Abstract
Non-small cell lung cancer (NSCLC) is the most frequent type of lung cancer accounting up to 80-85% of all lung cancer cases. Gemcitabine (Gem), a pyrimidine nucleoside antimetabolite, is widely used chemotherapy offering several months survival benefit in patients with NSCLC. The emergence of Gem resistance is a main clinical concern in cancer treatment and thus a continuous demand for development of new therapeutic strategies to improve its antitumor activity. Hence, we report an adjuvant therapeutic regimen based on natural compound, gambogic acid (GA) which has been shown to enhanced Gem induced inhibition of cancer cell growth, arrest cell cycle, induce apoptosis by enhanced accumulation of Gem. The in vitro cell viability, clonogenicity, invasion, and migration assays demonstrate a significantly higher therapeutic effect of Gem when it was combined with GA in A549 and H1299 cells. A better access of internalization of drug molecules achieved in Rhodamine 123 assay when GA was used as adjuvant treatment. Further, GA and Gem combination significantly reduced tubular formation of HUVEC cells indicates lowering angiogenesis potential. Microarray and western blot studies confirm that GA+Gem co-treatment strategy promotes cancer cell death by downregulating anti-apoptosis proteins, chemoresistance-associated proteins, and upregulation of apoptosis proteins. More importantly, a significant higher therapeutic benefit was noticed for GA and Gem combination in A549 xenograft mice model. Together, these results offer a rationale to evaluate the clinical translational possibility of GA as adjuvant therapy to overcome Gem resistance. This combination regimen can be a new therapeutic concept to eradicate this devastating disease.
Keywords: Gambogic acid, gemcitabine, chemotherapy, drug resistance, combination therapy
Graphical Abstract

1. Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer accounting for 80-85% of the LC cases (Siegel et al., 2018, 2020). Surgery, radiation, chemotherapy, and immunotherapy are the current therapeutic options for NSCLC (Baker et al., 2016; Baxevanos and Mountzios, 2018; Blakely and Jahan, 2011; Schrank et al., 2018; Zhao et al., 2018). Chemotherapeutic agents, such as, cisplatin, docetaxel, gemcitabine (Gem), irinotecan, paclitaxel, vinorelbine, etc., are the most effective therapeutic regimen for NSCLC (Favaretto et al., 2009; Miller et al., 1995; Sweeney and Sandler, 1998). However, these agents provide good initial response and offer slight increment in overall survival but patients eventually will develop drug resistance (Formisano et al., 2018; Rotow and Bivona, 2017). As a result therapy needs increased drug dosage for effective treatment which offers severe side effects (Chua et al., 2004; MacDonagh et al., 2016; Ramalingam and Belani, 2004; Sekine et al., 2004; Zhao et al., 2018). Thus, drug resistance is a major challenge for NSCLC therapy.
Gem (2′,2′-difluorodeoxycytidine), a pyrimidine analogue of the nucleoside antimetabolite, has been widely used in the treatment of NSCLC (Hayashi et al., 2011; Toschi and Cappuzzo, 2009). Gem exerts its anti-tumor activity through its triphosphate metabolite (dFdCTP) which arrests the DNA replication/synthesis and eventually precedes to apoptosis (Huang and Plunkett, 1995). Gem can increase the objective response rate of patients by 20-25% (4-9 months) with relatively low toxicity profile (Hayashi et al., 2011). Gem chemoresistance also hinders the maximum capacity of Gem therapeutic outcome (d’Amato et al., 2007). In 3,042 NSCLC patient tumor cohort, ~72% tumors found to be either extreme or intermediate resistance against Gem (d’Amato et al., 2006). Intrinsic resistance is caused by metabolic regulation of Gem uptake and other anti-apoptotic pathways (Achiwa et al., 2004; Bergman et al., 2002).
Gambogic acid (GA, chemical formula; C38H44O8), a xanthonoid derived from the resin of Garcinia hanburyi tree (Kale et al., 2018; Zhao et al., 2010). Previous reports have identified GA as a potent apoptosis inducer, an anticancer agent, and inhibitor of tumor growth (Kashyap et al., 2016). GA has shown to be potent molecule with very low half-maximal inhibitory concentrations (IC50) (nM range) against cancer cell lines (Banik et al., 2018; Zhu et al., 2009). GA has apoptotic effects on various cancer cells by modulating different signaling pathway such as MAPK/ERK, PI3K/AKT, and NF-κB (Banik et al., 2018). GA also show chemosensitization effects on various cancer types, by inhibiting P-glycoprotein (P-gp) and suppressing survivin expression (Wang et al., 2015). GA alone and in combination with various therapeutic agents show promising chemosensitization and synergistic therapeutic benefits (Banik et al., 2018; Kashyap et al., 2016). Phase IIa clinical trial was approved by Chinese Food and Drug Administration with GA as single agents for lung cancer (Chi et al., 2013) and other solid tumors (Chi et al., 2013; Wang et al., 2014b).
In this study, we report an innovative combination strategy to induce elevated anticancer effects which may ultimately reduce tumor burden of NSCLC. A chemotherapeutic agent (gemcitabine) and natural compound (gambogic acid) (Fig. 1A) are composited at various ratios in treatment regimen to achieve the synergistic effect of GA with Gem in in vitro assays and in vivo. This superior effect was observed since GA sensitizes NSCLC tumor cells to Gem therapy by inhibiting drug resistance phenomenon in cancer cells while inducing higher apoptosis potential.
Fig. 1. The synergistic inhibitory effect of GA and Gem combination on the cell viability of NSCLC cells.

(A) Chemical structure of GA and Gem. (B-E) Cell viability was assessed by MTT assay and IC50 was calculated. (B) A549 and H1299 NSCLC cell lines and were incubated with different concentration of GA, (C) lung epithelial (BEAS-2B) was incubated with GA and Gem for 48 h, (D-E) A549 and H1299 cell lines were incubated with GA and Gem combination and Gem alone for 48 h. (F) Heat map of combination index using Chou and Talalay method for different concentration ratio of GA and Gem on A549 and H1299 NSCLC. Values represent the mean ± S.E.M. (n =3).
2. Materials and methods
2.1. Chemicals and regents
GA was purchased from Gaia Chemical Corporation (Gaylordsville, CT, USA) and dissolved in in dimethyl sulfoxide (DMSO) and restored in −20 °C and diluted further for usage, where DMSO was less than 0.1% in the final dilutions. Gem was purchased from Fisher Scientific (Waltham, MA, USA) and dissolved in sterile 1X PBS. All the other reagents, solvents, chemicals, and cell culture plastics were obtained from Fisher Scientific (Pittsburgh, PA, USA) and Sigma–Aldrich Co. (St. Louis, MO, USA), unless otherwise stated.
2.2. Cell lines and culture conditions
BEAS-2B (ATCC® CRL-9609™, lung, bronchus epithelial virus transformed, normal cells), HUV-EC-C [HUVEC] (ATCC® CRL-1730™, vascular endothelium normal cells), A549 (ATCC® CCL-185™, about 22 hours, lung epithelial cells derived from lung carcinomatous tissue from a 58-year-old, Caucasian male), and NCI-H1299 (ATCC® CRL-5803™, lung epithelial derived from metastatic site lymph node; male 43 years adult, Caucasian with non-small cell lung cancer) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained as described earlier (Hatami et al., 2018). A549 and H1299 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) and Roswell Park Memorial Institute medium (RPMI-1640) (Gibco, Thermo Fisher Scientific, Grand Island, NY, USA), while BEAS-2B and HUVEC cells were cultured in bronchial epithelial cell growth medium (BEGM) and endothelial cell growth medium-2 (EGM™-2) (Lonza, Morristown, NJ, USA) contained 10% fetal bovine serum (FBS) and 1% (w/v) penicillin–streptomycin (Gibco, Thermo Fisher Scientific). These cell lines were grown in humidified incubator at 37 °C with a 5% CO2 environment (Thermo Fisher Scientific, Waltham, MA, USA). All cell lines were regularly monitored for their typical morphology and contamination under the microscope. These cells in log-phase were trypsinized and seeded for various in vitro studies.
2.3. Cell viability assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell growth assay kit (#CT02, Sigma) was employed to determine cell viability following treatment with GA, Gem, and the combination of GA and Gem in NSCLC cells. While the effect of GA and Gem were also investigated in normal human lung bronchial epithelial cells (BEAS-2B). For this study, cells (5×103) were seeded into 96-well sterile culture plates and left for overnight to attach the plates. Cells were treated with varying concentrations of GA (25, 50, 100, 200, and 400 nM) and Gem (25, 50, 100, and 200 nM) as well as the combination of both GA and Gem for 48 h. Every treatment was performed in triplicate per experiment and each experiment performed three times. After 48 h treatment, 20 μl of MTT solution (5 mg/ml) was added to 100 μl of the culture medium in each well, and plates were incubated at 37 °C for another for 3 h. The culture medium containing MTT solution was discarded and the formed formazan crystals were dissolved in 100 μl DMSO solution in each well for 15 min at room temperature (RT) under shaking. After this step, the absorbance was measured using a microplate reader (Cytation™ 5, BioTek Instruments, Winooski, VT, USA) at 490 nm according to the manufacturer’s instructions. The cell viability of treated cells was normalized to absorbance readings in untreated control cells (considered to have 100% viability). The IC50 calculation was performed using GraphPad Prism 6.07 software. The IC50 was referred as the concentration required for 50% cell growth inhibition. The combination index (CI) was calculated using the Chou–Talalay method (Zhao et al., 2017), as presented below;
Where Ds1 and Ds2 represent the IC50 of GA and Gem, respectively, while Dc1 and Dc2 are the IC50 values of the drugs applied in combination. Combination index designated as: CI < 0.9, synergism, 0.9-1.1, additive effect, >1.1 antagonism, respectively.
2.4. Colony formation
For this assay, A549 and H1299 cells (~300 cells/well) were seeded in 12-well sterile culture plates. Cells were allowed 48 h to initiate colonies (3-4 cells per colony). Then cells were treated with and without 25 and 50 nM GA, 25 and 50 nM Gem, and the combination of both drugs with respective concentrations. The drug-containing medium was renewed every 3 days, for 15 days. After 15 days of incubation, grown colonies were washed with 1X PBS, fixed with pure cold methanol, and stained with 0.05% crystal violet at RT. Images of the colonies were acquired using a ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, USA) (Nagesh et al., 2018b). All colonies were counted electronically using the NIH ImageJ software (www.imagej.nih.gov/ij/).
2.5. Cell migration
Cell migration experiments were performed in Corning® Transwell® 96-well Boyden chambers (8 μm) (#3374, Corning®, NY, USA) with each well separated by microporous membrane (8 μm pores) as per manufacturer’s instructions (Chowdhury et al., 2019). Briefly, starved cells (overnight starvation by culturing in serum-free medium) (5×104 cells/well) were seeded in upper chambers of the plate containing serum-free culture medium. These cells were exposed to 100 nM and 200 nM of GA; 100 nM and 200 nM of Gem; and combination of GA and Gem drugs for 18 h, respectively. Cells without treatment served as controls. Cells were allowed to migrate from upper chambers with serum free medium towards lower chambers with complete medium containing 10% FBS. The migrated cells at the lower side of the inserts were fixed with cold 4% paraformaldehyde for 30 min, rinsed with 1X PBS, and stained with crystal violet for 45 min. Cells in the upper chamber were completely removed using cotton swabs. The experiments were performed in triplicate and three random fields were imaged from replicate wells using a Keyence BZ-X800 microscope (Itasca, IL, USA). The number of migrated cells were quantified using ImageJ software.
2.6. Cell invasion
The ability of cells to invade was evaluated using BioCoat Matrigel Invasion Chambers (BD Biosciences, Bedford, MA, USA) (Chowdhury et al., 2019). After starvation, cells (3.5×104 cells/well) were seeded in the upper chamber of the trans-well plate, containing serum free medium. Cells were left 24 h for adhesion. After 24 h, they were exposed to treatments with (100 and 200 nM) GA, (100 nM and 200 nM) Gem, and combination of both drugs, for 24 h. Cells without treatment served as controls. Cells invaded to the lower chamber were fixed, stained, and imaged and quantified as mentioned above. This experiment was done in triplicate.
2.7. P-gp function by Rhodamine 123 assay
To investigate the effect of GA to facilitate Gem higher accumulation inside the cells for its potential activity, the P-gp function in cancer cells was determined by Rhodamine 123 (Rh123) accumulation (Chowdhury et al., 2019; Nagesh et al., 2018b). Higher accumulation of Rh123 indicator for lower in P-gp function. For this study, A549 and H1299 cells (5×105 cells/well) were cultured in a 6-well plate and left overnight to adhere to the plate. The next day, cells were treated with 100 and 200 nM GA, 100 nM Gem, and the combination treatment. After 24 h of treatment, 2.62 mM Rh123 was added to each well and cells were placed back into the CO2 incubator for 30 min. Cells were then rinsed twice with sterile 1X PBS, in order to wash any undesirable Rh123 dye adhering to the cell surface. Next, the medium was replaced with fresh phenol red-free medium and subjected to imaging using an EVOS® FL Imaging System (AMF4300, Life Technologies, Carlsbad, CA, USA) for qualitative examination. After that, cells were trypsinized and collected in phenol red-free medium which were subjected for quantitative evaluation of extent of Rh123 accumulation by using the NovoCyte Flow Cytometer (ACEA NovoCyte® 1000, ACEA Biosciences, Inc. San Diego, Ca, USA). An FITC channel (fluorescence measurements at λex: 485 nm and λem: 520 nm) was used to measure the mean fluorescence intensity (MFI) of Rh123 dye that was internalized in the cells.
2.8. Cell cycle analysis
For the cell cycle analysis, A549 and H1299 cells (5×105 cells/well) were seeded into 6-well plates for culture overnight. Cells were then treated with 100 and 200 nM GA, 200 nM Gem, and the combination of GA and Gem for 24 h, respectively. These cells were washed with 1XPBS, trypsinized, and collected by centrifugation at 1000 g for 5 min and fixed with 70% pure ethanol and kept at least for 48 h at −20 °C. Cells were washed with 1X PBS and incubated with propidium iodide (PI) and FxCycle™PI/RNase Staining solutions at 37 °C for 1 h in the dark following manufacturer’s instructions. Cell cycle analysis was followed by using a Bio-Rad ZE5 FACS flow cytometer (Bio-Rad Laboratories, Hercules, CA, USA) (Hafeez et al., 2017). Cell cycle distribution and the different cell cycle phase of cells were analyzed by the ModFit software (Verity Software House, USA).
2.9. Apoptosis detection
The dead cell apoptosis kit with Annexin V Alexa Fluor™ 488 and PI (Thermo Fisher Scientific, Pittsburgh, PA, USA) was used to detect apoptosis by flow cytometer. For this study, A549 and H1299 cells (5×105 cells/well) were plated in 6-well plates and cultured overnight. The cells were incubation with 200 nM GA, 200 nM Gem, and 200 nM GA + 200 nM Gem separately for 48 h. These cells were harvested and washed twice in cold 1X PBS and pelleted by centrifugation at 500 g for 10 min. Then resuspended 100 μl in 1X Annexin-binding buffer. Stained with 5 μl Alexa Fluor® 488 Annexin V reagent and 1 μl of 100 μg/ml PI, for each 100 μl of cell suspension and incubated in the dark for 15 min at RT (Hafeez et al., 2017; Wang et al., 2015). They were then mixed gently with 400 μl of binding buffer and kept on ice while immediately processing them with a Bio-Rad ZE5 FACS flow cytometer.
2.10. Western blotting
Protein extractions from A549 cells treated with 200 nM GA, 200 nM Gem, and 200 nM GA+200 nM Gem combination were performed using standard protocols as previously reported (Nagesh et al., 2018a). For the western blotting, equal amounts of cellular protein (40 μg) were denatured in sample lysis buffer and subjected to 4-20% SDS–PAGE. Proteins were transferred from gel to a nitrocellulose membrane using the trans-blot electrophoretic transfer cell containing Tris-glycine buffer, pH 8.3, and methanol, the transfer procedure was performed 0-4 °C for 150 min at 55 V (400 mA). For blocking purposes, 3% BSA was used for 1 h at RT. Nitrocellulose membranes were probed with primary antibodies overnight incubation at 4 °C on a rocker. The following primary antibodies, cleaved caspase 3 (#9665), Cl-PARP (#5625), Bcl-2 (#2872), Bak (#3814), RRM2 (#65939) and β-Actin (#4970) (Cell Signaling Technologies, Danvers, MA, USA) were used. Next, membranes were washed three times with PBST (each time for 5 min) and incubated with respective secondary antibodies for 45 min. Finally, the blots were subjected to immunoreactive proteins on nitrocellulose membrane using Bio-Rad ECL Western Blotting Substrate Solution (Bio-Rad Laboratories, Hercules, CA, USA) (Nagesh et al., 2018a). Blots were imaged using a Bio-Rad computer-based gel imaging instrument with ImageLab™ software (Bio-Rad Laboratories, Hercules, CA, USA).
2.11. Angiogenesis assay
For examining in vitro angiogenesis, the tube formation ability of HUVEC cells were assessed in presence and absence of the treatments (Chen et al., 2003). HUVECs (1×104 cells/well) with passages number less than five, were seeded in pre-coated Matrigel flat-bottomed 96-well plates (Corning, NY, USA). After incubation for 4 h with the medium containing 200 nM GA, 200 nM Gem, and 200 nM GA + 200 nM Gem combination, HUVEC tubular formation was evaluated by phase-contrast at 10X magnification and photographed at 3 random fields by using the EVOS® FL Imaging System. The length of tubules was analyzed inside 2×2 square area at the center of each well (Chen et al., 2003). Each experiment was repeated in triplicate.
2.12. Microarray analysis
The total mRNA from A548 cell line upon treatment with 200 nm GA, 200 nm Gem and 200+200 nm GA+Gem was extracted using RNA Isolation Kit (Qiagen, Inc., Hilden, Germany). Quality and purification steps of mRNA were followed as reported earlier (Nagesh et al., 2018a; Nagesh et al., 2019). The purified mRNA samples were subjected and hybridized to gene microarray studies using Affymterix Clariom S Human gene array or GeneChip® Human gene 2.0 ST array (Affymterix, Santa Clara, CA, USA). Each sample was triplicated for mRNA expression profile. The obtained results were validated iPathway guide analysis (Advaita Corporation, Plymouth, MI, USA) (Nguyen et al., 2016) and the gene expression fold changes between the treatments and no treatment groups were resulted from Impact Analysis (Draghici et al., 2003; Khatri et al., 2007).
2.13. In vivo anti-tumor effects of GA and Gem combination in A549 xenograft mice model
For this pilot study, 12 athymic male nude mice (6-8-week-old) were supplied by Jackson laboratory (Bar Harbor, ME USA) to generate subcutaneous A549 cell line derived xenograft tumors. Mice were housed under specific pathogen-free condition in accordance with the recommendation of the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. The experiments were performed by following a protocol (#18-031.0) approved by Animal Use and Care Administrative Advisory Committee at the University of Tennessee Health Science Center, Memphis, TN, USA. The tumor xenografts were established by injecting 2×107 A549 cells per mouse in 100 μl DMEM medium and Matrigel (Corning Inc., Corning, NY) in 1:1 ratio on the dorsal flank. Mice allowed to develop tumors and tumor volumes were measured using a digital caliper [tumor volumes, V = (0.5238 x L x W x H), where L, W, and H are length, width, and height of the tumor, respectively]. Upon reaching tumor volumes ~ 100 mm3, mice were treated intraperitoneally with (saline), GA (2 mg/kg mice), Gem (20 mg/kg mice), and GA+Gem (2+20 mg/kg mice). Each time the administered dose was maintained in 50 μl total volume. These treatments were twice a week. We used less concentration of GA in this study because higher concentration may lead to significant tumor growth reduction which may not offer to see its chemosensitization potential. After treatments, tumors were excised and formalin fixed and paraffin embedded for immunohistochemistry for testing proliferation potential using PCNA marker (# 8109, Cell Signaling Technologies, Danvers, MA, USA). The extent of immunostaining was viewed under Keyence microscope at 20X magnification.
2.14. Statistical analysis
The GraphPad Prism (GraphPad Software, Inc, La Jolla, CA, USA) software was used for statistical analysis. Data was presented as mean ± standard deviation. To compare the means of pairs of groups, Studen’s t-test was applied, while to compare three or more groups a one-way ANOVA was used. *P < 0.05, **P < 0.01, and ***P < 0.001 was considered statistically significant compared to control group and #P < 0.05 was considered statistically significant compared to GA and Gem treatment groups.
3. Results
3.1. Combination of GA and Gem synergistically reduce the growth of NSCLC cells
The MTT assay showed that 25-400 nM of GA had no significant effect on cell viability of lung cancer (A549 and H1299) (Fig. 1B) and lung epithelial (BEAS-2B) cell lines (Fig. 1C). This confirmed useful concentrations that can be applied in combination therapies without any toxicity on normal cells. GA can sensitize tumor cells to various chemotherapy agents. Thus, the potential for synergy between GA and Gem was evaluated in two NSCLC cell lines through proliferation assay. Gem (0-200 nM) showed dose dependent effects on cell viability (Fig. 1D–E). This effect was significantly enhanced when combined with 25-400 nM of GA treatments. The IC50 of Gem was drastically reduced to 4.4, 2.2, and 0.63 nM with 100, 200, and 400 nM GA treatments in A549 cell lines. It was also followed similar trend observed in reduction of IC50 of Gem to 6.1, 3.6, and 1.2 nM with GA combination treatment in H1299 cells. Such shifts in lowering of the dose–response effects were clearly seen in Fig. 1D–E. This cell viability data was used to calculate the combination index (CI) using the Chou–Talalay (CompuSyn) method. As displayed in (Fig. 1F), the combination of GA and Gem showed a synergetic effect (CI < 1) in both A549 and H1299 cell lines. Moreover, CI values obtained at higher concentration of GA were lower, indicating more of a synergistic effect on cells.
3.2. GA plus Gem treatment promotes the suppression of colony formation, migration and invasion ability of NSCLC cells
Clonogenic assay represents an in vitro quantitative technique to evaluate the ability of a cancer cell to sprout into a larger colony formation by expansion of colonies. The clonogenic potential is an indicator for undifferentiated cancer cells to grow into tumors. Thus, the synergy of GA and Gem combination was tested using long-term colony formation assay and colonies were stained by 0.05% crystal violet for visualization (Fig. 2A). Clones were larger and more prevalent in control wells and the clone size and number of colonies were reduced in the GA or Gem treated groups, later represented graphically (Fig. 2B). Whereas strong inhibitory colony formation was noticed with GA and Gem combination and this effect was being synergistic compared with the single drugs and controls.
Fig. 2. The significant inhibitory effect of GA and Gem combination on the colony formation of NSCLC cells.
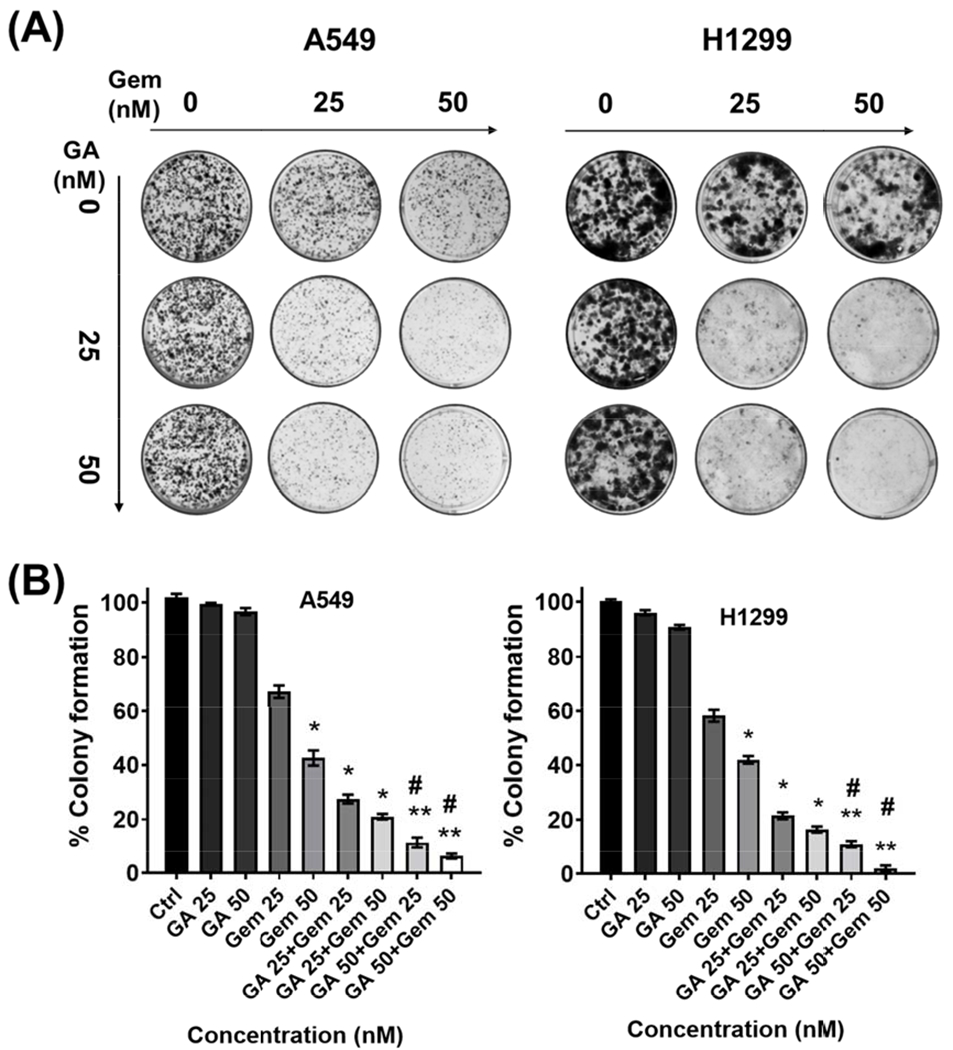
(A) Colony formation capability of NSCLC cells were analyzed for the above-mentioned individual GA and Gem concentration, and their combination for 15 days. (B) Graphical representation of quantitative data analyzed with ImageJ; untreated cells were taken as control (100%). Values represent the mean ± S.E.M. (n=3). The significance level was *P < 0.05 and **P < 0.01 compared to controls cells, and #P < 0.05 compared to their individual drugs.
Migration characteristic of NSCLC cells is crucial for distant metastases. Cancer cell’s ability to reorganize its cytoskeleton and movement is required for invasion. Cancer cells which can’t migrate towards a source of nutrition and invade through an organ may not survive. Therefore, the influence of this new treatment strategy was examined on both migration and invasion properties of NSCLC cells (Fig. 3).
Fig. 3. GA and Gem combination significantly inhibited cell migration and invasion abilities.

(A-B) Migration ability was analyzed using a Transwell assay, migration cell images and graphical representation of quantitative data showing the number of migrated cells. (C-D) The cell invasion abilities were analyzed using a Boyden chamber assay, invasion cell images and graphical representation of quantitative data showing the number of invaded cells. Both the data were analyzed by ImageJ software. Values represent the mean ± S.E.M. (n = 3). The significance level was *P < 0.05 and **P < 0.01 compared to controls cells, and #P < 0.05 compared to their individual drugs.
As shown in Fig. 3A and C, A549 and H1299 cells treated with Gem alone showed a ~50-20% migration and invasion compared to their untreated control groups. GA treatment did not show any inhibitor effects on migration and invasion characteristics of cells. When these cell lines were treated with GA+Gem combination strongly restrict their migration and invasiveness. This reduction in migrated and invaded cell data analyzed by ImageJ software was found to be a synergistic effect of combination treatment than that of control as well as the GA and Gem alone treatment (*P < 0.05, **P < 0.01, and #P < 0.05 Fig. 3B and D) in both cell lines.
3.3. GA and Gem combination reduced P-gp activity in NSCLC cells
To learn possible reason of significant anticancer potential of GA and Gem combination compared to GA or Gem treatments, a P-gp activity assay was applied. In general, less P-gp expression offers more accumulation of drug(s) to act on cells. Rhodamine 123 is a fluorescent dye and acts as P-gp substrate. Thus, it can be used as indicator to evaluate the ability of drug efflux by P-gp transporters. To determine the effect of GA on inhibition of P-gp efflux activity, A549 and H1299 cells were exposed to GA and Gem (alone and in combination). The higher accumulation of Rh123 in cells indicates lower in P-gp expression. As shown in Fig. 4A, cells treated with GA showed higher fluorescence intensity due to Rh123 accumulation in comparison with cells without GA treatment (Fig 4B). Intracellular Rh123 accumulation in NSCLC cells was dose-dependent, as higher GA concentrations showed higher Rh123 accumulation, while lower accumulation was observed with Gem treatment. This may be due to the higher expression of P-gp, which suggest that GA has an inhibitory effect on P-gp efflux activity that resulted in the higher fluoresce inside cells. This result signifies the chemo sensitization ability of GA on NSCLC cells and shows that GA prevents drug efflux from cancer cells by decreasing the function of P-gp.
Fig. 4. Effect of GA on intracellular Rh123 accumulation in A549 and H1299 cells.
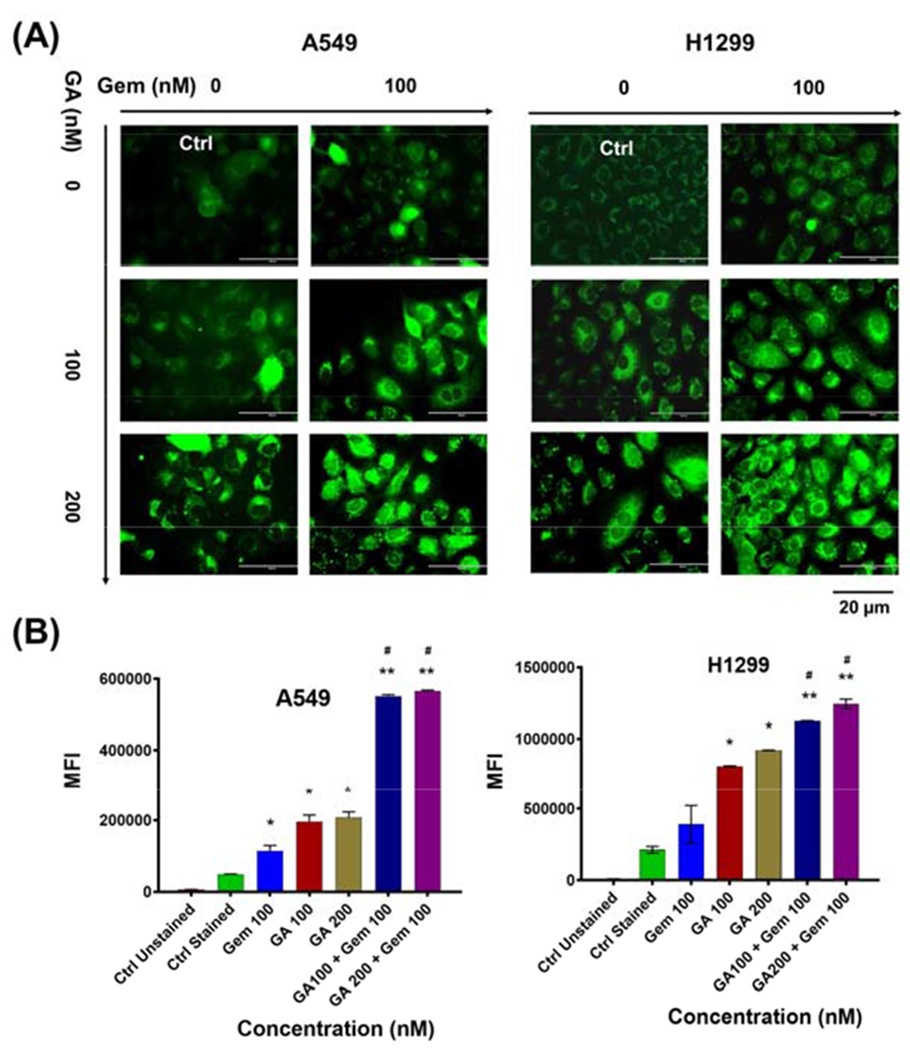
(A) Cells were incubated with medium containing 2.62 mM Rh123 pretreated with GA and Gem with the respective combination dosage for 24 h and without pretreatment. After 30 min, mean fluorescent intensity (MFI) associated with intracellular Rh123 accumulation was assessed. Representative images were presented. (B) A graphical representation of quantitative data analyzed by flow cytometry. Values represent the mean ± S.E.M. (n = 3). The significance level was *P < 0.05 and *P < 0.01 compared to controls cells, and #P < 0.05 compared to GA and Gem.
3.4. Combined GA and Gem treatment induces significant apoptosis in NSCLC cells
Analysis of the cell cycle revealed that GA arrested cells S and G2 phases while Gem treatment arrested cells in S phase cycle. GA and Gem combination treatment related their combined effect on cell cycle distribution (Fig. 5A). The percent of G2 phase arrest remains almost similar around ~40% in both 100 and 200 nM GA treatments. This was observed because the employed GA concentration for treatment was low. To achieve a significant change in cell cycle analysis, at least 500 nm, 1, or 2 μM of GA need to be used (Xia et al., 2017). The combination treatment significantly increased the apoptotic cell population in the cell cycle process compared to treatment with either single agent, in both A549 and H1299 cells. A detailed cell cycle distribution was presented in Table 1.
Fig. 5. Effect of GA and Gem combination on cell cycle and apoptosis induction in NSCLC.

(A) GA and Gem combination increased the apoptosis population on A549 cells, after 24 hr treatment incubation for cell cycle analysis. (B) A549 and H1299 cells were incubated with GA and Gem and respective combination concentrations of GA+Gem for 48 h. The X-axis shows Annexin V-FITC staining and Y-axis indicates Propidium iodide staining; Lower left (LL) quadrant: viable cells; lower right (LR) quadrant: early apoptotic cells; upper left (UL) quadrant: necrotic cells, upper right (UR) quadrant: late apoptotic cells. Data representative of triplicate (n = 3).
Table 1.
Summary of cell cycle distribution of A549 cells.
| Cell cycle | Ctrl | GA 100 | GA 200 | Gem 200 | GA 100+Gem 200 | GA 100+Gem 200 |
|---|---|---|---|---|---|---|
| G0 / G1 | 60.32±1.32 | 21.84 ± 0.65 | 43.25 ± 1.32 | 33.26 ± 0.98 | — | — |
| G2 | 11.34 ± 1.56 | 32.01 ± 0.30 | 16.01 ± 0.85 | 0.15 ± 1.62 | — | — |
| S | 26.87 ± 0.88 | 45.62 ± 1.01 | 41.32 ± 1.32 | 65.32 ± 1.03 | — | — |
| Apoptosis | — | — | — | — | 15.22 ± 0.65 | 26.09 ± 0.74 |
Apoptosis is truly differentiated from necrosis, or accidental cell death, by distinctive morphological changes in conjunction with biochemical changes, as it is known well by specific nuclear and cytoplasmic properties, and loss of membrane alignments and asymmetry. The transference of phosphatidylserine protein (PS) to the outer surface of the cell membrane is one of the primary features of apoptosis, thus in order to distinguish the early or late apoptotic cells, the Annexin-V/PI double staining assay was employed. Additionally, dual staining with PI allows differentiation of early apoptotic cells with undisturbed membranes (positive Annexin V and negative PI) from late apoptotic/necrotic cells with permeable membranes (positive Annexin V and positive PI) and normal cells (negative Annexin V and negative PI). The Annexin-V-Alexa Fluor/PI dual staining assay indicated that GA induced a decrease in the percentage of live cells (bottom left) with a concomitant increase in the percentage of early apoptotic cells (upper left) and late apoptotic cells (upper right) as compared to untreated cells, in a dose-dependent manner (Fig. 5B). GA+Gem cotreatment significantly induced approximately 6% and 18% of early and late apoptotic cells, respectively. A detailed quadrat cell population of this analysis is presented in Table 2.
Table 2.
Summary of cell death analysis (Annexin V-PI) of A549 and H1299 cells.
| A549 | H1299 | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Ctrl | GA | Gem | GA+Gem | Ctrl | GA | Gem | GA+Gem |
| Live Cells | 98.3±0.3 | 95.9±0.4 | 88.5±0.05 | 68.1±0.6 | 98.1±0.5 | 95.9±1.0 | 87.6±0.9 | 68.4±1.0 |
| Annexin V | 1.0±0.1 | 1.4±0.2 | 3.9±1.0 | 5.8±0.6 | 0.8±1.0 | 1.5±0.4 | 5.0 ±0.3 | 7.4±0.5 |
| PI | 0.9±0.3 | 0.7±0.9 | 3.6±0.6 | 5.5±0.1 | 0.4±0.3 | 0.7±0.2 | 4.1±0.8 | 6.1±0.1 |
| Annexin V + PI | 0.4±0.1 | 0.7±0.3 | 3.1±0.9 | 19.3±1.0 | 0.7±1.1 | 0.7±0.1 | 4.2±0.3 | 19.0±1.1 |
3.5. Combination of GA and Gem inhibit tube formation in HUVEC cells
Angiogenesis is another important characteristic that is often linked to the development, progression and drug resistance phenomenon in many cancers including NSCLC. The tube formation assay mimics the in vivo angiogenesis condition that represents the cellular growth in the form of capillary-like and branched networks on the surface of extracellular matrix. Suppressing the angiogenesis and causing damage to the tumor vascular network, leads to the inhibition of tumor growth by abducting supply of oxygen and nutrients. The effect of GA and Gem, either as monotherapy or in combination, was assessed for their ability to inhibit tube formation using a HUVEC line (Pingwara et al., 2017). GA+Gem combination treatment led to significant decrease not only in tube network lengths but their network junction and size than that of the GA or Gem treatments. This can be clearly viewed in phase contrast images (Fig. 6A). Semi-quantitative analysis demonstrates that treatment of 200 nM GA, 100 nM Gem, and their combination (200 nM GA, 100 nM Gem) reduced the total master segments length by ~33, 71, and 97%, respectively, compared to the vehicle control (Fig. 6B). Combining these two compounds achieved the highest inhibition compared to single drugs and the difference was statistically significant.
Fig. 6. Inhibition of tube formation by GA and Gem combination.

HUVEC cells were treated with GA, Gem, and their combination for 4 h and total master segments lengths were measured with ImageJ. (A) Representative images of HUVEC cell line treated and untreated (control). Magnification = 10× (scale bar = 500 μm). (B) Results of three replicates are shown as the mean master segments length (Unit: Pixels) compared to the control. Values represent the mean ± S.E.M. (n =3); *P < 0.05, **P < 0.01, and ***P < 0.001 treatments compared to control groups and #P < 0.05 combination treatment compared to GA and Gem treatments.
3.6. Synergistic mechanisms of GA+Gem in promoting apoptosis in NSCLC cells
The superior anticancer potential of this combination treatment was determined through western blot analysis. The Bcl-2 expression was drastically reduced while Bak was induced during combination than alone GA and Gem treatments. Additionally, the expression of cl-PARP and cl-caspase 3 was profoundly elevated during combinatorial treatments than GA and Gem alone exposure (Fig. 7A). Also, we observed MDR and RRM2 expression was decreased by synergistically by drug combination. Thus, the combination treatment was effective over single drug treatment through synergistic mechanisms in A549 cells.
Fig. 7. Molecular effects of combination treatment.

(A) Western blot analysis confirms the significant induction of apoptotic signaling proteins after the treatment with GA+Gem compare to alone drugs and control. Protein extractions from A549 cells treated with different concentrations of 200 nM GA and 200 nM Gem, and equivalent combination of these two agents, cl-PARP and cl-caspase 3 indicates cleaved PARP and cleaved caspase 3, respectively, β-actin was probed for as internal equal protein control. (B-E) Heap map analysis of the differential expression of genes in A549 cells after the GA, Gem and GA+Gem treatments. (B) Apoptotic signaling, (C) ABC family/Nuclear translocation signaling, (D) EMT signaling, and (E) Angiogenic signaling. Data represented from triplicates.
3.7. Microarray analysis confirms potential synergistic action of GA and Gem combination
Microarray analysis contributes to the expression of thousands of genes simultaneously in cells. In fact, this precise gene expression data can establish a scoring system to predict synergy of GA+Gem therapy in NSCLC. Apparently, the microarray data collectively validated the in vitro functional assays and western blot studies. A detailed analysis indicated that up-regulation of genes associated to apoptosis while down-regulation of genes belongs to pro-survival, angiogenic, metastasis, and drug resistance associated, in GA+Gem treated A549 cells compared to GA/Gem or vehicle treated cells. Fig. 7B–E summarizes these results in the form of heat maps utilizing heatmapper software (University of Alberta, Edmonton, AB, Canada). Altogether, microarray data confirms that GA+Gem combination treatment indeed enhance Gem-induced anticancer activity in NSCLC cells via downregulation of drug-resistance phenomenon while inducing excessive apoptosis features.
3.8. GA and Gem combination significantly reduce tumor burden in A549 xenograft mouse model
Considering our in vitro results, we anticipate that Gem with a combination treatment of GA may cause increased therapeutic activity in A549 NSCLC xenograft mouse model. Up on reaching a volume of 100-150 mm3, mice were randomly assigned and treated with a combination of GA and Gem. Since GA is used as a dietary supplement with chemosensitization and anticancer activity, we proposed a low concentration as possible (2 mg/kg mice). Treatments were performed twice a week for 4-6 weeks and tumor growth was measured using digital Vernier calipers. As anticipated, the pilot combination therapy (GA+Gem) resulted in a significantly lower tumor growths in mice compared with monotherapies (GA or Gem) and control mice (Fig. 8). A negligible tumor growth was observed with combination treatment compared to their individual treatment (Fig 8B). Interestingly, dissected tumors show visible blood vessels in control, GA, and Gem treatment groups whereas it was significantly lower in GA+Gem combination treatment (Fig. 8C). This suggest possible role in reducing angiogenesis with the combination treatment. Altogether, GA+Gem combination therapy induce synergistic and superior anti-tumor benefit in NSCLC. To observe GA+Gem combination effect on tumor growth, a PCNA was probed in IHC study (Fig. 8D). It clearly indicates that PCNA marker staining is minimal in GA+Gem (~27%) combination while control (~95%), GA (~86%) and Gem (~42%) treatments exhibits significant amount of PCNA staining.
Fig. 8. GA+Gem combination treatment significantly induce anticancer efficacy in A549 tumor xenografts.

(A) Representative images of A549 tumor bearing male mice treated with control vehicle (no therapy), GA (2 mg/kg mice), Gem (20 mg/kg mice), and GA+Gem (2+20 mg/kg mice), respectively. Treatments were given twice a week for 3-4 weeks. Arrow represents starting of administration time. (B) Graphical representation of tumor volumes (mm3) as a function of time in control, GA, Gem, and GA+Gem treatments mice bearing A549 NSCLC tumors. Values represent the mean ± S.E.M. (n =3); The significance level was *P < 0.05 and **P < 0.01 compared to controls cells, and #P < 0.05 compared to GA and Gem. (C) Representative tumor images of treatment groups indicating apparent change in angiogenic blood vessels with combination treatment. (D) Immunohistochemistry images of mice tumors upon treatment. A greater reduction of PCNA staining in GA+Gem indicate cancer cell proliferation inhibition.
4. Discussion
Non-small cell lung cancer is the most commonly diagnosed (approximately 228,820) and exhibiting a high annual mortality about 23% (72,500 cancer deaths) annually in the United States (Siegel et al., 2020). NSCLC still incurable and treatments have aided to inhibit the tumor growth that helps to prolong survival. Chemotherapy is considered as the promising treatment option for patients with NSCLC, either alone or in combination with resection or radiotherapy. Clinical treatment regiments often include platinum, cisplatin, doxorubicin, 5-fluorouracil, paclitaxel, docetaxel, Gem, etc. for lung cancer (Lee, 2019). Gem is a cornerstone and widely employed chemotherapeutic agent to improve survival of lung cancer patients with minimal known systemic toxicity over other chemotherapeutic agents. Multiple studies advice that Gem treatment exhibited 16–38.5% response rate with 6.6–7.7 months overall survival. Therapeutic efficacy of Gem needs further improvement. Clinically, Gem-related resistance often noticed as a prime impediment in lung cancer treatment. The present study aimed to assess a clinically relevant chemo sensitization approach which can potentiate Gem induced anticancer efficacy.
GA, a caged xanthone natural compound, is known for its potent anti-cancer and chemosensitization properties. To date a couple of studies have already shown that the anticancer effect of GA on NSCLC (Zhao et al., 2017). Collective literature evidence demonstrates that GA in combination with adriamycin, cisplatin, doxorubicin, 5-fluorouracil, and gefitinib, promotes therapeutic benefit via sensitizing cancer cells (Kale et al., 2018). However, GA’s chemosensitization effect on Gem therapy in NSCLC has not been examined. In this study, we investigate the role of GA as synergistic chemosensitizer to potentiate Gem therapeutic efficacy and can result in reduced resistance and enhanced anti-cancer potential of Gem both in vitro (Fig. 1–7) and in vivo (Fig. 8).
Our study for the time reports an enhanced anticancer effect of GA+Gem combination on NSCLC. The results of the cell viability assay reveled that cotreatment of GA and Gem on A549 and H1299 inhibited proliferation in a dose-dependent manner (Fig 1). The combination of GA and Gem resulted in synergistic growth inhibition of A549 and H1299 cells which is validated with the Chou–Talalay method (Fig. 1) (Wang et al., 2014a; Zhao et al., 2017; Zhu et al., 2019). This was confirmed through combination indexes that were lower than 0.9 (CI<0.9). It is noteworthy to mention that the GA concentrations chosen in the present study were not toxic on normal and cancer cells (Fig. 1B). But GA promotes Gem activity against cancer cells. Furthermore, our data indicates an inhibitory effect on the number of colonies, the invasive, and migratory properties of cell lines (Fig. 2–3), in the presence of the combination treatments in a synergistic manner. A similar reduction of tumor growth with GA and Gem combination was noticed in a pilot study in xenograft mouse model (Fig. 8). Such chemosensitization effect of GA observed in our studies concurs with findings from previous investigation with GA and other chemotherapeutic agents such as doxorubicin and 5-fluorouracil on ovarian cancer (Wang and Yuan, 2013) and colorectal respectively (Wei et al., 2017). Altogether, this advice that the Gem concentration required for its efficient function is reduced enormously with the use of GA as adjuvant in the treatment. The dose of Gem or number of treatment cycles can be minimized with such combination treatment regimen.
GA has ability to accelerate apoptosis in cancer cells. Previous finding suggests that agents modulating and promoting G2 phase arrest strengthen the cytotoxicity effect of agents (Xia et al., 2017). Our cell cycle analysis revealed that the G2 cell population increased in response to an increase in the dose of GA and induced increased S phase arrest with Gem combination which acts on DNA (the main mechanism by which Gem causes cell death is via apoptosis). This suggests our combination strategy inhibited the growth and proliferation of NSCLC cells by inducing apoptosis via promoting cell cycle arrest (Fig. 5A). This phenomenon was further proved higher apoptotic population in co-treated cells through Annexin-V/propidium iodide staining data (Fig. 5B).
Chemoresistance in cancer cells is often developed by the overexpression of ATP-binding cassette (ABC) efflux transporters such as the P-glycoprotein (P-gp/ABCB1) (Wang et al.,2014a). Gem resistance is considered to be consequence of the reduction in drug concentration in the cancer cells due to either drug efflux or decrease in drug influx (Rudin et al., 2011; Xue and Liang, 2012). A previous study showed the dependency of reducing P-glycoprotein transport expression for reversing the Gem resistance (Bergman et al., 2003; Wu et al., 2008). Our data showed that GA and Gem combination treatment increased the Rh 123 accumulation in cancer cells which is an indication for feasibility of higher access to the drug transport (Fig. 4). This is further confirmed by down-regulation in MDR1/ABCB1 protein (Fig. 7A). Earlier studies proved that GA can enhance the drug intake by suppression of P-gp efflux activity (Wang et al., 2015). The prime anticancer mechanism of action of Gem is inhibiting DNA synthesis (Toschi et al., 2005). This is achieved indirectly by suppressing the ribonucleotide reductase (RR) enzyme activity. RR enzyme converts ribonucleotide 5’-diphosphates to deoxyribonucleotide 5’-diphosphates, one of the essential steps in DNA replication (Bergman et al., 2002). Moreover, the RR enzyme is responsible for cell cycle process regulation (Montano et al., 2017). The high levels of the subunits of RR expression not only indicate the poor prognosis (Grossi et al., 2015) and Gem resistance (Achiwa et al., 2004; Jia and Xie, 2015) in lung cancer. Previous studies have shown that low expression of two subunits of the RR enzyme relates to the sensitivity to Gem (Nakano et al., 2007). Our co-treatment showed a significant decrease in the expression level of RRM2 (the catalytic subunit of the RR enzyme). We determined that this synergistical effect cause is due to intrinsic differences in molecular mechanism and leads to apoptosis induction and reverse the multi-drug resistance. Gem therapy resistance was observed by high expression of Bcl-2 associated proteins which is also apoptosis defection. Our study delineates that GA able to decrease the expression anti-apoptosis proteins such as Bcl-2 indicates possibility to enhance the sensitivity of NSCLC cells to Gem therapy (Fig. 7A). Further, proteomic profiling further supports elevated levels of apoptosis in cells subjected to simultaneous co-treatment with both drugs was associated with upregulation of pro-apoptotic proteins such as Bax, cleaved PARP and cleaved caspase-3 (known key markers for apoptosis regulation). Similar, previous study done on GA a potent apoptotic inducer, demonstrated downregulation of Bcl-2 and Bax expression on platinum resistance NSCLC (Luo et al., 2019).
Microarray analysis reports the expression of thousands of genes simultaneously. It can be used to delineate outperform of GA+Gem combination synergy over their single drug counterparts. This further confirms the treatment action, either an additive or synergistic. Our microarray data predicted that A549 cells were indeed very sensitive to the GA+Gem combination treatments over the single compounds (GA/Gem) (Fig. 7B–E). Through microarray analysis, we affirmed the combinatorial treatments were influential regulated over angiogenic, metastatic and ABC family of proteins besides the activation of apoptosis in NSCLC cells. From the literature and our gene analysis, GA plays a critical role in combination or adjuvant therapy for reducing the occurrence of NSCLC.
NSCLC can be fatal when it becomes metastatic disease. Angiogenesis is the biological process that involves development of new blood vessel growth commonly observed in cancer and metastasis disease. This process also enables the excessive supplementation of necessary oxygen and nutrients for growth of cancer cells. Thus, inhibition of such process is clinically viable treatment options, for example, bevacizumab and ramucirumab. However, these treatments often associated with severe side effects: hypertension and risk of stroke. Our treatment strategy indicates an elevated inhibitory effect on endothelial HUVEC cells tube formation (Fig. 6). A similar observation was made on angiogenesis inhibition effects of GA (Wan et al., 2019; Yi et al., 2008).
Various reports presented GA significantly enhances Gem anti-cancer potential in different ways (Hatami et al., 2020). It may be possible due to bridged cased structure of GA play a key role to encapsulate amino groups of Gem (Ren et al., 2011) thus deliver Gem in an efficient manner to cancer cells. Other version is that GA possibly target cancer cells by various pathways which possibly support such improved Gem activity (Banik et al., 2018). Therefore, an appropriate ratio of GA and Gem can efficiently serve as combination regimen for efficiently control the proliferation of tumor cells. However, our future interest of this combination study is to use a nanoparticle formulation of GA for targeted delivery and sensitize to Gem chemotherapy (Hatami et al., 2020).
The prognosis for patients with NSCLC, particularly those with metastasis disease remains poor. Overall chemotherapy outcome was not satisfactory in NSCLC. Although, Gem treatments revealed 16–38.5% response rate with 6.6–7.7 months overall survival benefit. Considering such limited efficacy of this treatment primarily may be due to both intrinsic and acquired resistance to the therapy and dose-limiting side effects. All these supports further new efficient adjuvant or drug combination therapies are urgently needed. GA synergistically promotes therapeutic effects of Gem in multiple in vitro assays. Further, our results demonstrated GA co-treatment improves the effects of Gem in A549 derived xenograft tumor mice (Fig. 8). Altogether, this study suggests that GA can act as an adjunct therapy along with Gem for NSCLC by inhibiting growth, clonogenicity, metastasis, angiogenesis potential of NSCLC cells (Scheme 1).
Scheme 1.

Schematic representation of adjuvant therapy of GA for enhancing Gem therapy in NSCLC.
Overall, as the concentration GA (2 mg/kg) used in this combinatorial dosage is relatively low. In many studies, 10-30 mg GA/kg is being used for therapeutic benefit. Besides, GA has favorable safety and therapeutic profile (Hatami et al., 2020) in lung cancer. We also did not observe any signs of cytotoxicity due to this combinatorial dosage since we have not found major weight loss of mice and the vital organs. However, additional studies will delineate its superior anti-cancer potential of this combination regimen. In the future, we would consider delineating the role and validation of GA in Gem-resistance cell line and tumor mice models. Together, these results promote possibility to implement GA as an adjuvant treatment module to improve the therapeutic benefit of Gem in NSCLC patients.
5. Conclusion
This study reports a new therapeutic regimen based on a combination of a natural compound and Gem. GA and Gem combination treatment demonstrated synergistic anti-tumor effects through various functional assays in NSCLC cells. Our results confirmed that GA has potential to potentiate Gem-induced cell cycle arrest and cellular apoptosis. Co-treatment strategy enhanced the anti-tumor effects of Gem by downregulating MDR1, RRM1, and anti-apoptosis protein (Bcl-2) and upregulation of apoptosis proteins (Bax) expression. Importantly, in vivo evaluation in xenograft tumor mouse model revealed enhanced efficiency of Gem by GA co-treatment. Overall, this study suggests that GA can act as an adjunct therapy along with Gem for NSCLC. Further investigations are warranted in large scale animal models to establish this as an efficient combination regimen for NSCLC.
Acknowledgments
The study was partially supported by the National Institute of Health of United States of America (K22 CA174841, R01 CA210192, R01 CA206069, and R01 CA204552), Faculty Stat up fund from UTRGV (to M.M.Y., M.J., and S.C.C.), and Herb Kosten Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Achiwa H, Oguri T, Sato S, Maeda H, Niimi T, Ueda R, 2004. Determinants of sensitivity and resistance to gemcitabine: the roles of human equilibrative nucleoside transporter 1 and deoxycytidine kinase in non-small cell lung cancer. Cancer Sci 95, 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S, Dahele M, Lagerwaard FJ, Senan S, 2016. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat Oncol 11, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik K, Harsha C, Bordoloi D, Lalduhsaki Sailo B, Sethi G, Leong HC, Arfuso F, Mishra S, Wang L, Kumar AP, Kunnumakkara AB, 2018. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett 416, 75–86. [DOI] [PubMed] [Google Scholar]
- Baxevanos P, Mountzios G, 2018. Novel chemotherapy regimens for advanced lung cancer: have we reached a plateau? Ann Transl Med 6, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman AM, Pinedo HM, Peters GJ, 2002. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine). Drug Resist Updat 5, 19–33. [DOI] [PubMed] [Google Scholar]
- Bergman AM, Pinedo HM, Talianidis I, Veerman G, Loves WJ, van der Wilt CL, Peters GJ, 2003. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br J Cancer 88, 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely C, Jahan T, 2011. Emerging antiangiogenic therapies for non-small-cell lung cancer. Expert Rev Anticancer Ther 11, 1607–1618. [DOI] [PubMed] [Google Scholar]
- Chen X, Beutler JA, McCloud TG, Loehfelm A, Yang L, Dong HF, Chertov OY, Salcedo R, Oppenheim JJ, Howard OM, 2003. Tannic acid is an inhibitor of CXCL12 (SDF-1alpha)/CXCR4 with antiangiogenic activity. Clin Cancer Res 9, 3115–3123. [PubMed] [Google Scholar]
- Chi Y, Zhan XK, Yu H, Xie GR, Wang ZZ, Xiao W, Wang YG, Xiong FX, Hu JF, Yang L, Cui CX, Wang JW, 2013. An open-labeled, randomized, multicenter phase IIa study of gambogic acid injection for advanced malignant tumors. Chin Med J (Engl) 126, 1642–1646. [PubMed] [Google Scholar]
- Chowdhury P, Nagesh PKB, Hatami E, Wagh S, Dan N, Tripathi MK, Khan S, Hafeez BB, Meibohm B, Chauhan SC, Jaggi M, Yallapu MM, 2019. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J Colloid Interface Sci 535, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua YJ, Steer C, Yip D, 2004. Recent advances in management of small-cell lung cancer. Cancer Treat Rev 30, 521–543. [DOI] [PubMed] [Google Scholar]
- d’Amato TA, Landreneau RJ, McKenna RJ, Santos RS, Parker RJ, 2006. Prevalence of in vitro extreme chemotherapy resistance in resected nonsmall-cell lung cancer. Ann Thorac Surg 81, 440–446; discussion 446–447. [DOI] [PubMed] [Google Scholar]
- d’Amato TA, Landreneau RJ, Ricketts W, Huang W, Parker R, Mechetner E, Yu IR, Luketich JD, 2007. Chemotherapy resistance and oncogene expression in non-small cell lung cancer. J Thorac Cardiovasc Surg 133, 352–363. [DOI] [PubMed] [Google Scholar]
- Draghici S, Khatri P, Bhavsar P, Shah A, Krawetz SA, Tainsky MA, 2003. Onto-Tools, the toolkit of the modern biologist: Onto-Express, Onto-Compare, Onto-Design and Onto-Translate. Nucleic Acids Res 31, 3775–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaretto A, Pasello G, Magro C, Schettino C, Gridelli C, 2009. Second and third line treatment in non-small cell lung cancer. Crit Rev Oncol Hematol 71, 117–126. [DOI] [PubMed] [Google Scholar]
- Formisano L, Jansen VM, Marciano R, Bianco R, 2018. From Biology to Therapy: Improvements of Therapeutic Options in Lung Cancer. Anticancer Agents Med Chem 18, 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi F, Dal Bello MG, Salvi S, Puzone R, Pfeffer U, Fontana V, Alama A, Rijavec E, Barletta G, Genova C, Sini C, Ratto GB, Taviani M, Truini M, Merlo DF, 2015. Expression of Ribonucleotide Reductase Subunit-2 and Thymidylate Synthase Correlates with Poor Prognosis in Patients with Resected Stages I-III Non-Small Cell Lung Cancer. Dis Markers 2015, 302649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez BB, Ganju A, Sikander M, Kashyap VK, Hafeez ZB, Chauhan N, Malik S, Massey AE, Tripathi MK, Halaweish FT, Zafar N, Singh MM, Yallapu MM, Chauhan SC, Jaggi M, 2017. Ormeloxifene Suppresses Prostate Tumor Growth and Metastatic Phenotypes via Inhibition of Oncogenic beta-catenin Signaling and EMT Progression. Mol Cancer Ther 16, 2267–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatami E, Jaggi M, Chauhan SC, Yallapu MM, 2020. Gambogic acid: A shining natural compound to nanomedicine for cancer therapeutics. Biochim Biophys Acta Rev Cancer, 188381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatami E, Nagesh PKB, Chowdhury P, Chauhan SC, Jaggi M, Samarasinghe AE, Yallapu MM, 2018. Tannic Acid-Lung Fluid Assemblies Promote Interaction and Delivery of Drugs to Lung Cancer Cells. Pharmaceutics 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Kurata T, Nakagawa K, 2011. Gemcitabine: efficacy in the treatment of advanced stage nonsquamous non-small cell lung cancer. Clin Med Insights Oncol 5, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Plunkett W, 1995. Induction of apoptosis by gemcitabine. Semin Oncol 22, 19–25. [PubMed] [Google Scholar]
- Jia Y, Xie J, 2015. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes Dis 2, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale VP, Gilhooley PJ, Phadtare S, Nabavizadeh A, Pandey MK, 2018. Chapter 8 - Role of Gambogic Acid in Chemosensitization of Cancer, in: Bharti AC, Aggarwal BB (Eds.), Role of Nutraceuticals in Cancer Chemosensitization. Academic Press, pp. 151–167. [Google Scholar]
- Kashyap D, Mondal R, Tuli HS, Kumar G, Sharma AK, 2016. Molecular targets of gambogic acid in cancer: recent trends and advancements. Tumour Biol 37, 12915–12925. [DOI] [PubMed] [Google Scholar]
- Khatri P, Draghici S, Tarca AL, Hassan SS, Romero R, 2007. A System Biology Approach for the Steady-State Analysis of Gene Signaling Networks. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 32–41. [Google Scholar]
- Lee SH, 2019. Chemotherapy for Lung Cancer in the Era of Personalized Medicine. Tuberc Respir Dis (Seoul) 82, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu L, Zhao M, Liu Q, Cheng Z, Zou J, Yao P, Gao C, Wei J, Ung COL, Wang S, Zhong Z, Wang Y, 2019. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med 14, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonagh L, Gray SG, Breen E, Cuffe S, Finn SP, O’Byrne KJ, Barr MP, 2016. Lung cancer stem cells: The root of resistance. Cancer Lett 372, 147–156. [DOI] [PubMed] [Google Scholar]
- Miller VA, Rigas JR, Grant SC, Pisters KM, Kris MG, 1995. New chemotherapeutic agents for non-small cell lung cancer. Chest 107, 306S–311S. [DOI] [PubMed] [Google Scholar]
- Montano R, Khan N, Hou H, Seigne J, Ernstoff MS, Lewis LD, Eastman A, 2017. Cell cycle perturbation induced by gemcitabine in human tumor cells in cell culture, xenografts and bladder cancer patients: implications for clinical trial designs combining gemcitabine with a Chk1 inhibitor. Oncotarget 8, 67754–67768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesh P, Hatami E, Chowdhury P, Kashyap V, Khan S, Hafeez B, Chauhan S, Jaggi M, Yallapu M, 2018a. Tannic Acid Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Prostate Cancer. Cancers 10, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesh PKB, Chowdhury P, Hatami E, Boya VKN, Kashyap VK, Khan S, Hafeez BB, Chauhan SC, Jaggi M, Yallapu MM, 2018b. miRNA-205 Nanoformulation Sensitizes Prostate Cancer Cells to Chemotherapy. Cancers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesh PKB, Chowdhury P, Hatami E, Kumari S, Kashyap VK, Tripathi MK, Wagh S, Meibohm B, Chauhan SC, Jaggi M, Yallapu MM, 2019. Cross linked polyphenol-based drug nano-self assemblies engineered to blockade prostate cancer senescence. ACS Appl Mater Interfaces. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y, 2007. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer 96, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Diaz D, Tagett R, Draghici S, 2016. Overcoming the matched-sample bottleneck: an orthogonal approach to integrate omic data. Scientific Reports 6, 29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingwara R, Witt K, Ulewicz K, Mucha J, Tonecka K, Pilch Z, Taciak B, Zabielska-Koczyws K, Mori M, Berardozzi S, Botta B, Rygiel T, Krol M, 2017. Interferon lambda 2 promotes mammary tumor metastasis via angiogenesis extension and stimulation of cancer cell migration. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society 68, 573–583. [PubMed] [Google Scholar]
- Ramalingam S, Belani CP, 2004. State-of-the-art chemotherapy for advanced non-small cell lung cancer. Semin Oncol 31, 68–74. [DOI] [PubMed] [Google Scholar]
- Ren Y, Yuan C, Chai HB, Ding Y, Li XC, Ferreira D, Kinghorn AD, 2011. Absolute configuration of (−)-gambogic acid, an antitumor agent. Journal of natural products 74, 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotow J, Bivona TG, 2017. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 17, 637–658. [DOI] [PubMed] [Google Scholar]
- Rudin D, Li L, Niu N, Kalari KR, Gilbert JA, Ames MM, Wang L, 2011. Gemcitabine Cytotoxicity: Interaction of Efflux and Deamination. J Drug Metab Toxicol 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank Z, Chhabra G, Lin L, Iderzorig T, Osude C, Khan N, Kuckovic A, Singh S, Miller RJ, Puri N, 2018. Current Molecular-Targeted Therapies in NSCLC and Their Mechanism of Resistance. Cancers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine I, Yamamoto N, Kunitoh H, Ohe Y, Tamura T, Kodama T, Saijo N, 2004. Treatment of small cell lung cancer in the elderly based on a critical literature review of clinical trials. Cancer Treat Rev 30, 359–368. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2018. Cancer statistics, 2018. CA Cancer J Clin 68, 7–30. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2020. Cancer statistics, 2020. CA Cancer J Clin 70, 7–30. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Sandler AB, 1998. Treatment of advanced (stages III and IV) non-small-cell lung cancer. Curr Probl Cancer 22, 85–132. [DOI] [PubMed] [Google Scholar]
- Toschi L, Cappuzzo F, 2009. Gemcitabine for the treatment of advanced nonsmall cell lung cancer. Onco Targets Ther 2, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F, 2005. Role of gemcitabine in cancer therapy. Future Oncol 1, 7–17. [DOI] [PubMed] [Google Scholar]
- Wan L, Zhang Q, Wang S, Gao Y, Chen X, Zhao Y, Qian X, 2019. Gambogic acid impairs tumor angiogenesis by targeting YAP/STAT3 signaling axis. Phytother Res 33, 1579–1591. [DOI] [PubMed] [Google Scholar]
- Wang J, Yuan Z, 2013. Gambogic acid sensitizes ovarian cancer cells to doxorubicin through ROS-mediated apoptosis. Cell Biochem Biophys 67, 199–206. [DOI] [PubMed] [Google Scholar]
- Wang LH, Li Y, Yang SN, Wang FY, Hou Y, Cui W, Chen K, Cao Q, Wang S, Zhang TY, Wang ZZ, Xiao W, Yang JY, Wu CF, 2014a. Gambogic acid synergistically potentiates cisplatin-induced apoptosis in non-small-cell lung cancer through suppressing NF-kappaB and MAPK/HO-1 signalling. Br J Cancer 110, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Yang JY, Yang SN, Li Y, Ping GF, Hou Y, Cui W, Wang ZZ, Xiao W, Wu CF, 2014b. Suppression of NF-kappaB signaling and P-glycoprotein function by gambogic acid synergistically potentiates adriamycin -induced apoptosis in lung cancer. Curr Cancer Drug Targets 14, 91–103. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang L, Chen M, Wang Y, 2015. Gambogic acid sensitizes resistant breast cancer cells to doxorubicin through inhibiting P-glycoprotein and suppressing survivin expression. Chem Biol Interact 235, 76–84. [DOI] [PubMed] [Google Scholar]
- Wei J, Yang P, Li W, He F, Zeng S, Zhang T, Zhong J, Huang D, Chen Z, Wang C, Chen H, Hu H, Cao J, 2017. Gambogic acid potentiates the chemosensitivity of colorectal cancer cells to 5-fluorouracil by inhibiting proliferation and inducing apoptosis. Exp Ther Med 13, 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CP, Calcagno AM, Ambudkar SV, 2008. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol 1, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Wang H, Song Z, Meng Q, Huang X, Huang X, 2017. Gambogic acid sensitizes gemcitabine efficacy in pancreatic cancer by reducing the expression of ribonucleotide reductase subunit-M2 (RRM2). J Exp Clin Cancer Res 36, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Liang XJ, 2012. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer 31, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, Liu M, 2008. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res 68, 1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ren D, Liu H, Chen J, 2018. Comparison and discussion of the treatment guidelines for small cell lung cancer. Thorac Cancer 9, 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhen C, Wu Z, Hu R, Zhou C, Guo Q, 2010. General pharmacological properties, developmental toxicity, and analgesic activity of gambogic acid, a novel natural anticancer agent. Drug Chem Toxicol 33, 88–96. [DOI] [PubMed] [Google Scholar]
- Zhao T, Wang HJ, Zhao WW, Sun YL, Hu LK, 2017. Gambogic acid improves nonsmall cell lung cancer progression by inhibition of mTOR signaling pathway. Kaohsiung J Med Sci 33, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Jiang Y, Wu H, Shi W, Lu G, Cong D, Liu K, Song S, Ren J, 2019Gambogic Acid Shows Anti-Proliferative Effects on Non-Small Cell Lung Cancer (NSCLC) Cells by Activating Reactive Oxygen Species (ROS)-Induced Endoplasmic Reticulum (ER) Stress-Mediated Apoptosis. Med Sci Monit 25, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhang H, Lin Y, Chen P, Min J, Wang Z, Xiao W, Chen B, 2009. Mechanisms of gambogic acid-induced apoptosis in non-small cell lung cancer cells in relation to transferrin receptors. J Chemother 21, 666–672. [DOI] [PubMed] [Google Scholar]


