Abstract
Salivary gland neoplasms of the buccal mucosa are relatively rare and often present with an unusual histopathologic profile when compared with other intraoral locations. We present a series of minor salivary gland neoplasms of the buccal mucosa and discuss demographics, clinical presentation, and histologic findings. An IRB approved retrospective search of University of Florida Oral Pathology Biopsy Service archive from 1994 to 2018 for all salivary gland neoplasms of the buccal mucosa was undertaken. Data related to age, gender, clinical presentation, diagnosis, and category of neoplasm recorded. Review for consensus of diagnosis and immunohistochemical (IHC) testing on current diagnostic standards was performed and diagnoses updated based on results. Of 66 cases identified majority were females (72.7%) and age mean was 63 years. Benign tumors were 56.06% and 43.94% malignant, with Mucoepidermoid carcinoma (MEC) being commonest (26/66, 39.4%), followed by canalicular adenoma (CLA) (14/66, 21.2%), ductal papilloma (DP) (10/66, 15.2%), cystadenoma (CA) (8/66, 12.1%), basal cell adenoma (BCA) (4/66, 6.1%), and 1(1.5%) each for pleomorphic adenoma (PA), secretory carcinoma (SC), adenoid cystic carcinoma (ACC) and adenocarcinoma not otherwise specified (ACNOS). This study with respect to demographics and percentage of benign and malignant buccal mucosal salivary gland neoplasms is in conformity with the literature. It underscores the fact that both benign and malignant salivary gland neoplasms should be included in the differential diagnosis of submucosal buccal masses. Future larger multicenter studies with detailed treatment and outcomes data may aid and assist in further understanding the behavior, diverse histomorphology and prognosis of these neoplasms.
Keywords: Minor salivary gland neoplasms, Buccal mucosa, Pleomorphic adenoma, Adenoid cystic carcinoma, Mucoepidermoid carcinoma, Secretory carcinoma, Mucocele
Introduction
Salivary gland neoplasms of the buccal mucosa (SGNBM) are relatively rare and may present a wide variation in histology and complex clinical behavior. These factors may sometimes make accurate initial diagnosis particularly challenging.
This study aims to describe the clinical presentation, histologic features and demographics of SGNBM collected over a period of twenty-five years. The data included within this study is based on a single institution’s biopsy service record and is not a representation of the countrywide demographics of buccal mucosal salivary gland tumors.The purpose of this paper is to contribute toward better understanding of potentially challenging and morphologically diverse salivary gland neoplasms of the buccal mucosa.
To our knowledge, this is the first article to specifically evaluate salivary gland neoplasms of buccal mucosa as a distinct location, thus providing a novel perspective on this unusual subset of lesions.
Patients and Methods
The study identified sixty-nine cases of salivary gland tumors arising in the buccal mucosa, selected from the archives of the University of Florida Oral Pathology Biopsy Service encompassing the time frame from1994 to 2018. The following parameters were analyzed: patient age, gender, specific histologic diagnosis, and diagnostic category (benign or malignant). Three cases were excluded due to incomplete clinical or pathologic data. The results were statistically analyzed using descriptive and quantitative analysis, the arithmetic mean (X) and standard deviation (SD). The difference in the average values was calculated using the t-test for two independent samples. The cases then were microscopically reviewed. In those cases where features of newly described salivary tumors (not known at the time of original sign-out) or where overlapping histologic features were noted during review of slides, additional confirmatory IHC studies were performed, including mammaglobin and S-100.
Results
An IRB approved retrospective search of the archives of the University of Florida Oral Pathology Biopsy Service spanning 24 years (1994–2018) was performed and a total of sixty-six cases of salivary gland tumors arising in the buccal mucosa were identified. Thirty-seven (56.06%) were benign and 29 (43.94%) malignant neoplasms. Mucoepidermoid (MEC) was the most frequent histological type (n = 26/66, 39.4%), followed by canalicular adenoma (CLA) (n = 14/66, 21.2%), ductal papilloma (DP) (n = 10/66, 15.2%), cystadenoma (CA) (n = 8/66, 12.1%), basal cell adenoma (BCA) (n = 4/66, 6.1%), and pleomorphic adenoma (PA), adenoid cystic carcinoma (ACC), secretory carcinoma (SC) and adenocarcinoma not otherwise specified (ACNOS) were uncommon, each accounting for (1.5%) of cases.
Forty-eight (72.7%) of the patients were females (26 benign and 22 malignant), and eighteen (27.3%) were males (11 benign and 7 malignant). SGNBM showed a female predilection, yielding an almost 3:1 female to male proportion. The mean age of the patients was 67.5 years for benign tumors, and 58.3 years for malignant tumors. In the group of benign tumors, the youngest patient was a 23-year-old female diagnosed with BCA. The oldest patient was a 93-year-old male diagnosed with BCA. Among malignant tumors, the youngest and the oldest patients were 11 and 89-year-old female, respectively, both diagnosed with MEC.
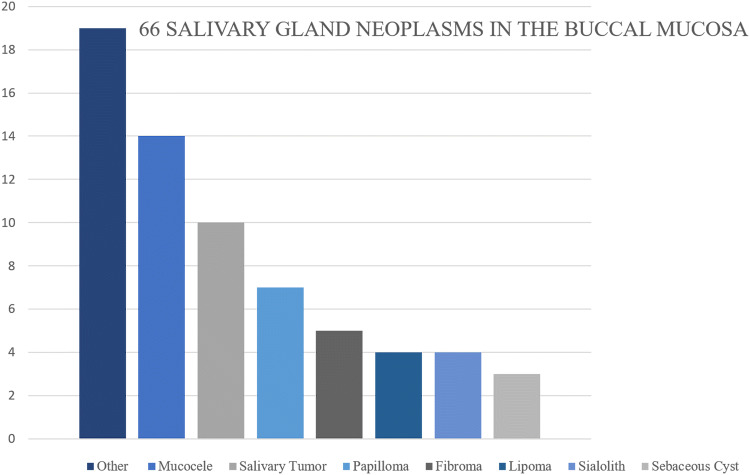
Review of the submitting clinician provided clinical impression of these lesions revealed that only 15.2% of the cases (n = 10/66) included salivary gland related lesions in the differential diagnoses. (Fig. 1).
Fig. 1.
Distribution of the clinical differential diagnoses
The clinical presentation was most frequently smooth, non-ulcerated, asymptomatic, mucosal colored mass and did not significantly differ between benign and malignant tumors (Figs. 2a and 3a). However, pain was more frequently found in malignant tumors. The time elapsed from onset of lesion to histopathological diagnosis ranged between a few weeks to 40 years. These are summarized in Tables 1 and 2. There was no difference when comparing lesions between left or right buccal mucosa. The mean size of the excisional samples for benign tumors was larger than the malignant neoplasms.
Fig. 2.
PA. a, clinical view of a submucosal nodule in the left buccal mucosa covered by normal mucosa. b, chondroid material (left) with adjacent ductal epithelium and myoepithelial cells, magnification X40 (H&E*). c, the myoepithelial cells arranged in the form of ducts and sheets in the myxomatous stroma, magnification X100 (H&E*). *Hematoxylin-eosin staining
Fig. 3.
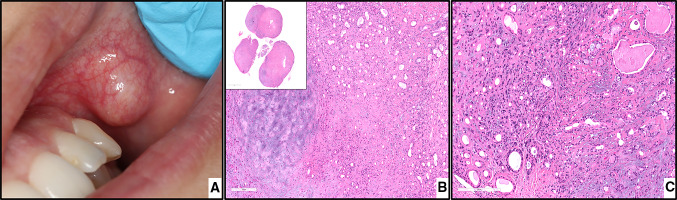
MEC. a, preoperative intraoral view. b, specimen of the excised tumor. c, MEC clear cell variant, magnification X200 (H&E*). d, numerous large mucous cells intermixed with clear cells, magnification X100 (H&E*). *Hematoxylin-eosin staining
Table 1.
Distribution of malignant salivary gland neoplasms according to patient age and gender, histological diagnosis, clinical symptoms, duration of the lesions, clinical impression, and biopsy type
| Case | Age | Gender | Diagnosis | Clinical symptoms* | Duration | Clinical impression | Biopsy type |
|---|---|---|---|---|---|---|---|
| 1 | 36 | F | MEC, low grade | - | 1 year | Salivary gland neoplasm | Excisional |
| 2 | 89 | F | MEC, low grade | - | 40 years | Adenoma | Excisional |
| 3 | 40 | M | MEC, intermediate grade | - | Years | PGCG** | Excisional |
| 4 | 11 | F | MEC, low grade | - | Years | Mucocele | Excisional |
| 5 | 71 | F | MEC, low grade | - | 5 years | Hodgkin lymphoma | Excisional |
| 6 | 52 | F | MEC, low grade | - | 1 year | Sialadenitis | Excisional |
| 7 | 69 | F | MEC | - | 1 month | Fibroma | Excisional |
| 8 | 74 | F | MEC, low grade | - | Months | Mucocele | Excisional |
| 9 | 72 | M | MEC, high grade | +, Trismus | 3 weeks | Fibroma | Excisional |
| 10 | 43 | F | MEC, intermediate grade | + | 3 months | Salivary gland neoplasm | Incisional |
| 11 | 81 | M | MEC, low grade | - | 6 months | Lipoma | Excisional |
| 12 | 36 | F | MEC, low grade | - | 7 years | Fibroma | Excisional |
| 13 | 75 | M | MEC, low grade | + | 2 years | Salivary gland neoplasm | Excisional |
| 14 | 35 | F | MEC, low grade | - | Unknown | Not specified | Excisional |
| 15 | 80 | F | MEC, low grade | - | Years | Mucocele | Excisional |
| 16 | 47 | M | SC | - | Years | Mucocele | Excisional |
| 17 | 68 | F | MEC, low grade | - | Unknown | Lipoma | Excisional |
| 18 | 51 | F | MEC, low grade | - | 2 months | Mucocele | Excisional |
| 19 | 70 | F | MEC, low grade | + | Months | Sebaceous cyst | Excisional |
| 20 | 88 | F | MEC, low grade | - | 1 week | Sialolith | Incisional |
| 21 | 51 | F | MEC, intermediate grade | - | Years | Not specified | Excisional |
| 22 | 67 | F | MEC, intermediate grade | + | 1 year | Salivary gland neoplasm | Excisional |
| 23 | 50 | M | MEC, intermediate grade | - | 5 years | Salivary gland neoplasm | Incisional |
| 24 | 38 | F | MEC, intermediate grade | + | Unknown | Adenoma | Excisional |
| 25 | 74 | F | MEC | - | Unknown | Mucocele | Excisional |
| 26 | 50 | M | ACNOS | - | 2 months | Not specified | Incisional |
| 27 | 78 | F | MEC, low grade | - | 3 months | Salivary gland neoplasm | Excisional |
| 28 | 41 | F | MEC, intermediate grade | - | Unknown | Mucocele | Excisional |
| 29 | 55 | F | ACC | + | 2 months | Lymphoid hyperplasia | Excisional |
Clinical Symptoms*; Presence of Pain: yes +, no –, PGCG**: peripheral giant cell granuloma
MEC mucoepidermoid carcinoma, SC secretory carcinoma, ACNOS adenocarcinoma not otherwise specified, ACC adenoid cystic carcinoma
Table 2.
Distribution of benign salivary gland neoplasms according to patient age and gender, histological diagnosis, clinical symptoms, duration of the lesions, clinical impression, and biopsy type
| Case | Age | Gender | Diagnosis | Clinical symptoms* | Duration | Clinical impression | Biopsy type |
|---|---|---|---|---|---|---|---|
| 1 | 83 | F | CLA | - | 2 years | Not specified | Excisional |
| 2 | 70 | F | DP | - | 2 years | Papilloma | Excisional |
| 3 | 71 | F | CLA | - | Unknown | Not specified | Excisional |
| 4 | 62 | F | PA | - | Unknown | Sebaceous cyst | Excisional |
| 5 | 83 | F | DP | - | 1 week | Mucocele | Incisional |
| 6 | 73 | F | CLA | - | Unknown | Cystadenoma | Excisional |
| 7 | 66 | F | CA | - | Unknown | Papilloma | Excisional |
| 8 | 22 | F | BCA | - | 5 months | Not specified | Excisional |
| 9 | 54 | M | CLA | - | 6 months | Monomorphic adenoma | Excisional |
| 10 | 52 | F | CLA | - | 1 year | Mucocele | Excisional |
| 11 | 66 | F | CLA | - | 20 years | Lipoma | Excisional |
| 12 | 93 | F | BCA | - | 20 years | Not specified | Excisional |
| 13 | 88 | M | CLA | - | Unknown | Salivary gland neoplasm | Excisional |
| 14 | 85 | F | BCA | - | 2 months | Sebaceous cyst | Excisional |
| 15 | 62 | F | CLA | - | 3 weeks | Lipoma | Excisional |
| 16 | 84 | F | BCA | + | Months | Salivary duct cyst | Excisional |
| 17 | 80 | M | CLA | +, ulceration | Unknown | Necrosis secondary to ductal blockage | Incisional |
| 18 | 58 | F | CLA | - | Months | Not specified | Excisional |
| 19 | 64 | M | CA | - | Unknown | Not specified | Excisional |
| 20 | 62 | F | CLA | - | Unknown | Hemangioma | Excisional |
| 21 | 69 | M | CLA | - | Months | Salivary duct cyst | Excisional |
| 22 | 75 | F | CLA | - | Unknown | Mucocele | Excisional |
| 23 | 58 | M | CLA | - | 1 month | Ductal blockage reaction | Excisional |
| 24 | 68 | F | CA | - | 1 month | Mucocele | Excisional |
| 25 | 76 | F | CA | + | Months | Sialadenitis | Excisional |
| 26 | 62 | M | CA | - | 2 months | Accessory salivary gland tissue | Excisional |
| 27 | 60 | F | CA | - | Unknown | Mucocele | Excisional |
| 28 | 74 | M | CA | - | 2 months | Mucocele | Excisional |
| 29 | 66 | F | CA | - | Unknown | Not specified | Excisional |
| 30 | 65 | F | DP | - | 6 months | Inflamed minor salivary gland | Excisional |
| 31 | 73 | M | DP | - | 1 year | Papilloma | Excisional |
| 32 | 82 | F | DP/SP** | - | Unknown | Papilloma | Excisional |
| 33 | 57 | M | DP | - | 3 months | Papilloma | Excisional |
| 34 | 53 | F | DP | - | Unknown | Papilloma | Excisional |
| 35 | 80 | F | DP | - | Unknown | Papilloma | Excisional |
| 36 | 53 | M | DP/SP** | - | 4 months | Not specified | Excisional |
| 37 | 50 | F | DP | - | Months | Fibroma | Excisional |
Clinical Symptoms*; Presence of Pain: yes +, no –, SP**: sialadenoma papilliferum
CLA canalicular adenoma, DP ductal papilloma, PA pleomorphic adenoma, CA cystadenoma, BCA basal cell adenoma
The microscopic features were reviewed. Mucicarmine stain was performed on three cases (case 6, 15, and 20) at the time of sign-out, and was positive. Two cases originally were diagnosed as MEC (Case 6/Case 16 (Table 1)); however, diagnostic features of SC were identified, which was not yet described as an entity at the time of original sign-out. The tumor cells displayed an acinic cell carcinoma like appearance but lacked zymogen granules and contained granular eosinophilic cytoplasm. Also seen were distinct, macrocystic and/or microcystic structures and tubular, papillary cystic areas filled with eosinophilic secretory “colloid-like material” giving a “bubble-like” appearance (Fig. 4a,b). Therefore, S-100 protein and mammaglobin stains were performed on those two cases. IHC revealed strong positive reaction in the neoplastic cells to mammaglobin and S100 protein in one case (16) (Fig. 4c, d) and a negative reaction in the other (6). Accordingly, case (16) has been reclassified as secretory carcinoma (SC). Perineural invasion was present only in one case of MEC.
Fig. 4.
SC. a-b, the tumor cells exhibit bland, vesicular nuclei surrounded by slightly granular or vacuolated cytoplasm, magnification X200 (H&E*). c, the tumor cells show diffuse positive immunoreactivity for S-100, magnification X200 (IHC**). d, the tumor cells show diffuse immunoreactivity for mammaglobin, magnification X200 (IHC**). *Hematoxylin-eosin staining, ** Immunohistochemical staining
Discussion
This study describes the demographics, clinical presentation, and histopathological features of 66 cases of SGNBM. Our results are similar to other reports where SGNBM were included with respect to female gender predilection and an age range of sixth and seventh decades [1–4].
Benign tumors appear at a higher mean age when compared to malignant tumors in our series, which is consistent with previous studies as well [2]. Jansisyanont et al. [2] reported that malignant tumors can occur in younger patients, and this is in agreement with the present study. Previous literature has reported different peaks of age for these tumors depending on the histological type [1–6] .
Signs and symptoms did not differ between benign and malignant neoplasms, contributing to the difficulty in developing a pertinent diagnosis of SGNBM clinically. Delay in the diagnosis of SGNBM most likely relates to the clinical presentation of an asymptomatic, smooth, mucosal colored, submucosal mass or nodule, rarely ulcerated unless it had been previously biopsied [4] Jansisyanont et al. [2] observed that 27.95% of malignant tumors were present for more than 1 year, and 13.1% were asymptomatic. Although pain was present more frequently in malignant tumors in our study in comparison to benign tumors, it is not clear if pain is a common sign in malignant neoplasms of minor salivary glands. We believe the available data in the literature is insufficient to prove that pain is more common or definitive finding in malignant neoplasms of minor salivary glands.
The most frequent differential diagnosis for buccal mucosa masses in our study was a mucocele, which is frequently seen as fluctuant mass or swelling. Deep mucoceles which are often surrounded by a fibrous tissue wall may not be fluctuant. When located at sites other than the lower lip these cannot reliably be differentiated clinically from salivary gland tumors, especially those that present with a predominantly cystic nature. Other differential diagnoses include buccal space abscess, hemangioma, dermoid cyst, lipoma, and foreign body reaction [5, 7] Therefore, a salivary gland tumor should be a consideration when dealing with deep seated, firm, soft tissue masses of the buccal mucosa.
In terms of frequency, our data significantly varies from the literature. Our study documented a higher number of benign lesions in contrast to the study by Weber et al. [4] and Jansisyanont et al. [2] who reported exclusively malignant SGNBM including 31 and 13 cases of a total of 50 and 80 cases studied from all oral minor salivary gland neoplasms respectively.
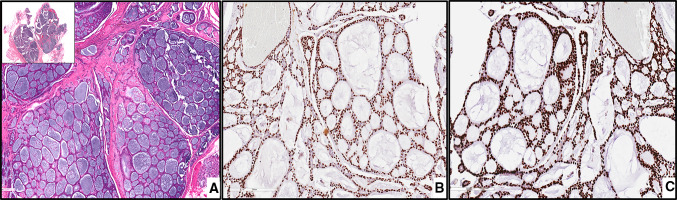
A higher incidence of benign tumors was seen in our study, compared to the results of other authors [2, 4], could be potentially explained by study size, geographic and/or racial factors. In our study the most common benign tumor was CLA (Fig. 5) (21.2%), though the incidence reported in the literature is lower (between 0 and 10%) [3, 4] DP was the second most frequent benign tumor in our series (15.2%), followed by CA (12.1%) and BCA (6.1%). PA (Fig. 3) was the least common benign neoplasm in our study. This is in agreement with the literature and various textbook chapters that concludes that the buccal mucosa is an uncommon site of occurrence for intraoral PA [5].
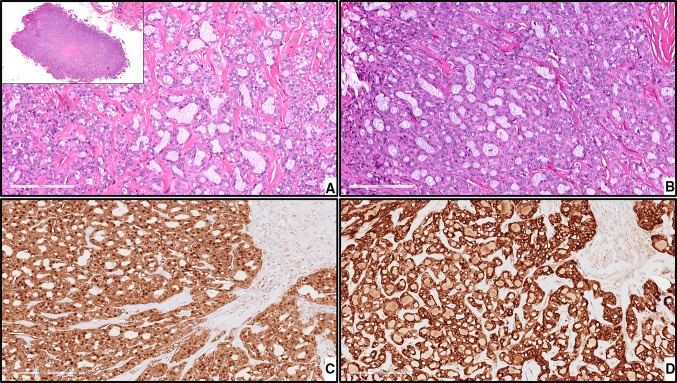
Fig. 5.
CLA. The tumor cells are uniform, columnar, and hyperchromatic forming canal-like ductal structures. The tumor cells are supported by a loose connective tissue stroma with prominent vascularity, magnification X100 (H&E*). *Hematoxylin-eosin staining
We found that malignant salivary gland neoplasms of the buccal mucosa are less common than the benign tumors. This is in alignment with results from other studies [2] Wyszyńska-Pawelec et al. [8] reported 11 cases from the buccal mucosa, and only 7 (63.6%) were benign. Vicente et al. [6] in their study on intraoral minor salivary gland neoplasms reported only 2 buccal mucosal lesions and both were benign.
According to the results of this study, MEC (Fig. 6) remained the most common salivary gland tumor overall, with 26 cases identified. The youngest patient was an 11-year- old female. Although salivary gland malignancies are rare in children, MEC is the most common malignant salivary gland tumor in the pediatric population [ 9].
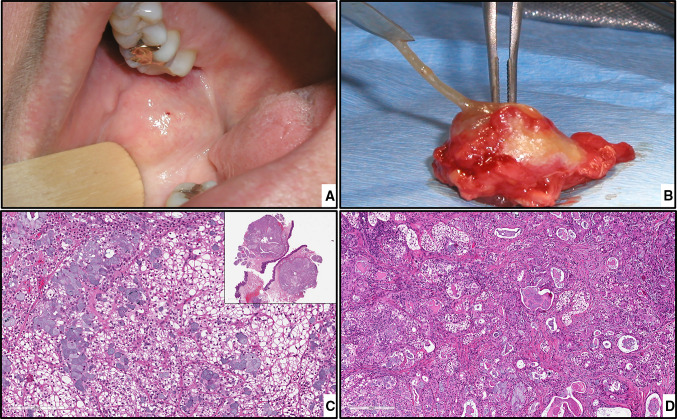
Fig. 6.
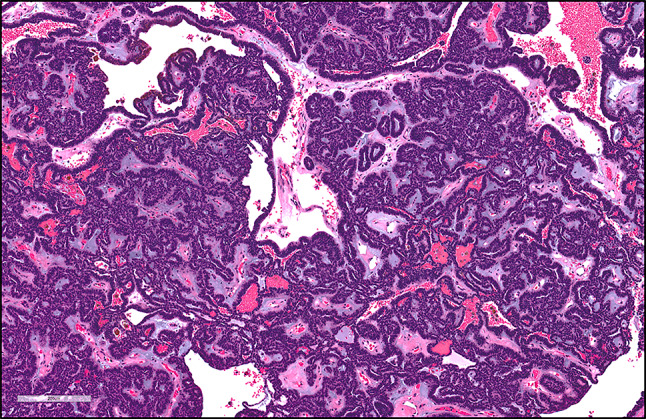
ACNOS. a, an infiltrative growth pattern of tumor cells, magnification X200 (H&E*). b, glandular/ductal differentiation with evidence of cellular pleomorphism, magnification X400 (H&E*). * Hematoxylin-eosin staining
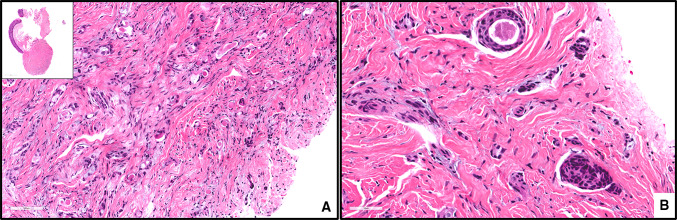
Other malignant neoplasms such as ACC, SC and ACNOS (Fig. 7) were far less frequently identified in our study. Weber et al. [4] reported that ACC is the most common malignant neoplasm of the minor salivary gland in the buccal mucosa followed by MEC. In the present study only one case of ACC (Fig. 1) was reported.
Fig. 7.
ACC. a, islands of hyperchromatic cells forming cribriform structures and surrounded by hyalinized material, magnification X100 (H&E*). b, P40- positive neoplastic cells, magnification X100 (IHC**). c, tumor cells stained with P63, magnification X100 (IHC**). * Hematoxylin-eosin staining, ** Immunohistochemical staining
Secretory carcinoma of salivary glands has been recently included in the fourth edition of the World Health Organization classification of head and neck tumors [10–13]. Since its description by Skalova et al. in 2010, some salivary tumors, including acinic cell carcinoma, have been reclassified as SC [11, 13] Paudel et al. [14] in 2019 documented 15 cases of SC in the buccal mucosa, with only one recurrent case that exhibited lymph node metastases. In our study, only one case was retrospectively reclassified secretory carcinoma.
No recurrent tumors were reported among our cases; this may be attributed to limited follow-up information available in our series and IRB restrictions. Recurrence in SGNBM can be related to growth of the tumor around the facial nerve, which complicates/restricts its complete surgical extirpation [1]. Therefore, radical surgery with wide/assured margins remains the mainstay of treatment for minor SGNBM. Other vital determinants such as histological type and clinical stage are extremely important in predicting tumor progression, effective therapy and patient outcome/prognosis.
Conclusions
Patient demographics and percentage of benign and malignant buccal mucosal salivary gland neoplasms were in conformity with previously published studies. However, benign neoplasms occurring in the buccal mucosa were more diverse than those found in other locations. Furthermore, benign neoplasms were more common than malignant ones and mucoepidermoid carcinoma was the predominant tumor overall. In addition, lesions in this location are less likely to be recognized as possible salivary gland neoplasms. Therefore, both benign and malignant salivary gland neoplasms should be included in the differential diagnosis of submucosal, soft-tissue buccal masses. Biopsy is required to avoid delay in diagnosis. Additional larger multicentric studies with more detailed treatment, outcome and follow-up data would assist in further understanding of any histologic diversity and differences in clinical behavior of salivary neoplasms of the buccal mucosa.
Compliance with Ethical Standards
Conflict of interest
No financial support was provided for this work and authors do not have conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trenkić Božinović M, Krasić D, Katić V, Krstić M. A retrospective review of 139 major and minor salivary gland tumors. Med Glas (Zenica) 2015;12(1):73–8. [PubMed] [Google Scholar]
- 2.Jansisyanont P, Blanchaert RH, Jr, Ord RA. Intraoral minor salivary gland neoplasm: a single institution experience of 80 cases. Int J Oral Maxillofac Surg. 2002;31(3):257–61. doi: 10.1054/ijom.2002.0223. [DOI] [PubMed] [Google Scholar]
- 3.Sarmento DJ, Morais ML, Costa AL, Silveira ÉJ. Minor intraoral salivary gland tumors: a clinical-pathological study. Einstein (Sao Paulo) 2016;14(4):508–12. doi: 10.1590/S1679-45082016AO3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber RS, Palmer JM, el-Naggar A, McNeese MD, Guillamondegui OM, Byers RM. Minor salivary gland tumors of the lip and buccal mucosa. Laryngoscope. 1989;99(1):6–9. doi: 10.1288/00005537-198901000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Geetha NT, Deepa BV, Umashankara KV, Kithikumar R. Pleomorphic adenoma of minor salivary gland in the cheek. Int J Oral Health Sci. 2015;5:117–20. doi: 10.4103/2231-6027.178497. [DOI] [Google Scholar]
- 6.Pons Vicente O, Almendros Marqués N, Berini Aytés L, Gay Escoda C. Minor salivary gland tumors: A clinicopathological study of 18 cases. Med Oral Patol Oral Cir Bucal. 2008;13(9):E582–8. [PubMed] [Google Scholar]
- 7.Kulkarni DG, Shetty L, Zurange V. Mucoepidermoid carcinoma of minor salivary gland in buccal mucosa: A rare case report. J Dent Res Rev. 2014;1:97–9. doi: 10.4103/2348-2915.133958. [DOI] [Google Scholar]
- 8.Wyszyńska-Pawelec G, Gontarz M, Zapała J, Szuta M. Minor salivary gland tumours of upper aerodigestive tract: a clinicopathological study. Gastroenterol Res Pract. 2012;2012:780453. doi: 10.1155/2012/780453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krolls SO, Trodahl JN, Boyers RC. Salivary gland lesions in children. A survey of 430 cases. Cancer. 1972;30(2):459–469. doi: 10.1002/1097-0142(197208)30:2<459::aid-cncr2820300225>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Skalova A, Bell D, Bishop JA, Inagaki H, Seethala R, Vielh P. Secretory carcinoma: tumors of salivary glands. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO Classification of Head and Neck Tumors. 4. Lyon: IARC; 2017. pp. 177–8. [Google Scholar]
- 11.Skalova A. Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol. 2013;7(Suppl 1):30–6. doi: 10.1007/s12105-013-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skálová A, Vanecek T, Simpson RH, et al. Mammary Analogue Secretory Carcinoma of Salivary Glands: Molecular Analysis of 25 ETV6 Gene Rearranged Tumors With Lack of Detection of Classical ETV6-NTRK3 Fusion Transcript by Standard RT-PCR: Report of 4 Cases Harboring ETV6-X Gene Fusion. Am J Surg Pathol. 2016;40(1):3–13. doi: 10.1097/PAS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 13.Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 14.Paudel D, Nishimura M, Adhikari BR, et al. Secretory Carcinoma of Minor Salivary Gland in Buccal Mucosa: A Case Report and Review of the Literature. Case Rep Pathol. 2019;2019(2074504):201927. doi: 10.1155/2019/2074504. [DOI] [PMC free article] [PubMed] [Google Scholar]