Abstract
Pain produced by bone cancer is often severe and difficult to treat. Here we examined effects of Resolvin D1 (RvD1) or E1 (RvE1), antinociceptive products of ω−3 polyunsaturated fatty acids, on cancer-induced mechanical allodynia and heat hyperalgesia. Experiments were performed using a mouse model of bone cancer produced by implantation of osteolytic ficrosarcoma into and around the calcaneus bone. Mechanical allodynia and heat hyperalgesia in the tumor-bearing paw were assessed by measuring withdrawal responses to a von Frey monofilament and to radiant heat applied on the plantar hind paw. RvD1, RvE1, and cannabinoid receptor antagonists were injected intrathecally. Spinal content of endocannabinoids was evaluated using UPLC-MS/MS analysis. RvD1 and RvE1 had similar antinociceptive potencies. ED50s for RvD1 and RvE1 in reducing mechanical allodynia were 0.2 pg (0.53 fmol) and 0.6 pg (1.71 fmol), respectively, and were 0.3 pg (0.8 fmol) and 0.2 pg (0.57 fmol) for reducing heat hyperalgesia. Comparisons of dose-response relationships showed equal efficacy for reducing mechanical allodynia, however, efficacy for reducing heat hyperalgesia was greater for of RvD1. Using UPLC-MS/MS we determined that RvD1, but not RvE1, increased levels of the endocannabinoids Anandamide and 2-Arachidonoylglycerol in the spinal cord. Importantly, Resolvins did not alter acute nociception or motor function in naïve mice.
Our data indicate, that RvD1 and RvE1 produce potent antiallodynia and antihyperalgesia in a model of bone cancer pain. RvD1 also triggers spinal upregulation of endocannabinoids that produce additional antinociception predominantly through CB2 receptors.
Keywords: resolvin D1, resolvin E1, endocannabinoids, bone cancer pain, spinal cord, mice
Introduction
Resolvins (RVs) are endogenous derivatives of ω−3 polyunsaturated fatty acids (ω−3-PUFAs). Resolvin D1 (RvD1) belongs to D-series RVs and is synthetized from docosahexaenoic acid (DHA), whereas Resolvin E1 (RvE1) belongs to the E-series and is derived from eicosapentaenoic acid (EPA). It was shown initially that they are synthetized in inflamed tissues. RVs inhibit inflammatory and activate pro-resolving pathways, leading to the resolution of inflammation, tissue healing, and antihyperalgesia [1]. It was also demonstrated that RVs produce antinociception following intrathecal (i.t.) administration [2]. Potent analgesia following i.t. administration of RVs indicated that they also play a role in reducing mechanisms of neuronal sensitization.
The effectiveness of RvD1 and RvE1 in the treatment of cancer pain has not been extensively studied. Only a single study reported that systemic administration of RvD1 inhibited heat hyperalgesia, but not mechanical allodynia in a model of oral squamous carcinoma [3]. This underscores the need for additional investigation for a use of RVs to treat cancer pain, which currently is hard to manage.
The mechanisms underlying antihyperalgesia produced by RVs are not fully understood, although they act through G protein-coupled receptors (GPCRs). RvD1 binds to ALX/FPR2 and orphan GPR32 receptors [4], whereas RvE1 binds to Chem23 and human BLT1 receptors [5, 6]. These receptors are expressed on primary afferent neurons, spinal neurons, and glia [7], but binding to them is not specific for RvD1 and RvE1, since other mediators also have high affinity for these targets. For example, Lipoxin A4, Annexin A1, Serum Amyloid A (SAA) protein bind to ALX/FPR2 and orphan GPR32 receptors with affinities comparable to RvD1 [8–12]. Leukotriene B4 and Chemerin bind to Chem23 and BLT1 receptors with affinity similar to RvE1 [5, 6, 13, 14].
RVs belong to a class of lipid autacoids. Endocannabinoids are members of another class of autacoids derived from arachidonic acid (AA), which is belong to ω−6 polyunsaturated fatty acids (ω−6-PAFAs). Endocannabinoids also are produced in tissues, disseminated through extracellular space, and act in close proximity, inducing multiple therapeutic effects including antinociception [15]. We hypothesized that RVs could increase signaling of Anandamide (N-arachidonoylethanolamine; AEA) and 2-Arachidonoylglycerol (2-AG), the most abundant endocannabinoids in the nervous system [16, 17]. AEA and 2-AG act through their GPCRs, Cannabinoid-1 and Cannabinoid-2 (CB1R and CB2R), that are expressed on central terminals of nociceptive primary afferents, central neurons, and glia [18–20]. Activation of CB1R and CB2R decreased cancer-evoked hyperalgesia [21]. We demonstrated previously that content of AEA is decreased in nociceptors that innervate the tumor bearing paw [22, 23], contributing to hyperalgesia. Furthermore, intraplantar administration of the non-selective cannabinoid receptor agonist, WIN 55,212–2, decreased evoked responses of C-fiber nociceptors in tumor bearing mice [24]. These studies suggest that upregulation of endocannabinoid signaling by RVs can enhance their antinociception during cancer pain.
The present study investigated the effects of RvD1 and RvE1 on mechanical allodynia and heat hyperalgesia in an established murine model of bone cancer pain and determined the contribution of cannabinoid receptors to RVs antinociception.
2. Materials and methods
2.1. Animals
A total of 165 adult male C3H/HeNCr mice (National Cancer Institute, Frederick, MD) were used. Mice were housed 4 per cage, allowed free access to food and water, and maintained on a 12-hour light/dark schedule. At the end of experiments, mice were euthanized by inhalation of CO2. All experiments and procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
2.2. Bone cancer model
Murine NCTC clone 2472 fibrosarcoma cells (ATCC, Manassas, VA) were grown as previously described [25]. Tumors was generated by injecting fibrosarcoma cells (2 × 105 cells in 10 μl of PBS pH 7.3) unilaterally into and around the calcaneus bone under isoflurane anesthesia (2–2.5% in room air) as described previously [26–28]. None of the mice showed signs of motor dysfunction at any time following cancer implantation.
2.3. Drugs and intrathecal injection
Stocks of RvD1 and RvE1 dissolved in ethanol were obtained from Cayman Chemical (Ann Arbor, MI, Cat. #10012554 and #10007848, respectively), stored at −80°C and diluted for i.t. injection in sterile saline just prior to the experiment. Ethanol (0.3% in saline, 5 μl) was used as a vehicle control. The CB1R antagonist AM281 (10μg/kg) and CB2R antagonist AM630 (4μg/kg) were obtained from Tocris (Bristol, UK) and dissolved in saline with 3% DMSO. The vehicle control consisted of 3% DMSO in saline. All drugs were administered i.t. in not anaesthetized mice. A volume of 5 lμ was injected by lumbar puncture using a 50-μl luer tip syringe (Hamilton, Reno, NY) connected to a sterile 30-gauge ½ inch needle that was inserted between L5 and L6 vertebrae [29]. The CB1/CB2R antagonists were administered 1hour before administration of RvD1 or RvE1.
2.4. Measurement of mechanical allodynia and heat hyperalgesia
To measure mechanical allodynia, mice were placed on an elevated mesh platform and a von Frey monofilament (Stoelting Co, Wood Dale, IL) with a bending force of 3.4 mN was applied to the plantar surface of each hind paw 10 times for 2 sec each with ~5 sec interstimulus interval. Paw withdrawal frequency was expressed as the percent of applications that evoked a withdrawal response. Tumor-evoked mechanical allodynia was defined as withdrawal frequency ≥ 50%. The average frequency of withdrawal for the hind paw contralateral to the tumor-bearing paw did not exceed 30%.
To measure heat hyperalgesia, mice were placed on an elevated temperature controlled glass platform (30°C) and radiant heat was applied to the middle of the plantar surface of the hind paw. The intensity of heat was adjusted to produce paw withdrawal latencies of approximately 12 – 14 sec in naïve mice. A cutoff time of 20 sec was imposed to avoid tissue damage. Radiant heat was applied 4 times to each hind paw with at least a 60 s interstimulus interval. Withdrawal latencies of the last 3 trials were averaged. Heat hyperalgesia was defined as a decrease in paw withdrawal latency (sec).
Baseline values for paw withdrawal frequency and paw withdrawal latency were determined for each animal for 3 consecutive days before cancer cell implantation and every other day beginning the third day after implantation.
2.5. Measurement of acute nociception in naïve mice
To determine if RVs increased or decreased sensitivity to mechanical stimuli in naïve mice, we measured the 50% mechanical paw withdrawal threshold (g) using the up-down method [30] with an adjustment for mouse paw sensitivity. Briefly, a series of 8 VF monofilaments (0.07, 0.16, 0.4, 0.6, 1, 1.2, 2, and 4 g) was used. Testing was initiated with a monofilament that delivered 0.6 g. In the absence of a withdrawal response, a stronger monofilament was applied. If a withdrawal occurred, a weaker stimulus was presented. The resulting pattern was tabulated and the 50% paw withdrawal threshold was calculated. The interstimulus interval was 5 sec.
To determine if RVs altered thermal sensitivity in naïve mice, paw withdrawal latencies to heat were obtained as described above for tumor-bearing mice. Paw withdrawal thresholds and paw withdrawal latencies were averaged for both hind paws.
2.6. Rotarod assay of motor function
The effects of RVs on motor function were determined using the rotarod test as described previously [31]. Mice were placed on a rotarod treadmill at an initial speed of 3.75 rpm with progressive acceleration to 5 rpm. The time spent on the treadmill before falling off was recorded for each mouse on each day of testing. A cutoff time of 300 s was used. Baseline measurements were obtained for all mice on 3 consecutive days. Motor performance was determined before, at 2, and 24 hours following i.t. injection.
2.7. Measurement of endogenous AEA and 2-AG
Samples of lumbar spinal cord were removed from naïve and tumor-bearing mice at 2 hours after injection of RVs, frozen in liquid nitrogen, and kept frozen at −80°C until the time of processing. Lipids were extracted from spinal cord tissue prepared for LC-MS/MS analysis as we previously described [32]. Briefly, samples were homogenized by sonication in 3 ml of acetone/saline (2:1 by volume) with 100 ng of AEA-d8 and 2-AG-d8 (Cayman Chemicals, Ann Arbor, MI) as internal standards and centrifuged at 2000g for 10 min. The supernatant was extracted 3 times with hexane. The hexane extracts were pooled, evaporated with a gentle stream of nitrogen, redissolved in 20μl of acetonitrile/water (1:1 by volume), and 10μl was analyzed on UPLC-MS/MS.
The separation of AEA and 2-AG was based on the UPLC method described previously [32, 33]. For analytes retention, a Waters ACUITY UPLC HSS T3 column (1.8 μM, 100 Å pore diameter, 2.1×150mm, Waters, Milford, MA) with an ACUITY UPLC HSS T3 precolumn (1.8 μM, 100 Å pore diameter, 2.1×5mm, Waters) was used at 55°C. The LC system consisted of a Waters ACUITY Class1 UPLC pump with a FTN sampler manager operated at 8 °C. Mobile phase A was 0.1% formic acid in water and phase B was 0.1% formic acid in acetonitrile. The flow rate was 0.45 ml/min with initial 39% B that was maintained for 0.5 min. Phase B was increased to 40.5% over 6.88 min, followed by an increase to 70% over 1.62 min, then increased to 75% over 3 min, and further increased to 98% over 1.5 min where it was held for 5.3 min. Finally, solvent B was returned to initial conditions over 0.2 minutes to re-equilibrate the column for 2min.
For MS/MS analysis, a triple quadrupole mass spectrometer (Xevo TQ-S, Waters) with electrospray ionization operated in positive ion mode was used. The capillary voltage was 3.29 kV and the cone voltage was 61V. The desolvation temperature was 500 °C and the source temperature was 150 °C. The desolvation gas flow was 1000 l/h, the cone gas flow was 150 l/h, and the nebulizer gas was at 5.0 Bar. MassLynx V4.1 software (Waters) was used for instrument control, acquisition, and sample analysis.
The analytes were monitored in MRM mode using 348.5/62.2 (collision energy (CE) 6V) and 348.5/287.5 (CE 12V) mass transitions for AEA, 356.5/62.2 (CE 6V) and 356.5/294.5 (CE 12V) for AEA-d8, 379.3/287.7(CE 13V) for 2-AG, and 387.5/294.4 (CE 13V) for 2-AGd8. AEA and 2-AG were quantified against deuterated internal standards using an isotope-dilution approach.
2.8. Experimental design
The antinociceptive effects of RvD1 and RvE1 were determined in tumor-bearing mice at 16–19 days after implantation of fibrosarcoma cells, when mice exhibit maximal and stable mechanical allodynia and heat hyperalgesia. Separate groups of mice (6 mice/group) received one i.t. injection of vehicle, or RvD1 and RvE1 at doses of 0.0001, 0.001, 0.003, 0.03, 0.1, 1, and 3 ng (~ 0.3, 3, 9, 90, 300 fmol, 3 pmol, and 9 pmol). Measures of paw withdrawal frequency followed by paw withdrawal latency were obtained before any injection, every hour for 4 hours after injection, and at 24 hours after injection. Heat hyperalgesia was tested 10 min after obtaining withdrawal responses to mechanical stimuli. Since a single i.t. injection of RvD1 and RvE1 changed responses for up to 4 hours, effects of RVs were calculated as the area under the curve (AUC) during the testing period of 4 hours. A reduction in mechanical allodynia was inversely related to AUC (decrease in withdrawal frequency), while a reduction in heat hyperalgesia was directly related to AUC (decrease in withdrawal latency). For calculations of median effective dose (ED50), values of AUC from each experiment were used.
To determine the contribution of CB1R and CB2R to RVs-induced antinociception, tumor-bearing mice received one i.t. injection of the selective CB1R antagonist AM281 (10μg/kg in 5 μl), CB2R antagonist AM630 (4μg/kg in 5μl), or vehicle (3% DMSO in saline, 5 μl) 1 hour prior to i.t. administration of RvD1, RvE1, or vehicle. Paw withdrawal frequencies and paw withdrawal latencies were determined every hour for 4 hours after RVs administration. These doses of AM281 and AM630 were chosen because they effectively blocked the antihyperalgesic effects of endocannabinoids in a murine model of cancer pain [21, 23, 34]. For all behavioral studies, the experimenter was blinded to the treatment.
For measurement of AEA and 2-AG in the spinal cord, tumor-bearing mice received an i.t. injection 1 ng of RvD1, RvE1 (3 pmol) or vehicle 2 hours before harvesting the spinal cord. Naïve mice were used as a control.
2.9. Statistical analyses
Data are reported as the mean ± SEM. The effects of RvD1, RvE1 or vehicle on paw withdrawal frequencies and paw withdrawal latencies were determined using One- or Two-Way ANOVA, or unpaired two-tailed Student’s t-tests using SigmaPlot 11.2 statistical software (San Jose, CA). Post hoc comparisons were made using Bonferroni’s t-tests. ED50 were calculated using nonlinear regression and analyzed by consecutive extra sum of squares F test using GraphPad Prism 5 software (La Jolla, CA). For all statistical comparisons, a value of p<0.05 was considered significant.
3. Results
3.1. Mechanical allodynia and heat hyperalgesia produced by bone cancer
Growth of fibrosarcoma cells in and around the calcaneus bone produced mechanical allodynia that was defined as an increase in paw withdrawal frequency of the tumor-bearing paw (77 ± 1.4%) as compared to the contralateral paw (24.2 ± 2.81%) (t-test, p<0.001). Cancer growth also produced heat hyperalgesia. Mean paw withdrawal latency for the tumor-bearing paw (5.0 ± 0.16 sec) was lower than the contralateral paw (12.4 ± 0.5 sec) (t-test, p<0.001).
3.2. Effects of RvD1 and RvE1 on mechanical allodynia and heat hyperalgesia: dose-response relationships
Intrathecal administration of RVs decreased mechanical allodynia and heat hyperalgesia in the tumor-bearing paw, whereas mechanical and heat sensitivity of the contralateral paw were not altered. Figure 1 A and B demonstrates examples of effects of vehicle or 0.03 and 1 ng RvD1 and RvE1 on mechanical pain sensitivity. RVs, but not vehicle, decreased mechanical allodynia of the tumor-bearing paw, while paw withdrawal frequency of contralateral paw was not affected. The same doses of RvD1 or RvE1, but not vehicle, also increased paw withdrawal latency of the tumor-bearing paw, while the contralateral hind paw was not affected (Figure 1 C, D). These changes persisted during the 4-hour test period. Mechanical allodynia and heat hyperalgesia fully recovered by 24 hours after i.t. injections.
Figure 1.
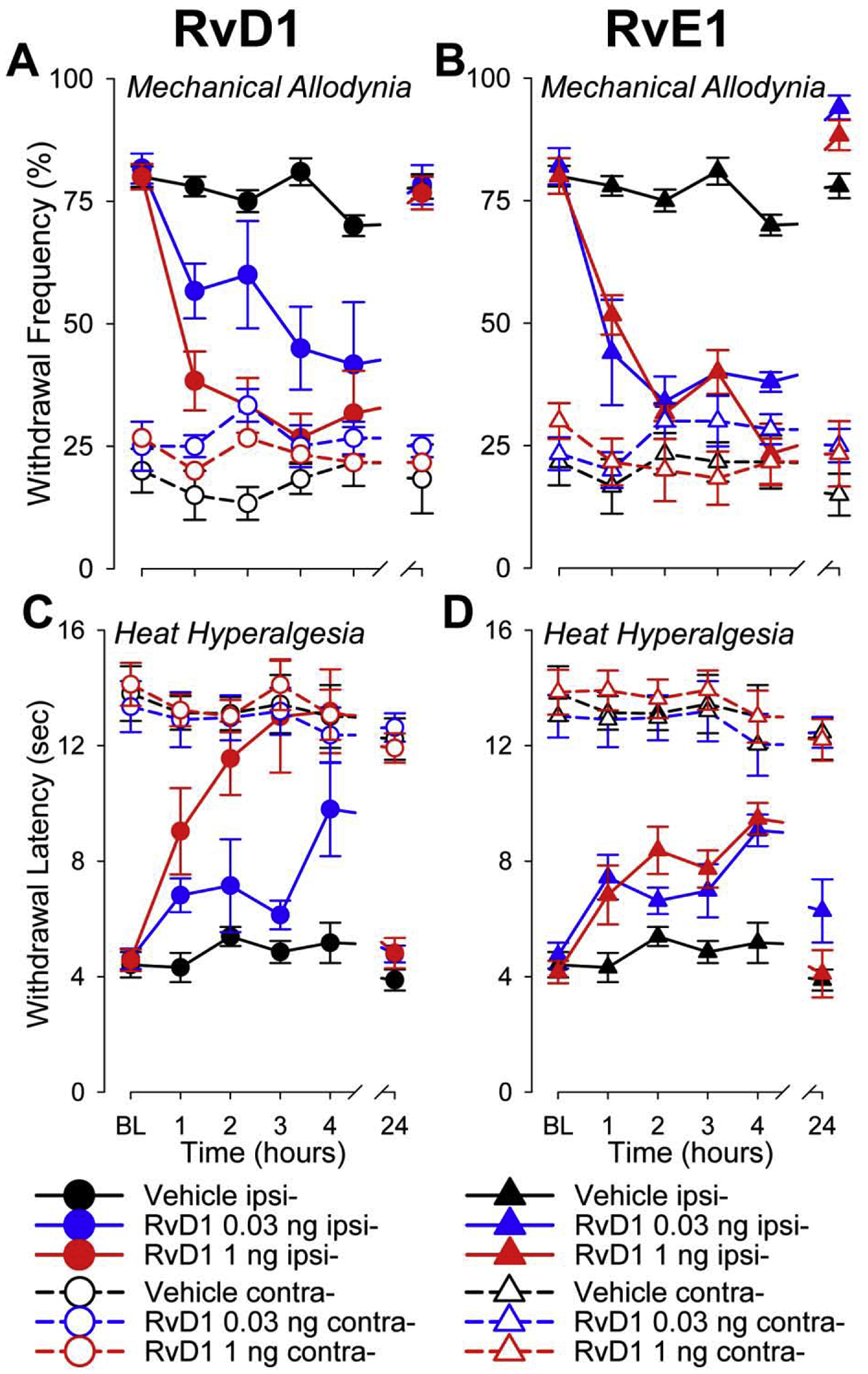
Examples of antinociceptive effects produced by RvD1 and RvE1. Effects on mechanical allodynia are represented by changes in mean (±SEM) paw withdrawal frequency (%) evoked by 10 applications of VF filament with a bending force of 3.9 mN applied to the plantar surface of tumor-bearing and contralateral hind paws. Measurements were made at baseline (before i.t. injections (BL), during the first 4 hours after injection, and at 24 hours after injections of RvD1 (A) and RvE1 (B). Effects on heat hyperalgesia are represented as mean (±SEM) paw withdrawal latency (sec) to radiant heat stimuli applied on the plantar surface in tumor-bearing and contralateral paws at BL, during the first 4 hours and at 24 hours after injections of RvD1 (C) and RvE1 (D). Vehicle was used as a control and did not alter withdrawal frequencies or latencies. RvD1 and RvE1 0.03 and 1 ng significantly decreased mechanical allodynia and heat hyperalgesia compared to vehicle for several hours after administration. (Two-Way repeated measures ANOVA p<0.01). Allodynia and hyperalgesia fully recovered by 24 hours after administration. Withdrawal response frequencies and latencies for the contralateral hind paw were not altered. Ipsi- refers to ipsilateral tumor-bearing paw; contra- refers to contralateral control paw.
We determined dose-response relationships by calculating the mean AUC ± SEM following i.t. administration. AUC was chosen for analysis because it provides an aggregate level of antiallodynia and antihyperalgesia over the 4-hour test period. Mice received RvD1 or RvE1 at doses of 0.0001, 0.001, 0.003, 0.03, 0.1, 1, and 3 ng, vehicle was used as a control (n = 6/group).
Mechanical allodynia.
The reduction in mechanical allodynia following RVs was manifested as a decrease in AUC (% × hours) and was compared to the vehicle control. Figure 2 A shows that doses of RvD1 and RvE1 ≥ 0.001ng significantly decreased mean ± SEM of the AUC. Both RVs had similar potencies for reducing paw withdrawal frequency. The ED50 for RvD1 was 0.0002 ng, 95% CI: 0.000005 – 0.008 ng (equal to 0.53 fmol). ED50 for RvE1 was 0.0006 ng, 95% CI: 0.00008 – 0.005 ng (equal to 1.71 fmol). These values did not differ (Extra Sum of Squares F test, p=0.9183).
Figure 2.

Dose-dependent decrease in mechanical allodynia and heat hyperalgesia produced by RvD1 and RvE1 evaluated as area under the curve (AUC). AUC is expressed as % × hours for mechanical allodynia (A) and sec × hours for heat hyperalgesia (B). Doses ≥ 0.001 significantly attenuated allodynia and hyperalgesia compared to vehicle. (One-Way ANOVA). * indicates a significant difference between RvD1 or RvE1 and vehicle: *p<0.05, **p<0.01, ***p<0.005, ****p<0.001. # indicates a significant difference between RvD1 and RvE1: #p<0.05, ###p<0.005.
Dose-response relationships for the effects of RvD1 and RvE1 in reducing mechanical allodynia demonstrated similarities (Two-Way ANOVA, p=0.086). Findings indicate that RvD1 and RvE1 reduced mechanical allodynia with similar potencies and efficacies.
Heat hyperalgesia.
The reduction in heat hyperalgesia following RVs was exhibited as an increase in the AUC (sec × hours) as compared to vehicle controls. Figure 2 B demonstrates that doses ≥ 0.001ng increased the AUC. As for mechanical allodynia, RvD1 and RvE1 exhibited similar potencies for reducing heat hyperalgesia (increasing paw withdrawal latency). The ED50 values for RvD1 and RvE1 were 0.0003 ng, 95% CI: 0.000007 – 0.009 ng (equal to 0.8 fmol), and 0.0002 ng, 95% CI: 0.000007 – 0.008 ng (equal to 0.57 fmol) respectively (Extra Sum of Squares F test, p=0.9878).
However, dose-response relationships for RvD1 and RvE1 in reducing heat hyperalgesia differed. Changes in paw withdrawal latency following high doses of RvD1 (1 and 3 ng) were greater than those following similar doses of RvE1 (Two-Way ANOVA, p>0.05). Therefore, RvD1 has a higher efficacy than RvE1 for reducing heat hyperalgesia.
3.3. RvD1 and RvE1 did not alter acute nociception or motor function in naïve mice
Intrathecal administration of 5 μl of vehicle (n=5), or 1 ng of RvD1 (n=6) or RvE1 (n=6) did not alter the 50% paw withdrawal threshold (Two-Way ANOVA, p=0.853) (Figure 3 A) or paw withdrawal latency (Two-Way ANOVA, p=0.994) (Figure 3 B) in naïve mice. Therefore, RvD1 and RvE1 did not modulate nociceptive processing under normal conditions.
Figure 3.

RvD1, or RvE1 did not alter acute pain sensitivity or motor control. Intrathecal administration of 1 ng of RvD1, RvE1 or vehicle in naïve mice did not alter mean (±SEM) mechanical response thresholds (A) or heat withdrawal latencies (B) over a period of 24 hours after injection. (C) 3 ng of RvD1, RvE1, or vehicle did not affect the mean (±SEM) amount of time (sec) spent on the treadmill at 2 and 24 hours after i.t. injections.
To determine whether RVs alter motor function, we compared performance of naïve mice (n=5/group) on the rotarod test before, and at 2 and 24 hours following i.t. administration of vehicle, 3 ng RvD1 or RvE1 (in 5 μl). Figure 3 C shows that time spent on the treadmill did not differ between groups (One-Way ANOVA, p=0.670). These data indicate that neither RvD1 nor RvE1 impair motor function.
3.4. Alteration of endocannabinoid content in the spinal cord
Measurement of endocannabinoid levels in the spinal cord was performed using LC–MS/MS. AEA and 2-AG were measured in naïve and tumor-bearing mice 2 hours after i.t. administration of vehicle, RvD1, or RvE1 (n=6/group) and were expressed as ng/g of wet weight (ng/g ww). Figure 4 A shows that spinal levels of AEA in naïve mice and vehicle-treated tumor-bearing mice did not differ. Spinal applications of 1 ng of RvD1, but not 1 ng of RvE1, increased AEA by 65.0 ± 9.5 % compared to vehicle treated animals. Spinal content of AEA in tumor-bearing mice treated with RvD1 was higher than in all other experimental groups (One-Way ANOVA p<0.001). Levels of 2-AG in the spinal cord were one hundred times higher that AEA (Fig. 4 B). Cancer pain by itself did not affect spinal content of 2-AG since its concentration was unaltered by i.t. injection of vehicle. Intrathecal administration of 1 ng RvD1, but not RvE1, increased 2-AG in the spinal cord by 44.2 ± 5.68 % compared to vehicle treated tumor-bearing mice (One-Way ANOVA p<0.001). Therefore, among these two classes of RVs only RvD1 increased endocannabinoids in the spinal cord.
Figure 4.

Effects of RvD1 and RvE1 on levels of endocannabinoids in the spinal cord. Intrathecal administration of 1 ng RvD1, but not RvE1 or vehicle, increased levels of AEA (A) and 2-AG (B) in tumor-bearing mice. Content of endocannabinoids is expressed as mean (±SEM) ng/g ww. * indicates a significant difference from RvD1: ***p<0.005, ****p<0.001.
3.5. Cannabinoid signaling contributes to antinociception produced by resolvins
AEA and 2-AG produce their effects via activation of CB1R and CB2R. Therefore, we determined if these receptors are involved in the antinociception produced by RVs. In tumor-bearing mice, vehicle (3% DMSO), the CB1R antagonist AM281 (10μg/kg), or the CB2R antagonist AM630 (4μg/kg) were given i.t. one hour prior to i.t. administration of 1 ng RvD1 (n=6 mice/group) or RvE1 (n=6 mice/group). Intrathecal administration of vehicle for CB1 and CB2R antagonists followed by i.t. administration of vehicle for RVs (0.3% ethanol) 1 hour later served as a control for i.t. injections (n=6). Administration of the CB1R, or CB2R antagonists, or vehicle, alone did not alter mechanical allodynia or heat hyperalgesia in the tumor-bearing paw as well as mechanical and heat sensitivity in contralateral paw (Table 1, comparisons by paired t-test). AUCs were determined from i.t. injections of RvD1, RvE1, or vehicle for 4 hours and used for comparisons.
Table 1.
CB1R and CB2R antagonists did not alter responses to mechanical and heat stimuli applied on tumor-bearing and contralateral paws.
| Test | Paw | Injection | Before | After | p = |
|---|---|---|---|---|---|
| Mechanical (%) | Ipsi- | CB1R antag | 76.3±3.24 | 73.8±3.75 | 0.622 |
| CB2R antag | 83.8±4.2 | 82.5±2.50 | 0.802 | ||
| Vehicle | 74.1±2.22 | 75.2±2.35 | 0.732 | ||
| Contra- | CB1R antag | 18.8±4.41 | 16.3±4.98 | 0.713 | |
| CB2R antag | 12.5±4.53 | 18.8±2.95 | 0.267 | ||
| Vehicle | 10.4±1.67 | 9.6±1.41 | 0.744 | ||
| Heat (sec) | Ipsi- | CB1R antag | 6.2±0.52 | 6.2±0.56 | 0.989 |
| CB2R antag | 5.5±0.31 | 5.5±0.35 | 0.978 | ||
| Vehicle | 5.8±0.18 | 5.8±0.23 | 0.929 | ||
| Contra- | CB1R antag | 10.4±0.51 | 10.7±0.57 | 0.684 | |
| CB2R antag | 11.6±0.53 | 11.1±0.59 | 0.528 | ||
| Vehicle | 11±0.3 | 11.0±0.32 | 0.863 |
Mechanical allodynia.
AUCs (% × hours) were calculated from the paw withdrawal frequencies obtained for the tumor-bearing and control paws. Figure 5 A shows that RvD1 given 1 hour after vehicle produced a smaller AUC (indication of antiallodynia) compared to that, when i.t. injection of vehicle was followed by another vehicle injection that was used as a control (p<0.001). Intrathecal administration of the CB1R antagonist did not alter the effect of RvD1 on allodynia (p<0.05). In contrast, pretreatment with the CB2R antagonist reduced the antiallodynic effect of RvD1 as evidenced by a higher AUC compared to that when RvD1 followed vehicle pretreatment (p<0.05). However, the AUC remained smaller than that in the control group (p<0.05), indicating that antiallodynia is preserved without CB2 receptor signaling. In contrast, RvE1 given after vehicle decreased allodynia compared to control and this effect was not altered by pretreatment with CB1R or CB2R antagonists. Figure 5 B shows the absence of effects on AUCs produced by stimulation of the contralateral paw, in which allodynia did not develop.
Figure 5.

Role of endocannabinoid signaling in antiallodynia produced by RVs. Intrathecal administration of the CB2R antagonist, AM630 (4 μg/kg), but not the CB1R antagonist AM281 (10 μg/kg), attenuated the antiallodynia produced by RvD1 (A). Nociceptive mechanical sensitivity of the contralateral paw was unaltered (B). Mechanical withdrawal responses were expressed as mean (±SEM) AUC (% × hours). * indicates a significant difference from control (vehicle): *p<0.05, ****p<0.001. # indicates a significant difference from RvD1 injected 1 hour after vehicle: p<0.05.
Heat hyperalgesia.
AUCs (sec × hours) were calculated from the paw withdrawal latencies obtained for the tumor-bearing and control paws. Figure 6 A shows that i.t. administration of RvD1 given 1 hour after vehicle produced a larger AUC (indication of antihyperalgesia) compared to that following control injections of the two vehicles with a 1 hour interval between injection (p<0.001). RvD1 produced a similar effect on heat hyperalgesia following pretreatment with the CB1R antagonist as compared to control (p<0.001). However, pretreatment with the CB2R antagonist decreased the AUC produced by RvD1 as compared to that after vehicle pretreatment (p<0.05), indicating a decrease in the antihyperalgesia produced by RvD1. Nevertheless, the AUC remained higher than that in the control group (p<0.05), indicating that antihyperalgesia is also preserved without CB2 receptor signaling. Antihyperalgesia produced by RvE1 was not affected by CB1R or CB2R antagonists. Also, paw withdrawal latencies for the contralateral hind paw were not altered by the CB1R or CB2R antagonists (Figure 6 B).
Figure 6.

Role of endocannabinoid signaling in antihyperalgesia produced by RVs. Intrathecal administration of the CB2R antagonist, AM630 (4 μg/kg), but not the CB1R antagonist AM281 (10 μg/kg), decreased the antihyperalgesia to heat produced by i.t. administration of RvD1 (A). No changes in sensitivity to heat stimuli occurred in the contralateral paw following any injection (B). Heat withdrawal latencies were expressed as mean (±SEM) AUC (sec × hours). * indicates a significant difference from vehicle control: *p<0.05, ***p<0.005, ****p<0.001. # indicates a significant difference from RvD1 followed by pretreatment with vehicle, p<0.05.
4. Discussion
Pain produced by bone cancer includes components of inflammatory and neuropathic pain together with the release of tumor-associated pain mediators as well as exosomes [35–38]. Clinically cancer pain is often associated with mechanical allodynia and thermal hyperalgesia [39–41]. Therefore, the occurrence of mechanical allodynia and heat hyperalgesia as a result of central sensitization in our mouse model [26] is relevant to the clinical situation. We demonstrated that i.t. application of RvD1 and RvE1 reduced tumor-evoked pain. RvD1 and RvE1 did not alter performance on the rotarod test, suggesting that antihyperalgesia produced by RVs is not attributed to motor impairment. Importantly, i.t. administration of RvD1 or RvE1 did not alter nociceptive responses in naïve mice, suggesting that cellular targets for RvD1- and RvE1-induced antinociception are normally in low abundance or unavailable, or are saturated by endogenous ligands.
RvD1 and RvE1 reduced mechanical allodynia and heat hyperalgesia at low doses when acting directly on the spinal cord. ED50 values for the reduction of mechanical allodynia were 0.2 pg and 0.6 pg for RvD1 and RvE1 respectively, which is equal to 0.53 and 1.71 fmol. ED50 values for the reduction of heat hyperalgesia were respectively 0.3 pg and 0.2 pg (0.8 and 0.57 fmol). Similarities in antinociceptive potencies are in line with similar affinity of RvD1 and RvE1 to membrane receptors in vitro [4, 6]. The antinociceptive potency of RVs can be considered high. For example, i.t. injection of morphine in a similar bone cancer model reduced cancer-induced mechanical allodynia with ED50 = 50 pmol [42].
Although RvD1 and RvE1 had comparable efficacies for inhibiting mechanical allodynia, RvD1 exhibited greater efficacy in reducing heat hyperalgesia than RvE1. Studies are needed to further elucidate the mechanisms by which RvD1 and RvE1 reduce pain to mechanical and heat stimuli. This is especially important since mechanical allodynia and heat hyperalgesia might respond differently to analgesics [43], possibly because transmission and encoding of different pain modalities in the spinal cord are performed through only partially overlapping neuronal pathways [44].
Hyperalgesia produced by bone cancer involves sensitization of nociceptors [27] and spinal neurons [26]. Membrane receptors for RvE1 are expressed on nociceptive primary afferent and spinal neurons [2, 45], while RvD1 receptors are mainly expressed on primary afferent neurons and infrequently on spinal neurons [46]. However, RvD1 receptors are abundant on astrocytes [46, 47]. Importantly, spinal astrocytosis is an important sign of pain induced by osteolytic bone cancer [48, 49]. Therefore receptors for RvD1 and RvE1 are in a position to reduce the development of central sensitization following i.t. injections.
Some mechanisms of antinociception by RVs have been described [7]. A novel finding of the present study regarding RvD1-induced antinociception is its ability to increase spinal levels of the endocannabinoids AEA and 2-AG. The mechanism underlying these effects are not clear, however our preliminary data indicate that RvD1 inhibits activity of the fatty acid amide hydrolase and monoacylglycerol lipase, enzymes that reduce tissues content of AEA and 2-AG, respectively. It is unclear which cells release endocannabinoids. Potential sources of endocannabinoids are neurons and glia [50–52], however, since RvD1 receptors are mostly located on spinal glia it could be suggested that they are a major target of RvD1-induced AEA and 2-AG upregulation.
Interestingly, with the exception of endocannabinoids, the majority of endogenous metabolites of ω−6-PUFAs are pronociceptive [53–56]. The absence of direct interactions between RVs and cannabinoid receptors [5, 6, 12] suggests that the involvement of CB2R in RvD1-induced antinociception results from RvD1-dependent upregulation of endocannabinoids.
This is the first report that antinociception produced by RvD1, a derivative of ω−3-PUFAs, occurs, at least in part, through endocannabinoids that are derivatives of ω−6-PUFAs. However, an opposite direction of interaction between antinociceptive D-series resolvins and cannabinoids was demonstrated in a human model of UV-killed E. coli-produced inflammation. Anabasum, a peripherally acting synthetic cannabinoid analog of Δ8-tetrahydrocannabinol which acts through CB2R [57], strongly increased the content of RvD1 and RvD3 in the area of inflammation [58]. Interestingly, this CB2 agonist also increased the content of the lipoxygenase interaction product Linoxin A4 [59]. However, this phenomena reflects interaction between derivatives of ω−6-PUFAs since Lipoxin A4 is also synthetized from AA [60].
Inactivation of CB1R in the spinal cord slightly reduced antiallodynia and did not alter antihyperalgesia produced by RvD1. In contrast, CB2R appears to play a substantial role in RvD1-dependent antiallodynia and antihyperalgesia. Blockade of CB2R reduced but did not abolish the antinociception produced by RvD1, suggesting that RvD1 triggers parallel endocannabinoid antinociception.
Under normal conditions, CB1R in the spinal cord are mainly expressed on spinal neurons while CB2R are mostly associated with glia [61] despite that minor presence of neuronal CB2R was also demonstrated [62, 63]. Upregulation of glial CB2R during persistent pain states plays important role in endocannabinoid antinociception [64–66].
Bone cancer pain is associated with increased cytokine levels in the spinal cord [67], which are released from spinal glia [68]. Spinal application of CB2R antagonists in tumor-bearing animals produced antihyperalgesia associated with a decrease in release of pro-inflammatory mediators [65, 68]. Therefore, CB2R is considered to be an important target for endocannabinoid antinociception during cancer pain [21, 34, 69]. Taking in account that receptors for RvD1 are also widely expressed on glial cells [46, 47] we suggest that these cellular elements are an important target for RvD1-induced CB2-dependent antinociception for cancer pain. However, the role of spinal neuron-glia interactions for RvD1-produced antinociception during cancer pain needs further investigation.
RVs and endocannabinoids could act synergistically to reduce sensitization and hyperalgesia. RvD1 and RvE1 decreased excitability of nociceptors and spinal neurons [2, 70] through downregulation of MAPK phosphorylation, decreased TNF-α signaling and NMDA receptor excitability, and reduced glutamate release [7]. Also, RvD1 and RvE1 inhibited spinal microglia and release of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α [47, 71, 72]. Important targets for RVs on primary afferent fibers are TRP channels. RvE1 inhibited TRPV1 channels [2], while RvD1 inhibited TRPA1 and TRPV4 channels [73]. RvD1 also inhibits TRPV3 channels but in mice this type are present only in skin keratinocytes [74].
Endocannabinoids decrease nociceptive input to the spinal cord and reduce hyperalgesia through peripheral [21, 23, 34, 37, 75, 76] and central [77, 78] mechanisms. They inhibit glutamate release from neurons [79, 80], NGF-induced sensitization of TRP channels, and glial activation [81]. Activation of CB2R also suppress release of cytokines from glia [82–84]. Therefore, the multi-targeting of RvD1 together with endocannabinoids might account for the strong reduction of tumor-induced hyperalgesia in the present study.
Our finding that for the reduction of heat hyperalgesia RvD1 exhibited a higher efficacy than RvE1 was unexpected. TRPV1 channels, that are inhibited exclusively by RvE1, are important transducers of noxious heat in peripheral nociceptors during cancer-induced neuropathy [85]. Perhaps the high efficacy of RvD1 is a result of inhibition of both TRPA1 and TRPV4 channels, which are sensitive to mechanical stimuli, temperature, tissue damage, and chemical irritants [86, 87]. They play an important role in the development of hyperalgesia produced by inflammation and tumor growth [88–91]. Also, we have suggested previously that cancer-induced mechanical allodynia develops due to central sensitization, while heat hyperalgesia is a result of peripheral sensitization followed by central sensitization [26, 27]. Therefore, the high efficacy of i.t. injected RvD1 for reduction of heat hyperalgesia could be a result of robust inhibition of central terminals primary nociceptors, spinal neurons, as well as glia due to a direct effect of RvD1 as well as indirect effects through endocannabinoids.
With rare exemption [92], most studies of fibrosarcoma-induced bone pain, including our previous studies, were performed only on male mice [26, 27, 37, 48, 93]. We have preliminary data indicating that mechanical allodynia in the fibrosarcoma model of cancer pain develops identically in male and female mice. However, the possibility of sex differences in analgesic responses in this model needs to be investigated. In this regard, sex differences in the antinociceptive effects of RVs has not been studied extensively. It was recently demonstrated that RvD1 decreased chemotherapy-induced mechanical allodynia independent of sex in contrast to RvD5 which decreased this allodynia and inflammatory pain only in male mice [94]. Consistent with the lack of a sex difference in the antinociception produced by RvD1 following chemotherapy, our preliminary data suggests that RvD1 also decreases cancer-induced mechanical allodynia independent of sex. However, it was found that RvD1 is upregulated to a greater extent in serum of female mice that developed Sjögren’s syndrome, an autoimmune disorder characterized by salivary gland dysfunction due to uncontrolled inflammation [95]. Moreover, sex differences for the source of endogenous D-series resolvins might exist. The content of DHA was found to be higher in the blood [96] and liver [97] of female rats and also in mononuclear blood cells, buccal cells, and the adipose tissue of women [98]. These data suggest that potential sex differences not only for effects of exogenous RVs but also for their endogenous syntheses need further investigation.
In summary, these data show that intrathecal administration of RvD1 and RvE1 potently decreased cancer pain without altering behavioral measures of nociception under normal conditions. It is also shown that antinociception produced byRvD1 involves the release of endocannabinoids and activation of CB2R. While opioids are often used to treat cancer pain, their use is limited due to their many undesirable side effects. Resolvins might offer an effective and alternative or adjunct approach for treating cancer pain without the side effects associated with traditional opioids.
Highlights.
Resolvin D1 and Resolvin E1, endogenous substances synthetized from ω−3 polyunsaturated fatty acids, produce potent spinal antinociception in a mouse model of bone cancer pain.
RvD1 and RvE1 decreased mechanical allodynia and heat hyperalgesia with similar potencies, however, RvD1 demonstrated higher efficacy for reduction of heat pain than RvE1.
RvD1, in parallel to its antinociceptive effect, increased spinal contents of endocannabinoids, derivatives of ω−6 polyunsaturated fatty acids. The combined potent action of these two endogenous antinociceptive systems could be an alternative for pain treatment with opioids, which is associated with severe side effects.
Funding
This work was supported by NIH grants CA241627, HL135895 (DAS), and NIH funded COBRE Mass Spec Core Facility Grant 5P30GM103329-05 (MG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
None
References
- [1].Serhan CN, Levy BD, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, J Clin Invest 128(7) (2018) 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR, Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions, Nat Med 16(5) (2010) 592–7, 1p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ye Y, Scheff NN, Bernabe D, Salvo E, Ono K, Liu C, Veeramachaneni R, Viet CT, Viet DT, Dolan JC, Schmidt BL, Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma, Neuropharmacology 139 (2018) 182–193. [DOI] [PubMed] [Google Scholar]
- [4].Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN, Resolvin D1 binds human phagocytes with evidence for proresolving receptors, Proc Natl Acad Sci U S A 107(4) (2010) 1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN, Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation, J Immunol 178(6) (2007) 3912–7. [DOI] [PubMed] [Google Scholar]
- [6].Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN, Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1, J Exp Med 201(5) (2005) 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ji RR, Xu ZZ, Strichartz G, Serhan CN, Emerging roles of resolvins in the resolution of inflammation and pain, Trends Neurosci 34(11) (2011) 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM, International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family, Pharmacol Rev 61(2) (2009) 119–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM, A seven-transmembrane G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells, J Exp Med 189(2) (1999) 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Back M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE, Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7, British journal of pharmacology 171(15) (2014) 3551–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brancaleone V, Gobbetti T, Cenac N, le Faouder P, Colom B, Flower RJ, Vergnolle N, Nourshargh S, Perretti M, A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation, Blood 122(4) (2013) 608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN, Resolvin D1 binds human phagocytes with evidence for proresolving receptors, Proceedings of the National Academy of Sciences of the United States of America 107(4) (2010) 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T, A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis, Nature 387(6633) (1997) 620–4. [DOI] [PubMed] [Google Scholar]
- [14].Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D, Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids, J Exp Med 198(7) (2003) 977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Keppel Hesselink JM, Fundamentals of and Critical Issues in Lipid Autacoid Medicine: A Review, Pain Ther 6(2) (2017) 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guasti L, Richardson D, Jhaveri M, Eldeeb K, Barrett D, Elphick MR, Alexander SP, Kendall D, Michael GJ, Chapman V, Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain, Mol Pain 5 (2009) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, Di Marzo V, Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats, Neuropharmacology 52(2) (2007) 415–22. [DOI] [PubMed] [Google Scholar]
- [18].Pertwee RG, Cannabinoid receptors and pain, Prog Neurobiol 63(5) (2001) 569–611. [DOI] [PubMed] [Google Scholar]
- [19].Pertwee RG, Pharmacology of cannabinoid CB1 and CB2 receptors, Pharmacol Ther 74(2) (1997) 129–80. [DOI] [PubMed] [Google Scholar]
- [20].Guindon J, Hohmann AG, Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain, British journal of pharmacology 153(2) (2008) 319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khasabova IA, Gielissen J, Chandiramani A, Harding-Rose C, Odeh DA, Simone DA, Seybold VS, CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain, Behav Pharmacol 22(5–6) (2011) 607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khasabova IA, Holman M, Morse T, Burlakova N, Coicou L, Harding-Rose C, Simone DA, Seybold VS, Increased anandamide uptake by sensory neurons contributes to hyperalgesia in a model of cancer pain, Neurobiol Dis 58 (2013) 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khasabova IA, Khasabov SG, Harding-Rose C, Coicou LG, Seybold BA, Lindberg AE, Steevens CD, Simone DA, Seybold VS, A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(44) (2008) 11141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Uhelski ML, Cain DM, Harding-Rose C, Simone DA, The non-selective cannabinoid receptor agonist WIN 55,212–2 attenuates responses of C-fiber nociceptors in a murine model of cancer pain, Neuroscience 247 (2013) 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clohisy DR, Ogilvie CM, Carpenter RJ, Ramnaraine ML, Localized, tumor-associated osteolysis involves the recruitment and activation of osteoclasts, J Orthop Res 14(1) (1996) 2–6. [DOI] [PubMed] [Google Scholar]
- [26].Khasabov SG, Hamamoto DT, Harding-Rose C, Simone DA, Tumor-evoked hyperalgesia and sensitization of nociceptive dorsal horn neurons in a murine model of cancer pain, Brain research 1180 (2007) 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, Simone DA, Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain, The Journal of neuroscience : the official journal of the Society for Neuroscience 21(23) (2001) 9367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL, Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain, Journal of Neuroscience 21(23) (2001) 9355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hylden JL, Wilcox GL, Intrathecal morphine in mice: a new technique, Eur J Pharmacol 67(2–3) (1980) 313–316. [DOI] [PubMed] [Google Scholar]
- [30].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, Quantitative assessment of tactile allodynia in the rat paw, Journal of neuroscience methods 53(1) (1994) 55–63. [DOI] [PubMed] [Google Scholar]
- [31].Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA, Seybold VS, Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy, The Journal of neuroscience : the official journal of the Society for Neuroscience 32(20) (2012) 7091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brose SA, Golovko SA, Golovko MY, Brain 2-Arachidonoylglycerol Levels Are Dramatically and Rapidly Increased Under Acute Ischemia-Injury Which Is Prevented by Microwave Irradiation, Lipids 51(4) (2016) 487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brose SA, Golovko MY, Eicosanoid post-mortem induction in kidney tissue is prevented by microwave irradiation, Prostaglandins Leukot Essent Fatty Acids 89(5) (2013) 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Khasabova IA, Chandiramani A, Harding-Rose C, Simone DA, Seybold VS, Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain, Pharmacol Res 64(1) (2011) 60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Falk S, Dickenson AH, Pain and nociception: mechanisms of cancer-induced bone pain, Journal of Clinical Oncology 32(16) (2014) 1647–54. [DOI] [PubMed] [Google Scholar]
- [36].Mantyh PW, Bone cancer pain: from mechanism to therapy, Curr Opin Support Palliat Care 8(2) (2014) 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Khasabova IA, Stucky CL, Harding-Rose C, Eikmeier L, Beitz AJ, Coicou LG, Hanson AE, Simone DA, Seybold VS, Chemical interactions between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain, The Journal of neuroscience : the official journal of the Society for Neuroscience 27(38) (2007) 10289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Khasabova IA, Morgan HR, Khasabov SG, Marti J, Seybold VS, Simone DA, Resolvins inhibit sensitization of nociceptors evoked by cancer cell exosomes., 16th International Conference on Bioactive Lipids in Cancer, Inflammation and Related Diseases, St. Petersburg, Florida, October 20 – 23., 2019. [Google Scholar]
- [39].Scott AC, McConnell S, Laird B, Colvin L, Fallon M, Quantitative Sensory Testing to assess the sensory characteristics of cancer-induced bone pain after radiotherapy and potential clinical biomarkers of response, Eur J Pain 16(1) (2012) 123–33. [DOI] [PubMed] [Google Scholar]
- [40].Paice JA, Mulvey M, Bennett M, Dougherty PM, Farrar JT, Mantyh PW, Miaskowski C, Schmidt B, Smith TJ, AAPT Diagnostic Criteria for Chronic Cancer Pain Conditions, J Pain 18(3) (2017) 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Martland ME, Rashidi AS, Bennett MI, Fallon M, Jones C, Rolke R, Mulvey MR, The use of quantitative sensory testing in cancer pain assessment: A systematic review, Eur J Pain 24(4) (2020) 669–684. [DOI] [PubMed] [Google Scholar]
- [42].Smeester BA, Lunzer MM, Akgun E, Beitz AJ, Portoghese PS, Targeting putative mu opioid/metabotropic glutamate receptor-5 heteromers produces potent antinociception in a chronic murine bone cancer model, Eur J Pharmacol 743 (2014) 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Marcuzzi A, Dean CM, Wrigley PJ, Hush JM, Early changes in somatosensory function in spinal pain: a systematic review and meta-analysis, Pain 156(2) (2015) 203–14. [DOI] [PubMed] [Google Scholar]
- [44].Honsek SD, Seal RP, Sandkuhler J, Presynaptic inhibition of optogenetically identified VGluT3+ sensory fibres by opioids and baclofen, Pain 156(2) (2015) 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Andoh T, Kuraishi Y, Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice, Brain Res Mol Brain Res 137(1–2) (2005) 263–6. [DOI] [PubMed] [Google Scholar]
- [46].Hu S, Mao-Ying QL, Wang J, Wang ZF, Mi WL, Wang XW, Jiang JW, Huang YL, Wu GC, Wang YQ, Lipoxins and aspirin-triggered lipoxin alleviate bone cancer pain in association with suppressing expression of spinal proinflammatory cytokines, J Neuroinflammation 9 (2012) 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bisicchia E, Sasso V, Catanzaro G, Leuti A, Besharat ZM, Chiacchiarini M, Molinari M, Ferretti E, Viscomi MT, Chiurchiu V, Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs, Mol Neurobiol 55(8) (2018) 6894–6905. [DOI] [PubMed] [Google Scholar]
- [48].Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW, Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons, Neuroscience 98(3) (2000) 585–98. [DOI] [PubMed] [Google Scholar]
- [49].Sabino MA, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW, Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system, International Journal of Cancer 104(5) (2003) 550–8. [DOI] [PubMed] [Google Scholar]
- [50].Walter L, Franklin A, Witting A, Moller T, Stella N, Astrocytes in culture produce anandamide and other acylethanolamides, J Biol Chem 277(23) (2002) 20869–76. [DOI] [PubMed] [Google Scholar]
- [51].Walter L, Dinh T, Stella N, ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase, The Journal of neuroscience : the official journal of the Society for Neuroscience 24(37) (2004) 8068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D, Formation and inactivation of endogenous cannabinoid anandamide in central neurons, Nature 372(6507) (1994) 686–91. [DOI] [PubMed] [Google Scholar]
- [53].Green D, Ruparel S, Gao X, Ruparel N, Patil M, Akopian A, Hargreaves K, Central activation of TRPV1 and TRPA1 by novel endogenous agonists contributes to mechanical allodynia and thermal hyperalgesia after burn injury, Mol Pain 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hargreaves KM, Ruparel S, Role of Oxidized Lipids and TRP Channels in Orofacial Pain and Inflammation, J Dent Res 95(10) (2016) 1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U, Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia, Proc Natl Acad Sci U S A 99(15) (2002) 10150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Svensson CI, Yaksh TL, The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing, Annu Rev Pharmacol Toxicol 42 (2002) 553–83. [DOI] [PubMed] [Google Scholar]
- [57].Tepper MA, Zurier RB, Burstein SH, Ultrapure ajulemic acid has improved CB2 selectivity with reduced CB1 activity, Bioorg Med Chem 22(13) (2014) 3245–51. [DOI] [PubMed] [Google Scholar]
- [58].Motwani MP, Bennett F, Norris PC, Maini AA, George MJ, Newson J, Henderson A, Hobbs AJ, Tepper M, White B, Serhan CN, MacAllister R, Gilroy DW, Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation, Clin Pharmacol Ther 104(4) (2018) 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zurier RB, Sun YP, George KL, Stebulis JA, Rossetti RG, Skulas A, Judge E, Serhan CN, Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4, FASEB J 23(5) (2009) 1503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Serhan CN, Oliw E, Unorthodox routes to prostanoid formation: new twists in cyclooxygenase-initiated pathways, J Clin Invest 107(12) (2001) 1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA, International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2), Pharmacol Rev 62(4) (2010) 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA, Identification and functional characterization of brainstem cannabinoid CB2 receptors, Science 310(5746) (2005) 329–32. [DOI] [PubMed] [Google Scholar]
- [63].Racz I, Nadal X, Alferink J, Banos JE, Rehnelt J, Martin M, Pintado B, Gutierrez-Adan A, Sanguino E, Manzanares J, Zimmer A, Maldonado R, Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(46) (2008) 12125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nent E, Nozaki C, Schmole AC, Otte D, Zimmer A, CB2 receptor deletion on myeloid cells enhanced mechanical allodynia in a mouse model of neuropathic pain, Sci Rep 9(1) (2019) 7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Curto-Reyes V, Llames S, Hidalgo A, Menendez L, Baamonde A, Spinal and peripheral analgesic effects of the CB2 cannabinoid receptor agonist AM1241 in two models of bone cancer-induced pain, British journal of pharmacology 160(3) (2010) 561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mecha M, Feliu A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutierrez S, de Sola RG, Guaza C, Endocannabinoids drive the acquisition of an alternative phenotype in microglia, Brain Behav Immun 49 (2015) 233–45. [DOI] [PubMed] [Google Scholar]
- [67].Bu HL, Xia YZ, Liu PM, Guo HM, Yuan C, Fan XC, Huang C, Wen YY, Kong CL, Wang T, Ma LT, Li XX, Zhang HW, Zhang LR, Ma MY, Ai YQ, Zhang W, The Roles of Chemokine CXCL13 in the Development of Bone Cancer Pain and the Regulation of Morphine Analgesia in Rats, Neuroscience 406 (2019) 62–72. [DOI] [PubMed] [Google Scholar]
- [68].Lu C, Liu Y, Sun B, Sun Y, Hou B, Zhang Y, Ma Z, Gu X, Intrathecal Injection of JWH-015 Attenuates Bone Cancer Pain Via Time-Dependent Modification of Pro-inflammatory Cytokines Expression and Astrocytes Activity in Spinal Cord, Inflammation 38(5) (2015) 1880–90. [DOI] [PubMed] [Google Scholar]
- [69].Lozano-Ondoua AN, Hanlon KE, Symons-Liguori AM, Largent-Milnes TM, Havelin JJ, Ferland HL 3rd, Chandramouli A, Owusu-Ankomah M, Nikolich-Zugich T, Bloom AP, Jimenez-Andrade JM, King T, Porreca F, Nelson MA, Mantyh PW, Vanderah TW, Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists, J Bone Miner Res 28(1) (2013) 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR, Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(50) (2011) 18433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Serhan CN, Chiang N, Van Dyke TE, Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators, Nature reviews.Immunology 8(5) (2008) 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N Jr., Serhan CN, Smith LE, Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis, Nature medicine 13(7) (2007) 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW, Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple antinociception, British journal of pharmacology 161(3) (2010) 707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A, Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin, Science 307(5714) (2005) 1468–72. [DOI] [PubMed] [Google Scholar]
- [75].Khasabova IA, Simone DA, Seybold VS, Cannabinoids attenuate depolarization-dependent Ca2+ influx in intermediate-size primary afferent neurons of adult rats, Neuroscience 115(2) (2002) 613–25. [DOI] [PubMed] [Google Scholar]
- [76].Khasabova IA, Harding-Rose C, Simone DA, Seybold VS, Differential effects of CB1 and opioid agonists on two populations of adult rat dorsal root ganglion neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 24(7) (2004) 1744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kelly S, Chapman V, Selective cannabinoid CB1 receptor activation inhibits spinal nociceptive transmission in vivo, J Neurophysiol 86(6) (2001) 3061–4. [DOI] [PubMed] [Google Scholar]
- [78].Johanek LM, Simone DA, Cannabinoid agonist CP 55,940, prevents capsaicin-induced sensitization of spinal cord dorsal horn neurons, J Neurophysiol 93(2) (2005) 989–97. [DOI] [PubMed] [Google Scholar]
- [79].Di S, Boudaba C, Popescu IR, Weng FJ, Harris C, Marcheselli VL, Bazan NG, Tasker JG, Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus, J Physiol 569(Pt 3) (2005) 751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU, Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization, Science 325(5941) (2009) 760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].McDowell TS, Wang ZY, Singh R, Bjorling D, CB1 cannabinoid receptor agonist prevents NGF-induced sensitization of TRPV1 in sensory neurons, Neurosci Lett 551 (2013) 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sun Y, Zhang W, Liu Y, Liu X, Ma Z, Gu X, Intrathecal injection of JWH015 attenuates remifentanil-induced postoperative hyperalgesia by inhibiting activation of spinal glia in a rat model, Anesth Analg 118(4) (2014) 841–53. [DOI] [PubMed] [Google Scholar]
- [83].Burgos E, Gomez-Nicola D, Pascual D, Martin MI, Nieto-Sampedro M, Goicoechea C, Cannabinoid agonist WIN 55,212–2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells, Eur J Pharmacol 682(1–3) (2012) 62–72. [DOI] [PubMed] [Google Scholar]
- [84].Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, Kuhn MN, Thakur GA, Makriyannis A, Milligan ED, Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels, Pain 153(5) (2012) 1091–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Maqboul A, Elsadek B, Expression profiles of TRPV1, TRPV4, TLR4 and ERK1/2 in the dorsal root ganglionic neurons of a cancer-induced neuropathy rat model, PeerJ 6 (2018) e4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Martinez-Rojas VA, Garcia G, Noriega-Navarro R, Guzman-Priego CG, Torres-Lopez JE, Granados-Soto V, Murbartian J, Peripheral and spinal TRPA1 channels contribute to formalin-induced long-lasting mechanical hypersensitivity, J Pain Res 11 (2018) 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A, A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition, Mol Pain 3 (2007) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ruparel S, Bendele M, Wallace A, Green D, Released lipids regulate transient receptor potential channel (TRP)-dependent oral cancer pain, Mol Pain 11 (2015) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, Gibbs JL, Schmidt BL, Nerve growth factor links oral cancer progression, pain, and cachexia, Mol Cancer Ther 10(9) (2011) 1667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Heo MH, Kim JY, Hwang I, Ha E, Park KU, Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain, Korean J Intern Med 32(6) (2017) 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Antoniazzi CTD, Nassini R, Rigo FK, Milioli AM, Bellinaso F, Camponogara C, Silva CR, de Almeida AS, Rossato MF, De Logu F, Oliveira SM, Cunha TM, Geppetti P, Ferreira J, Trevisan G, Transient receptor potential ankyrin 1 (TRPA1) plays a critical role in a mouse model of cancer pain, Int J Cancer (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Vit JP, Ohara PT, Tien DA, Fike JR, Eikmeier L, Beitz A, Wilcox GL, Jasmin L, The analgesic effect of low dose focal irradiation in a mouse model of bone cancer is associated with spinal changes in neuro-mediators of nociception, Pain 120(1–2) (2006) 188–201. [DOI] [PubMed] [Google Scholar]
- [93].Wacnik PW, Kehl LJ, Trempe TM, Ramnaraine ML, Beitz AJ, Wilcox GL, Tumor implantation in mouse humerus evokes movement-related hyperalgesia exceeding that evoked by intramuscular carrageenan, Pain 101(1–2) (2003) 175–86. [DOI] [PubMed] [Google Scholar]
- [94].Luo X, Gu Y, Tao X, Serhan CN, Ji RR, Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy, Front Pharmacol 10 (2019) 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Parashar K, Schulte F, Hardt M, Baker OJ, Sex-mediated elevation of the specialized pro-resolving lipid mediator levels in a Sjogren’s syndrome mouse model, FASEB J 34(6) (2020) 7733–7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lin YH, Brown JA, DiMartino C, Dahms I, Salem N Jr., Hibbeln JR, Differences in long chain polyunsaturates composition and metabolism in male and female rats, Prostaglandins Leukot Essent Fatty Acids 113 (2016) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Extier A, Langelier B, Perruchot MH, Guesnet P, Van Veldhoven PP, Lavialle M, Alessandri JM, Gender affects liver desaturase expression in a rat model of n-3 fatty acid repletion, J Nutr Biochem 21(3) (2010) 180–7. [DOI] [PubMed] [Google Scholar]
- [98].Walker CG, Browning LM, Mander AP, Madden J, West AL, Calder PC, Jebb SA, Age and sex differences in the incorporation of EPA and DHA into plasma fractions, cells and adipose tissue in humans, Br J Nutr 111(4) (2014) 679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


