Abstract
Predictive, non-invasive tools are needed to monitor key features of nonalcoholic fatty liver disease (NAFLD) in children that relate to improvement in liver histology. The purpose of this study was to evaluate the relationship between liver chemistries and liver histology using data from the CyNCh clinical trial. This study included 146 children. Improvement in liver histology, defined as decrease in NAFLD Activity Score ≥ 2 points without worsening of fibrosis, occurred in 43 participants (30%). There were 46 participants with borderline zone 1 nonalcoholic steatohepatitis (NASH) at baseline, with resolution in 28% (12/46). Multivariate models were constructed using baseline and change in ALT, AST, and GGT at 52 weeks, for improvement in 1) liver histology primary outcome 2) borderline zone 1 NASH, and 3) fibrosis. For improvement in histology, the model (p < 0.0001) retained baseline and change in GGT (AUROC 0.79; 95% CI 0.71 – 0.87). For borderline zone 1 NASH, the model (p = 0.0004) retained baseline and change in ALT (AUROC 0.80; 95% CI 0.67 – 0.93). For fibrosis, the model (p<0.001) retained baseline and change in ALT (AUROC 0.80, 95% CI 0.67–0.93). Additional clinical parameters were added to the models using Akaike’s Information Criteria selection, and significantly boosted performance: improvement in histology with AUROC of 0.89 (95% CI 0.82 – 0.95), borderline zone 1 NASH with AUROC of 0.91 (95% CI 0.83 – 0.99) and fibrosis with AUROC of 0.89 (95% CI 0.82–0.94). Models were validated using data from the TONIC trial. Conclusion: In children with NAFLD, dynamic changes in serum ALT and GGT are associated with change in liver histology and appear to be powerful indicators of histologic response.
Keywords: nonalcoholic fatty liver disease, children, obesity, body mass index, LDL cholesterol
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children in the United States with an estimated prevalence of 5–10%. (1–3) Current guidelines recommend lifestyle modifications to improve diet and increase physical activity as the mainstay of treatment. (4) Despite advice and attempts with these interventions, NAFLD can progress to nonalcoholic steatohepatitis (NASH), and end stage liver disease. NASH is now the most common indication for liver transplant among young adults. (5) The current reference standard to assess disease status is liver histology. The American Association for the Study of Liver Disease (AASLD) currently recommends liver histology as the primary outcome measure for phase 2b and phase 3 clinical trials in adults. (6, 7) However, percutaneous liver biopsy, although demonstrably safe in children (8), is an invasive test with rare but known complications; the procedure also requires sedation, may be costly and therefore presents a limitation in terms of diagnostic or prognostic acceptance. Thus, there is a pressing need for reasonably predictive, non-invasive tools that can be used to monitor key features of NAFLD response to therapy that relate to important components of histology.
As a pragmatic approach for clinical practice, NAFLD is most often detected and monitored using readily available biochemical tests such as serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT). Serum ALT is most sensitive and liver-specific and is the preferred surrogate marker for the presence of hepatocellular injury. (9) As such, ALT has been commonly used as an outcome measure in early phase clinical trials in NAFLD, because it is readily obtained, inexpensive, and gives presumptive information about the degree of liver injury. However, prevailing evidence has illustrated that ALT at a single time point is an imprecise marker of liver injury in pediatric NAFLD, as children with even slightly elevated ALT may have advanced disease, including severe fibrosis. (10) Nonetheless, whether the relative or absolute change in ALT from baseline over variable time intervals is an accurate measure of improvement or progression in NAFLD prompts further investigation.
There have been two studies in children with NAFLD that have demonstrated correlation between decrease in ALT over time, and increase in odds of improvement in liver histology. (11, 12) However, there is relatively limited understanding of how closely changes in ALT relate to particular changes in liver histology. Moreover, the relative merits of utilizing AST and GGT in conjunction with ALT to assess NAFLD status also requires further investigation. Therefore, the aim of this study was to evaluate the relationship between changes in serum ALT, AST and GGT with improvement in selected aspects of liver histology. In order to do this, we utilized data from the Cysteamine Bitartrate Delayed-Release for the Treatment of NAFLD in Children (CyNCh) trial, a multicenter randomized clinical trial conducted by the NASH Clinical Research Network (NASH CRN) at 10 clinical centers, that compared 52 weeks treatment with daily oral cysteamine bitartrate delayed release (CBDR) or placebo to improve liver histology in children with NAFLD (NCT01529268). (13) In addition, although serum ALT, AST, and GGT are measures commonly used to evaluate the liver, the interplay between other clinical variables that may also have clinical relevance requires further exploration. Therefore, an additional aim of this study was to evaluate specific clinical factors that are related to change in ALT, AST and GGT over 52 weeks.
METHODS
Study Overview
The CyNCh study included children 8–17 years of age with NAFLD and a NAFLD Activity Score (NAS) of ≥ 4. The full protocol, including inclusion and exclusion criteria for study participants, has been published. (13) In CyNCh, children were randomized to either cysteamine bitartrate microspheronized, delayed-release enteric-coated, core beads (CBDR) or matching placebo capsules given orally in weight adjusted doses: the target dose was 300 mg twice daily for children ≤65 kg, 375 mg twice daily for >65–80 kg, or 450 mg twice daily for >80kg. Treatment duration was 52 weeks. Follow-up study visits were scheduled at weeks 4, 12, 24, 36, and 52. The primary outcome was a change in liver histology and was assessed through liver biopsy performed at week 52. Liver histology was evaluated centrally by the NASH CRN Pathology Committee using published criteria. (14) Histologic activity was assessed using the NAS on a scale of 0 to 8. Components of the NAS include grades of steatosis (0–3), lobular inflammation (0–3) and hepatocellular ballooning (0–2). Fibrosis was scored by stage from 0–4, and portal inflammation was scored on a scale from 0–2 (not included in NAS scores). Biopsies were interpreted as: “not NAFLD”; “NAFLD but not steatohepatitis”; “borderline steatohepatitis with Zone 3 pattern”; “borderline steatohepatitis with Zone 1 pattern”; or definite steatohepatitis. The primary outcome measure in CyNCh was the proportion of children with histologic improvement in NAFLD between the baseline liver biopsy and follow-up biopsy after 52 weeks of treatment, where improvement was defined as decrease in NAS of 2 points or more without worsening of fibrosis. No worsening of fibrosis was defined as either no change or any decrease in the fibrosis stage. In this study, participants were classified as responders or non-responders based on the primary outcome.
Specific Aims
There were four specific aims. The first was to evaluate how changes in ALT, AST and GGT associate with improvement in overall liver histology, defined as a decrease in NAFLD activity score by ≥ 2 points without worsening fibrosis controlling for treatment group. The second aim was to evaluate how changes in ALT, AST and GGT associate with improvement of borderline zone 1 NASH controlling for treatment group; borderline zone 1 NASH is a characteristic pattern in children not often seen in adults, and is more often associated with fibrosis than other subtypes. The third aim was to evaluate how changes in ALT, AST and GGT associate with improvement in fibrosis controlling for treatment group; fibrosis improvement was defined as a decrease by one or more stage, with change from stage 1b to 1a also considered improvement. The fourth aim was to evaluate which clinical factors were associated with the change in selected serum biochemistries ALT, AST, and GGT over 52 weeks.
Statistical Methods
Baseline characteristics of the CyNCh study participants with paired biopsies at baseline and 52-weeks were compared by histologic improvement using means (SD) or N (%). Liver chemistry values (ALT, AST, and GGT) were presented as medians and interquartile ranges due to non-normality. P-values were derived from Fisher’s exact test for categorical measures, two-sample t-tests for continuous measures, and robust linear regression using iteratively re-weighted least squares for ALT, AST, and GGT to reduce the dependence of the estimates and standard errors on normality and on extreme data points.
Aims 1, 2, and 3 evaluated how changes in liver chemistries (AST, ALT and GGT) associated with improvement in overall liver histology, with resolution of borderline zone 1 NASH, and with improvement in fibrosis respectively. Logistic regression analysis of histologic improvement on liver chemistries (ALT, AST, and GGT) was performed; the odds ratio for histologic improvement was determined based on 10 u/L incremental changes in ALT, AST, and GGT. Clinical prediction models were developed for three histologic outcomes: 1) histologic improvement 2) resolution of zone 1, periportal pattern and 3) improvement in fibrosis stage. Multiple logistic regression models with Akaike’s Information Criteria (AIC), a penalized likelihood method that is a trade-off between goodness of fit versus model size, with smaller AICs corresponding to models with more information about the outcome, were used for model selection. (12, 13) We fit a model including all baseline ALT, AST, GGT and 52-week changes; using AIC selection, we determined that GGT (baseline and change) was the best predictor of histologic improvement, and ALT (baseline and change) was the best predictor of resolution of Zone 1, periportal pattern. ALT (baseline and change) was also the best predictor for improvement in fibrosis stage. These measures were then included in a multivariable logistic regression analysis, in which additional variables were selected, using AIC selection, from a candidate set of 24 variables: age, gender, race (white vs. non-white), Hispanic/Latino ethnicity, diabetes, hypertension, hyperlipidemia, histologic features (steatosis, lobular inflammation, portal inflammation, ballooning, fibrosis), BMI z-score, waist circumference, systolic and diastolic blood pressure, total bilirubin, glucose, insulin, HOMA-IR, total cholesterol, triglycerides, HDL, and LDL.
To validate these models, we performed cross-validation, and we validated the models using data from the TONIC clinical trial (Treatment of Nonalcoholic Fatty Liver Disease in Children) also conducted by the NASH CRN (ClinicalTrials.gov Identifier: NCT00063635).(15) Performance characteristics for the prediction models, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were estimated for specificity fixed at 90%, and Youden’s index (sensitivity+specificity-1) fixed at the maximum value. Area Under the Receiver Operating Characteristic curves (AUROCs) and 95% confidence intervals were compared for each model, and the percentages of participants that could be correctly classified as having histologic improvement and resolution of borderline zone 1 NASH utilizing liver chemistry parameters were calculated, using the maximum Youden’s Index as the probability cutoff.
The relationship between clinical factors and the change in liver chemistry is a different question than to what extent change in liver chemistry relates to improvement in liver histology. Both of these questions are clinically relevant. Therefore, the fourth aim of this study was to evaluate which clinical factors were associated with the change in liver chemistry over 52 weeks. For aim 4, three separate multiple linear regression analyses were performed, where the 52-week change in serum ALT, AST, and GGT were the outcome measures, and histologic improvement was the primary covariate, controlling for treatment group, baseline value of ALT, AST, or GGT, baseline age (years), sex (male vs. female), baseline BMI z-score, and 52-week change in BMI z-score. Nominal (i.e., no adjustments for multiple comparisons), 2-sided p-values were considered significant if P<0.05. Analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC) and Stata (Release 15.1, Stata Corporation, College Station, TX).
RESULTS
Study participants
Baseline characteristics for study participants who completed the study with follow-up biopsy by histologic improvement are shown in Table 1. There were 146 children with liver biopsies at baseline and at week 52 included in this analysis (of the 169 children who enrolled). The overall mean age was 13.6 ± 2.6 years. A majority of the participants were male (71%). The overall mean weight was 84 ± 26 kg. Liver histology improvement per the primary outcome occurred in 43 participants (30%). There were 46 participants with a diagnosis of borderline zone 1 NASH at baseline and this resolved in 28% (12/46). Fibrosis was present in 101/146 (69%) of participants at baseline and improved in 35% (35/101). Characteristics by treatment group are shown in Supplemental Table 1.
Table 1.
Baseline characteristics of CyNCh patients with baseline and end of treatment biopsies
| Histological Improvement | ||||
|---|---|---|---|---|
| Improved (N=43) |
Not improved (N=103) |
Total (N=146) |
P | |
| Demographics | ||||
| Age (years) | 13.2 (2.6) | 13.7 (2.6) | 13.6 (2.6) | 0.25 |
| Male | 30 (70%) | 73 (71%) | 103 (71%) | 1.00 |
| Race | 0.82 | |||
| White | 26 (60%) | 60 (58%) | 86 (59%) | |
| Non-white | 5 (12%) | 17 (17%) | 22 (15%) | |
| Refusal/not stated | 12 (28%) | 26 (25%) | 38 (26%) | |
| Hispanic ethnicity | 35 (81%) | 73 (71%) | 108 (74%) | 0.22 |
|
Treatment group CBDR Placebo |
25 (58%) 18 (42%) |
46 (45%) 57 (55%) |
71 (49%) 75 (51%) |
0.15 |
|
Weight stratum
≤65 kg >65–80 kg >80 kg |
15 (35%) 2 (5%) 26 (60%) |
27 (26%) 16 (16%) 60 (58%) |
42 (29%) 18 (12%) 86 (59%) |
0.15 |
| Liver chemistries – median (IQR) | ||||
| Alanine aminotransferase (U/L) | 94 (57–179) | 84 (62–130) | 86 (62–140) | 0.53 |
| Aspartate aminotransferase (U/L) | 53 (39–92) | 50 (38–74) | 51 (38–79) | 0.67 |
| γ-glutamyl transpeptidase (U/L) | 38 (27–67) | 34 (26–54) | 35 (27–62) | 0.30 |
| Metabolic factors | ||||
| Weight (kg) | 81 (23) | 86 (27) | 84 (26) | 0.28 |
| Body-mass index (kg/m2) | 31 (6) | 32 (7) | 32 (6) | 0.23 |
| Body-mass index z-score | 2.1 (0.4) | 2.2 (0.5) | 2.2 (0.4) | 0.40 |
| Liver histology findings | ||||
| NAFLD activity score* | 5.4 (1.2) | 4.4 (1.3) | 4.7 (1.4) | <0.001 |
| Steatosis score | 2.4 (0.8) | 2.4 (0.7) | 2.4 (0.7) | 0.92 |
| Lobular inflammation score | 2.1 (0.7) | 1.5 (0.6) | 1.7 (0.7) | <0.001 |
| Hepatocellular ballooning score | 0.9 (0.8) | 0.5 (0.7) | 0.6 (0.7) | 0.007 |
| Portal inflammation score† | 1.1 (0.5) | 1.2 (0.5) | 1.1 (0.5) | 0.16 |
| Fibrosis stage | 0.18 | |||
| 0, none | 8 (19%) | 37 (36%) | 45 (31%) | |
| 1a, mild, zone 3 perisinusoidal | 3 (7%) | 8 (8%) | 11 (8%) | |
| 1b, moderate, zone 3 perisinusoidal | 4 (9%) | 5 (5%) | 9 (6%) | |
| 1c, portal/periportal only | 12 (28%) | 25 (24%) | 37 (25%) | |
| 2, zone 3 and periportal, any combination | 8 (19%) | 10 (10%) | 18 (12%) | |
| 3, bridging | 7 (16%) | 18 (17%) | 25 (17%) | |
| 4, cirrhosis | 1 (2%) | 0 (0%) | 1 (1%) | |
| Steatohepatitis | 0.03 | |||
| No | 6 (14%) | 34 (33%) | 40 (27%) | |
| Borderline Zone 3 pattern | 8 (19%) | 14 (14%) | 22 (15%) | |
| Borderline Zone 1 pattern | 12 (28%) | 34 (33%) | 46 (32%) | |
| Definite | 17 (40%) | 21 (20%) | 38 (26%) | |
Data are n (%) or mean (SD), unless otherwise noted.
NAFLD activity score was assessed on a scale of 0–8, with higher scores showing more severe disease (the components of this measure are steatosis [assessed on a scale of 0–3], lobular inflammation [assessed on a scale of 0–3], and hepatocellular ballooning [assessed on a scale of 0–2]).
Portal inflammation was assessed on a scale of 0–2, with higher scores showing more severe inflammation.
Characteristics of change in liver chemistries
The values of liver chemistry levels at baseline, 52 weeks, and overall change are demonstrated in Figure 1 and Supplemental Table 2. ALT was not significantly different at baseline between histologic responders and non-responders (geometric mean 105 U/L, versus 90 U/L; p= 0.53). In contrast, the change in ALT was significantly greater in histologic responders compared to histologic non-responders (−75 U/L versus −11 U/L; p< 0.001). There was also a significant difference in the change in AST (p< 0.001) and GGT (p< 0.001) between responder and non-responder groups. (Figure 1 and Supplemental Table 2) The largest change in liver chemistries was seen over the initial 12 weeks of the study period (Figure 2). Importantly, changes in ALT, AST, and GGT at 12-weeks correlated with changes at 52-weeks (r=0.81, 0.79, 0.71, respectively). (Supplemental Table 3, Supplemental Table 4, Supplemental Figure 1)
Figure 1.
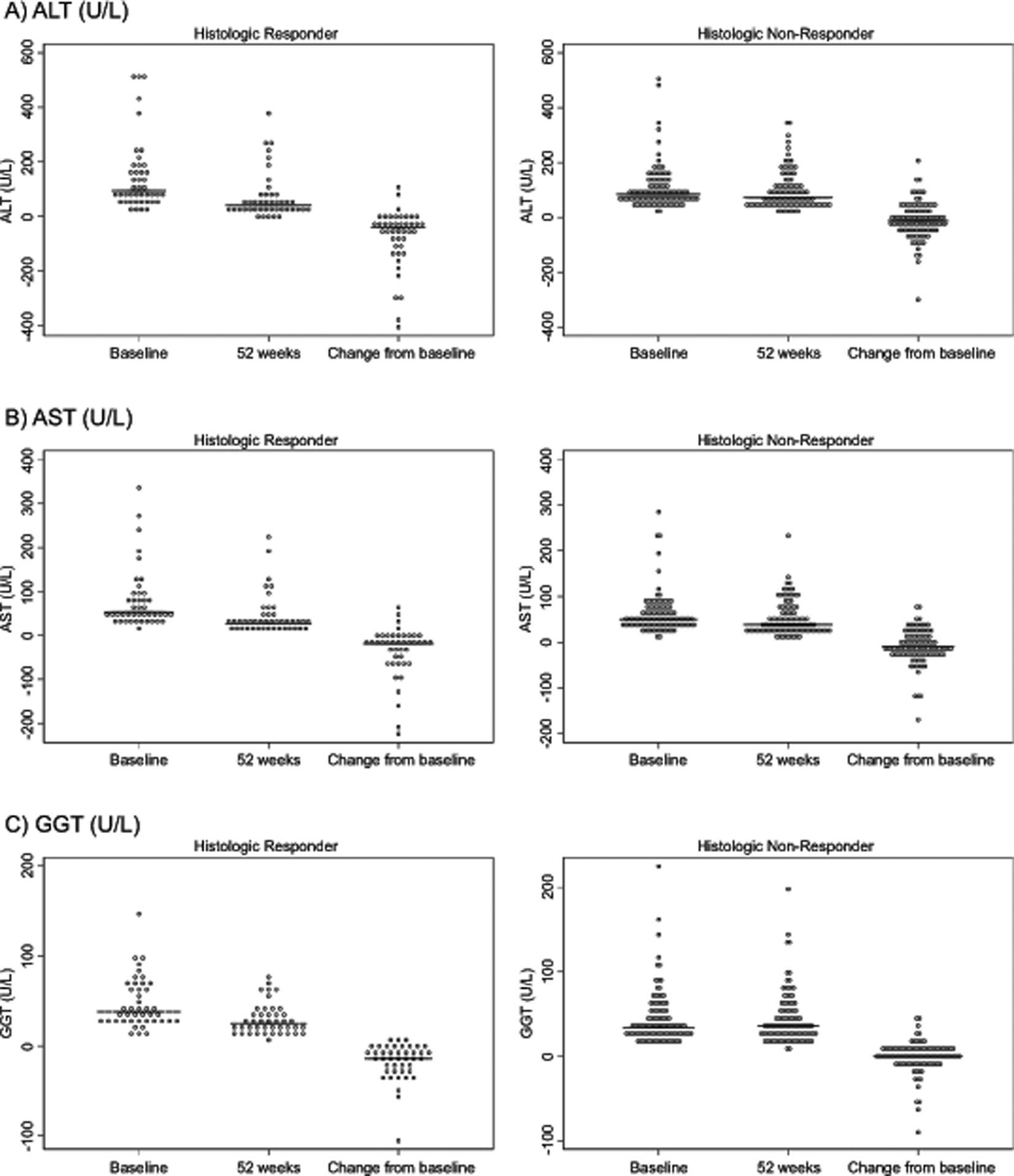
Figure 2.
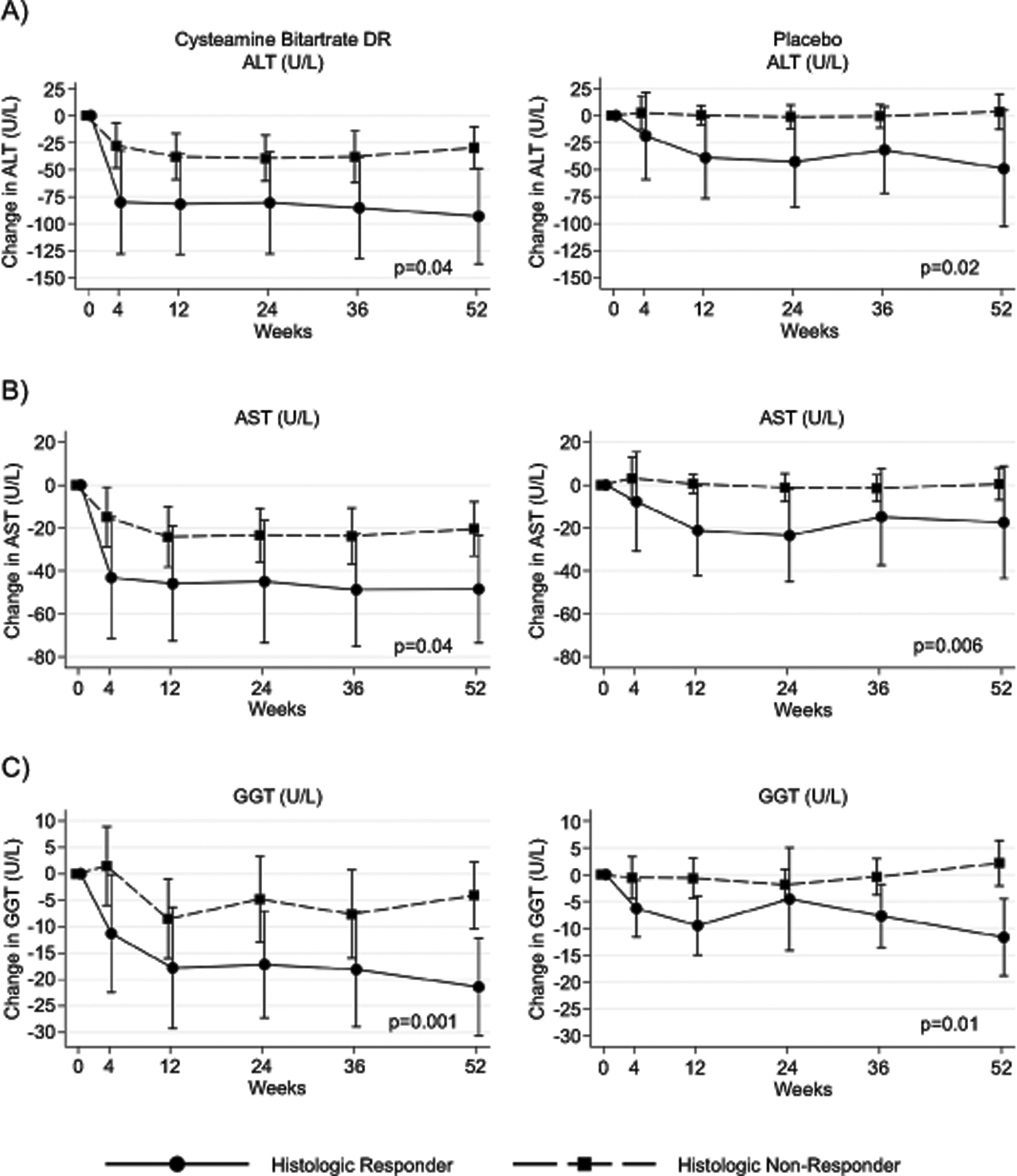
Relationship between histologic improvement and liver chemistries
Histologic improvement was significantly associated with the relative change in liver chemistries from baseline in ALT, AST, and GGT. For every 10% decrease in ALT over 52 weeks, there were 1.24 times greater odds of histologic improvement (95% CI 1.10 – 1.39, p<0.001) after controlling for baseline ALT, treatment group, age, sex, and body mass index (BMI) z-score. Similar results were noted for AST (OR 1.16, 95% CI 1.04−−1.29, p=0.006) and GGT (OR 1.51, 95% CI 1.28–1.79, p<0.001). (Table 2)
Table 2.
Multiple logistic regression analysis of histologic improvement on the 52-week relative changes in liver chemistries, controlling for treatment group, baseline liver chemistries, age, sex, and BMI z-score
| Odds Ratio | 95% CI | P | AUROC | |
|---|---|---|---|---|
| Outcome: Histologic improvement (yes vs. no) | 0.74 | |||
| Relative change in ALT (U/L), per 10% decrease from baseline to 52 weeks | 1.24 | 1.10 – 1.39 | <0.001 | |
| Treatment group (Cyst vs. Plbo) | 1.08 | 0.48 – 2.43 | 0.85 | |
| Baseline ALT (U/L) | 1.00 | 1.00 – 1.01 | 0.39 | |
| Baseline age (years) | 0.98 | 0.84 – 1.14 | 0.78 | |
| Sex (male vs. female) | 0.90 | 0.38 – 2.12 | 0.80 | |
| Baseline BMI z-score | 1.14 | 0.46 – 2.83 | 0.78 | |
| Outcome: Histologic improvement (yes vs. no) | 0.69 | |||
| Relative change in AST (U/L), per 10% decrease from baseline to 52 weeks | 1.16 | 1.04 – 1.29 | 0.006 | |
| Treatment group (Cyst vs. Plbo) | 1.16 | 0.53 – 2.57 | 0.71 | |
| Baseline AST (U/L) | 1.00 | 1.00 – 1.01 | 0.47 | |
| Baseline age (years) | 0.95 | 0.82 – 1.10 | 0.52 | |
| Sex (male vs. female) | 0.95 | 0.41 – 2.17 | 0.90 | |
| Baseline BMI z-score | 0.93 | 0.39 – 2.24 | 0.87 | |
| Outcome: Histologic improvement (yes vs. no) | 0.81 | |||
| Relative change in GGT (U/L), per 10% decrease from baseline to 52 weeks | 1.51 | 1.28 – 1.79 | <0.001 | |
| Treatment group (Cyst vs. Plbo) | 1.40 | 0.61 – 3.23 | 0.43 | |
| Baseline GGT (U/L) | 0.99 | 0.98 – 1.01 | 0.26 | |
| Baseline age (years) | 0.98 | 0.84 – 1.15 | 0.83 | |
| Sex (male vs. female) | 0.92 | 0.37 – 2.32 | 0.87 | |
| Baseline BMI z-score | 1.04 | 0.41 – 2.63 | 0.93 |
Three separate multiple logistic regression models were fit for the outcome of histologic improvement. Covariates included relative change in liver chemistry values (separate models fit for ALT, AST, and GGT), the baseline liver chemistry value, baseline age, sex, and baseline BMI z-score.
The liver chemistry effects were similar in Cysteamine Bitartrate DR and placebo groups. Interaction p-values 0.23, 0.37, 0.09 for ALT, AST, and GGT models, respective
Model for primary outcome: histologic improvement
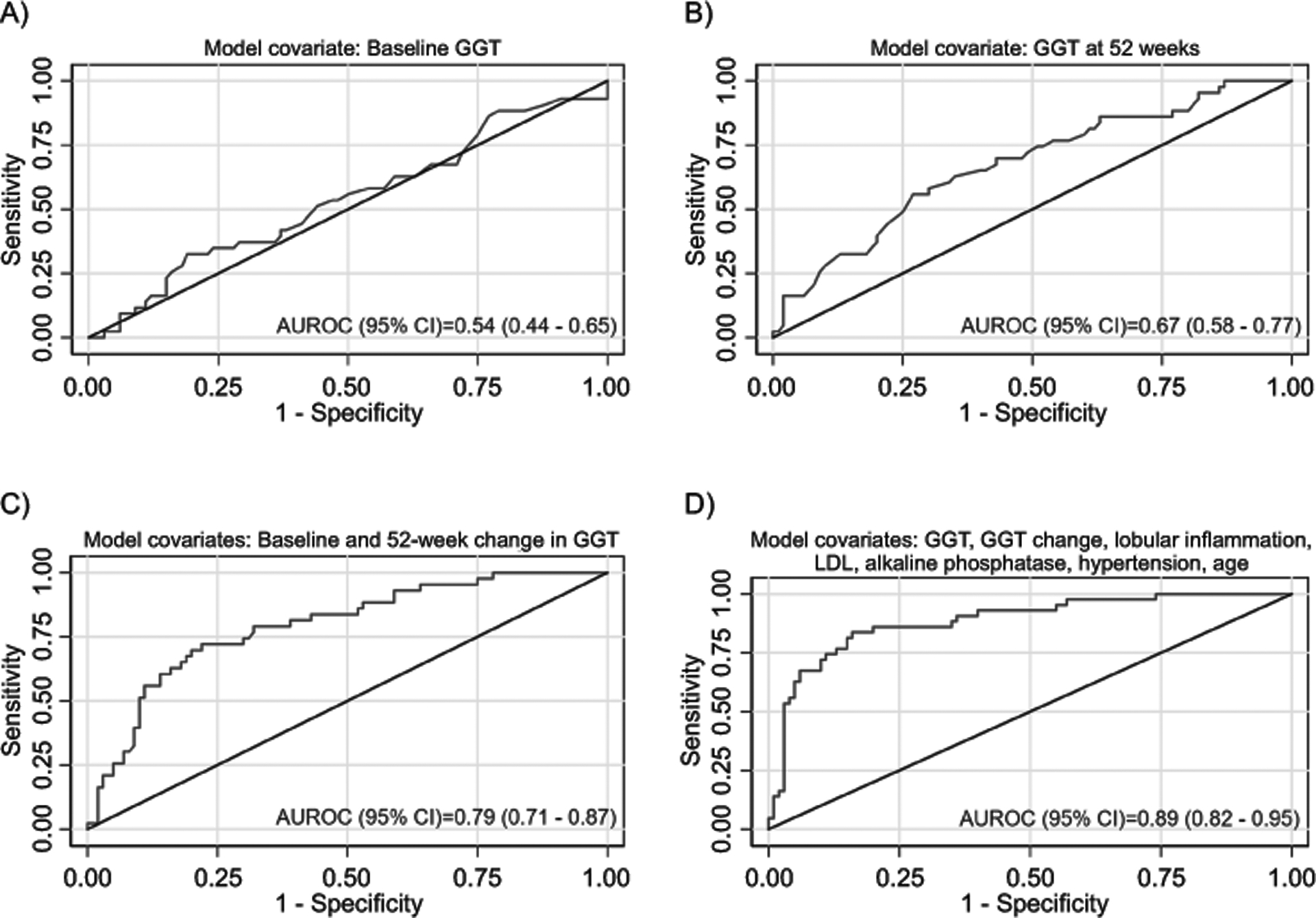
Models were developed for the CyNCh study’s primary outcome of improvement in overall liver histology, defined as a decrease in NAFLD activity score by ≥ 2 points without worsening fibrosis. (Table 3, Figure 3, Supplemental Table 5) A multivariate model was constructed based upon the baseline values of ALT, AST, and GGT and the change in ALT, AST, and GGT at 52 weeks. The strongest model (p < 0.0001) retained only baseline GGT and change in GGT at 52 weeks with an AUROC of 0.79 (95% CI 0.71 – 0.87). (Figure 3 panel c) A multivariate model was then constructed for histologic improvement using AIC selection, from a candidate set of 24 variables forcing GGT and change in GGT in the model. The best AIC model included the following variables: baseline GGT, change in GGT, baseline alkaline phosphatase, baseline LDL, baseline lobular inflammation, hypertension, and age. The AUROC of the model for predicting histologic improvement was 0.89 (95% CI 0.82 – 0.95), which was significantly better than the model including only baseline and 52-week change in GGT (P=0.01). (Figure 3 Panel d) When the model was fixed at the maximum Youden’s Index, the sensitivity was 84%, specificity was 84%, PPV was 69%, and NPV was 92%. When the model was validated using data from the TONIC trial, the AUROC was 0.84 (95% CI 0.77 – 0.91), and the sensitivity, specificity, PPV and NPV were 85%, 72%, 65%, and 89% respectively. The values of baseline GGT along with change in GGT were able to correctly classify presence of histologic improvement in 77.2% of participants. The clinical model and additional diagnostic statistics are shown in Table 3.
Table 3.
Diagnostic statistics for clinical predication models
| Model fixed at 90% specificity | Model fixed at maximum Youden’s Index* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUROC (95% CI) | Sens | PPV† | NPV‡ | Sens | Spec | PPV† | NPV‡ | |||
| Outcome: Histologic Improvement | ||||||||||
| Clinical Model | 0.89 (0.82 – 0.95) | 0.67 | 0.74 | 0.87 | 0.84 | 0.84 | 0.69 | 0.92 | ||
| Cross-validated Model | 0.85 (0.77 – 0.92) | 0.65 | 0.74 | 0.86 | 0.81 | 0.80 | 0.64 | 0.91 | ||
| Model validated using TONIC dataset | 0.84 (0.77 – 0.91) | 0.56 | 0.79 | 0.77 | 0.85 | 0.72 | 0.65 | 0.89 | ||
| Clinical Model: log(P/1-P)= −8.64 – 0.06 × baseline GGT (U/L) −0.08 × change in GGT (U/L) + 1.84 × lobular inflammation grade + 0.02 × baseline LDL (mg/dL) + 0.008 × baseline alkaline phosphatase (U/L) + 1.48 if hypertensive + 0.20 × age (years) | ||||||||||
| Outcome: Resolution of Zone 1, periportal pattern | ||||||||||
| Clinical Model | 0.91 (0.83 – 0.99) | 0.42 | 0.80 | 0.69 | 0.95 | 0.82 | 0.78 | 0.96 | ||
| Cross-validated Model | 0.83 (0.71 – 0.95) | 0.32 | 0.75 | 0.66 | 0.79 | 0.82 | 0.75 | 0.85 | ||
| Model validated using TONIC dataset | 0.92 (0.81 – 1.00) | 0.10 | 0.67 | 0.46 | 1.00 | 0.82 | 0.88 | 1.00 | ||
| Clinical model: log(P/1-P)= 6.23 – 0.05 × baseline ALT (U/L) – 0.07 × change in ALT (U/L) − 0.06 × baseline LDL (mg/dL) + 3.10 if white | ||||||||||
| Outcome: Fibrosis Improvement | ||||||||||
| Clinical Model | 0.89 (0.83 – 0.94) | 0.63 | 0.77 | 0.83 | 0.90 | 0.77 | 0.65 | 0.94 | ||
| Cross-validated Model | 0.85 (0.78 – 0.91) | 0.56 | 0.75 | 0.81 | 0.94 | 0.64 | 0.56 | 0.96 | ||
| Model validated using TONIC dataset | 0.82 (0.75 – 0.89) | 0.43 | 0.74 | 0.72 | 0.87 | 0.69 | 0.64 | 0.90 | ||
| Clinical model: log(P/1-P)= 0.09 – 0.03 × baseline ALT (U/L) – 0.02 × change in ALT (U/L) + 1.71 × baseline fibrosis stage – 1.16 × baseline BMI z-score – 0.68 × baseline ballooning score + 0.92 × baseline lobular inflammation score + 0.95 if white | ||||||||||
Youden’s Index=sensitivity+specificity-1
PPV = Positive Predictive Value: the probability that the disease is present when the test is positive
NPV = Negative Predictive Value: the probability that the disease is not present when the test is negative.
In CyNCh, 43/146 (29%) had histologic improvement; in TONIC 56/147 (38%) had histologic improvement.
In CyNCh, 19/46 (41%) had resolution of borderline Zone 1 steatohepatitis; in TONIC 22/40 (55%) had resolution of borderline, zone 1 steatohepatitis.
In CyNCh, 48/146 (33%) had fibrosis improvement; in TONIC 58/146 (40%) had fibrosis improvement.
TONIC clinical model for P, probability of histologic improvement: log(P/1-P)= −1.22 – 0.04 × baseline GGT (U/L) −0.06 × change in GGT (U/L) + 0.54 × lobular inflammation grade + 0.008 × baseline LDL (mg/dL) + 0.007 × baseline alkaline phosphatase (U/L) – 0.46 if hypertensive − 0.13 × age (years)
TONIC clinical model for P, probability of resolution of zone 1, periportal pattern: log(P/1-P)=5.88 – 0.09 × baseline ALT (U/L) – 0.08 × change in ALT (U/L) + 0.005 × baseline LDL (mg/dL) – 1.56 if white
TONIC clinical model for P, probability of fibrosis improvement: log(P/1-P)= +0.79 – 0.02 × baseline ALT (U/L) – 0.01 × change in ALT (U/L) + 1.13 × baseline fibrosis stage – 0.37 × baseline BMI z-score – 0.35 × baseline ballooning score + 0.47 × baseline lobular inflammation score – 1.30 if whit
Figure 3.
Models for resolution of borderline zone 1 NASH
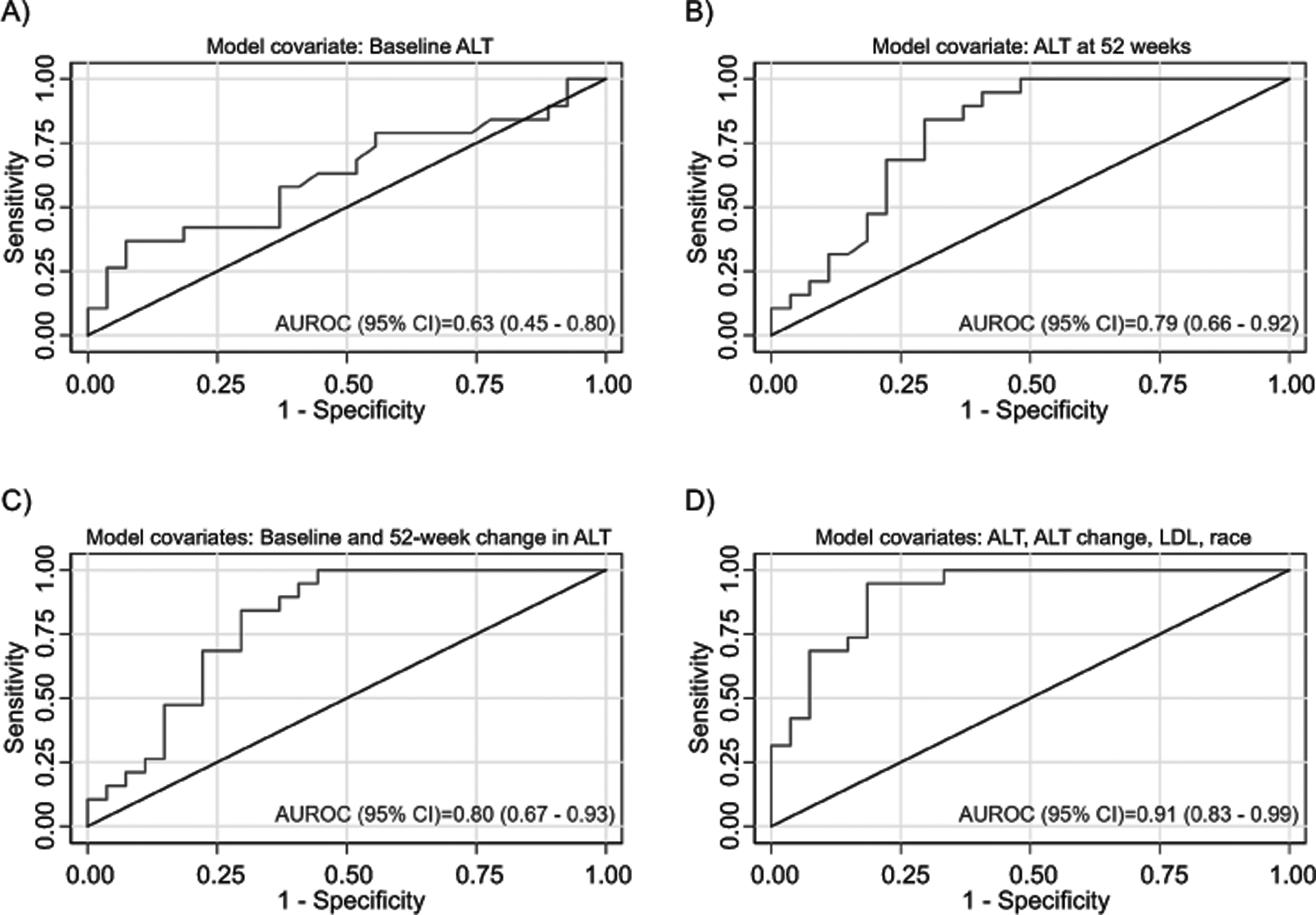
Models were created for the histologic outcome of resolution of borderline zone 1 NASH. (Table 3, Figure 4, Supplemental Table 6) A multivariate model was constructed using both the baseline values and the 52 week change in ALT, AST, and GGT. The strongest model (p = 0.0004) retained only baseline ALT and change in ALT at 52 weeks and had an AUROC of 0.80 (95% CI 0.67 – 0.93). (Figure 4, Panel c) Next, a multivariable model for resolution of borderline zone 1 NASH was built from a candidate set of 24 variables and using AIC selection, forcing baseline and 52-week change in ALT in the model. In addition to baseline ALT and change in ALT, the variables selected were race, and baseline LDL. The AUROC of the model was 0.91 (95% CI 0.83 – 0.99), which was significantly better than the model that included only baseline and 52-week change in ALT (P=0.03). (Figure 4, Panel d) When the model was fixed at the maximum Youden’s Index, the sensitivity was 95%, specificity was 82%, PPV was 78%, and NPV was 96%. When the model was validated using data from the TONIC trial, the AUROC was 0.92 (95% CI 0.81 – 1.00), and the sensitivity, specificity, PPV and NPV were 100%, 82%, 88%, and 100%. The values of baseline ALT and change in ALT were able to correctly classify resolution of borderline zone 1 NASH in 71.7% of participants. The clinical model and additional diagnostic statistics are shown in Table 3.
Figure 4.
Models for improvement in fibrosis
Models were created for the histologic outcome of improvement in fibrosis. (Table 3, Supplemental Figure 3) A multivariate model was constructed using both the baseline values and the 52 week change in ALT, AST, and GGT. The strongest model ((p<0.001) retained only baseline and change of ALT at 52 weeks and had an AUROC of 0.80 (95% CI 0.67– 0.93). (Supplemental Figure 3 Panel d) Next, a multivariable model for improvement in fibrosis was built from a candidate set of 24 variables using AIC selection. In addition to baseline ALT and change in ALT, the best model retained baseline BMI z-score, race, along with baseline histologic features of fibrosis stage, ballooning grade, and lobular inflammation grade. The AUROC of the model was 0.89 (95% CI 0.83– 0.94). (Supplemental Figure 3, Panel d) When the model was fixed at the maximum Youden’s Index, the sensitivity was 90%, specificity was 77%, PPV was 65%, and NPV was 94%. When the model was validated using data from the TONIC trial, the AUROC was 0.85 (95% CI 0.78 – 0.91), and the sensitivity, specificity, PPV and NPV were 94%, 64%, 56%, and 96%. The clinical model and additional diagnostic statistics are shown in Table 3.
Model for change in liver chemistries
Multiple linear regression models of the change in each liver chemistry (ALT, AST, GGT) over 52 weeks on histologic improvement, controlling for age, sex, baseline BMI z-score, baseline liver chemistry (ALT, AST, or GGT) and treatment group are presented in Table 4. Histologic improvement was found to be significantly associated with change in ALT (p=0.006) and change in GGT at 52 weeks (p<0.001) but not with change in AST (p=0.08). Factors that were independently associated with change in all three liver chemistry parameters were baseline liver chemistry value, change in BMI z-score, and treatment group. For ALT and AST, but not GGT, baseline BMI z-score was also significant (p= 0.002 for ALT, p=0.01 for AST, and p= 0.27 for GGT). Age and sex were not significantly associated with change in liver chemistry.
Table 4.
Multiple linear regression analysis of the 52-week changes in liver chemistries on histologic improvement, controlling for treatment group, baseline liver chemistries, age, sex, and BMI z-score
| β | 95% CI | P | |
|---|---|---|---|
| Histologic improvement (yes vs. no) | −29.9 | −51.3, −8.5 | 0.006 |
| Treatment group (Cyst vs. Plbo) | −20.4 | −39.4, −1.3 | 0.04 |
| Baseline ALT (U/L) | −0.5 | −0.6, −0.4 | <0.001 |
| Baseline age (years) | −0.01 | −3.6, 3.6 | 1.00 |
| Sex (male vs. female) | −10.6 | −31.0, 9.9 | 0.31 |
| Baseline BMI z-score | 33.3 | 12.1, 54.4 | 0.002 |
| 52-week change in BMI z-score | 61.3 | 17.3, 105.4 | 0.007 |
| Intercept | −11.8 | ||
| Outcome: Δ AST (52-week-BL, U/L) | |||
| Histologic improvement (yes vs. no) | −10.8 | −22.7, 1.2 | 0.08 |
| Treatment group (Cyst vs. Plbo) | −13.6 | −24.3, −2.8 | 0.01 |
| Baseline AST (U/L) | −0.6 | −0.7, −0.4 | <0.001 |
| Baseline age (years) | −0.8 | −2.8, 1.2 | 0.42 |
| Sex (male vs. female) | −7.8 | −19.3, 3.7 | 0.18 |
| Baseline BMI z-score | 15.3 | 3.4, 27.1 | 0.01 |
| 52-week change in BMI z-score | 26.2 | 1.5, 50.9 | 0.04 |
| Intercept | 14.2 | ||
| Outcome: Δ GGT (52-week-BL, U/L) | |||
| Histologic improvement (yes vs. no) | −12.8 | −18.9, −6.6 | <0.001 |
| Treatment group (Cyst vs. Plbo) | −6.1 | −11.5, −0.6 | 0.03 |
| Baseline GGT (U/L) | −0.3 | −0.4, −0.2 | <0.001 |
| Baseline age (years) | 0.7 | −0.3, 1.8 | 0.17 |
| Sex (male vs. female) | 0.3 | −5.6, 6.2 | 0.92 |
| Baseline BMI z-score | 3.5 | −2.7, 9.7 | 0.27 |
| 52-week change in BMI z-score | 14.0 | 1.3, 26.8 | 0.03 |
| Intercept | −3.4 |
DISCUSSION
We performed a secondary analysis of data from the CyNCh clinical trial, a randomized, double-blind, placebo-controlled trial of CBDR for the treatment of children with NAFLD, to evaluate the relationship between change in liver chemistry and improvement in liver histology. We noted that changes in liver chemistry at 12 weeks strongly correlated with changes in liver chemistry at 52 weeks. Moreover, changes in liver chemistry showed strong relationships with histologic improvement. For improvement in NAS, baseline and change in GGT was the most predictive indicator, while for resolution of borderline zone 1 NASH and improvement in fibrosis, baseline and change in ALT were most predictive. In addition to improvement in liver histology, changes in ALT, AST and GGT over 52 weeks were also strongly related to their baseline value, treatment group and changes in BMI z-score.
Of the common clinical laboratory tests, GGT had the strongest relationship with improvement in NAS in the CyNCh clinical trial. Notably, this finding was replicated utilizing data from the TONIC trial and is consistent with other available cross-sectional and basic science data. GGT is present in the bile canaliculi of hepatocytes and in biliary epithelial cells, and has an important role in the metabolism of glutathione, the principal thiol antioxidant in humans. As oxidized glutathione increases, hepatic GGT is induced, and thus GGT levels are a marker of oxidative stress. (16, 17) Furthermore, GGT has been shown to correlate with more severe liver histology in both children and adults with NAFLD. (18–20) Moreover, GGT is a marker of extrahepatic comorbidities that associate with NAFLD such as type 2 diabetes mellitus, hypertension, and dyslipidemia. (21) Thus, our data suggest that GGT may be useful for non-invasively monitoring the improvement of NAFLD histology in children due to its relationship to the underlying pathophysiology of NAFLD, its value in predicting histological severity based on NAS, and its relevance to commonly associated comorbidities seen in NAFLD that influence NAFLD severity.
The relationship between laboratory parameters and various subtypes of pediatric NAFLD is of particular interest. Borderline zone 1 NASH, the dominant pattern in children, is more often associated with fibrosis than other subtypes. (22) As fibrosis is a major determinant of morbidity and mortality related to liver disease, there is a need for accurate monitoring of progression of this phenotype in children. In the CyNCh clinical trial, although ALT improved in children receiving the study drug, it was only children with borderline zone 1 NASH that also had significant corresponding histologic improvement. (13) In this secondary analysis, baseline and change in ALT predicted improvement in borderline zone 1 NASH, demonstrating that the most important marker to follow in a given patient may depend upon baseline histology. Furthermore, it was not only the change in ALT that was predictive, but the combination of baseline ALT along with change in ALT, thus illustrating the importance of interpretation of a given change in a liver chemistry in the context of its starting value. Interestingly, this relationship between ALT and improvement in borderline zone 1 NASH was independent of BMI. In summary, accurate interpretation of how change in liver chemistry reflects histologic change requires an appreciation of the various patient and laboratory specific parameters that may influence this laboratory-histology relationship.
The ability to assess fibrosis presence and severity in NAFLD is of particular importance, given that fibrosis is a key predictor of NAFLD prognosis and the risk of progression to cirrhosis and end-stage liver disease. Thus, an important outcome to consider in pediatric NAFLD clinical trials is improvement in fibrosis stage. To date, there are no non-invasive measures that have been validated in children to assess change in liver fibrosis stage. Therefore, our model, with good performance characteristics, is a promising development. Interestingly, both baseline and change in liver chemistry is required to predict fibrosis improvement which is similar to models predicting improvement in NAS score, borderline zone 1 NASH. Additionally, all of the parameters in the model to predict histologic improvement other than change in ALT, were baseline histologic parameters, including baseline fibrosis stage. Therefore, comprehensive characterization pretherapy, including liver histology, is necessary in order for this model to be utilized. This is consistent with recommendations from AASLD that a liver biopsy to establish a diagnosis of NASH should be obtained before starting children on pharmacological therapy for NASH (23).
NASPGHAN and AASLD clinical practice guidelines emphasize the need for noninvasive biomarkers to detect and accurately measure change in NAFLD and NASH longitudinally. (4, 23) This secondary analysis suggests that readily available laboratory tests, particularly ALT and GGT, may aid in the prediction of histologic improvement over time. Based upon the baseline values of ALT and GGT and the change in ALT and GGT, one may correctly classify histologic improvement in about three-quarters of children with NAFLD. Further biomarker development should be targeted on closing that remaining gap. Therefore, these laboratory tests may be useful both for clinical monitoring and for use as outcomes in clinical trials. The choice of which liver chemistry should be the primary outcome may depend on the outcome variable of interest; for example, GGT would be the best liver chemistry to predict improvement in NAS score. In addition, the manner in which we consider these parameters requires consideration of both the baseline value and the relative change from baseline in the interpretation.
The relationship between liver chemistry and disease severity is complex and multifactorial. In this study, factors found to be independently associated with change in ALT, AST, and GGT were baseline liver chemistry values, change in BMI z-score and treatment group assignment. Baseline liver chemistry values determine the potential for change in liver chemistry, in part by setting mathematical constraints on how much room for change there is, thus affecting the relationship between relative and absolute change. There are also corresponding physiologic constraints; the degree of liver chemistry elevation is associated with the severity of hepatocellular injury (24) and with more inflammation at outset, there is greater potential for improvement with intervention. In addition, liver chemistry change was also significantly associated with change in BMI. This finding is supported by previous studies which have associated reduction in BMI with improvement in metabolic function, decreasing both insulin resistance (25) and oxidative stress (26), known contributors to hepatic inflammation. The potential impact of oxidative stress on liver chemistry is further illustrated by the fact that treatment with cysteamine, a glutathione precursor with antioxidant properties, was significantly associated with liver chemistry improvement (13). Thus, the difficulty in interpreting change in liver chemistry is due in part to the divergent factors that influence this change.
Strengths of the study included that data were from a randomized placebo-controlled trial conducted by the NASH CRN, which has a diverse geographic representation of children with accurate and rigorously characterized NAFLD. Liver histology was evaluated via central review by a Pathology Committee reading slides in consensus without awareness of clinical data. This study included analysis of liver chemistries which are readily available, affordable, and are routinely used in clinical practice. This study was able to validate the models by applying models to data from the only other multi-center trial using liver histology in children, TONIC. A limitation to this study is that this was a secondary analysis. (27) An additional limitation is that the constructed models may not apply to individuals who have normal liver chemistry, however, those with normal liver chemistries are uncommon participants in treatment trials. We encourage these findings to be tested in other studies with different patient populations.
In conclusion, in children with NAFLD, the dynamic changes in serum ALT and GGT are strongly associated with change in liver histology and may be useful as an indicator of histologic response. More specifically, GGT may best address improvement in NAS score, and ALT may best address improvement in borderline zone 1 NASH and improvement in fibrosis. There are important implications to understanding this specific relationship between liver chemistry and histologic change, both in terms of clinical care and in the context of clinical trials, as children with borderline zone 1 NASH may have a differential risk for advanced fibrosis and also may respond differently to interventions.
Supplementary Material
Source of funding
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713). Additional support is received from the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000077, UL1TR000150, UL1TR000424, UL1TR000006, UL1TR000448, UL1TR000040, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000454). The CyNCh trial was conducted by the NASH CRN and supported in part by the Intramural Research Program of the National Cancer Institute and by a Collaborative Research and Development Agreement (CRADA) between NIDDK and Raptor Pharmaceuticals.
List of Abbreviations:
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- AASLD
American Association for the Study of Liver Disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
gamma-glutamyl transferase
- CyNCh
Cysteamine Bitartrate Delayed-Release for the Treatment of NAFLD in Children
- NASH CRN
NASH Clinical Research Network
- CBDR
cysteamine bitartrate delayed release
- NAS
NAFLD Activity Score
- AIC
Akaike’s Information Criteria
- BMI
body mass index
- PPV
positive predictive value
- NPV
negative predictive value
- AUROC
Area Under the Receiver Operating Characteristic
REFERENCES
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. [DOI] [PubMed] [Google Scholar]
- 2.Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. The Journal of pediatrics. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes DM, Pantangi V, Azam M, Salomao M, Iuga AC, Lefkowitch JH, et al. Pediatric Nonalcoholic Fatty Liver Disease in New York City: An Autopsy Study. The Journal of pediatrics. 2018;200:174–80. [DOI] [PubMed] [Google Scholar]
- 4.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). Journal of pediatric gastroenterology and nutrition. 2017;64(2):319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. Journal of clinical gastroenterology. 2018;52(4):339–46. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A, Neuschwander-Tetri BA, Kleiner DE, Schabel E, Rinella M, Harrison S, et al. Defining Improvement in Nonalcoholic Steatohepatitis for Treatment Trial Endpoints: Recommendations from the Liver Forum. Hepatology. 2019. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Newton KP, Awai HI, Choi LJ, Garcia MA, Ellis LL, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Alimentary pharmacology & therapeutics. 2013;38(10):1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. The American journal of gastroenterology. 2017;112(1):18–35. [DOI] [PubMed] [Google Scholar]
- 10.Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. The Journal of pediatrics. 2014;164(4):707–13 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arsik I, Frediani JK, Frezza D, Chen W, Ayer T, Keskinocak P, et al. Alanine Aminotransferase as a Monitoring Biomarker in Children with Nonalcoholic Fatty Liver Disease: A Secondary Analysis Using TONIC Trial Data. Children. 2018;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuppalanchi R, Jain AK, Deppe R, Yates K, Comerford M, Masuoka HC, et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(12):2121–30 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Lavine JE, Wilson LA, Neuschwander-Tetri BA, Xanthakos SA, Kohli R, et al. In Children With Nonalcoholic Fatty Liver Disease, Cysteamine Bitartrate Delayed Release Improves Liver Enzymes but Does Not Reduce Disease Activity Scores. Gastroenterology. 2016;151(6):1141–54 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. [DOI] [PubMed] [Google Scholar]
- 15.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. Jama. 2011;305(16):1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993;32(24):6302–6. [DOI] [PubMed] [Google Scholar]
- 17.Hochwald SN, Harrison LE, Rose DM, Anderson M, Burt ME. gamma-Glutamyl transpeptidase mediation of tumor glutathione utilization in vivo. Journal of the National Cancer Institute. 1996;88(3–4):193–7. [DOI] [PubMed] [Google Scholar]
- 18.Irie M, Sohda T, Iwata K, Kunimoto H, Fukunaga A, Kuno S, et al. Levels of the oxidative stress marker gamma-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. The Journal of international medical research. 2012;40(3):924–33. [DOI] [PubMed] [Google Scholar]
- 19.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135(6):1961–71 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52(3):913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndrepepa G, Kastrati A. Gamma-glutamyl transferase and cardiovascular disease. Annals of translational medicine. 2016;4(24):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Africa JA, Behling CA, Brunt EM, Zhang N, Luo Y, Wells A, et al. In Children With Nonalcoholic Fatty Liver Disease, Zone 1 Steatosis Is Associated With Advanced Fibrosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(3):438–46 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. [DOI] [PubMed] [Google Scholar]
- 24.Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367–84. [DOI] [PubMed] [Google Scholar]
- 25.Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111(15):1985–91. [DOI] [PubMed] [Google Scholar]
- 26.Mohn A, Catino M, Capanna R, Giannini C, Marcovecchio M, Chiarelli F. Increased oxidative stress in prepubertal severely obese children: effect of a dietary restriction-weight loss program. The Journal of clinical endocrinology and metabolism. 2005;90(5):2653–8. [DOI] [PubMed] [Google Scholar]
- 27.Curran-Everett D, Milgrom H. Post-hoc data analysis: benefits and limitations. Current opinion in allergy and clinical immunology. 2013;13(3):223–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


