Abstract
Cancer is one of the main causes of mortality in the world. Many cancer cells produce ATP through high-level lactic acid fermentation catalyzed by lactate dehydrogenase (LDH), which converts pyruvic acid to lactic acid. LDH plays a dominant role in the Warburg effect, wherein aerobic glycolysis is favored over oxidative phosphorylation. Due to the high lactic acid production level in cancer cells, LDH-targeting could be a potential cancer treatment strategy. A few approaches, such as drug treatment, reportedly inhibited LDH activity. In this study, we describe new 1,3-benzodioxole derivatives that might be potential small molecule candidates for LDHA inhibition. The synthesis was carried out by trans-esterification between aryl ester and alcohol groups from piperonyl alcohol. Compounds 2 and 10 exhibited a selective LDHA IC50 value of 13.63 µM and 47.2 µM, respectively. Whereas only compound 10 showed significant cytotoxicity in several lines of cancer cells, especially in human pancreatic cancer PANC-1 cells. These synthesized compounds possess 2 aromatic rings and –CF3 moiety, which expectedly contributes to LDHA inhibition. The presented products have the potential to become a promising LDHA inhibitor drug candidate.
Subject terms: Small molecules, Cancer prevention
Introduction
Cancer is one of the biggest health concerns for humans, which takes place at a tissue level1–3. Cancer develops through a series of genetic mutations that result in a change in cell fate. The Warburg effect is a phenomenon wherein cancer cells consume more glucose than healthy cells do to ensure ATP supply for energy production and its catabolites as building blocks simultaneously. In particular, ATP and the precursors of lipid, protein, and nucleotide synthesis are produced through glucose conversion during aerobic glycolysis in cancer cells with lactic acid as the primary end product4.
Lactate dehydrogenase (LDH) is an enzyme with a tetrameric structure that catalyzes pyruvate conversion to lactate and vice versa. LDH has two known isoforms. LDHA mainly converts pyruvic acid to lactic acid, while LDHB catalyzes the reverse reaction5. Several studies have reported that LDHB is constitutively expressed in various cancer cell types, while LDHA is proposedly important for tumor initiation as it is often overexpressed in cancer. Reduced LDHA levels were related to less cellular transformation and delayed tumor formation6,7.
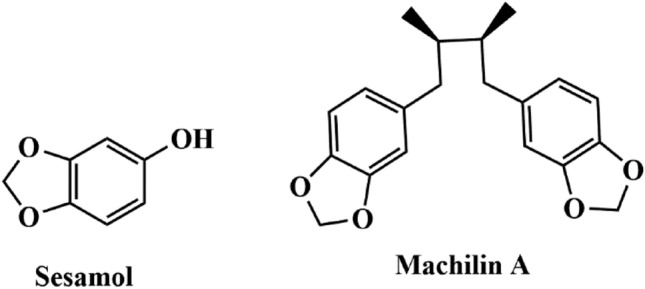
Chemical approaches are among the common strategies to inhibit LDHA activity in cancer cells. The 1,3-benzodioxole ring was described as a component of many natural compounds with various biological activities. These compounds and their derivatives are widely-used pesticides and herbicides8. Certain studies reported that the 1,3-benzodioxole ring possesses antitumor, antiparasitic, antifungal, antioxidant, and antibacterial bioactivities9–13, such as the antioxidant sesamol (Fig. 1)14. In addition, 1,3-benzodioxole derivatives could act as carcinogenesis-associated histone deacetylase inhibitors during cancer treatment15.
Figure 1.
Chemical structure of natural product contained 1,3-benzodioxole rings.
Several 1,3-benzodioxole ring-containing chemical compounds have been developed with the aim to inhibit LDHA activity. These compounds include 1,3-benzodioxole derivatives, such as Machilin A (Fig. 1), which are efficient competitive inhibitors that function by blocking the nicotinamide adenine dinucleotide (NAD) binding site of LDHA, suppressing lactate production and cancer cell growth16. Furthermore, benzodioxole ring-containing thiazolyl-pyrazoline derivatives were tested against MCF-7 and B16-F10 tumor cells and showed significant antiproliferative activity in vitro17. In addition, we previously reported that a selenobenzene compound harboring trifluoromethyl group, 1-(phenylseleno)-4-(trifluoromethyl) benzene, showed an anti-tumor effect thorough suppressing LDHA activity18. Based on the aforementioned results, we developed new 1,3-benzodioxole and trifluoromethyl derivatives through trans-esterification and evaluated the in vitro LDHA inhibitor activity of the synthesized compounds through decreasing NADH intensity.
Results and discussion
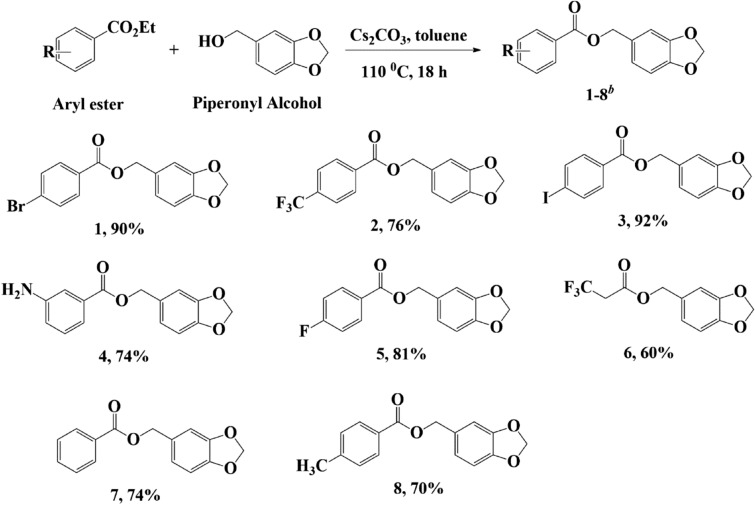
The main chemical reaction in this study was a trans-esterification reaction between the ester group of ethyl 4-bromobenzoate (1a) and the alcohol groups of piperonyl alcohol (1b). A base was used to abstract the protons from the alcohol groups, creating an anion that could directly abstract the carbonyl in the ester groups, and release the alcohol moiety19. The reaction between ethyl 4-bromobenzoate and piperonyl alcohol was carried out as presented in Table 1. Initially, the study was carried out using pyridine as a base in toluene at 110 °C under air condition and the reaction mixture was stirred for 12 h. The yield of the product was 39% (Table 1, entry 1). The reaction using K2CO3 as a base resulted in a lower yield compared to that using pyridine, which yielded 32% (Table 1, entry 2). By increasing the degree of basicity, the product could be obtained with yields of 45% and 53% in the case of NaOH and KOH, respectively (Table 1, entry 3–4). The highest yield (74%) was obtained when Cs2CO3 was used as a base under these reaction conditions (Table 1, entry 5). Therefore, Cs2CO3 was selected as a reaction base for further optimization due to its higher activity, yield, and catalytic speed compared to acid catalysts20. The transesterification mechanism using Cs2CO3 has proposed21. The carbonyl group coordinates with a metal ion to make the carbon center more electrophilic, while the alcohol group is activated by carbonate ion to make a negative charge on the oxygen of the hydroxyl group. This anion directly abstracts the activated carbonyl to form the ester group and release ethanol.
Table 1.
Optimization of reaction conditions for synthesis of compound 1 to 8.
|
| |||||
|---|---|---|---|---|---|
| Entry | Base | Solvent | T (°C) | t (h) | Yielda (%) |
| 1 | Pyridine | Toluene | 110 | 12 | 39 |
| 2 | K2CO3 | Toluene | 110 | 12 | 32 |
| 3 | NaOH | Toluene | 110 | 12 | 45 |
| 4 | KOH | Toluene | 110 | 12 | 53 |
| 5 | Cs2CO3 | Toluene | 110 | 12 | 74 |
| 6 | Cs2CO3 | DMSO | 110 | 12 | 40 |
| 7 | Cs2CO3 | DCM | 40 | 12 | Trace |
| 8 | Cs2CO3 | THF | 60 | 12 | 20 |
| 9 | Cs2CO3 | Toluene | 25 | 12 | Trace |
| 10 | Cs2CO3 | Toluene | 60 | 12 | 29 |
| 11 | Cs2CO3 | Toluene | 110 | 18 | 91 |
| 12 | Cs2CO3 | Toluene | 110 | 24 | 94 |
Reaction conditions: ethyl 4-bromobenzoate (1 mmol), piperonyl alcohol (1.1 mmol), Cs2CO3 (1 mmol), and toluene (5 mL).
aIsolated yields.
We then screened for the solvent effect in this reaction. When an aprotic polar solvent, such as DMSO, was used, the product yield decreased to 40% (Table 1, entry 6). No reaction could be observed when DCM was used as a solvent (Table 1, entry 7), whereas reaction using THF was less successful (Table 1, entry 8). We could conclude that toluene was the best solvent for this reaction. The solvent effect plays an important role in organic equilibrium reactions, such as tautomerization, electron transfer reaction, isomerization, and acid–base balance22.
Furthermore, the reaction was tested at different conduction temperatures. At room temperature, no product was observed (Table 1, entry 9). We could only detect a yield of 29% at 60 °C (Table 1, entry 10). We also aimed at optimizing the reaction time. When the reaction time was increased to 18 h, an excellent yield (91%) was obtained (Table 1, entry 11). We observed no significant difference when the reaction time was increased to 24 h (Table 1, entry 12). Based on the optimization results, we selected Cs2CO3 as a base, toluene as a solvent, 110 °C as reaction temperature, and 18 h as the reaction time for further experiments.
Under these optimized conditions, we synthesized other 1,3-benzodioxole derivatives by changing the R substituent. This method can tolerant some of the substituents in good to excellent yield (Fig. 2). Aryl ester as a substrate with halogen substituents (p-Br, p-I, and p-F) resulted in excellent yield, between approximately 81 and 92% (compound 1, 3, and 5). Furthermore, reactions with trifluoromethyl (p-CF3) and amine (m-NH2) as substituents also proceeded smoothly and resulted in the products 2 and 4, with a yield of 76% and 74%, respectively. The addition of electron-donating groups, such as in the case of compounds 7 and 8, also resulted in good yields of 74% and 70%, respectively. However, the use of –CF3 as a substituent did not provide a favorable result when it was not attached to the aromatic ring (compound 5). This suggested that the aromatic ring played a role in the reaction efficiency as it could delocalize electrons that would result in more electrophilic carbonyl groups.
Figure 2.
Substrate scope. Reaction conditions: Arylethyl ester (1 mmol), piperonyl alcohol (1.1 mmol), Cs2CO3 (1 mmol), and toluene (5 mL). bIsolated yields.
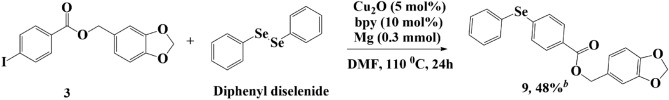
Selenide compounds reportedly exhibit various bioactivities, such as antioxidant, antibacterial, and anticancer effects23–25. In our study, we also developed 1,3-benzodioxole-modified selenide compounds as we expected that such modifications would further increase the bioactivity of the compound. The modification of the selenide compound and 1,3-benzodioxole was achieved by reacting aryl iodide with diphenyl diselenide via the cleavage of the Se–Se bond (Fig. 3). Our results showed that compound 3, the product of the trans-esterification from the previous reaction, acted as an aryl iodide compound that reacted with diphenyl diselenide to form an asymmetrical diaryl chalcogenide compound. Using this method, we successfully synthesized target product 9 with a 48% yield.
Figure 3.
Synthesis of compound 9. Reaction conditions: Compound 3c (0.6 mmol), diphenyl diselenide (0.3 mmol), and DMF (1 mL). bIsolated yields.
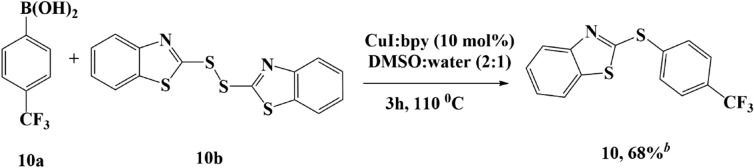
Apart from the selenide compound modification, we also synthesized an aryl-heteroatom C–S bond, with a heterocyclic group in order to study its bioactivity as an LDHA inhibitor. This compound was used as a comparison for a 1,3-benzodioxole ring and p-CF3 moiety in the structure of compound 2. N and S heterocyclic compounds are well-known for their diverse biological activities and are used in several diseases treatments26–28. In our study, we synthesized such a compound using (trifluoromethyl)phenylboronic acid and 2,2′-dithiobis(benzothiazole) via S–S cleavage, resulting in a C–S bond with an N and S heterocyclic ring 10, with a 68% yield (Fig. 4).
Figure 4.
Synthesis of compound 10. Reaction conditions: phenyl boronic acid (1.3 mmol), 2,2-dithiobis(benzothiazole) (0.6 mmol), and DMSO:water (2:1). bIsolated yields.
The in vitro evaluation of the LDHA inhibitor activities of the synthesized compounds was determined by the NADH intensity decrease through oxidation in a solution of HEPES-K+, NADH, and pyruvate at a pH = 7.2. The NADH oxidation fluorescence intensity was measured using a spectrofluorometer at 340 nm excitation and 460 nm emission wavelengths, representing the NADH-specific fluorescence spectrum. Furthermore, the in vitro evaluation of the LDHB inhibitor activity was determined as a reverse reaction, which converts lactate to pyruvate by determining the amount of NAD+ converted to NADH using the above-mentioned spectrophotometric experimental setting (Table 2). Based on this analysis, the half-maximal inhibitory concentration (IC50) of the synthesized compounds was obtained.
Table 2.
Bioactivity of synthesized compounds in inhibits LDHA and LDHB.
| Entry | Compounds | LDHA IC50 | LDHB IC50 | LDHB/LDHA |
|---|---|---|---|---|
| 1 | 1 | > 1000 µM | 79.05 µM | ND |
| 2 | 2 | 13.63 µM | 395.3 µM | 29.00 |
| 3 | 3 | > 1000 µM | 150.8 µM | ND |
| 4 | 4 | 182.5 µM | 7.87 µM | 0.043 |
| 5 | 5 | 477.5 µM | > 1000 µM | 2.094 |
| 6 | 6 | 452.5 µM | 129.8 µM | 0.29 |
| 7 | 7 | 842.6 µM | > 1000 µM | > 1.18 |
| 8 | 8 | > 1000 µM | 248 µM | ND |
| 9 | 9 | > 1000 µM | 151.1 µM | ND |
| 10 | 10 | 47.20 µM | > 1000 µM | > 21.18 |
| 11 | GSK2837808A | 2.6 nM | 130.3 nM | 50.12 |
| 12 | GNE140 | 59.9 nM | 151.1 nM | 2.52 |
ND not determined.
We used GSK2837808A and GNE140 as standard compounds in this measurement that is a routinely used potential LDH inhibitor with an IC50 of 2.6 nM and 59.9 nM, respectively. Since LDHA is often overexpressed in cancer, LDHB was used to evaluate the LDHA-selectivity of the synthesized compounds. We tested various aryl esters, presented in Fig. 2, of which compound 1 (p-Br), 3 (p-I), and 8 (p-CH3) exhibited the highest LDHA IC50 values of over 1000 µM. However, compound 1 exhibited the lowest LDHB IC50 value of 79.05 µM, indicating that compound 1 is a selective LDHB inhibitor compare to the other 3 compounds. Moreover, compound 7 also showed high LDHA IC50 value (842.6 µM) and LDHB IC50 value (over 1000 µM) values. These values were too high to be compared with the standards; thus, we concluded that these 4 aryl ester compounds were inactive LDHA inhibitors.
Furthermore, compound 5, with 2 aromatic rings and a p-F moiety, exhibited a moderate LDHA IC50 value of 477.5 µM and was a selective LDHA inhibitor due to its LDHB IC50 value, which was greater than 1000 µM. Besides compound 5, compound 6, with only one aromatic ring and a –CF3 moiety, also had a moderate LDHA IC50 value of 452.5 µM. However, compound 6 was not selective for LDHA based on its LDHB IC50 value, which was lower than its LDHA IC50 value. In addition, compound 4, containing an m-NH2 moiety, was not selective for LDHA either, as we measured the lowest LDHB IC50 value (7.87 µM) in the case of this compound. The lowest and selective LDHA inhibitor was compound 2 (13.63 µM) with 2 aromatic rings and p-CF3 moiety. This compound was selective as LDHA inhibitor compares to its LDHB inhibitor activity (LDHB IC50 value of 395.3 µM). These values were still higher compared to the IC50 values of the GSK2837808A and GNE140 standards but lower compared to other established LDHA inhibitors, such as galloflavin, which was also used as a reference compound since it is routinely used in enzymatic LDH assays, with an IC50 value of approximately 110 µM29. This result suggested that it could potentially become LDHA inhibitor drug candidate. Moreover, we also synthesized selenide compounds, containing 1,3-benzodioxole (9) and benzothiazole (10) groups. Compound 9 was not efficient with LDHA and LDHB IC50 values of 1000 µM, while compound 10 exhibited a favorable selectivity as an LDHA inhibitor with an LDHA IC50 value of 47.20 µM and an LDHB IC50 value of over 1000 µM.
To assess the anticancer potency of these compounds in several cancer cells, we determined the cell toxicities of these compounds using an MTT assay. As shown in Table 3, the compound 2 showed was too weak. It was over 1000 µM of the concentration of half-maximal growth inhibition (GI50) value. On the other hand, compound 10 has higher cytotoxicity compared to other compounds in various cancer cell lines. Among these cell lines, compound 10 is most effective in reducing the cell viability in human pancreatic cancer PANC-1 cells (GI50 value of 12.19 µM). In addition, we analyzed the cytotoxicity of two standard compounds, GSK2837808A and GNE140. The calculated GI50 values of GSK2837808A and GNE140 were 11.31 µM and 11.59 µM, respectively. There is a small difference between standard compounds and compound 10 in growth inhibition of PANC-1 cells.
Table 3.
The 50% growth inhibition concentration (GI50) of compounds on various human cancer cell lines.
| Entry | Compounds | Cell lines | ||||
|---|---|---|---|---|---|---|
| PANC-1 (GI50) (µM) | A549 (GI50) (µM) | MCF-7 (GI50) (µM) | MiaPaCa-2 (GI50) (µM) | U87 (GI50) (µM) | ||
| 1 | 1 | > 1000 | > 1000 | > 1000 | > 1000 | > 1000 |
| 2 | 2 | > 1000 | > 1000 | > 1000 | > 1000 | > 1000 |
| 3 | 3 | 269.3 | > 1000 | > 1000 | > 1000 | > 1000 |
| 4 | 4 | 203.4 | > 1000 | 729 | > 1000 | 379.5 |
| 5 | 5 | 643.9 | > 1000 | > 1000 | > 1000 | > 1000 |
| 6 | 6 | > 1000 | > 1000 | > 1000 | > 1000 | > 1000 |
| 7 | 7 | 243.3 | > 1000 | > 1000 | > 1000 | > 1000 |
| 8 | 8 | 86.46 | > 1000 | > 1000 | > 1000 | > 1000 |
| 9 | 9 | 21.11 | 924.8 | 455.1 | 108 | > 1000 |
| 10 | 10 | 12.19 | 56.46 | 61.38 | 88.88 | 343.4 |
| GSK2837808A | 11.31 | ND | ND | ND | ND | |
| GNE140 | 11.93 | ND | ND | ND | ND | |
ND not determined.
In addition to intracellular anticancer activity, to provide the basic in vitro drug-like data of compound 10, we performed basically in vitro assays, such as human liver microsomal stability, plasma stability, and CYP inhibition (Table 4). The compound 10 was rapidly metabolized by human and rat microsomes; only 9.5% and 5.2% of compound 10 remained after 30 min of incubation. However, a plasma stability test showed compound 10 was stable in both human and rat plasma. The compound 10 is a moderate perpetrator of drug–drug interactions based on their inhibition of the most abundant CYP450 enzymes, such as 1A2 and 2C19.
Table 4.
Summary of in vitro ADME for compound 10.
| Compounds | CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4 |
|---|---|---|---|---|---|
| CYP isozyme activity (% of control activity) | |||||
| Compound 10 | 36.7 | 97.4 | 12.1 | 95.1 | > 100 |
| Ketoconazole (reference) | 99.1 | 97.5 | > 100 | 99.0 | 25.0 |
| Ketoconazole: CYP3A4 inhibitor (0.1 µM) | |||||
| Compounds | Human (%) | Rat (%) | |||
|---|---|---|---|---|---|
| Human and rat liver microsomal stability (% remaining during 30 min) | |||||
| Compound 10 | 9.5 | 5.2 | |||
| Verapamil (reference) | 15.3 | – | |||
| Compounds | Human | Rat | |||
|---|---|---|---|---|---|
| 30 min | 120 min | 30 min | 120 min | ||
| Human and rat plasma stability (% remaining) | |||||
| Compound 10 | 98.1 | 91.6 | 93.8 | 87.5 | |
| Procaine (reference) | 1.5 (5 min) | 0.4 (10 min) | 89.8 | 52.6 | |
| Enalapril (reference) | 95.0 | 93.7 | 37.7 | 1.8 | |
The compound 2 exhibits a simple structure with one 1,3-benzodioxole group compared to the previously reported Machilin A16, containing two 1,3-benzodioxole groups. Furthermore, it has a lower LDHA IC50 value compared to Machilin A (84 µM). Sada et al., also presented the LDHA inhibitory activity of stiripentol analogs at a higher dose (500 µM)30. However, piperonyl alcohol and 1,3-benzodioxole did not exhibit an LDHA inhibitory activity even at concentrations up to 1 mM16. However, unlike the previously reported compounds harboring 1,3-benzodioxole group, compound 2 did has an anti-cancer effect in several lines of cancer cells. It could be a result of its chemical properties including low stability or poor cellular uptake. Compound 10, a benzothiazole compound harboring a –CF3 moiety, could also be considered as a potential LDHA inhibitor. Although higher IC50 in vitro LDHA assay, the GI50 value of compound 10 is lower than that of previously reported selenobenzene compound, 1-(phenylseleno)-4-(trifluoromethyl) benzene18. The GI50 value of compound 10 in PANC-1 cells is comparable to that of standard compounds, GSK2837808A and GNE140.
From these results, compound 2 and 10 among the synthesized compounds, with the simple structure and comparable activity, could be potentially used as an LDHA inhibitor and should be further investigated. These synthesized compounds possess 2 aromatic rings and –CF3 moiety, which is expected to contribute to LDHA inhibition. The compounds have the potential to become a promising LDHA inhibitor for the anticancer drug candidate. To improve the in vitro LDHA inhibition and intracellular activity of these compounds, it is needed to conduct an extensive structure–activity relationship study, including substitutions in a different position, bioisosteres replacement, and scaffold hopping.
Conclusions
Here we described 1,3-benzodioxole derivatives, synthesized through trans-esterification between aryl ester and piperonyl alcohol groups. Through this approach, the substrate scope of this reaction was also investigated and could tolerate many substituents, with good to excellent yields. In addition, we also synthesized benzothiazole derivatives and 1,3-benzodioxole-modified selenide compounds. Compound 2, containing a benzodioxole ring and a –CF3 moiety, showed a potent inhibitory action in vitro assay, however, failed to show anticancer effect in human cancer cells. Compound 10, a benzothiazole harboring a –CF3 group, showed both activities of in vitro LDHA inhibition and intracellular cytotoxicity. These compounds could potentially be used as an LDHA inhibitor due to its optimal activity and selectivity based on the decrease in the NADH intensity and as it has the smallest IC50 among all the compounds. Thus, compound 10 could be considered a potent LDHA inhibitor for further in vivo evaluations.
Methods
General methods
All chemicals and reagents were purchased from Tokyo Chemical Industry (Tokyo, Japan) and Sigma-Aldrich (St. Louis, MO) and used without further purification. Fourier-transform infrared (FT-IR) spectra were recorded on NICOLET 380. 1H (400 MHz) and 13C (100 MHz) NMR of all synthesized compounds were recorded on Bruker Magnet System 400′54 Ascend. Gas Chromatography–Mass Spectrometry spectra were performed on Shimadzu GC-1010 Plus GCMS-QP2010 SE. High-resolution Mass Spectrometry (HR-MS) data were performed on 6530Accurate-Mass Q-TOF LC/MS. Spectrofluorometer spectra were performed on Spectramax M2; Molecular Devices, Sunnyvale, CA, USA.
Synthesis of compound 1–8
Arylethyl ester (1 mmol) was reacted with piperonyl alcohol (1.1 mmol) in 5 mL toluene. Cesium carbonate (1 mmol) was added to the mixture as a base. The reaction mixture was stirred at reflux under air condition for 18 h. The reaction was monitored by TLC and GC–MS for completion reaction. After the completion reaction, the mixture was cooled to room temperature. The mixture was diluted by dichloromethane and evaporate the solvent. The crude product was stored in a refrigerator for 24 h to conduct white solid. The crude product was purified by column chromatography over silica gel.
Synthesis of compound 9
The reactions referred to31 with modification. Compound 3 (0.6 mmol) was reacted with diphenyl diselenide (0.3 mmol) in 1 mL DMF. Cu2O (5 mol%), bpy (10 mol%), and Mg (0.6 mmol) were added to the reaction mixture. The mixture was stirred at 110 °C for 24 h. The reaction was monitored by TLC and GC–MS. After the completion reaction, the mixture was cooled to room temperature. Then, the crude product was separated by an extraction process using dichloromethane and brine solution. The organic layer was evaporated and the crude product was purified by column chromatography over silica gel.
Synthesis of compound 10
The reactions referred to32 with modification. Trifluoromethyl phenyl boronic acid 10a (1.3 mmol) was reacted with 2,2-dithiobis(benzothiazole) 10b (0.6 mmol) in DMSO:water (2:1). CuI;bpy (10 mol%) was added to the reaction mixture. The mixture was stirred at 110 °C for 3 h. The reaction was monitored by TLC and GC–MS. After the completion reaction, the mixture was cooled to room temperature. Then, the crude product was separated by an extraction process using dichloromethane and brine solution. The organic layer was evaporated and the crude product was purified by column chromatography over silica gel.
In vitro evaluation on LDHA inhibitor activity. LDHA activity assay was performed in accordance with previous studies16,18. Briefly, the various concentrations of compounds were incubated with reaction buffer containing 20 mM HEPES-K+, 20 μM NADH, 2 mM pyruvate, and 100 ng of purified recombinant human LDHA protein (Abcam, Cambridge, UK). The fluorescence of NADH, which has an excitation wavelength of 340 nm and an emission wavelength of 460 nm, was detected using a microplate spectrofluorometer (Spectramax M2; Molecular Devices, Sunnyvale, CA).
In vitro LDHB Activity Assay
In vitro analysis was determined the amount of NAD+ converted to NADH33. The assay mixture composed of 100 mM Tris–HCl buffer (pH 8.0), 200 mM sodium L-lactate, 2.5 mM NAD+, and 100 ng of purified recombinant human LDHB protein (Abcam). The fluorescence intensity of NADH was measured using a spectrofluorometer (Spectramax M2) at 340 nm as excitation wavelength and 460 nm as emission wavelength which is the specific fluorescence of NADH.
Cell culture
The human pancreatic cancer cell lines, PANC-1 and MiaPaCa-2, human lung cancer A549 cells, human breast cancer MCF-7, and human glioma U87 cells were obtained from the Korean cell line bank (Seoul, Korea). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; ThermoFisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich) and 1% penicillin/streptomycin (ThermoFisher Scientific). The cells were cultured at 37 °C in an atmosphere containing 5% CO2.
Intracellular LDHA activity assay
To observe the LDH activities from the lysates of cells, we performed in accordance with previous studies16,18. Briefly, Total protein from lysate (1 μg) was mixed with containing 20 mM HEPES-K+, 20 μM NADH, 2 mM pyruvate. The fluorescence of NADH, which has an excitation wavelength of 340 nm and an emission wavelength of 460 nm, was detected using a microplate spectrofluorometer (Spectramax M2).
Cell viability
The potential cytotoxicity of compounds at different concentrations was evaluated using the MTT assay. Briefly, cancer cell lines were pre-incubated in 96-well plates with compounds for 48 h. Subsequently, MTT working solution (2 mg/mL in phosphate buffer solution) was added to each well and the plate was incubated for 4 h at 37 °C in an atmosphere containing 5% CO2. Then, the conditioned media were aspirated, and the formed formazan crystals in living cells were quantified using the microplate reader (Spectramax M2) at 540 nm. The concentrations that produce 50% cell growth inhibition (GI50) were calculated by curves constructed by the plot of cell survival by an assistant of PRISM software (GraphPad, San Diego, CA).
LC–MS/MS analysis
Chromatographic separation was performed on the Shimadzu Nexera XR system (Kyoto, Japan). Detection was performed on a Thermo TSQ Vantage triple quadrupole LC–MS/MS (MA, USA). The analytes were separated on a Phenomenex Kinetex C18 (2.1 × 100 mm, 2.6 µm particle size) column (Torrance, USA). The mobile phase system consisted of water containing 0.1% formic acid (mobile phase A, MPA), and acetonitrile containing 0.1% formic acid (mobile phase B, MPB). Xcalibur 1.6.1 software (Thermo Scientific) was used for data acquisition and processing.
Plasma stability
Human and rat plasma (Sigma-Aldrich) are incubated at 37 °C with test compounds. During the incubation, aliquots are withdrawn at 0, 30, 120 min time points and acetonitrile solution (containing chlorpropamide) is added. After vortexing, the aliquots are centrifuged and the supernatant is withdrawn for analysis by LC–MS/MS.
CYP isozymes activity assay
Pooled Human liver microsomes (Sigma-Aldrich; 0.25 mg/mL), 0.1 M phosphate buffer solution (pH 7.4), the five most commonly used substrate cocktails, such as 50 µM phenacetin (CYP1A2), 10 µM diclofenac (CYP), 100 µM S-mephenytoin (CYP2C19) 5 µM dextromethorphan (CYP2D6), and 2.5 µM midazolam (CYP3A4), and compound 10 are pre-incubated at 37 °C for 5 min, then incubated with NADPH generation system solution for 15 min. To finish the enzymatic reaction, an acetonitrile solution (containing terfenadine) is added. The reaction tubes are centrifuged and the supernatant is withdrawn for analysis by LC–MS/MS.
Microsomal stability
The assay use liver microsomes from two species (human, rat, 0.5 mg/mL). liver microsomes preincubated with 0.1 M PBS (pH 7.4) and 1 µM compound 10 at 37 °C for 5 min, then incubated with NADPH regeneration system solution for 30 min. To finish the reaction, an acetonitrile solution (involved in chlorpropamide) is added. The reaction tubes are centrifuged and the supernatant is withdrawn for analysis by LC–MS/MS.
Statistical analysis
Results were presented as mean ± standard deviation (SD) of triplicate experiments. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post hoc test, and p values of lesser than 0.05 were considered statistically significant.
Supplementary information
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2020R1I1A3067208 to K.H.P and 2014R1A5A20009936 to K.-T.H).
Author contributions
D.A. performed synthesized, analyze the organic compounds and manuscript writing; M.Y. performed analyze the synthesized compounds; S.Y.C. and S.J.B. performed most of the in vitro experiments and manuscript writing; K.-T.H. and K.H.P. designed the study and wrote the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information Files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dicky Annas and Se-Yun Cheon.
Contributor Information
Ki-Tae Ha, Email: hagis@pusan.ac.kr.
Kang Hyun Park, Email: chemistry@pusan.ac.kr.
Supplementary information
is available for this paper at 10.1038/s41598-020-77056-4.
References
- 1.Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017;4:127–129. doi: 10.1016/j.jcrpr.2017.07.001. [DOI] [Google Scholar]
- 2.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer. 2013;108:479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty JR, Cleveland JL, Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics find the latest version: Review series targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman RD, Kaplan NO, Hall TC. Lactic dehydrogenase in human neoplastic tissues. Cancer Res. 1964;24:389–399. [PubMed] [Google Scholar]
- 6.Le A, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie H, et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol. Cancer Ther. 2009;8:626–635. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugolini L, Della Noce I, Trincia P, Borzatta V, Palmieri S. Benzodioxole derivatives as negative effectors of plant proteases. J. Agric. Food Chem. 2005;53:7494–7501. doi: 10.1021/jf0580418. [DOI] [PubMed] [Google Scholar]
- 9.Wei PL, et al. The in vivo antitumor effects on human COLO 205 cancer cells of the 4,7-dimethoxy-5-(2-propen-1-yl)-1,3-benzodioxole (apiole) derivative of 5-substituted 4,7-dimethoxy-5-methyl-l,3-benzodioxole (SY-1) isolated from the fruiting body of Antrodia camphorate. J. Cancer Res. Ther. 2012;8:532–536. doi: 10.4103/0973-1482.106529. [DOI] [PubMed] [Google Scholar]
- 10.Leite ACL, Peixoto Da Silva K, De Souza IA, Magali De Araújo J, Brondani DJ. Synthesis, antitumour and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur. J. Med. Chem. 2004;39:1059–1065. doi: 10.1016/j.ejmech.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Kamau E, et al. A novel benzodioxole-containing inhibitor of Toxoplasma gondii growth alters the parasite cell cycle. Antimicrob. Agents Chemother. 2011;55:5438–5451. doi: 10.1128/AAC.00455-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson FR, Hoosseintehrani B. Effects of benzylphenol and benzyl-1,3-benzodioxole derivatives on fertility and longevity of the yellow fever mosquito (Diptera:Culicidae) J. Econ. Entomol. 1982;75:877–878. doi: 10.1093/jee/75.5.877. [DOI] [PubMed] [Google Scholar]
- 13.Bakhite EAG, Radwan SM. Synthesis, reactions and biological activity of some new thieno[2,3-f]-1,3-benzodioxoles. Pharmazie. 1999;54:491–498. [PubMed] [Google Scholar]
- 14.Shenoy RR, et al. Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamum indicum (L.) in albino rats. J. Ethnopharmacol. 2011;133:608–612. doi: 10.1016/j.jep.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Kumar N, et al. Preclinical evaluation and molecular docking of 1,3-benzodioxole propargyl ether derivatives as novel inhibitor for combating the histone deacetylase enzyme in cancer. Artif. Cells Nanomed. Biotechnol. 2018;46:1288–1299. doi: 10.1080/21691401.2017.1369423. [DOI] [PubMed] [Google Scholar]
- 16.Chung TW, et al. Machilin a inhibits tumor growth and macrophage m2 polarization through the reduction of lactic acid. Cancers (Basel) 2019;11:1–21. doi: 10.3390/cancers11070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HH, et al. Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives containing benzodioxole as potential anticancer agents. Bioorg. Med. Chem. 2013;21:448–455. doi: 10.1016/j.bmc.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Kim EY, et al. A novel lactate dehydrogenase inhibitor, 1-(phenylseleno)-4-(trifluoromethyl) benzene, suppresses tumor growth through apoptotic cell death. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karmee SK, Mahesh P, Ravi R, Chadha A. Kinetic study of the base-catalyzed transesterification of monoglycerides from Pongamia oil. J. Am. Oil Chem. Soc. 2004;81:425–430. doi: 10.1007/s11746-004-0917-4. [DOI] [Google Scholar]
- 20.Yusup S, Khan MA. Base catalyzed transesterification of acid treated vegetable oil blend for biodiesel production. Biomass Bioenergy. 2010;34:1500–1504. doi: 10.1016/j.biombioe.2010.04.027. [DOI] [Google Scholar]
- 21.Xiong Y, Zhang X. Significant heterogeneous carbonate salt catalyzed acetylation of alcohols via a transesterification process with carbonate salt-activated alcohol 1H NMR evidence. Chin. J. Chem. 2011;29:1143–1148. doi: 10.1002/cjoc.201190214. [DOI] [Google Scholar]
- 22.Reichardt C. Solvents and solvent effects: An introduction. Org. Process Res. Dev. 2007;11:105–113. doi: 10.1021/op0680082. [DOI] [Google Scholar]
- 23.Sentkowska A, Pyrzyńska K. Investigation of antioxidant activity of selenium compounds and their mixtures with tea polyphenols. Mol. Biol. Rep. 2019;46:3019–3024. doi: 10.1007/s11033-019-04738-2. [DOI] [PubMed] [Google Scholar]
- 24.Álvarez-Pérez M, Ali W, Marć MA, Handzlik J, Domínguez-Álvarez E. Selenides and diselenides: A review of their anticancer and chemopreventive activity. Molecules. 2018;23:628–646. doi: 10.3390/molecules23030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, et al. Selenium-containing naphthalimides as anticancer agents: Design, synthesis and bioactivity. Bioorg. Med. Chem. 2012;20:2558–2563. doi: 10.1016/j.bmc.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Ardiansah B. Chalcones bearing N, O, and S-heterocycles: Recent notes on their biological significances. J. Appl. Pharm. Sci. 2019;9:117–129. [Google Scholar]
- 27.Chauvière G, et al. Synthesis and biological activity of nitro heterocycles analogous to megazol, a trypanocidal lead. J. Med. Chem. 2003;46:427–440. doi: 10.1021/jm021030a. [DOI] [PubMed] [Google Scholar]
- 28.Saleh SS, Al-Salihi SS, Mohammed IA. Biological activity study for some heterocyclic compounds and their impact on the gram positive and negative bacteria. Energy Procedia. 2019;157:296–306. doi: 10.1016/j.egypro.2018.11.194. [DOI] [Google Scholar]
- 29.D’Andrea F, et al. Synthesis and biological evaluation of new glycoconjugated LDH inhibitors as anticancer agents. Molecules. 2019;24:3520. doi: 10.3390/molecules24193520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–1368. doi: 10.1126/science.aaa1299. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi N, Onami T. Magnesium-induced copper-catalyzed synthesis of unsymmetrical diaryl chalcogenide compounds from aryl iodide via cleavage of the Se–Se or S–S bond. J. Org. Chem. 2004;69:915–920. doi: 10.1021/jo030300+. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi N. Convenient synthesis of unsymmetrical organochalcogenides using organoboronic acids with dichalcogenides via cleavage of the S–S, Se–Se, or Te–Te bond by a copper catalyst. J. Org. Chem. 2007;72:1241–1245. doi: 10.1021/jo062131+. [DOI] [PubMed] [Google Scholar]
- 33.Dave KK, Punekar NS. Expression of lactate dehydrogenase in aspergillus Niger for L-lactic acid production. PLoS ONE. 2015;10:1–16. doi: 10.1371/journal.pone.0145459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information Files).