Abstract
Interdomain electron transfer (IET) between the flavin mononucleotide (FMN) and heme centers is an obligatory step in nitric oxide synthase (NOS) enzymes. An isoform-specific pivotal region near Leu406 in the heme domain of human inducible NOS (iNOS) was proposed to mediate the FMN-heme domain-domain alignment [Sheng, Y. et al. (2015) J. Inorg. Biochem. 153, 186-196]. The FMN - heme IET rate is a measure of the interdomain FMN/heme complex formation. In this work, the FMN - heme IET kinetics in the wild type (wt) human iNOS oxygenase/FMN (oxyFMN) construct were directly measured by CO photolysis with added synthetic peptide related to the pivotal region, in comparison with the wt construct alone. The IET rates were decreased by the iNOS HKL peptide in a dose-saturable fashion, and the effect was abolished by a single L406→E mutation in the peptide. A similar trend in change of the NO synthesis activity of wt iNOS holoenzyme by the peptides was observed. These results, along with the kinetics and modelling results for the L406T and L406F mutant oxyFMN proteins, indicated that the Leu406 residue modulates the FMN - heme IET through hydrophobic interactions. Moreover, the IET rates were analyzed for the wt iNOS oxyFMN protein in the presence of nNOS or eNOS-derived peptide related to the equivalent pivotal heme domain site. These results together indicate that the isoform-specific pivotal region at the heme domain specifically interacts with the conserved FMN domain surface, to facilitate proper interdomain docking for the FMN - heme IET in NOS.
Keywords: Nitric oxide synthase, Electron transfer, Kinetics, Laser flash photolysis
1. Introduction
Mammalian nitric oxide synthase (NOS) catalyzes the conversion of L-arginine (L-Arg) to citrulline and nitric oxide (NO), with NADPH and O2 as co-substrates [1, 2]. Ca2+-sensing protein, calmodulin (CaM), exquisitely controls the NOS enzyme in vivo. Each subunit of the homodimeric NOS protein consists of two domains joined by a CaM-binding linker: a C-terminal reductase domain, which is made up of smaller NADPH/FAD and FMN (sub)domains, and an N-terminal heme-containing oxygenase domain; the terms “oxygenase domain” and “heme domain” are interchangeable in the NOS field. Three NOS isoforms exist in mammals: inducible, neuronal, and endothelial NOS (iNOS, nNOS, and eNOS, respectively). Binding of CaM to nNOS or eNOS requires an increase in intracellular [Ca2+], while iNOS tightly associates with CaM at basal Ca2+ concentration.
At the basic level, NO production by the NOS enzyme occurs through the combination of catalytic chemical reactions at heme active sites [3, 4] with the enzyme-spanning electron transport “fueling” the reactions [5]. CaM binding to NOS activates the NO synthesis by enabling the NADPH-derived electron transport from the flavin cofactors to the catalytic heme site (where the NO biosynthesis occurs) [6]. The intraprotein interdomain electron transfer (IET) processes (following the flow of NADPH → FAD → FMN → heme) are crucial steps in NO production by coupling the reactions between the NOS domains [1, 7–9]. A stringent control of the IET processes is necessary for the usual function of NOS because an uncoupled NOS (in which the normal electron flow within the enzyme is diverted to molecular oxygen to yield superoxide and other related species) generates excessive reactive oxygen and nitrogen species, resulting in deleterious effects [10–13]. It is of current interest to investigate control mechanisms of the NOS enzymes at the molecular level, both experimentally [14–17] and computationally [18–21]. Despite intensive efforts, the molecular mechanism of NOS regulation remains unclear because the large, modular enzyme is intrinsically complex, involving not only sophisticated substrate oxidation pathways, but also protein dynamics [2, 5, 6].
Evidence from the nNOS reductase domain structure [22] and our earlier work [23] leads to the awareness that the FMN domain motion is required for the electron transport across the NOS domains [24]. A tethered shuttle model defines this motion [24, 25]: the tethered FMN domain moves back and forth between the electron-accepting “input” state and the electron-donating “output” state through the free/open states, to transfer the NADPH-derived electrons to the NOS heme site. The establishment of the NOS output state largely encompasses two stages: a) CaM-NOS binding induced release of the FMN (sub)domain from the rest of the reductase domain, and b) subsequent docking of the FMN domain onto the heme domain [24]. The FMN – heme IET within the docked FMN-heme complex is indispensable in delivering the electrons required for dioxygen activation and the consequent NO synthesis at the heme domain. It is thus important to decipher the mechanism through which the FMN-heme docking is modulated [26, 27].
We recently conducted molecular dynamics (MD) simulations on human iNOS protein models in the pre- and post-IET redox states to elucidate the structural rearrangements and the domain docking interactions prior to and after the FMN – heme IET [28]. Guided by the computational models, we have experimentally examined some of the specific residues of the NOS heme domain and CaM that are involved in optimal FMN-heme docking [29]. Interestingly, our MD simulations predicted a L406-mediated pivot at the FMN-heme interdomain interface (Figure 1), which is maintained in both the computational pre- and post-IET conformations to restrain the FMN domain motions [28]. This pivot region is predicted to correlate with existence of a bottleneck in the FMN domain’s sampling of the conformational space that allows for the IET-competent state [28]. It is of note that these pivotal interacting residues at the NOS heme domain are isoform-specific, while the pairing residues at the FMN domain are conserved (Figure 1c).
Figure 1.
(A) A docking model of human iNOS oxyFMN at the redox state of [Fe(III)][FMNH−], taken from molecular dynamics simulations [28]. The heme domain in the same subunit is in purple (and labeled Heme A), and the bound CaM is green. Note that the FMN domain docks to the heme domain (light blue) of the other subunit (labeled Heme B) to allow for the inter-subunit FMN – heme IET. The heme and FMN cofactors are displayed in ball and stick. (B) A close-up view of the pivotal residues displayed in stick. (C) Sequence alignment of the pivotal regions in the three NOS isoforms. Note that the pivotal residues HKL in human iNOS heme domain (highlighted in purple) differs among the NOS isoforms (RKT and RTT for nNOS and eNOS, respectively), while the pairing residues in the FMN domain (e.g., E661, L662, Q665, E666 in human iNOS) are conserved (colored in orange).
Herein we experimentally studied the role of this isoform-specific pivot in the FMN – heme IET. A human iNOS bi-domain oxygenase/FMN (oxyFMN) construct was utilized. The truncated oxyFMN construct is made up with only the heme-containing oxygenase and FMN domains that are connected by the CaM-binding linker [30]. This was originally realized by Ghosh and Salerno [30] in 2006 to exclude the FAD/FMN interdomain interactions and favor the FMN/heme interdomain interactions. The oxyFMN construct was also constructed by other laboratories later on [31, 32], and has become an established model of minimal electron transfer complex of the NOS output state [8]. Rate of the FMN – heme IET process can be used as a measure of the interdomain FMN/heme complex formation [8]. In the present work, we have determined the FMN – heme IET kinetics of human iNOS oxyFMN construct and the NO synthesis activities of iNOS holoenzyme with added synthetic peptide that is related to the L406 region (Fig. 1). These experimental perturbation data, along with the IET kinetic results and in silico modelling for the L406 mutant proteins, provided new evidence for the role of the L406-pivot in facilitating the FMN – heme IET through specific hydrophobic interactions.
2. Experimental procedures
2.1. Overexpression and purification of recombinant human iNOS oxyFMN and full-length NOS proteins
The human iNOS L406 mutant plasmids were constructed on a pCWori+ vector containing cDNA of the wild type (wt) iNOS oxyFMN construct [31] by site-directed mutagenesis. The primers for the mutations are listed in Table S1 in Supporting Information. The mutated plasmids were confirmed by sequencing the DNA at Genewiz LLC (New Jersey, USA). The wt or the mutant oxyFMN plasmid was co-transfected with a CaM expression vector (p209) into E. coli BL21 (DE3) cells on an electroporator (MicroPulser, Bio-Rad) [31]. The purification and characterization of the human iNOS oxyFMN proteins were then conducted by using the reported protocol [33].
Preparation of wt full-length human iNOS protein were performed as described [31]. CaM coexists in the isolated iNOS oxyFMN construct and holoprotein.
2.2. Laser flash photolysis
The CO photolysis experiments were conducted on a LP920 laser flash photolysis spectrometer (Edinburgh Instruments, Edinburgh, UK) [8]. A 446 nm laser pulse was focused onto the sample to trigger the FMN – heme IET process. As needed, the synthetic peptide was added into the sample to certain concentration. All the experiments were performed at least twice at 21 °C [34]. The transient traces were averaged and fitted using OriginPro 2020 (OriginLab, M.A.).
2.3. Steady-state NOS enzymatic activity assays
Steady-state rates of NO production by iNOS holoprotein was measured in a pH 7.4 buffer (50 mM Tris, 5 μM H4B, 100 mM NaCl, and 200 μM CaCl2) [35]. The assay solution contained 8 μM oxyhemoglobin, 100 μM L-Arg, and100 μM NADPH. As needed, the synthetic peptide was added into the assay mixture. The reaction was initiated by adding iNOS holoprotein to a 20 nM final concentration. The rate of NO production was obtained using extinction coefficients of 60 mM−1cm−1 at 401 nm [35].
2.4. Modeling of the pivotal region in the L406 mutants
The models of the pivotal region of the L406 mutant proteins were built by starting with the docked model structure of wt human iNOS oxyFMN [28]. Leu406 was replaced by Phe, Thr, or Lys using the Rotamers tool in UCSF Chimera ( http://www.cgl.ucsf.edu/chimera) [36]. The selected rotamer was based on probability using the Dunbrack 2010 Rotamer library [37]. The clashes/contacts were examined for the 406-residue using surface/binding analysis tools in the Chimera software.
3. Results and Discussions
3.1. The FMN – heme IET kinetics and NO production activities of human iNOS oxyFMN with added L406-related peptide
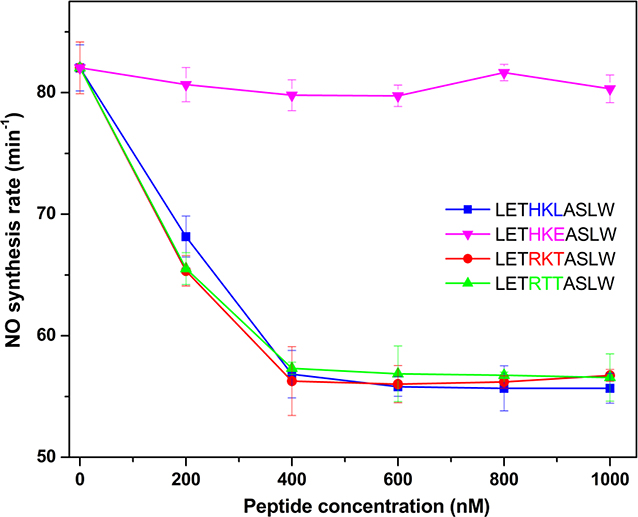
We first measured the FMN – heme IET kinetics of wt human iNOS oxyFMN construct with added synthetic peptide LETHKLASLW that corresponds to the endogenous sequence of the human iNOS L406 region; the pivotal site is underlined. A plot of typical absorbance traces at 580 nm from the laser flash photolysis experiments is shown in Figure 2. The best single-exponential fit to the decay phase yielded the rate of the FMN – heme IET [31]. The HKL peptide slows the IET notably in a dose-saturable fashion (see Table S2 and the line and symbol plot in Figure 3). For example, the IET rate in the presence of 6-fold concentration of the peptide is 240 ± 10 s−1, which is ~ 25% lower than that of wt protein alone (321 ± 8 s−1). It is also of note that the HKL peptide inhibits the NO production activity of wt iNOS holoenzyme to similar extent (Figure 4 and Table S3).
Figure 2.
Transient absorbance traces at 580 nm obtained for the [Fe(II)–CO][FMNH•] form of wild type human iNOS oxyFMN in the presence of LETHKLASLW peptide. The IET process was monitored by the loss of absorbance of FMNH• at 580 nm, which decayed below the pre-flash baseline upon a laser excitation. Single exponential model was used to fit to the data, as shown in red solid line. Plot of residual from the fitting to a single exponential function is shown in the bottom panel; note that the fitting gives an excellent normal distribution of the residuals. Anaerobic solution contained 15 μM iNOS oxyFMN, 90 μM LETHKLASLW peptide, ~ 20 μM 5-deazariboflavin and 5 mM fresh semicarbazide in a pH 7.6 buffer (40 mM bis-Tris propane, 400 mM NaCl, 2 mM L-Arg, 20 μM H4B, 1 mM Ca2+ and 10 % glycerol). The experiment was conducted at 21 °C.
Figure 3.
Plot of the observed FMN – heme IET rates of wild type human iNOS oxyFMN protein with added iNOS-HKL peptide (LETHKLASLW), iNOS-HKE peptide (LETHKEASLW), nNOS-RKT peptide (LETRKTASLW) or eNOS-RTT peptide (LETRTTASLW). At [peptide]:[iNOS] ratio of 6, inhibitory effect of the iNOS-HKL peptide on the kinetics has been saturated. Since the goal of these experiments is to compare the inhibitory effects/trend of the various peptides, it is not necessary to examine all peptides at other lower concentrations.
Figure 4.
Plot of the NO production rates of wild type human iNOS holoprotein in the presence of various concentrations of the iNOS-HKL peptide (LETHKLASLW), iNOS-HKE peptide (LETHKEASLW), nNOS-RKT peptide (LETRKTASLW) or eNOS-RTT peptide (LETRTTASLW).
To further define the site of the HKL peptide that is responsible for the observed inhibitions, we measured the kinetics in the presence of a L406E mutant form of the iNOS HKL peptide (LETHKEASLW). Notably, the inhibitions of the FMN – heme IET and the NO production by the HKL peptide are both eliminated by the single L→E mutation in the peptide (Table 1; see also Figures 3 and 4 and Table S3), which indicates the decisive nature of L406 relative to the other amino acids in the HKL peptide. Note that there are two glutamate residues (E661 and E666) in the HKL-pairing residues on the FMN domain (Figure 1c), and the L406E mutant peptide thus may not effectively compete with the endogenous site of the heme(iNOS) domain for explicit interaction with the pivot-partnering region of the FMN domain, due to the repulsion between the negatively charged E406 and the other glutamates on the FMN domain. This leads to its diminished inhibitory effect on the IET and NO production observed here. Therefore, it is the L406 residue that underlies the competition of the HKL peptide with the pivotal heme domain region for interacting with the partnering site of the FMN domain. This may likely result in lower docked FMN-heme state population (i.e., a shift of conformational equilibrium) and slower FMN – heme IET. In addition to the conformational equilibrium, the conformational change rates determine the bulk IET rate measured in the laser flash photolysis experiments [38]. We thus cannot exclude the possibility of the peptide affecting the association and dissociation rates of the interdomain FMN-heme docking complex, if the peptide binds to the FMN domain relatively strongly. These potential pathways merit further investigation.
Table 1.
| Peptide | [iNOS] (μM) | [Peptide] (μM) | [peptide]:[iNOS] | kIET (s−1) |
|---|---|---|---|---|
| 15 | 0 | 0 | 321 ± 8 | |
| LETHKLASLW | 15 | 90 | 6 | 240 ± 10 |
| LETHKEASLW | 15 | 90 | 6 | 317 ± 16 |
| LETRKTASLW | 15 | 90 | 6 | 205 ± 9 |
| LETRTTASLW | 15 | 90 | 6 | 226 ± 10 |
Rates are the average of at least two experiments conducted on separate dates.
All the peptides were synthesized by Genemed Synthesis, Inc.; purity > 95%. The varying segment among the peptides is underlined.
We recognize that the maximum inhibitions of the NO production and IET activities by the iNOS-HKL peptide are not complete (~ 25 – 33 %), showing that the competitive inhibition is not strong. This is not unexpected since the design of small peptide is based on the endogenous protein sequence only and has not considered its conformational fitness into the interfacial space at the docking site. Similar extent of inhibition was observed for other peptides targeting the dynamic interface in human iNOS [29].
3.2. The IET kinetics of L406F and L406T mutants of iNOS oxyFMN
To determine whether the FMN – heme IET is affected by mutation at the L406 site in the iNOS protein, we measured the kinetics in a L406F mutant of iNOS oxyFMN. The FMN – heme IET rate for the L406F mutant (365 ± 13 s−1) is slightly larger (~ 13%) than that of wt (321 ± 8 s−1); the two-tailed P value equals 0.0552 in unpaired t test, and the difference is not quite statistically significant. On the other hand, the FMN – heme IET rate for a L406T mutant (389 ± 14 s−1) is noticeably faster than that of the wt protein. The IET rate values for the wt iNOS and L406T mutant samples are statistically different: unpaired t test gives a two-tailed P value of 0.0270. Overall, the mutation (L → F or T) still allows for efficient FMN – heme IET at a similar level of the wt protein. This is not unexpected because, for example, Thr in the L406T mutant contains a polar hydroxyl group, but it is still uncharged and of smaller size (compared to Leu), which may presumably allow for proper FMN-heme domain docking/alignment at this region.
We further evaluated the effects of the individual mutants on the IET rates by generating model structures based on the computational structure of wt iNOS oxyFMN. We conducted in silico mutation and modelling using UCSF Chimera to explore the interacting region; the resulting model structures are represented in Figure 5. For both mutants’ models, the highest probability rotamers have the 406 residue (F or T) fit into the space without clashing with the neighboring residues. This would unlikely disturb the hydrophobic interacting network in the pivot region (Figure 1), which would account for a rate constant that is comparable to the wt protein.
Figure 5.
Comparison of the interactions between the heme domain 406 residue and the FMN domain L662 residue (colored in magenta and yellow, respectively). The Leu406 residue was mutated to Thr, Phe, or Glu, using Chimera software.
It is desirable to introduce a charge at this position in human iNOS protein to more significantly modify the L406-mediated hydrophobic interactions. However, the L406K mutation diminished the oxyFMN protein overexpression significantly, and we could not acquire enough amount of the iNOS mutant. The in silico L→K mutation resulted in 28 clashes with Leu662 (Figure 5), which may explain why the L406K mutant did not produce well. The kinetics results and modelling for these mutants together suggest that the L406 residue acts through hydrophobic interactions with aliphatic residues on the FMN domain, as previous molecular dynamics simulations predicted [28].
We are aware that the extents of the changes in the IET rate are noticeable but rather small. This is not unexpected because the docking very likely involves more than one site/interface and the FMN-heme pivotal interface area is rather small. Of note is that the intra-subunit pivotal interacting region is among multiple interfaces involved in facilitating the FMN-heme domain-domain alignment (Figure 6): besides hydrophobic interactions in the pivotal region, electrostatic interactions between surface ionic pairing residues at the CaM-heme(NOS) [29] and inter-subunit FMN-heme [39, 40] docking sites favor formation of the IET-competent conformations, bestowing dynamic, regulated FMN – heme IET. These interactions at multiple sites together restrain the FMN domain motion so that the FMN domain can readily reach the IET-competent docking positions out of a three-dimensional conformational space. Specifically, the L406-pivot was projected to remain intact in both the pre- and post-IET states [28]; this prevents the FMN domain from becoming completely free of binding to the heme domains. The existence of such a pivot presumably reduces the randomness of the FMN domain motions and allows the FMN domain to more readily approach the heme domain with an appropriate degree of freedom, and eventually dock at proper positions, augmenting the FMN – heme process [28]. Our kinetic results for the iNOS mutants and iNOS-related peptides together indicate that the FMN-heme pivot region indeed plays an auxiliary but still meaningful role in modulating the FMN–heme IET and NO production in iNOS.
Figure 6.
A close-up view of multiple domain-domain interacting sites in the docking complex model. The FMN/heme/CaM complex is shown in the same orientation as Figure 1. Only surface of the intra-subunit pivotal region (colored in parent color) is depicted, while the rest is displayed in line ribbon. Selected interacting residues are labelled for the CaM-heme and inter-subunit FMN-heme sites; these NOS and CaM interface residues have been reported in the literature [29, 39–41].
3.3. The FMN – heme IET kinetics and NO production activities of iNOS proteins in the presence of nNOS or eNOS peptide
To determine whether the inhibitory effect of iNOS L406-pivotal region is isoform-specific, we utilized peptides LETRKTASLW and LETRTTASLW that contain the nNOS and eNOS pivotal sequence (RKT and RTT) respectively; see Figure 1c. We did not fully go with the rest of their endogenous sequences since these three residues are the most different in their amino acid types, while the other amino acids among the NOS isoforms are basically identical/conserved or at least similar (Figure 1c). This design also allows a more straightforward comparison of the experimental results to elucidate the roles of specific residues in changing the IET and enzymatic kinetics. The observed FMN – heme IET and NO production rates for wt human iNOS proteins with the nNOS or eNOS peptide are listed in Tables 1 and S3, respectively. The IET rate of wt human iNOS oxyFMN was decreased upon adding the RKT(nNOS) or RTT(eNOS) peptide, and the extent was similar to that of the HKL(iNOS) peptide (Figure 3). The rate of NO production by wt iNOS holoprotein was similarly decreased upon adding the nNOS or eNOS peptide (Figure 4). These results indicate that the RKT(nNOS) and RTT(eNOS) peptides, like the HKL(iNOS) peptide, can also effectively compete for the FMN interacting region. This is because the partnering FMN domain region is conserved among the NOS isoforms (Figure 1c) and the pivot likely operates in all the NOS isoforms. Therefore, the nNOS and eNOS peptides may still be able to recognize the iNOS FMN domain pivotal region, resulting in slower kinetics. The results for the mutant peptides also assisted in assessing the roles of other residues in the pivotal region. For the RKT peptide, the L→T mutation should have enhanced the IET (see the data for the L406T mutant iNOS oxyFMN above); however, the observed effect is opposite, suggesting that the Arg residue in the RKT peptide is responsible for the disturbed FMN/heme docking. Given that there are two Glu residues in the partnering FMN domain region (Figure 1c), the RKT peptide may more effectively compete with the pivotal ‘HKL’ region for the FMN domain site, slowing down the FMN – heme IET. In comparison with the RKT peptide, the RTT peptide did not further inhibit the IET (Table 1), indicating that the middle Thr residue may be relatively insignificant in the inhibitory effect within experimental error, in relative to the Arg residue. These experimental observations are consistent with the previous computational model, in which specific interactions at the predicted pivotal site between the heme and FMN domains mediate the interdomain docking (Figure 1). Moreover, these results suggest that specific interactions at the pivotal region may differ among the three NOS isoforms because of the intrinsic difference in the pivotal region sequence of the heme domain.
In summary, our kinetics results of the discrete FMN – heme electron transfer step and NO production have provided experimental evidence for the role of an isoform-specific pivotal region in specifically interacting with the conserved FMN domain surface, to facilitate proper domain docking for the FMN – heme IET. These results have thus provided new insights into the similarities and differences in the pivotal region among the NOS isoforms. This work should motivate future combined experimental and computational studies of the dynamic interface governing the electron transfer between modules in redox proteins.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health (GM081811 and GM133973 to C.F.).
Abbreviations
- NO
nitric oxide
- NOS
NO synthase
- iNOS
inducible NOS
- nNOS
neuronal NOS
- eNOS
endothelial NOS
- IET
intraprotein interdomain electron transfer
- CaM
calmodulin
- dRF
5-deazariboflavin
- H4B
6R-5,6,7,8-tetrahydrobiopterin
- L-Arg
L-arginine
- oxyFMN
a truncated oxygenase/FMN bi-domain construct of NOS that consists of only the heme-containing oxygenase domain and the FMN domain, along with the CaM-binding linker in between the two domains
Footnotes
Conflict of Interest Statement. The authors declare no conflict of interest.
Supporting Information
Primers for constructing the iNOS mutants; the FMN – heme IET and NO production rates of human iNOS proteins in the presence of the synthetic peptide.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Alderton WK, Cooper CE and Knowles RG (2001) Biochem J 357:593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuehr DJ and Haque MM (2019) Br J Pharmacol 176:177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y and Silverman RB (2008) Biochemistry 47:2231–2243 [DOI] [PubMed] [Google Scholar]
- 4.Lang J, Santolini J and Couture M (2011) Biochemistry 50:10069–10081 [DOI] [PubMed] [Google Scholar]
- 5.Leferink NGH, Hay S, Rigby SEJ and Scrutton NS (2015) FEBS J 282:3016–3029 [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zheng H and Feng C (2018) Frontiers in bioscience (Landmark edition) 23:1803–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuehr DJ, Santolini J, Wang ZQ, Wei CC and Adak S (2004) J Biol Chem 279:36167–36170 [DOI] [PubMed] [Google Scholar]
- 8.Feng C (2012) Coord Chem Rev 256:393–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedison TM, Hay S and Scrutton NS (2017) Nitric Oxide 63:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo S, Lei H, Qin H and Xia Y (2014) Curr Pharm Des 20:3548–3553 [DOI] [PubMed] [Google Scholar]
- 11.Zweier JL, Chen C-A and Druhan LJ (2011) Antioxid Redox Signal 14:1769–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BSS, Karoui H, Tordo P and Pritchard KA (1998) Proc Natl Acad Sci U S A 95:9220–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CA, Druhan LJ, Varadharaj S, Chen YR and Zweier JL (2008) J Biol Chem 283:27038–27047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng H, Li J and Feng C (2020) FEBS Lett 594: 2904–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tejero J, Hunt AP, Santolini J, Lehnert N and Stuehr DJ (2019) J Biol Chem 294:7904–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedison TM, Leferink NGH, Hay S and Scrutton NS (2016) ACS Catalysis 6:5170–5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang J, Maréchal A, Couture M and Santolini J (2016) Biophys J 111:2099–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bignon E, Rizza S, Filomeni G and Papaleo E (2019) Computational and Structural Biotechnology Journal 17:415–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinet JJ, Cho K-B and Gauld JW (2008) J Am Chem Soc 130:3328–3334 [DOI] [PubMed] [Google Scholar]
- 20.de Visser SP and Tan LS (2008) J Am Chem Soc 130:12961–12974 [DOI] [PubMed] [Google Scholar]
- 21.Cho KB, Derat E and Shaik S (2007) J Am Chem Soc 129:3182–3188 [DOI] [PubMed] [Google Scholar]
- 22.Garcin ED, Bruns CM, Lloyd SJ, Hosfield DJ, Tiso M, Gachhui R, Stuehr DJ, Tainer JA and Getzoff ED (2004) J Biol Chem 279:37918–37927 [DOI] [PubMed] [Google Scholar]
- 23.Feng CJ, Tollin G, Hazzard JT, Nahm NJ, Guillemette JG, Salerno JC and Ghosh DK (2007) J Am Chem Soc 129:5621–5629 [DOI] [PubMed] [Google Scholar]
- 24.Feng CJ, Tollin G, Holliday MA, Thomas C, Salerno JC, Enemark JH and Ghosh DK (2006) Biochemistry 45:6354–6362 [DOI] [PubMed] [Google Scholar]
- 25.Ghosh DK and Salerno JC (2003) Front Biosci 8:D193–D209 [DOI] [PubMed] [Google Scholar]
- 26.Astashkin AV, Li J, Zheng H and Feng C (2019) The Journal of Physical Chemistry A 123:7075–7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Zheng H and Feng C (2019) Biochemistry 58:3087–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng Y, Zhong L, Guo D, Lau G and Feng C (2015) J Inorg Biochem 153:186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Zheng H, Wang W, Miao Y, Sheng Y and Feng C (2018) FEBS Lett 592:2425–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh DK, Holliday MA, Thomas C, Weinberg JB, Smith SME and Salerno JC (2006) J Biol Chem 281:14173–14183 [DOI] [PubMed] [Google Scholar]
- 31.Feng CJ, Dupont A, Nahm N, Spratt D, Hazzard JT, Weinberg J, Guillemette J, Tollin G and Ghosh DK (2009) J Biol Inorg Chem 14:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilagan RP, Tejero JS, Aulak KS, Ray SS, Hemann C, Wang Z-Q, Gangoda M, Zweier JL and Stuehr DJ (2009) Biochemistry 48:3864–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, Fan W, Elmore BO and Feng C (2011) FEBS Lett 585:2622–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Chen L, Fan W and Feng C (2012) FEBS Lett 586:159–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panda SP, Li W, Venkatakrishnan P, Chen L, Astashkin AV, Masters BSS, Feng C and Roman LJ (2013) FEBS Lett 587:3973–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC and Ferrin TE (2004) J Comput Chem 25:1605–1612 [DOI] [PubMed] [Google Scholar]
- 37.Shapovalov Maxim V. and Dunbrack Roland L. (2011) Structure 19:844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astashkin AV and Feng C (2015) J Phys Chem A 119:11066–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tejero J, Hannibal L, Mustovich A and Stuehr DJ (2010) J Biol Chem 285:27232–27240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Chen L, Lu C, Elmore BO, Astashkin AV, Rousseau DL, Yeh S-R and Feng C (2013) Inorg Chem 52:4795–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith BC, Underbakke ES, Kulp DW, Schief WR and Marletta MA (2013) Proc Natl Acad Sci U S A 110:E3577–E3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.