Abstract
Cognitive behaviors, such as episodic memory formation, are complex processes involving coordinated activity in multiple brain regions. However, much of the research on hormonal regulation of cognition focuses on manipulation of one region at a time or provides a single snapshot of how a systemic treatment affects multiple brain regions without investigating how these regions might interact to mediate hormone effects. Here, we use estrogenic regulation of episodic memory as an example of how circuit-based approaches may be incorporated into future studies of hormones and cognition. We first review basic episodic memory circuitry, rapid mechanisms by which 17β-estradiol can alter circuit activity, and current knowledge about 17β-estradiol’s effects on episodic memory. Next, we outline approaches that researchers can employ to consider circuit effects in their estrogen research and provide examples of how these methods have been used to examine hormonal regulation of memory and other behaviors.
Keywords: 17β-estradiol, hippocampus, prefrontal cortex, genetic tools, chemogenetic, optogenetic
1. Introduction
Nearly five decades of research has demonstrated that learning and memory are modulated by estrogens, most notably 17β-estradiol (E2) (see Bean et al., 2014; Daniel et al., 2015; Tuscher et al., 2015; Hamson et al., 2016; Frick and Kim, 2018; Sheppard et al., 2018; Luine and Frankfurt, 2020 for recent reviews). In trying to identify the neurobiological mechanisms underlying this regulation, most investigators have used static approaches that examine brain regions in isolation, either by infusing E2 directly to a single brain region or injecting E2 systemically and examining alterations in dissected tissue from one or more brain regions. The majority of studies have focused, at least in part, on the hippocampus, given the importance of this brain region to many forms of memory and the well-established effects of E2 on hippocampal dendritic morphology, synaptic plasticity, biochemistry, epigenetics, and gene expression (see Frick, 2015; Frick et al., 2015 for reviews). Numerous studies have also examined how infusion of E2 into other regions like the prefrontal cortex, temporal cortices, and amygdala influences memory function (e.g., Almey et al., 2014; Tuscher et al., 2019; Gervais et al., 2013, 2016; Mitchnick et al., 2019; Lymer et al., 2018). Nevertheless, studies examining estrogenic regulation of memory function rarely consider how these brain regions interact to regulate memory, despite increasing evidence that the systems-level neural circuits formed by their reciprocal connections are essential for intact memory function. Identifying the brain regions that form memory circuits will not only further understanding of how those circuits form systems-level networks that mediate memory, but also how neuromodulators like E2 influence the function of these circuits and networks. The recent application of new tools, such as optogenetics and chemogenetics, to identify and manipulate these connections now make it possible to examine how a modulator like E2 can influence memory by mediating activity of entire circuits and networks, rather than just a single brain region. Such circuit-based approaches will be necessary in future studies to truly understand regulation of memory by E2 and other hormones.
Understanding how estrogens like E2 regulate memory is particularly important in the context of aging and Alzheimer’s disease, where estrogen loss is thought to contribute to the risk and magnitude of memory dysfunction (Launer et al., 1999; Yaffe et al., 2007; Fox et al., 2013; Jacob et al., 2016). Therefore, mechanistic insights into estrogenic memory regulation could aid in the development of treatments for memory dysfunction. Because systemically-delivered therapies that cross the blood-brain barrier (e.g., pills, patches, creams) will affect the entire brain, it is critical to determine how estrogens affect multiple brain regions within the memory circuitry rather than just a single brain region. More immediately, millions of women already take exogenous estrogens as part of existing hormone therapies (e.g., hormonal contraceptives, menopausal hormone therapy), and thus, appreciating how estrogens affect global brain function may provide insights into effects of these treatments on cognition (Griksiene and Ruksenas, 2011; Peragine et al., 2019).
Our goal here is to encourage increased attention to brain circuitry and systems-level effects in studies of hormones and memory. We will use E2 as a representative neuromodulator, given the wealth of data available regarding the mnemonic effects of this hormone, and episodic memory will serve as an archetypical behavioral outcome due to the abundance of evidence that E2 influences this type of memory. Episodic memory is a form of explicit memory that binds sensory, affective, and contextual information into a cohesive unit that allows for explicit recall of events and experiences (Dickerson and Eichenbaum, 2010; Allen and Fortin, 2013). Because episodic memory can be impaired by many conditions, including aging and Alzheimer’s disease (Leyhe et al., 2009; Small et al., 2011), decades of research have been devoted to understanding this essential cognitive process. In this review, we will first provide an overview of the literature pinpointing brain regions involved in episodic memory, estrogenic effects on brain regions involved in episodic memory, and estrogenic modulation of episodic memory. Next, we will discuss methodological approaches for investigating circuit-level effects of estrogen treatment. Unless otherwise specified, the data described below comes from studies of young (2-5 month-old) rodents because the majority of studies related to neural mechanisms underlying E2’s effects on episodic memory use rats and mice in this age group. Although E2 and episodic memory will be the focus throughout, the principles discussed here should apply to other hormones and types of cognition.
2. Overview of brain regions that mediate episodic memory
Historically, studies investigating estrogenic modulation of brain function have focused on the hippocampus because: 1) the dorsal hippocampus is one of the primary brain regions that mediates episodic memory encoding (Dickerson and Eichenbaum, 2010; Eichenbaum, 2017), 2) episodic memory encoding and recall is impaired in aging and numerous disorders (Gallagher and Koh, 2011; Dickerson and Eichenbaum, 2010; Burke and Barnes, 2010), and 3) E2 regulates numerous aspects of dorsal hippocampal function (see Section 2.2.). However, it should be noted that most of our existing knowledge about the neurobiology of episodic memory comes from studies in which males or subjects of unspecified sex were used. With the 2016 implementation of a National Institutes of Health policy requiring sex to be considered as a variable in vertebrate animal research (Clayton, 2016; Brooks and Clayton, 2017; Clayton, 2018) , sex differences have increasingly been reported in numerous types of episodic memory throughout the adult rodent (Jonasson, 2005; Koss and Frick, 2017; Koss et al., 2018; Keiser et al., 2017) and human lifespan (Rentz et al., 2017; Laws et al., 2018; Asperholm et al., 2019). As such, delineating how brain regions interact on the circuit and network levels in females, especially with respect to estrogenic modulation of episodic memory, is an important future direction for episodic memory research.
The acquisition, consolidation, and retrieval of episodic memory is a complex process that requires activity in many different brain regions (Fig. 1; Dickerson and Eichenbaum, 2010; Allen and Fortin, 2013). Early evidence from amnesic patients and lesion studies in nonhuman primates and rodents suggested a uniquely important role for the hippocampus (particularly the dorsal hippocampus in rodents) and adjacent medial temporal lobe cortices in encoding spatial and certain nonspatial components of episodic memory (Eichenbaum et al., 1990; Squire, 1992; Zola et al., 2000). The hippocampus is one of the most thoroughly studied brain regions involved in episodic memory function because it is integral for binding neocortical and subcortical inputs into richly detailed representations that include both semantic (e.g., facts) and contextual (e.g., sensory, affect, arousal) information (Squire, 1992; Dickerson and Eichenbaum, 2010). Incoming sensory information undergoes higher-order processing in sensory association areas throughout the neocortex and is then integrated in the temporal lobe’s perirhinal, parahippocampal, and entorhinal cortices. Afferents from the entorhinal cortex transmit this information to the dentate gyrus of the hippocampus, which then sends it for further processing in the CA3 and CA1 subfields (Lavenex and Amaral, 2000; Eichenbaum, 2000). Subcortical information is integrated into the memory representation via inputs from the basal forebrain, amygdala, and other limbic structures (Hepler et al., 1985; Yonelinas and Ritchey, 2015; Kitamura et al., 2017). These complex contextual memories are thought to be consolidated with aid from the prefrontal cortex and midline thalamic nuclei (Warburton and Brown, 2015; Eichenbaum, 2017; Mitchell, 2015; Dolleman-van der Weel et al., 2019). As such, episodic memory formation “takes a village” of coordinated activity among a network of regions throughout the brain.
Figure 1. Schematic illustration of several key brain regions involved in episodic memory formation.
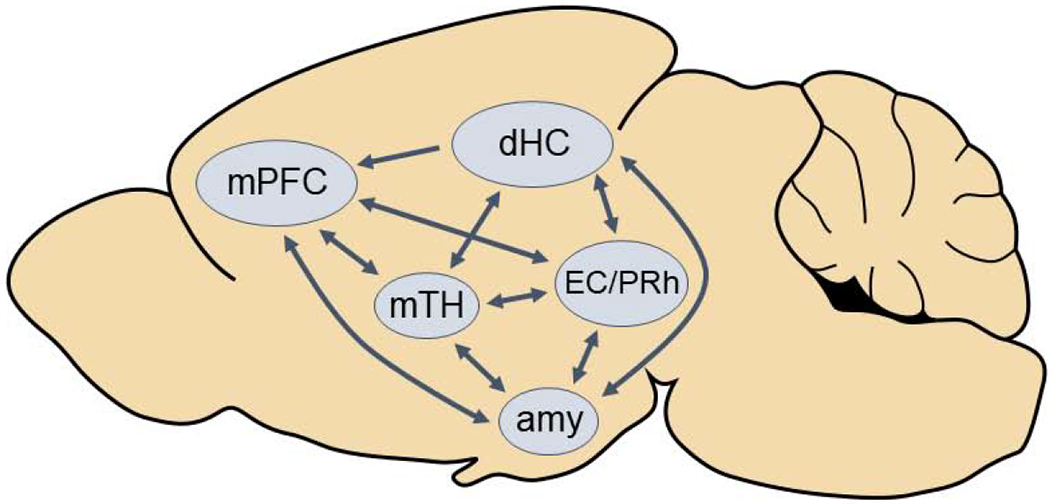
mPFC = medial prefrontal cortex, dHC = dorsal hippocampus, mTH = midline thalamic nuclei, EC/PRh = entorhinal and perirhinal cortices, amy = amygdala
Evidence to support the importance of coordination among multiple brain regions to episodic memory formation comes from several recent rodent studies. Data from male and female rodents indicate that coordinated activity, either concurrently or in succession, between the hippocampus and mPFC is necessary for the formation of various forms of episodic memory, including object recognition, spatial memory, and contextual fear memory (Warburton and Brown, 2015; Kitamura et al., 2017; Tuscher et al., 2018). Findings also suggest that activity in other brain regions, including dorsal and ventral midline thalamic nuclei, mediate hippocampal-prefrontal communication and are necessary for cognitive tasks requiring the hippocampus and mPFC (Jin and Maren, 2015; Mitchell, 2015; Dolleman-van der Weel et al., 2019). The amygdala is also essential when emotion is a key aspect of the learning process, as indicated by hundreds of studies using contextual fear conditioning to examine episodic memory (e.g., Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Maren and Quirk, 2004). In sum, although the hippocampus and adjacent temporal lobe cortices have historically received the lion’s share of attention, successful acquisition, consolidation, and retrieval of episodic memories require a complex network of numerous cortical and subcortical brain regions.
3. Neural mechanisms underlying E2 effects on episodic memory
E2 acts by binding to intracellular estrogen receptors (ER) alpha and beta (ERα and ERβ) as well as the membrane-bound G-protein-coupled estrogen receptor (GPER). In the nucleus, ERα and ERβ act as transcription factors to influence gene expression. However, these ERs and GPER can act at the membrane to exert rapid effects on many aspects of cellular function including cell signaling, epigenetic processes, neurotransmitter release, synaptic plasticity, and dendritic spine morphology (e.g., Avila et al., 2017; Bi et al., 2001; Boulware et al., 2005, Boulware et al., 2013; Fernandez et al., 2008; Hasegawa et al., 2015; Kim et al., 2016; Kramár et al., 2009; Zhao et al., 2010; Oberlander and Woolley, 2016; Tuscher et al., 2016a). Estrogen receptors are present in many regions throughout the forebrain, including the hippocampus, prefrontal cortex, temporal cortex, amygdala, basal forebrain, and other regions critical for episodic memory (Shughrue et al., 1997; Mitra et al., 2003; Milner et al., 2001, 2005; Mitterling et al., 2010; Waters et al., 2015). Estrogen receptors have been detected both pre- and post-synaptically in the prefrontal cortex (Almey et al., 2014) and hippocampus (Milner et al., 2001, 2005), as well as on perisynaptic astrocytes (Garcia-Segura et al., 1999). Many of these cells also express aromatase, the enzyme that synthesizes E2 from testosterone (Kretz et al., 2004; Vierk et al., 2014). Blocking aromatase activity within the rodent hippocampus prevents the formation of spatial and object recognition memories, indicating a key role for brain-derived E2 in episodic memory formation (Tuscher et al., 2016b; Koss and Frick, 2019). Because research on the neurobiology underlying E2’s effects on episodic memory has largely focused on the rodent hippocampus, this brain region is the primary emphasis below.
Much attention has focused on the postsynaptic effects of E2 in the hippocampus, due to seminal findings that endogenous and exogenous E2 regulate the density of dendritic spines on CA1 pyramidal neurons in female rats. Initial reports compared CA1 spine density in the female rat dorsal hippocampus during phases of the natural estrous cycle, and found that spine density was approximately 30% higher during proestrus (elevated E2) relative to estrus (low E2) (Woolley and McEwen, 1993). In ovariectomized rats, chronic or acute treatment with exogenous E2 increased CA1 dendritic spine density (Gould et al., 1990; Woolley and McEwen, 1992), an effect associated with enhanced E2-induced synaptic plasticity, long-term potentiation (LTP), and memory (for reviews see Woolley, 2007; Frankfurt and Luine, 2015; Luine et al., 2018). E2 also enhances CA1 spine density in gonadectomized male rats (Jacome et al., 2016), although it is unclear whether sex differences exist in nature, magnitude, or mechanisms underlying the response in adults. Interestingly, E2-induced spinogenesis in the hippocampus of both sexes seems limited to CA1, as reports indicate no effect or even reduced spine density in CA3 or the dentate gyrus of young adult rats and mice (Tsurugizawa et al., 2005; Tuscher et al., 2016a). E2 treatment also increases spine density in the mPFC of young adult ovariectomized mice (Tuscher et al., 2016a) and rats (Inagaki et al., 2012; Ye et al., 2019), as well as aged female rhesus monkeys (Tang et al., 2004; Hao et al., 2006). Interestingly, emerging in vivo and in vitro research indicates that E2 does not uniformly influence all spines along the length of a dendrite (Oberlander and Woolley, 2017; Jain et al., 2019; Ye et al., 2019), suggesting that only certain synapses may be responsive to E2. This finding could suggest that E2 can modify circuits at the level of the individual synapse. This possibility is supported by data from Phan et al. (2015) showing that E2-induced spine formation in CA1 was associated with decreased excitatory input to CA1, suggesting that formation of immature or silent synapses may be a key postsynaptic mechanism through which E2 modulates memory (Srivastava et al., 2008).
Evidence linking E2-induced dendritic spine changes to episodic memory comes from studies of hippocampal cell signaling, where such signaling plays a key role in the ability of E2 to increase spine density in both the DH and mPFC. E2 activates numerous cell signaling cascades in the DH within five minutes of bilateral DH infusion, including ERK (extracellular signal-regulated kinase), PI3K (phosphatidylinositol 3-kinase), and mTOR (mammalian target of rapamycin). Rapid activation of these cell signaling pathways in the DH by E2 is necessary for E2 to enhance spatial and object recognition memory consolidation in ovariectomized mice (Fernandez et al., 2008; Fan et al., 2010; Fortress et al., 2013). Activation of ERK and mTOR in the DH are also necessary for E2 to increase dendritic spine density on pyramidal neurons in the DH and mPFC (Tuscher et al., 2016a), supporting a link between E2’s effects on spine density and episodic memory formation. These effects may be mediated by ERα and ERβ in the DH, as activation of either receptor enhances spatial and object recognition memory consolidation in ovariectomized mice in an ERK-dependent manner (Boulware et al., 2013). Activation of GPER enhances both types of memory and facilitates CA1 dendritic spine density in ovariectomized mice by promoting actin filament polymerization (Kim et al., 2016, 2019). However, these effects appear to be independent of E2, as a GPER antagonist does not block the effects of E2 on ERK signaling, spines, or memory (Kim et al., 2016, 2019). Additional information about E2’s effects on DH cell signaling, epigenetic processes, and gene expression can be found in other reviews that describe this work in more detail (e.g., Frick, 2015; Frick and Kim, 2018; Sheppard et al., 2018).
Although the bulk of research on rapid effects of E2 has focused on postsynaptic effects, E2 can also act presynaptically by influencing neurotransmitter release. For example, in slice recordings from the CA1 of ovariectomized female rats, E2 increased the probability of glutamate release and decreased the probability of GABA release, resulting in increased excitatory postsynaptic currents and reduced inhibitory currents (Smejkalova and Wooley, 2010; Oberlander and Woolley, 2017; Huang and Woolley, 2012; Tabatadze et al., 2015). Outside of the hippocampus, E2 acts in the dorsal striatum to enhance amphetamine-evoked dopamine release in ovariectomized female rats (Becker, 1990; Shams et al., 2016). E2 can also enhance intrinsic excitability of neurons in the CA1 (Wu et al., 2011) or infralimbic medial PFC of ovariectomized rats (Yousuf et al., 2019).
4. Estrogenic regulation of episodic memory
The effects of exogenous E2 and other estrogens on episodic memory function have been tested extensively in rodents, primarily using hippocampus-dependent tasks that assess spatial memory, working memory, and object or social recognition. Although results can vary based on sex, gonadal status, age, species, timing of treatment, and the task being used to measure memory, the balance of studies report that E2 facilitates the acquisition and/or consolidation of episodic memories in both female and male rodents (Frick, 2009; Bimonte-Nelson et al., 2010; Daniel, 2013; Luine, 2014; Tuscher et al., 2015; Hamson et al., 2016; Koss and Frick, 2017; Sheppard et al., 2018). For example, considerable research has shown that acute or chronic systemic E2 administration improves spatial memory among ovariectomized female rats and mice in traditional hippocampus-dependent tasks like the Morris water maze, radial arm maze, and T-maze, as well as in tests of object or social recognition, object location, inhibitory avoidance, and trace eyeblink conditioning (e.g., O’Neal et al. 1996; Daniel et al. 1997; Fader et al. 1998, 1999; Luine et al. 1998; Bimonte and Denenberg 1999; Gibbs 1999; Daniel and Dohanich 2001; Sandstrom and Williams 2001, 2004; Bowman et al. 2002; Heikkinen et al. 2002; Holmes et al. 2002; Garza-Meilandt et al. 2006; Bohacek and Daniel 2007; Hammond et al. 2009; Singh et al. 1994; Frye and Rhodes 2002; Leuner et al. 2004). Furthermore, acute systemic E2 given immediately post-training to ovariectomized rats and mice enhances memory consolidation in the Morris water maze and object recognition tasks (e.g., Luine et al., 2003; Gresack and Frick, 2006; Walf et al. 2006; Frye et al. 2007; Fernandez et al. 2008; Inagaki et al. 2010). Pre and/or post-training treatment with other forms of estrogen, including estriol, estrone, and 17α-estradiol, can also enhance numerous forms of episodic memory such as object recognition and spatial memory tested via object placement or the radial arm maze in young or middle-aged female rats (Luine et al., 2003; Engler-Chiurazzi et al., 2011; Acosta et al., 2009), although continuous estrone treatment has been found to impair spatial memory in middle-aged female rats (Engler-Chiurazzi et al., 2012).
Collectively, the data from systemic administration studies suggest that E2 and other estrogens generally enhance both the acquisition and consolidation of various types of episodic memories. However, effects of direct intracranial infusions can reveal considerably more about the role of E2 in specific brain regions within the episodic memory circuit. Thus, the sections below will provide an overview of the effects of direct intracranial infusions of E2 on episodic memory.
4.1. Hippocampus
Studies involving direct intracranial infusion of E2 have largely focused on the dorsal hippocampus (DH). To avoid tissue damage from repeated daily infusion, episodic memory in these studies is typically assessed using tasks in which training involves a single trial or day, with treatments often administered immediately after training to pinpoint effects on memory consolidation. For example, E2 infused bilaterally into the DH immediately after one-day Morris water maze training enhanced spatial memory consolidation in male and ovariectomized rats (Packard et al., 1996; Packard and Teather, 1997). Numerous other studies have used one-trial object-based tasks that take advantage of rodent’s inherent preference for novelty. The tasks consist of a training phase in which animals explore two identical objects in an open field, and a testing phase in which one object is moved to another location (object placement/location) or replaced with a new object (object recognition). Rodents that remember the identity and location of the training objects spend significantly more time than chance exploring the moved or novel object. When infused bilaterally into the DH prior to or immediately after training, E2 enhances memory consolidation in both tasks among male and ovariectomized female rats and mice, such that E2-treated subjects demonstrate longer-lasting memory for the training objects and locations than vehicle-infused mice (e.g., Fernandez et al., 2008; Zhao et al., 2010, 2012; Fortress et al., 2013; Boulware et al., 2013; Pereira et al., 2014; Phan et al., 2015; Kim et al., 2016; Koss et al., 2018).
Pre-training DH E2 infusions similarly enhance social recognition in ovariectomized mice (Phan et al., 2015). Effects of E2 in object and social memory tasks among ovariectomized mice can be mediated by ERα, ERβ, and/or GPER, as DH infusion of ER agonists or antagonists enhance and impair, respectively, episodic memory formation (Boulware et al., 2013; Pereira et al., 2014; Phan et al., 2015; Kim et al., 2016, Kim and Frick, 2017; Lymer et al., 2017). In ovariectomized mice, activation of ERα or ERβ, in association with metabotropic glutamate receptor 1a, or of GPER, triggers rapid increases in cell signaling, histone acetylation, and DNA methylation that lead to downstream changes in local protein synthesis, actin polymerization, dendritic spinogenesis, and gene transcription, upon which E2-mediated episodic memory enhancement depend (see Frick, 2015a, 2015b; Frick and Kim, 2018 for recent reviews).
E2 is an important mediator of other types of hippocampal-dependent episodic memories as well. For example, DH E2 infusion in ovariectomized rats enhances extinction of contextual fear conditioning, another form of episodic learning that requires the DH (Chang et al., 2009). Interestingly, E2 in the hippocampus and other brain regions also appears to influence strategy selection and competition between brain regions. One series of studies (Zurkovsky et al., 2006, 2007, 2011) compared roles of E2 in the hippocampus and striatum in versions of a T-maze alternation task that encouraged spatial or response learning. In ovariectomized rats, infusions of E2 into the hippocampus, but not striatum, enhanced spatial learning, whereas infusion of E2 into the striatum, but not hippocampus impaired response learning, particularly when few visual cues were present. This work suggests that the effects of E2 may differentially affect spatial and response learning via independent actions within the hippocampus and striatum.
As E2 and other steroid hormones are synthesized within the hippocampus and other brain regions (Kawato et al., 2002; Kretz et al., 2004; Hojo et al., 2004, 2011; Kimoto et al., 2011; Vierk et al., 2014), it is important to note that hippocampally-synthesized E2 is also essential for episodic memory formation. For example, post-training infusion of the aromatase inhibitor letrozole, which prevents the synthesis of E2 from testosterone, blocks episodic memory consolidation in the object recognition and object placement tasks among gonadectomized male and female mice (Tuscher et al., 2016b; Koss and Frick, 2019). Interestingly, however, letrozole does not block memory formation in these tasks among gonadally-intact males (Koss et al., 2019), suggesting that circulating gonadal steroids protect against the loss of hippocampally-synthesized E2. Subsequent work showed that androgen receptors in the DH play a key role in this neuroprotection, as letrozole does block episodic memory formation after infusion of an androgen receptor antagonist into the DH of intact male mice (Koss et al., 2019).
4.2. Temporal cortices and amygdala
Relative to the DH, much less is known about E2’s effects in medial temporal lobe brain regions important for episodic memory including the perirhinal cortex (PRh), entorhinal cortex (EC), and amygdala. In rodents, ERα, and ERβ can be found in the temporal cortices, and are particularly abundant in the amygdala (Shughrue et al., 1997; Österlund et al., 1998; Kritzer, 2002; Mitra et al., 2003). Similar to the DH, pre-training infusion of E2 into the medial amygdala enhanced social recognition memory in ovariectomized mice (Lymer et al., 2018). This effect was mimicked by agonists of ERα, ERβ, and GPER (Lymer et al., 2018), suggesting that E2 may act through any of the ERs in the medial amygdala to facilitate social recognition. The effects of E2 in the amygdala on other forms of episodic memory is unknown, so this is an area ripe for future investigation.
The modulatory effects of E2 in the nearby PRh and EC have been tested in a series of studies using various object recognition protocols, including an object-based delayed non-match to sample (DNMS) task. Acute pre- or post-training infusion of E2 into a region comprising the PRh-EC enhanced object recognition in ovariectomized rats (Gervais et al., 2013, 2016). This effect was mimicked by PRh-EC infusion of an ERβ agonist (Gervais et al., 2016). However, in the DNMS version of the task, which required extensive training and rewarded rats with a food reward for displacing novel objects, PRh-EC infusions of E2 impaired object recognition memory. More recently, a study using a 4-object object-in-place task found that E2 infusion into the PRh enhanced both short- and long-term object memory in male rats (Mitchnick et al., 2019). This effect was mimicked by PRh infusion of an ERβ agonist, but not an ERα agonist, indicating a key role for ERβ in the PRh. Memory in the object-in-place task was blocked by PRh infusion of the GPER antagonist G-15 or the aromatase inhibitor letrozole, also suggesting roles for rapid effects mediated by membrane ER signaling and de novo hippocampal E2 synthesis. However, only G-15 blocked a learning-induced increase in the phosphorylation of c-Jun N-terminal kinase (JNK) in the PRh. In the DH of ovariectomized mice, JNK phosphorylation is necessary for GPER to enhance object recognition and object placement memory consolidation (Kim et al., 2016), supporting a possible role for JNK signaling in the memory-enhancing effects of GPER in the PRh.
4.3. Prefrontal cortex
ERα, ERβ, and GPER are widely expressed in the rodent and non-human primate mPFC (Almey et al., 2014; Crimins et al., 2017), where they are predominantly located presynaptically in axons and terminals, but are also associated with the post-synaptic density in dendritic spines. Systemic E2 treatment in ovariectomized rats increased both pyramidal neuron dendritic spine density and enhanced object recognition and object placement memory consolidation (Luine et al., 2003; Inagaki et al., 2010, 2012; Jacome et al., 2010). Similarly, infusion of E2 into the prelimbic (PL) mPFC enhanced object recognition and object placement memory consolidation and increased mPFC apical dendritic spine density in ovariectomized mice (Tuscher et al., 2019). These findings further support a link between E2-induced spinogenesis and episodic memory consolidation. Systemic E2 or infusion of E2 into the PL mPFC or DH also dose-dependently improved spatial working memory in a win-shift radial arm maze task (Sinopoli et al., 2006) and increased use of a spatial memory strategy in a T-maze navigation task (Almey et al., 2014). The effects of E2 on episodic memory in the mPFC may be due to altered intrinsic excitability, as infralimbic (IL) mPFC E2 infusion in ovariectomized rats was recently shown to increase IL-mPFC intrinsic neuronal excitability and facilitate extinction of cocaine seeking in a manner dependent on activation of TrkB receptors (Yousuf et al,. 2019).
5. Methodological approaches for investigating circuit-level estrogenic effects
As reviewed above, two developing bodies of literature suggest that: a) episodic memory formation is a complex process involving an extensive network of brain regions, and b) E2 regulates brain function and episodic memory through multiple mechanisms in several brain areas. Thus, to fully understand the mechanisms through which E2 regulates episodic memory formation, it will be vital to conduct cell-type-, region-specific, and circuit-specific investigations that determine how, when, and in which cells E2 influences memory formation. Such circuit-level investigations of brain connectivity involve a two-pronged approach; first identify the brain regions that facilitates the behavior of interest and then determine how hormonal regulation of the brain regions in the circuit, alone and in concert, influence behavior. As such, the remainder of this review will discuss commonly used modern methods for examining circuit-level brain function and how they may be applied to study hormonal regulation of episodic memory. The sections below will first outline several current methods used to elucidate brain circuitry and then discuss how some of these techniques have been used to address circuit-level questions about hormones and behavior.
5.1. Defining neural circuitry involved in episodic memory and other behaviors
As summarized above, the neural circuitry supporting episodic memory is complex and not yet fully elucidated. Understanding the role that hormones play in mediating interactions among brain regions first necessitates that these connections be defined. This can be accomplished by: 1) empirically establishing the brain regions that comprise the circuit, 2) adapting the published work of others, or 3) collaborating with others who have expertise in the circuit you want to study. Pinpointing the brain regions involved is an important first step, but it is also useful to know, if possible, the cell types involved within each brain region, as well as their topographical organization and connectivity within and outside the region. Neuroanatomical tracing methods including anterograde, retrograde, and transsynaptic tracing have helped identify connections between regions (reviewed in Lanciego and Wouterlood, 2020). As most of this work is likely to have been conducted by investigators outside of behavioral neuroendocrinology, endocrinologists will often have to rely on research conducted by other labs that have published such information. It is important to note, however, that if you are collaborating with or building upon the research of others, it may first be necessary to validate that the proposed brain regions and projections are relevant in your own animal model under your unique experimental conditions in your particular behavioral task. This validation is especially important for female subjects, given that much of the research on the neural circuitry of episodic memory was conducted in males only. Many classical and novel techniques have been used to dissect neural circuitry, so a thorough discussion is beyond the scope of this review. The sections below provide a brief overview of four general approaches for investigating the brain circuitry involved in episodic memory or other behaviors, and we refer readers to two particularly excellent reviews for more detailed information on these and other methods (Luo et al., 2018; Navabpour et al., 2020).
5.2. Single brain regions
Determining how brain regions coordinate to mediate behavior usually begins by manipulating activity in a single brain region to determine whether that area contributes to a given behavior. Classically, drugs have been infused intracranially to broadly inactivate a brain region of interest. Examples include drugs that potentiate γ-aminobutyric acid (GABA)-induced hyperpolarization (e.g., the GABAA agonist muscimol), inhibit depolarization through antagonism of glutamate receptors (e.g., the N-Methyl-D-aspartate (NMDA) receptor antagonist APV ((2R)-amino-5-phosphonovaleric acid; (2R)-amino-5-phosphonopentanoate)), or block voltage-gated sodium channels (e.g., the inhibitors bupivacaine and lidocaine). However, newer techniques allow for reversible activation or inhibition of neuronal activity with greater spatial and temporal resolution (Fig. 2A).
Figure 2. Diagrammatic representation of various genetic approaches to dissecting brain circuit connectivity.
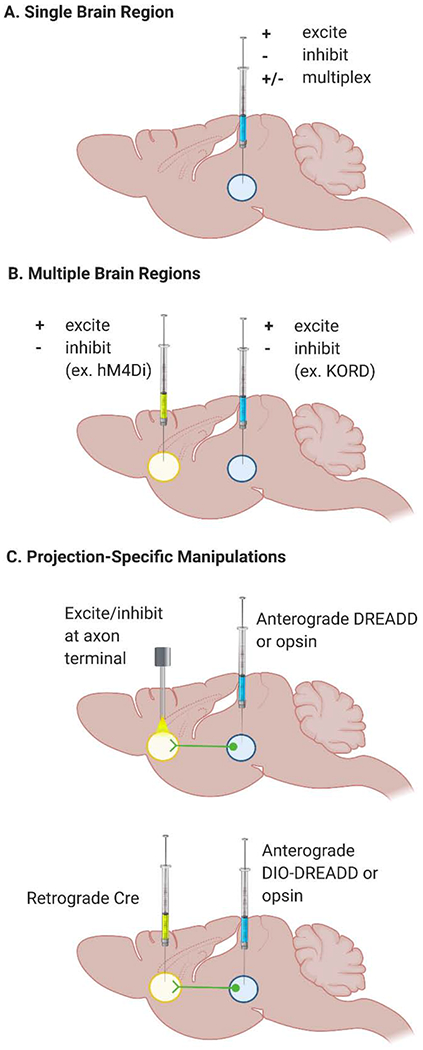
A. Infusion of one or more virally-delivered DREADD or optogenetic constructs to a single brain region can be used to determine its role in episodic memory. Infusion of a single viral construct can be used to either excite or inhibit the region, or multiple constructs can be multiplexed to concurrently excite and inhibit the same brain region using different actuators. B. Different viral constructs can also be used to multiplex across multiple brain regions. For example, expression of the CNO-activated hM4Di DREADD in one region can be paired with expression of the SALB-activated KORD in another region, allowing for separate or simultaneous inactivation of the two regions. C. Two methods for targeting projection neurons. In the first, an anterograde DREADD or opsin is delivered to the region containing the projection cell bodies, whose axon terminals in the target region can be selectively excited or inhibited via light delivery (for optogenetics) or infusion of a DREADD ligand into the target region. In the second method, a Cre-dependent anterograde DIO-DREADD or opsin is delivered to the cell body region, while retrograde virally-delivered Cre is administered to the region containing the axon terminals. The actuator can then be delivered systemically or, for optogenetics, light can be applied to the cell body region.
Chemogenetic inhibition of a brain region is primarily conducted using viral vector-induced expression of DREADDs (designer receptors exclusively activated by designer drugs), which are adeno-associated virus (AAV)-encoded synthetic (“designer”) receptors that are infused into the brain, allowed time for neuronal transfection (usually 2+ weeks), and then later activated by a synthetic ligand, or “actuator” (“designer drug”) that has minimal off-target effects. The primary “designer receptors” are human muscarinic receptors M3 (hM3) or M4 (hM4) and k-opioid receptor (KOR). The most commonly used chemical actuators are clozapine-N-oxide (CNO) and salvinorin-B (SALB). The hM3 receptor is Gq-coupled (thus, is termed “hM3Dq”), whereas the hM4 receptor is Gi-coupled (termed “hM4Di”), as is the KOR DREADD (termed “KORD”). Therefore, when the appropriate chemical actuator is administered (CNO for hM3Dq/hM4Di, SALB for KORD), it binds to the synthetic receptor and causes excitation/depolarization (hM3Dq) or inhibition/hyperpolarization (hM4Di, KORD) of neurons that express the virus (Armbruster et al., 2007; Alexander et al., 2008; Vardy et al., 2015). DREADDs have an advantage over classical pharmacological inhibitors in that they can be used to manipulate subpopulations of cells based on cell-type specific promoters (for primers on DREADDs, see Urban and Roth, 2015; Roth et al., 2016; Campbell and Marchant, 2018). The sustained inhibition offered by DREADDs can be advantageous for studying episodic memory, where the encoding, consolidation, and retrieval phases typically take place on the order of minutes to hours. However, when using tasks with discrete encoding phases or when manipulation on the order of milliseconds to seconds is needed, virally-delivered optogenetic methods can be used to excite or inhibit neuronal activity (Tye and Deisseroth, 2012; Guru et al., 2015). These methods use genetically encoded, light-sensitive ion channels or proton pumps, called opsins, which are activated by specific wavelengths of light delivered intracranially via a fiber cable. Bidirectional optogenetic modulation is possible through the use of excitatory opsins (e.g., the cation channel channelrhodopsin-2) or inhibitory opsins (e.g., the chloride channel halorhodopsin), often within the same brain region.
Both DREADDs and optogenetics have now been used in hundreds of studies to more precisely define a role for the hippocampus, prefrontal cortex, temporal cortices, amygdala and others in episodic memory (Smith et al., 2016; Barnett et al., 2018), building upon decades of research using broad techniques such as lesioning and pharmacological inactivation. In just one recent example, our lab showed that immediate post-training inactivation of excitatory neurons in the DH or mPFC via the KORD and hM4Di DREADDs, respectively, blocked memory consolidation in the object recognition and object placement tasks in ovariectomized mice (Tuscher et al., 2018). Similarly, pre-training hM4Di-mediated inactivation of the dorsal hippocampus impaired object placement in male mice (López et al., 2016), confirming a key role for the dorsal hippocampus in episodic memory in both sexes.
5.3. Multiple brain regions
The aforementioned viral technologies can also be leveraged to examine interactions between brain regions by combined use of (i.e., “multiplexing”) multiple viruses (e.g., Vardy et al., 2015). For example, employing two inhibitory DREADDs that use different actuators (e.g., CNO for hM4Di and SALB for KORD) allows for discrete inactivation of two regions with systemic administration of each actuator (Fig. 2B). Alternatively, using KORD with CNO-dependent hM3Dq allows excitation in one brain region and inhibition in another. A similar multiplexing approach can be taken for optogenetics by using different opsins activated by different wavelengths of light. For example, halorhodopsin is excited by green/yellow light with a peak absorbance at about 570 nm and channelrhodopsin-2 is excited by blue light at around 470 nm. Thus, these opsins can be infused within the same brain region to alternately excite or inhibit neurons within the brain region, depending on the wavelength of light to which its exposed. Additionally, because light stimulation is spatially precise, the same opsin can be used in two different brain regions via the implantation of multiple fibers.
As an example of the multiplexing approach, our laboratory used two DREADDs with different chemical actuators to investigate the role of DH and mPFC both independently, and concurrently, in object recognition and object placement memory consolidation. In ovariectomized mice, we virally expressed KORD in the DH and hM4Di in the mPFC; both DREADDs use a CaMKII promoter to target excitatory neurons, which are hyperpolarized after administration of SALB and CNO, respectively. As mentioned above, inhibition of excitatory neuron activity in the mPFC or DH alone impaired memory consolidation in the object recognition and object placement tasks (Tuscher et al., 2018). However, memory was also impaired in both tasks by simultaneous subthreshold inactivation of these brain regions with lower actuator doses that had no effect on memory alone (Tuscher et al., 2018). These data suggest that concurrent activity of the DH and mPFC supports memory consolidation. By combining brain region-specific expression of different DREADDs that are activated by systemic injection of DREADD-specific activators, multiplexing allows investigators to assess the roles of multiple brain regions in mediating episodic memory within the same animal, while avoiding the challenges of multiple cannulations and infusions necessary to inhibit multiple brain regions with inhibitor or antagonist drugs.
5.4. Projection-specific manipulations
To gain deeper insights about the function of cell-type specific neuronal connections between brain regions, the aforementioned genetic tools can be used in two main ways to specifically target projections from one brain region to the other (Fig. 2C). First, because both DREADDs and opsins can be expressed on the membrane of axon terminals, intracranial infusion of a chemogenetic actuator or delivery of light into the terminal region of interest will selectively manipulate only those cells that project to the region of interest, even though many others may express the opsin or DREADD. A second, more novel approach for chemogenetics makes use of the flip-excision (FLEX)-switch system, which uses a recombinase (such as Cre) to conditionally control gene expression (reviewed in Urban and Roth, 2015; Navabpour et al., 2020). In this approach, a retrograde AAV for Cre is delivered to the terminal region of interest and an anterograde AAV for the FLEX DREADD is delivered to the cell body region of interest (for example, see Boender et al., 2014). Both viral constructs are necessary for expression of the DREADD, ensuring that only those specific projection cells will be manipulated upon systemic injection of the actuator or in the case of optogenetics, light delivery to the cell body region. For example, Paretkar et al. (2018) found that chemogenetic activation of the corticotropin-releasing hormone neurons that project from the central amygdala to the locus coeruleus enhanced preference for the novel object in the object recognition test. Although these projection neurons are generally associated with mediation of anxiety, this study revealed a novel role in modulating episodic memory.
5.5. Recording brain activity
In some instances, brain activity recordings provide insight into the function and connectivity of nodes within a circuit that cannot be accomplished with neuronal manipulation alone. For example, decades of work using single unit recording have identified the hippocampal, temporal, and parietal cells underlying spatial memory encoding. These groups of neurons processes information at several levels, including lower-level sensory cortices where neurons fire at precise times in response to specific environmental stimuli, as well as highly integrated spatial information encoded by place cells and head direction cells in the hippocampus (reviewed in Burgess, 2008). Additionally, the impact of altered activity in one brain region on that of other regions can be recorded to study how the two areas are functionally connected. For example, Nakamura et al. (2010) stimulated CA1/subiculum projections to the PFC and recorded activity in cingulate and prelimbic pyramidal neurons to study how this projection modulates PFC plasticity in response to nociception. More recently, the development of the genetically encoded fluorescent calcium sensor GCaMP has paved the way for quantification of neuronal calcium activity in cell populations through fiber photometry (Cui et al., 2014) or within individual cells using two-photon imaging. These experiments can be conducted in restrained animals whose head is fixed beneath a microscope or in freely moving animals using head-mounted miniature fluorescent microscopes (Ghosh et al., 2011). Because GCaMP is genetically encoded, these calcium imaging methods can target specific cell types and allow for the study of neural connectivity at the level of individual cells (Girven and Sparta, 2017).
6. Investigating the contribution of E2 to the episodic memory circuitry
Once the brain regions involved in episodic memory have been identified, the next step is to investigate the behavioral consequences of hormone action both within each region and across the entire network. As discussed above, a growing arsenal of tools exists to study brain connectivity in memory, but they have not been generally employed thus far to study how E2 regulates episodic memory. To our knowledge, only a single study has used genetic methods like DREADDs to examine how functional interactions among brain regions influence the mnemonic response to E2 (Tuscher et al., 2019). These findings will be described later in this section, but we will first highlight how circuit-based methods have been used to address hormonal regulation of other neural systems.
One circuit in which a role for E2 in regulating synaptic transmission and behavior is well understood is lordosis, the postural behavior in females that indicates sexual receptivity and is necessary for copulation (Micevych and Meisel review, 2017). Lordosis is mediated by the nucleus accumbens and several hypothalamic nuclei, including the arcuate nucleus, medial preoptic nucleus (MPN), and ventromedial nucleus, which highly express ERs on neuronal cell membranes (Micevych et al., 2015). Importantly, ERα-expressing proopiomelanocortin (POMC)/β-endorphin neurons in the arcuate project to an area of the MPN rich in μ-opioid receptors. Although E2 facilitates lordosis, it initially inhibits lordosis via a mechanism thought to involve β-endorphin-mediated μ-opioid receptor activation in the MPN (Sirinathsinghji, 1986; Wiesner and Moss, 1986). To determine if β-endorphin-releasing POMC projections from the arcuate are vital for regulating lordosis and μ-opioid receptors in the MPN, a recent study combined Pomc-cre mice with an AAV-FLEX system. Optogenetic stimulation of β-endorphin-releasing terminals in the MPN attenuated expression of lordosis and activated μ-opioid receptors in E2- and progesterone-primed mice (Johnson et al., 2020), suggesting a primary role for POMC/β-endorphin projections from the arcuate to μ-opioid receptors in the MPN in lordosis. This finding illustrates the utility of circuit-based techniques to address questions relevant to hormonal regulation of the brain regions that regulate behavior.
Similar gene-specific tools could be used to study estrogenic regulation of episodic memory, as these methods can be adapted to target genes for ERα, ERβ, and GPER (Esr1, Esr2, Gper1, respectively), as well as aromatase (Cyp19a1). As in the β-endorphin study above, Cre/LoxP or other recombinase systems (reviewed in Tsien, 2016; Luo et al., 2018) can be used to knock in or knock out ERs or aromatase in specific projection neurons to study the role of estrogen signaling in particular aspects of brain circuit function. For example, to demonstrate that neuron-synthesized E2 in the forebrain is important for synaptic function and memory, Lu et al. (2019) used a site-specific Cre/LoxP and flippase recombinase/flippase recognition target (FLP/FRT) recombination system to delete the Cyp19a1 gene, and then crossed these mice with CaMKIIα-Cre mice to specifically reduce aromatase activity in excitatory forebrain neurons. Male and female mice lacking E2 synthesis in excitatory forebrain neurons exhibited reduced ERK, Akt, and CREB signaling and dendritic spine density in CA1 and cortex, as well as impaired object recognition, spatial reference, and contextual fear memory. Systemic E2 treatment reversed these deficits in both sexes (Lu et al., 2019), suggesting a key role for forebrain-synthesized E2. Cre recombinase can also be virally delivered for situations in which a region-specific gene knockout is required. Herber et al. (2019) used this method to selectively knockout ERα in Kisspeptin-1 neurons in the arcuate nucleus of female mice to show that estrogenic signaling via ERα in this node of the circuit is an important regulator of bone homeostasis.
Temporally specific recombination may be achieved through the use of tamoxifen-inducible Cre. However, we caution researchers using this method to study estrogen signaling to employ appropriate controls (Patel et al., 2017) or consider alternative strategies. Tamoxifen is a selective estrogen receptor modulator with either agonistic or antagonistic effects depending on tissue type (Duterte and Smith, 2000), and research on the physiological effects of the low doses of tamoxifen typically used for Cre induction have yielded mixed results. One study found no effect of a single tamoxifen injection on hippocampal neurogenesis or spatial memory in the Morris water maze among nestin-CreERT2/R26R-YFP mice of either sex (Rotheneichner et al., 2017), whereas another study using a five-day injection protocol found no long-term changes in gene expression in the cortex or hippocampus in C57BL6 mice of both sexes (Chucair-Elliott et al., 2019). In contrast, a single low dose of tamoxifen caused lasting detrimental effects to the reproductive system and endocrine function in juvenile male Nestin-Cre-ERT2 mice (Patel et al., 2017). In addition, tamoxifen altered metabolic function in female, but not male, C57BL/6 mice by increasing adipose tissue browning and thermogenesis (Zhao et al., 2020). Thus, investigators using CreER mice to study estrogenic regulation of behavior must consider possible confounding effects of tamoxifen.
Instead of targeting a general cell type (e.g., all excitatory neurons), Cre can be expressed only in ER- or aromatase-positive neurons, allowing for precise manipulation of cellular processes in ER-responsive cells. Cre lines under hormone-related promoters such as Esr1 (Lee et al., 2014) and Esr2 are becoming increasingly commercially available (e.g., through The Jackson Laboratory, https://www.jax.org). Cells expressing Cre via genetic manipulation or delivered by AAV, can then be transduced with a Cre-dependent optogenetic or chemogenetic construct to activate or inactivate the genetically-targeted subpopulation. For example, aromatase-expressing neurons in the medial amygdala were identified and, using genetic ablation and chemogenetic inhibition, shown to facilitate sexually dimorphic aggression behaviors but not other forms of estrogen-dependent social behavior (Unger et al., 2016). Similarly, Yang et al. (2017) used mice expressing Cre in progesterone receptor-expressing cells, combined with Cre-dependent hM3Dq-mediated activation, to study the role of ventromedial hypothalamus progesterone signaling in male mouse aggression.
Studies of hormonal regulation of episodic memory have not yet employed Cre-based methods. However, our laboratory recently used DREADDs to examine whether the DH and mPFC work in concert to mediate the effects of E2 on spatial and object recognition memory in female mice. This interaction was suggested by a previous report from our lab showing that, 1) infusion of E2 into the DH of ovariectomized mice increased spine density in the mPFC within 2 h, and 2) the ability of intracerebroventricular E2 infusion to increase spine density in the mPFC was blocked by inhibition of ERK or mTOR signaling in the DH (Tuscher et al., 2016a). Moreover, our subsequent work with multiplexed DREADDs infused into the mPFC (hM4Di) and DH (KORD) demonstrated that concurrent mPFC and DH activity is required for memory consolidation in the object recognition and object placement tasks (Tuscher et al., 2018). Given the nature of the observed mPFC-DH interactions, we hypothesized that both brain regions must be active concomitantly for DH-infused E2 to enhance memory consolidation. To test this hypothesis, we used the hM4Di DREADD in ovariectomized mice to inhibit excitatory neuronal activity in the mPFC while simultaneously infusing E2 into the DH immediately after object training (Tuscher et al., 2019). Blocking mPFC activity using a dose of CNO that does not impair memory on its own prevented DH-infused E2 from enhancing consolidation in the object recognition and object placement tasks, demonstrating that DH-mPFC interactions are essential for E2 in the DH to facilitate memory consolidation (Tuscher et al., 2019). These data imply that alterations in E2 levels in one brain area may reverberate throughout interconnected regions in the episodic memory circuit, and beg the question of how E2 influences the other regions alone or in combination. This kind of methodological approach, in which neuronal activity and E2 administration are simultaneously manipulated, is just one example of how researchers may leverage readily available genetic tools to uncover E2’s effects on memory circuitry.
6.1. Additional considerations
Although developed to precisely target specific cell populations, genetic-based techniques used to manipulate brain activity can produce inadvertent off-target effects. Thus, it is critical that studies using the genetic techniques we have discussed include adequate controls to account for potentially unintended effects of delivery systems or chemical/optical actuators. For example, the supposedly inert chemogenetic actuator CNO is readily converted to the anti-psychotic drug clozapine (Gomez et al., 2017). When injected systemically, it is clozapine, not CNO, that crosses the blood brain barrier and binds to synthetic DREADD receptors (Gomez et al., 2017). Via its conversion to clozapine, CNO influences discrimination learning in rats and mice (Manvich et al., 2018) and working memory in non-human primates (Upright and Baxter, 2020). Thus, it is critical that studies using the hM3Dq and hM4Di DREADDs include groups treated with CNO alone and use doses of CNO that have no effect on memory on their own (as in Tuscher et al., 2018, 2019).
Although optogenetic methods avoid the confounds inherent to chemical actuators such as CNO and SALB, long-periods of light exposure can alter cellular function. For example, the heat produced by longer periods optogenetic stimulation can affect activity in the striatum on its own (Owen et al., 2019), and hour-long exposure of cultured cortical neurons to the blue light used to stimulate some opsins increased activity-related gene expression (Tyssowski and Gray, 2019) . Care must also be taken to ensure that the viral vectors used to deliver opsins to the brain have no adverse effects on their own. Similar steps must be taken with viruses used for Cre/LoxP manipulations, as virally-delivered Cre can be cytotoxic in sufficiently high doses, creating brain lesions and impacting behavior (Rezai Amin et al., 2019). Thus, it is essential to use proper controls with all of these techniques to avoid these potential confounds (Baba et al., 2005).
As we have discussed, many brain regions involved in episodic memory are highly interconnected and functional changes in one region may affect others that receive strong input from that region. When developing research designs and interpreting data, it is important to consider potential effects of E2 on regions outside of the one directly under study. As illustrated above, our lab previously found that infusion of E2 into the DH increased spine density not only on apical and basal dendrites of CA1 pyramidal neurons, but also on basal dendrites of pyramidal neurons in the mPFC (Tuscher et al., 2016a). This finding was unexpected, but led to our subsequent study showing that DH-mPFC interactions are necessary for DH-infused E2 to enhance memory (Tuscher et al., 2019). Thus, even when experimental manipulations are localized to one region, functional changes may be detected in other regions that affect the behavior under study. To this end, it is worthwhile to assay, blot, or record from other regions involved in the behavior of interest to determine the extent to which manipulations in one region reverberate through the system.
Additionally, researchers should consider cell-type specific mechanisms when examining hormone-mediated circuitry. The majority of studies examining how E2 regulates hippocampal function presume neuronal mechanisms. However, given that hippocampal glial cells express estrogen receptors (Azcoitia et al, 1999) and are responsive to E2 treatment (Chaban et al., 2004; Micevych et al., 2009; Acaz-Fonseca et al., 2014), they may play a role in estrogenic regulation of memory enhancement, although this has not yet been demonstrated. E2 treatment of astrocytes in culture increases expression of glutamate-transporter 1 (Lee et al., 2012) and stimulates intracellular Ca2+ signaling which causes release of glutamate into the synapse (Parpura and Haydon, 2000; Santello and Volterra, 2009), suggesting that estrogenic signaling in astrocytes may in turn regulate glutamate signaling in synapses. Given emerging research demonstrating a key role for astrocytes in memory formation (Suzuki et al., 2011; Ota et al., 2013; Adamsky et al., 2018), regulation of astrocyte function is a potentially important way through which E2 impacts episodic memory.
Lastly, although it may be obvious to many neuroendocrinology researchers, it is also critical to examine potential sex differences in the effects of E2 and other hormones on brain circuitry, as sex differences in the physiological response to hormones in one brain region could produce functionally discrepant effects that influence other brain regions in a sex-specific manner. For example, Hwang et al. (2015) compared network activity during a fear conditioning task in men, women taking oral contraceptives, and naturally cycling women grouped according to serum E2 levels. The data indicated sex differences in activation of various nodes in the fear conditioning network that were particularly apparent when E2 levels were elevated in women. Recently discovered sex differences in the role of certain ERs and cell-signaling pathways in the effects of E2 on glutamatergic neurotransmission and episodic memory consolidation in the hippocampus (Oberlander and Woolley, 2017; Koss et al., 2018; Jain et al., 2019) may have implications not only for this brain region but also for other parts of the episodic memory circuit. It is not yet known if these sex differences influence the functioning of the mPFC and other interconnected brain regions, but such disparities could be translationally relevant for future development of treatments to reduce memory dysfunction (Frick et al., 2018).
7. Conclusions
Based on recent developments in the field, we encourage researchers to begin studying estrogenic effects on episodic memory with the rich connectivity of the brain in mind. Not only can estrogens, especially E2, exert rapid effects on cells and synapses in several regions integral to episodic memory function, but they may also influence the physiology of cells in other interconnected nodes of the circuit. A systems-level circuit-based approach is now facilitated by technological advances that have yielded well-validated tools for selectively targeting specific cellular subpopulations and projections, and will allow researchers to manipulate both brain activity and estrogen signaling in these populations. Thus, this is an exciting time to study hormonal regulation of brain circuitry. Doing so will provide a more comprehensive understanding of how estrogens regulate whole-brain physiology and function, which could pave the way for the future development of more effective therapeutics for improving or maintaining memory function.
Table 1.
Effects of intracranial E2 infusion on various forms of episodic memory.
| Brain region into which E2 was infused | Type of memory enhanced |
|---|---|
| Hippocampus | Spatial memory (object placement) Spatial strategy use Object recognition memory Social recognition memory Contextual fear conditioning extinction |
| Temporal cortex | Spatial memory (object-in-place) Object recognition memory |
| Amygdala | Social recognition memory |
| Prefrontal cortex | Spatial memory (working memory) Spatial memory (object placement) Object recognition memory Extinction of cocaine seeking |
Highlights:
Episodic memory is mediated by complex circuitry involving multiple brain regions
Studies of estrogen effects on episodic memory often focus on single brain regions
The role of 17β-estradiol in regulating episodic memory circuitry is reviewed
Approaches to study interactions among brain regions are described
Use of circuit-based methods to study estrogenic regulation of memory is discussed
Acknowledgements:
The authors gratefully thank the following for their support: the National Institutes of Health (R01MH107886, 2R15GM118304-02, R01AG022525, F31MH118822), the Alzheimer’s Association (SAGA-17-419092), University of Wisconsin-Milwaukee Research Growth Initiative Awards 101x334 and 101x240, University of Wsconsin-Milwaukee Research Catalyst Awards, the University of Wisconsin-Milwaukee College of Letters and Science, and the University of Wsconsin-Milwaukee Office of Undergraduate Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acaz-Fonseca E, Sanchez-Gonzalez R, Azcoitia I, Arevalo MA, Garcia-Segura LM, 2014. Role of astrocytes in the neuroprotective actions of 17β-estradiol and selective estrogen receptor modulators. Mol. Cell. Endocrinol, 389, 48–57. [DOI] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA, 2009. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav 55, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, London M, Goshen I, 2018. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174, 59–71. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL, 2009. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Fortin NJ, 2013. The evolution of episodic memory. Proc. Natl. Acad. Sci. U S A 110, 10379–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG, 2014. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology 155, 4422–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U S A 104, 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asperholm M, Hogman N, Rafi J, Herlitz A, 2019. What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychol. Bull 145, 785–821. [DOI] [PubMed] [Google Scholar]
- Avila JA, Alliger AA, Carvajal B, Zanca RM, Serrano PA, Luine VN, 2017. Estradiol rapidly increases GluA2-mushroom spines and decreases GluA2-filopodia spines in hippocampus CA1. Hippocampus 27, 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Nakano M, Yamada Y, Saito I, Kanegae Y, 2005. Practical range of effective dose for Cre recombinase-expressing recombinant adenovirus without cell toxicity in mammalian cells. Microbiol. Immunol 49, 559–570. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Perry B. a. L., Dalrymple-Alford JC, Parr-Brownlie LC, 2018. Optogenetic stimulation: Understanding memory and treating deficits. Hippocampus 28, 457–470. [DOI] [PubMed] [Google Scholar]
- Bean LA, lanov L, Foster TC, 2014. Estrogen receptors, the hippocampus, and memory. Neuroscientist 20, 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, 1990. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci. Lett 118, 169–171. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba R-M, Thompson RF, Baudry M, 2001. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc. Natl. Acad. Sci. U S A 98, 13391–13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH, 1999. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24, 161–173. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS, 2010. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules 15, 6050–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MCM, van der Plasse G, Adan RAH, 2014. Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM, 2007. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm. Behav 52, 237–243. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM, 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci 33, 15184–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG, 2005. Estradiol activates group i and ii metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci 25, 5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN, 2002. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience 113, 401–410. [DOI] [PubMed] [Google Scholar]
- Brooks CE, Clayton JA, 2017. Sex/gender influences on the nervous system: Basic steps toward clinical progress. J. Neurosci. Res 95, 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, 2008. Spatial cognition and the brain. Ann. N. Y. Acad. Sci 1124, 77–97. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA, 2010. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 33, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Marchant NJ, 2018. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br. J. Pharmacol 175, 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P, 2004. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology 145, 3788–3795. [DOI] [PubMed] [Google Scholar]
- Chang Y-J, Yang C-H, Liang Y-C, Yeh C-M, Huang C-C, Hsu K-S, 2009. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus 19, 1142–1150. [DOI] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Ocanas SR, Stanford DR, Hadad N, Wronowski B, Otalora L, Stout MB, Freeman WM, 2019. Tamoxifen induction of Cre recombinase does not cause long-lasting or sexually divergent responses in the CNS epigenome or transcriptome: implications for the design of aging studies. GeroScience 41, 691–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, 2018. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav 187, 2–5. [DOI] [PubMed] [Google Scholar]
- Clayton JA, 2016. Studying both sexes: a guiding principle for biomedicine. FASEB J. 30, 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimins JL, Wang AC-J, Yuk F, Puri R, Janssen WGM, Hara Y, Rapp PR, Morrison JH, 2017. Diverse synaptic distributions of G protein-coupled estrogen receptor 1 in monkey prefrontal cortex with aging and menopause. Cereb. Cortex 27, 2022–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Luo G, Pham MD, Lovinger DM, Vogel SS, Costa RM, 2014. Deep brain optical measurements of cell type-specific neural activity in behaving mice. Nat. Protoc 9, 1213–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP, 2001. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J. Neurosci 21, 6949–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP, 1997. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm. Behav 32, 217–225. [DOI] [PubMed] [Google Scholar]
- Daniel JM, 2013. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm. Behav 63, 231–237. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Witty CF, Rodgers SP, 2015. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm. Behav 74, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H, 2010. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 35, 86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Griffin AL, Ito HT, Shapiro ML, Witter MP, Vertes RP, Allen TA, 2019. The nucleus reuniens of the thalamus sits at the nexus of a hippocampus and medial prefrontal cortex circuit enabling memory and behavior. Learn. Mem 26, 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre M, Smith CL, 2000. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J. Pharmacol. Exp. Ther 295, 431–437. [PubMed] [Google Scholar]
- Eichenbaum H, 2017. Prefrontal-hippocampal interactions in episodic memory. Nat. Rev. Neurosci 18, 547–558. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, 2000. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci 1, 41–50. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris R, 1990. Hippocampal representation in place learning. J. Neurosci 10, 3531–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA, 2011. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol. Aging 32, 680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Talboom JS, Braden BB, Tsang CWS, Mennenga S, Andrews M, Demers LM, Bimonte-Nelson HA, 2012. Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat. Horm. Behav 62, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP, 1998. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol. Learn. Mem 69, 225–240. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP, 1999. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol. Biochem. Behav 62, 711–717. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM, 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci 30, 4390–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci 28, 8660–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM, 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem 20, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Berzuini C, Knapp LA, 2013. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer’s risk in a cohort of British women. Psychoneuroendocrinology 38, 2973–2982. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Luine V, 2015. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm. Behav 74, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, 2015. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav 74, 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, 2009. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm. Behav 55, 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, 2018. Mechanisms underlying the rapid effects of estradiol and progesterone on hippocampal memory consolidation in female rodents. Horm. Behav 104, 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM, 2015. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn. Mem 22, 472–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Tuscher JJ, Koss WA, Kim J, Taxier LR, 2018. Estrogenic regulation of memory consolidation: a look beyond the hippocampus, ovaries, and females. Physiol. Behav 187, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Waif AA, 2007. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem 88, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, 2002. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 956, 285–293. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Koh MT, 2011. Episodic memory on the path to Alzheimer’s disease. Curr. Opin. Neurobiol 21, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA, 1999. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol. 40, 574–584. [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Claiborne BJ, 2006. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behav. Neurosci 120, 905–916. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Hamel LM, Brake WG, Mumby DG, 2016. Intra-perirhinal cortex administration of estradiol, but not an ERβ agonist, modulates object-recognition memory in ovariectomized rats. Neurobiol. Learn. Mem 133, 89–99. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Jacob S, Brake WG, Mumby DG, 2013. Systemic and intra-rhinal-cortical 17-β estradiol administration modulate object-recognition memory in ovariectomized female rats. Horm. Behav 64, 642–652. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ, 2011. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, 1999. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm. Behav 36, 222–233. [DOI] [PubMed] [Google Scholar]
- Girven KS, Sparta DR, 2017. Probing deep brain circuitry: new advances in in vivo calcium measurement strategies. ACS Chem. Neurosci. 8, 243–251. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M, 2017. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley C, Frankfurt M, McEwen B, 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci 10, 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM, 2006. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol. Biochem. Behav 84, 112–119. [DOI] [PubMed] [Google Scholar]
- Griksiene R, Ruksenas O, 2011. Effects of hormonal contraceptives on mental rotation and verbal fluency. Psychoneuroendocrinology 36, 1239–1248. [DOI] [PubMed] [Google Scholar]
- Guru A, Post RJ, Ho Y-Y, Warden MR, 2015. Making sense of optogenetics. Int. J. Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB, 2009. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm. Behav 56, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Roes MM, Galea LAM, 2016. Sex hormones and cognition: Neuroendocrine influences on memory and learning. Compr. Physiol 6, 1295–1337. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WGM, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH, 2006. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci 26, 2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung B-C, Yamazaki T, Kawato S, 2015. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: involvement of kinase networks. Brain Res. 1621, 147–161. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Liu L, Rissanen A, Tanila H, 2002. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm. Behav 41, 22–32. [DOI] [PubMed] [Google Scholar]
- Hepler DJ, Wenk GL, Cribbs BL, Olton DS, Coyle JT, 1985. Memory impairments following basal forebrain lesions. Brain Res. 346, 8–14. [DOI] [PubMed] [Google Scholar]
- Herber CB, Krause WC, Wang L, Bayrer JR, Li A, Schmitz M, Fields A, Ford B, Zhang Z, Reid MS, Nomura DK, Nissenson RA, Correa SM, Ingraham HA, 2019. Estrogen signaling in arcuate Kissl neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nat. Commun 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori T-A, Enami T, Furukawa A, Suzuki K, Ishii H-T, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S, 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U S A 101, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-lkeda M, Mukai H, Kimoto T, 2011. Hippocampal synthesis of sex steroids and corticosteroids: Essential for modulation of synaptic plasticity. Front. Endocrinol 2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LAM, 2002. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav. Neurosci 116, 928–934. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS, 2012. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74, 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, Marin MF, Milad MR, 2015. Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry 15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V, 2010. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm. Behav 58, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Weiss BK, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM, 2016. Impact of sex and menopausal status on episodic memory circuitry in early midlife. J. Neurosci 36, 10163–10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN, 2016. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology 157, 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V, 2010. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol. Learn. Mem 94, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Huang GZ, Woolley CS, 2019. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci 39, 1552–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Maren S, 2015. Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci 9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Hong W, Micevych P, 2020. Optogenetic activation of β-endorphin terminals in the medial preoptic nucleus regulates female sexual receptivity. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z, 2005. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci. Biobehav. Rev 28, 811–825. [DOI] [PubMed] [Google Scholar]
- Kawato S, Hojo Y, Kimoto T, 2002. Histological and metabolism analysis of P450 expression in the brain. Meth. Enzymol 357, 241–249. [DOI] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC, 2017. Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology 42, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Frick KM, 2017. Distinct effects of estrogen receptor antagonism on object recognition and spatial memory consolidation in ovariectomized mice. Psychoneuroendocrinology 85, 110–114. [DOI] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, Frick KM, 2016. 17β-estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms. J. Neurosci 36, 3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Schalk JC, Koss WA, Gremminger RL, Taxier LR, Gross KS, Frick KM, 2019. Dorsal hippocampal actin polymerization is necessary for activation of G-protein-coupled estrogen receptor (GPER) to increase CA1 dendritic spine density and enhance memory consolidation. J. Neurosci 39, 9598–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, 1992. Modality-specific retrograde amnesia of fear. Science 256, 675–677. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S, 2001. Neurosteroid synthesis by cytochrome P450-containing systems localized in the rat brain hippocampal neurons: N-methyl-d-aspartate and calcium-dependent synthesis. Endocrinology 142, 3578–3589. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, Tonegawa S, 2017. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Frick KM, 2019. Activation of androgen receptors protects intact male mice from memory impairments caused by aromatase inhibition. Horm. Behav 111, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Frick KM, 2017. Sex differences in hippocampal function. J. Neurosci. Res 95, 539–562. [DOI] [PubMed] [Google Scholar]
- Koss WA, Haertel JM, Philippi SM, Frick KM, 2018. Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-estradiol. eNeuro 5(5):ENEURO.0267-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G, 2009. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J. Neurosci 29, 12982–12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM, 2004. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci 24, 5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, 2002. Regional, laminar, and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact, adult male and female rats. Cereb. Cortex 12, 116–128. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Wouterlood FG, 2020. Neuroanatomical tract-tracing techniques that did go viral. Brain Struct. Funct. 225, 1193–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A, 1999. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology 52, 78–84. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG, 2000. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 10, 420–430. [DOI] [PubMed] [Google Scholar]
- Laws KR, Irvine K, Gale TM, 2018. Sex differences in Alzheimer’s disease. Curr. Opin. Psychiatry 31, 133–139. [DOI] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Wang N, Webb A, Son D-S, Lee K, Aschner M, 2012. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J. Biol. Chem 287, 26817–26828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim D-W, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ, 2014. Scalable control of mounting and attack by Esr1 + neurons in the ventromedial hypothalamus. Nature 509, 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ, 2004. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology 29, 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T, Müller S, Milian M, Eschweiler GW, Saur R, 2009. Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 47, 2464–2469. [DOI] [PubMed] [Google Scholar]
- López AJ, Kramár E, Matheos DP, White AO, Kwapis J, Vogel-Ciernia A, Sakata K, Espinoza M, Wood MA, 2016. Promoter-specific effects of DREADD modulation on hippocampal synaptic plasticity and memory formation. J. Neurosci 36, 3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sareddy GR, Wang J, Wang R, Li Y, Dong Y, Zhang Q, Liu J, O’Connor JC, Xu J, Vadlamudi RK, Brann DW, 2019. Neuron-derived estrogen regulates synaptic plasticity and memory. J. Neurosci 39, 2792–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M, 2020. Estrogenic regulation of memory: the first 50 years. Horm. Behav 121, 104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Serrano P, Frankfurt M, 2018. Rapid effects on memory consolidation and spine morphology by estradiol in female and male rodents. Horm. Behav 104, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, 2014. Estradiol and cognitive function: past, present and future. Horm. Behav 66, 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ, 2003. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144, 2836–2844. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD, 1998. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm. Behav 34, 149–162. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K, 2018. Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymer J, Robinson A, Winters BD, Choleris E, 2017. Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice. Psychoneuroendocrinology 77, 131–140. [DOI] [PubMed] [Google Scholar]
- Lymer JM, Sheppard PAS, Kuun T, Blackman A, Jani N, Mahbub S, Choleris E, 2018. Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 89, 30–38. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, Weinshenker D, 2018. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci. Rep 8(1):3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ, 2004. Neuronal signalling of fear memory. Nat. Rev. Neurosci 5, 844–852. [DOI] [PubMed] [Google Scholar]
- Micevych P, Kuo J, Christensen A, 2009. Physiology of membrane estrogen receptor signaling in reproduction. J. Neuroendocrinol 21, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Meisel RL, 2017. Integrating neural circuits controlling female sexual behavior. Front. Syst. Neurosci 11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Wong AM, Mittelman-Smith MA, 2015. Estradiol membrane-initiated signaling and female reproduction. Compr. Physiol 5, 1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE, 2005. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J. Comp. Neurol 491, 81–95. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE, 2001. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol 429, 355–371. [PubMed] [Google Scholar]
- Mitchell AS, 2015. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci. Biobehav. Rev 54, 76–88. [DOI] [PubMed] [Google Scholar]
- Mitchnick KA, Mendell AL, Wideman CE, Jardine KH, Creighton SD, Muller A-M, Choleris E, MacLusky NJ, Winters BD, 2019. Dissociable involvement of estrogen receptors in perirhinal cortex-mediated object-place memory in male rats. Psychoneuroendocrinology 107, 98–108. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE, 2003. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144, 2055–2067. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA, 2010. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol 518, 2729–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Katayama Y, Kawakami Y, 2010. Hippocampal CA1/subiculum-prefrontal cortical pathways induce plastic changes of nociceptive responses in cingulate and prelimbic areas. BMC Neurosci. 11, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabpour S, Kwapis JL, Jarome TJ, 2020. A neuroscientist’s guide to transgenic mice and other genetic tools. Neurosci. Biobehav. Rev 108, 732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS, 2017. 17β--estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci 37, 12314–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal MF, Means LW, Poole MC, Hamm RJ, 1996. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology 21, 51–65. [DOI] [PubMed] [Google Scholar]
- Österlund M, G.J.M.Kuiper G, Gustafsson J-Å, Hurd YL, 1998. Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Mol. Brain Res. 54, 175–180. [DOI] [PubMed] [Google Scholar]
- Ota Y, Zanetti AT, Hallock RM, 2013. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast. 2013:185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM, 1996. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav. Neurosci 110, 626–632. [DOI] [PubMed] [Google Scholar]
- Packard MG, leather LA, 1997. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport 8, 3009–3013. [DOI] [PubMed] [Google Scholar]
- Paretkar T, Dimitrov E, 2018. The central amygdala corticotropin-releasing hormone (CRH) neurons modulation of anxiety-like behavior and hippocampus-dependent memory in mice. Neuroscience 390, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Haydon PG, 2000. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. USA 97, 8629–8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SH, O’Hara L, Atanassova N, Smith SE, Curley MK, Rebourcet D, Darbey AL, Gannon A-L, Sharpe RM, Smith LB, 2017. Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: implications for inducible transgenics. Sci. Rep 7(1 ):8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peragine D, Simeon-Spezzaferro C, Brown A, Gervais NJ, Hampson E, Einstein G, 2019. Sex difference or hormonal difference in mental rotation? The influence of ovarian milieu. Psychoneuroendocrinology 115:104488. [DOI] [PubMed] [Google Scholar]
- Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS, 2014. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor a. Neurobiol. Learn. Mem 114, 1–9. [DOI] [PubMed] [Google Scholar]
- Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CDC, Kow L-M, MacLusky NJ, Pfaff DW, Choleris E, 2015. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc. Natl. Acad. Sci. USA 112, 16018–16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE, 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci 106, 274–285. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Weiss BK, Jacobs EG, Cherkerzian S, Klibanski A, Remington A, Aizley H, Goldstein JM, 2017. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause 24, 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai Amin S, Gruszczynski C, Guiard BP, Callebert J, Launay J-M, Louis F, Betancur C , Vialou V, Gautron S, 2019. Viral vector-mediated Cre recombinase expression in substantia nigra induces lesions of the nigrostriatal pathway associated with perturbations of dopamine-related behaviors and hallmarks of programmed cell death. J. Neurochem 150, 330–340. [DOI] [PubMed] [Google Scholar]
- Roth BL, 2016. DREADDs for neuroscientists. Neuron 89, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheneichner P, Romanelli P, Bieler L, Pagitsch S, Zaunmair P, Kreutzer C, Konig R, Marschallinger J, Aigner L, Couillard-Després S, 2017. Tamoxifen activation of Cre-recombinase has no persisting effects on adult neurogenesis or learning and anxiety. Front. Neurosci 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL, 2004. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm. Behav 45, 128–135. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Wiliams CL, 2001. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci 115, 384–393. [PubMed] [Google Scholar]
- Santello M, Volterra A, 2009. Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 158, 253–259. [DOI] [PubMed] [Google Scholar]
- Shams WM, Sanio C, Quinlan MG, Brake WG, 2016. 17β--Estradiol infusions into the dorsal striatum rapidly increase dorsal striatal dopamine release in vivo. Neuroscience 330, 162–170. [DOI] [PubMed] [Google Scholar]
- Sheppard P. a. S., Koss WA, Frick KM, Choleris E, 2018. Rapid actions of oestrogens and their receptors on memory acquisition and consolidation in females. J. Neuroendocrinol 30(2): 10.1111/jne.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I, 1997. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol 388, 507–525. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW, 1994. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 644, 305–312. [DOI] [PubMed] [Google Scholar]
- Sinopoli KJ, Floresco SB, Galea LAM, 2006. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol. Learn. Mem 86, 293–304. [DOI] [PubMed] [Google Scholar]
- Sirinathsinghji DJ, 1986. Regulation of lordosis behaviour in the female rat by corticotropin-releasing factor, beta-endorphin/corticotropin and luteinizing hormone-releasing hormone neuronal systems in the medial preoptic area. Brain Res. 375, 49–56. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Wtter MP, Barnes CA, 2011. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci 12, 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS, 2010. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J. Neurosci 30, 16137–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV, 2016. DREADDS: use and application in behavioral neuroscience. Behav. Neurosci 130, 137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, 1992. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J. Cogn. Neurosci 4, 232–243. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Woolfrey K, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P, 2008. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc. Natl. Acad. Sci. USA 105, 14650–14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM, 2011. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS, 2015. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J. Neurosci 35, 11252–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Janssen WGM, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH, 2004. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb. Cortex 14, 215–223. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, 2016. Cre-lox neurogenetics: 20 years of versatile applications in brain research and counting.… Front. Genet 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugizawa T, Mukai H, Tanabe N, Murakami G, Hojo Y, Kominami S, Mitsuhashi K, Komatsuzaki Y, Morrison JH, Janssen WGM, Kimoto T, Kawato S, 2005. Estrogen induces rapid decrease in dendritic thorns of CA3 pyramidal neurons in adult male rat hippocampus. Biochem. Biophys. Res. Commun 337, 1345–1352. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Fortress AM, Kim J, Frick KM, 2015. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav. Brain Res. 285, 140–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM, 2016a. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J. Neurosci 36, 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM, 2016b. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm. Behav 83, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Taxier LR, Fortress AM, Frick KM, 2018. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol. Learn. Mem 156, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Taxier LR, Schalk JC, Haertel JM, Frick KM, 2019. Chemogenetic suppression of medial prefrontal-dorsal hippocampal interactions prevents estrogenic enhancement of memory consolidation in female mice. eNeuro 6(2):ENEURO.0451-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K, 2012. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci 13, 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyssowski KM, Gray JM, 2019. Blue light increases neuronal activity-regulated gene expression in the absence of optogenetic proteins. eNeuro, 6(5):ENEURO.0085-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EK, Burke KJ, Yang CF, Bender KJ, Fuller PM, Shah NM, 2015. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 10, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upright NA, Baxter MG, 2020. Effect of chemogenetic actuator drugs on prefrontal cortex-dependent working memory in nonhuman primates. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Roth BL, 2015. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol 55, 399–417. [DOI] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, Sassano FM, Huang X-P, Zhu H, Urban DJ, White KL, Rittiner JE, Crowley NA, Pleil KE, Mazzone CM, Mosier PD, Song J, Kash TL, Malanga CJ, Krashes MJ, Roth BL, 2015. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Brandt N, Rune GM, 2014. Hippocampal estradiol synthesis and its significance for hippocampal synaptic stability in male and female animals. Neuroscience 274, 24–32. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA, 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem 86, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Brown MW, 2015. Neural circuitry for rat recognition memory. Behav. Brain Res. 285, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA, 2015. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J. Neurosci 35, 2384–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner JB, Moss RL, 1986. Behavioral specificity of beta-endorphin suppression of sexual behavior: Differential receptor antagonism. Pharmacol. Biochem. Behav 24, 1235–1239. [DOI] [PubMed] [Google Scholar]
- Woolley C, McEwen B, 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci 12, 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, 2007. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol 47, 657–680. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol 336, 293–306. [DOI] [PubMed] [Google Scholar]
- Wu WW, Adelman JP, Maylie J, 2011. Ovarian hormone deficiency reduces intrinsic excitability and abolishes acute estrogen sensitivity in hippocampal CA1 pyramidal neurons. J. Neurosci 31, 2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, Satterfield S, Harris T, Cummings SR, 2007. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neruobiol. Aging 28, 171–178. [DOI] [PubMed] [Google Scholar]
- Yang T, Yang CF, Chizari MD, Maheswaranathan N, Burke KJ, Borius M, Inoue S, Chiang MC, Bender KJ, Ganguli S, Shah NM, 2017. Social control of hypothalamus-mediated male aggression. Neuron 95, 955–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Cudmore RH, Linden DJ, 2019. Estrogen-dependent functional spine dynamics in neocortical pyramidal neurons of the mouse. J. Neurosci 39, 4874–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Ritchey M, 2015. The slow forgetting of emotional episodic memories: an emotional binding account. Trends. Cogn. Sci 19, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf H, Smies CW, Hafenbreidel M, Tuscher JJ, Fortress AM, Frick KM, Mueller D , 2019. Infralimbic estradiol enhances neuronal excitability and facilitates extinction of cocaine seeking in female rats via a BDNF/TrkB mechanism. Front. Behav. Neurosci 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wang B, Gomez NA, de Avila JM, Zhu M-J, Du M, 2020. Even a low dose of tamoxifen profoundly induces adipose tissue browning in female mice. Int. J. Obes. (Lond) 44, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM, 2012. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J. Neurosci 32, 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM, 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. USA 107, 5605–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE, 2000. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci 20, 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL, 2006. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol. Learn. Mem 86, 336–343. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL, 2007. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience 144, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Serio SJ, Korol DL, 2011. Intra-striatal estradiol in female rats impairs response learning within two hours of treatment. Horm. Behav 60, 470–477. [DOI] [PubMed] [Google Scholar]


