Abstract
Purpose
The gut–brain axis could be a possible key factor in the pathophysiology of anorexia nervosa. The neuropeptide peptide YY3–36, secreted by endocrine L cells of the gastrointestinal tract, is a known regulator of appetite and food intake. The objective of this study was to investigate peptide YY3–36 plasma concentrations at different stages of anorexia nervosa in a combined cross-sectional and longitudinal design to differentiate between effects of acute undernutrition and more enduring characteristics.
Methods
We measured fasting plasma peptide YY3–36 concentrations in young patients with acute anorexia nervosa (n = 47) and long-term recovered patients (n = 35) cross-sectionally in comparison to healthy control participants (n = 58), and longitudinally over the course of inpatient treatment. Physical activity was controlled as it may modulate peptide YY secretion.
Results
There was no group difference in peptide YY3–36 concentration among young acutely underweight anorexia nervosa patients, long-term recovered anorexia nervosa patients, and healthy control participants. Longitudinally, there was no change in peptide YY3–36 concentration after short-term weight rehabilitation. For acute anorexia nervosa patients at admission to treatment, there was a negative correlation between peptide YY3–36 concentration and body mass index.
Conclusions
The current study provides additional evidence for a normal basal PYY3–36 concentration in AN. Future studies should study multiple appetite-regulating peptides and their complex interplay and also use research designs including a food challenge.
Electronic supplementary material
The online version of this article (10.1007/s00394-020-02210-7) contains supplementary material, which is available to authorized users.
Keywords: Peptide YY, PYY, PYY3–36, Anorexia nervosa, Recovered anorexia nervosa, Gut–brain axis
Introduction
Anorexia nervosa (AN) is a life-threatening eating disorder typically beginning in adolescence and characterized by an intense fear of weight gain and a relentless pursuit of weight loss, mostly by self-starvation. Only less than half of AN patients fully recover from the disorder, one-third shows improvement with residual symptoms, and one fifth remains long-term chronically ill [1]. The underlying mechanisms of AN are still largely unclear and a better understanding of factors contributing to the development and progression of this enigmatic disorder would be the foundation for improving therapeutic interventions. Recently, the gut–brain axis, which describes the bidirectional communication between gut and brain via neurons, immune mediators, gut hormones, and influence of the gut microbiota [2], has been discussed as a possible key factor in the pathophysiology of AN.
Peptide YY (PYY) belongs to the neuropeptide Y family of biologically active peptides that are considered important mediators of the gut–brain axis [2]. It is a 36-amino acid peptide that is thought to be a satiety factor inhibiting food intake, gastrointestinal motility, and secretion, and may interact with the gut microbiota [2–6]. More recently, PYY has also been implicated as a possible regulator of glucose homeostasis [7]. PYY is expressed by endocrine cells of the digestive system, mostly by the endocrine L cells of the gastrointestinal tract in co-secretion with the incretin hormone glucagon-like-peptide 1 but also by endocrine cells in the pancreas and enteric neurons of the stomach [2, 7]. Its release from L cells into the blood stream is triggered by meal intake in proportion to energy intake and depending on meal composition, as well as stimulated by gastric acid secretion and a number of metabolites (for example, cholecystokinin or short chain fatty acids produced by the gut microbiota) [2, 8]. After its release, part of the parent peptide PYY1–36 is cleaved enzymatically by dipeptidyl peptidase 4 (DPP-4) to the main circulating form PYY3–36 [2, 9]. This cleavage is accompanied by a shift in the stimulated Y receptor subtypes leading to possibly divergent actions of PYY1–36 and PYY3–36 regarding energy and glucose homeostasis [2, 7, 10, 11]. Out of the two human forms of PYY, PYY3–36 is considered to be the one relevant to energy homeostasis [7]. The effects of PYY3–36 are mostly mediated through agonism at Y2 receptors on neurons both in the gastrointestinal tract and in the central nervous system [2]. Translational research implicates the arcuate nucleus of the hypothalamus and certain brain stem regions as key areas of central appetite-regulating circuits influenced by PYY3–36 [12]. Batterham et al. [13] found that peripherally infused PYY3–36 leads to a significant reduction in appetite and food intake in healthy, non-obese volunteers. Evidence from functional magnetic resonance imaging (fMRI) studies points to an influence of PYY concentration on neural activity in brain regions involved in reward processing (such as the orbito-frontal cortex), implicating that PYY may modulate the reward value of food [14, 15].
Relatively few studies have investigated PYY concentration in AN yielding heterogeneous results, as highlighted in Table 1, and only one study has investigated PYY in peripheral blood in long-term recovered AN patients. While a number of studies found a normal fasting PYY concentration in acutely underweight AN [16–20], some studies reported an elevated PYY concentration [21–25]. From a physiological perspective, one would expect the anorexigenic PYY to have low levels in this underweight and undernourished population. An elevated PYY concentration would be a maladaptive response as it may facilitate restrictive eating in AN. However, when interpreting previous studies, some confounding factors should be taken into consideration. For instance, physical exercise was shown to significantly increase the peripheral PYY concentration. Suggestive of short-term effects, Larson-Meyer et al. [26] reported elevated PYY concentrations following a 60-min run which gradually returned to baseline over the span of 120 min. Short aerobic exercise (3 × 10 min of rope skipping or cycling) also led to a brief PYY increase [27]. Similarly, Broom et al. [28] found an increase in PYY concentration after aerobic exercise (60-min run) but no difference in PYY levels among the exercise and non-exercise groups 7 h post-exercise. The majority of acutely underweight AN patients engage in excessive physical activity (reported prevalences range from 31 to 80% [29]) and it seems possible that exercise effects bias the assessment of PYY concentrations in AN. Furthermore, only a few studies assessed the biologically more relevant isoform PYY3–36 instead of total PYY [16, 21, 24, 30].
Table 1.
PYY research on anorexia nervosa in humans
| Author (year) | Sample size AN group (n) | Group comparison | Correlation with BMI | Assay | Tested PYY form (in peripheral blood) |
|---|---|---|---|---|---|
| Eddy et al. (2015) [21] | 75 |
PYY total: AN > HC PYY3-36: AN > HC |
All: yes (−) |
RIA a RIA a |
PYY total PYY3-36 |
| Fernández-Aranda et al. (2016) [16] | 64 | AN = HC = OB | Not reported | ELISA a | PYY3-36 |
| Germain et al. (2007) [17] | 12 |
AN = HC AN < CT |
Not reported | RIA c | PYY total |
| Germain et al. (2010) [30] | 32 | AN < HC | Not reported | ELISA c | PYY3-36 |
| Lawson et al. (2011) [42] | 16 |
AN > OB (trend: AN > HC) |
Not reported | RIA a | PYY total |
| Misra et al. (2006) [23] | 23 |
AN > HC (trend: AN T1 > AN T2) |
All: yes (−) | RIA d | PYY total |
| Misra et al. (2008) [22] | 34 | AN > HC | Not reported | RIA d | PYY total |
| Nakahara et al. (2007) [24] | 14 |
AN > HC AN T1 = AN T2 |
Not reported | RIA b | PYY3-36 |
| Otto et al. (2007) [18] | 16 | AN = HC | No | ELISA b | PYY total |
| Pfluger et al. (2007) [25] | 18 |
AN > HC AN T1 = AN T2 |
All: yes (−) | ELISA b | PYY total |
| Rigamonti et al. (2011) [43] |
7 acAN 4 recAN |
AN = recAN AN > OB |
All: yes (−) | RIA a | PYY total |
| Sedlackova et al. (2012) [19] | 14 | HC = AN | Not reported | RIA a | PYY total |
| Stock et al. (2005) [20] | 10 | HC = AN = OB | No | RIA c | PYY total |
| Utz et al. (2008) [45] | 12 | AN only | AN: yes (−) | RIA d | PYY total |
The presented group comparisons are for fasting PYY3–36 or total PYY concentrations only. Some of the listed studies additionally investigated PYY response to potentially stimulating factors such as food intake and some included additional patient groups
AN anorexia nervosa, CT constitutionally thin, ELISA enzyme-linked immunosorbent assay, HC healthy control participants, OB obese/overweight, recAN recovered from anorexia nervosa, RIA radioimmunoassay, T1 timepoint 1 (longitudinal design), T2 timepoint 2 (longitudinal design). Assays: RIA a Linco Research/Millipore Corp., MO, USA; b Peninsula Laboratories, CA, USA; c “established in-house”, d Phoenix Pharmaceuticals, CA, USA, ELISA a BioVendor Research and Diagnostic Products, Czech Republic, b Diagnostic Systems Laboratories Inc., TX, USA, c Phoenix, CA, USA
Gaining a broader understanding of the role of gut hormones such as PYY in the etiology and maintenance of AN holds the potential of identifying prognostic biomarkers and opening up new avenues for treatment. To date, no study on PYY in AN has taken physical activity into account, even though it is likely to be of great importance as a confounding factor in this patient sample. Furthermore, there is a considerably research gap regarding the persistence of possible changes after short-term and long-term weight recovery. Thus, the objective of the current study was to investigate PYY3–36 concentrations of patients with acutely underweight AN, before and after partial weight recovery (longitudinal sample), as well as in long-term recovered patients in comparison to healthy control participants (cross-sectional sample). The inclusion of long-term recovered patients allows the differentiation of state markers (i.e., related to acute undernutrition) from factors that might confer vulnerability towards AN. To control for possible exercise-related bias on PYY in AN, blood samples were obtained shortly after patients were admitted to an intensive treatment program which helps patients resist the drive to exercise.
Materials and methods
Participants
A total of 140 female subjects participated in this study: 47 underweight patients with acute AN (acAN, 12–23 years old), 35 patients long-term recovered from AN (recAN, 15–28 years old), and 58 healthy control participants (HC, 12–26 years old). For the longitudinal arm of our study, 32 acAN patients were reassessed after short-term weight rehabilitation. Participants with acAN were admitted to intensive treatment of an eating disorder program at a child and adolescent psychiatry and psychosomatic medicine department of a tertiary care university hospital. Newly admitted patients spent their first days under close supervision by specialized nursing staff and continuous heart rate monitoring at night. Therefore, we can ensure an exercise-free time period of at least 18 h before blood sample collection. Diagnosis of AN was established using the expert form of the Structured Interview for Anorexia and Bulimia Nervosa (SIAB-EX) [31] and required a body mass index (BMI) below the 10th age percentile (if younger than 15.5 years) or below 17.5 kg/m2 (if older than 15.5 years). RecAN participants were required to have had a diagnosis of AN in the past and, for at least 6 months before the study, (1) to have maintained a BMI above the 10th age percentile (if younger than 18 years) or above 18.5 kg/m2 (if older than 18 years), (2) to menstruate, and (3) to not have binged, purged, or engaged in substantial restrictive eating patterns. HC had to be of normal weight, eumenorrhoeic, and without any history of psychiatric illness. All HC were assessed with the SIAB-EX [31] and excluded if they showed any abnormal eating behavior. HC were recruited through advertisement among middle school, high school, and university students. While recAN and HC participants were only assessed once, acAN participants were assessed within 96 h of admission to intensive treatment (timepoint 1, acAN-T1) and reassessed after short-term weight rehabilitation (timepoint 2, acAN-T2; after BMI increase of at least 13%) for the longitudinal arm of this study.
Information regarding exclusion criteria and possible confounding variables, including menstrual cycle and use of contraceptive medication, was obtained from all participants (acAN, recAN, and HC) using the SIAB-EX [31], supplemented by our own semi-structured interview and medical records. Comorbid diagnoses were taken according to standard practice from medical records and confirmed by an expert clinician with over 10 years of experience. Participants of all groups were excluded if they had a history of any of the following diagnoses: organic brain syndrome, schizophrenia, substance dependence, psychosis not otherwise specified, bipolar disorder, bulimia nervosa, or binge-eating disorder. Further exclusion criteria for all participants were an IQ below 85; current substance abuse; inflammatory, neurologic, or metabolic illness; chronic medical or neurological illness that could affect appetite, eating behavior or body weight; clinically relevant anemia; pregnancy or breast feeding. Psychotropic medication within 4 weeks before the study was an additional exclusion criterion for all groups, with the exception of selective serotonin reuptake inhibitors in the acAN and the recAN group.
Clinical measures
Additional to the evaluation with the SIAB-EX [31], eating disorder-specific psychopathology was assessed with the German version of the self-report questionnaire Eating Disorder Inventory-2 (EDI-2) [32]. Depressive symptoms were explored using the German version of the Beck Depression Inventory-II (BDI-II) [33] and general levels of psychopathology and anxiety symptoms using the revised Symptom Checklist 90 (SCL-90-R) [34]. Pre-treatment physical activity within the 3 months before inclusion into our study was assessed using the corresponding module of the SIAB-EX as previously described by Holtkamp et al. [35] and Ehrlich et al. [36]. The intelligence quotient (IQ) was estimated with short versions of the German adaptation of the Wechsler Adult Intelligence Scale (Wechsler Intelligenztest für Erwachsene) [37] or the Wechsler Intelligence Scale for Children (Hamburg-Wechsler Intelligenztest für Kinder IV) [38] for study participants aged 15 years or younger. Demographic and clinical study data were collected and managed using the secure, web-based electronic data capture tool REDCap (Research Electronic Data Capture) [39].
Procedure
Venous blood samples were collected into vacutainer tubes between 7 and 9 a.m. after an overnight fast, for the acAN group at the first timepoint within 96 h after initiating intensive treatment. Aprotinin (Sigma-Aldrich, St. Louis, Missouri, USA) was added during blood sampling to prevent protein degradation by serine proteases. Samples were immediately centrifuged (800×g for 15 min) in a pre-cooled centrifuge (5 °C), aliquoted, and stored at − 80 °C. Plasma PYY3–36 and leptin concentrations were measured according to the manufacturer's instructions. To prevent enzymatic in-vitro degradation of PYY, DPP-4 inhibitor (Millipore S.A.S, Molsheim, France) was added before thawing of samples in a concentration of 10 µl/ml. Plasma PYY3–36 concentration was analyzed in duplicate using the commercially available human PYY3–36-specific radioimmunoassay (RIA, EMD Millipore Corporation, St. Louis, Missouri, USA) with an intra-assay coefficient of variation (CV) of 6–11%, inter-assay CV of 7–15%, and a lower limit of detection of 20 pg/ml. The employed RIA utilizes 125I-labeled PYY and a PYY3–36 antiserum to determine the PYY3–36 concentrations in the research samples and is specific to human PYY3–36, while PYY1–36 is not detectable. Plasma leptin concentration was measured using a commercially available enzyme-linked immunoabsorbent assay with an intra-assay CV of 4.2%, inter-assay CV of 6.7%, and a lower limit of detection of 0.2 ng/ml (ELISA, BioVendor Research and Diagnostic Products, Brno, Czech Republic) and served as a control variable.
Statistical analysis
For all samples, PYY3–36 was measured twice and the mean was used for all statistical analyses. Due to deviations from normality, the non-parametric Kruskal–Wallis test was used to analyze PYY3–36 concentrations for the cross-sectional group comparison (acAN-T1, recAN, HC). The Wilcoxon signed-rank test was employed to analyze PYY3–36 concentrations longitudinally in the acAN group (acAN-T1, acAN-T2). Correlation analyses were performed using Spearman correlation and p values were corrected for multiple comparisons using the False Discovery Rate correction method of Benjamini and Hochberg [40]. Statistical analyses were performed using SPSS 25 (SPSS, Chicago, Illinois) and JASP for Bayesian statistics [41] with the goal to estimate the evidence in favor of the null hypothesis.
Results
Sample characteristics
Demographic and clinical characteristics are summarized in Table 2 for the cross-sectional sample (acAN-T1, recAN, HC) and in Table 3 for the longitudinal sample (acAN-T1, acAN-T2). As expected, patients with acAN-T1 had significantly lower BMI and BMI standard deviation scores (BMI-SDS) and higher levels of psychopathology (EDI-2 total score, BDI-II total score, SCL-R-90 global severity index). RecAN patients still had some residual psychopathology. As anticipated, leptin concentration was suppressed in the acAN-T1 group, increasing significantly after short-term weight rehabilitation (acAN-T2), while it was in the expected range for the recAN and the HC groups. There was no difference in IQ among groups. Physical activity was increased in inpatients with acAN-T1 in comparison to HC and recAN, and decreased significantly over the course of short-term weight rehabilitation (acAN-T2).
Table 2.
Cross-sectional study sample: demographic and clinical characteristics
| N | acAN-T1 | recAN | HC | F | p | Post hoc tests | |
|---|---|---|---|---|---|---|---|
| Age (years) | 47/35/58 | 15.8 ± 2.4 | 22.0 ± 3.0 | 18.6 ± 4.0 | 34.85 | < 0.001 |
acAN < recAN acAN < HC recAN > HC |
| IQ | 42/35/57 | 110.3 ± 13.7 | 109.3 ± 10.0 | 111.5 ± 9.6 | 0.44 | 0.646 | – |
| BMI (kg/m2) | 47/35/58 | 14.8 ± 1.4 | 20.9 ± 1.9 | 21.0 ± 2.7 | 133.04 | < 0.001 |
acAN < recAN acAN < HC |
| BMI-SDS | 47/35/58 | − 3.2 ± 1.9 | − 0.5 ± 0.6 | − 0.1 ± 0.8 | 84.31 | < 0.001 |
acAN < recAN acAN < HC |
| Minimal lifetime BMI (kg/m2) | 47/35/57 | 14.3 ± 1.5 | 14.3 ± 1.8 | 19.9 ± 2.3 | 135.59 | < 0.001 |
acAN < HC recAN < HC |
| EDI-2 (total score) | 44/34/58 | 195.8 ± 48.8 | 164.0 ± 44.0 | 138.4 ± 26.8 | 26.35 | < 0.001 |
acAN > recAN acAN > HC recAN > HC |
| BDI-II (total score) | 47/35/58 | 18.8 ± 11.2 | 8.4 ± 8.0 | 4.8 ± 5.4 | 37.42 | < 0.001 |
acAN > recAN acAN > HC |
| SCL-90-R (global severity index) | 47/35/58 | 0.79 ± 0.65 | 0.44 ± 0.39 | 0.32 ± 0.40 | 12.16 | < 0.001 |
acAN > recAN acAN > HC |
| Leptin (µg/l) | 47/35/58 | 2.1 ± 3.3 | 9.7 ± 5.8 | 12.1 ± 9.1 | 29.16 | < 0.001 |
acAN < recAN acAN < HC |
| Physical activity | 45/35/57 | 2.4 ± 1.5 | 1.5 ± 1.0 | 1.7 ± 0.9 | 8.42 | < 0.001 |
acAN > recAN acAN > HC |
Mean values ± standard deviation for each variable are shown separately for each sample. Group differences were tested using ANOVA and post-hoc Scheffé
acAN-T1 acute anorexia nervosa participants at timepoint 1 (admission), BDI-II Beck Depression Inventory, BMI body mass index, BMI-SDS body mass index standard deviation score, EDI-2 Eating Disorder Inventory-2, HC healthy control participants, recAN long-term recovered anorexia nervosa participants, SCL-90-R Symptom Checklist-90-Revised
In the acAN group, the mean age of illness onset was 13.5 ± 1.9 years and the mean duration of illness in the acAN group was 17.9 ± 25.0 months. In the acAN group, 42 (89.4%) were of the restrictive subtype and 5 (10.6%) were of the binge/purge subtype. The mean duration since weight normalization in the recAN group was 50.3 ± 34.1 months. In the recAN group, 27 (77.1%) used to be of the restrictive AN subtype and 8 (22.9%) used to be of the binge/purge AN subtype. Of the acAN participants at T1, 5/47 had psychiatric comorbidities: 3/47 had a depressive disorder, 1/47 reported selective mutism, and 1/47 had a major depressive disorder, a generalized anxiety disorder and social phobia, an obsessive–compulsive disorder, and a combined personality disorder. Of the recAN participants, 8/35 had psychiatric comorbidities: 7/35 had a depressive disorder, and 1/35 had an obsessive–compulsive disorder. None of the acAN-T1, recAN, or HC participants were on psychoactive medication
Table 3.
Longitudinal sample: demographic and clinical characteristics
| N | acAN-T1 | acAN-T2 | t | p | |
|---|---|---|---|---|---|
| Age (years) | 32/32 | 15.3 ± 2.5 | 15.6 ± 2.5 | − 11.23 | < 0.001 |
| BMI (kg/m2) | 32/32 | 14.8 ± 1.1 | 18.6 ± 1.1 | − 20.01 | < 0.001 |
| BMI-SDS | 32/32 | − 2.8 ± 1.1 | − 0.7 ± 0.7 | − 16.9 | < 0.001 |
| EDI-2 (total score) | 28/28 | 190.5 ± 43.4 | 183.1 ± 43.2 | 1.24 | 0.225 |
| BDI-II (total score) | 30/30 | 17.9 ± 10.3 | 9.7 ± 8.5 | 5.10 | < 0.001 |
| SCL-90-R (global severity index) | 31/31 | 0.72 ± 0.56 | 0.42 ± 0.36 | 4.53 | < 0.001 |
| Leptin (µg/l) | 32/32 | 2.6 ± 3.7 | 12.7 ± 7.1 | − 7.17 | < 0.001 |
| Physical activity | 31/31 | 2.4 ± 1.4 | 1.6 ± 1.2 | 2.88 | 0.007 |
Mean values ± standard deviation for each variable are shown separately for each timepoint. Differences between timepoints were tested using paired-sample t tests
acAN acute anorexia nervosa participants, BDI-II Beck Depression Inventory, BMI body mass index, EDI-2 Eating Disorder Inventory-2, HC healthy control participants, recAN long-term recovered anorexia nervosa participants, SCL-90-R Symptom Checklist-90-Revised, T1 timepoint 1 (at admission), T2 timepoint 2 (after short-term weight rehabilitation). Regarding psychiatric comorbidities, 2/32 acAN participants in the longitudinal sample had a depressive disorder. None of the acAN participants in the longitudinal sample were on psychoactive medication
PYY3–36 concentration
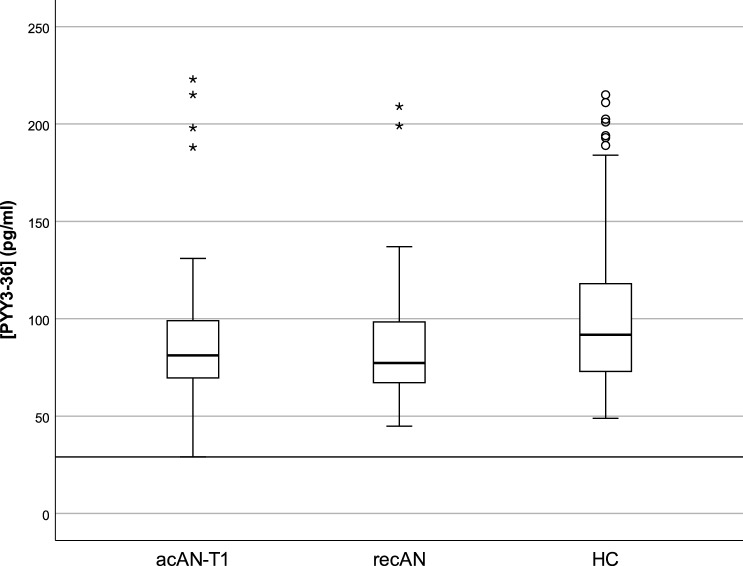
There was no significant difference in PYY3–36 concentration among the acAN-T1, recAN, and HC groups, H(2) = 4.04, p = 0.133 (Fig. 1). Bayesian statistics provided additional support for the null hypothesis (Supplemental Material 1.1). In our longitudinal sample of acAN participants, the PYY3–36 concentration did not change over the course of short-term weight rehabilitation, T = 178, p = 0.170, r = − 0.17 (Fig. 2).
Fig. 1.
PYY3–36 concentrations in the cross-sectional sample. acAN-T1 acute anorexia nervosa participants at timepoint 1 (admission), recAN long-term recovered anorexia nervosa participants; HC, healthy control participants. Boxplots showing the median, upper and lower quartiles, mild outliers (depicted as circles, values deviating more than 1.5 times the interquartile range from the upper or lower quartile) and extreme outliers (depicted as asterisks, values deviating more than 3 times the interquartile range from the upper or lower quartile), and the lower measurement limit indicated as a horizontal line. PYY3-36 concentrations: acAN-T1: median (Mdn) = 81.2 pg/ml, interquartile range (IQR) = 30.5, recAN: Mdn = 77.3 pg/ml, IQR = 32.9, HC: Mdn = 91.8 pg/ml, IQR = 46.2. 2/47 acAN-T1 participants, but none of the recAN or the HC participants had PYY3-36 concentrations below the lower measurement limit (29 pg/ml). The figure was created with SPSS 25 (SPSS, Chicago, Illinois)
Fig. 2.

PYY3–36 concentrations in the longitudinal sample. acAN-T1 acute anorexia nervosa participants at timepoint 1 (admission), acAN-T2 acute anorexia nervosa participants at timepoint 2 (after short-term weight rehabilitation). Boxplots showing the median, upper and lower quartiles, mild outliers (depicted as circles, values deviating more than 1.5 times the interquartile range from the upper or lower quartile), and extreme outliers (depicted as asterisks, values deviating more than 3 times the interquartile range from the upper or lower quartile), and the lower measurement limit indicated as a horizontal line. PYY3–36 concentrations: acAN-T1: median (Mdn) = 85.4 pg/ml, interquartile range (IQR) = 31.1, acAN-T2: Mdn = 76.9 pg/ml, IQR = 34.9. One participant had PYY3–36 concentrations below the lower measurement limit (29 pg/ml) for both timepoints. The figure was created with SPSS 25 (SPSS, Chicago, Illinois)
Associations of PYY3–36 with BMI, BMI-SDS, age, leptin concentration, pre-treatment physical activity (for the acAN group), duration of recovery (for the recAN group), and measures of psychopathology (BDI-II total score, EDI-2 total score, SCL-90-R global severity index) were tested for each group using Spearman correlations. For the acAN-T1 group, there was a negative correlation between PYY3–36 and BMI (rs = -0.45, p = 0.001, Benjamini–Hochberg corrected p = 0.009), but no significant correlation of PYY3–36 with any of the other parameters including age-adjusted BMI-SDS and pre-treatment physical activity. There were no significant associations between PYY3–36 and demographic and clinical variables for the acAN group at T2, the recAN, or the HC groups. An exploratory analysis to test a possible influence of the duration of realimentation and BMI change over the course of treatment on PYY3–36 concentrations in the longitudinal acAN study population revealed no significant influence of these factors (Supplemental Material 1.2).
Discussion
In the present study, we investigated plasma PYY3–36 concentrations in patients with acute AN and individuals after long-term recovery from AN cross-sectionally in comparison to healthy control participants, and longitudinally over the course of inpatient treatment. To specifically measure the isoform most relevant to energy homeostasis [7], we chose a PYY3–36-specific RIA. There was no group difference in PYY3–36 concentrations. Our longitudinal analysis in acute AN patients also yielded no change in PYY3–36 concentration after short-term weight rehabilitation. At admission to treatment, we found a negative correlation between PYY3–36 concentration and BMI.
The results of the present study are in line with a number of studies reporting a normal PYY concentration in acute AN [16–20]. However, others have found elevated PYY levels in acute AN [21–25] and one study, measuring circadian levels of PYY, reported lower concentrations in AN in comparison to a healthy control group [30]. This heterogeneity of results may be explained at least in part by the use of assays targeting total PYY (sum of PYY1–36 and PYY3–36) instead of PYY3–36 [17–20, 22, 23, 42, 43]. The importance of differentiating between PYY1–36 and PYY3–36 is further underlined by a recent study demonstrating different physiological effects of the two isoforms [10]. Last but not least, physical activity may be a possible confounding factor especially relevant for this study population as patients in the acute stage of the disorder often engage in excessive exercise. Multiple studies have reported an exercise-induced increase of PYY concentrations in healthy subjects [26–28]. The duration of the exercise-related effect on PYY concentration appears to depend on the type and intensity of the physical activity, but does not seem to exceed a few hours. Given the intensive treatment setting at the time of venipuncture in our study, we were able to minimize acute effects of excessive physical activity on PYY. Our finding of a lack of correlation between the PYY concentration and pre-treatment physical activity supports this assumption.
Two longitudinal studies, which reported no significant change in PYY concentrations after weight gain, are in accordance with our findings [24, 25]. To our knowledge, there are only two studies investigating PYY in long-term recovered AN patients, with study designs that significantly limit comparability to our results [43, 44]. In line with our findings, Rigamonti et al. [43] did not report a significant difference in total PYY concentrations among acAN and recAN, but the sample size was very small (acAN n = 7, recAN n = 4) and no healthy control group was included in the study. Gendall et al. [44] also reported normal total PYY levels for recAN patients, but PYY was measured in cerebrospinal fluid instead of blood.
Our finding of a negative correlation between PYY concentration and BMI in the acute AN group before treatment is consistent with the results of a number of studies [21, 23, 25, 43, 45]. While our result should be interpreted cautiously as it was not confirmed for age-adjusted BMI-SDS, it is possible that a higher PYY concentration contributes to the severity of the disorder by reducing appetite and food intake. On the other hand, a low PYY concentration might be a protective factor against severe undernutrition in a subgroup of patients as it may counteract self-starvation by increasing appetite and food intake. In this context, Misra et al. [23] remarked a strong negative association between fasting PYY levels and fat intake in girls with AN.
When considering our findings, some limitations have to be taken into account. First, the sample size may not have been sufficient to draw definite conclusions regarding basal PYY3–36 concentrations in AN. Second, taking into account the complexity of the function of PYY as a regulator of energy homeostasis, measurement of fasting concentrations is only one approach to investigate its role in the etiology and progression of AN and future studies in acute AN should also target the PYY3–36 response to standard meals. To date, only four studies investigated PYY in response to meal intake in AN with mixed results [18–20, 24] and only one of these measured PYY3–36 instead of total PYY [24]. Third, because of the possibly divergent actions of the two human isoforms PYY1–36 and PYY3–36, we chose an assay that targets only the isoform PYY3–36, which is assumed to be more relevant for energy homeostasis. Thus, our results allow no conclusions regarding the physiological effects of PYY1–36 and measurement of both, total PYY (the sum of PYY1–36 and PYY3–36) and PYY3–36, yielding their ratio (i.e., total PYY/PYY3–36 or PYY1–36/PYY3–36), would allow for a more comprehensive picture of the peptide’s metabolism in AN.
In conclusion, the current study provides additional evidence for a normal basal PYY3–36 concentration in AN. Future studies should further measure both PYY1–36 and PYY3–36, explore their levels in response to food intake in AN, and take physical activity as a possible confounding factor into consideration. Another promising approach would be to simultaneously study multiple appetite-regulating peptides and their complex interplay.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open Access funding provided by Projekt DEAL. This work was supported by the Deutsche Forschungsgemeinschaft (Grant numbers EH 367/5-1, EH 367/7-1 and SFB 940/2), the Swiss Anorexia Nervosa Foundation and the B. Braun Foundation.
Author contributions
FT acquired and analyzed the data and wrote the manuscript. MS contributed to the design of the study and data acquisition and critically revised the manuscript. FR and IB were involved in participant selection and recruitment, made conceptional suggestions, and critically revised the manuscript. RB conducted all chemical analyses and critically revised the manuscript. KB and KW were involved in participant selection and recruitment and critically revised the manuscript. VR contributed to the design of the study and critically revised the manuscript. SE contributed to the design of the study and data analysis, and critically revised the manuscript. All authors read and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
VR has received payment for consulting and writing activities from Lilly, Novartis, and Shire Pharmaceuticals; lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals, and Medice Pharma; and support for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with Novartis, Shire, and Otsuka. VR has no financial relationship with the organizations that sponsored the research. The other authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committee of the Technische Universität Dresden and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants (or their guardians, if under 18 years old) gave written informed consent prior to their inclusion in the study after full explanation of the purpose and nature of all procedures used.
References
- 1.Steinhausen H-C. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159:1284–1293. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 2.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut–brain axis. Neuropeptides. 2012;46:261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adrian TE, Savage AP, Sagor GR, Allen JM, Bacarese-Hamilton AJ, Tatemoto K, Polak JM, Bloom SR. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89:494–499. doi: 10.1016/0016-5085(85)90442-1. [DOI] [PubMed] [Google Scholar]
- 4.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wettergren A, Petersen H, Orskov C, Christiansen J, Sheikh SP, Holst JJ. Glucagon-like peptide-1 7–36 amide and peptide YY from the L-cell of the ileal mucosa are potent inhibitors of vagally induced gastric acid secretion in man. Scand J Gastroenterol. 1994;29:501–505. doi: 10.3109/00365529409092462. [DOI] [PubMed] [Google Scholar]
- 6.Yang H. Central and peripheral regulation of gastric acid secretion by peptide YY. Peptides. 2002;23:349–358. doi: 10.1016/S0196-9781(01)00611-8. [DOI] [PubMed] [Google Scholar]
- 7.Manning S, Batterham RL. The role of gut hormone peptide YY in energy and glucose homeostasis: twelve years on. Annu Rev Physiol. 2014;76:585–608. doi: 10.1146/annurev-physiol-021113-170404. [DOI] [PubMed] [Google Scholar]
- 8.McGowan BMC, Bloom SR. Peptide YY and appetite control. Curr Opin Pharmacol. 2004;4:583–588. doi: 10.1016/j.coph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Chandarana K, Batterham R. Peptide YY. Curr Opin Endocrinol Diabetes Obes. 2008;15:65–72. doi: 10.1097/MED.0b013e3282f3f4b1. [DOI] [PubMed] [Google Scholar]
- 10.Sloth B, Davidsen L, Holst JJ, Flint A, Astrup A. Effect of subcutaneous injections of PYY1–36 and PYY3–36 on appetite, ad libitum energy intake, and plasma free fatty acid concentration in obese males. Am J Physiol Endocrinol Metab. 2007;293:E604–609. doi: 10.1152/ajpendo.00153.2007. [DOI] [PubMed] [Google Scholar]
- 11.Chandarana K, Gelegen C, Irvine EE, Choudhury AI, Amouyal C, Andreelli F, Withers DJ, Batterham RL. Peripheral activation of the Y2-receptor promotes secretion of GLP-1 and improves glucose tolerance. Mol Metab. 2013;2:142–152. doi: 10.1016/j.molmet.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berner LA, Brown TA, Lavender JM, Lopez E, Wierenga CE, Kaye WH. Neuroendocrinology of reward in anorexia nervosa and bulimia nervosa: beyond leptin and ghrelin. Mol Cell Endocrinol. 2018 doi: 10.1016/j.mce.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 14.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SCR. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 15.De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD, Dhillo WS. The gut hormones PYY3-36 and GLP-17-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández-Aranda F, Agüera Z, Fernández-García JC, Garrido-Sanchez L, Alcaide-Torres J, Tinahones FJ, Giner-Bartolomé C, Baños RM, Botella C, Cebolla A, de la Torre R, Fernández-Real JM, Ortega FJ, Frühbeck G, Gómez-Ambrosi J, Granero R, Islam MA, Jiménez-Murcia S, Tárrega S, Menchón JM, Fagundo AB, Sancho C, Estivill X, Treasure J, Casanueva FF. Smell–taste dysfunctions in extreme weight/eating conditions: analysis of hormonal and psychological interactions. Endocrine. 2016;51:256–267. doi: 10.1007/s12020-015-0684-9. [DOI] [PubMed] [Google Scholar]
- 17.Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, Bloom SR, Estour B. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr. 2007;85:967–971. doi: 10.1093/ajcn/85.4.967. [DOI] [PubMed] [Google Scholar]
- 18.Otto B, Cuntz U, Otto C, Heldwein W, Riepl RL, Tschöp MH. Peptide YY release in anorectic patients after liquid meal. Appetite. 2007;48:301–304. doi: 10.1016/j.appet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Sedlackova D, Kopeckova J, Papezova H, Hainer V, Kvasnickova H, Hill M, Nedvidkova J. Comparison of a high-carbohydrate and high-protein breakfast effect on plasma ghrelin, obestatin, NPY and PYY levels in women with anorexia and bulimia nervosa. Nutr Metab. 2012;9:52. doi: 10.1186/1743-7075-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stock S, Leichner P, Wong ACK, Ghatei MA, Kieffer TJ, Bloom SR, Chanoine JP. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 21.Eddy KT, Lawson EA, Meade C, Meenaghan E, Horton SE, Misra M, Klibanski A, Miller KK. Appetite regulatory hormones in women with anorexia nervosa: binge-eating/purging versus restricting type. J Clin Psychiatry. 2015;76:19–24. doi: 10.4088/JCP.13m08753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, Klibanski A. Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. J Clin Endocrinol Metab. 2008;93:1292–1297. doi: 10.1210/jc.2007-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027–1033. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- 24.Nakahara T, Kojima S, Tanaka M, Yasuhara D, Harada T, Sagiyama K, Muranaga T, Nagai N, Nakazato M, Nozoe S, Naruo T, Inui A. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J Psychiatr Res. 2007;41:814–820. doi: 10.1016/j.jpsychires.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Pfluger PT, Kampe J, Castaneda TR, Vahl T, D’Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AFH, Koebnick C, Weickert MO, Otto B, Spranger J, Tschöp MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab. 2007;92:583–588. doi: 10.1210/jc.2006-1425. [DOI] [PubMed] [Google Scholar]
- 26.Larson-Meyer DE, Palm S, Bansal A, Austin KJ, Hart AM, Alexander BM. Influence of running and walking on hormonal regulators of appetite in women. J Obes. 2012;2012:730409. doi: 10.1155/2012/730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawano H, Mineta M, Asaka M, Miyashita M, Numao S, Gando Y, Ando T, Sakamoto S, Higuchi M. Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite. 2013;66:26–33. doi: 10.1016/j.appet.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296:R29–35. doi: 10.1152/ajpregu.90706.2008. [DOI] [PubMed] [Google Scholar]
- 29.Hebebrand J, Exner C, Hebebrand K, Holtkamp C, Casper RC, Remschmidt H, Herpertz-Dahlmann B, Klingenspor M. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol Behav. 2003;79:25–37. doi: 10.1016/S0031-9384(03)00102-1. [DOI] [PubMed] [Google Scholar]
- 30.Germain N, Galusca B, Grouselle D, Frere D, Billard S, Epelbaum J, Estour B. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J Clin Endocrinol Metab. 2010;95:3057–3062. doi: 10.1210/jc.2009-2196. [DOI] [PubMed] [Google Scholar]
- 31.Fichter M, Quadflieg N. Strukturiertes Inventar für anorektische und bulimische Essstörungen (SIAB); Fragebogen (SIAB-S) und Interview (SIAB-EX) nach DSM-IV und ICD-10; Handanweisung. Göttingen: Hogrefe; 1999. [Google Scholar]
- 32.Thiel A, Jacobi C, Horstmann S, Paul T, Nutzinger DO, Schüßler G. Eine deutschsprachige Version des Eating Disorder Inventory EDI-2. [German translation of the Eating Disorder Inventory EDI-2.] PPmP Psychother Psychosom Med Psychol. 1997;47:365–376. [PubMed] [Google Scholar]
- 33.Hautzinger M, Kühner C, Keller F. Beck Depressions-Inventar (BDI-II) Frankfurt: Pearson Assessment and Information GmbH; 2009. [Google Scholar]
- 34.Franke G, Derogatis L. Symptom-Checkliste von LR Derogatis: SCL-90-R; deutsche Version. Göttingen: Beltz Test; 2002. [Google Scholar]
- 35.Holtkamp K, Herpertz-Dahlmann B, Mika C, Heer M, Heussen N, Fichter M, Herpertz S, Senf W, Blum WF, Schweiger U, Warnke A, Ballauff A, Remschmidt H, Hebebrand J. Elevated physical activity and low leptin levels co-occur in patients with anorexia nervosa. J Clin Endocrinol Metab. 2003;88:5169–5174. doi: 10.1210/jc.2003-030569. [DOI] [PubMed] [Google Scholar]
- 36.Ehrlich S, Burghardt R, Schneider N, Broecker-Preuss M, Weiss D, Merle JV, Craciun EM, Pfeiffer E, Mann K, Lehmkuhl U, Hebebrand J. The role of leptin and cortisol in hyperactivity in patients with acute and weight-recovered anorexia nervosa. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:658–662. doi: 10.1016/j.pnpbp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 37.von Aster M, Neubauer A, Horn R. WIE - Wechsler Intelligenztest für Erwachsene. Bern, Switzerland: Huber; 2006. [Google Scholar]
- 38.Petermann F, Petermann U (2010) HAWIK IV. Hamburg-Wechsler-Intelligenztest für Kinder IV. Übersetzung und Adaptation der WISC-IV von David Wechsler. Hogrefe, Göttingen, Germany
- 39.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 41.JASP Team (2019) JASP (Version 0.11.1) [Computer software]
- 42.Lawson EA, Eddy KT, Donoho D, Misra M, Miller KK, Meenaghan E, Lydecker J, Herzog D, Klibanski A. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol. 2011;164:253–261. doi: 10.1530/EJE-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigamonti AE, Cella SG, Bonomo SM, Mancia G, Grassi G, Perotti M, Agosti F, Sartorio A, Müller EE, Pincelli AI. Effect of somatostatin infusion on peptide YY secretion: studies in the acute and recovery phase of anorexia nervosa and in obesity. Eur J Endocrinol. 2011;165:421–427. doi: 10.1530/EJE-11-0312. [DOI] [PubMed] [Google Scholar]
- 44.Gendall KA, Kaye WH, Altemus M, McConaha CW, La Via MC. Leptin, neuropeptide Y, and peptide YY in long-term recovered eating disorder patients. Biol Psychiatry. 1999;46:292–299. doi: 10.1016/S0006-3223(98)00292-3. [DOI] [PubMed] [Google Scholar]
- 45.Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, Klibanski A, Miller KK. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43:135–139. doi: 10.1016/j.bone.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.