Abstract
Diapause is a mechanism necessary for survival in arthropods. Often diapause induction and resurrection is light-dependent and therefore dependent on the photoperiod length and on the number of consecutive short-days. In many organisms, including the microcrustacean Daphnia magna, one functional entity with the capacity to measure seasonal changes in day-length is the circadian clock. There is a long-standing discussion that the circadian clock also controls photoperiod-induced diapause. We tested this hypothesis in D. magna, an organism which goes into a state of suspended animation with the shortening of the photoperiod. We measured gene expression of clock genes in diapause-destined embryos of D. magna in the initiation, resting and resurrection phases and checked it against gene expression levels of continuously developing embryos. We demonstrate that some genes of the clock are differentially expressed during diapause induction but not during its maintenance. Furthermore, the photoreceptor gene cry2 and the clock-associated gene brp are highly expressed during induction and early diapause, probably in order to produce excess mRNA to prepare for immediate resurrection. After resurrection, both types of embryos show a similar pattern of gene expression during development. Our study contributes significantly to the understanding of the molecular basis of diapause induction, maintenance and termination.
Subject terms: Developmental biology, Ecology, Limnology
Introduction
Diapause is widespread in insects and crustaceans and has undoubtedly contributed to their enormous ecological and evolutionary success by allowing them to exploit resources in favourable seasons and to evade cold winters, desiccation, starvation, predators and parasites (reviewed1). The crustacean Daphnia is a keystone organism in the carbon transfer from primary producers to secondary consumers, and is a model organism in genetic and (eco)toxicological studies. In Daphnia, diapause is a phenotypically plastic trait which is dependent on environmental conditions2. Factors inducing diapause in Daphnia include food availability, high density of conspecifics, photoperiod, low temperature, predation and desiccation3–6. Cyclical parthenogenetic Daphnia females can switch from asexual to sexual reproduction in order to produce resting stages (ephippia containing up to two diapausing eggs5) that can persist in lake sediments and be resurrected after years or even decades (cf.7–9).
The regulation of diapause is an intriguing developmental problem, because development is brought to a halt before being resumed a long time later. Diapause in arthropods can be categorized into three different phases: Induction, maintenance and termination10; the molecular signals and biochemical mechanisms that drive development through these phases are only partly understood1.
Growth, development and metabolism are also arrested in crustaceans during diapause, while tolerance to environmental and physiological stress is increased11. In order to maintain this state, a specific pattern of differentially expressed genes is governed (reviewed in12,13): The stress-inducible transcription co-factor p8 is up-regulated in the crustacean Artemia franciscana both in the induction and in the maintenance of diapause. This is also the case for three small heat shock proteins which might promote diapause maintenance by enhancing stress tolerance. Furthermore, genes that suggest hormonal influence on Artemia diapause (i.e. genes that are involved in metabolism or that inhibit cell growth and division) are differentially expressed. Also a low intracellular pH was discussed as being a possible mechanism that inhibits metabolism in dormant cysts of Artemia14. Specifically for D. magna, Pauwels et al.15 observed higher levels of glycerol and a heat shock protein in dormant than in parthenogenetic eggs.
Photoperiodic induction of winter diapause requires a mechanism for measuring day-length (a clock) and a mechanism for counting the number of short days (a counter). Two rhythms of light exist on earth: The daily rhythm due to the Earth’s rotation around its axis and the seasonal rhythm caused by the Earth’s rotation around the sun. Therefore, Bünning16 proposed the functional involvement of the circadian clock in seasonal time measurement. In line with this proposition, the involvement of genes of the circadian clock in photoperiodism was verified in several insect species (e.g.17–20.). In other cases, it has been more controversially discussed whether the circadian clock perceives photoperiod21,22. Emerson et al.23 found that the circadian clock and the photoperiodic clock that controls diapause can evolve independently, and there is an ongoing debate as to if and/or to what extent the circadian clock and the timer of photoperiod have the same underlying genetic mechanism22,24. In the case of Daphnia, Roulin et al.25 have demonstrated in a QTL study that a variation in a rhodopsin photoreceptor gene plays a significant role in the variation of timing of resting stage induction that is not part of the circadian clock.
It is not known whether the expression of the circadian clock genes persists during diapause in Daphnia. It is well imaginable that the counting of the number of elapsed clock cycles contributes to the timing of diapause termination; in addition, ephippia often rest in sediments that are not reached by light. However, a light stimulus is needed to initiate development in resting eggs. Daphnia diapause is most effectively terminated by blue and UV-light stimuli26. Interestingly, cryptochrome 2 (cry2) is a gene in Daphnia’s putative circadian clock system27 that has been shown to be expressed in a cyclic manner over a 24-h day-night cycle28,29. In other organisms (e.g. spider mites30) the photoperiodic clock necessary for termination of diapause is probably not identical to the circadian clock. However, the involvement of cry 2 in diapause termination of Daphnia is likely: In a QTL study, Czypionka et al.31 have identified three isoforms of an ELKS/Rab6 interacting/Cast 396 family member protein (ERC; homologous to the gene bruchpilot (brp) in insects) to be potentially involved in diapause termination. Interestingly, brp interacts with the circadian clock although it is not part of the core circadian system32. Analogous to the light-dependent degradation of the circadian clock gene timeless (tim), it is degraded by cryptochrome33. Therefore, the circadian clock might play a significant role in diapause termination in Daphnia.
We hypothesize that the core genes of Daphnia’s circadian clock in ephippia are expressed differently in the initiation, resting, and termination phases of diapause. We further hypothesize that cry 2 and brp are highly expressed in ephippia either during initiation or termination of diapause in order to provide enough mRNA and/or photoreceptor molecules for immediate diapause termination and thus for a quick resumption of development of resting eggs. Therefore, we measured expression of brp and five core clock genes (cry 2, tim, period (per), clock (clk) and cycle (cyc)) that had previously been demonstrated to show a day-time dependent expression in D. pulex28. Gene expression of these genes was measured in sexually produced embryos of D. magna that are destined to go into a phase of suspended animation34. We selected developmental stages based on cell count in which diapause is initiated, maintained and terminated. Asexually produced embryos develop continuously, and also here we measured gene expression in the comparative developmental stages. This allowed us to determine clock gene expression during continuous development and development intermitted by a phase of suspended animation.
Material and methods
Culture conditions
We raised a population of the D. magna clone ‘Elias’ from Mount Sinai, Egypt, and a D. magna clone FT442 from Finland (kindly provided by Dieter Ebert) as published in34: All animals of the culture and the experiments were raised in 1 L glass jars (WECK, Germany) filled with ADaM medium35) in temperature-controlled incubators at 20 °C ± 0.1 °C and under different light conditions (for asexually produced embryos: 16:8 day:night; for sexually produced embryos: 8:16 day:night). Animals were fed the algae Acutodesmus obliquus ad libitum, > 1.5 mg C/L. To ensure clonal reproduction, females were kept at low densities (about 30 adult females per 800 mL of ADaM in a 1 L jar, Weck; Germany). Sexual reproduction was induced by maintaining the clones under shortened photoperiodic conditions (8 h: 16 h light: dark cycle) at 20 °C ± 0.1 °C, with low food levels and via crowding. To create the crowded conditions, we cultured more than 50 adult male and female animals in 800 mL ADaM under conditions of limited food supply, < 1 g C/L. Food concentration was determined by measuring the algae’s optical density (at 800 nm). Carbon content was adjusted to a standard curve available in the lab.
We collected asexually produced embryos from clone ‘FT442’ and sexually produced clones were crosses of ‘FT442′ females and ‘Elias’ males.
Ovulation monitoring of sexually and asexually reproducing D. magna
We monitored sexually and asexually reproducing females (as described in34; Table 1) in order to collect timely staged sexually and asexually produced Daphnia embryos. Sexually and asexual produced embryos can already be distinguished within the ovary (for details see34). Upon the appearance of either type, each female was individually transferred into a 50 mL snap cap vial filled with 40 mL ADaM and fed the algae Acutodesmus obliquus ad libitum. Asexual females were in their vials, while one male of a different clone was transferred to the sexually reproducing female. We then checked all females at 15 min intervals to determine the time point of ovulation. From this time point onwards, we collected time-dependent stages of asexually and sexually produced embryos (listed in Table 1) during the light phase of the respective photoperiod 8 h:16 h light: dark cycle (sexually produced embryos) and 16 h:8 h light: dark cycle (asexually produced embryos) and during daytime from 10 a.m. to 4 p.m. (Central European Standard Time). Sexually produced embryos that had entered diapause were transferred to dark and cold conditions; these conditions are necessary to prevent hatching. Resurrection was initiated by exposure to daylight (Osram Biolux L, 30 W/965) in an acclimatized room at 20° C ± 0.1 °C (in a long photoperiod with a 16:8 light: dark cycle), and respective stages were again collected during the daytime from 10 a.m. to 4 p.m. (Table 1). Target stages were selected with respect to the developmental time point based on cell count or explicit morphological features as published in34. All work was performed at 20 °C ± 0.1 °C to ensure timely correlated development of biological replicates.
Table 1.
Comparative sampling stages based on cell number of sexually and asexually produced embryos collected for qPCR.
| Stage | Time of collection in sexually produced embryos | Time of collection in asexually produced embryos |
|---|---|---|
| 1000 cell stage | < 24 h-mitotic active stage | 10 h-mitotically active stage |
| 3500 cell stage | 48 h-deceleration stage, pre-diapause | 15 h-mitotically active stage |
| 3500 cell stage | 74 h-stationary phase | – |
| 3500 cell stage | 1 month in diapause | – |
| 3500 cell stage | 11 months in diapause | – |
| Unknown | 1 d reactivation | – |
| Unknown | 5 d reactivation | – |
| Unknown | 12 d reactivation | – |
| Unknown | 19 d reactivation | – |
| > 7000 cells | Revived 1-appearance of the 2nd antennae | 24 h- mitotically active stage; appearance of the 2nd antennae |
| > 7000 cell stage | Revived 2-appearance of bright red eye spots | Appearance of bright red eye spots |
| Revived 3-eye spots fused and black | Eye spots fused and black |
Fixation of sampling stages
When the animals reached the respective developmental stage, they were flash-frozen in liquid nitrogen and stored at − 80 °C until RNA extraction was conducted. When sexually produced embryos were encapsulated in an ephippium, they were dissected using a fine forceps and flash frozen without the ephippium. We collected three timely correlated biological replicates consisting of 30 embryos (stages with < 3500 cells) and 15 embryos (stages with > 3500 cells).
RNA extraction
Tissue samples were thawed on ice, homogenized using a pistil and then extracted with the ReliaPrep RNA Miniprep system (Promega, Germany) for tissues as according to the manufacturer’s instructions. The RNA was quality checked; only samples with A260/280 ~ 2.0 and A260/230 ~ 2.0–2.2 were used. The RNA integrity index was determined with the help of an Experion microchip reader (Biorad, Germany) and a StdSens RNA kit. Only samples with a RIN > 8.0 were taken for qPCR. RNA quantity was determined with a Qubit RNA broad range assay kit (Thermo Fisher Scientific, Germany).
One step RT-qPCR
Reverse transcription quantitative PCR (RT-qPCR) was performed using the Luna Universal One Step RT-qPCR kit (New England Biolabs, Germany) as according to the manufacturer’s protocol. In total, 10 ng RNA was added to a total reaction volume of 10 µl so that 1 ng RNA was reverse transcribed and amplified with specific primers. Primer pairs (Table 2) were added at a concentration of 0.4 µM, and amplification was performed at 60 °C with 40 cycles. All reactions were finalized by a melting curve step, giving constant melting peaks but in the non-template and the non-reverse transcription controls. Plates were set up in technical duplicates. Due to the number of samples, replicates and genes, multiple plates were used. These plates were controlled for comparative results by adding a two standard RNA samples that were run with the reference gene primer tbp36. Differences between these Cqs of both RNAs of all the individual plates were lower than 0.2%.
Table 2.
qPCR primers for Daphnia magna clock genes.
| Gene | Abbreviation | Primer forward (5′-3′) | Primer reverse (5′-3′) | Tmelt | Amplicon size (bp) | Gene origin |
|---|---|---|---|---|---|---|
| Clock | clk | tccttttgaagttctcgggaca | gcttcatgacaggtagaaactttc | 60 °C | 80 | scaffold00547 |
| Cycle | cyc | ttttattcgtcgtgggctgc | aataattgagcacttgagacaccg | 60 °C | 75 | scaffold03242 |
| Cryptochrome 2 | cry2 | tgctactagacgcagattggtc | actttcctgccaaatctgacag | 60 °C | 115 | scaffold00687 |
| Timeless | tim | tccgcatcattggctacact | cgatggctgtgattactgatgc | 60 °C | 111 | scaffold03376 |
| Period | per | cggccggaattcaacagatg | tgctcggcttccatttctgt | 60 °C | 117 | scaffold02670 |
| Bruchpilot | brp | cacaacgatggcgttcacgtatt | gtcttctcagccacttctgacgt | 56 °C | 149 | Dm_Bassoon |
| Tata-box binding protein | tbp | gcagggaagtttagtttctgga | tggtatgcacaggagcaaag | 60 °C | 88 | Heckmann et al. 2006 |
Listed are gene names, abbreviations, primer sequences, melting temperature (Tmelt), amplicon sizes and the origin of D. magna sequences for which D. pulex sequences (for gene IDs see Schwarzenberger & Wacker 2015) were blasted against the D. magna genome v.2.4 (wfleabase.org). Delineated are the scaffolds on which the blast hits were positioned. Tata-box binding protein (Heckmann et al. 2006) was used as reference based on result obtained from RefFinder38.
Data analysis
Primer efficiency was determined using LinReg36. The reference gene tbp37 was validated using RefFinder38 and found to be stably expressed over all tested stages. We had to rely on this single reference gene only, as other standard reference genes (i.e. STX16, actin WARS, 18S) were strongly regulated between developmental stages and between sexually and asexually produced embryos. Unfortunately, tbp expression was not stable between sexually and asexually produced embryos, a fact which prevented us from directly comparing gene expression between the embryo types. Differential gene expression between all tested stages was analyzed as according to the Pfaffl method in REST39. The mathematical model used is based on the correction for exact PCR efficiencies and the mean crossing point deviation between sample group(s) and control group(s). Subsequently, the expression ratio results of the investigated transcripts are tested for significance by a Pair Wise Fixed Reallocation Randomisation Test.
Results
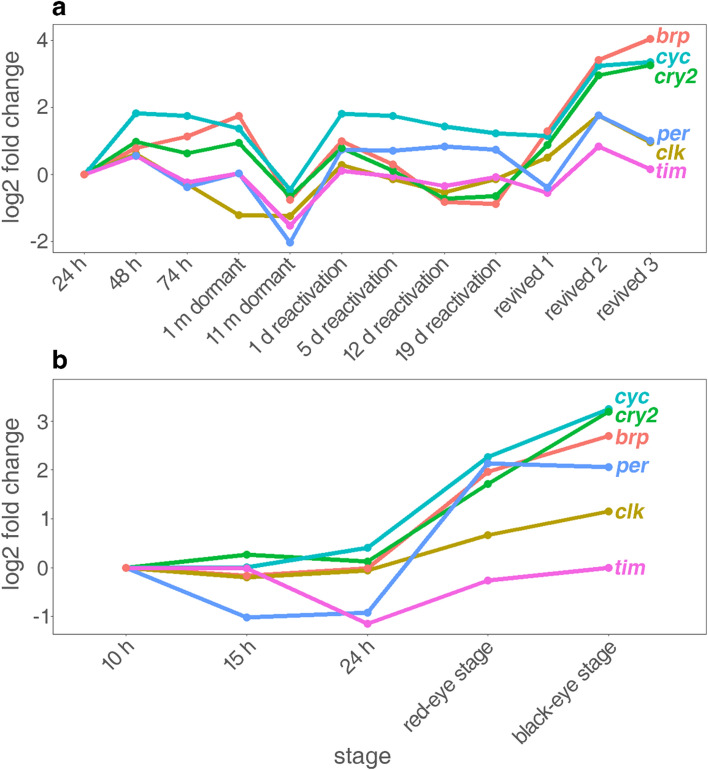
We analyzed log2 fold changes in gene expression of circadian clock genes and a putatively associated gene during the development of sexually produced embryos that are about to go into diapause. Moreover, we resurrected these embryos and screened for gene expression changes (Fig. 1a). In order to see diapause-associated changes in circadian clock gene expression, we compared these gene expression patterns to gene expression patterns in asexually produced embryos (Fig. 1b). More detailed information on gene expression differences between all stages and p-values can be found in the supplemented heatmaps (Supplementary 1).
Figure 1.
mRNA expression of all six genes across developmental stages in (a) sexually and (b) asexually produced D. magna embryos. Displayed is the log2 fold change of each stage relative to the first stage, i.e. 24 h in sexually produced embryos and 10 h in asexually produced embryos. For more details see supplemented heatmaps (Supplementary 1).
Gene expression patterns during diapause preparation in sexually produced embryos
In the preparation phase of diapause at 48 h post ovulation (Fig. 1a), clk and cyc mRNA is significantly upregulated in comparison to 24 h post ovulation. In comparison, tim shows a significant weaker log2-fold expression change when 48 h sexual embryos are compared with 24 h embryos, whereas per gene expression was not changed. Cry2 mRNA is significantly upregulated 48 h post ovulation in comparison to 24 h. Brp shows a tendency of being upregulated in this developmental stage.
Gene expression patterns during diapause of sexually produced embryos
In stages when morphological development has come to a halt, i.e. at 74 h and 1-month dormant (Fig. 1a), clk, per and tim expression does not change significantly in comparison to the 24 h stage, but was downregulated in comparison to the 48 h stage (except per). Clk, per and tim expression is significantly downregulated in 11-month dormant embryos compared with all previous developmental stages. Cyc expression remains at a stable expression level until 1-month domant. In 11-months dormant embryos, cyc expression is not significantly different from cyc expression in 24 h embryos, but is significantly reduced in comparison to the other previous developmental stages. In comparison to 24 h, cry2 expression is significantly upregulated in all stages until 1-month dormant, but is significantly downregulated in 11-months dormant embryos. In 1-month dormant embryos, brp expression is significantly increased in comparison to the previous developmental stages, but is significantly downregulated in 11-months dormant embryos.
Gene expression patterns during resurrection of sexually produced embryos
Upon resurrection through light exposure (Fig. 1a), cyc, per and tim gene expression is significantly increased in reactivated embryos in comparison to 11-months dormant embryos and either reached similar (tim) or significantly higher gene expression levels than before diapause (cyc: 1 and 19 d reactivation; per: 1 to 19 d reactivation). Clk, cry2 and brp gene expression is also significantly increased in reactivated embryos in comparison to 11-months dormant embryos. With ongoing reactivation, gene expression levels are lower than in pre-diapause stages (clk: 12 d reactivation; cry2: 12 to 19 d reactivation; brp: 19 d reactivation).
Gene expression patterns in resurrected and developing sexually produced embryos
Active development in sexually produced embryos was determined based on the appearance of morphological features, i.e. the second antennae (revived 1), red eye stage (revived 2) and black eye stage (revived 3; Table 1). Gene expression of clk, cry2 and brp increases significantly after reactivation to similar (clk, brp) or higher levels (cry2) than before diapause. Cyc gene expression first decreases to similar levels as before diapause (revived 1) and then significantly increases in gene expression (revived 2 and 3). Per and tim gene expression decreases after resurrection (revived 1), then increase in gene expression (revived 2), before returning to similar levels as before diapause (revived 3).
Gene expression patterns across development in asexually produced embryos
In asexually produced embryos, clk and cyc, brp, cry2 become significantly upregulated in the red- and black-eye stages (Fig. 1b), whereas tim is stably expressed across all developmental stages except in the 24 h post ovulation stage when gene expression is significantly reduced.
Discussion
Daphnia’s core clock shows a 24-h pattern of gene expression in response to changes in day and night28,29. Daphnia can also adjust its clock gene expression to different photoperiods (i.e. one clone of D. pulex shows higher and longer per gene expression during longer nights; Schwarzenberger, A. & Wacker, A. unpublished data). This suggests that the clock is not only circadian but also that it measures photoperiod. Therefore, it is reasonable to assume that Daphnia’s core clock genes act not only as a clock (a preceptor of day-length), but also as a counter of shortening days in order to induce diapause.
We found that induction of diapause in Daphnia magna involves a general expression increase of the core clock genes and the clock-associated gene brp. We have recently elucidated that at the point in time when the embryos are still in the mother’s brood pouch, mitotic activity decelerates and comes to a halt 50 h post ovulation34. In line with this, we found that the up-regulation of tim, per and clk is completed after 48 h post ovulation, and we have previously found and again find here that diapause is prepared until 48 h post ovulation. The halt of mitotic activity might be caused by an arrest of the circadian clock. Since clk is no longer expressed, translated clk and cyc can no longer form into a hetero-dimer. This hetero-dimer is necessary as a transcription factor binding to the E-box of per and tim40, and so gene expression is also downregulated. The circadian clock probably stops without per and tim transcription.
In the cases of cyc, cry2 and brp, increased gene expression lasts until 74 h post ovulation. At this stage, development is completely suspended and embryos are encapsulated in ephippia which are shed during the mother’s next molting cycle34. Since expression of these genes is still stably increased even at developmental arrest and until one month of diapause (or in case of brp is even further increased), this allocation of additional mRNA molecules might allow continuous mRNA translation into proteins also during developmental arrest. By this, receptor molecules of e.g. cry2 can be continuously synthesized to be functional as a blue light sensor that may enable diapause termination upon stimulation. Similarly, since cry2 and brp are connected (i.e. brp is degraded by cry2 after light stimulation33), a provision of brp mRNA or translated protein at the time point of diapause termination is then necessary. We therefore anticipate that cry2 is another photoreceptor gene (besides a rhodopsin gene25) that plays a significant role in the variation of timing of resting-stage induction in Daphnia. Interestingly, cry2 seems to be involved in diapause in insects as well: In Drosophila, allelic differences in cry2 (and tim) were associated with differences in the incidence of diapause41. In the case of cyc, a continuous provision of mRNA or translated proteins might be necessary to prepare the restart of the circadian clock immediately after diapause termination. If—at the time point of diapause termination—the gene expression of clk is initiated, the formation of the clk-cyc hetero-dimer is possible in a short amount of time.
During deep diapause (i.e. 11 months post ovulation), expression of all clock genes is down-regulated. This is in line with findings for per and cry expression in diapausing adult females of an insect (Pyrrhocoris apterus42). Therefore, sustaining Daphnia’s clock gene expression is not necessary for active maintenance of diapause and is arrested similarly to other genes involved in growth, development and metabolism. Furthermore, the clock does not act as a counter of days until diapause termination, because the daily rhythmicity is probably arrested during diapause. At the time point of reactivation with day-light, all core clock genes and the associated gene brp increase in expression levels. This has also been observed in adults of the insect Pyrrhocoris apterus, for which per and cry increase after diapause termination42. However, in the pupae of another insect species, Rhagoletis pomonella, no change in clock gene expression has been found between early and late diapause and diapause termination43. In Daphnia, all genes show a strong increase in gene expression in comparison to deep diapause which levels out (or slightly decreases) during all stages of reactivation (where there are no signs of morphological differentiation). This level probably represents the onset of the daily cycling of the clock which is necessary for metabolism and other physiological responses during reactivation.
Both revived and asexually produced embryos grew in the same photoperiod and developed into parthenogenetic females. Therefore, it is not surprising that gene expression of the clock is similar during development from embryo to the black-eye stage. In both cases, gene expression increases continuously over developmental progression, or—in case of tim—gene expression first decreases and then increases both in sexual and asexual embryos. A higher clock gene expression is probably necessary in order to maintain the increasing circadian metabolic activity of growing embryos.
To our knowledge, this is the first report describing the expression of the genes of the core clock of embryos of a crustacean over a whole diapause cycle (i.e. before, during and after diapause). We found that the clock is differentially expressed during diapause induction but not during its maintenance; furthermore, the photoreceptor cry2 and the downstream brp are highly expressed in the late induction and early diapause phase, probably in order to store mRNA or molecules necessary for immediate diapause termination due to a light stimulus. After reactivation, both sexually and asexually produced embryos show a similar pattern of gene expression during development to parthenogenetic females.
Diapause is an essential phase during the life cycle of many arthropods; survival is not possible in deleterious living conditions without the sexual production of resting stages. Our study is a crucial addition to the understanding of the molecular basis of diapause induction, maintenance and termination. Furthermore, based on our findings, RNAi (reverse genetics) knock-down of certain clock genes is the next logical step to test whether diapause or its termination is still possible with reduced gene expression in vivo.
Supplementary information
Acknowledgements
The authors would like to thank Frederic Bartlett for the English correction of the manuscript.
Author contributions
A.S. initiated this study. L.C.W. and A.S. were responsible for the experimental design. L.C. and L.C.W. conducted the experiment and analyzed the data. All authors discussed the results and read and contributed to the manuscript that was written by A.S. and L.C.W.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by a grant from the German Research Foundation (DFG) to AS (SCHW 1830/4-1) and the China Scholarship Council.
Data availability
Data are provided in the appendix.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77065-3.
References
- 1.Hand SC, Denlinger DL, Podrabsky JE, Roy R. Mechanisms of animal diapause: Recent developments from nematodes, crustaceans, insects, and fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R1193–R1211. doi: 10.1152/ajpregu.00250.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stross RG, Hill JC. Diapause induction in Daphnia requires two stimuli. Science. 1965;150:1463–1464. doi: 10.1126/science.150.3702.1462. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho GR, Hughes RN. The effect of food availability, female culture-density and photoperiod on ephippia production in Daphnia magna Straus (Crustacea, Cladocera) Freshw. Biol. 1983;13:37–46. doi: 10.1111/j.1365-2427.1983.tb00655.x. [DOI] [Google Scholar]
- 4.Schwartz SS, Hebert PD. Methods for the activation of the resting eggs of Daphnia. Freshw. Biol. 1987;17:373–379. doi: 10.1111/j.1365-2427.1987.tb01057.x. [DOI] [Google Scholar]
- 5.Alekseev V, Lampert W. Maternal control of resting-egg production in Daphnia. Nature. 2001;414:899–901. doi: 10.1038/414899a. [DOI] [PubMed] [Google Scholar]
- 6.Šlusarczyk M. Food threshold for diapause in Daphnia under the threat of fish predation. Ecology. 2001;82:1089–1096. doi: 10.1890/0012-9658(2001)082[1089:FTFDID]2.0.CO;2. [DOI] [Google Scholar]
- 7.Hairston NG, et al. Rapid evolution revealed by dormant eggs. Nature. 1999;401:446. doi: 10.1038/46731. [DOI] [Google Scholar]
- 8.Kerfoot WC, Robbins JA, Weider LJ. A new approach to historical reconstruction: Combining descriptive and experimental paleolimnology. Limnol. Oceanogr. 1999;44:1232–1247. doi: 10.4319/lo.1999.44.5.1232. [DOI] [Google Scholar]
- 9.Frisch D, et al. A millennial-scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecol. Lett. 2014;17:360–368. doi: 10.1111/ele.12237. [DOI] [PubMed] [Google Scholar]
- 10.Koštál V. Eco-physiological phases of insect diapause. J. Ins. Physiol. 2006;52:113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Hairston NG. Time travelers: what's timely in diapause research? Arch Hydrobiol. Spec. Issues Adv. Limnol. 1998;52:1–15. [Google Scholar]
- 12.Qiu Z, MacRae TH. A molecular overview of diapause in embryos of the crustacean, Artemia franciscana. In: Lubzens E, Cerda J, Clark M, editors. Dormancy and Resistance in Harsh Environments. Topics in Current Genetics. Berlin: Springer; 2010. pp. 165–187. [Google Scholar]
- 13.Denlinger DL. Regulation of diapause. Ann. Rev. Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- 14.Hand SC, Carpenter JF. pH-induced metabolic transitions in Artemia embryos mediated by a novel hysteretic trehalase. Science. 1986;232:1535–1537. doi: 10.1126/science.232.4757.1535. [DOI] [PubMed] [Google Scholar]
- 15.Pauwels K, Stoks R, Verbiest A, De Meester L. Biochemical adaptation for dormancy in subitaneous and dormant eggs of Daphnia magna. Hydrobiol. 2007;594:91–96. doi: 10.1007/s10750-007-9091-4. [DOI] [Google Scholar]
- 16.Bünning E. Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber. Dtsch. Bot. Ges. 1936;54:590–607. [Google Scholar]
- 17.Nunes MV, Saunders D. Photoperiodic time measurement in insects: A review of clock models. J. Biol. Rhythms. 1999;14:84–104. doi: 10.1177/074873049901400202. [DOI] [PubMed] [Google Scholar]
- 18.Pittendrigh CS, Kyner WT, Takamura T. The amplitude of circadian oscillations: Temperature dependence, latitudinal clines, and the photoperiodic time measurement. J. Biol. Rhythms. 1991;6:299–313. doi: 10.1177/074873049100600402. [DOI] [PubMed] [Google Scholar]
- 19.Pittendrigh CS, Elliott J, Takamura T. The circadian component in photoperiodic induction. In: Porter R, Collins GM, editors. Photoperiodic regulation of insect and molluscan hormones. Trowbridge: Pitman; 1984. pp. 26–47. [Google Scholar]
- 20.Syrova Z, Doležel D, Šaumann I, Hodkova M. Photoperiodic regulation of diapause in linden bugs: Are period and Clock genes involved? Cell. Mol. Life Sci. 2003;60:2510–2515. doi: 10.1007/s00018-003-3227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeno T, Tanaka SI, Numata H, Goto SG. Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol. 2010;8:2. doi: 10.1186/1741-7007-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw WE, Holzapfel CM. What season is it anyway? Circadian tracking vs photoperiodic anticipation in insects. J. Biol. Rhythms. 2010;25:155–165. doi: 10.1177/0748730410365656. [DOI] [PubMed] [Google Scholar]
- 23.Emerson KJ, Dake SJ, Bradshaw WE, Holzapfel CM. Evolution of photoperiodic time measurement is independent of the circadian clock in the pitcher-plant mosquito Wyeomyia smithii. J. Comp. Physiol. A. 2009;195:385–391. doi: 10.1007/s00359-009-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denlinger DL, Hahn DA, Merlin C, Holzapfel CM, Bradshaw WE. Keeping time without a spine: what can the insect clock teach us about seasonal adaptation? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372:20160257. doi: 10.1098/rstb.2016.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roulin AC, Bourgeois Y, Stiefel U, Walser J-C, Ebert D. A photoreceptor contributes to the natural variation of diapause induction in Daphnia magna. Mol. Biol. Evol. 2016;33:3194–3204. doi: 10.1093/molbev/msw200. [DOI] [PubMed] [Google Scholar]
- 26.Davison J. Activation of the ephippial egg of Daphnia pulex. J. Gen. Physiol. 1969;53:562–575. doi: 10.1085/jgp.53.5.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilden AR, McCoole MD, Harmon SM, Baer KN, Christie AE. Genomic identification of a putative circadian system in the cladoceran crustacean Daphnia pulex. Comp. Biochem. Physiol. D Genom. Proteom. 2011;6:282–309. doi: 10.1016/j.cbd.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzenberger A, Wacker A. Melatonin synthesis follows a daily cycle in Daphnia. J. Plankton Res. 2015;37:636–644. doi: 10.1093/plankt/fbv029. [DOI] [Google Scholar]
- 29.Bernatowicz PP, et al. Temporal expression of the clock genes in the water flea Daphnia pulex (Crustacea: Cladocera) J. Exp. Zool. A Ecol. Genet. Physiol. 2016;325:233–254. doi: 10.1002/jez.2015. [DOI] [PubMed] [Google Scholar]
- 30.Veerman A, Koveos DS. Separation of photoperiodic and circadian effects on the termination of diapause in the spider mite Tetranychus urticae. Experientia. 1998;45:1143–1146. doi: 10.1007/BF01950183. [DOI] [Google Scholar]
- 31.Czypionka T, et al. The genetic architecture underlying diapause termination in a planctonic crustacean. Mol. Ecol. 2018;28:998–1008. doi: 10.1111/mec.15001. [DOI] [PubMed] [Google Scholar]
- 32.Górska-Andrzejak J, et al. Circadian expression of the presynaptic active zone protein Bruchpilot in the lamina of Drosophila melanogaster. Dev. Neurobiol. 2013;73:14–26. doi: 10.1002/dneu.22032. [DOI] [PubMed] [Google Scholar]
- 33.Damulewicz M, et al. Cryptochrome is a regulator of synaptic plasticity in the visual sytem of Drosophila melanogaster. Front. Mol. Neurosci. 2017;10:2. doi: 10.3389/fnmol.2017.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, et al. Mitotic activity patterns and cytoskeletal changes throughout the progression of diapause developmental program in Daphnia. BMC Cell Biol. 2018;19(1):30. doi: 10.1186/s12860-018-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klüttgen B, Dülmer U, Engels M, Ratte HT. ADaM, an artificial fresh-water for the culture of zooplankton. Wat. Res. 1994;28:743–746. doi: 10.1016/0043-1354(94)90157-0. [DOI] [Google Scholar]
- 36.Ruijter JM, et al. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucl. Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heckmann LH, et al. Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genom. 2006;7:175–182. doi: 10.1186/1471-2164-7-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012;80:75–84. doi: 10.1007/s11103-012-9885-2. [DOI] [PubMed] [Google Scholar]
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucl. acids res. 2001;29(9):e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foulkes NS, Borjigin J, Snyder SH, SassoneCorsi P. Rhythmic transcription: The molecular basis of circadian melatonin synthesis. Trends Neurosci. 1997;20:487–492. doi: 10.1016/S0166-2236(97)01109-0. [DOI] [PubMed] [Google Scholar]
- 41.Yamada H, Yamamoto M-T. Association between Circadian Clock genes and Diapause Incidence in Drosophila triauraria. PLoS ONE. 2011;6:e27493. doi: 10.1371/journal.pone.0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koštál V, Tollarová M, Doležel D. Dynamism in physiology and gene transcription during reproductive diapause in a heteropteran bug Pyrrhocoris apterus. J. Insect Physiol. 2008;54:77–88. doi: 10.1016/j.jinsphys.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Ragland GJ, Egan SP, Feder JL, Berlocher SH, Hahn DA. Developmental trajectories of gene expression reveal candidates for diapause termination: a key life-history transition in the apple maggot fly Rhagoletis pomonella. J. exp. Biol. 2011;214:3948–3960. doi: 10.1242/jeb.061085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided in the appendix.