Abstract
Lymphoepithelial carcinoma of salivary glands (LECSG) are rare neoplasms, reported in endemic populations (southeastern Chinese) with a strong Epstein-Barr virus (EBV) association. A retrospective series comparing EBV status within an ethnically diverse population (endemic vs. non-endemic patients) has not been reported. Sixteen LECSG were equally distributed between males (n = 8) and females (n = 8) with a median age of 54 years (range 18 to 85 years) at initial diagnosis. Ten patients were white, 4 Asian, and 2 black. The patients typically presented with swelling or mass for an average of 11.6 months. Tumors affected only major salivary glands: parotid (n = 13); submandibular (n = 3). Tumors were an average of 2.9 cm (range 1.5 to 5.8 cm). Nine of 16 (56%) patients had cervical lymph node metastases at presentation. No patients had nasopharyngeal or oropharyngeal tumors. Microscopically, the tumors were widely infiltrative, characterized by large polygonal to spindled cells arranged in a syncytial, lattice-like network in a background of lymphoplasmacytic cells. The neoplastic cells showed an open-vesicular nuclear chromatin to a more basaloid-morphology, the latter showing hyperchromatic nuclei and less cytoplasm, while nearly all of the cases had associated lymphoepithelial lesions/sialadenitis. By in situ hybridization, 8 of 16 cases had a strong, diffuse EBER expression (4 of 4 Asians; 4 of 12 non-Asians), while with immunohistochemistry all cases tested were pan-cytokeratin, CK5/6 and p63 reactive; none of the cases tested were p16 reactive. All patients were managed with wide or radical excision, 4 with concurrent chemoradiation, and 6 with radiation alone. Distant metastasis (lung, brain, and bone) developed in 2 patients. Overall follow-up (mean 3.8 years) revealed 12 patients alive and 2 dead, none with evidence of disease (mean 4.3 years); one white male alive with disease at 1.9 years, and one Asian female dead of disease at 4.2 years; both of these latter patients had Group IV stage disease. High stage (Group IV) patients had a shorter mean survival than lower stage patients: 3.1 versus 4.8 years, respectively. In conclusion, LECSG are uncommon primary neoplasms. Concurrent lymphoepithelial lesions may help suggest a primary tumor. The tumors, irrespective of race or ethnicity, may express EBER. There is an overall good survival, perhaps better for EBV-negative patients and for those with lower stage disease.
Keywords: Lymphoepithelial carcinoma, Salivary glands, Immunohistochemistry, EBER, Ethnic groups
Introduction
Lymphoepithelial carcinoma (LEC) is a category of malignancy that encompasses an undifferentiated appearing squamous cell carcinoma that is associated with a prominent, nonneoplastic lymphoplasmacytic cell infiltrate [1]. Morphologically it is indistinguishable from undifferentiated nasopharyngeal carcinoma (NPC), which is the prototypical LEC [2–5]. LEC of salivary glands (LECSG) has been reported to develop with the same high frequency in endemic populations who have the highest incidence of NPC, where nearly all cases are documented to be associated with Epstein-Barr virus (EBV). The endemic populations include South East Asian and Artic Inuit populations (Greenland, northern Canada, Alaska) [6–15], with LECSG rates of up to 92% of all malignant salivary gland tumors [16]. By contrast, in non-endemic regions (such as the United Kingdom and the United States of America), the tumors represented 0.3 to 0.7% of all malignant salivary gland tumors [17, 18]. Even in the non-endemic regions, whites only represent about 7% of all reported cases, and as such a comment about EBV-association in non-endemic patients has not been well documented. This study was undertaken to report EBV-association in a cohort of patients from the United States of America and Central America (non-endemic regions) who had LECSG.
Methods
Sixteen cases of salivary gland tumors diagnosed as “lymphoepithelial carcinoma,” “anaplastic carcinoma,” “malignant lymphoepithelial lesion,” “lymphoepithelioma-like carcinoma,” and “carcinoma ex lymphoepithelial lesion” were retrospectively selected from the clinical files of the authors between 2008 and 2019. Ten cases were identified within one healthcare delivery system treating more than 4 million patient members. Materials within the files were supplemented by a review of the patient demographics (sex, age, race) and symptoms at presentation (mass, nerve symptoms, hearing changes, ulceration, other findings), including duration. Smoking and alcohol history and any other concurrent findings were identified. In addition, we reviewed the medical history, surgical pathology and operative reports, specifically noting exact tumor location, lateralization, and tumor size (greatest dimension in centimeters). Documentation of evaluation of the nasopharynx and oropharynx was included. Imaging findings, when performed, were reviewed. Follow-up data included information regarding the specific treatment, the presence or absence of recurrent or persistent disease, and the current status of the disease and patient. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization (#5968) performed under the direction of Southern California Permanente Medical Group relating to human subjects in research.
Hematoxylin and eosin-stained slides from all cases were reviewed. The following specific macroscopic and histologic observations were recorded for each tumor: tumor bosselation or lobulation; presence of salivary gland tissue; capsular invasion; perineural invasion; lymphovascular invasion; lymphoepithelial sialadenitis; mitotic index; pattern of growth; granuloma formation; amyloid deposition; lymph node number and affected lymph node number; and the presence of other microscopic pathologic findings. Staging was defined by American Joint Committee on Cancer (AJCC) Staging Manual 8th edition.
Immunophenotypic analysis was performed in all cases by a standardized Envision™ method employing 4 µm-thick, formalin fixed, paraffin embedded sections. Antibodies included AE1/AE3 (pan-cytokeratin, Dako and Becton-Dickson), CK5/6 (Dako), p63 (Leica Microsystems), p40 (BioCare), 34βE12 (K903 by Sigma), p16 (MTM Laboratories), among others (the unspecified studies were not performed on all cases). Epitope retrieval was performed, as required by the manufacturer guidelines. Standard positive controls were used throughout, with serum used as the negative control. In situ hybridization for Epstein-Barr encoding region (EBER) for small RNAs was performed using an automated benchmark XT system (Ventana Medical Systems, Inc., Tucson, AZ) with the reaction developed using a Hybrid Ready Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ). Positive signals included punctate or diffuse reactivity within tumor nuclei, with RNA controls and negative controls included.
Results
Clinical
The clinicopathologic information is summarized in Tables 1 and 2. The patients included 8 females and 8 males who ranged in age from 18 to 85 years, with a mean age at presentation of 55.4 years. There was no difference in mean age at presentation between men and women, but the Asian patients presented at a younger age than the non-Asian patients, although not statistically significant (p = 0.078). There were 2 black, 4 Asian and 10 white patients. All of the patients presented with a mass, and 3 had documented facial nerve weakness and/or nerve paralysis. There were no changes in hearing and no surface ulceration. Other findings included a history of childhood leukemia managed by radiation and chemotherapy; dermatomyositis; atopic dermatitis; and rheumatoid arthritis. Three patients had previous parotid gland tumors: pleomorphic adenoma in two patients, 5 and 8 years previously; and a Warthin tumor 16 years before in the same affected gland. One patient had a history of lung and colon carcinoma. Symptoms were present from 3 to 36 months, with an average of 11.6 months. There was no difference between men and women (p = 0.784) or Asian and non-Asian patients (p = 0.512). Tobacco use was reported in 5 patients and alcohol abuse in 4, but this parameter was not recorded in all patients. All patients had concurrent evaluation of the nasopharynx and oropharynx by endoscopic evaluation and/or imaging, without tumor documented in these sites. All tumors developed in major salivary glands, with 13 in the parotid gland and 3 in the submandibular gland. Imaging studies (documented in 13 patients as computed tomography, magnetic resonance imaging and/or ultrasound) demonstrated a solid to multilobular cystic mass within the salivary gland parenchyma, often showing heterogenous mixed enhancement, while also documenting lymph node involvement of the cervical lymph node chains (Fig. 1a).
Table 1.
Patient Information for Lymphoepithelial Carcinomas of Salivary Gland
| No | Age (years)/sex/race | Site/side/size (cm) | Symptom duration (months) | Unique clinical findings | LESA | pT | pN | AJCC8 Stage Group | EBER | Treatment | Status (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 F A | S R 2.6 | 3 | + | 2 | 1 | 3 | + | WE | Alive, NED, 6.6 | |
| 2 | 52 F A | P L 3.3 | 4 | Nerve symptoms; atopic dermatitis; pleomorphic adenoma 8 years previous | + | 4 | 2b | 4 | + | RE | Dead, disseminated disease (lung, brain), 4.2 |
| 3 | 60 F A | P L 3.0 | 3 | Ruptured cyst; Warthin tumor 16 years previous | + | 2 | 2b | 4 | + | RE, Rads, Chemo | Alive, NED, 9.4 |
| 4 | 46 M A | S R 5.8 | 18 | Hypertension | + | 3 | 0 | 3 | + | RE, Rads, Chemo | Alive, NED, 8.0 |
| 5 | 56 F B | P R 2.1 | 3 | Facial weakness | + | 2 | 0 | 2 | − | RE, Rads | Alive, NED, 1.0 |
| 6 | 50 M B | P L 3.2 | 60 | − | 2 | 0 | 2 | − | WE, Rads | Alive, NED, 0.3 | |
| 7 | 50 F W | P L 1.5 | 12 | Dermatomyositis | + | 1 | 0 | 1 | + | WE, Rads | Alive, NED, 1.6 |
| 8 | 53 F W | P L 1.6 | 11 | + | 1 | 0 | 1 | + | RE | Dead, NED, 6.1 | |
| 9 | 60 F W | P L 1.1 | 12 | Facial weakness; fell with facial injury | − | 1 | 2b | 4 | − | WE, Rads | Alive, NED, 2.0 |
| 10 | 77 F W | S L 3.0 | 36 | Skin basal cell carcinomas; pleomorphic adenoma 5 years previous; history of lung and colon carcinomas | + | 2 | 1 | 3 | − | WE | Dead, NED, 4.4 |
| 11 | 42 M W | P L 0.7 | 1 | Childhood leukemia at age 8 treated by radiation and chemotherapy | − | 1 | 0 | 1 | − | WE, Rads | Alive, NED, 6.0 |
| 12 | 54 M W | P R 3.0 | 2 | + | 2 | 0 | 2 | − | RE | Alive, NED, 4.0 | |
| 13 | 54 M W | P R 4.5 | 3 | + | 3 | 3b | 4 | + | RE, Rads | Alive, NED, 0.6 | |
| 14 | 59 M W | P R 4.2 | 7 | + | 4 | 3b | 4 | + | RE, Rads, chemo | Alive, disseminated disease (bone), 1.9 | |
| 15 | 71 M W | P L 2.5 | 3 | + | 2 | 2b | 4 | − | RE, Rads | Alive, NED, 0.8 | |
| 16 | 85 M W | P L 4.0 | 8 | Rheumatoid arthritis | + | 3 | 1 | 3 | − | RE | Alive, NED, 10.2 |
Patients ordered by race, sex and then age. pT, primary tumor category; pN, regional lymph node category; AJCC8, American Joint Committee on Cancer staging manual 8th edition
LESA lymphoepithelial sialadenitis, EBER Epstein-Barr virus encoding region, F female, M male, A Asian, B black, W white, S submandibular, P parotid, R right, L left, WE wide excision, RE radical excision, Rads radiotherapy, chemo chemotherapy, NED no evidence of disease
Table 2.
Aggregated findings of 16 Lymphoepithelial Carcinomas of Salivary Gland (LECSG)
| Characteristicsa | Number (n = 16) |
|---|---|
| Sex | |
| Female | 8 |
| Male | 8 |
| Age (years) | |
| Range | 18–85 |
| Mean | 55.4 |
| Median | 54.0 |
| Asian patients (mean) | 44.0 |
| White and black patients (mean) | 59.3 |
| Ethnicity | |
| White | 10 |
| Asian | 4 |
| Black | 2 |
| Symptom duration (months) | |
| Range | 3–36 |
| Mean | 11.6 |
| Clinical presentation | |
| Parotid mass | 13 (81%) |
| Submandibular mass | 3 (19%) |
| Lymph node involvement at presentation | 9 (56%) |
| Laterality | |
| Left | 10 |
| Right | 6 |
| Tumor size (cm) | |
| Range | 1.5–5.8 |
| Mean | 2.9 |
| Females (mean) | 2.3 |
| Males (mean) | 3.5 |
| Asians (mean) | 3.7 |
| Non-Asians (mean) | 2.6 |
| EBV status | |
| EBER positive (4 of 4 Asians; 4 Whites) | 8 |
| EBER negative | 8 |
| Group stage (AJCC8) | |
| I (NED, 4.5 years) | 3 |
| II (NED, 1.8 years) | 3 |
| III (NED, 7.3 years) | 4 |
| IV (2 with disease, 3.0 years; 4 NED, 3.2 years) | 6 |
| Therapy | |
| Surgery only (average follow-up: 5.9 years) | 6 |
| Surgery and radiation (average follow-up: 1.8 years) | 6 |
| Chemoradiation (average follow-up: 5.2 years) | 4 |
| Patients with follow up (n = 16) (mean years of follow-up) | |
| No evidence of disease | 14 (4.3) |
| Alive with disease | 1 (1.9) |
| Died of disease | 1 (4.2) |
| Follow up (years) | |
| Range | 0.3–10.2 |
| Mean | 4.2 |
| Follow up (years) | |
| Submandibular gland primaries (n = 3) | NED, 6.3 |
| Parotid gland primaries (n = 13) |
2 with disease, 3.0 years; 11 NED, 3.8 years |
| EBER positive (n = 8) |
2 with disease, 3.0 years; 6 NED, 5.4 years |
| EBER negative (n = 8) | NED, 3.6 |
aNot stated in all cases; AJCC8, American Joint Committee on Cancer staging manual 8th edition
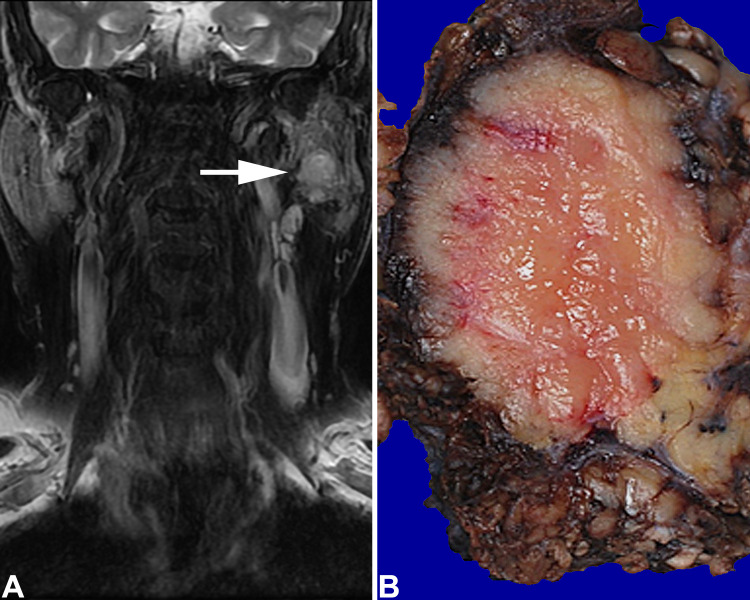
Fig. 1.
a A left parotid gland well defined hyperintense mass (arrow) on T2 weighted coronal MR, fat suppressed image. b There is a fish-flesh pale cut surface to a mass replacing much of the parotid gland
Pathologic Features
Macroscopic
The tumors ranged in size from 1.5 up to 5.8 cm, with a mean of 2.9 cm. Tumors in female patients were smaller than in male patients (p = 0.067), and smaller in non-Asians than in Asian patients (p = 0.162), but these findings were not significant. On gross examination, the tumors were unencapsulated, showing a lobular, firm, tan-white cut appearance, infiltrative into the adjacent parenchyma (Fig. 1b).
Microscopic
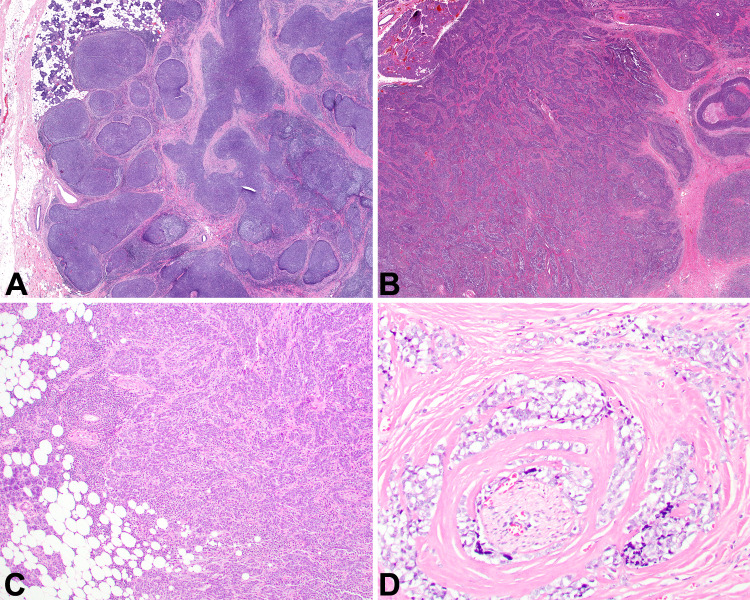
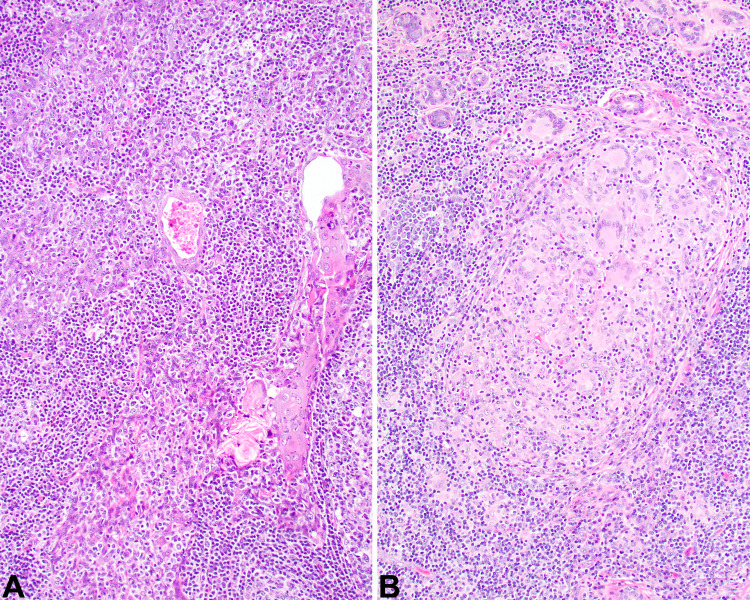
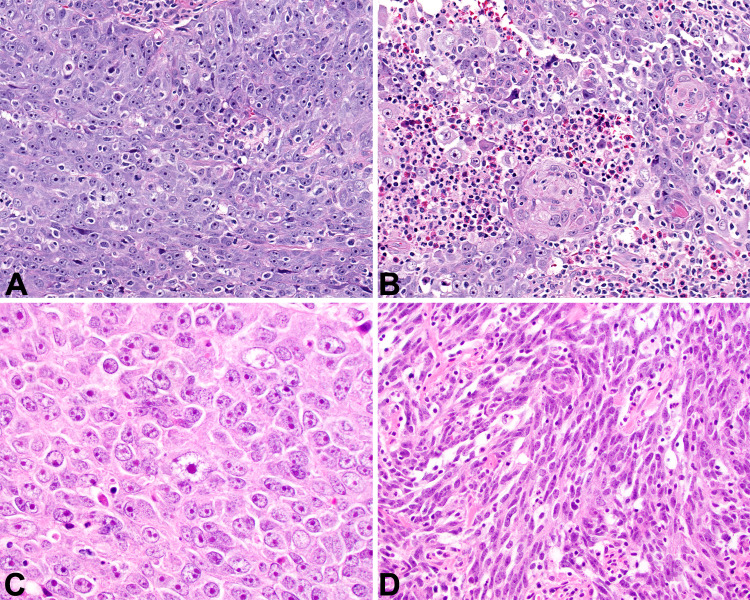
The tumors were infiltrative into the adjacent salivary gland tissue (Fig. 2a, b) and soft tissues (Fig. 2c). Perineural invasion was noted in several cases (Fig. 2d). Background lymphoepithelial sialadenitis (LESA) was noted in the majority of cases (n = 13; Fig. 3a), while granulomatous inflammation was also noted (n = 3; Fig. 3b). The tumor cells were arranged as isolated undifferentiated neoplastic cells with an intimate relationship to the lymphoid elements (Fig. 4a), or more in sheets, cords and nests or islands of syncytial neoplastic cells surrounded by the lymphoid stroma (Fig. 4b, c). A jigsaw puzzle appearance was noted, but was not the dominant finding (Fig. 4d). Mitotic activity was greatly increased and included atypical forms. The neoplastic cells were crowded, large, undifferentiated cells with a very high nuclear to cytoplasmic ratio. Their nuclei were irregular to oval with open to vesicular nuclear chromatin with prominent, hypereosinophilic nucleoli (Fig. 5a), while in more basaloid areas, the nuclei were more hyperchromatic. Isolated areas of keratinization were seen in a few cases (Fig. 5b). Pleomorphism was conspicuous (Fig. 5c). Tumor cell spindling was noted in several cases (Fig. 5d). The cytoplasm was sparse, delicate, eosinophilic to amphophilic with indistinct cell borders.
Fig. 2.
a Broad sheets and nests of neoplastic cells invade into the parotid gland as large islands (10x). b Smaller islands of epithelial cells associated with sclerosis and tumor necrosis (20x). c Infiltration of the neoplastic cells into the adjacent adipose tissue (100x). d Perineural invasion by the neoplastic cells (400x)
Fig. 3.
a Squamous metaplasia of a duct, with an associated lymphoepithelial lesion showing an intimate relationship between the lymphoid elements and the epithelium, but without atypia (400x). b Numerous foreign-body type giant cells are seen at the advancing edge of the tumor, representing a granulomatous reaction (400x)
Fig. 4.
a The neoplastic cells are difficult to identify as they blend with the lymphoid stroma (400x). b Islands of interconnecting neoplastic cells (200x). c A sheet-like distribution of syncytial neoplastic cells (400x). d A jigsaw puzzle-like architecture with prominent sclerotic stroma (100x)
Fig. 5.
a Syncytium of pleomorphic cells with high nuclear to cytoplasmic ratio (400x). b Focal areas of squamous differentiation and adjacent tumor necrosis (400x). c Pleomorphic tumor cells showing prominent nucleoli within vesicular nuclei (400x). d Tumor cell spindling was noted in a few cases (400x)
Immunohistochemical and In Situ Hybridization Results
All tumors tested demonstrated a squamous epithelial phenotype, with reactions to CK5/6 (Fig. 6a), AE1/AE3 (Fig. 6b), p40, p63 (Fig. 6c), EMA and CK903. EBER ISH was positive in 8 of 16 cases tested (Fig. 6d). While these EBV studies confirmed the diagnosis is endemic patients, they were not always seen in non-endemic patients (Table 1). Thus, in this series, 8 of 16 tumors tested lacked EBER and/or LMP1, a finding seen in 6 white patients and 2 black patients (Tables 1 and 2). The overall histologic pattern and epithelial reactivity was still characteristic of LECSG in these non-EBV-associated eight patients.
Fig. 6.
a There is a strong, diffuse, membranous and cytoplasmic reaction with CK5/6 (100x). b There is a wispy, lattice-like cytoplasmic reaction with AE1/AE3 (400x). c The nuclei of the neoplastic cells are strongly reactive with p63 (400x). d A strong, diffuse, nuclear reaction in all of the neoplastic epithelial cells with EBER ISH (200x)
Treatment and Follow-up
All patients were managed by surgery, including wide excision and total parotidectomy (Table 1). At the time of presentation and initial management, 7 patients (4 EBV-negative) had no lymph node disease. The remaining 9 patients had lymph node involvement: 3 patients with pN1; 4 patients with pN2b; and 2 patients with pN3b disease. Based on the tumor size and extent of invasion, there were 4 pT1, 7 pT2, 3 pT3 and 2 pT4 tumors. Thus, overall, based on the AJCC 8th edition staging, there were 3 Group I; 3 Group II; 4 Group III; and 6 Group IV patients, respectively. Irrespective of treatment, all Group I, II, and III patients were either alive or had died of unrelated causes, and were without evidence of disease at last follow-up (mean follow-up, 4.3 years). In Group IV patients (overall mean, 3.1 years), 2 patients were alive or had died with disease (mean 3.0 years) with the remaining 4 patients alive without evidence of disease (mean 3.2 years). Therefore, patients with higher stage disease were more likely to develop metastatic disease or die with disease than lower stage patients. There was no statistically significant survival difference between submandibular gland primaries and parotid gland primaries. There does not appear to be a difference in outcome based on ethnicity: 1 Asian patient had died with disease (4.2 years), and 1 white patient was alive with disease (1.9 years), while all others had no evidence of disease. Similarly, there is no difference in outcome between females (mean 4.4 years) or males (mean 4.0 years), with one each having disease at last follow-up. All of the 8 EBER-negative patients were without evidence of disease at last follow-up (mean 3.6 years), while 2 of the EBER-positive patients had disease (mean 3.0 years) and 6 patients were without evidence of disease (mean 5.4 years).
Of the patients managed by surgery alone, 1 had died with disease (4.2 years), while the remaining 5 were alive or dead without disease (average 6.3 years). Six patients were managed with surgery and radiation and all were alive without evidence of disease, but only followed for a mean of 1.8 years. Of the 4 patients managed with combination surgery, radiation and chemotherapy, 1 was alive with disease (1.9 years), and the other 3 were alive without evidence of disease (mean 6.3 years).
Discussion
In the vast majority of LECSG cases, the etiologic role of EBV is well documented, with EBV identified in the neoplastic epithelial cells by in situ hybridization or latent membrane protein (LMP) reactivity [6, 8, 15, 16, 19, 20]. The EBV-association is particularly well developed in patients who are also well known to develop NPC, with remarkably overlapped incidence of the endemic patients: South eastern Chinese, Arctic region natives (Eskimos/Inuits from Alaska, Canada, Greenland), Japanese, northern Africans, and Mongolians [6, 8–15, 21–25]. Whites and blacks make up only a small proportion of patients [17, 18], without a well-documented EBV-association. In this cohort of patients, all Asian patients' tumors were EBV-associated, but neither of the black patients' tumors were EBV-associated, and only 4 of 10 white patients' tumors showed EBV-association. Further, when tested, these EBV-negative cases did not show p16 immunoreactivity. Thus, there is clearly a much more complicated interaction between genetic (ethnic), environmental, geographical, behavioral and viral (EBV) factors in the oncogenic process of LEC [18, 25, 26].
There was no sex predilection in our patients, similar to reported data. In this case series, there is a wide age range at presentation, although when separated by ethnicity, non-endemic patients tend to be older (mean 59.3 years) than endemic patients (mean 44.0 years), a finding similarly reported in the literature for non-endemic patients (median, 62 years) [18, 27–32].
A mass is the usual clinical presentation, with symptoms usually present for about 12 months. Major salivary gland sites are most commonly affected, and account for 93% of the cases in the literature, similar to our results. More than half of the patients in this series had cervical lymph node involvement at the time of presentation, a finding that is higher than reported in the literature (about 15%) [10, 33–36].
LEC shows a histologic appearance that is quite indistinguishable from NPC, non-keratinizing (undifferentiated) type, although the ratio of epithelial to lymphoid component is quite variable cases to case. In this series, 81% (13 of 16) of cases showed a background of lymphoepithelial sialadenitis (LESA). Whether it is a reaction to the tumor or a precursor of the neoplasm, when it is seen adjacent to or within the tumor, it favors a primary lesion over a metastatic tumor [2, 5, 31, 37–41]. None of the patients in this series had laboratory findings supporting Sjögren syndrome. The pattern of either individual cells or islands-sheets of cells, arranged as a syncytium of crowded, large, undifferentiated cells with vesicular to open nuclear chromatin, adjacent to or blended with a lymphoplasmacytic stroma is classical and characteristic [10, 22, 42]. Definitive squamous differentiation may be present, but is usually limited, while a basaloid morphology can be identified [43]. Noncaseating granulomatous inflammation including multinucleated giant cells and even amyloid may rarely be seen [42, 44–46]. A stromal desmoplastic reaction may be focally noted, but is not usually a prominent finding. Tumor cell spindling may be prominent, and when present brings to mind basal cell adenoma/adenocarcinoma and myoepithelial carcinoma [5, 8, 31, 47, 48]. However, none of these other tumors are reactive with nuclear EBER by ISH [8, 9, 25, 28, 47]. While it could go without saying, importantly, no EBER nuclear reaction was identified in the adjacent lymphocytes nor in the epithelial cells of LESA. Even though 8 cases in this series were EBER-negative, the overall histologic pattern and epithelial reactivity was still characteristic of LECSG, and the outcome is identical to the EBER-positive cases. Thus, the diagnosis should still be applied in these cases.
A variety of reactive and neoplastic conditions may be brought to mind when evaluating this type of “undifferentiated” lesion, but a lymphoepithelial lesion, reactive tumor associated lymphoid proliferation (TALP), lymphadenoma, and Warthin tumor generally lack a rapid clinical onset, any significant atypia, are not destructive, maintain a lobular or even distribution of epithelial elements, an oncocytically altered epithelium or have a papillary architecture, and are negative with EBER [49–53]. Generally, lymphomas of the salivary gland are either extranodal marginal zone B-cell lymphomas (which are not EBV-associated) or diffuse large B-cell lymphomas, some of which may be EBV-associated [54–58]. In lymphomas, the lack of any epithelial markers will aid in diagnosis, whether EBV-associated or not [55, 58–60]. Exclusion of a nasopharyngeal primary has already been stated, but clinical, endoscopic, imaging and even biopsy results from the nasopharynx must be incorporated to exclude this possibility. Rarely, a lymphoepithelial pattern may be seen in HPV-associated oropharyngeal squamous cell carcinoma, and salivary gland metastasis may be seen. A strong, diffuse, nuclear and cytoplasmic p16 reaction in > 70% of the neoplastic cells and/or ISH for high risk human papillomavirus may be helpful in this setting to confirm an oropharyngeal primary [61–63]. Poorly differentiated squamous cell carcinoma (SCC) may present as metastatic disease to the parotid gland and associated lymph nodes. Most are not a lymphoepithelial pattern, but show well developed keratinization and squamous pearl formation. Skin primaries are not EBER reactive, but would be difficult to separate in EBV-negative LECSG. Just like excluding a nasopharyngeal primary site, careful clinical correlation with known skin or mucosal primary SCC must be achieved. Large cell undifferentiated carcinoma (lacking evidence of glandular, squamous, or neuroendocrine differentiation) has an organoid growth, minimal differentiation, high mitotic rate, and coagulative necrosis, but lacks the lymphoid infiltrate and by definition lacks EBV [10, 64–66]. Thus, in EBV-negative LECSG, the lack of lymphoid infiltrate would help make this distinction, a challenge if reviewing only core needle samples. Metastatic melanoma is non-cohesive, but will be reactive with various melanocytic markers [67–69].
The optimal management of LECSG is complete surgical resection with clear surgical margins followed by adjuvant radiotherapy to the tumor bed and neck. Wider surgical margins may not be beneficial, associated with decreased progression free survival and neck fibrosis [5, 15, 18, 70]. As lymph node metastasis is frequently present, elective neck dissections should be performed when biopsy proven metastases or suspicious imaging findings are documented [10, 15, 23, 25, 71]. While there is a stage-related outcome difference, lymph node status at presentation may not adversely affect prognosis [18]. It seems that combination therapies, including chemotherapy, may yield the best overall patient outcome, as it is well known that LEC is highly radiosensitive with high rates of locoregional tumor control [31]. Similar to the literature, when disseminated disease is documented, there is a strong correlation with death from disease [10, 15, 18, 25, 65, 71–73]. The overall 81% 5-year raw survival [10, 15, 18, 22, 36, 74–76] is quite similar to this cohort of patients, the majority of whom are alive without evidence of disease (mean, 4.3 years).
Conclusions
LECSG irrespective of race or ethnicity, may express EBER, but a significant proportion of non-endemic patients may not be EBV-associated. Patients are usually middle aged without a sex bias, presenting with a mass in a major salivary gland site. Concurrent lymphoepithelial lesions may help suggest a primary tumor. Still, it must be emphasized that thorough clinical, endoscopic and imaging studies of the nasopharynx must be completed prior to implementing therapy. The undifferentiated histology associated with a nonneoplastic lymphoplasmacytic cell infiltrate is quite classical for LEC, with EBER easily identified by ISH when tumors are EBV-associated. As the tumors are exquisitely radiosensitive, combination multimodality therapy will yield an excellent outcome, whether EBV-associated or not and whether lymph node metastases are present or not.
Acknowledgements
Data presented as Abstract #3, Poster #96 at the 109th Annual Meeting of the United States and Canadian Academy of Pathology, March 3, 2020.
Funding
No external funding was obtained for this study.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB #5968), which did not require informed consent. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lewis JS, El-Mofty SK, Nicolai P. Lymphoepithelial Carcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumors. Lyon: IARC; 2017. pp. 181–182. [Google Scholar]
- 2.Cleary KR, Batsakis JG. Undifferentiated carcinoma with lymphoid stroma of the major salivary glands. Ann Otol Rhinol Laryngol. 1990;99:236–238. [PubMed] [Google Scholar]
- 3.Thompson LD. Update on nasopharyngeal carcinoma. Head Neck Pathol. 2007;1:81–86. doi: 10.1007/s12105-007-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson LDR. Head and neck cancer. In: Stewart BW, Wild CP, editors. World cancer report. Lyon: IARCPress; 2014. pp. 422–435. [Google Scholar]
- 5.Wenig BM. Lymphoepithelial-like carcinomas of the head and neck. Semin Diagn Pathol. 2015;32:74–86. doi: 10.1053/j.semdp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Arthaud JB. Anaplastic parotid carcinoma ("malignant lymphoepithelial lesion") in seven Alaskan natives. Am J Clin Pathol. 1972;57:275–286. doi: 10.1093/ajcp/57.3.275. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen NH, Mikkelsen F, Hansen JP. Incidence of salivary gland neoplasms in Greenland with special reference to an anaplastic carcinoma. Acta Pathol Microbiol Scand A. 1978;86:185–193. doi: 10.1111/j.1699-0463.1978.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagao T, Ishida Y, Sugano I, et al. Epstein-Barr virus-associated undifferentiated carcinoma with lymphoid stroma of the salivary gland in Japanese patients. Comparison with benign lymphoepithelial lesion. Cancer. 1996;78:695–703. doi: 10.1002/(SICI)1097-0142(19960815)78:4<695::AID-CNCR1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Saku T, Cheng J, Jen KY, et al. Epstein-Barr virus infected lymphoepithelial carcinomas of the salivary gland in the Russia-Asia area: a clinicopathologic study of 160 cases. Arkh Patol. 2003;65:35–39. [PubMed] [Google Scholar]
- 10.Wang CP, Chang YL, Ko JY, Lou PJ, Yeh CF, Sheen TS. Lymphoepithelial carcinoma versus large cell undifferentiated carcinoma of the major salivary glands. Cancer. 2004;101:2020–2027. doi: 10.1002/cncr.20614. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Qing J, Wei MW. Guo ZM [Clinical analysis of sixteen cases of lymphoepithelial carcinoma of salivary gland] Ai Zheng. 2005;24:1384–1387. [PubMed] [Google Scholar]
- 12.Li LJ, Li Y, Wen YM, Liu H, Zhao HW. Clinical analysis of salivary gland tumor cases in West China in past 50 years. Oral Oncol. 2008;44:187–192. doi: 10.1016/j.oraloncology.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Tian Z, Li L, Wang L, Hu Y, Li J. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg. 2010;39:235–242. doi: 10.1016/j.ijom.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Wang YL, Zhu YX, Chen TZ, et al. Clinicopathologic study of 1176 salivary gland tumors in a Chinese population: experience of one cancer center 1997–2007. Acta Otolaryngol. 2012;132:879–886. doi: 10.3109/00016489.2012.662715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Zhu G, Wang Y, Wang Y, Chen T, Ji Q. A clinical analysis of 37 cases with lymphoepithelial carcinoma of the major salivary gland treated by surgical resection and postoperative radiotherapy: a single institution study. Med Oncol. 2014;31:957. doi: 10.1007/s12032-014-0957-9. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurthy S, Lanier AP, Dohan P, Lanier JF, Henle W. Salivary gland cancer in Alaskan natives, 1966–1980. Hum Pathol. 1987;18:986–996. doi: 10.1016/s0046-8177(87)80214-9. [DOI] [PubMed] [Google Scholar]
- 17.Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44:407–417. doi: 10.1016/j.oraloncology.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhan KY, Nicolli EA, Khaja SF, Day TA. Lymphoepithelial carcinoma of the major salivary glands: Predictors of survival in a non-endemic region. Oral Oncol. 2016;52:24–29. doi: 10.1016/j.oraloncology.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Chan JK, Yip TT, Tsang WY, Poon YF, Wong CS, Ma VW. Specific association of Epstein-Barr virus with lymphoepithelial carcinoma among tumors and tumorlike lesions of the salivary gland. Arch Pathol Lab Med. 1994;118:994–997. [PubMed] [Google Scholar]
- 20.Hanji D, Gohao L. Malignant lymphoepithelial lesions of the salivary glands with anaplastic carcinomatous change. Report of nine cases and review of literature. Cancer. 1983;52:2245–2252. doi: 10.1002/1097-0142(19831215)52:12<2245::aid-cncr2820521214>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Albeck H, Nielsen NH, Hansen HE, et al. Epidemiology of nasopharyngeal and salivary gland carcinoma in Greenland. Arctic Med Res. 1992;51:189–195. [PubMed] [Google Scholar]
- 22.Sheen TS, Tsai CC, Ko JY, Chang YL, Hsu MM. Undifferentiated carcinoma of the major salivary glands. Cancer. 1997;80:357–363. [PubMed] [Google Scholar]
- 23.Tsai CC, Chen CL, Hsu HC. Expression of Epstein-Barr virus in carcinomas of major salivary glands: a strong association with lymphoepithelioma-like carcinoma. Hum Pathol. 1996;27:258–262. doi: 10.1016/s0046-8177(96)90066-0. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton-Dutoit SJ, Therkildsen MH, Neilsen NH, Jensen H, Hansen JP, Pallesen G. Undifferentiated carcinoma of the salivary gland in Greenlandic Eskimos: demonstration of Epstein-Barr virus DNA by in situ nucleic acid hybridization. Hum Pathol. 1991;22:811–815. doi: 10.1016/0046-8177(91)90210-g. [DOI] [PubMed] [Google Scholar]
- 25.Leung SY, Chung LP, Yuen ST, Ho CM, Wong MP, Chan SY. Lymphoepithelial carcinoma of the salivary gland: in situ detection of Epstein-Barr virus. J Clin Pathol. 1995;48:1022–1027. doi: 10.1136/jcp.48.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mozaffari HR, Ramezani M, Janbakhsh A, Sadeghi M. Malignant salivary gland tumors and epstein-barr virus (EBV) infection: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017;18:1201–1206. doi: 10.22034/APJCP.2017.18.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelkrim SB, Trabelsi A, Hammedi F, et al. Primary lymphoepithelial carcinoma of the parotid gland in a North African woman. Rare Tumors. 2009;1:e16. doi: 10.4081/rt.2009.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bialas M, Sinczak A, Choinska-Stefanska A, Zygulska A. EBV-positive lymphoepithelial carcinoma of salivary gland in a woman of a non-endemic area–a case report. Pol J Pathol. 2002;53:235–238. [PubMed] [Google Scholar]
- 29.Gallo O, Santucci M, Calzolari A, Storchi OF. Epstein-Barr virus (EBV) infection and undifferentiated carcinoma of the parotid gland in Caucasian patients. Acta Otolaryngol. 1994;114:572–575. doi: 10.3109/00016489409126107. [DOI] [PubMed] [Google Scholar]
- 30.Larbcharoensub N, Tubtong N, Praneetvatakul V, Pongtippan A, Leopairat J, Sirikulchayanonta V. Epstein-Barr virus associated lymphoepithelial carcinoma of the parotid gland; a clinicopathological report of three cases. J Med Assoc Thai. 2006;89:1536–1541. [PubMed] [Google Scholar]
- 31.Manganaris A, Patakiouta F, Xirou P, Manganaris T. Lymphoepithelial carcinoma of the parotid gland: is an association with Epstein-Barr virus possible in non-endemic areas? Int J Oral Maxillofac Surg. 2007;36:556–559. doi: 10.1016/j.ijom.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Mrad K, Ben Brahim E, Driss M, Abbes I, Marakchi M, Ben RK. Lymphoepithelioma-like carcinoma of the submandibular salivary gland associated with Epstein-Barr virus in a North African woman. Virchows Arch. 2004;445:419–420. doi: 10.1007/s00428-004-1072-7. [DOI] [PubMed] [Google Scholar]
- 33.Ban X, Wu J, Mo Y, et al. Lymphoepithelial carcinoma of the salivary gland: morphologic patterns and imaging features on CT and MRI. AJNR Am J Neuroradiol. 2014;35:1813–1819. doi: 10.3174/ajnr.A3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Yang J, Yu Q. Lymphoepithelial carcinoma of salivary glands: CT and MR imaging findings. Dentomaxillofac Radiol. 2017;46:20170053. doi: 10.1259/dmfr.20170053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao L, Zhang Y, Chen Q, et al. Diagnosis of lymphoepithelial carcinoma in parotid gland with three dimensional computed tomography angiography reconstruction: a case report. J Xray Sci Technol. 2018;26:155–164. doi: 10.3233/XST-17347. [DOI] [PubMed] [Google Scholar]
- 36.Zhang G, Tang J, Pan Y, Zhuang Q, Wu C. CT features and pathologic characteristics of lymphoepithelial carcinoma of salivary glands. Int J Clin Exp Pathol. 2014;7:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis GL. Lymphoid lesions of salivary glands: malignant and benign. Med Oral Patol Oral Cir Bucal. 2007;12:E479–485. [PubMed] [Google Scholar]
- 38.Kim KI, Kim YS, Kim HK, Chae YS, Yoem BW, Kim I. The detection of Epstein-Barr virus in the lesions of salivary glands. Pathol Res Pract. 1999;195:407–412. doi: 10.1016/S0344-0338(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 39.James PD, Ellis IO. Malignant epithelial tumours associated with autoimmune sialadenitis. J Clin Pathol. 1986;39:497–502. doi: 10.1136/jcp.39.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiGiuseppe JA, Corio RL, Westra WH. Lymphoid infiltrates of the salivary glands: pathology, biology and clinical significance. Curr Opin Oncol. 1996;8:232–237. doi: 10.1097/00001622-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Schneider M, Rizzardi C. Lymphoepithelial carcinoma of the parotid glands and its relationship with benign lymphoepithelial lesions. Arch Pathol Lab Med. 2008;132:278–282. doi: 10.5858/2008-132-278-LCOTPG. [DOI] [PubMed] [Google Scholar]
- 42.Saw D, Lau WH, Ho JH, Chan JK, Ng CS. Malignant lymphoepithelial lesion of the salivary gland. Hum Pathol. 1986;17:914–923. doi: 10.1016/s0046-8177(86)80641-4. [DOI] [PubMed] [Google Scholar]
- 43.Friborg J, Hamilton-Therkildsen M, Homoe P, et al. A spectrum of basaloid morphology in a subset of EBV-associated "lymphoepithelial carcinomas" of major salivary glands. Head Neck Pathol. 2012;6:445–450. doi: 10.1007/s12105-012-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace AC, MacDougall JT, Hildes JA, Lederman JM. Salivary gland tumors in Canadian Eskimos. Cancer. 1963;16:1338–1353. doi: 10.1002/1097-0142(196310)16:10<1338::aid-cncr2820161015>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 45.Hilderman WC, Gordon JS, Large HL, Jr, Carroll CF., Jr Malignant lymphoepithelial lesion with carcinomatous component apparently arising in parotid gland. A malignant counterpart of benign lymphoepithelial lesion? Cancer. 1962;15:606–610. doi: 10.1002/1097-0142(196205/06)15:3<606::aid-cncr2820150322>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 46.Kuo T, Hsueh C. Lymphoepithelioma-like salivary gland carcinoma in Taiwan: a clinicopathological study of nine cases demonstrating a strong association with Epstein-Barr virus. Histopathology. 1997;31:75–82. doi: 10.1046/j.1365-2559.1997.5830814.x. [DOI] [PubMed] [Google Scholar]
- 47.Christiansen MS, Mourad WA, Hales ML, Oldring DJ. Spindle cell malignant lymphoepithelial lesion of the parotid gland: clinical, light microscopic, ultrastructural, and in situ hybridization findings in one case. Mod Pathol. 1995;8:711–715. [PubMed] [Google Scholar]
- 48.Nagao K, Matsuzaki O, Saiga H, et al. A histopathologic study of benign and malignant lymphoepithelial lesions of the parotid gland. Cancer. 1983;52:1044–1052. doi: 10.1002/1097-0142(19830915)52:6<1044::aid-cncr2820520620>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Prasad ML, Chiosea S, Ihrler S, Skalova A. Tumours of salivary glands: benign neoplasms: lymphadenoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO Classification of head and neck tumours. Lyon: IARC; 2017. pp. 190–191. [Google Scholar]
- 50.Liu G, He J, Zhang C, Fu S, He Y. Lymphadenoma of the salivary gland: report of 10 cases. Oncol Lett. 2014;7:1097–1101. doi: 10.3892/ol.2014.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seethala RR, Thompson LD, Gnepp DR, et al. Lymphadenoma of the salivary gland: clinicopathological and immunohistochemical analysis of 33 tumors. Mod Pathol. 2012;25:26–35. doi: 10.1038/modpathol.2011.135. [DOI] [PubMed] [Google Scholar]
- 52.Patel DK, Morton RP. Demographics of benign parotid tumours: Warthin's tumour versus other benign salivary tumours. Acta Otolaryngol. 2016;136:83–86. doi: 10.3109/00016489.2015.1081276. [DOI] [PubMed] [Google Scholar]
- 53.Schmitt AC, Cohen C, Siddiqui MT. Expression of SOX10 in salivary gland oncocytic neoplasms: a review and a comparative analysis with other immunohistochemical markers. Acta Cytol. 2015;59:384–390. doi: 10.1159/000441890. [DOI] [PubMed] [Google Scholar]
- 54.Vazquez A, Khan MN, Sanghvi S, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the salivary glands: a population-based study from 1994 to 2009. Head Neck. 2015;37:18–22. doi: 10.1002/hed.23543. [DOI] [PubMed] [Google Scholar]
- 55.Agaimy A, Wild V, Markl B, et al. Intraparotid classical and nodular lymphocyte-predominant Hodgkin lymphoma: pattern analysis with emphasis on associated lymphadenoma-like proliferations. Am J Surg Pathol. 2015;39:1206–1212. doi: 10.1097/PAS.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 56.Mian M, Capello D, Ventre MB, et al. Early-stage diffuse large B cell lymphoma of the head and neck: clinico-biological characterization and 18 year follow-up of 488 patients (IELSG 23 study) Ann Hematol. 2014;93:221–231. doi: 10.1007/s00277-013-1856-4. [DOI] [PubMed] [Google Scholar]
- 57.Laviv A, Sohani AR, Troulis MJ. Lymphoma mimics obstructive sialadenitis: three cases. J Oral Maxillofac Surg. 2014;72(7):1325.e1–1325.e11. doi: 10.1016/j.joms.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 58.Feinstein AJ, Ciarleglio MM, Cong X, Otremba MD, Judson BL. Parotid gland lymphoma: prognostic analysis of 2140 patients. Laryngoscope. 2013;123:1199–1203. doi: 10.1002/lary.23901. [DOI] [PubMed] [Google Scholar]
- 59.Roh JL, Huh J, Suh C. Primary non-Hodgkin's lymphomas of the major salivary glands. J Surg Oncol. 2008;97:35–39. doi: 10.1002/jso.20901. [DOI] [PubMed] [Google Scholar]
- 60.Tiplady CW, Taylor PR, White J, Arullendran P, Proctor SJ. Lymphoma presenting as a parotid tumour: a population-based study of diagnosis, treatment and outcome on behalf of the Scotland and Newcastle Lymphoma Group. Clin Oncol (R Coll Radiol) 2004;16:414–419. doi: 10.1016/j.clon.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Thompson LDR, Burchette R, Iganej S, Bhattasali O. Oropharyngeal squamous cell Carcinoma in 390 patients: analysis of clinical and histological criteria which significantly impact outcome. Head Neck Pathol. 2019 doi: 10.1007/s12105-019-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carpenter DH, El-Mofty SK, Lewis JS., Jr Undifferentiated carcinoma of the oropharynx: a human papillomavirus-associated tumor with a favorable prognosis. Mod Pathol. 2011;24:1306–1312. doi: 10.1038/modpathol.2011.87. [DOI] [PubMed] [Google Scholar]
- 63.Singhi AD, Stelow EB, Mills SE, Westra WH. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of HPV-related head and neck carcinoma. Am J Surg Pathol. 2010;34:800–805. doi: 10.1097/PAS.0b013e3181d9ba21. [DOI] [PubMed] [Google Scholar]
- 64.Batsakis JG, Luna MA. Undifferentiated carcinomas of salivary glands. Ann Otol Rhinol Laryngol. 1991;100:82–84. doi: 10.1177/000348949110000115. [DOI] [PubMed] [Google Scholar]
- 65.Hatta C, Terada T, Okita J, Kakibuchi M, Kubota A, Sakagami M. Clinicopathological study of undifferentiated carcinoma of the parotid gland. Auris Nasus Larynx. 2003;30:273–277. doi: 10.1016/s0385-8146(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 66.Hui KK, Luna MA, Batsakis JG, Ordonez NG, Weber R. Undifferentiated carcinomas of the major salivary glands. Oral Surg Oral Med Oral Pathol. 1990;69:76–83. doi: 10.1016/0030-4220(90)90271-s. [DOI] [PubMed] [Google Scholar]
- 67.Wick MR. Primary lesions that may imitate metastatic tumors histologically: a selective review. Semin Diagn Pathol. 2018;35:123–142. doi: 10.1053/j.semdp.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Den Hondt M, Starr MW, Millett MC, et al. Surgical management of the neck in patients with metastatic melanoma in parotid lymph nodes. J Surg Oncol. 2019;120:1462–1469. doi: 10.1002/jso.25732. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Hoda RS, Faquin W, et al. FNA biopsy of secondary nonlymphomatous malignancies in salivary glands: a multi-institutional study of 184 cases. Cancer Cytopathol. 2017;125:91–103. doi: 10.1002/cncy.21798. [DOI] [PubMed] [Google Scholar]
- 70.Kim YJ, Hong HS, Jeong SH, Lee EH, Jung MJ. Lymphoepithelial carcinoma of the salivary glands. Medicine (Baltimore) 2017;96:e6115. doi: 10.1097/MD.0000000000006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao W, Deng N, Gao X, et al. Primary lymphoepithelioma-like carcinoma of salivary glands: a clinicopathological study of 21 cases. Int J Clin Exp Pathol. 2014;7:7951–7956. [PMC free article] [PubMed] [Google Scholar]
- 72.Bosch JD, Kudryk WH, Johnson GH. The malignant lymphoepithelial lesion of the salivary glands. J Otolaryngol. 1988;17:187–190. [PubMed] [Google Scholar]
- 73.Lanier AP, Clift SR, Bornkamm G, Henle W, Goepfert H, Raab-Traub N. Epstein-Barr virus and malignant lymphoepithelial lesions of the salivary gland. Arctic Med Res. 1991;50:55–61. [PubMed] [Google Scholar]
- 74.Hsiung CY, Huang CC, Wang CJ, Huang EY, Huang HY. Lymphoepithelioma-like carcinoma of salivary glands: treatment results and failure patterns. Br J Radiol. 2006;79:52–55. doi: 10.1259/bjr/17905092. [DOI] [PubMed] [Google Scholar]
- 75.Abdulla AK, Mian MY. Lymphoepithelial carcinoma of salivary glands. Head Neck. 1996;18:577–581. doi: 10.1002/(SICI)1097-0347(199611/12)18:6<577::AID-HED13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 76.Ma Q, Song H. Diagnosis and management of lymphoepithelial lesion of the parotid gland. Rheumatol Int. 2011;31:959–962. doi: 10.1007/s00296-010-1617-9. [DOI] [PubMed] [Google Scholar]