Abstract
CD73 (ecto-5′-nucleotidase) is a novel immunoinhibitory protein that plays a key role for tumor growth and metastasis. Its main function is to convert extracellular ATP to immunosuppressive adenosine in concert with CD39 in normal tissues to limit excessive immune response. However, tumors take advantage of the CD73-mediated adenosinergic mechanism to protect them from immune attack. In particular, inducible expression of CD73 along with other adenosinergic molecules on both cancer cells and host cells sustains immunosuppressive tumor microenvironment by affecting multiple aspects of the immune response. Owing to its multifaceted capacity to tumor promotion as an emerging immune checkpoint, CD73 is an ideal therapeutic target for cancer treatment especially in combination with conventional therapy and/or other immune checkpoint inhibitors. In this review, we will discuss the roles of CD73 on tumor and immune cells and will highlight the therapeutic value of CD73 for combination therapy.
Keywords: CD73, Immunotherapy
Introduction
CD73, also known as ecto-5′-nucleotidase (ecto-5′-NT, EC 3.1.3.5) is a glycosylphosphatidyl inositol (GPI)-anchored cell surface protein that is encoded by NT5E gene. CD73 is widely expressed on different tissues [1,2] and cell types including, but not limited to the subsets of T cells and B cells [1,3–5], endothelial cells [4,6] and epithelial cells [7].
The balance between ATP and adenosine is crucial to prevent uncontrolled tissue damage due to excessive inflammatory responses. CD73, as a rate-limiting enzyme for adenosine production, plays a critical role to maintain tissue homeostasis by converting/switching ATP-triggered immune activation to adenosine-mediated immunosuppression, although the relative contribution of non-canonical adenosinergic pathways led by alkalike phosphatases and/or NAD+ ectohydrolase CD38 may need consideration. The extracellular ATP level is elevated in stressful situations such as inflammation, malignancy, and ischemia [8,9]. While ATP mediates inflammatory responses through their P2 purinergic receptors, i.e. P2XRs and P2YRs, it is rapidly hydrolyzed by the enzymatic cascade via CD39 (NTPDases) and CD73 (ecto-5’-nucleotidase) to generate adenosine that acts as an anti-inflammatory mediator to downregulate the immune cell function through its four receptors (A1, A2A, A2B, and A3). As such, CD73, by degrading extracellular AMP to adenosine, is a key player for the establishment of an immunosuppressive tumor microenvironment (TME). In this review, the roles of CD73 on tumor and immune cells, as well as its therapeutic potential will be discussed.
CD73 on tumor cells
CD73 expression level is higher in the majority of human solid tumors. Its expression and activity are closely associated with tumor invasiveness and metastasis [10]. We [11] and others [12] have demonstrated that extracellular adenosine generated by CD73 on tumor cells is sufficient to mediate immune evasion, facilitating tumor growth and metastasis. The importance of CD73 on tumor cells versus host cells in tumorigenesis has been further documented using multiple CD73-deficient tumor models [11,13–16]. Besides the immune regulation of CD73 by tumor cells [11,12], CD73 affects multiple aspects of tumorigenesis such as proliferation, adhesion/migration, angiogenesis and metastasis. It promotes proliferation of tumor cells by regulating cell cycle, apoptosis, and signaling pathways such as EGFR, β-catenin/cyclin D1, VEGF, and AKT/ERK[17–21]. Independent of its enzymatic function, CD73 can also promote cell-to-cell adhesion, migration, invasion of cancer cells as well as stemness [17,20,22–24]. Interestingly, CD73 on both tumor cells and host cells is required for tumor angiogenesis [25,26]. It has been also demonstrated the importance of CD73-A2AR signaling for tumor-associated lymphangiogenesis [27,28], further supporting the potential use of adenosine blocking agents to inhibit pathological lymphangiogenesis in cancers and prevent tumor dissemination. In addition, two recent studies reported that cancer cell-intrinsic CD73 expedited metastasis by driving epithelial-to-mesenchymal transition (EMT) through PI3K/AKT signaling pathway [29] and RICS/Rho GTPase signaling pathway [30], respectively. In support, CD73 expression is often associated with worse prognosis [18,21,31–33] and poor response to therapeutic agents [34,35]. However, CD73 is not always upregulated in cancers and its expression has been reported to be correlated with a positive prognosis [36,37]. In fact, aberrantly glycosylated CD73 [38], as well as a human specific CD73 isoform (CD73s) [39] have been identified in human hepatocellular carcinoma (HCC), leading to the functional suppression of tumor CD73. CD73 was also downregulated in advance stage prostate [40], laryngeal [41] and high grade colon carcinomas [42]. Lower expression levels of CD73 were observed in poorly-differentiated and advanced stage of endometrial carcinomas compared to normal and well-differentiated, early state tumors, and higher CD73 expression was associated with better overall survival [43]. CD73-generated adenosine was further shown to protect epithelial integrity via actin polymerization in early-stage endometrial tumors [43]. Thus, the role of CD73 in cancers seems to be complex possibly due to the non-tumor promoting effects mediated by CD73.
In addition, evidence supports the existence of a soluble form of CD73 (sCD73) [44] and its increased levels in the plasma of cancer patients compared to healthy individuals (Q Huang et al., abstract 1538, 106th American Association for Cancer Research, Philadelphia, April 2015). Although the role of sCD73 is less explored, high levels of sCD73 enzyme activity in serum, before nivolumab (anti-PD-1 Ab) treatment, was found to be associated with poor survival of metastatic melanoma patients [45], indicating sCD73 as a potential prognostic marker for cancer immunotherapy.
Interestingly, CD73 together with CD39 were found on exosomes isolated from mesothelioma patients [46] and CD73+ exosome suppressed immune cell function [46,47]. Furthermore, prostate cancer cell–derived exosome was able to induce CD73 expression on dendritic cells (DC), thereby inhibiting T cell function [48]. Thus, CD73 by tumor cells or their derived exosomes exerts its immunosuppressive function in an adenosine-dependent manner.
CD73 on immune cells
CD73 along with other adenosinergic molecules play critical roles in the establishment of an immunosuppressive TME by affecting multiple types of immune cells [11,13–16] (see Figure1). Thereafter we will summarize the roles of CD73 on the following major immune cell populations.
Figure 1. CD73-mediated immunosuppression in the TME.
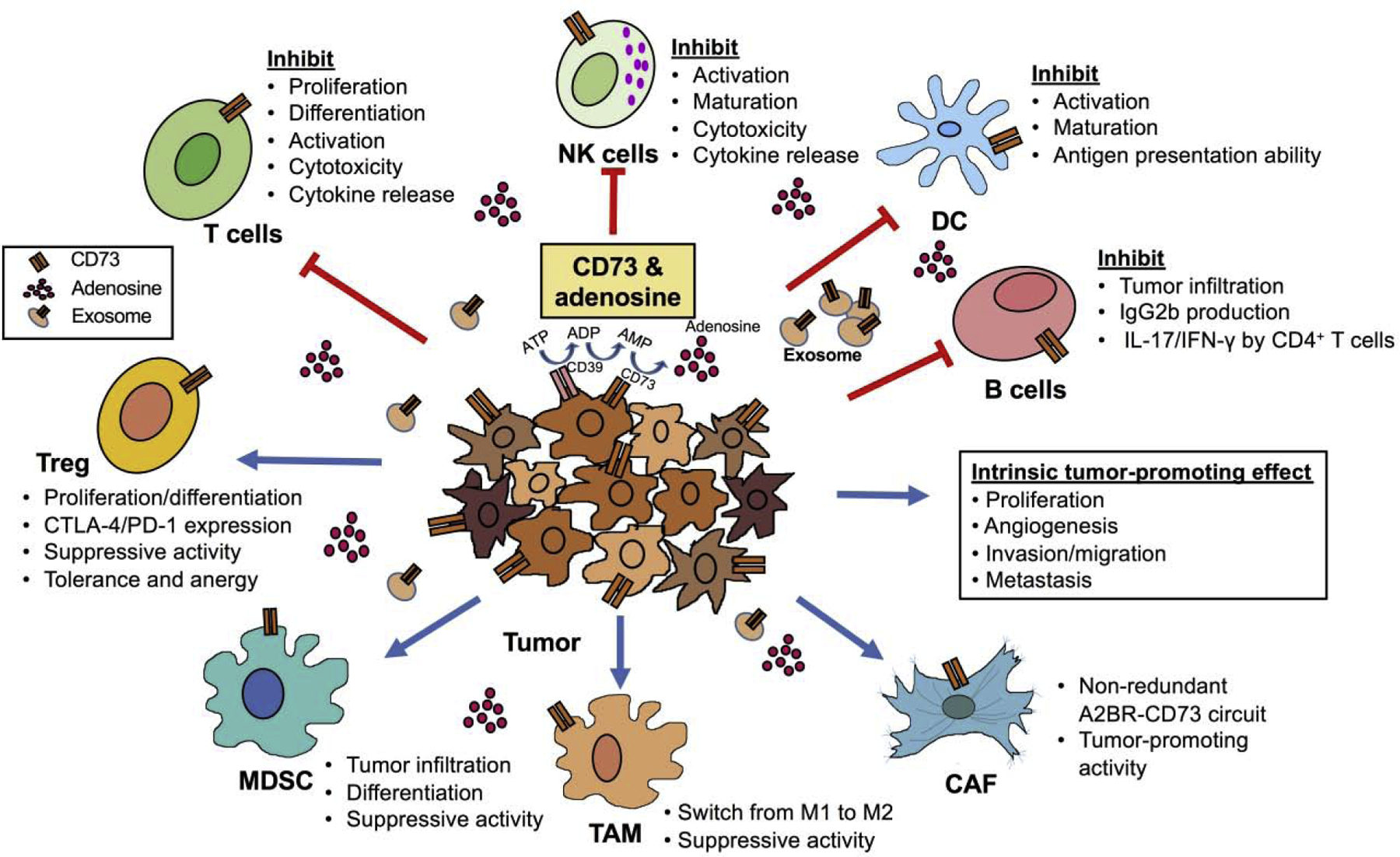
CD73 serve as a major immune suppressive mediator in TME mainly through generation of extracellular adenosine. Besides the effect of cancer cell-intrinsic CD73 on tumor cell proliferation, angiogenesis, invasion/migration and metastasis, CD73 expression by tumor cells and immune cells impairs anti-tumor immunity by suppressing the function of protective immune cells (e.g. effector T cells, NK cells, DC and B cells), while maintaining the function of regulatory immune cells (e.g. Treg, MDSC, TAM and CAF).
Regulatory T (Treg) cell:
In mice, CD73 is expressed on different subsets of T lymphocytes, but it is particularly abundant in Foxp3+ Tregs [49,50]. CD73 is crucial for Treg-mediated inhibition of effector T cell function as shown by impaired immunosuppressive capability of Treg cells in CD73–deficient tumor-bearing mice [14,15]. These effects are mediated mainly through A2AR on T effector cells. A2AR activation in naive CD4+ T cells promotes their differentiation towards Foxp3+ and LAG-3+ Treg cells and induce a long-term anergy [51,52]. In human, the CD73 expression level in Tregs is low, but increased in certain cancer patients [53,54]. especially after high-dose IL-2 therapy in melanoma patients [55]. Similar to mouse cell system, CD73 inhibition decreases Treg-mediated immunosuppressive function [56].
Effector T cell:
High level of CD73 is associated with the exhausted or anergic T cell phenotype [57,58]. Th17 cells express CD73 and CD39, and suppress effector T cell function dependent on the enzymatic activity of CD39/CD73 [59]. Furthermore, genetic ablation of CD73 or reducing CD73 by reprogramming Th17 cells improves antitumor effects by increasing their effector function [60]. As expected, A2AR agonist treatment inhibits T cell activation and proliferation, and induces T cell anergy [61–63]. Despite the role of CD73 by effector CD8+ T cells remains elusive, a recent study supported a prognostic value of CD8+ T cells expressing CD73 particularly after immunotherapy [64].
Natural killer (NK) cell:
The CD73 expression level in NK cells is low, but increased under specific conditions. For example, CD73 was induced on NK when co-cultured with human mesenchymal stem cells [65]. In gastrointestinal stromal tumors, tumor-infiltrating NK cells express higher levels of CD73 than those in PBMCs [66]. CD73 was also found on NK cells isolated from mouse melanoma [67], suggesting that tumor-infiltrating NK cells might acquire CD73 expression. CD73-produced adenosine suppresses NK cell functions primarily through A2AR [68,69]. A2AR activation hinders NK cell maturation, activation and cytotoxic function [67,68,70–72]. In contrast, loss of A2AR signaling in NK cells ameliorates CD73+ tumor metastasis and enhances anti-tumor immune response [66,73]. A recent study demonstrated that NK cells underwent phenotypic and functional switch to immunosuppressive population through acquiring CD73 in the TME [74], suggesting the importance of targeting CD73 for NK-based immunotherapy.
Myeloid derived suppressor cell (MDSC):
CD39 and CD73 levels on MDSC are higher in cancer patients [75–77]. CD73-mediated adenosine promotes MDSC function mainly through A2BR. A2BR antagonist inhibited the accumulation of tumor-infiltrating MDSCs in TME and this led to the delayed tumor growth in a mouse model [78]. In contrast, mice treated with a A2BR agonist accelerated tumor growth through enhanced MDSC infiltration to tumor and angiogenesis [79]. Tumor-derived TGF-β induced CD39 and CD73 on MDSCs through mTOR/HIF-1α pathway and CD39+CD73+ MDSCs represented a distinct inflammatory subpopulation associated with immunosuppressive signatures and chemotherapeutic response in the NSCLC patients [76]. On the other hand, metformin was found to reduce CD39 and CD73 via activation of AMP-activated protein kinase α, thereby blocking MDSC activity in patients with ovarian cancer [77]. These data suggest the targeting CD39/CD73 improves antitumor immunity in part through inhibition of MDSCs.
Macrophages:
In mice, CD39 and CD73 are expressed on resident macrophages [80,81], and their expression level changes depending on the activation state of the macrophage [82]. Modulation of CD73 activity determines macrophage function by switching M1 and M2 phenotype [13,82,83]. Notably, tumor-associated macrophages (TAMs) express CD39/CD73 that suppress CD4+ T cell proliferation through adenosine generation [84]. Furthermore, fasting-mediated tumor inhibition was related to reduced M2 polarization of TAMs with less CD73 and lower adenosine level in the TME [85]. Together, these data support the idea that activity of CD73 together with CD39 is required for fine-tuning of TAM function during tumor progression. However, more studies are needed for further clarification due to the conflicting report [86].
B cell:
The CD73 expression level is considered as an indication of B cell maturity [1,5]. In adult human, majority of B cells express CD73 [1], but neonatal B cells are deficient in CD73, and this deficiency seems to be responsible for impaired B cell function in early life [87]. Moreover, CD73 is required for class switch recombination in B cells [88,89]. In a murine melanoma model, CD73 activity in B cells was reported to play an important role in tumor growth [90]. Treatment with adenosine 5’-(α,β-methylene) diphosphate (APCP), a CD73 specific inhibitor, induced IL17A and facilitated the presence of B cells and the production of IgG2b within the melanoma [90].
CD73 as a novel therapeutic target for combination therapy
CD73 has recently emerged as a promising target for novel immunotherapy due to its critical role for anti-tumor immunity. In fact, inhibition of CD73 using either monoclonal antibodies (mAb) or small molecule inhibitor such as APCP have demonstrated antitumor effects in preclinical tumor mouse models [10,91]. Furthermore, a number of anti-CD73 mAbs (MEDI9447, BMS986179, SRF373/NZV930, CPI-006/CPX-006, IPH5301, TJ004309) and selective small molecule inhibitors (LY3475070, AB680, CB-708) are being tested in early phase clinical trials [92–94]. Notably, CD73 expression and activity can be increased upon different therapeutics. We will thus review the evidence below to support feasibility of targeting CD73 in combination with chemotherapy, radiation therapy, and other immunotherapies (see Figure 2).
Figure 2. Co-targeting CD73 with other therapies as an attractive therapeutic strategy for cancer treatment.
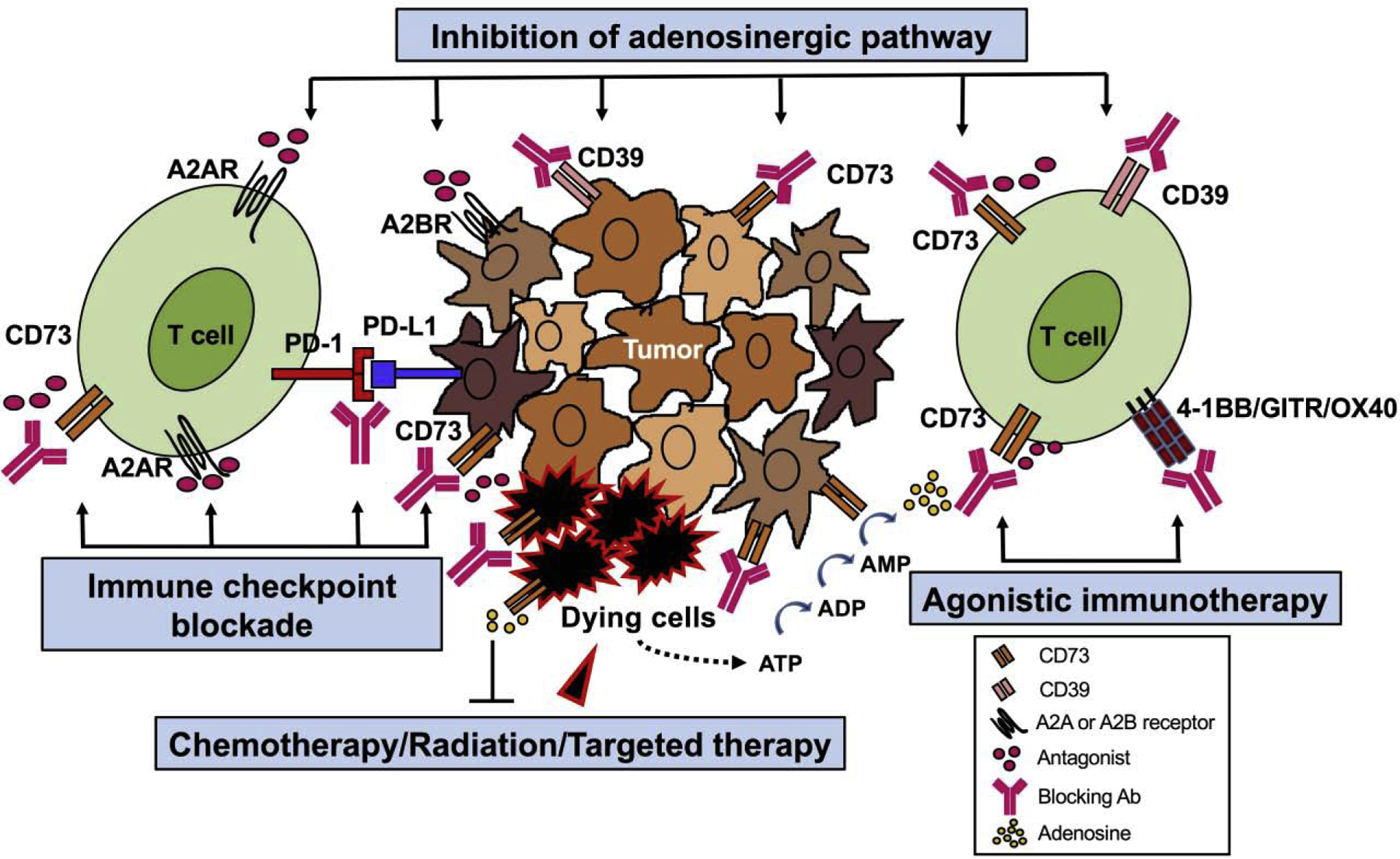
Based on their distinct expression pattern and nonredundant functionality, CD73 along with other molecules of adenosinergic pathway (e.g.CD39, A2AR and A2BR) can be targeted together to achieve synergy in antitumor efficacy by modulating both tumor cells and immune cells (e.g. T cells) in many ways. In addition, CD73 expression and activity seem to be upregulated to confer tumor resistance to therapies. Thus, targeting CD73 with blocking antibodies or small molecule inhibitors in combination with other therapies such as immune checkpoint blockade, adoptive T cell therapy, agonistic immunotherapy, chemotherapy, and radiation therapy is a rational strategy to enhance therapeutic benefit in various cancers. The different combination therapies involving inhibition of CD73 and/or A2AR are already under evaluation in early phase clinical trials.
Inhibition of the adenosinergic pathway:
Stimulated by the seminal work of Sitkovsky group showing the promise of A2AR inhibition for cancer immunotherapy [95], we [10] and others [91] have further demonstrated the therapeutic potential of targeting CD73-A2AR axis in multiple types of cancer. Inhibition of both CD73 and A2AR showed synergy in anti-tumor response in several mouse tumor models [67]. Importantly, a promising clinical response was reported in renal cell cancer (RCC) patients receiving A2AR antagonist (ciforadenant) alone or in combination with an anti-PD-L1 antibody (atezolizumab) [96]. Moreover, ciforadenant-mediated antitumor activity was associated with high levels of adenosine gene signature expression before treatment, suggesting that adenosine gene signature might serve as a predictive biomarker for adenosine blockade [67]. Although blockade of CD73 and A2AR is likely the most efficient strategy to neutralize tumor-driving adenosine effects, co-inhibition of CD73 and other adenosinergic members (e.g. A2BR, and CD39) is also a viable option, given their distinct expression pattern and nonredundant functionality. For example, blockade of CD73 and CD39 enzymatic activities resulted in greater inhibitory effect on human MDSC-mediated suppressive function [77]. Moreover, the anti-CD39/CD73 mAb combination at suboptimal doses acted in synergy to promote the proliferation of T cells from healthy donors and cancer patients [94]. A recent study also showed a synergistic anti-metastatic effect between anti-CD39 mAb and A2AR antagonists in mouse models of experimental and spontaneous metastases [97]. Different from other agents targeting the adenosinergic pathway, CD39 enzyme blockade-mediated anti-tumor effect was attributed to the critical role of an eATP-P2X7-ASC-NALP3-inflammasome-IL-18 pathway as mechanism of action [97,98]. On the other hand, it was reported that a non-canonical adenosinergic pathway led by CD38 might contribute to the immunosuppressive TME, especially serving as a mechanism of tumor cell escape from PD-1/PD-L1 blockade [99]. In addition, CD73 was upregulated via adenosine-A2BR circuit in cancer-associated fibroblasts (CAF), and inhibition of A2AR and A2BR together with CD73 blockade significantly enhanced antitumor immunity in murine CAF-rich tumors [100].
Immune checkpoint blockade (ICB):
In several murine tumor models, combination therapy of anti-CD73 with PD-L1 and/or anti-CTLA-4 enhanced therapeutic activity compared to monotherapy [101–104], Likewise, co-targeting A2AR and ICB showed therapeutic synergy [105]. Although ICB is effective in certain cancer patients, many patients do not respond (innate/primary resistance) or acquire resistance after initial response (acquired resistance). This might be due to the existence of alternative and/or therapy-induced immunosuppressive pathways in the TME. Supporting this notion, CD73 level was found to be upregulated in melanoma patients who received PD-1 immunotherapy [106]. Furthermore, comprehensive immune profiling indicated a unique CD73high macrophage population that persisted in glioblastoma patients after anti-PD-1 therapy [107]. CD73 deficiency enhanced the efficacy of anti-PD-1 and anti-CTLA-4 in a murine model of glioblastoma [107].
Adoptive cell therapy:
CD73 knockdown on tumor cells was sufficient to facilitate T cell effector function following tumor-specific T cell transfer, leading to tumor regression [11]. We further demonstrated that inhibition of CD73 activity by APCP or anti-CD73 mAb improved the efficacy of adoptive T cell therapy (ACT) using B16-SIY melanoma model and peritoneal ID8 tumor models [14]. CD73 upregulation was also observed in melanoma patients during ACT therapy [106]. Furthermore, CD73 was induced in relapsed melanomas in a mouse model of T-cell immunotherapy [106], providing the potential foundation for combining ACT therapy with CD73 blockade. Similarly, inhibition of A2AR pathway in T cells also increased the efficacy of ACT [95] and chimeric antigen receptor (CAR)-T cell therapy [108]. In addition, targeting CD73 activity with anti-CD73 antibody enhanced therapeutic efficacy of engineered CAR-NK cells against CD73+ tumors in human lung cancer xenograft models [109]. Thus, CD73 blockade could inhibit tumor growth in vivo dependent of both adaptive and innate immunity of ACT.
Agonistic immunotherapy:
Similar to blocking immune inhibitory molecules, activating immune co-stimulatory molecules on T cells is an open area to explore. Using preclinical models, we recently demonstrated that CD73 expression by T cells conferred tumor resistance to agonistic immunotherapy targeting 4–1BB, an inducible costimulatory molecule in the TNFR superfamily, while anti-4–1BB therapy preferentially mediated CD73-negative effector T cell response for tumor inhibition [110]. In addition, the synergistic antitumor effect was achieved by combination of CD73 blockade with anti-GITR (another potent T cell costimulatory molecule) as well [110]. Based on this exciting result, we infer that immunotherapeutic agonists targeting TNFR costimulatory receptors such as 4–1BB, GITR, OX40, or CD40 in combination with CD73 and/or other adenosinergic signaling molecules may be attractive for clinical development. Indeed, combination of CD73/A2AR blockade and anti-OX40 is being exploited in early phase clinical trials [110].
Chemotherapy:
CD73 has been shown to contribute to multidrug–resistance [34,111]. For instance, CD73 especially in TNBC patients was correlated with resistance to doxorubicin [35]. Doxorubicin treatment increased CD73 expression that led to the suppression of CD8+ T cells [35]. Increased frequency of CD47+CD73+PD-L1+ cell population in TNBC cells was also reported after treatment of other chemotherapeutic reagents such as carboplatin, gemcitabine, and paclitaxel [112]. By analyzing the sensitivity of NCI-60 cell lines to a panel of chemotherapeutic drugs, CD73 expression was found to be negatively associated with sensitivity to several chemotherapeutic reagents. And CD73 level was indeed elevated in platinum resistant ovarian cancer cells [113]. It was also reported that mesenchymal stem/stromal cells-derived IL-6 promoted cisplatin resistance by upregulating CD73 in nasopharyngeal carcinoma [114]. CD73 upregulation after chemotherapy seemed to be an attempt to counterbalance excess ATP released from dying tumor cells after therapy [115,116]. Additionally, CD73-mediated adenosine signaling seems to downregulate ABC transporters and P-glycoprotein, a drug efflux transporter, thereby contributing to drug resistance [111,117,118].
Radiation therapy:
Beside direct cancer cell killing by radiation, radiation therapy can also affect immune response [119]. For example, radiation induced apoptosis of NK cells and T cells, and B cells [120], but recruited and activated DCs. This differential effect of radiation on immune regulation is likely dependent on the ratio of ATP to adenosine. Interestingly, enhanced CD73 enzymatic activity was observed in irradiated lung tissue, and involved in pulmonary fibrosis [121], which is a severe side effect of thoracic irradiation. In particular, treatment with anti-CD73 mAb significantly reduced radiation-induced lung fibrosis [121], suggesting that CD73 inhibition might be a promising mean in limiting lung toxicities associated with the treatment of thoracic malignancies. Radiation also increased CD73 expression on human breast cancer cells, and CD73 inhibition combined with radiotherapy showed better antitumor response due to enhanced antitumor T cell response [122]. However, pharmacological inhibition or knocking out CD73 was found to rescue proliferative capacity of T24 human bladder cancer cells, thereby reducing their sensitivity to radiation [123].
Targeted therapy:
High levels of CD73 gene expression were found to link significantly with poor outcome in a randomized phase III clinical trial evaluating the activity of the anti-HER2/ErbB2 mAb trastuzumab, indicating the potential role of CD73 in conferring tumor resistance to targeted therapies [124]. Indeed, anti-CD73 mAb therapy augmented the efficacy of anti-ErbB2 mAb in immunocompetent mouse models of HER2/ErbB2-driven breast cancer. Furthermore, it is of note that clinical trials are ongoing with anti-CD73 mAb in combination with anti-EGFR therapy or A2AR inhibitors in non-small cell lung cancer (NCT03381274). Similarly, more advanced clinical stage disease was associated with increased CD73 expression despite CD73 expression was not an independent prognostic factor in melanoma [125]. Interestingly, activating MAPK mutations and growth factors drove CD73 expression [106], while BRAF and MEK inhibition potently reduced CD73 expression [125]. Inhibition of adenosine signaling with A2AR antagonist along with BRAF and MEK inhibition enhanced antitumor effects of BRAF-mutated melanoma in mice [125]. These studies together open new avenues for developing targeting CD73-mediated adenosine signaling in combination with targeted therapies and provide insights into how CD73 is regulated in cancer treatment.
Conclusion
CD73 is an ideal therapeutic target of cancer therapy for the following reasons; (i) CD73 expression by cancer cells and host cells including, but limited to, a variety of immune cell populations, creates immunosuppressive adenosine-rich TME. Evolving data support the tumor-promoting role of cancer cell-intrinsic CD73. (ii) CD73 promotes tumor growth and metastasis primarily via its enzymatic activity. The role of CD73 independent of its enzymatic activity in tumorigenesis warrants intensive investigation, providing novel insight into the regulatory function of CD73 in cancer. (iii) As CD73 expression and activity seem to be modulated upon many therapies, co-targeting CD73 with other therapeutic reagents represents a rational strategy. CD73 inhibition in general is expected to boost immune response to keep the tumor cells in control. However, certain concerns on undesirable side effects remain due to ubiquitous expression of CD73 on multiple cell types in various tissues. Notably, there were patients that received BMS-986179, an anti-CD73 mAb that experienced cardiac events while on the clinical study; this led to a change in the inclusion criteria for recruitment of patients. Nevertheless, BMS-986179 in combination with nivolumab appears to have the same toxicity as nivolumab alone (LL Siu et al., abstract CT180, 109th American Association for Cancer Research, Chicago, April 2018). With several clinical trials currently evaluating inhibitors of the adenosine pathway in cancers, the pathophysiological role of adenosine with a focus on effects on antitumor immunity has been comprehensively reviewed [126]. We believe that designing anti-CD73 mAbs that incorporate alternative action mechanism such as Fc receptor engagement to maximize anti-tumor effects and development of more potent and selective small molecule inhibitors with longer half-life would greatly enhance therapeutic efficacy. More attention should be paid especially to important avenues of clinical studies in the future including evaluation of membrane and soluble CD73 as a potential prognostic or/and predictive biomarker, and clarification of the mechanisms of action for CD73 blockade and in combination therapy together with adverse effects.
Highlights.
CD73 is a multifunctional ectoenzyme affecting both tumor cells and immune cells.
CD73 has been hijacked by TME to promote tumor growth and metastasis.
Targeting CD73 and other adenosinergic molecules orchestrates anti-tumor immunity.
CD73 blockade achieves synergy in combination with conventional therapy and/or ICB.
Acknowledgments
This research was in part supported by National Institutes of Health grant CA149669, CA208354 and CA222963, and Northwestern University RHLCCC NCI CCSG P30 CA060553. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
- This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
- The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Resta R, Yamashita Y, Thompson LF: Ecto-enzyme and signaling functions of lymphocyte cd73. Immunol Rev 1998, 161: 95–109. [DOI] [PubMed] [Google Scholar]; * This review describes the function of CD73 including histological perspective of how CD73 came to attention of immunologist and distribution of CD73 on lymphoid tissues in both human and murine.
- 2.Colgan SP, Eltzschig HK, Eckle T, Thompson LF: Physiological roles for ecto-5’-nucleotidase (cd73). Purinergic Signal 2006, 2(2):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson LF, Ruedi JM, Glass A, Low MG, Lucas AH: Antibodies to 5’-nucleotidase (cd73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood t cells to proliferate. J Immunol 1989, 143(6):1815–1821. [PubMed] [Google Scholar]
- 4.Airas L, Niemela J, Salmi M, Puurunen T, Smith DJ, Jalkanen S: Differential regulation and function of cd73, a glycosyl-phosphatidylinositol-linked 70-kd adhesion molecule, on lymphocytes and endothelial cells. J Cell Biol 1997, 136(2):421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita Y, Hooker SW, Jiang H, Laurent AB, Resta R, Khare K, Coe A, Kincade PW, Thompson LF: Cd73 expression and fyn-dependent signaling on murine lymphocytes. Eur J Immunol 1998, 28(10):2981–2990. [DOI] [PubMed] [Google Scholar]
- 6.Koszalka P, Ozuyaman B, Huo Y, Zernecke A, Flogel U, Braun N, Buchheiser A, Decking UK, Smith ML, Sevigny J, Gear A et al. : Targeted disruption of cd73/ecto-5’-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res 2004, 95(8):814–821. [DOI] [PubMed] [Google Scholar]
- 7.Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL: Surface expression, polarization, and functional significance of cd73 in human intestinal epithelia. J Clin Invest 1997, 99(11):2588–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S: The p2×7 receptor in infection and inflammation. Immunity 2017, 47(1):15–31. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Galluzzi L, Kepp O, Zitvogel L: Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013, 31:51–72. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Wainwright DA, Wu JD, Wan Y, Matei DE, Zhang Y, Zhang B: Cd73: An emerging checkpoint for cancer immunotherapy. Immunotherapy 2019, 11(11):983–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B: Cd73 on tumor cells impairs antitumor t-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Res 2010, 70(6):2245–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrates a potential mechanism for tumor CD73-mediated immune evasion and developing an immunotherapy strategy by targeting the enzymatic activity of CD73.
- 12.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ: Anti-cd73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 2010, 107(4):1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first preclinical study showing that therapeutic potential of anti-CD73 mAb therapy to improve adaptive antitumor immunity and inhibit metastasis of breast cancer.
- 13.Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemela J, Laurila JP, Elima K, Jalkanen S, Salmi M: Altered purinergic signaling in cd73-deficient mice inhibits tumor progression. Eur J Immunol 2011, 41(5):1231–1241. [DOI] [PubMed] [Google Scholar]; * Using CD73-deficient mice, this study shows that the host CD73 can shift the purinergic signaling toward anti-tumor immunity and tumor progression through reduced accumulation of Tregs and type 2 like macrophages.
- 14.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B: Cd73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest 2011, 121(6):2371–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first study demonstrating the specific contribution of tumor or host CD73 to tumor growth and optimal antitumor effects that can be achieved by blocking both tumor and host CD73.
- 15.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ: Cd73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 2011, 71(8):2892–2900. [DOI] [PubMed] [Google Scholar]; ** This paper shows that the role of host-derived CD73 on anti-tumor immunity and metastasis and the importance of CD73 expression on Tregs for Treg-mediated tumor growth.
- 16.Stagg J, Beavis PA, Divisekera U, Liu MC, Moller A, Darcy PK, Smyth MJ: Cd73-deficient mice are resistant to carcinogenesis. Cancer Res 2012, 72(9):2190–2196. [DOI] [PubMed] [Google Scholar]
- 17.Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z, Yin L: Rna interference of ecto-5’-nucleotidase (cd73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis 2007, 24(6):439–448. [DOI] [PubMed] [Google Scholar]
- 18.Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DM, Haibe-Kains B, Karn T, Rahimi K et al. : Cd73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res 2015, 75(21):4494–4503. [DOI] [PubMed] [Google Scholar]
- 19.Wu R, Chen Y, Li F, Li W, Zhou H, Yang Y, Pei Z: Effects of cd73 on human colorectal cancer cell growth in vivo and in vitro. Oncol Rep 2016, 35(3):1750–1756. [DOI] [PubMed] [Google Scholar]
- 20.Gao ZW, Wang HP, Lin F, Wang X, Long M, Zhang HZ, Dong K: Cd73 promotes proliferation and migration of human cervical cancer cells independent of its enzyme activity. BMC Cancer 2017, 17(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Jia S, Chen Y, Wang W, Wu Z, Yu W, Zhang M, Ding G, Cao L: The distinct role of cd73 in the progression of pancreatic cancer. J Mol Med (Berl) 2019, 97(6):803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadej R, Spychala J, Skladanowski AC: Expression of ecto-5’-nucleotidase (en, cd73) in cell lines from various stages of human melanoma. Melanoma Res 2006, 16(3):213–222. [DOI] [PubMed] [Google Scholar]
- 23.Koszalka P, Golunska M, Stanislawowski M, Urban A, Stasilojc G, Majewski M, Wierzbicki P, Skladanowski AC, Bigda J: Cd73 on b16f10 melanoma cells in cd73-deficient mice promotes tumor growth, angiogenesis, neovascularization, macrophage infiltration and metastasis. Int J Biochem Cell Biol 2015, 69:1–10. [DOI] [PubMed] [Google Scholar]
- 24.Lupia M, Angiolini F, Bertalot G, Freddi S, Sachsenmeier KF, Chisci E, Kutryb-Zajac B, Confalonieri S, Smolenski RT, Giovannoni R, Colombo N et al. : Cd73 regulates stemness and epithelial-mesenchymal transition in ovarian cancer-initiating cells. Stem Cell Reports 2018), 10(4):1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Tang S, Wang Y, Xu S, Yu J, Zhi X, Ou Z, Yang J, Zhou P, Shao Z: Ecto-5’-nucleotidase (cd73) promotes tumor angiogenesis. Clin Exp Metastasis 2013, 30(5):671–680. [DOI] [PubMed] [Google Scholar]
- 26.Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J: Anti-cd73 therapy impairs tumor angiogenesis. Int J Cancer 2014, 134(6):1466–1473. [DOI] [PubMed] [Google Scholar]
- 27.Ghalamfarsa G, Rastegari A, Atyabi F, Hassannia H, Hojjat-Farsangi M, Ghanbari A, Anvari E, Mohammadi J, Azizi G, Masjedi A, Yousefi M et al. : Anti-angiogenic effects of cd73-specific sirna-loaded nanoparticles in breast cancer-bearing mice. J Cell Physiol 2018, 233(10):7165–7177. [DOI] [PubMed] [Google Scholar]
- 28.Allard B, Cousineau I, Allard D, Buisseret L, Pommey S, Chrobak P, Stagg J: Adenosine a2a receptor promotes lymphangiogenesis and lymph node metastasis. Oncoimmunology 2019, 8(8):1601481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma XL, Shen MN, Hu B, Wang BL, Yang WJ, Lv LH, Wang H, Zhou Y, Jin AL, Sun YF, Zhang CY et al. : Cd73 promotes hepatocellular carcinoma progression and metastasis via activating pi3k/akt signaling by inducing rap1-mediated membrane localization of p110beta and predicts poor prognosis. J Hematol Oncol 2019, 12(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z, Gu C, Yao X, Guo W, Wang H, Lin T, Li F, Chen D, Wu J, Ye G, Zhao L et al. : Cd73 promotes tumor metastasis by modulating rics/rhoa signaling and emt in gastric cancer. Cell Death Dis 2020, 11(3):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteiro I, Vigano S, Faouzi M, Treilleux I, Michielin O, Menetrier-Caux C, Caux C, Romero P, de Leval L: Cd73 expression and clinical significance in human metastatic melanoma. Oncotarget 2018, 9(42):26659–26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang T, Xu X, Qiao M, Li X, Zhao C, Zhou F, Gao G, Wu F, Chen X, Su C, Ren S et al. : Comprehensive evaluation of nt5e/cd73 expression and its prognostic significance in distinct types of cancers. BMC Cancer 2018, 18(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buisseret L, Pommey S, Allard B, Garaud S, Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA, Di Leo A et al. : Clinical significance of cd73 in triple-negative breast cancer: Multiplex analysis of a phase iii clinical trial. Ann Oncol 2018, 29(4):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ujhazy P, Berleth ES, Pietkiewicz JM, Kitano H, Skaar JR, Ehrke MJ, Mihich E: Evidence for the involvement of ecto-5’-nucleotidase (cd73) in drug resistance. Int J Cancer 1996, 68(4):493–500. [DOI] [PubMed] [Google Scholar]; * This is the first study showing that CD73 is involved in drug resistance through its role in the extracellular adenosine salvage pathway.
- 35.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J: Cd73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013, 110(27):11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supernat A, Markiewicz A, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J, Sejda A, Szade J, Czapiewski P, Biernat W, Zaczek A: Cd73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol 2012, 20(2):103–107. [DOI] [PubMed] [Google Scholar]
- 37.Oh HK, Sin JI, Choi J, Park SH, Lee TS, Choi YS: Overexpression of cd73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory t cell infiltration. J Gynecol Oncol 2012, 23(4):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcedo KP, Guerrero A, Basrur V, Fu D, Richardson ML, McLane JS, Tsou CC, Nesvizhskii AI, Welling TH, Lebrilla CB, Otey CA et al. : Tumor-selective altered glycosylation and functional attenuation of cd73 in human hepatocellular carcinoma. Hepatol Commun 2019, 3(10):1400–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snider NT, Altshuler PJ, Wan S, Welling TH, Cavalcoli J, Omary MB: Alternative splicing of human nt5e in cirrhosis and hepatocellular carcinoma produces a negative regulator of ecto-5’-nucleotidase (cd73). Mol Biol Cell 2014, 25(25):4024–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rackley RR, Lewis TJ, Preston EM, Delmoro CM, Bradley EL Jr., Resnick MI, Pretlow TP, Pretlow TG: 5’-nucleotidase activity in prostatic carcinoma and benign prostatic hyperplasia. Cancer Res 1989, 49(13):3702–3707. [PubMed] [Google Scholar]
- 41.Durak I, Isik AC, Canbolat O, Akyol O, Kavutcu M: Adenosine deaminase, 5’ nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in cancerous and noncancerous human laryngeal tissues. Free Radic Biol Med 1993, 15(6):681–684. [DOI] [PubMed] [Google Scholar]
- 42.Eroglu A, Canbolat O, Demirci S, Kocaoglu H, Eryavuz Y, Akgul H: Activities of adenosine deaminase and 5’-nucleotidase in cancerous and noncancerous human colorectal tissues. Med Oncol 2000, 17(4):319–324. [DOI] [PubMed] [Google Scholar]
- 43.Bowser JL, Blackburn MR, Shipley GL, Molina JG, Dunner K Jr., Broaddus RR: Loss of cd73-mediated actin polymerization promotes endometrial tumor progression. J Clin Invest 2016, 126(1):220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klemens MR, Sherman WR, Holmberg NJ, Ruedi JM, Low MG, Thompson LF: Characterization of soluble vs membrane-bound human placental 5’-nucleotidase. Biochem Biophys Res Commun 1990, 172(3):1371–1377. [DOI] [PubMed] [Google Scholar]
- 45.Morello S, Capone M, Sorrentino C, Giannarelli D, Madonna G, Mallardo D, Grimaldi AM, Pinto A, Ascierto PA: Soluble cd73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J Transl Med 2017, 15(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z: Cancer exosomes express cd39 and cd73, which suppress t cells through adenosine production. J Immunol 2011, 187(2):676–683. [DOI] [PubMed] [Google Scholar]; * This is the first study showing that adenosine in the TME can be generated through the enzymatic activity of CD39 and CD73 on exosome, thereby inhibiting T cell function.
- 47.Schuler PJ, Saze Z, Hong CS, Muller L, Gillespie DG, Cheng D, Harasymczuk M, Mandapathil M, Lang S, Jackson EK, Whiteside TL: Human cd4+ cd39+ regulatory t cells produce adenosine upon co-expression of surface cd73 or contact with cd73+ exosomes or cd73+ cells. Clin Exp Immunol 2014, 177(2):531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, Tabi Z: Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles 2017, 6(1):1368823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR: T regulatory and primed uncommitted cd4 t cells express cd73, which suppresses effector cd4 t cells by converting 5’-adenosine monophosphate to adenosine. J Immunol 2006, 177(10):6780–6786. [DOI] [PubMed] [Google Scholar]; * This is the first study showing a potential mechanism by which CD73 enzymactic activity by Treg and unprimed CD4+ T cells suppresses effector CD4+ T cell function.
- 50.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK et al. : Adenosine generation catalyzed by cd39 and cd73 expressed on regulatory t cells mediates immune suppression. J Exp Med 2007, 204(6):1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD: A2a receptor signaling promotes peripheral tolerance by inducing t-cell anergy and the generation of adaptive regulatory t cells. Blood 2008, 111(1):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M: The development and immunosuppressive functions of cd4(+) cd25(+) foxp3(+) regulatory t cells are under influence of the adenosine-a2a adenosine receptor pathway. Front Immunol 2012, 3:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, Gorelik E, Lang S, Johnson JT, Whiteside TL: Increased ectonucleotidase expression and activity in regulatory t cells of patients with head and neck cancer. Clin Cancer Res 2009, 15(20):6348–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Gennaro P, Gerlini G, Caporale R, Sestini S, Brandani P, Urso C, Pimpinelli N, Borgognoni L: T regulatory cells mediate immunosuppresion by adenosine in peripheral blood, sentinel lymph node and tils from melanoma patients. Cancer Lett 2018, 417:124–130. [DOI] [PubMed] [Google Scholar]
- 55.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P et al. : Il-2 therapy promotes suppressive icos+ treg expansion in melanoma patients. J Clin Invest 2014, 124(1):99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL: Generation and accumulation of immunosuppressive adenosine by human cd4+cd25highfoxp3+ regulatory t cells. J Biol Chem 2010, 285(10):7176–7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, Mueller DL: Arthritogenic self-reactive cd4+ t cells acquire an fr4hicd73hi anergic state in the presence of foxp3+ regulatory t cells. J Immunol 2012, 188(1):170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng WW, Li YC, Ma SR, Mao L, Yu GT, Bu LL, Kulkarni AB, Zhang WF, Sun ZJ: Specific blockade cd73 alters the “exhausted” phenotype of t cells in head and neck squamous cell carcinoma. Int J Cancer 2018, 143(6):1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, Robson SC et al. : Stat3 and gfi-1 transcription factors control th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 2012, 36(3):362–373. [DOI] [PubMed] [Google Scholar]
- 60.Chatterjee S, Thyagarajan K, Kesarwani P, Song JH, Soloshchenko M, Fu J, Bailey SR, Vasu C, Kraft AS, Paulos CM, Yu XZ et al. : Reducing cd73 expression by il1beta-programmed th17 cells improves immunotherapeutic control of tumors. Cancer Res 2014, 74(21):6048–6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang S, Apasov S, Koshiba M, Sitkovsky M: Role of a2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of t-cell activation and expansion. Blood 1997, 90(4):1600–1610. [PubMed] [Google Scholar]
- 62.Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH: Activation of th1 and tc1 cell adenosine a2a receptors directly inhibits il-2 secretion in vitro and il-2-driven expansion in vivo. Blood 2005, 105(12):4707–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lappas CM, Rieger JM, Linden J: A2a adenosine receptor induction inhibits ifn-gamma production in murine cd4+ t cells. J Immunol 2005, 174(2):1073–1080. [DOI] [PubMed] [Google Scholar]
- 64.Capone M, Fratangelo F, Giannarelli D, Sorrentino C, Turiello R, Zanotta S, Galati D, Madonna G, Tuffanelli M, Scarpato L, Grimaldi AM et al. : Frequency of circulating cd8+cd73+t cells is associated with survival in nivolumab-treated melanoma patients. J Transl Med 2020, 18(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chatterjee D, Tufa DM, Baehre H, Hass R, Schmidt RE, Jacobs R: Natural killer cells acquire cd73 expression upon exposure to mesenchymal stem cells. Blood 2014, 123(4):594–595. [DOI] [PubMed] [Google Scholar]
- 66.Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, Lin G, Coudert JD, Stannard KA, Zitvogel L, Degli-Esposti MA et al. : A2ar adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res 2018, 78(4):1003–1016. [DOI] [PubMed] [Google Scholar]
- 67.Young A, Ngiow SF, Barkauskas DS, Sult E, Hay C, Blake SJ, Huang Q, Liu J, Takeda K, Teng MWL, Sachsenmeier K et al. : Co-inhibition of cd73 and a2ar adenosine signaling improves anti-tumor immune responses. Cancer Cell 2016, 30(3):391–403. [DOI] [PubMed] [Google Scholar]; ** This is the first study showing the non-redundant activity of CD73 and A2AR inhibition to promote therapeutic response by co-targeting adenosinergic signaling molecules.
- 68.Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E: Adenosine-mediated inhibition of cytotoxic activity and cytokine production by il-2/nkp46-activated nk cells: Involvement of protein kinase a isozyme i (pka i). Immunol Res 2006, 36(1–3):91–99. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Matosevic S: Adenosinergic signaling as a target for natural killer cell immunotherapy. J Mol Med (Berl) 2018, 96(9):903–913. [DOI] [PubMed] [Google Scholar]
- 70.Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK: Blockade of a2a receptors potently suppresses the metastasis of cd73+ tumors. Proc Natl Acad Sci U S A 2013,110(36):14711–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, Sethumadhavan S, Philbrook P, Ko K, Cannici R, Thayer M et al. : Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med 2015, 7(277):277ra230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chambers AM, Wang J, Lupo KB, Yu H, Atallah Lanman NM, Matosevic S: Adenosinergic signaling alters natural killer cell functional responses. Front Immunol 2018, 9: 2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin L, Thompson LF, Kuzel TM, Zhang B: Requirement of nk cells for selective a2a receptor blockade to suppress cd73+ tumor metastasis. Immunotherapy 2014, 6(1):19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neo SY, Yang Y, Record J, Ma R, Chen X, Chen Z, Tobin NP, Blake E, Seitz C, Thomas R, Wagner AK et al. : Cd73 immune checkpoint defines regulatory nk cells within the tumor microenvironment. J Clin Invest 2020, 130(3):1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrates that NK cells can be converted to immunosuppressive cells for tumor escape by upregulating CD73 expression.
- 75.Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, Vincent J, Bengrine-Lefevre L et al. : Accumulation of mdsc and th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a folfox-bevacizumab drug treatment regimen. Cancer Res 2016, 76(18):5241–5252. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Wang L, Chen X, Li L, Li Y, Ping Y, Huang L, Yue D, Zhang Z, Wang F, Li F et al. : Cd39/cd73 upregulation on myeloid-derived suppressor cells via tgf-beta-mtor-hif-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 2017, 6(6):e1320011. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first study demonstrating that human tumor-infiltrating MDSCs express CD39 and CD73 and their enzymatic activities are required for MDSC-mediated immunosuppressive effects and associated with chemotherapeutic response in the NSCLC patients.
- 77.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, Zhang C, Yue D, Qin G, Zhang T, Li F et al. : Metformin-induced reduction of cd39 and cd73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res 2018, 78(7):1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iannone R, Miele L, Maiolino P, Pinto A, Morello S: Blockade of a2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia 2013, 15(12):1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorrentino C, Miele L, Porta A, Pinto A, Morello S: Myeloid-derived suppressor cells contribute to a2b adenosine receptor-induced vegf production and angiogenesis in a mouse melanoma model. Oncotarget 2015, 6(29):27478–27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamidzadeh K, Mosser DM: Purinergic signaling to terminate tlr responses in macrophages. Front Immunol 2016, 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy PS, Wang J, Bhagwat SP, Munger JC, Janssen WJ, Wright TW, Elliott MR: Cd73 regulates anti-inflammatory signaling between apoptotic cells and endotoxin-conditioned tissue macrophages. Cell Death Differ 2017, 24(3):559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zanin RF, Braganhol E, Bergamin LS, Campesato LF, Filho AZ, Moreira JC, Morrone FB, Sevigny J, Schetinger MR, de Souza Wyse AT, Battastini AM: Differential macrophage activation alters the expression profile of ntpdase and ecto-5’-nucleotidase. PLoS One 2012, 7(2):e31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ponce NE, Sanmarco LM, Eberhardt N, Garcia MC, Rivarola HW, Cano RC, Aoki MP: Cd73 inhibition shifts cardiac macrophage polarization toward a microbicidal phenotype and ameliorates the outcome of experimental chagas cardiomyopathy. J Immunol 2016, 197(3):814–823. [DOI] [PubMed] [Google Scholar]
- 84.Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, Dietl J, Lutz MB, Mittelbronn M, Wischhusen J, Hausler SFM: Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, cd39- and cd73-dependent mechanism for tumor immune escape. J Immunother Cancer 2016, 4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun P, Wang H, He Z, Chen X, Wu Q, Chen W, Sun Z, Weng M, Zhu M, Ma D, Miao C: Fasting inhibits colorectal cancer growth by reducing m2 polarization of tumor-associated macrophages. Oncotarget 2017, 8(43):74649–74660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eichin D, Laurila JP, Jalkanen S, Salmi M: Cd73 activity is dispensable for the polarization of m2 macrophages. PLoS One 2015, 10(8):e0134721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pettengill MA, Levy O: Circulating human neonatal naive b cells are deficient in cd73 impairing purine salvage. Front Immunol 2016, 7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schena F, Volpi S, Faliti CE, Penco F, Santi S, Proietti M, Schenk U, Damonte G, Salis A, Bellotti M, Fais F et al. : Dependence of immunoglobulin class switch recombination in b cells on vesicular release of atp and cd73 ectonucleotidase activity. Cell Rep 2013, 3(6):1824–1831. [DOI] [PubMed] [Google Scholar]
- 89.Allard D, Charlebois R, Gilbert L, Stagg J, Chrobak P: Cd73-a2a adenosine receptor axis promotes innate b cell antibody responses to pneumococcal polysaccharide vaccination. PLoS One 2018, 13(1):e0191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S: Inhibition of cd73 improves b cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol 2012, 189(5):2226–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This is the first study showing the critical role of B cells for the anti-tumor activity of a APCP, a CD73-specific inhibitor.
- 91.Allard D, Chrobak P, Allard B, Messaoudi N, Stagg J: Targeting the cd73-adenosine axis in immuno-oncology. Immunol Lett 2019, 205: 31–39. [DOI] [PubMed] [Google Scholar]
- 92.Geoghegan JC, Diedrich G, Lu X, Rosenthal K, Sachsenmeier KF, Wu H, Dall’Acqua WF, Damschroder MM: Inhibition of cd73 amp hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. MAbs 2016, 8(3):454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Overman MJ, LoRusso P, Strickler JH, Patel SP, Clarke SJ, Noonan AM et al. : Safety, efficacy and pharmacodynamics (pd) of medi9447 (oleclumab) alone or in combination with durvalumab in advanced colorectal cancer (crc) or pancreatic cancer (panc). . Journal of Clinical Oncology 2018, 36:15_suppl, 4123–4123. [Google Scholar]
- 94.Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, Courtois R, Dejou C, Jecko D, Becquart O, Rispaud-Blanc H et al. : Blocking antibodies targeting the cd39/cd73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep 2019, 27(8):2411–2425 e2419. [DOI] [PubMed] [Google Scholar]
- 95.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF et al. : A2a adenosine receptor protects tumors from antitumor t cells. Proc Natl Acad Sci U S A 2006, 103(35):13132–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first preclinical study showing the therapeutic potential of A2AR using its genetic ablation and pharmacological inhibition.
- 96.Fong L, Hotson A, Powderly JD, Sznol M, Heist RS, Choueiri TK, George S, Hughes BGM, Hellmann MD, Shepard DR, Rini BI et al. : Adenosine 2a receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov 2020, 10(1):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan J, Li XY, Roman Aguilera A, Xiao C, Jacoberger-Foissac C, Nowlan B, Robson SC, Beers C, Moesta AK, Geetha N, Teng MWL et al. : Control of metastases via myeloid cd39 and nk cell effector function. Cancer Immunol Res 2020, 8(3):356–367. [DOI] [PubMed] [Google Scholar]
- 98.Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, Madore J, Lepletier A, Aguilera AR, Sundarrajan A, Jacoberger-Foissac C et al. : Targeting cd39 in cancer reveals an extracellular atp- and inflammasome-driven tumor immunity. Cancer Discov 2019, 9(12):1754–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, Villalobos PA, Cascone T, Liu X, Tan L, Lorenzi PL et al. : Cd38-mediated immunosuppression as a mechanism of tumor cell escape from pd-1/pd-l1 blockade. Cancer Discov 2018, 8(9):1156–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu M, Guo G, Huang L, Deng L, Chang CS, Achyut BR, Canning M, Xu N, Arbab AS, Bollag RJ, Rodriguez PC et al. : Cd73 on cancer-associated fibroblasts enhanced by the a2b-mediated feedforward circuit enforces an immune checkpoint. Nat Commun 2020, 11(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study identifies the importance of CD73 by cancer-associated fibroblasts (CAF) for cancer immunotherapies.
- 101.Allard B, Pommey S, Smyth MJ, Stagg J: Targeting cd73 enhances the antitumor activity of anti-pd-1 and anti-ctla-4 mabs. Clin Cancer Res 2013, 19(20):5626–5635. [DOI] [PubMed] [Google Scholar]; ** This is the first preclinical study showing that anti-CD73 mAb can enhance the therapeutic activity of anti-PD-1 and anti-CTLA blockade.
- 102.Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ: Antimetastatic effects of blocking pd-1 and the adenosine a2a receptor. Cancer Res 2014, 74(14):3652–3658. [DOI] [PubMed] [Google Scholar]
- 103.Iannone R, Miele L, Maiolino P, Pinto A, Morello S: Adenosine limits the therapeutic effectiveness of anti-ctla4 mab in a mouse melanoma model. Am J Cancer Res 2014, 4(2):172–181. [PMC free article] [PubMed] [Google Scholar]
- 104.Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, Hammond SA, Rothstein R, Rios-Doria J, Poon E, Holoweckyj N et al. : Targeting cd73 in the tumor microenvironment with medi9447. Oncoimmunology 2016, 5(8):e1208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK: Adenosine receptor 2a blockade increases the efficacy of anti-pd-1 through enhanced antitumor t-cell responses. Cancer Immunol Res 2015, 3(5):506–517. [DOI] [PubMed] [Google Scholar]
- 106.Reinhardt J, Landsberg J, Schmid-Burgk JL, Ramis BB, Bald T, Glodde N, Lopez-Ramos D, Young A, Ngiow SF, Nettersheim D, Schorle H et al. : Mapk signaling and inflammation link melanoma phenotype switching to induction of cd73 during immunotherapy. Cancer Res 2017, 77(17):4697–4709. [DOI] [PubMed] [Google Scholar]
- 107.Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, Vence L, Blando J, Zhao H, Yadav SS, Ott M et al. : Immune profiling of human tumors identifies cd73 as a combinatorial target in glioblastoma. Nat Med 2020, 26(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, Davenport AJ, John LB, Mardiana S, Slaney CY, Johnstone RW et al. : Targeting the adenosine 2a receptor enhances chimeric antigen receptor t cell efficacy. J Clin Invest 2017, 127(3):929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Lupo KB, Chambers AM, Matosevic S: Purinergic targeting enhances immunotherapy of cd73(+) solid tumors with piggybac-engineered chimeric antigen receptor natural killer cells. J Immunother Cancer 2018, 6(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen S, Fan J, Zhang M, Qin L, Dominguez D, Long A, Wang G, Ma R, Li H, Zhang Y, Fang D et al. : Cd73 expression on effector t cells sustained by tgf-beta facilitates tumor resistance to anti-4–1bb/cd137 therapy. Nat Commun 2019, 10(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first preclinical study indicating a synergistic combination of anti-CD73 mAb and agonist anti-4–1BB or anti-GITR therapy.
- 111.Ujhazy P, Klobusicka M, Babusikova O, Strausbauch P, Mihich E, Ehrke MJ: Ecto-5’-nucleotidase (cd73) in multidrug-resistant cell lines generated by doxorubicin. Int J Cancer 1994, 59(1):83–93. [DOI] [PubMed] [Google Scholar]
- 112.Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL: Chemotherapy induces enrichment of cd47(+)/cd73(+)/pdl1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A 2018, 115(6):E1239–E1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nevedomskaya E, Perryman R, Solanki S, Syed N, Mayboroda OA, Keun HC: A systems oncology approach identifies nt5e as a key metabolic regulator in tumor cells and modulator of platinum sensitivity. J Proteome Res 2016, 15(1):280–290. [DOI] [PubMed] [Google Scholar]
- 114.Zeng J, Chen S, Li C, Ye Z, Lin B, Liang Y, Wang B, Ma Y, Chai X, Zhang X, Zhou K et al. : Mesenchymal stem/stromal cells-derived il-6 promotes nasopharyngeal carcinoma growth and resistance to cisplatin via upregulating cd73 expression. J Cancer 2020, 11(8):2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ: Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011, 71(14):4809–4820. [DOI] [PubMed] [Google Scholar]
- 116.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S et al. : Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334(6062):1573–1577. [DOI] [PubMed] [Google Scholar]
- 117.Quezada C, Garrido W, Oyarzun C, Fernandez K, Segura R, Melo R, Casanello P, Sobrevia L, San Martin R: 5’-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells. J Cell Physiol 2013, 228(3):602–608. [DOI] [PubMed] [Google Scholar]
- 118.Kim DG, Bynoe MS: A2a adenosine receptor modulates drug efflux transporter p-glycoprotein at the blood-brain barrier. J Clin Invest 2016, 126(5):1717–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Antonioli L, Novitskiy SV, Sachsenmeier KF, Fornai M, Blandizzi C, Hasko G: Switching off cd73: A way to boost the activity of conventional and targeted antineoplastic therapies. Drug Discov Today 2017, 22(11):1686–1696. [DOI] [PubMed] [Google Scholar]
- 120.Park B, Yee C, Lee KM: The effect of radiation on the immune response to cancers. Int J Mol Sci 2014, 15(1):927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wirsdorfer F, de Leve S, Cappuccini F, Eldh T, Meyer AV, Gau E, Thompson LF, Chen NY, Karmouty-Quintana H, Fischer U, Kasper M et al. : Extracellular adenosine production by ecto-5’-nucleotidase (cd73) enhances radiation-induced lung fibrosis. Cancer Res 2016, 76(10):3045–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wennerberg E, Spada S, Rudqvist NP, Lhuillier C, Gruber S, Chen Q, Zhang F, Zhou XK, Gross SS, Formenti SC, Demaria S: Cd73 blockade promotes dendritic cell infiltration of irradiated tumors and tumor rejection. Cancer Immunol Res 2020, [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrates CD73 may be a radiation-induced checkpoint.
- 123.Dietrich F, Figueiro F, Filippi-Chiela EC, Cappellari AR, Rockenbach L, Tremblay A, de Paula PB, Roesler R, Filho AB, Sevigny J, Morrone FB et al. : Ecto-5’-nucleotidase/cd73 contributes to the radiosensitivity of t24 human bladder cancer cell line. J Cancer Res Clin Oncol 2018, 144(3):469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Turcotte M, Allard D, Mittal D, Bareche Y, Buisseret L, Jose V, Pommey S, Delisle V, Loi S, Joensuu H, Kellokumpu-Lehtinen PL et al. : Cd73 promotes resistance to her2/erbb2 antibody therapy. Cancer Res 2017, 77(20):5652–5663. [DOI] [PubMed] [Google Scholar]
- 125.Young A, Ngiow SF, Madore J, Reinhardt J, Landsberg J, Chitsazan A, Rautela J, Bald T, Barkauskas DS, Ahern E, Huntington ND et al. : Targeting adenosine in braf-mutant melanoma reduces tumor growth and metastasis. Cancer Res 2017, 77(17):4684–4696. [DOI] [PubMed] [Google Scholar]
- 126.Allard B, Allard D, Buisseret L, Stagg J: The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol 2020. [DOI] [PubMed] [Google Scholar]


