Abstract
NUT midline carcinoma (NMC) is a recently described entity with a predilection for young individuals, characterised by a rearrangement of NUT, most commonly with BRD4. It usually involves midline structures, with a minority of cases presenting outside the midline axis. Given its dismal prognosis, new molecularly targeted therapies (eg, HDAC inhibitors) are gaining ground, but the HDAC expression pattern remains unknown. We describe the exceptional evolution of a NMC arising in the parotid gland. A 34-year-old male presented with a rapidly growing 35 mm left-parotid mass. Parotidectomy and lymphadenectomy were performed. The tumour invaded the surrounding soft tissue and lay adjacent to the surgical margin. No lymph node metastases were identified. Histology revealed blue nests of undifferentiated cells merging with foci of necrosis and occasional abrupt foci of keratinising squamous epithelium. FISH analysis confirmed a rearrangement of NUT, but not of BRD4. A diagnosis of NMC was rendered. Currently, after adjuvant chemoradiotherapy and 47 months after diagnosis, the patient is alive and well. The tumour was found to have increased immunoexpression of HDAC2, 4 and 6 and phospho-HDAC4/5/7. This case emphasises the importance of considering NMC in the differential diagnosis of poorly differentiated carcinomas of the head and neck region in young adults, even away from midline structures. As molecular targets hold the promise of successful therapy for the vast majority of NMC patients, the knowledge of their HDAC expression patterns will probably be relevant.
Keywords: NUT midline carcinoma, NUT gene, Salivary gland, Histone deacetylase, Histone deacetylase inhibitors, Bromodomain and extraterminal protein
Introduction
NUT midline carcinoma (NMC) is a rare, poorly differentiated carcinoma with a median overall survival of 6.7 months, or lower still (5 months, in a recent large systematic review [1]), and a predilection for young individuals, although it has also been recognised in adults [2]. Most NMCs involve supradiaphragmatic midline structures, namely the sinonasal tract and mediastinum [3]. A few unusual cases have been reported to occur below the diaphragm [4] and outside the midline axis [5, 6]. To date, only ten cases have been described involving the salivary glands (parotid, submandibular and sublingual glands) [7–14].
These tumours are defined by the presence of acquired chromosomal rearrangements of the Nuclear Protein in Testis (NUT, also known as NUTM1) gene at 15q14 locus [15]. Given the overlapping histologic features with other poorly undifferentiated carcinomas, the diagnosis needs confirmation of the rearrangement either by FISH or immunohistochemistry against the NUT protein [16–18].
Given the dismal prognosis of this genetically defined tumour with current therapeutic options, new molecularly targeted therapies—namely bromodomain and extraterminal motif (BET) and histone deacetylase (HDAC) inhibitors—are promising alternatives, with ongoing clinical trials. HDAC inhibitors have shown encouraging results in xenograft models and in two cases of paediatric NMC [19–21]. Nonetheless, the level of expression of HDAC in NMC remains to be determined.
We describe a case of parotid NMC with extraordinarily long survival and increased HDAC2, 4 and 6 and phospho-HDAC4, 5 and 7 (pHDAC457) immunoexpression. Written informed consent to this report was duly obtained from the patient.
Case Report
The case concerns a 34-year-old non-smoking male with no significant past medical history who presented with a rapidly growing left-sided neck mass for the past 6 months. Magnetic resonance imaging (MRI) revealed an isolated infiltrative intra-accessory lesion of the left parotid gland measuring 30 × 28 × 21 mm. Subsequently left radical parotidectomy with ipsilateral cervical lymphadenectomy was performed. Gross examination revealed a 35 mm white, solid and infiltrative tumour that invaded the surrounding soft tissue and was adjacent to the surgical margin. No lymphovascular or perineural invasions were observed. No lymph node metastases were identified. Histological examination showed a poorly differentiated carcinoma with nests and anastomosing strands of undifferentiated cells merging with foci of necrosis and occasional abrupt foci of well-differentiated squamous epithelium with keratinisation (Fig. 1). Immunohistochemical staining revealed positivity for cytokeratins (CAM5.2 and AE1/AE3), p63 and NUT protein. Fluorescent in situ hybridisation (FISH) analysis performed on sections cut from formalin-fixed paraffin-embedded tissue blocks—using bacterial artificial chromosome (BAC) clones as break-apart probes for both NUT and BDR4, as previously described [4]—showed rearrangement of NUT but not of BDR4. Finally, a definitive diagnosis of NMC arising in the parotid gland was rendered. Follow-up 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET) was performed with no abnormal metabolic foci.
Fig. 1.
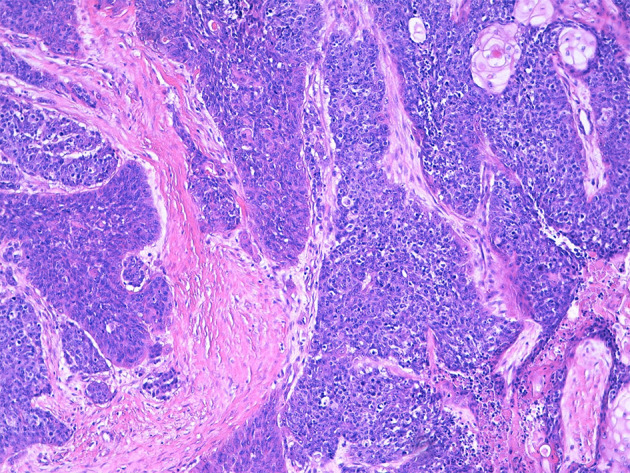
The carcinoma grows in thick anastomosing strands, with foci of abrupt keratinisation (upper right corner) and dirty necrosis (lower right corner)
The patient underwent adjuvant therapy with cisplatin and simultaneous integrated boost by intensity-modulated radiotherapy (66 Gy on the surgical bed and 54 Gy on the ipsilateral IB-III lymph node chains). As of today, almost 4 years after diagnosis (and three additional negative FDG-PET scans), he is alive and well.
The tumour immunoexpression of C-MYC, P53, HDAC1, HDAC2, HDAC3, phospho-HDAC3 (pHDAC3), HDAC4, HDAC6, HDAC7, HDAC8 and pHDAC457 was evaluated (Table 1). Since no counterpart to NMC’s tumour cells in the salivary gland is known to exist, a HDAC positive staining (ie, “overexpression”) was arbitrarily considered when the tumour had a stronger staining than any component, either ducts or acini, of the surrounding normal gland; a negative staining was considered when the tumour and the surrounding normal salivary gland had at most similar-intensity staining. HDAC2, 4 and 6 and pHDAC457 were strongly to moderately expressed in the neoplastic cells (Fig. 2). About 70% of tumour cells expressed C-MYC (Fig. 3). P53 was weakly expressed in a patchy pattern. Both were negative in the surrounding parotid gland.
Table 1.
Grading of HDAC expression in the parotid gland’s ducts and acini and the tumour, according to the relative intensity of staining by each antiserum, from 0 to 3, 0 meaning no expression (N. nucleus; C. cytoplasm; *grade 3 staining was also observed in surrounding reactive lymphoid follicles and endothelial cells)
| HDAC | 1 | 2 | 3 | p3 | 4 | 6 | 7 | 8 | p457 |
|---|---|---|---|---|---|---|---|---|---|
| Salivary ducts | 2 | 1 | 3 | 1 | 1 | 2 | 0 | 2 | 1 |
| Acini | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 1 |
| Tumour | 2* | 3 | 3* | 1 | 3* | 3 | 0* | 2 | 2* |
| Localisation | N | N | N | N | C | C | C | N | C |
Fig. 2.
a HDAC2 is strongly and diffusely expressed in all tumour nuclei, but weakly so in reactive lymphocytes’ and the parotid gland’s. b, c HDAC4 and pHDAC457 have a strong and diffuse cytoplasmic expression in all tumour cells as well as in reactive lymphocytes, but not in the parotid gland. d HDAC6 has a diffuse cytoplasmic expression in all tumour cells, stronger than in reactive lymphocytes and in the parotid gland
Fig. 3.
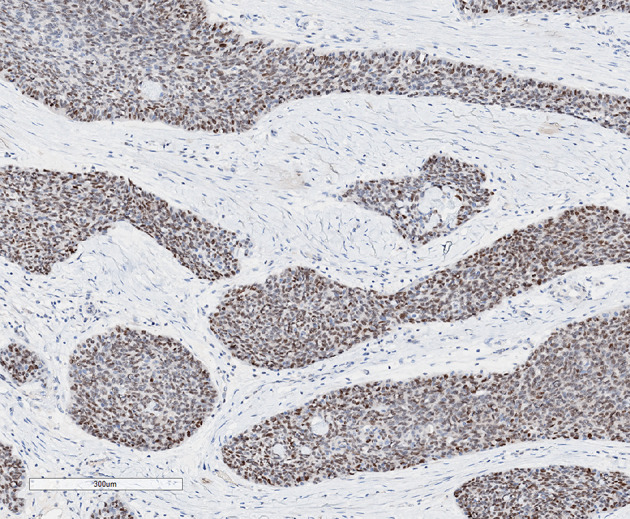
C-MYC is expressed in about 70% of the tumour cells
The immunohistochemical study was performed according to standard procedures in the BenchMark ULTRA IHC/ISH automatic-staining platform (Ventana Medical Systems, Inc.). The technical details of the antibodies are specified in Table 2.
Table 2.
Technical specifications of the antibodies used
| Target | Clone | Manufacturer | Dilution | Catalog number |
|---|---|---|---|---|
| NUT | C52B1 | Cell signaling | 1:10 | 3625S |
| MYC | Y69 | Ventana | Prediluted | 790–4628 |
| P53 | DO7 | Cell marque | 1:150 | 453 M |
| HDAC1 | 10E2 | Cell signaling | 1:2500 | 5356P |
| HDAC2 | 3F3 | Cell signaling | 1:200 | 5113P |
| HDAC3 | 7G6C5 | Cell signaling | 1:400 | 3949S |
| pHDAC3 | S424 | Cell signaling | 1:150 | 3815S |
| HDAC4 | D15C3 | Cell signaling | 1:400 | 7628S |
| HDAC6 | D2E5 | Cell signaling | 1:500 | 7558P |
| HDAC7 | D4E1L | Cell signaling | 1:400 | 33418S |
| HDAC8 | Polyclonal | Atlas antibodies | 1:250 | HPA048560 |
| pHDAC457 | S246, S259 and S155 | Cell signaling | 1:300 | 3443P |
Discussion
NMC is a rare and highly aggressive malignancy with no sex or racial predilection that primarily affects young individuals [2]. As its name implies, it occurs chiefly in midline structures, most commonly in the head, neck and mediastinum [3]. It often presents in a locally advanced stage or with distantly metastatic disease [2].
Due to its rarity, it is a frequently underdiagnosed entity, possibly misdiagnosed as a poorly differentiated conventional squamous-cell carcinoma (SCC). A high level of suspicion is therefore required: recognition of the characteristic histological features in combination with the use of specific immunohistochemistry or molecular testing is key to perform this diagnosis. In the salivary glands, NMC’s differential diagnoses include other poorly undifferentiated malignancies, such as primary or secondary SCC, high-grade basal cell adenocarcinoma and undifferentiated carcinoma [14].
These tumours are defined by an acquired chromosomal rearrangement of NUT (also known as NUTM1) at 15q14 locus [15]. Most commonly NUT is involved in a translocation with BRD4 on chromosome 19p13 and, less commonly, with BRD3, NSD3 or other still-uncharacterised genes (termed NUT variants) [15, 22]. French et al. have claimed NUT-variant carcinomas to carry a less fulminant clinical course than those with BRD4-NUT fusions [4], but this has not been confirmed in the largest study published to date [2]. If true, however, this hypothesis might explain the unexpected favourable clinical evolution shown by our patient, which is otherwise inexplicable.
The median overall survival is 6.7 months despite conventional treatment with diverse combined chemotherapies [2]. In a recent statistical analysis of 119 patients from 64 publications (including Bauer et al.’s [2]), the median survival was 5 months [1]. Hence, molecularly targeted therapies hold a promise of successful treatment for these patients.
BET inhibitors (BETi) are acetyl-histone mimetic drugs that prevent the activation of proto-oncogenes by competitively inhibiting the binding of both bromodomains (BD1 and BD2) of all BET proteins (BRD4 or BRD3) to chromatin [23]. NUT-fusion proteins drive tumour growth and block differentiation through the interaction with hyperacetylated chromatin “megadomains”, which crucially include the regulatory regions of MYC and TP63 [24]. Accordingly, we observed C-MYC overexpression by most of the tumour cells in our case.
Stathis et al. offered the first proof of BETi efficacy (OTX015/MK-8628) in four NMC patients, and also noticed the eventual emergence of resistance [25]. Secondary resistance to BETi has been hypothesised to emerge as a consequence of restoration of MYC expression through the WNT pathway or BDR4 phosphorylation, upregulation of cyclin D1, an oncogenic cyclin D3 mutant or even RB1 loss [26]. There is currently an expanded access trial available for testing the efficacy of BETi molibresib (GSK525762) in individual patients (ClinicalTrials.gov Identifier: NCT03702036).
On the other hand, HDAC inhibitors (HDACi) have exhibited preclinical and clinical activity in NMC. HDACs are known to play a central role in the regulation of several cellular properties intimately linked with the development and progression of cancer [27]. The rationale for the use of HDACi in NMC patients is the global hypoacetylation of the genome due to enhanced HDAC activity, which results in transcriptional repression of genes responsible for cell differentiation. The potential therapeutic utility of HDACi was successfully tested in three different NMC xenograft models [19] and this led to the use of pan-HDACi vorinostat in two cases of paediatric metastatic NMC [20, 21]. In both cases, objective response to HDACi was achieved but treatment had to be stopped due to severe thrombocytopenia, one of the most common adverse effects associated with these drugs [20, 21]. Additionally, the molecular interplay between BET proteins and HDACs holds a promise for effective dual inhibition in haematologic and possibly solid malignancies, as already demonstrated in vitro [28].
HDAC expression, namely of class I enzymes (HDACs 1, 2, 3 and 8), has proven to be abnormal in diverse cancer types, such as gastric, colorectal, prostate, ovarian and endometrial cancers [29]. The correlation between HDAC expression and therapeutic response to HDACi, however, remains to be elucidated. One study on urothelial carcinoma has shown that although upregulation of HDAC2 and 8 may be common, it does not predict response to vorinostat [30]. Aberrant HDAC expression has also been reported in head-and-neck SCC. Increased HDAC6 expression was found to be associated with advanced-stage oral SCC [31] and with poor prognosis in oesophageal SCC, which exhibited significant proliferation arrest and apoptosis under selective HDAC6 inhibitor ricolinostat (ACY-1215) [32]. Also, HDAC2 and 4 overexpression has been shown to play an oncogenic role in oesophageal SCC [33, 34]. HDAC8 inhibition by primarily class I-selective HDACi apicidin has demonstrated antineoplastic activity, both in vitro and in vivo murine models of oral SCC, possibly hinting to HDAC8 overexpression as a therapeutic target [35].
In conclusion, this is the first report of HDAC2, 4 and 6 and pHDAC457 overexpression in a case of parotid NMC associated with one of the longest survivals on record in medical literature. The NUT-variant rearrangement might explain this extraordinary survival, as proposed by French et al. [4]. The case also emphasises the importance of considering NMC as a differential diagnosis when a rapidly growing, poorly differentiated carcinoma of the head and neck region is identified, even when it occurs outside the midline. HDAC overexpression in NMC must be further investigated to uncover its true biological meaning and impact on clinical response to HDACi.
Funding
This work had no specific funding.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gonçalo Esteves and Joana Ferreira contributed equally to the work.
References
- 1.Giridhar P, Mallick S, Kashyap L, et al. Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. Eur Arch Otorhinolaryngol. 2018;275(3):815–821. doi: 10.1007/s00405-018-4882-y. [DOI] [PubMed] [Google Scholar]
- 2.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18(20):5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2008;63:492–496. doi: 10.1136/jcp.2007.052902. [DOI] [PubMed] [Google Scholar]
- 4.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 5.Samples S, Gleditsch K, Polimenakos A. Intrapericardial NUT midline carcinoma: unusual presentation of a rare tumor and literature review with management considerations. Pediatr Cardiol. 2016;37:208–211. doi: 10.1007/s00246-015-1313-3. [DOI] [PubMed] [Google Scholar]
- 6.Shehata BM, Steelman CK, Abranowsky CR, et al. NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a 2-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol. 2010;13:481–485. doi: 10.2350/09-10-0727-CR.1. [DOI] [PubMed] [Google Scholar]
- 7.Den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33:1253–1258. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 8.Ziai J, French CA, Zambrano E. NUT gene rearrangement in a poorly differentiated carcinoma of the submandibular gland. Head Neck Pathol. 2010;4:163–168. doi: 10.1007/s12105-010-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HS, Bae YS, Yoon SO, et al. Usefulness of nuclear protein in testis (NUT) immunohistochemistry in the cytodiagnosis of NUT midline carcinoma: a brief case report. Korean J Pathol. 2014;48:335–338. doi: 10.4132/KoreanJPathol.2014.48.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klijanienko J, Le Tourneau C, Rodriguez J, et al. Cytological features of NUT midline carcinoma arising in sino-nasal tract and parotid gland: report of two new cases and review of the literature. Diagn Cytopathol. 2016;44:753–756. doi: 10.1002/dc.23506. [DOI] [PubMed] [Google Scholar]
- 11.Vulsteke C, Lurquin E, Debiec-Rychter M, et al. First evidence of treatment efficacy in metastatic carcinoma of the parotid gland with BRD4/NUT translocation. J Chemother. 2016;28(3):242–246. doi: 10.1179/1973947815Y.0000000046. [DOI] [PubMed] [Google Scholar]
- 12.Andreasen S, French CA, Josiassen M, et al. NUT carcinoma of the sublingual gland. Head Neck Pathol. 2016;10(3):362–366. doi: 10.1007/s12105-015-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seim NB, Philips RHW, Schoenfield L, et al. NUT midline carcinoma of the sublingual gland: clinical presentation and review. Head Neck Pathol. 2017;11(4):460–468. doi: 10.1007/s12105-017-0809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agaimy A, Fonseca I, Martins C, et al. NUT carcinoma of the salivary glands: clinicopathologic and molecular analysis of 3 cases and a survey of NUT expression in salivary gland carcinomas. Am J Surg Pathol. 2018;42(7):877–884. doi: 10.1097/PAS.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French CA, Miyoshi I, Kubonishi I, et al. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 16.French CA, Miyoshi I, Aster JC, et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19) Am J Pathol. 2001;159(6):1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engleson J, Soller M, Panagopoulos I, et al. Midline carcinoma with t(15;19) and BRD4-NUT fusion oncogene in a 30-year-old female with response to docetaxel and radiotherapy. BMC Cancer. 2006;6:69. doi: 10.1186/1471-2407-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, Hong SM, Schwartz BE, Cameron MJ, Rubin MA, Chang MC, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertens F, Wiebe T, Adlercreutz C, et al. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49:1015–1017. doi: 10.1002/pbc.20755. [DOI] [PubMed] [Google Scholar]
- 21.Maher OM, Christensen AM, Yedururi S, et al. Histone deacetylase inhibitor for NUT midline carcinoma. Pediatr Blood Cancer. 2015;62:715–717. doi: 10.1002/pbc.25350. [DOI] [PubMed] [Google Scholar]
- 22.French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 23.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alekseyenko AA, Walsh EM, Wang X, et al. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015;29(14):1507–1523. doi: 10.1101/gad.267583.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stathis A, Zucca E, Bekradda M, et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6(5):492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitano M, Venturelli M, Molinaro E, et al. NUT midline carcinoma of the head and neck: current perspectives. Onco Targets Ther. 2019;12:3235–3244. doi: 10.2147/OTT.S173056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280(2):168–176. doi: 10.1016/j.canlet.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Manzotti G, Ciarrocchi A, Sancisi V. Inhibition of BET proteins and histone deacetylase (HDACs): crossing roads in cancer therapy. Cancers (Basel) 2019;11(3):304. doi: 10.3390/cancers11030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Shang YP, Chen HY, et al. Histone deacetylases function as novel potential therapeutic target for cancer. Hepatol Res. 2017;47(2):149–159. doi: 10.1111/hepr.12757. [DOI] [PubMed] [Google Scholar]
- 30.Niegish G, Knievel J, Koch A, et al. Changes in histone deacetylase (HDAC) expression patterns and activity of HDAC inhibitors in urothelial cancers. Urol Oncol. 2013;31(8):1770–1779. doi: 10.1016/j.urolonc.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Sakuma T, Uzawa K, Onda T, et al. Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. Int J Oncol. 2006;29(1):117–124. [PubMed] [Google Scholar]
- 32.Cao J, Lv W, Wang L, et al. Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell Death Dis. 2018;9(8):817. doi: 10.1038/s41419-018-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng LS, Yang XZ, Wen YF, et al. Overexpressed HDAC4 is associated with poor survival and promotes tumor progression in esophageal carcinoma. Aging (Albany, NY) 2016;8(6):1236–1248. doi: 10.18632/aging.100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Wang F, Qu Y, et al. HDAC2 regulates cell proliferation, cell cycle progression and cell apoptosis in esophageal squamous cell carcinoma EC9706 cells. Oncol Lett. 2017;13(1):403–409. doi: 10.3892/ol.2016.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn MY. HDAC inhibitor apicidin suppresses murine oral squamous cell carcinoma cell growth in vitro and in vivo via inhibiting HDAC8 expression. Oncol Lett. 2018;16(5):6552–6560. doi: 10.3892/ol.2018.9468. [DOI] [PMC free article] [PubMed] [Google Scholar]



