Abstract
Data on the occurrence and clinicopathological characteristics of actinic cheilitis (AC) and lip squamous cell carcinoma (LSCC) are well studied; however, they are based on studies limited to a single centre. Herein, we described the frequency of AC and LSCC submitted to microscopic examination from representative geographic regions of Brazil. A retrospective multicentre study was performed on biopsies obtained from 1953 to 2018 at 10 Brazilian oral and maxillofacial pathology centres. A total of 198,709 biopsy specimens were surveyed. Sociodemographic data and clinicopathologic characteristics were analysed. A total of 2017 cases of ACs (1.0%) and 850 cases of LSCCs (0.4%) were recorded. A strong fair-skinned (> 87%) male (> 70%) predilection was observed in both conditions. The mean age was 54.8 ± 18.7 for individuals with AC and 57.8 ± 19.0 for individuals with LSCC. The most commonly affected site was the lower lip (> 90%). This is a large multicentre study of AC and LSCC from Brazil. The frequency and clinicopathological features of AC and LSCC were similar to those described worldwide. This study provides robust and representative epidemiological data of these conditions for the scientific community.
Keywords: Actinic cheilitis, Epidemiology, Lip neoplasms, Oral cancer, Squamous cell carcinoma, UV radiation
Introduction
Actinic cheilitis (AC) is a chronic inflammatory condition that has been described as a potentially malignant oral disorder. AC affects the vermilion region of the lower lips in 95% of cases [1–4]. The worldwide occurrence of AC ranges from 4.6 to 43.2% [5–11]. Data from a recent systematic review and meta-analysis, in which only studies with the histopathological diagnosis of this condition were included, demonstrated the prevalence of AC at 2.08% [12]. High-risk populations, including those living in tropical regions under excessive exposure to ultraviolet (UV) radiation, fair-skinned male individuals in their forties, and smokers are the most affected by this condition [11, 13]. Clinically, AC is characterized by dryness, fissures/erosions, atrophy (characterized by smooth, blotchy, pale areas) and erythematous and/or leucoplastic areas with blurring of the margin between vermilion and the adjacent skin [1, 4].
Nearly 95% of LSCC cases may be preceded by AC [14]; therefore, malignant transformation of AC is indeed a public health concern [15]. According to the World Health Organization (WHO) [16], lip squamous cell carcinoma (LSCC) is one of the most frequent malignant lesions of the oral and maxillofacial region, accounting for 25–30% of all oral cancers, and 12% of all head and neck cancers [11, 17, 18]. A total of 14,700 new cases of oral cancer have been estimated in Brazil in 2019 [19]. In particular, there are no national data depicting the incidence of lip cancer, since, in general, the data reported are regarded as oral cavity cancer [19]. LSCC has also predilection for fair-skinned men, with a peak incidence between the sixth and seventh decades of life. The lower lip is affected in 95% of cases. Prolonged exposure to sunlight also is an etiological factor for LSCC [4, 20, 21]. Long-term consumption of alcohol and tobacco associated with the carcinogenic action of UV radiation greatly increases the risk of developing this malignancy [2]. Sociodemographic conditions such as social vulnerability, and genetic susceptibility, or immunosuppression, are also factors that may contribute to the development of this disease [2, 22]. The initial clinical signs of LSCC may include asymptomatic crusts or ulcerations that may be quite equivalent to those observed in AC. At advanced stages, a painful exudative ulcer may occur, covered with scales and crusts, with indurated borders and an infiltrated base that does not heal [1, 2].
Although the clinicopathological characteristics and aetiological factors of AC and LSCC are well studied, there are no investigations reporting representative data about these conditions in all regions of Brazil. In addition, the prevalence rates for AC and LSCC are derived from epidemiological studies with specific populations [4, 6–8, 23–25]. Considering the importance of AC and LSCC for public health, and the concern regarding the 5898 deaths from cancer of the oral cavity in Brazil in 2015 [26], the purpose of the present multicentre study was to determine the frequency of AC and LSCC in a Brazilian population from 10 different centres of oral and maxillofacial pathology.
Materials and Methods
Study Design and Ethical Issues
A total of 198,709 histopathological records of oral and maxillofacial biopsies were analysed in this retrospective study. The guidelines for Strengthening the Reporting of Observational studies in Epidemiology [27] were followed. The records were obtained from a consortium of 10 referral centres of oral and maxillofacial pathology across the five Brazilian regions. This study was approved by the Ethics Committee of Universidade Federal de Minas Gerais (No. 2936807). Patient anonymity was ensured in conformity with the Declaration of Helsinki.
Clinicopathologic Data
Affected individuals were analysed concerning gender, age, skin colour (fair-skinned/brown-skinned), occupation (indoors/outdoors) and habits (tobacco smoking, alcohol consumption and chronic solar exposure). Lesions were analysed in terms of anatomical location (upper/lower lip), evolution time (months), symptoms (symptomatic/asymptomatic), manifestation type (primary/recurrent), and previous history of AC for cases of LSCC. Records with lack of specific information regarding histopathological diagnosis of AC and LSCC and records of histologic variants other than conventional SCC were excluded.
Data Analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) software, version 23.0 (SPSS Inc., Armonk, NY, USA). Descriptive statistics was performed to characterise the cases of AC and LSCC.
Results
A total of 198,709 histopathological records of oral and maxillofacial biopsies had been diagnosed at the 10 centres; of these, 2,017 were cases of AC (1.0%) and 850 were cases of LSCC (0.4%). The distribution of AC and LSCC cases by centre is depicted in Table 1. General data regarding the sociodemographic characteristics and clinicopathological features of cases of AC and LSCC are shown in Table 2. Figure 1 displays the temporal distribution of cases of AC and LSCC per decade.
Table 1.
Information regarding the sources of the reviewed cases of actinic cheilitis (AC) and lip squamous cell carcinoma (LSCC)
| Centre | State | Population (million) | Period | Number of biopsied lesions | AC (n) | AC (%)a | LSCC (n) | LSCC (%) |
|---|---|---|---|---|---|---|---|---|
| UFPAb | Pará | 8,513,497 | 2007–2018 | 5689 | 25 | 0.4 | 19 | 0.3 |
| UFRNc | Rio Grande do Norte | 3,479,010 | 1990–2018 | 12,528 | 207 | 1.6 | 43 | 0.3 |
| UEPBd | Paraíba | 3,996,496 | 2011–2018 | 3112 | 83 | 2.7 | 26 | 0.8 |
| UPEe | Pernambuco | 9,496,294 | 1990–2018 | 6911 | 42 | 0.6 | 11 | 0.2 |
| UFRGSf | Rio Grande do Sul | 11,329,605 | 1994–2018 | NAl | 125 | -m | 197 | -m |
| UFPelg | Rio Grande do Sul | 11,329,605 | 1968–2018 | 24,910 | 218 | 0.9 | 341 | 1.4 |
| UFMGh | Minas Gerais | 21,040,662 | 1953–2018 | 37,363 | 200 | 0.5 | 113 | 0.3 |
| UFRJi | Rio de Janeiro | 17,159,960 | 1979–2018 | 15,232 | 75 | 0.5 | 65 | 0.4 |
| USPj | São Paulo | 12,176,866 | 1991–2018 | 82,406 | 965 | 1.2 | NAn | –o |
| UFGk | Goiás | 6,921,161 | 1999–2018 | 10,558 | 77 | 0.7 | 35 | 0.3 |
| Total | – | – | – | 198,709 | 2017 | 1.0 | 850 | 0.4 |
aPercentage of the whole sample
bService of Oral Pathology, Hospital Universitário João de Barros Barreto, Universidade Federal do Pará (North region)
cPostgraduate Program in Oral Pathology, Universidade Federal do Rio Grande do Norte (North-East region)
dDepartment of Dentistry, School of Dentistry, Universidade Estadual da Paraíba (North-East region)
eDepartment of Oral and Maxillofacial Surgery and Pathology, School of Dentistry, Universidade de Pernambuco (North-East region)
fDepartment of Oral Medicine, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul (South region)
gDiagnostic Centre for Oral Diseases, School of Dentistry, Universidade Federal de Pelotas (South region)
hDepartment of Oral Surgery and Pathology, School of Dentistry, Universidade Federal de Minas Gerais (South-East region)
iDepartment of Oral Diagnosis and Pathology, School of Dentistry, Universidade Federal do Rio de Janeiro (South-East region)
jDepartment of Oral and Maxillofacial Pathology, School of Dentistry, Universidade de São Paulo (South-East region)
kDepartment of Stomatology (Oral Pathology), School of Dentistry, Universidade Federal de Goiás (Mid-East region)
lData were not available (NA) because they were from a primary centre; therefore, lesions affecting the maxillofacial region were not separated from the others
mData not calculated due to the absence of the total number of biopsied lesions
nData about this lesion were not collected at this centre
oData not calculated due to the absence of the number of LSSC lesion
Table 2.
Sociodemographic characteristics, habits and clinical and histopathological features of the individuals with actinic cheilitis (AC) and lip squamous cell carcinoma (LSCC)
| Regions | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North | North–East | South | South–East | Mid-East | ||||||||
| AC | LSCC | AC | LSCC | AC | LSCC | AC | LSCC | AC | LSCC | AC | LSCC | |
| Gender | ||||||||||||
| Male | 15 | 14 | 251 | 56 | 248 | 444 | 861 | 132 | 64 | 27 | 1439 | 673 |
| Female | 10 | 5 | 81 | 24 | 93 | 93 | 378 | 46 | 13 | 8 | 575 | 176 |
| NI | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 3 | 1 |
| Age (years/decades) | Mean: 59.8 ± 11.2; range: 43–97 | Mean: 67.1 ± 16.9; range: 17–99 | Mean: 54.6 ± 14.0; range: 12–85 | Mean: 65.1 ± 15.5; range: 26–103 | Mean: 57.1 ± 13.9; range: 4-100 | Mean: 60.0 ± 13.6; range: 18–102 | Mean: 59.0 ± 14.0; range: 3–93 | Mean: 60.0 ± 15.6; range: 7–91 | Mean: 55.8 ± 12.6; range: 3–80 | Mean: 58.7 ± 17.0; range: 35–95 | Mean: 54.8 ± 18.7; range: 3-100 | Mean: 57.8 ± 19.0; range: 7-103 |
| 0–9 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 3 | 2 |
| 10–19 | 0 | 1 | 1 | 0 | 2 | 1 | 3 | 0 | 1 | 0 | 7 | 2 |
| 20–29 | 0 | 0 | 19 | 1 | 9 | 11 | 28 | 2 | 1 | 0 | 57 | 14 |
| 30–39 | 0 | 0 | 27 | 3 | 19 | 31 | 94 | 14 | 2 | 4 | 142 | 52 |
| 40–49 | 1 | 0 | 59 | 11 | 46 | 48 | 154 | 26 | 16 | 8 | 276 | 93 |
| 50–59 | 14 | 3 | 85 | 8 | 99 | 152 | 253 | 39 | 24 | 6 | 475 | 208 |
| 60–69 | 7 | 6 | 87 | 21 | 96 | 153 | 360 | 37 | 20 | 5 | 570 | 222 |
| 70–79 | 0 | 3 | 38 | 18 | 44 | 83 | 217 | 39 | 10 | 7 | 309 | 150 |
| 80–89 | 1 | 4 | 5 | 11 | 6 | 32 | 54 | 10 | 1 | 1 | 67 | 58 |
| 90–99 | 1 | 1 | 0 | 0 | 0 | 1 | 4 | 3 | 0 | 2 | 5 | 7 |
| 100–109 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 3 |
| NI | 1 | 1 | 11 | 6 | 18 | 24 | 72 | 6 | 2 | 2 | 104 | 39 |
| Skin colour | ||||||||||||
| Fair-skinned | 7 | 8 | 220 | 52 | 324 | 507 | 1031 | 135 | 58 | 24 | 1640 | 726 |
| Brown-skinned | 9 | 5 | 68 | 16 | 5 | 10 | 149 | 30 | 8 | 6 | 239 | 69 |
| NI | 9 | 6 | 44 | 12 | 14 | 21 | 60 | 13 | 11 | 5 | 138 | 55 |
| Symptomatology | ||||||||||||
| Symptomatic | 2 | 6 | 39 | 29 | 1 | 14 | 133 | 10 | 2 | 2 | 177 | 62 |
| Asymptomatic | 8 | 0 | 252 | 47 | 2 | 42 | 750 | 11 | 29 | 7 | 1041 | 107 |
| NI | 15 | 13 | 41 | 4 | 340 | 482 | 357 | 157 | 46 | 26 | 799 | 681 |
| Anatomical location | ||||||||||||
| Upper lip | 0 | 2 | 3 | 3 | 15 | 13 | 8 | 5 | 1 | 0 | 27 | 23 |
| Lower lip | 25 | 17 | 329 | 77 | 328 | 525 | 1232 | 173 | 76 | 35 | 1990 | 827 |
| NI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Occupation | ||||||||||||
| Indoor | 10 | 3 | 121 | 35 | 123 | 201 | 124 | 76 | 26 | 12 | 404 | 329 |
| Outdoor | 7 | 10 | 130 | 27 | 101 | 168 | 48 | 35 | 24 | 9 | 310 | 249 |
| NI | 8 | 6 | 81 | 18 | 119 | 169 | 1068 | 67 | 27 | 14 | 1303 | 272 |
| Evolution time (months) | Median: 12; range: 2–120 | Median: 12; range: 5–144 | Median: 12; range: 0.5–420 | Median: 12; range: 1–72 | Median: 12; range: 0.25–480 | Median: 12; range: 0.5–720 | Median: 12; range: 0.25–600 | Median: 6; range: 0.25–240 | Median: 24; range: 0.25–480 | Median: 6.5; range: 1-144 | Median: 12; range: 0.25–600 | Median: 12; range: 0.25–720 |
| NI | 3 | 173 | 28 | 205 | 308 | 586 | 49 | 32 | 3 | 999 | 391 | |
| Previous history of ACa | ||||||||||||
| Yes | – | 1 | – | 22 | – | 35 | 9 | 5 | – | 72 | ||
| NI | – | 18 | – | 58 | – | 503 | – | 169 | – | 30 | – | 778 |
| Manifestation type | ||||||||||||
| Primary | 23 | 18 | 166 | 64 | 257 | 415 | 231 | 157 | 58 | 31 | 735 | 685 |
| Recurrent | 2 | 0 | 64 | 0 | 13 | 27 | 24 | 13 | 5 | 2 | 108 | 42 |
| NI | 0 | 1 | 102 | 16 | 73 | 96 | 985 | 8 | 14 | 2 | 1174 | 123 |
| Habits | ||||||||||||
| Tobacco smoking | ||||||||||||
| Yes | 6 | 9 | 14 | 5 | 22 | 83 | 28 | 14 | 10 | 10 | 80 | 121 |
| No | 0 | 0 | 0 | 0 | 3 | 24 | 0 | 0 | 0 | 0 | 3 | 24 |
| Ex-smoker | 2 | 0 | 2 | 0 | 9 | 28 | 4 | 4 | 6 | 2 | 23 | 34 |
| NI | 17 | 10 | 316 | 75 | 309 | 403 | 1208 | 160 | 61 | 23 | 1911 | 671 |
| Alcohol consumption | ||||||||||||
| Yes | 9 | 8 | 1 | 5 | 3 | 23 | 12 | 8 | 8 | 5 | 33 | 49 |
| No | 0 | 0 | 0 | 1 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | 23 |
| Ex-consumer | 1 | 1 | 0 | 0 | 0 | 24 | 2 | 0 | 0 | 0 | 3 | 25 |
| NI | 15 | 10 | 331 | 74 | 340 | 469 | 1226 | 170 | 69 | 30 | 1981 | 753 |
| Chronic solar exposure | ||||||||||||
| Yes | 0 | 1 | 27 | 21 | 23 | 54 | 21 | 10 | 4 | 5 | 75 | 91 |
| No | 0 | 0 | 6 | 0 | 1 | 18 | 0 | 0 | 0 | 0 | 7 | 18 |
| NI | 25 | 18 | 299 | 59 | 319 | 466 | 1219 | 168 | 73 | 30 | 1935 | 741 |
NI not informed
aThis variable does not apply to AC
Fig. 1.
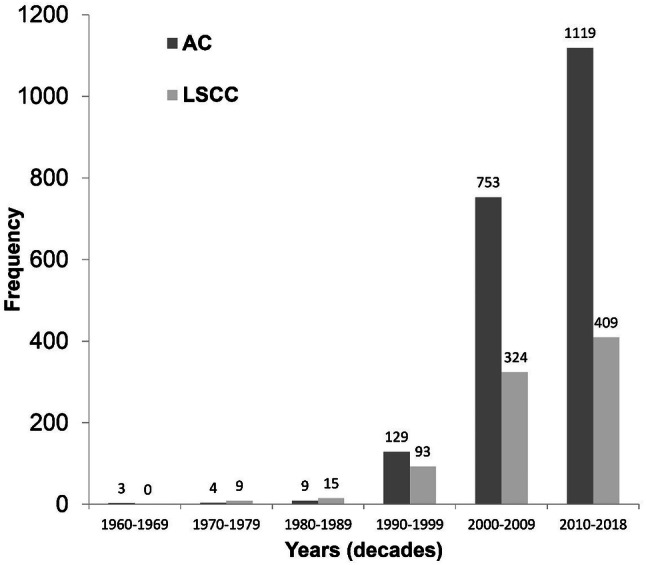
Temporal distribution of cases of actinic cheilitis (AC) and lip squamous cell carcinoma (LSCC) per decade.
Data of Individuals with AC
A total of 1,439 (71.4%) cases occurred in males and 575 (28.6%) in females (male-to-female ratio of 2.5:1). Older subjects (≥ 60 years) accounted for 49.8% of the survey. Most individuals were fair-skinned (87.3%), reported an indoor occupation (56.6%) and had no symptoms (78.8%). Of the individuals who had answered positively regarding habits of tobacco smoking, alcohol consumption and chronic solar exposure, 75.5% were smokers, 91.7% were alcohol users and 91.5% were chronically exposed to the sun.
The most common site of AC was the lower lip (98.7%) and 87.2% of the lesions presented as a primary manifestation with a median evolution time of 12 months. Figure 2 illustrates the main clinicopathologic features of individuals with AC. In 55.1% of the cases, the histopathological diagnosis confirmed the clinical diagnosis of AC. The other clinical diagnostic hypotheses were oral leukoplakia (25.2%) and oral squamous cell carcinoma (5.6%).
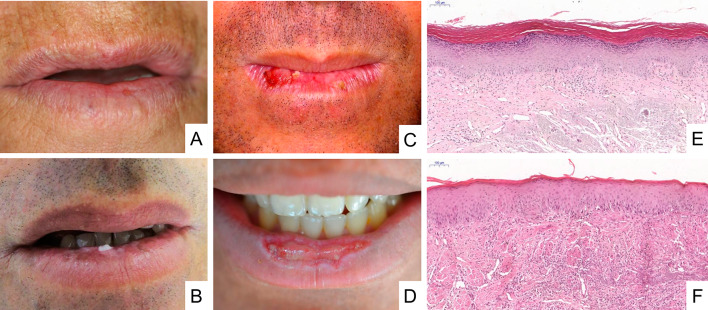
Fig. 2.
Clinical aspects and histopathological features of actinic cheilitis. a Atrophic regions with blurring of the margin between the affected area and the vermilion border of the lower lip. b Leucoplastic and pigmented areas in the lower lip. c Ulcerated, crusty and leucoplastic lesions with presence of oedema and blurring of the margin between the affected area and the vermilion border of the lower lip. d Presence of an exuberant ulcer in the central region of the lower lip with a leucoplastic border. e Low-grade epithelial dysplasia represented by hyperkeratosis with lack of polarization of basal cells, abnormal variations in nuclear size and shape, and hiperchromatism (H&E, 6 ×). f High-grade epithelial dysplasia represented by irregular epithelial stratification, loss of polarity of basal cells, drop-shaped rete ridges, and loss of epithelial cells cohesion. In addition, abnormal variation is observed in nuclear shape and size, in cell shape and size, hyperchromasia, and increased nuclear/cytoplasmic ratio (H&E, 2 ×). In both images (e, f), it is also possible to observe basophilic degeneration of collagen (solar elastosis) in connective tissue.
Data of Individuals with LSCC
A total of 673 (79.3%) cases occurred in males and 176 (20.7%) in females (male-to-female ratio of 3.8:1). The majority of cases consisted of older individuals (54.2%), fair-skinned (91.3%), with an indoor occupation (56.9%) and asymptomatic (63.3%). Of the individuals who had reported on the habits evaluated, 67.6% were smokers, 50.5% were alcohol users and 83.5% were chronically exposed to the sun.
The lower lip was also the most common anatomical location of LSCC (97.3%). Regarding the type of manifestation, 94.2% of the lesions were primary and 91.5% of the patients did not report a previous history of AC. The median follow-up time was 12 months. Figure 3 shows the main clinicopathologic aspects of LSCCs. In 74.2% of the cases, the histopathological diagnosis confirmed the clinical diagnosis of LSCC. The other clinical diagnostic hypotheses were AC (11.5%) and oral leukoplakia (4.4%).
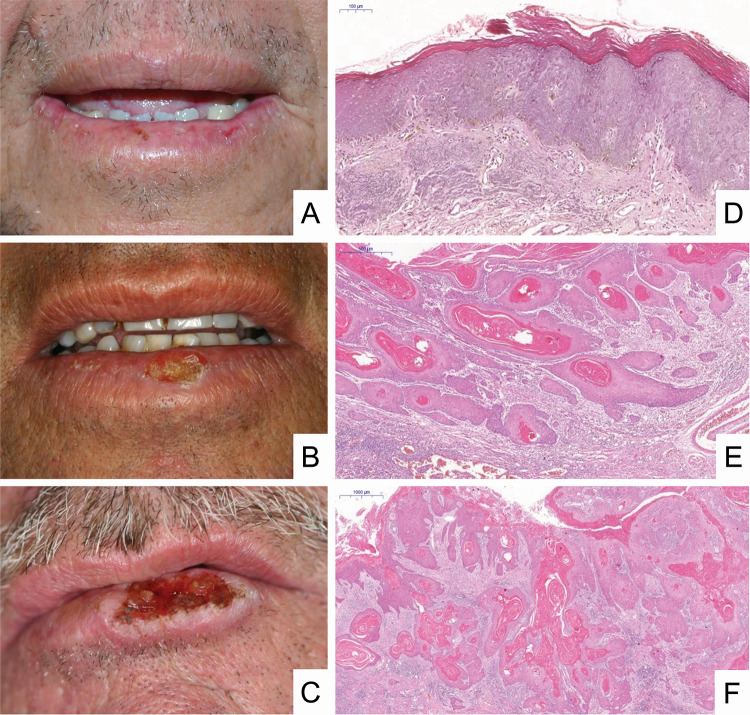
Fig. 3.
Clinical aspects and histopathological features of lip squamous cell carcinoma. a A small ulceration of the lower lip vermilion mimicking actinic cheilitis. Note lesions with crust and ulceration, presence of atrophic regions, oedema, and blurring of the margin between the vermilion and the adjacent skin. b Presence of a crusted lesion with areas of atrophy oedema and blurring of the margin between the vermilion and the adjacent skin. c Presence of an exuberant ulcer with a leucoplastic border, raised and hardened, and beginning of crust formation on the lesion. d In situ carcinoma represented by irregular epithelial stratification, loss of polarity of basal cells, drop-shaped rete ridges and loss of epithelial cell cohesion. In addition, abnormal variation in size and shape is observed in both nucleus and cell, with hyperchromasia, and an increased nucleus/cell ratio [haematoxylin & eosin (H&E), 6 ×]. e Superficially invasive carcinoma represented by nests and cordons of malignant epithelial cells is observed, starting invasion of the lamina propria with formation of keratin pearls and showing an intense inflammatory infiltrate (H&E, 4 ×). f Invasive carcinoma represented by islands of malignant epithelial cells in the lamina propria with intense formation of keratin pearls and an inflammatory infiltrate (H&E, 4 ×).
Discussion
Actinic cheilitis has been widely studied and its clinical importance stands out due to the possibility of malignant transformation of this disorder to lip cancer [28]. Brazilian investigations have demonstrated a frequency of AC ranging from 3.9 to 28.4% [4, 10]. For LSCC, the occurrence ranges from 3.0 to 13.5/100,000 individuals annually [17, 29]. Nevertheless, most investigations about AC are observational studies or case series in which the diagnosis of such condition was based exclusively on clinical parameters. Furthermore, these studies are restricted to specific populations of individuals constantly exposed to the aetiological factor (UV radiation), such as rural workers, sugarcane workers, beach workers, and fishermen. The findings of these studies must also be interpreted with caution due to the assessments performed in a particular city or state of the country [4, 6–9, 14, 23–25, 30]. Therefore, comparisons with the results of our study are worth doing, but should be made with caution since the present study is the first evaluation of a representative survey of AC and LSCC that have been submitted to microscopic examination from all regions of Brazil, reporting sociodemographic data and clinicopathologic aspects of these conditions. Interestingly, an increase in the number of cases of AC and LSCC over the last decades, mainly since 2000 has been observed. Indeed, these outcomes may have occurred due to the expansion of access to dental care and the intensification of oral cancer prevention campaigns and early diagnosis throughout the country [31, 32].
Regarding sociodemographic data about AC and LSCC, the findings obtained herein are in accordance with previous reports, showing a high frequency among fair-skinned men. In line with the literature, nearly 82% of AC cases and 80% of LSCC affect men and 85% of the individuals with AC and 87% of those with LSCC are fair-skinned individuals [4]. These features strengthen the statement that fair-skinned individuals are more susceptible to the development of diseases whose etiological factor is UV radiation, because of the lower amount of melanin present in their skin [28, 33]. Moreover, tropical countries such as Brazil, where a large number of male individuals work in outdoor occupations or have the habit of prolonged exposure to UV radiation, tend to have a larger number of cases of these lesions due to high solar incidence and the failure to use protective agents such as sunscreens and hats [30, 34]. However, cases of AC and LSCC have been reported predominantly among brown-skinned individuals in Africa [35, 36] and no imbalance in the male to female ratio has been observed [35].
Individuals affected by AC and LSCC are usually middle-aged or older, with a mean age of more than 40 years [1, 2, 4, 33]. Few studies have reported cases of young people affected by LSCC [36, 37]. Moreover, there is a difference in the age at diagnosis between individuals with AC and individuals with LSCC. Cases of LSCC have been diagnosed at a later age (sixth and seventh decades of life) [1, 17, 18, 21, 22] when compared to cases of AC (fifth decade of life), showing the chronic character of acquisition of the malignant phenotype [1, 4, 11, 30]. Our findings are in contrast to those of the aforementioned reports, in which the concentration of diagnosis was highest among individuals between the sixth and eighth decades of life, suggesting a late diagnosis in cases of AC. These data reinforce the increasing need for the continuity and expansion of campaigns for the prevention and early diagnosis of these lesions, thus reducing the neglect of AC by the population by disseminating knowledge about the importance of prevention and the search for a professional for diagnosis and treatment.
As regards the occupation of individuals, previous studies have reported that the prevalence of AC and LSCC is higher among subjects with outdoor occupations [4, 7–9, 11, 14, 17, 18, 24, 25]. Herein, most affected individuals with AC and LSCC had indoor occupations. However, a way out of this apparent paradox exists. Nearly 25% of the individuals who provided information about their occupation were retired or unemployed or were housewives at the time when data were collected. Retired or unemployed individuals may have had an outdoor occupation in the past. Therefore, the effects of the previous occupation on the participants may have contributed to the increase of cases of individuals with AC and LSCC who had an indoor occupation at the time of data collection. The past outdoor occupation may not only have contributed to the appearance and progression of these lesions, but may also have encouraged the individuals to develop habits such as smoking, alcohol consumption and chronic exposure to the sun, regardless of their occupation [1, 4, 15, 18]. Although many patients in the present study had not reported data regarding habits; among those who had, the frequency of habits was quite high, with more than 75% of the individuals confirming they were smokers and more than 90% reporting alcohol consumption. Similarly, these high rates have also been observed in other investigations, [4, 21, 37]. In addition, although the association of the primary aetiological factor (UV radiation) with secondary factors such as smoking and/or alcohol consumption is still unclear, smoking has been suggested as one of the main secondary factors for the development of LSCC [22, 38]. Therefore, further studies are needed to clarify the potential contribution of tobacco and alcohol consumption to the aetiopathogenesis of LSCC.
With respect to the most frequent anatomical site of the lesions studied herein, the lower lip was undoubtedly the most affected, with a rate of almost 95% of the cases reported in previous studies [1, 4, 7, 11, 13, 18]. The present study reinforces these findings, since more than 97% of the cases were in the lower lip. Though visible, these early-stage lesions are often neglected due to the lack of symptoms in most cases [1, 28]. In this study, there was a high frequency of individuals with AC or LSCC who were asymptomatic. Absence of symptoms is relevant information for the clinical diagnosis of AC and LSCC. In nearly 55% of cases of AC and 74% of LSCC, the clinical diagnosis was confirmed histopathologically, as also reported by Melo et al. [4]. Thus, this data pool reinforces the importance of knowledge about these lesions by clinicians and dermatologists, who should be concerned with an early diagnosis and timely referral to adequate treatment. The evolution time of these lesions is slow [17, 20, 28, 26], demonstrating a potential for malignant transformation and a much lower aggression pattern when compared to other lesions, such as intraoral erythroplakia and oral squamous cell carcinoma [1, 3, 12, 28]. Although the study conducted by Kaugars et al. [39] showed no difference in the pattern of differentiation and invasion between primary and recurrent cases, definitive conclusions are limited by the small number of cases analysed.
Histologically, the profile of AC changes is located in epithelial and connective tissues [30]. In LSCC, malignant changes are observed. Basophilic collagen degeneration is a histopathological feature common to both lesions [1, 16]. Currently, there are several classification systems for epithelial dysplasia [16, 40, 41] and oral squamous cell carcinoma [16, 42–44]. Among the classification systems, the one recommended by the WHO [16] is the most widely used. However, uniformity in the classification of these conditions is an inter-examiner challenge and, even for intra-examiner agreement, kappa values reveal poor to moderate agreement, proving that these classification systems are highly subjective [45–48]. For these reasons and because in the present study we did not perform a uniform histological classification of cases, these data are not shown. Therefore, we acknowledge that this topic is very important and should be assessed in further studies.
Concerning the malignant transformation rate of AC, the literature is quite controversial. The various studies did not use the same methodology to estimate this rate and no certainty about the progression of AC to LSCC exists [28]. A rate of 3.07% has been reported in a systematic review [28]. On the other hand, information about the LSCC survival rate is well-documented in the literature [20, 36, 49]. Individuals affected by this malignancy have higher survival rates than individuals with other head and neck cancers [49] due to the anatomical location and the clinical behaviour of the lesion. The five-year survival rate for patients diagnosed with LSCC ranges from 62–79% [20, 49].
Our study has limitations that should be recognized. The first is the retrospective design of the study with participants recruited from secondary care facilities. Thus, the follow-up of the clinical course of the individuals in order to analyse the rate of malignant transformation of AC and the rate of survival in cases of LSCC was impossible. Although our large series consisted of cases of AC and LSCC obtained from 10 different centres of oral and maxillofacial pathology for over 60 years, information on the grading of epithelial dysplasia of AC and the invasion pattern of LSCC was unreported, as there could be some bias due to the non-uniformity of dysplasia criteria/classification among different evaluators (intra-observer agreement). Inter-observer agreement across time could also be difficult [47, 48].
Conclusion
In summary, data of the Brazilian individuals with AC and LSCC reported herein agree with findings of case series and retrospective studies reported elsewhere. There is a predilection of white men in their seventh decade of life. In most cases, the lower lip is affected.
Acknowledgements
This work was supported by the Brazilian National Council for Scientific and Technological Development (CNPq #305493/2018-3, #455644/2018-1). The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES, Finance code 001), Brazil. L.V.O.S. and J.A.A.A. are the recipients of fellowships. We would like to extend our thanks to Dr. L. Barnabé for technical assistance with histopathological photomicrographs. Mrs. E. Greene provided English editing of the manuscript.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001), Brazil.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Human Research Ethics Committee of the study institution (No. 2.936.807).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vieira RA, Minicucci EM, Marques ME, Marques SA. Actinic cheilitis and squamous cell carcinoma of the lip: clinical, histopathological and immunogenetic aspects. An Bras Dermatol. 2012;87:105–14. doi: 10.1590/s0365-05962012000100013. [DOI] [PubMed] [Google Scholar]
- 2.Lopes ML, Silva Júnior FL, Lima KC, Oliveira PT, Silveira ÉJ. Clinicopathological profile and management of 161 cases of actinic cheilitis. An Bras Dermatol. 2015;90:505–12. doi: 10.1590/abd1806-4841.20153848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:582–90. doi: 10.1016/j.oooo.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Mello FW, Melo G, Modolo F, Rivero ER. Actinic cheilitis and lip squamous cell carcinoma: Literature review and new data from Brazil. J Clin Exp Dent. 2019;11:62–9. doi: 10.4317/jced.55133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campisi G, Margiotta V. Oral mucosal lesions and risk habits among men in an Italian study population. J Oral Pathol Med. 2001;30:22–8. doi: 10.1034/j.1600-0714.2001.300104.x. [DOI] [PubMed] [Google Scholar]
- 6.Zanetti R, Flório FM, Moraes PC, Lima YBA, França FMG, Araújo VC. Prevalence of actinic cheilitis in an oral health campaign in the city of Campinas, SP. J Appl Oral Sci. 2007;15:353. [Google Scholar]
- 7.Martins-Filho PR, Da Silva LC, Piva MR. The prevalence of actinic cheilitis in farmers in a semi-arid northeastern region of Brazil. Int J Dermatol. 2011;50:1109–14. doi: 10.1111/j.1365-4632.2010.04802.x. [DOI] [PubMed] [Google Scholar]
- 8.de Souza Lucena EE, Costa DC, da Silveira EJ, Lima KC. Prevalence and factors associated to actinic cheilitis in beach workers. Oral Dis. 2012;18:575–9. doi: 10.1111/j.1601-0825.2012.01910.x. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira Ribeiro A, da Silva LC, Martins-Filho PR. Prevalence of and risk factors for actinic cheilitis in Brazilian fishermen and women. Int J Dermatol. 2014;53:1370–6. doi: 10.1111/ijd.12526. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira AM, de Souza Lucena EE, de Oliveira TC, da Silveira É, de Oliveira PT, de Lima KC. Prevalence and factors associated with oral potentially malignant disorders in Brazil's rural workers. Oral Dis. 2016;22:536–42. doi: 10.1111/odi.12488. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Blanco I, Flórez Á, Paredes-Suárez C, Rodríguez-Lojo R, González-Vilas D, Ramírez-Santos A, et al. Actinic cheilitis prevalence and risk factors: A cross-sectional, multicentre study in a population aged 45 years and over in North-west Spain. Acta Derm Venereol. 2018;98:970–4. doi: 10.2340/00015555-3014. [DOI] [PubMed] [Google Scholar]
- 12.Mello FW, Miguel AFP, Dutra KL, Porporatti AL, Warnakulasuriya S, Guerra ENS, et al. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J Oral Pathol Med. 2018;47:633–40. doi: 10.1111/jop.12726. [DOI] [PubMed] [Google Scholar]
- 13.Markopoulos A, Albanidou-Farmaki E, Kayavis I. Actinic cheilitis: clinical and pathologic characteristics in 65 cases. Oral Dis. 2004;10:212–6. doi: 10.1111/j.1601-0825.2004.01004.x. [DOI] [PubMed] [Google Scholar]
- 14.Miranda AM, Ferrari TM, Calandro TLL. Queilite actínica: aspectos clínicos e prevalência encontrados em uma população rural no interior do Brasil. Rev Saúde Pesq. 2011;4:67–72. [Google Scholar]
- 15.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 16.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization classification of head and neck tumours. Lyon: IARC Press; 2017
- 17.Moore SR, Johnson NW, Pierce AM, Wilson D. The epidemiology of lip cancer: a review of global incidence and aetiology. Oral Dis. 1999;5:185–95. doi: 10.1111/j.1601-0825.1999.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 18.Biasoli ÉR, Valente VB, Mantovan B, Collado FU, Neto SC, Sundefeld ML, et al. Lip cancer: a clinicopathological study and treatment outcomes in a 25-year experience. J Oral Maxillofac Surg. 2016;74:1360–7. doi: 10.1016/j.joms.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Brasil. Ministério da Saúde. Instituto Nacional de Câncer. Estimativa 2019: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2018. p. 39.
- 20.Czerninski R, Zini A, Sgan-Cohen H. Lip cancer: incidence, trends, histology and survival: 1970–2006. Br J Dermatol. 2010;162:1103–9. doi: 10.1111/j.1365-2133.2010.09698.x. [DOI] [PubMed] [Google Scholar]
- 21.Souza LR, Fonseca-Fonseca T, Oliveira-Santos CC, Corrêa GT, Santos FB, Cardoso CM, et al. Lip squamous cell carcinoma in a Brazilian population: Epidemiological study and clinicopathological associations. Med Oral Patol Oral Cirug Bucal. 2011;16:757–62. doi: 10.4317/medoral.16954. [DOI] [PubMed] [Google Scholar]
- 22.Perea-Milla López E, Miñarro-Del Moral RM, Martínez-García C, Zanetti R, Rosso S, Serrano S, et al. Lifestyles, environmental and phenotypic factors associated with lip cancer: a case-control study in southern Spain. Br J Cancer. 2003;88:1702–7. doi: 10.1038/sj.bjc.6600975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavalcante AS, Anbinder AL, Carvalho YR. Actinic cheilitis: clinical and histological features. J Oral Maxillofac Surg. 2008;66:498–503. doi: 10.1016/j.joms.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Piñera-Marques K, Lorenço SV, Silva LF, Sotto MN, Carneiro PC. Actinic lesions in fishermen's lower lip: clinical, cytopathological and histopathologic analysis. Clinics. 2010;65:363–7. doi: 10.1590/S1807-59322010000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos RFD, Oliveira RL, Gallottini M, Caliento R, Sarmento DJS. Prevalence of and factors associated with actinic cheilitis in extractive mining workers. Braz Dent J. 2018;29:214–21. doi: 10.1590/0103-6440201801605. [DOI] [PubMed] [Google Scholar]
- 26.Brasil. Ministério da Saúde. Departamento de Informática do SUS. Sistema de informações sobre mortalidade. Brasília. 2017. Available http://www.datasus.gov.br/DATASUS/index.php?area=060701. Accessed 2 June 2019.
- 27.Knottnerus A, Tugwell P. STROBE—a checklist to strengthen the reporting of observational studies in epidemiology. J Clin Epidemiol. 2008;61:323. doi: 10.1016/j.jclinepi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Dancyger A, Heard V, Huang B, Suley C, Tang D, Ariyawardana A, et al. Malignant transformation of actinic cheilitis: A systematic review of observational studies. J Investig Clin Dent. 2018;9:12343. doi: 10.1111/jicd.12343. [DOI] [PubMed] [Google Scholar]
- 29.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 30.De Santana Sarmento DJ, da Costa Miguel MC, Queiroz LM, Godoy GP, da Silveira EJ. Actinic cheilitis: clinicopathologic profile and association with degree of dysplasia. Int J Dermatol. 2014;53:466–72. doi: 10.1111/ijd.12332. [DOI] [PubMed] [Google Scholar]
- 31.Martins JS, Abreu SC, Araújo ME, Bourget MM, Campos FL, Grigoletto MV, et al. Strategies and results of the oral cancer prevention campaign among the elderly in São Paulo, Brazil, 2001 to 2009. Rev Panam Salud Public. 2012;31:246–52. doi: 10.1590/s1020-49892012000300010. [DOI] [PubMed] [Google Scholar]
- 32.Baumann E, Koller M, Wiltfang J, Wenz HJ, Möller B, Hertrampf K. Challenges of early detection of oral cancer: raising awareness as a first step to successful campaigning. Health Educ Res. 2016;31:136–45. doi: 10.1093/her/cyv099. [DOI] [PubMed] [Google Scholar]
- 33.Maruccia M, Onesti MG, Parisi P, Cigna E, Troccola A, Scuderi N. Lip cancer: a 10-year retrospective epidemiological study. Anticancer Res. 2012;32:1543–6. [PubMed] [Google Scholar]
- 34.Corrêa MdeP. Solar ultraviolet radiation: properties, characteristics and amounts observed in Brazil and South America. An Bras Dermatol. 2015;90:297–313. doi: 10.1590/abd1806-4841.20154089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goracci G, Colangelo G, Nini G. Incidence of actinic cheilitis in Somalia. Riv Ital Stomatol. 1981;50:1009–16. [PubMed] [Google Scholar]
- 36.Chidzonga MM. Lip cancer in Zimbabwe. Report of 14 cases. Int J Oral Maxillofac Surg. 2005;34:149–51. doi: 10.1016/j.ijom.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Salihu S, Güven O, Gllareva E, Prekazi M, Salihu L. A clinical study on survival rate of patients with squamous cell carcinoma of the lower lip in Kosovo. J Craniomaxillofac Surg. 2014;42:1773–7. doi: 10.1016/j.jcms.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Jadotte YT, Schwartz RA. Solar cheilosis: an ominous precursor: part I. Diagnostic insights. J Am Acad Dermatol. 2012;66:173–84. doi: 10.1016/j.jaad.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 39.Kaugars GE, Pillion T, Svirsky JA, Page DG, Burns JC, Abbey LM. Actinic cheilitis: a review of 152 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:181–6. doi: 10.1016/s1079-2104(99)70115-0. [DOI] [PubMed] [Google Scholar]
- 40.Zerdoner D. The Ljubljana classification: its application to grading oral epithelial hyperplasia. J Craniomaxillofac Surg. 2003;31:75–9. doi: 10.1016/s1010-5182(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 41.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42:987–93. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229–49. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 43.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders' grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432–7. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 44.Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375–81. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 45.Warnakulasuriya S. Histological grading of oral epithelial dysplasia:revisited. J Pathol. 2001;194:294–7. doi: 10.1002/1096-9896(200107)194:3<294::AID-PATH911>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 46.Ranganathan K, Kavitha L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J Oral Maxillofac Pathol. 2019;23:19–27. doi: 10.4103/jomfp.JOMFP_13_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pindborg JJ, Reibel J, Holmstrup P. Subjectivity in evaluating oral epithelial dysplasia, carcinoma in situ and initial carcinoma. J Oral Pathol. 1985;14:698–708. doi: 10.1111/j.1600-0714.1985.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 48.Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA, et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:188–91. doi: 10.1016/s1079-2104(05)80201-x. [DOI] [PubMed] [Google Scholar]
- 49.Guntinas-Lichius O, Wendt T, Buentzel J, Esser D, Lochner P, Mueller A, et al. Head and neck cancer in Germany: a site-specific analysis of survival of the Thuringian cancer registration database. J Cancer Res Clin Oncol. 2010;136:55–63. doi: 10.1007/s00432-009-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]