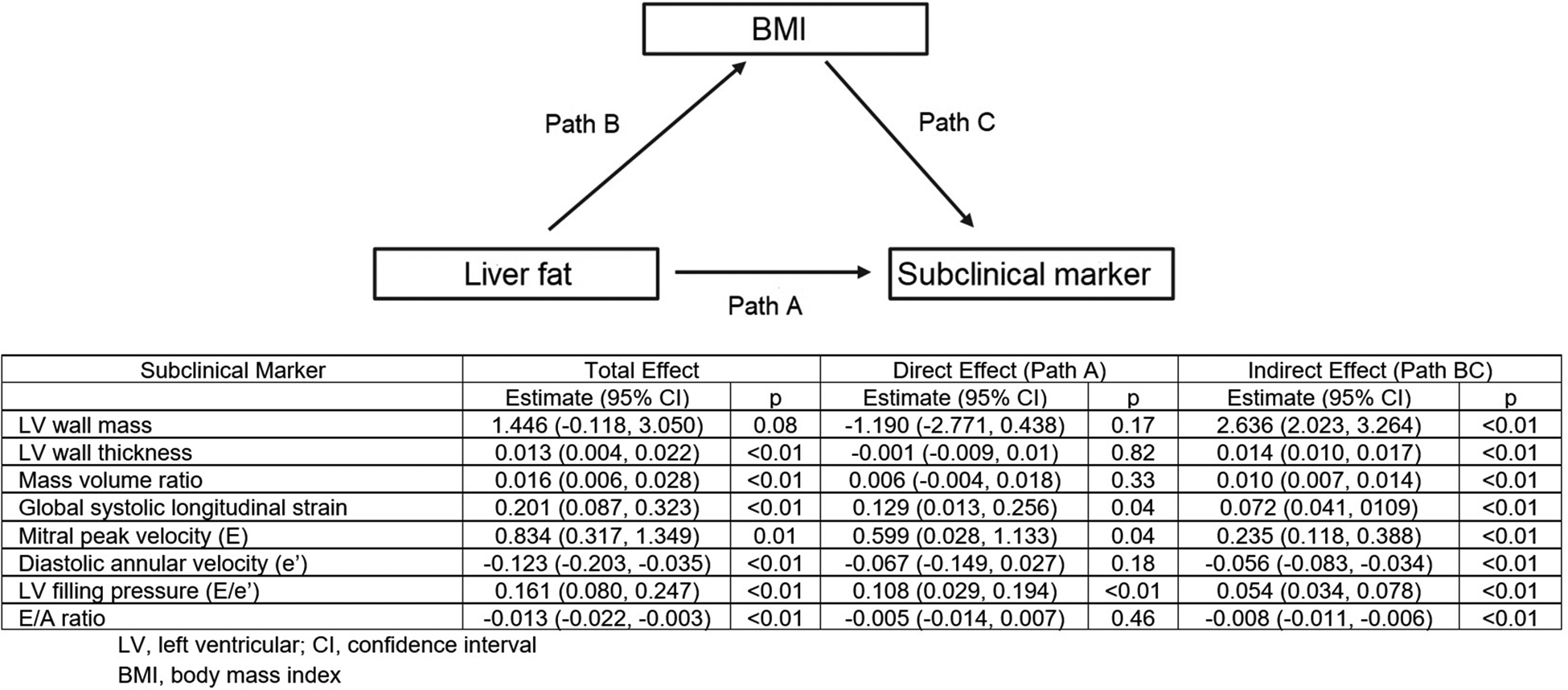
Mediation effect of BMI in the association between liver fat and subclinical markers of myocardial dysfunction.
Discussion
In our large, community-based sample from the FHS, liver fat was significantly associated with multiple subclinical cardiac structural and functional abnormalities after adjusting for a number of demographic and HF risk factors. Increased liver fat was associated with worse global systolic longitudinal strain, a sensitive prognostic marker of LV systolic dysfunction47. Many of the associations we observed were attenuated by obesity; however, liver fat remained associated with higher LV filling pressure (E/e’ ratio), a sensitive marker of diastolic heart dysfunction and potential precursor to clinical HF, in models further adjusted by general or visceral adiposity. Moreover, mediation analyses revealed that BMI appeared to only partially mediate the relation of liver fat with LV filling pressure and diastolic annular velocity; therefore, liver fat may contribute to diastolic dysfunction above and beyond general adiposity.
Robust evidence has shown that the presence and severity of NAFLD is associated with multiple markers of subclinical CVD, including decreased coronary flow, impaired flow-mediated vasodilation, increased carotid intima-media thickness, arterial thickness, and carotid atherosclerotic calcification independent of several cardiometabolic risk factors58–63. Previous studies have also observed an association between NAFLD and impaired diastolic function after adjusting for HF risk factors similar to our study12,29–37,40. Whereas prior studies were limited by small sample sizes30,32,34,37,40, the use of select populations, including men only36, those with diabetes30,40, bariatric surgery32, or patients with hypertension34, or were conducted outside of the US37.
Our study adds to the literature by evaluating the association between liver fat and multiple measures of cardiac structure and function in a large, US community-based sample. Additionally, few prior studies measured LV filling pressure, a potential precursor to clinical heart failure, in their investigation of diastolic impairment12,33,40. We employed novel speckle-tracking techniques that were applied by a limited number of prior studies12,40 to assess early markers of systolic dysfunction.
Our results are generally similar with the findings from the Coronary Artery Risk Development in Young Adults (CARDIA) study, with a few exceptions. In the CARDIA study, the association with liver fat and LV filling pressure was no longer significant after adjusting for general or visceral adiposity; however, in our study, this association persisted and was only partially mediated by BMI12. Interestingly, an updated analysis of the CARDIA study found that liver fat was prospectively associated with increased LV filling pressure compared to non-NAFLD patients independent of adiposity during a 30-year follow-up, strengthening the perspective that that liver fat may add additional risk for diastolic heart failure64. The prior CARDIA study also observed an association between NAFLD and worse global systolic longitudinal strain after accounting for similar HF risk factors as in our study12; however, the association persisted even after further adjusting for VAT in their study. Nearly half of the participants in the CARDIA study were black and the prevalence of diabetes was higher and that of NAFLD was lower compared to our sample. It is possible that differences in the study sample may account for some of the differences in results, although further investigation is needed.
Obesity, is a major contributor to numerous comorbidities, including NAFLD and CVD65–67. HF with preserved ejection fraction is a complex and heterogeneous clinical entity with multiple underlying pathophysiologic substrates, including obesity68. We add to the literature by testing BMI’s mediation effect on the relationship between NAFLD and measures of cardiac structure and function. Our findings support the hypothesis that general adiposity may lie in the causal pathway between fatty liver and early LV diastolic dysfunction. Future studies are needed to determine if early cardiac abnormalities could serve as predictive markers of HF risk in patients with NAFLD. Additional studies are also needed to determine if decreasing general adiposity reduces the risk of HF in patients with NAFLD. Interestingly, our results suggest that obesity alone does not completely account for the association between increased liver fat and LV filling pressure, a notable marker of diastolic dysfunction. Most individuals with NAFLD are also obese, but a significant proportion are lean69. Lean individuals with NAFLD have comparable risk for adverse CVD outcomes compared to those with both NAFLD and obesity70. The pathophysiologic mechanisms underlying the association between NAFLD and impaired LV filling pressure beyond obesity should be further explored.
Increased liver fat is associated with markers of inflammation and oxidative stress. Liver fat was associated with increased C-reactive protein, urinary isoprostanes, IL-6, intercellular adhesion molecule 1, and P-selectin even after adjustment for BMI or VAT in a prior FHS analysis71. NAFLD, as a chronic inflammatory condition, may contribute to the overproduction of systemic inflammatory mediators associated with impaired cardiac function29,41,72,73. Insulin resistance, marked by elevated insulin levels, which is a common feature of NAFLD, may also occur as a result of increased inflammation and oxidative stress. Inflammation may contribute to abnormal myocyte growth and fibrosis and activate the sympathetic nervous system through increased sodium retention, causing impaired cardiac performance25,74,75. Moreover, emerging literature suggest that NAFLD is associated with abnormal fatty acid oxidation and intestinal dysbiosis, which may alter lipid metabolism and inflammation, contributing to CVD8,76. Obesity is also associated with increased inflammatory mediator production and reduced levels of adiponectin77, a known anti-inflammatory factor, and may therefore be involved in the pro-inflammatory pathway leading to increased HF risk in NAFLD. Increased myocardial steatosis, common in obese patients, may additionally alter the efficiency of cardiac metabolism and lead to myocardial lipotoxicity and cardiac dysfunction in the NAFLD population78,79. Additional mechanistic studies are needed to deepen our understanding of how NAFLD contributes to subclinical myocardial impairment.
Major strengths of our investigation include using CT imaging to objectively evaluate liver fat and acquisition of comprehensive cardiovascular outcomes data using well-measured covariates. Our study also applies speckle-tracking echocardiography techniques, a sensitive tool for detection of subclinical myocardial dysfunction80. Our use of a large community sample size additionally provides more power to find significant differences not otherwise detectable in smaller studies.
The present study has certain limitations. Though measuring liver fat by CT imaging correlates well with histologic assessments of liver fat81, CT is insensitive to mild liver fat, so we likely underestimated the burden of hepatic steatosis in our sample and biased our results towards the null. Furthermore, hepatic steatosis may diminish as NAFLD progresses into steatohepatitis and fibrosis, reducing our ability to fully capture the entire NAFLD spectrum in our study. We acknowledge that the prevalence of NAFLD in our study is low compared to other studies, however we did have sufficient power to detect clinically meaningful differences between groups. The low prevalence of NAFLD in our cohort likely reflects the lower population prevalence of NAFLD in the early 2000s compared to more contemporary cohorts and the insensitivity of CT scans for detecting mild NAFLD. Additionally, LV filling pressure was not directly measured, however E/e’ ratio was used as a marker of LV filling pressure, which has been done in previous studies. While E/e’ is useful for assessing filling pressure noninvasively, it is less accurate at predicting LV filling pressures in certain clinical settings, including significant LV dilation and mitral regurgitation. Furthermore, our study is limited by the lack of information on other chronic liver diseases, including viral hepatitis, which can contribute to the features of liver fat on CT scan, leading to a misclassification bias. The generalizability of our findings to other ancestries is also not known. The mediation analyses were hypothesis generating; we cannot exclude the possibility that the attenuation of the associations were due to confounders associated with adiposity measures. As our study was exploratory, we opted to not correct for multiple testing; it is possible that the associations we observed are the result of random chance. Confirmatory studies are needed.
In summary, increased liver fat is associated with alterations in subclinical cardiac structure and function that may lead to clinical heart failure in the absence of overt liver disease and after accounting for notable HF risk factors. Many of the associations between NAFLD and early myocardial dysfunction may be related to adiposity; though NAFLD is associated with LV filling pressure after accounting for obesity. Further studies are warranted to gain mechanistic insights and to confirm our findings.
Supplementary Material
Acknowledgments
This work was supported by:
National Heart, Lung, and Blood Institute contracts N01-HC-25195 and HHSN268201500001l (Dr. Vasan) and grants R01HL126136 (Mitchell/Vasan), R01HL080124, R01HL077477, and 5R01AG047645 (Dr. Vasan), 1R01HL128914 and 2R01HL092577 (Dr. Benjamin), and the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252 and the Boston University School of Medicine Department of Medicine (Long). Dr. Cheng was supported by R01HL131532, R01HL134168, R01HL143227, and R01HL142983 from the NIH. Dr. McManus was supported by KL2RR031981, 5R01HL126977-02, 1R15HL121761-01-A1, and IUH2TR000921-02 from the NIH. Career Investment Award to Dr. Long. Dr. Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Boston University School of Medicine.
The authors disclose the following potential conflicts of interest:
Dr. Gary F. Mitchell is the owner of Cardiovascular Engineering, Inc., serves as a consultant to and receives honoraria from Novartis, Merck, Servier, and Philips Healthcare, and is funded by research grants from Novartis and the National Institutes of Health (NIH). Dr. Susan Cheng serves as a consultant to Zogenix. Dr. David D. McManus serves as a consultant to and receives honoraria from Bristol-Meyers Squibb, Pfizer, FlexCon, and Samsung Semiconductor and is an equity stakeholder in Mobile Sense Technologies, LLC. The remaining authors disclose no conflicts.
References
- 1.Fabbrini E, Sullivan S, Klein S. Obesity and Nonalcoholic Fatty Liver Disease: Biochemical, Metabolic and Clinical Implications. Hepatology. 2010;51:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the Risk of Heart Failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol &Amp; Hepatol. 2013;10:686. [DOI] [PubMed] [Google Scholar]
- 4.Nagarajan V, Kohan L, Holland E, Keeley EC, Mazimba S. Obesity paradox in heart failure: a heavy matter. ESC Hear Fail. 2016;3:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Zobair M, Koenig Aaron B, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2015;64:73–84. [DOI] [PubMed] [Google Scholar]
- 6.Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 8.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goya Wannamethee AS. Gerald Shaper, Lucy Lennon and Naveed Sattar PHW. γ-Glutamyltransferase, Hepatic Enzymes, and Risk of Incident Heart Failure in Older Men. Arterioscler Thromb Vasc Biol. 2012;32:830–835. [DOI] [PubMed] [Google Scholar]
- 10.Dhingra R, Gona P, Wang TJ, Fox CS, D’Agostino RB, Vasan RS. Serum Gamma Glutamyl Transferase and Risk of Heart Failure in the Community. Arterioscler Thromb Vasc Biol. 2010;30:1855–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanWagner LB, Rinella ME. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Curr Hepatol Reports. 2016;15:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanWagner LB, Wilcox JE, Colangelo LA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15:425–439. [DOI] [PubMed] [Google Scholar]
- 14.Miura Y, Fukumoto Y, Shiba N, et al. Prevalence and clinical implication of metabolic syndrome in chronic heart failure. Circ J. 2010;74:2612–21. [DOI] [PubMed] [Google Scholar]
- 15.Scherbakov N, Bauer M, Sandek A, et al. Insulin resistance in heart failure: differences between patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2015;17:1015–1021. [DOI] [PubMed] [Google Scholar]
- 16.von Bibra H, Paulus W, St John Sutton M. Cardiometabolic Syndrome and Increased Risk of Heart Failure. Curr Heart Fail Rep. 2016;13:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased Heart Failure Risk in Normal-Weight People With Metabolic Syndrome Compared With Metabolically Healthy Obese Individuals. J Am Coll Cardiol. 2011;58:1343–1350. [DOI] [PubMed] [Google Scholar]
- 18.Walker AM, Patel PA, Rajwani A, et al. Diabetes mellitus is associated with adverse structural and functional cardiac remodelling in chronic heart failure with reduced ejection fraction. Diabetes Vasc Dis Res. 2016;13:331–40. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Sarnola K, Ruotsalainen S, et al. The metabolic syndrome predicts incident congestive heart failure: a 20-year follow-up study of elderly Finns. Atherosclerosis. 2010;210:237–42. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Hu G. Individual and Joint Associations of Obesity and Physical Activity on the Risk of Heart Failure. Congest Hear Fail. 2010;16:292–299. [DOI] [PubMed] [Google Scholar]
- 21.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–443. [DOI] [PubMed] [Google Scholar]
- 23.Dela Cruz CS, Matthay RA. Role of obesity in cardiomyopathy and pulmonary hypertension. Clin Chest Med. 2009;30:509–23, ix. [DOI] [PubMed] [Google Scholar]
- 24.Cassidy S, Hallsworth K, Thoma C, et al. Cardiac structure and function are altered in type 2 diabetes and Non-alcoholic fatty liver disease and associate with glycemic control. Cardiovasc Diabetol. 2015;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fotbolcu H Duman D, Karaahmet T, Tigen K, Cevik C, Kurtoglu U, Dindar IYT. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457–463. [PubMed] [Google Scholar]
- 26.Kocabay G, Karabay CY, Colak Y, et al. Left atrial deformation parameters in patients with non-alcoholic fatty liver disease: a 2D speckle tracking imaging study. Clin Sci. 2013;126:297. [DOI] [PubMed] [Google Scholar]
- 27.Karabay CY, Kocabay G, Kalayci A, et al. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26:325–31. [DOI] [PubMed] [Google Scholar]
- 28.Karabay CY, Kocabay G, Kalayci A, et al. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26:325–31. [DOI] [PubMed] [Google Scholar]
- 29.Jung JY, Park SK, Ryoo J-H, et al. Effect of non-alcoholic fatty liver disease on left ventricular diastolic function and geometry in the Korean general population. Hepatol Res. 2016;n/a-n/a. doi: 10.1111/hepr.12770 [DOI] [PubMed] [Google Scholar]
- 30.Mantovani A, Pernigo M, Bergamini C, et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS One. 2015;10:e0135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widya RL, de Mutsert R, den Heijer M, et al. Association between Hepatic Ventricular Diastolic Function in a Population-based Cohort : The Netherlands Epidemiology of Obesity Study 1. Radiology. 2016;279:443–450. [DOI] [PubMed] [Google Scholar]
- 32.Simon TG, Bamira DG, Chung RT, Weiner RB, Corey KE. Nonalcoholic Steatohepatitis is Associated with Cardiac Remodeling and Dysfunction. Obesity. 2017;25:1313–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y ho, Kim KJ, Yoo M eun, et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol. 2018;68:764–772. [DOI] [PubMed] [Google Scholar]
- 34.Fallo F, Dalla Pozza A, Sonino N, et al. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646–653. [DOI] [PubMed] [Google Scholar]
- 35.Goland S Zornitzi T, Knobler H, Azoulai O, Lautaty G, Melzer E, Orr A, Caspi A, Malnick SSS. Cardiac abnormalities as a new manisfestation of nonalcoholic fatty liver disease: echocardiographc and tissue doppler imaging assessment. J Clin Gastroenterol. 2006;10:949–955. [DOI] [PubMed] [Google Scholar]
- 36.Granér M, Nyman K, Siren R, et al. Ectopic Fat Depots and Left Ventricular Function in Nondiabetic Men With Nonalcoholic Fatty Liver Disease. Circ Cardiovasc Imaging. 2015;8:e001979. [DOI] [PubMed] [Google Scholar]
- 37.Kim NH, Park J, Kim SH, et al. Non-alcoholic fatty liver disease, metabolic syndrome and subclinical cardiovascular changes in the general population. Heart. 2014;100:938–943. [DOI] [PubMed] [Google Scholar]
- 38.Petta S, Argano C, Colomba D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: Association with the severity of liver disease. J Hepatol. 2015;62:928–933. [DOI] [PubMed] [Google Scholar]
- 39.Trovato FM, Martines GF, Catalano D, Musumeci G, Pirri C, Trovato GM. Echocardiography and NAFLD (non-alcoholic fatty liver disease). Int J Cardiol. 221:275–279. [DOI] [PubMed] [Google Scholar]
- 40.Bonapace S, Perseghin G, Molon G, et al. Nonalcoholic Fatty Liver Disease Is Associated With Left Ventricular Diastolic Dysfunction in Patients With Type 2 Diabetes. Diabetes Care. 2012;35:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fotbolcu H, Zorlu E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J Gastroenterol. 2016;22:4079–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekler A, Gazi E, Erbag G, et al. Right ventricular function and its relationship with grade of hepatosteatosis in non-alcoholic fatty liver disease. Cardiovasc J Afr. 2015;26:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunbul M, Kivrak T, Durmus E, et al. Nonalcoholic Steatohepatitis Score is an Independent Predictor of Right Ventricular Dysfunction in Patients with Nonalcoholic Fatty Liver Disease. Cardiovasc Ther. 2015;33:294–299. [DOI] [PubMed] [Google Scholar]
- 44.Mantovani A, Zoppini G, Targher G, Golia G, Bonora E. Non-alcoholic fatty liver disease is independently associated with left ventricular hypertrophy in hypertensive Type 2 diabetic individuals. J Endocrinol Invest. 2012;35:215–218. [DOI] [PubMed] [Google Scholar]
- 45.Hallsworth K, Hollingsworth KG, Thoma C, et al. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J Hepatol. 2013;58:757–762. [DOI] [PubMed] [Google Scholar]
- 46.Psychari SN, Rekleiti N, Papaioannou N, et al. Epicardial Fat in Nonalcoholic Fatty Liver Disease: Properties and Relationships With Metabolic Factors, Cardiac Structure, and Cardiac Function. Angiology. 2016;67:41–8. [DOI] [PubMed] [Google Scholar]
- 47.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- 48.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 49.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 50.Speliotes EK, Massaro JM, Hoffmann U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham heart study. Hepatology. 2010;51:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng S, McCabe EL, Larson MG, et al. Distinct Aspects of Left Ventricular Mechanical Function Are Differentially Associated With Cardiovascular Outcomes and All‐Cause Mortality in the Community. J Am Heart Assoc. 2015;4:e002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- 54.Kaess BM, Rong J, Larson MG, et al. Relations of Central Hemodynamics and Aortic Stiffness with Left Ventricular Structure and Function: The Framingham Heart Study. J Am Hear Assoc. 2016;5:e002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng S, Larson MG, McCabe EL, et al. Reproducibility of Speckle-Tracking Based Strain Measures of Left Ventricular Function in a Community-Based Study. J Am Soc Echocardiogr. 2013;26: 10.1016/j.echo.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng S, McCabe EL, Larson MG, et al. Left ventricular mechanical function: clinical correlates, heritability, and association with parental heart failure. Eur J Heart Fail. 2015;17:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59:1–38.26917999 [Google Scholar]
- 58.Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism. 2016;65:1136–1150. [DOI] [PubMed] [Google Scholar]
- 59.Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. [DOI] [PubMed] [Google Scholar]
- 60.Volzke H, Robinson D-M, Kleine V, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, Musani SK, Bidulescu A, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim D, Choi S-Y, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.VanWagner LB, Wilcox JE, Ning H, et al. Longitudinal Association of Non‐Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J Am Heart Assoc. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duseja A, Chalasani N. Epidemiology and risk factors of nonalcoholic fatty liver disease (NAFLD). Hepatol Int. 2013;7:755–764. [DOI] [PubMed] [Google Scholar]
- 66.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arter Thromb Vasc Biol. 2006;26:968–976. [DOI] [PubMed] [Google Scholar]
- 67.Ahmed M Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Argulian E, Chandrashekhar Y, Shah SJ, et al. Teasing Apart Heart Failure With Preserved Ejection Fraction Phenotypes With Echocardiographic Imaging: Potential Approach to Research and Clinical Practice. Circ Res. 2018;122:23–25. [DOI] [PubMed] [Google Scholar]
- 69.Wei JL, Leung JC-F, Loong TC-W, et al. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306–1314. [DOI] [PubMed] [Google Scholar]
- 70.Sookian SPCJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46:85–95. [DOI] [PubMed] [Google Scholar]
- 71.Fricker ZP, Pedley A, Massaro JM, et al. Liver Fat is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin Gastroenterol Hepatol. 2018. doi: 10.1016/J.CGH.2018.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pacifico L, Di Martino M, De Merulis A, et al. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59:461–470. [DOI] [PubMed] [Google Scholar]
- 73.Targher G, Day CP, Bonora E. Risk of Cardiovascular Disease in Patients with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2010;363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 74.Di Bello V, Santini F, Di Cori A, et al. Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization study. J Am Soc Echocardiogr. 2006;19:1063–1071. [DOI] [PubMed] [Google Scholar]
- 75.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. [DOI] [PubMed] [Google Scholar]
- 76.Mantovani A, Ballestri S, Lonardo A, Targher G. Cardiovascular Disease and Myocardial Abnormalities in Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1246–1267. [DOI] [PubMed] [Google Scholar]
- 77.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perseghin G, Lattuada G, De Cobelli F, et al. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology. 2008;47:51–58. [DOI] [PubMed] [Google Scholar]
- 79.Rijzewijk LJ, Jonker JT, van der Meer RW, et al. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. J Am Coll Cardiol. 2010;56:225–233. [DOI] [PubMed] [Google Scholar]
- 80.Cimino S, Canali E, Petronilli V, et al. Global and regional longitudinal strain assessed by two-dimensional speckle tracking echocardiography identifies early myocardial dysfunction and transmural extent of myocardial scar in patients with acute ST elevation myocardial infarction and relatively. Eur Hear J Cardiovasc Imaging. 2013;14:805–811. [DOI] [PubMed] [Google Scholar]
- 81.Speliotes EK, Massaro JM, Hoffmann U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


