Abstract
Preeclampsia (PE) is associated with placental ischemia, hypertension (MAP), increased cytolytic natural killer (cNK) cells, tumor necrosis factor alpha (TNF-α) and mitochondrial Reactive Oxygen Species (mt ROS). The reduced uterine perfusion pressure (RUPP) rat model of PE, exhibits many characteristics of PE. We have shown that blockade of TNF-α, via etanercept (ETAN), decreases MAP, however, the effect of ETAN on cNK cells and mt ROS is unknown. We hypothesized that ETAN would decrease cNK cell and mt ROS in RUPP rats. Rats were divided into 3 groups: normal pregnant (NP) (n=20), RUPP (n=20), RUPP + ETAN (0.4 mg/kg) (n=20). On gestational day 14, RUPP surgery was performed, and ETAN was administered on day 18, MAP, blood and tissue mitochondria were collected on day 19. MAP was elevated in RUPP vs. NP (118 ± 2 vs. 102± 1 mmHg, p<0.05) which was reduced to 110 ± 2 in RUPP + ETAN (p<0.05). cNK cells were increased in circulation (5.9 ± 1.8 vs. 4.0 ± 1.4 % gated cells), placenta (4.8 ± 1.3 vs. 1.9 ± 0.7 %;), and kidney (3.1 ± 0.5 vs. 0.8 ± 0.5% gated cells; of RUPP vs. NP (p<0.05) and were lowered to 1.5 ± 0.5%, 1.7 ± 0.7%, 0.8 ± 0.5% gated cells in RUPP + ETAN ( p<0.05). Placental and renal mt ROS was reduced with ETAN treatment. TNF-α blockade lowered cNK cells, and mt ROS in placental ischemic rats, which could have contributed to lowered blood pressure observed in response to placental ischemia.
Keywords: Hypertension, inflammation and Oxidative Stress
Introduction
Women with preeclampsia have a 2–3-fold increase in plasma TNF-α concentrations compared to normal pregnant women.(1, 2) Serum and placental TNF-α levels are also elevated in rodent models of preeclampsia, such as in the reduced utero placental perfusion model (RUPP) in rats.(3–8) TNF-α is a potent pro-inflammatory cytokine secreted by a variety of cells during an inflammatory response.(1, 8) TNF-α can be secreted by vascular endothelial cells, smooth muscle cells, trophoblasts cells, and epithelial cells.(1, 9, 10) Specifically, in the kidneys, TNF-α can be derived from podocytes, mesangial cells, and epithelial cells of the proximal tubule, thick ascending limb, and collecting duct. TNF-α acts in an autocrine fashion on many immune cells that cause activation of downstream immune and cell signaling events.(10) Multiple studies suggest that TNF-α plays a causative role in hypertension by exerting effects to promote vasoconstriction, endothelial dysfunction, alterations in renal hemodynamics, renal damage, and cardiac injury.(1, 2, 9–13)
Earlier studies by LaMarca et al. and others showed that infusion of TNF-α (at 50ng/day) during the last five days of pregnancy in rats increased their blood pressure.(3–7, 14) Furthermore TNF-α administered during late pregnancy increased other plasma markers associated with preeclampsia, such as endothelin-1, soluble fms-like tyrosine kinase (sFlt-1), soluble endoglin (sEng), and ANG II type I receptor autoantibodies (AT1-AAs).(4, 6, 7, 15)
Anti-TNF-α agents are now widely available for the treatment of autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, and inflammatory bowel disease.(16) Etanercept (ETAN) binds to the TNF- α and blocks its interaction with the TNF-α cell surface receptors. It is a monoclonal antibody and has a large molecular weight of approximately 150kDa, and is therefore, not expected to cross the human placenta. One study compared the use of three different types of TNF-α inhibition and its association with development of congenital fetal anomalies, it was noted that there was no significant difference between the three groups exposed to anti-TNF-α medications during the first trimester (17).
We have previously shown that blockade of TNF-α by ETAN reduced circulating TNF-α levels and blood pressure by ~15mmHg in RUPP rats.(14) Furthermore these studies showed that HUVEC cells incubated with RUPP serum plus ETAN reduced endothelin-1 (a potent vasoconstrictor), suggesting that TNF-α secretion during preeclampsia is a major contributor to the vasoconstrictor responses that occurs in women with preeclampsia.(14) However, TNF-α blockade did not decrease ET-1 production in the kidney or the placenta of RUPP rats leaving the anti-hypertensive effect during pregnancy unclear. Moreover, it remains unknown what effect ETAN may have on AT1-AA production, however 24 hours post administration is likely not long enough to effect AT1-AA levels in the RUPP rat. A follow up study showed that TNF-α blockade in RUPP rats prevented the enlargement of cardiomyocytes, fibrosis, apoptosis, lowered the expression of mRNA for brain natriuretic peptide (a marker of cardiac hypertrophy), and decreased sFlt-1 amounts in RUPP rats.(11, 15).
It is important to note that preeclampsia is also associated with increased NK cell activation and mitochondrial reactive oxygen species (mt ROS) generation. (8, 18–20) Moreover, we have also demonstrated that RUPP rats with depleted NK cells have decreased serum and placental levels of TNF-α, supporting a role for NK cells to secrete TNF-α, indicating an autocrine effect between TNF-α and NK cells. Our lab has shown through numerous publications the important role of NK cell activation and mt ROS in the pathophysiology of preeclampsia, however little is known about the role of TNF-α in NK cell activation and mt ROS. (18, 19, 21) In summary, all these findings suggest and reinforce that TNF-α contributes to increases in antiangiogenic factors, endothelial dysfunction, oxidative stress and hypertension during pregnancy. Thus signifying that blockade of TNF-α could be an important pathway to target for future therapeutics for this disease. Therefore, the goal of this study was to determine if TNF-α blockade, via ETAN in placental ischemic (RUPP) rats will improve NK cell activation and renal or placental mt ROS.
Methods
The techniques and data that support the findings of this study are available from the corresponding author upon reasonable request.
Timed-pregnant Sprague Dawley (SD) rats purchased from Envigo (Indianapolis, IN) were used in this study. Rats were housed in a 12-hour light and dark cycle each day with temperature-controlled rooms (75°F), in which they were maintained on a normal diet with free access to food and water. All experiments performed were in accordance with the National Institutes of Health guidelines for use and care of animals and all animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Effects of TNF-α blockade on blood pressure in RUPP rats
On gestational day 14 of pregnancy, the reduced uterine perfusion pressure (RUPP) surgery was performed on rats as previously described.(22, 23) Briefly surgical clips were placed over the abdominal aorta and ovarian arteries on the left and right side to reduce blood flow by ~ 40 %.(22, 23) This surgical procedure is often used as a model of preeclampsia in the rat and mimics the pathophysiology of preeclampsia in women.(22, 23) Analgesics were used to provide comfort for the surgical rats, including 5mg/kg carprofen administered via subcutaneous injection, once daily for 2–3 days following RUPP surgical procedure and 0.25% sensor care administered topically after carotid catheter insertion. Pregnant SD rats were randomly divided into 3 groups: Normal Pregnant (NP, n= 20), Reduced Uterine Perfusion Pressure (RUPP, n=20), and RUPP + etanercept (ETAN). ETAN (Enbrel) was administered at a dose of 0.4 mg/kg/day in saline subcutaneously once on gestational day 18. No control saline vehicle was given to RUPP or NP rats, because saline has no physiological effects on rats and surgeries within the control group are unnecessary. This dose of ETAN was used in previous studies and shown to reduce circulating TNF-α levels, blood pressure, and endothelin-1 in RUPP rats.(14) Thus this dose was used in this study to determine its role in NK cell activation and mt ROS generation in RUPP rats. On gestational day 18, under isoflurane anesthesia, catheters were inserted into the carotid artery to measure the blood pressure. On gestational day 19, conscious blood pressure (MAP), litter size, and pup weights were recorded followed by the collection of blood, placentas, and kidneys for flow cytometry to determine NK cells and mitochondrial isolation for functional assays (24, 25).
The effect of TNF-α blockade on NK cells in RUPP rats
Lymphocytes were isolated from blood placenta, and kidney via centrifugation on a density gradient medium (Lymphoprep, Accurate Chemical & Scientific Corp., Westbury, NY). For flow cytometry, 1 X 106 cells were incubated for 10 minutes at 4 °C with antibodies against rat natural killer cell markers ANK61 and ANK44 as previously described.(26) Cells that stained for ANK61+ and ANK44+ were designated as activated NK cells respectively. The percent of (ANK44+/ANK61+) positive stained cells above the negative control was determined for individual rats and the mean values for each experimental group were calculated.(18)
The effect of TNF-α blockade on mitochondrial oxidative stress (mt ROS) in RUPP rats
Intact placental or renal mitochondria were isolated by differential centrifugation and used to determine mt ROS as previously described.(18) Briefly, once mt were isolated, hydrogen peroxide (H2O2) production in placental or renal RUPP mitochondria was determined by fluorescence (555/581 nm excitation/emission for 30 min) using the amplex red reagent (6, 30). All data were normalized per milligram of mitochondrial protein.
Statistical Analysis
All data are presented as mean ± SEM. Data were analyzed by one-way ANOVA with Bonferroni post hoc analysis. Mitochondria reactive oxygen species between RUPP and RUPP+ETAN was analyzed by student t-test. All statistical analysis was performed with GraphPad Prism 7 software (GraphPad Software, La Jolla, CA). P < 0.05 was considered statistically significant.
Results
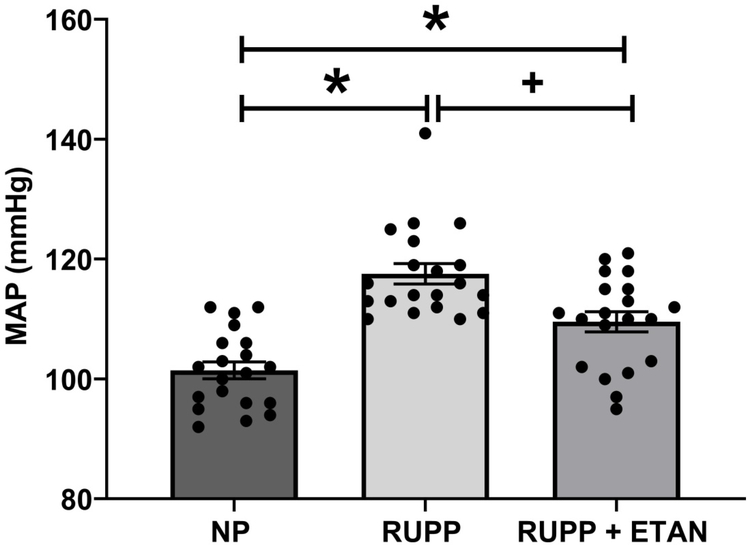
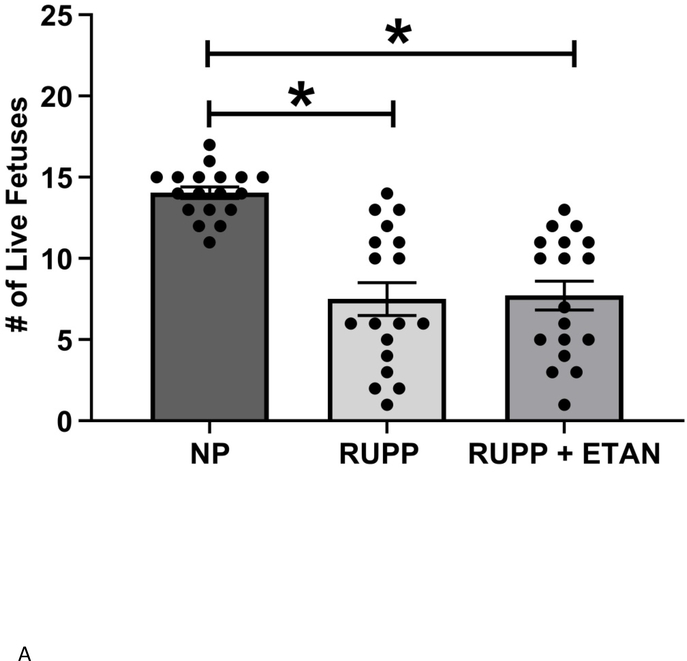
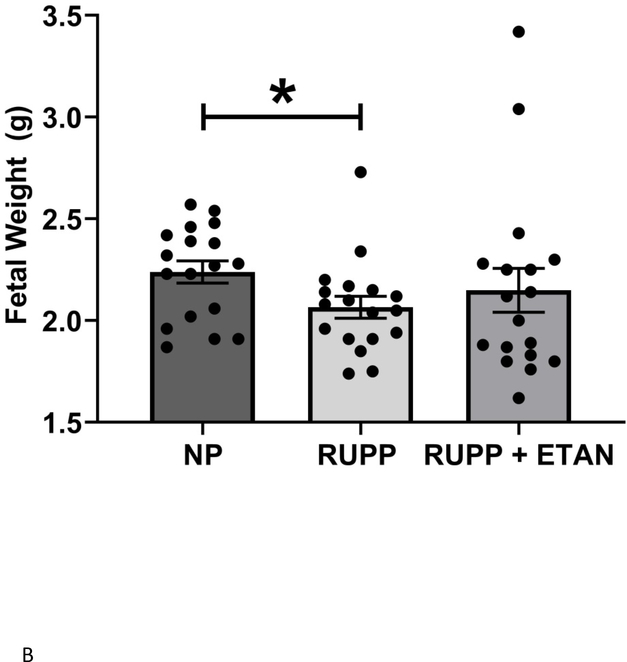
Mean arterial pressure (MAP) was elevated in RUPP vs NP (118 ± 2 vs. 102± 1 mmHg, p<0.05) rats as previously shown (Figure 1).(18, 22) MAP decreased in RUPP rats treated with ETAN vs RUPP rats alone (110 ± 2 vs. 118 ± 2 mmHg, p<0.05) (Figure 1). The number of live rat births was reduced for RUPP (8.0±1.0) and RUPP + ETAN (8.0±0.9) rats vs NP rats (14 ± 0.4 pups) (p<0.05) (Figure 2A). Pup weight was decreased in RUPP vs NP rats (2.07±0.05 vs. 2.24 ± 0.05 grams, p<0.05) (Figure 2B) and was not different between RUPP +ETAN vs NP rats (2.15±0.11 vs. 2.24 ± 0.05 grams) (Figure 2B), indicating improvements in pup weights in response to TNF-α inhibition in response to placental ischemia.
Figure 1.
MAP was measured in RUPP (n=20), normal pregnant (n=20) (NP), and ETAN administered to RUPP (n=20) rats.. Statistical differences were achieved using one-way ANOVA with Bonferroni post hoc analysis, *p < 0.05 vs NP and +p < 0.05 vs. RUPP.
Figure 2.
A) The number if live pups and B) Fetal weight was recorded NP, RUPP, and RUPP + ETAN ratsStatistical differences were achieved using one-way ANOVA with Bonferroni post hoc analysis, *p < 0.05 vs NP.
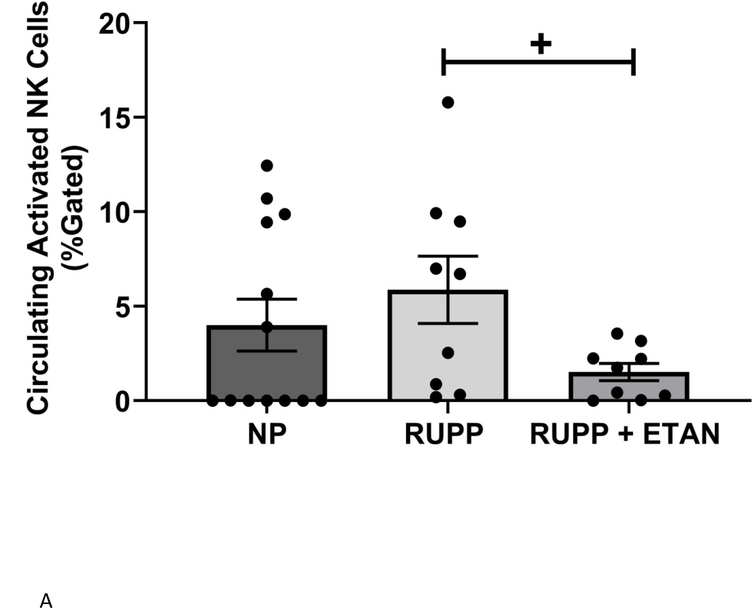
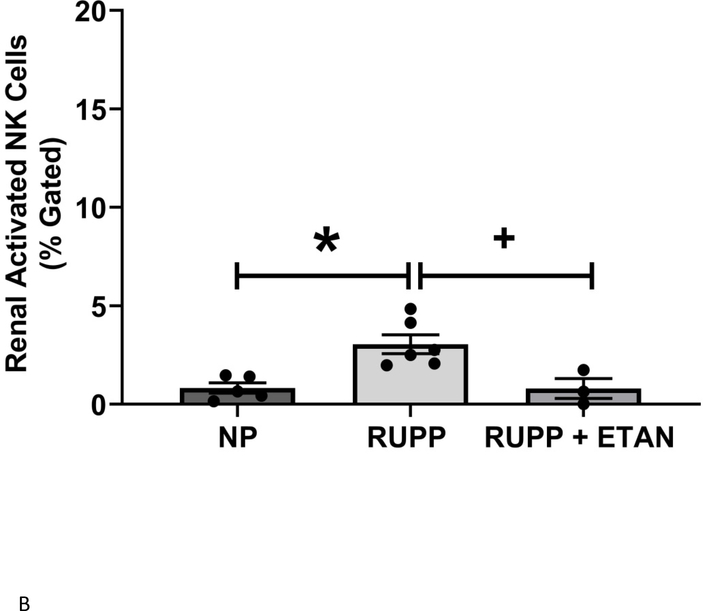
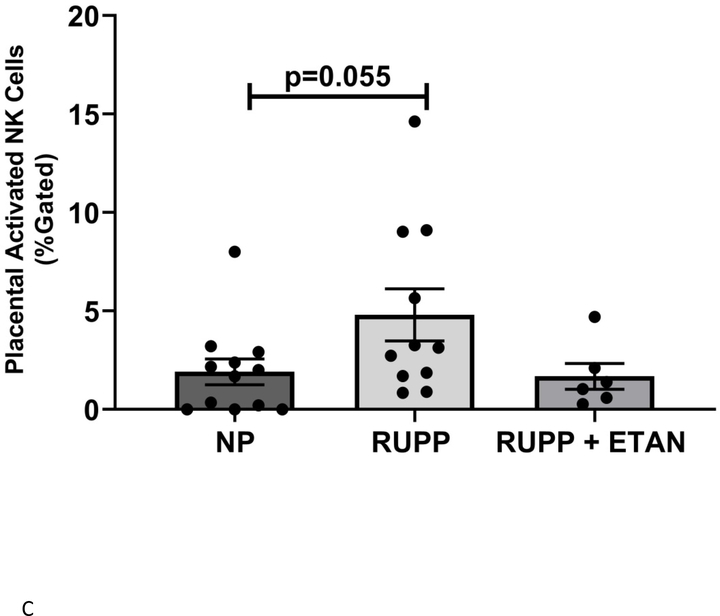
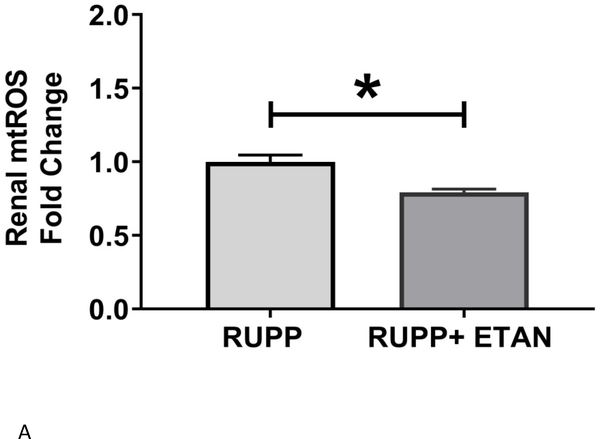
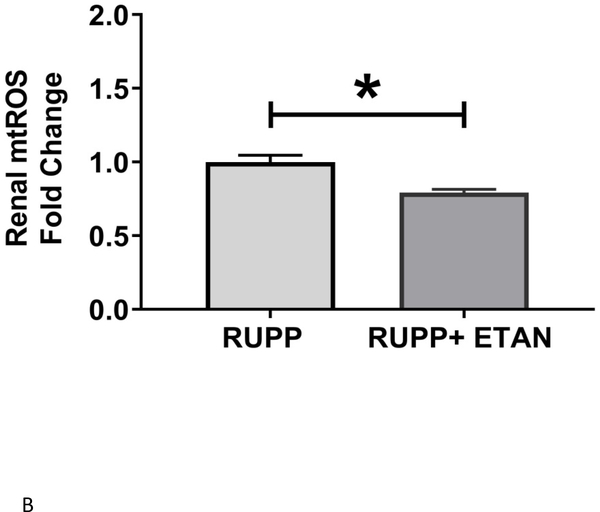
Circulating (5.9 ±1.8 vs 4.0±1.4 % gated) (Figure 3A), renal (3.1 ±0.5 vs 0.9±0.3 % gated, p<0.05) (Figure 3B), and placental (4.8 ±1.3 vs 1.9±0.7 % gated, p=0.055) (Figure 3C) activated NK cells are elevated in RUPP vs. NP rats. ETAN drastically decreased the circulating (1.5 ±0.5 vs 5.9±1.8 % gated, p<0.05) (Figure 3A), renal (0.8 ±0.5 vs 3.1±0.5 % gated, p<0.05) (Figure 3B), and placental (1.7 ±0.7 vs 4.8±1.3 % gated) (Figure 3C) activated NK cells in RUPP rats vs. RUPP. We have previously shown renal and placental respiration and mt ROS to be significantly different in RUPP rats compared to NP rats.(27) Mt ROS in the kidney was reduced by 21% and 29% in placentas in RUPP + ETAN vs RUPP rats; renal-0.79 ±0.02 vs 1.00±0.05 % gated, (p<0.05) (Figure 4A) and placenta0.71±0.06 vs 1.00±0.07 % gated, (p<0.05) (Figure 4B).
Figure 3.
A) Circulating NP (n=13), circulating RUPP (n=9), circulating RUPP + ETAN (n=9). B) Placental NP (n=5), placental RUPP (n=6), placental RUPP + ETAN (n=3). C) Renal NP (n=12), renal RUPP (n=11), renal RUPP + ETAN (n=6). Activated NK cells were quantified using flowcytometry. Statistical differences were achieved using one-way ANOVA with Bonferroni post hoc analysis, *p < 0.05 vs NP and +p < 0.05 vs. RUPP.
Figure 4.
A) Renal and B) Placental mitochondrial oxidative stress was measured in RUPP (n=3) and RUPP + ETAN (n=3) rats. Statistical differences were achieved using student t- test, *p < 0.05 vs NP.
Discussion
In our current study we demonstrate that TNF-α blockade with ETAN not only reduces blood pressure, but lowers circulating, renal, and placental activated NK cells, improves fetal weight to a level that is no longer significantly less than normal pregnant pup weights, and decreases placental and renal mitochondrial oxidative stress in the hypertensive pre-clinical rat model of preeclampsia.
Importantly, this study demonstrates that use of ETAN does not worsen the fetal outcomes that have been demonstrated with placental ischemia. This study also shows that treatment with ETAN improved hypertension, suggesting that it could be used as a potential therapeutic to prolong pregnancies complicated by preeclampsia. RUPP rats are shown to have an increase in circulating, placental, and renal activated NK cells (20, 24) however, it had not been previously demonstrated that ETAN treatment decreased activated NK cells in circulation or in tissues. This novel finding demonstrates that ETAN has a positive effect on the immune response during placental ischemia that improved the clinical characteristics observed in preeclampsia.
Another important finding of our study is that ETAN treatment reduced mt ROS in both the placenta and kidney, supporting a link between NK cell activation and TNF-α causing mt oxidative stress. Granzymes A & B, serine proteases, majorly secreted by activated NK cells, have been shown to cause mitochondrial dysfunction and mt ROS in in vitro as well as in isolated mitochondria. [28, 29]. In fact, Chiusolo et. al., published that granzyme A and B enter mitochondria via Tob55/Sam50 and Tim22 channels located on the outer and inner mitochondrial membranes and go on to cleave Complex I subunits. This ultimately results in mt dysfunction, mt ROS, and apoptosis.(28) (29) Collectively, these studies demonstrate that the ultimate targets of granzymes are located within the mitochondria and can cause mitochondrial injury, dysfunction, and ROS generation.
We have previously, and were the first to reveal, that RUPP rats exhibit mt dysfunction and mt ROS in both placenta and kidney,.(27) We further went on to demonstrate that NK cell activation is one of the mechanisms behind mt oxidative stress in RUPP rats.(18, 21) However, the downstream signaling pathways of NK cell activation that mediate mt dysfunction in our RUPP rats is still yet to be explored.
ETAN is prescribed to treat autoimmune diseases, such as rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis and juvenile rheumatoid arthritis, that is safe for women before pregnancy and during pregnancy (30,31). Treatment with ETAN has been shown to reduce activation of NK cell cytoxicity in patients with rheumatoid arthritis or psoriatic arthritis (RA/PsA)(32). The ability of ETAN to restore NK cell balance has been demonstrated in women with recurrent pregnancy losses (33). In the available literature to date clinical studies indicate no harm to the mother or fetus in women with inflammatory bowel disease taking anti-TNF agents during pregnancy (16,17,30–33). Based on our studies ETAN appears to be a therapeutic target for pre-eclampsia, because it reduces the blood pressure, NK cell activation, which results in improved mt ROS and fetal weight in response to placental ischemia. Furthermore, ETAN during pregnancy has also shown to have no increase in the risk of congenital malformations in infants, one possiblly due to ETAN having the shortest half-life of all TNF-α inhibitors (30). A few studies have shown low levels of ETAN in fetal cord blood and neonatal blood after delivery. It was not present in the fetal blood stream after delivery (31)
In summary ETAN reduced blood pressure, NK cell activation, and mt ROS in placental ischemic rats while improving fetal weight. Thus, ETAN could be used as a potential therapeutic for improving hypertension, inflammation, and mitochondrial oxidative stress during complicated pregnancies.
Acknowledgments
This study was supported by NIH grant RO1HD067541 (BL), NIH grants R00HL130456 and P20GM104357 (DCC), NIH P20GM121334 (BL, LMA), American heart association (AHA) early career award 19CDA34670055 (LMA), and the AHA early career grant award 18CDA34110264 (MWCJr).
Footnotes
No Conflicts of Interest
References
- 1.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37(3):240–9. [DOI] [PubMed] [Google Scholar]
- 2.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40(2):102–11. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15(2 Pt 1):170–5. [DOI] [PubMed] [Google Scholar]
- 4.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52(6):1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46(4):1022–5. [DOI] [PubMed] [Google Scholar]
- 6.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46(1):82–6. [DOI] [PubMed] [Google Scholar]
- 7.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens. 2010;23(8):911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, et al. Identifying immune mechanisms mediating the hypertension during preeclampsia. American journal of physiology Regulatory, integrative and comparative physiology. 2016;311(1):R1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70(2):427–51. [DOI] [PubMed] [Google Scholar]
- 10.Ramseyer VD, Garvin JL. Tumor necrosis factor-alpha: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304(10):F1231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: effect of tumor necrosis factor blockade. J Hypertens. 2011;29(6):1203–12. [DOI] [PubMed] [Google Scholar]
- 12.Lefer AM, Ma XL. Cytokines and growth factors in endothelial dysfunction. Crit Care Med. 1993;21(2 Suppl):S9–14. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Ba ZF, Chaudry IH. Administration of tumor necrosis factor-alpha in vivo depresses endothelium-dependent relaxation. Am J Physiol. 1994;266(6 Pt 2):H2535–41. [DOI] [PubMed] [Google Scholar]
- 14.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52(6):1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alpha. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304(2):R130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, Bonfa E. Immunogenicity of Anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol. 2010;38(2–3):82–9. [DOI] [PubMed] [Google Scholar]
- 17.Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, Ornoy A. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78–84. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham MW Jr., Vaka VR, McMaster K, Ibrahim T, Cornelius DC, Amaral L, et al. Renal natural killer cell activation and mitochondrial oxidative stress; new mechanisms in AT1-AA mediated hypertensive pregnancy. Pregnancy Hypertens. 2019;15:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaka VR, Cunningham MW, Deer E, Franks M, Ibrahim T, Amaral LM, et al. Blockade of endogenous angiotensin II type I receptor agonistic autoantibody activity improves mitochondrial reactive oxygen species and hypertension in a rat model of preeclampsia. American journal of physiology Regulatory, integrative and comparative physiology. 2020;318(2):R256–R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elfarra J, Amaral LM, McCalmon M, Scott JD, Cunningham MW Jr., Gnam A, et al. Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure. Clinical science. 2017;131(23):2753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaka VR, McMaster KM, Cornelius DC, Ibrahim T, Jayaram A, Usry N, et al. Natural killer cells contribute to mitochondrial dysfunction in response to placental ischemia in reduced uterine perfusion pressure rats. American journal of physiology Regulatory, integrative and comparative physiology. 2019;316(5):R441–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham MW Jr., Castillo J, Ibrahim T, Cornelius DC, Campbell N, Amaral L, et al. AT1-AA (Angiotensin II Type 1 Receptor Agonistic Autoantibody) Blockade Prevents Preeclamptic Symptoms in Placental Ischemic Rats. Hypertension. 2018;71(5):886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMarca B, Amaral LM, Harmon AC, Cornelius DC, Faulkner JL, Cunningham MW, Jr., Placental Ischemia and Resultant Phenotype in Animal Models of Preeclampsia. Current hypertension reports. 2016;18(5):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham MW Jr., Williams JM, Amaral L, Usry N, Wallukat G, Dechend R, et al. Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor Enhance Angiotensin II-Induced Renal Vascular Sensitivity and Reduce Renal Function During Pregnancy. Hypertension. 2016;68(5):1308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu F, Bytautiene E, Tamayo E, Gamble P, Anderson GD, Hankins GD, et al. Gender-specific effect of overexpression of sFlt-1 in pregnant mice on fetal programming of blood pressure in the offspring later in life. American journal of obstetrics and gynecology. 2007;197(4):418 e1–5. [DOI] [PubMed] [Google Scholar]
- 26.Elfarra JT, Cottrell JN, Cornelius DC, Cunningham MW Jr., Faulkner JL, Ibrahim T, et al. 17-Hydroxyprogesterone caproate improves T cells and NK cells in response to placental ischemia; new mechanisms of action for an old drug. Pregnancy Hypertens. 2020;19:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaka VR, McMaster KM, Cunningham MW, Jr.,, Ibrahim T, Hazlewood R, Usry N, et al. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension. 2018;72(3):703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiusolo V, Jacquemin G, Yonca Bassoy E, Vinet L, Liguori L, Walch M, et al. Granzyme B enters the mitochondria in a Sam50-, Tim22- and mtHsp70-dependent manner to induce apoptosis. Cell Death Differ. 2017;24(4):747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade F, Roy S, Nicholson D, Thornberry N, Rosen A, Casciola-Rosen L. Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity. 1998;8(4):451–60. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen OH, Loftus EV Jr., Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC medicine. July 31 2013;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. The American journal of gastroenterology. September 2013;108(9):1426–1438. [DOI] [PubMed] [Google Scholar]
- 32.Conigliaro P, Triggianese P, Perricone C, Chimenti MS, Di Muzio G, Ballanti E, et al. Restoration of peripheral blood natural killer and B cell levels in patients affected by rheumatoid and psoriatic arthritis during etanercept treatment. Clin Exp Immunol. 2014;177(1):234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jerzak M, Ohams M, Gorski A, Baranowski W. Etanercept immunotherapy in women with a history of recurrent reproductive failure. Ginekol Pol. 2012;83(4):260–4. [PubMed] [Google Scholar]