Abstract
Adenoid cystic carcinoma (AdCC) comprises of less than 1% of all head and neck cancers and less than 10% of all salivary gland neoplasms. Dedifferentiation/high-grade transformation (HGT) in AdCC is a rare but well known phenomenon which is associated with aggressive clinical behaviour and poor prognosis. We herein report the clinical, cytologic, histologic and immunohistochemical findings of a left submandibular gland AdCC with transformation to high grade carcinomatous and probable dedifferentiation to sarcomatoid component, occurring in a 64 year old male patient. To the author’s best knowledge, this is the first case report of such dual transformation occurring in adenoid cystic carcinoma.
Keywords: Adenoid cystic carcinoma, Dedifferentiation/high-grade transformation, Sarcomatoid differentiation
Introduction
Dedifferentiation/HGT is an unusual phenomenon that has been described in a variety of salivary gland carcinomas, including acinic cell carcinoma, epithelial-myoepithelial carcinoma, polymorphous low-grade adenocarcinoma, myoepithelial carcinoma, mucoepidermoid carcinoma, hyalinising clear cell carcinoma, mammary analogue secretory carcinoma, low grade cribriform cystadenocarcinoma and AdCC [1]. The term “dedifferentiation” has been applied traditionally to sarcomas and was first coined by Dahlin and Beabout in reference to the abrupt transition of low-grade chondrosarcoma to a histologically different high-grade sarcoma [2]. Previously, this terminology was used for salivary gland tumours where a low grade tumour is juxtaposed to a high grade tumour, in which the original line of differentiation is no longer apparent or it may acquire a new line of differentiation [3]. However, current authors often tend to use the term HGT instead of “dedifferentiation” as in majority of the epithelial tumours; the transformed component did not exhibit complete loss of phenotypical characteristics and could still be recognised as poorly differentiated adenocarcinoma/carcinoma or an undifferentiated carcinoma [4]. Since the first documentation of “dedifferentiated” AdCC by Cheuk et al. [3], a total of 48 AdCC cases with dedifferentiation/HGT have been described in the literature till date (Table 1). Only three cases of low grade AdCC transforming to sarcomatoid component have been reported in the literature (Table 2) [3, 5, 6]. Herein, we report the first case of AdCC arising in submandibular gland showing synchronous transformation to high grade carcinomatous and also to a sarcomatoid component.
Table 1.
Review of reported cases of adenoid cystic carcinoma with high-grade transformation/dedifferentiation
| Authors | Cases |
|---|---|
| Cheuke et al. [3] | 3 |
| Moles et al. [27] | 1 |
| Terasaki et al. [18] | 1 |
| Chau et al. [28] | 1 |
| Ide et al. [29] | 1 |
| Nagao et al. [16] | 6 |
| Brackrock et al. [30] | 1 |
| Sato et al. [17] | 1 |
| Seethala et al. [4] | 11 |
| Handra-Luca et al. [31] | 1 |
| Malhotra et al. [12] | 1 |
| Bonfitto et al. [32] | 7 |
| Costa et al. [10] | 1 |
| Panarelli et al. [5] | 1 |
| Boland et al. [33] | 3 |
| Argyris et al. [19] | 1 |
| Bavle et al. [34] | 1 |
| Ly et al. [13] | 1 |
| Sayar et al. [35] | 1 |
| Kusafuka et al. [36] | 1 |
| Noda et al. 37 | 1 |
| Tando et al. [6] | 1 |
| Bhardwaj et al. [14] | 1 |
Table 2.
Reported cases of HGT/dedifferentiation of AdCC with sarcomatoid differentiation
| Case | Author | Age, sex | Tumour site, size 9 cm) | Histology (dedifferentiated component) | IHC profile | Treatment and outcome | |
|---|---|---|---|---|---|---|---|
| 1 | Cheuk et al. [3] | 53, F | Soft palate, NA | Sarcomatoid (focal myoepithelial differentiation) | Focal positive: Cam5.2, EMA, S100, GFAP, SMA, CK14, Rb protein, Cyclin-D1. Ki67: 20% | Negative: Cytokeratin, p53 | Excision and curative radiotherapy; Local recurrence after 4 years, wide local excision and second course radiotherapy given, died after 9 months with extensive local recurrence and lung metastasis |
| 2 | Panarelli et al. [5] | 52, M | Right lacrimal gland, 3.2 × 3.2 × 2.9 | Sarcomatoid; focal osteoid formation present (focal myoepithelial differentiation) |
Positive: Vimentin, S100 Focal positive: SMA |
Negative: AE1/AE3, Cam5.2, p63 | Surgical debulking and proton beam radiotherapy; Local recurrence after one year, planned for right orbital exenteration |
| 3 | Tando et al. [6] | 42, F | Epipharynx, 3.1 × 2.5 | Sarcomatoid (myoepithelial carcinoma) | Positive: Pan-CK, CK5/6, LMWCK, Calponin, SMA, p63, vimentin, Focal positive: S100, p53, Cyclin-D1 Ki67: 50% | Negative: EMA, desmin, GFAP, HER2, Myc, beta catenin, synaptophysin, chromogranin A, CD56, MYB | Surgical resection; Died after 2 years and 9 months of acute myeloblasstic leukemia. Recurrence was not confirmed clinically |
| 4 | Present case | 64, M | Left submandibular gland, 6.5 × 4.5 × 4 | Sarcoma (complete loss of myoepithelial differentiation) | Positive: Vimentin, p53 Focal positive: EMA (weak), S100 (occasional positive cells) Ki67: 90% | Negative: AE1/AE3, CD117, p63, desmin, Her2 | Surgical resection and left selective neck dissection; Developed lung metastasis, hence referred to palliative care, after 4 months recurrence in alveolar region, refused palliative radiotherapy and was lost to follow up |
Case Report
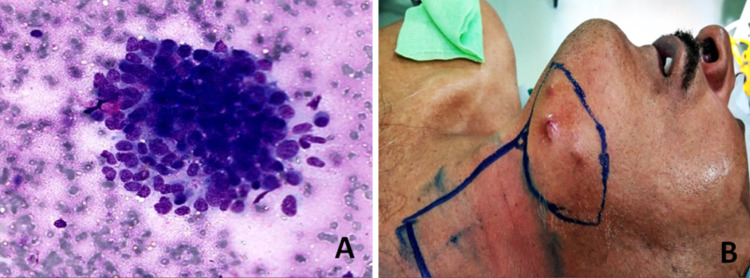
A 64 year old male presented with complaints of swelling in the left submandibular region for six months. Positron emission tomography–computed tomography (PET–CT) performed at another center showed a well defined nodular soft tissue mass in the left submandibular gland and multiple small left submandibular and intra parotid nodes. Multiple parenchymal nodules were also noted in the right lung basal zone. On clinical examination, a hard, mobile, bosselated 8.0 × 5.0 cm growth was felt in the left submandibular area, free from the mandible. Fine needle aspiration cytology (FNAC) was performed at another center and the slides were reviewed at our hospital. The smears were cellular and showed atypical basaloid cells in clusters and occasional clumps of matrix material (Fig. 1a). A diagnosis of basaloid neoplasm with adenoid cystic carcinoma and basal cell adenocarcinoma as differentials was rendered and histological correlation was suggested. Later on, a core biopsy performed at our center was non-representative and the patient was planned for surgery. Meanwhile, within a month, the tumour grew rapidly forming multiple skin nodules (Fig. 1b). Resection of the left submandibular mass with left selective neck dissection was performed. On gross examination, an infiltrative, grey-white, firm, vaguely nodular tumour measuring 6.5 × 4.5 × 4.0 cm was identified. The tumour infiltrated adjacent salivary gland, muscle and fat and overlying skin surface showed nodularity. The inked surgical resection margin was involved by tumour focally.
Fig. 1.
a FNA smears showing atypical basaloid cells in clusters (Giemsa stain, × 400); b Clinical picture showing multiple skin nodules over left submandibular region
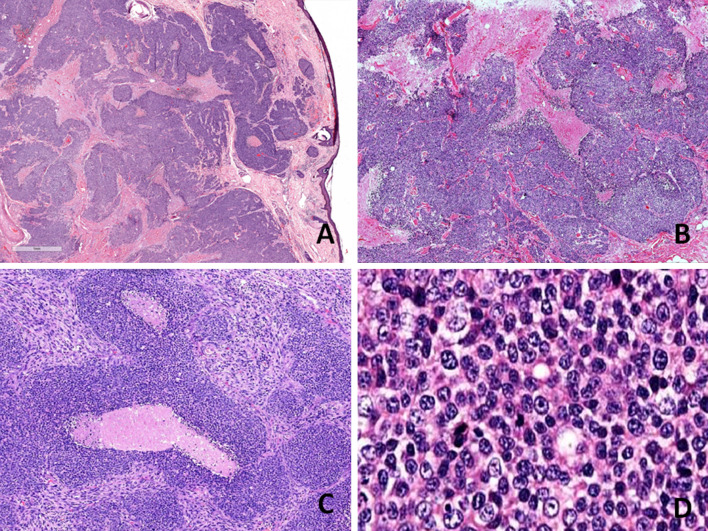
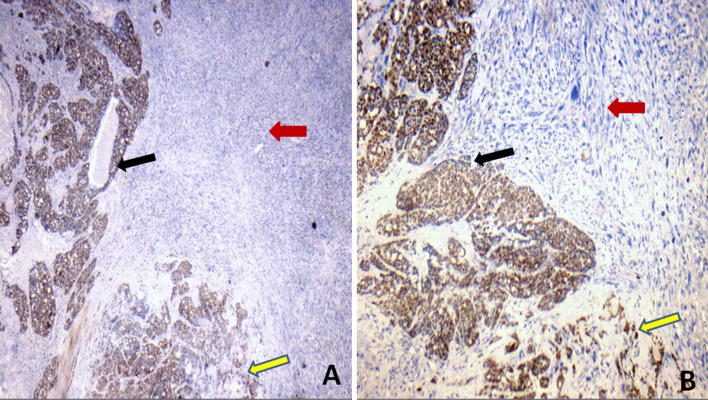
The histologic sections examined, showed an infiltrative tumour with a variegated appearance, comprising of three distinct histomorphologic patterns (Figs. 2, 3). The first and predominant pattern was arranged in solid confluent nests, islands and sheets of basaloid cells in a desmoplastic stroma. Punctuate to large zones of comedo necrosis was seen. The tumour cells were round to oval with scant to moderate amount of cytoplasm, enlarged, vesicular nuclei with prominent nucleoli and showed frequent mitoses. This component was identified as poorly differentiated carcinomatous component and amounted to 70% of the total tumour. The second pattern was composed of pleomorphic spindle cells arranged in vague fascicles and sheets, exhibiting marked nuclear anaplasia, conspicuous nucleoli and brisk mitotic activity. This was recognised as the sarcomatoid component and it comprised approximately 25% of the tumour. The third and minor component (approximately 5%) was conventional adenoid cystic carcinoma composed of basaloid cells arranged in cribriform pattern which was identified focally in one of the sections from the tumour. Perineural and lymphovascular invasion were seen. The tumour was seen infiltrating the adjacent salivary gland, muscle and dermis of overlying skin, however, the epidermis was free. The inked surgical margin was involved by tumour. Lymph nodes were free of metastasis.
Fig. 2.
Histological findings a Scanning power appearance of a section showing an infiltrative tumour involving dermis of overlying skin (H&E stain, × 10). b–c Low-power view showing the predominant poorly differentiated carcinomatous component of tumour arranged in solid confluent nests, islands and sheets of basaloid cells with interspersed comedo necrosis (H&E stain, b, × 100; c, × 200). d High-power appearance of the same component showing round to oval tumour cells with scant to moderate amount of cytoplasm, enlarged, vesicular nuclei with prominent nucleoli (H&E stain, × 400)
Fig. 3.
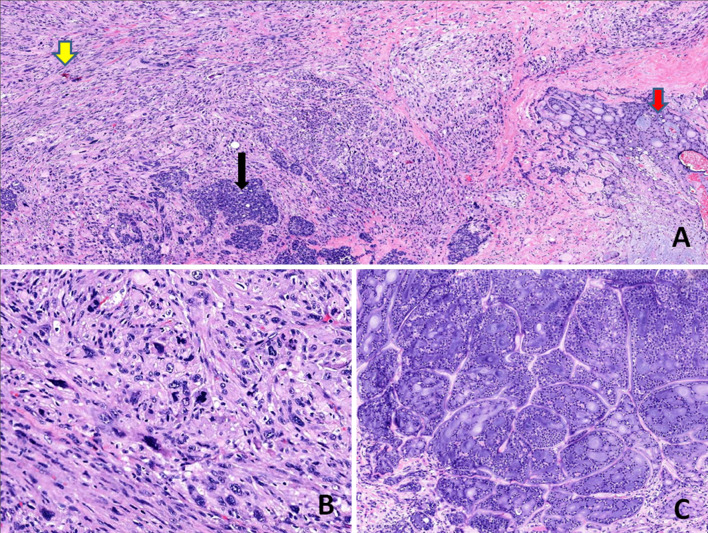
Histological findings a Low-power view showing three distinct components of the tumour: conventional cribriform-type AdCC (red arrow), sarcomatoid component (yellow arrow) and intervening poorly differentiated carcinoma component (black arrow) (H&E stain, × 100). b The sarcomatoid component is composed of pleomorphic spindle cells arranged in vague fascicles and sheets, exhibiting marked nuclear anaplasia and brisk mitotic activity (H&E stain, × 400). c The conventional AdCC composed of basaloid cells arranged in cribriform pattern (H&E stain, × 200)
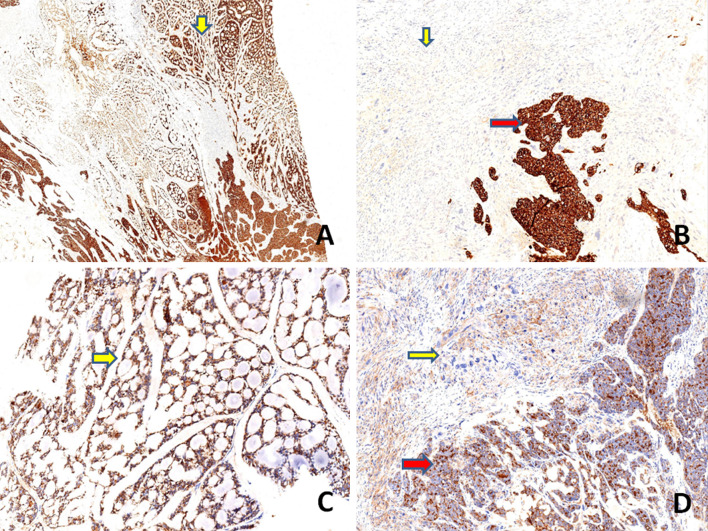
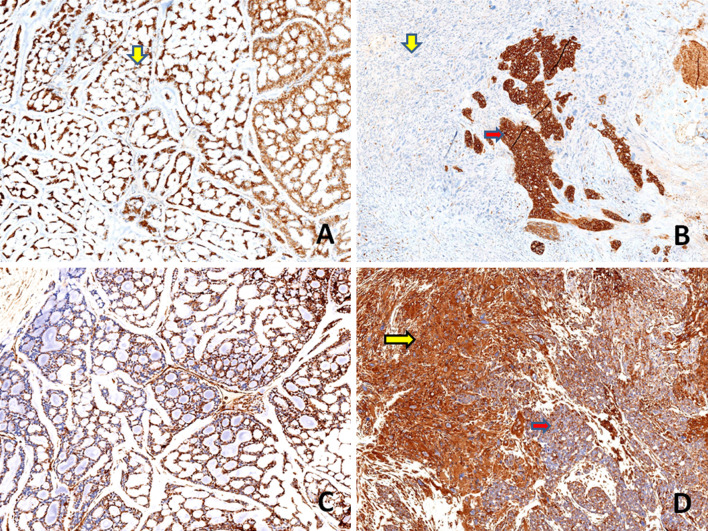
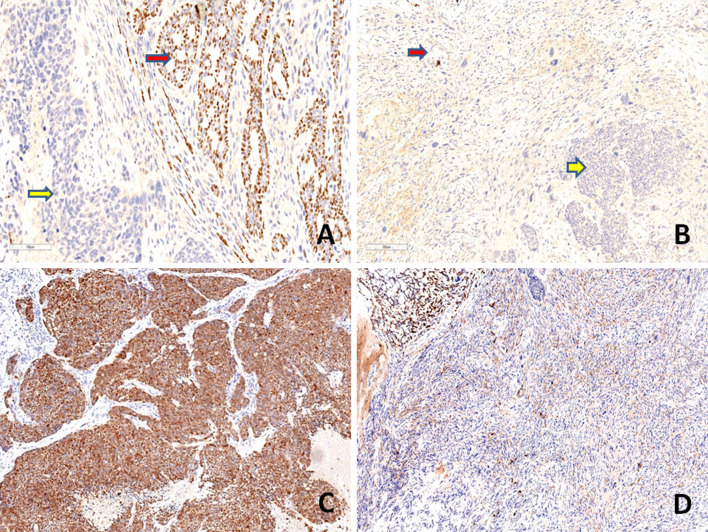
On immunohistochemistry, the conventional AdCC component was positive for cytokeratin (AE1/AE3), CD117(C-kit) and EMA (Epithelial Membrane Antigen). Immunostains: vimentin, S100 protein and p63 highlighted the myoepithelial cells in this component. The poorly differentiated carcinomatous component was diffusely positive for AE1/AE3, CD117, EMA and S100 protein while, an immunostain for p63 was negative and vimentin was focally positive. The sarcomatoid component was negative for AE1/AE3, CD117 and p63 while, diffuse positivity for vimentin was noted. EMA was weakly positive focally and occasional cells showed S100 protein positivity. Immunostaining with p53 displayed diffuse strong positivity in the poorly differentiated carcinomatous and sarcomatoid component (80–90%) while, it was focally positive in conventional AdCC component (20%). SOX10 (SRY-related HMG-box 10) protein was expressed in the low grade and high grade carcinomatous component, while there was loss of expression of the marker in the sarcomatoid component. The MIB-1 labeling index was approximately 90% in both high grade carcinomatous and sarcomatoid components while it was about 10% in the AdCC component. HER 2 staining was negative in all components. Table 3 and Figs. 4, 5, 6, 7 show the comparative immunohistochemical features of all three components.
Table 3.
Immunohistological findings of present case
| Histology | Conventional AdCC | High grade carcinomatous component | Sarcomatoid component |
|---|---|---|---|
| Cribriform pattern | Solid nests with comedo necrosis | Fascicles of spindle cells with brisk mitotic activity | |
| IHC | |||
| CK (AE1-AE3) | + | + | − |
| CD117 | + | + | − |
| P63 | + | − | − |
| Vimentin | + Myoepithelial cells | Focal + | Strongly + |
| P53 | 20% | 80–90% | 80–90% |
| Ki67 | 10% | 90% | 90% |
| S100 | + Myoepithelial cells | Diffuse + | Mostly negative. Occasional cells + |
| SOX10 | + | + | − |
| EMA | + | + | Weak focally positive |
| Desmin | − | − | − |
| Her2 | − | − | − |
Fig. 4.
Immunohistochemical findings a, b Both conventional AdCC (yellow arrow, a) and poorly differentiated carcinoma component (red arrow, b) showing strong and diffuse positivity for cytokeratin (AE1/AE3), whereas the sarcomatoid component is negative for the marker (yellow arrow, b) (a, b, × 100). c, d An immunostain for EMA highlights the conventional AdCC ( yellow arrow, c) and poorly differentiated carcinoma component (red arrow, d) and is weakly and focally positive in sarcomatoid component (yellow arrow, d) (c, d, × 100)
Fig. 5.
Immunohistochemical findings a, b Both conventional AdCC (yellow arrow, a) and poorly differentiated carcinoma component (red arrow, b) show strong and diffuse positivity for CD117, whereas the sarcomatoid component is negative (yellow arrow, b) (a, b, × 100). c, d Vimentin highlights the myoepithelial cells in the conventional AdCC. c The sarcomatoid component is diffusely positive for vimentin (yellow arrow) (d, × 100) and is focally and weakly positive in poorly differentiated carcinoma component (red arrow) (d, × 100)
Fig. 6.
Immunohistochemical findings. a, b Immunostaining with p63 highlights the myoepithelial cells in the conventional AdCC (red arrow, a) (a, × 100), while poorly differentiated carcinoma (yellow arrow, a, b) and sarcomatoid component (red arrow, b) are negative (b, × 100). c, d The poorly differentiated carcinoma component is diffusely positive for S100 protein (c, × 100) and the sarcomatoid component is mostly negative for S100 protein (d, × 100)
Fig. 7.
Immunohistochemical findings. The conventional AdCC and high grade carcinomatous components (yellow and black arrows respectively in both a, b) showing strong and diffuse nuclear positivity for SOX10, while the sarcomatoid component (red arrow, in both a, b) is negative for the marker (a, × 25, b, × 100)
A diagnosis of adenoid cystic carcinoma with HGT/dedifferentiation to high grade carcinomatous and sarcomatoid components was made and the tumour was staged as pT4aN0. C-Kit (Exon 9, 11, 13 and 17) gene sequencing was done using an ABI 3500 DNA analyzer which showed wild type for exon 9, 11, 13 and 17 in the KIT gene and was negative for C-Kit mutation. The initial plan to proceed with surgery in spite of radiologic evidence of lung metastasis was due to the fact that lung metastases from adenoid cystic carcinoma are indolent for long periods of time [7] or could be treated with local therapy like radiation or radiofrequency ablation if they progressed on follow up. However, in the renewed circumstances of an aggressive histology, lung metastases and considering the rapid growth in size in the period leading to surgery, a guarded prognosis was explained to the patient who declined adjuvant treatment. The patient was referred to palliative care for symptomatic management and within four months, the disease recurred with a growth in the alveolar region. Palliative radiotherapy was planned; however, the patient refused to undergo treatment and was lost to follow up.
Discussion
AdCC of the salivary gland is a malignant tumour characterised by a slow but relentless clinical course, with frequent local recurrences, late metastases, and an ultimately fatal outcome. Histologically, AdCC is a biphasic tumour composed of ductal (luminal) and myoepithelial (abluminal) cells and exhibits three basic growth patterns (tubular, cribriform and solid) [8]. The histomorphologic pattern and percentage of the solid component form the basis of the grading system defined by Szanto et al. [9]. AdCC with both tubular and cribriform patterns having a less aggressive clinical course than those with the solid pattern [8].
Cheuk et al., first reported three cases of “de-differentiated” AdCC [3]. In all the three cases, the de-differentiated component was a distinct population of anaplastic cells with more abundant cytoplasm, irregular-shaped tumour islands infiltrating a desmoplastic stroma and total loss of bicellular differentiation, characteristic of AdCC. Seethala et al. emphasized on using the term ‘high-grade transformation (HGT)’ instead of dedifferentiation in their case series of 11 cases of adenoid cystic carcinomas with high grade transformation (AdCC-HGT) because the so-called dedifferentiated component was still recognisable as carcinoma (either poorly differentiated adenocarcinoma or undifferentiated carcinoma) [4]. Since then, a fair number of cases with dedifferentiation or AdCC-HGT have been described in the literature (Table 1). The presence of a sarcomatoid component as seen in our case is actually suggestive of complete loss of original line of differentiation, that is, transition from epithelial differentiation to mesenchymal differentiation. Whether, the terminology ‘dedifferentiation’ is applicable to our case is a matter of contention. Moreover, Costa et al. and others have reported AdCCs which have undergone transformation to moderately differentiated adenocarcinomas indicating that the term, “high grade transformation” may not be sufficient to explain this phenomenon [10].
AdCC-HGT primarily affects older adults in the sixth decade (age range 32 to 88 years) with a slight female predominance (male to female ratio 1:1.14). The most common sites of involvement were the submandibular gland (12 cases), paranasal sinus (9 cases), followed by parotid gland and palate (7 cases each). Other rare involved sites were nasal cavity (3 cases), lacrimal gland (3 cases), tongue (2 cases), pterygopalatine (1 case), lip (1 case), pyriform (1 case) and epipharynx (1 case). The tumour is usually grossly aggressive with bone involvement at sinonasal or palatine sites and extraglandular extension for submandibular sites, often with positive surgical margins. The present case also had similar clinical features, occurring in an elderly male, arising in submandibular gland, showing rapid increase in size, skin involvement, infiltration of adjacent muscle and adipose tissue and a positive resection margin. Another significant clinical feature of AdCC-HGT is the high propensity for lymph node metastasis (41%) and distant metastasis (50%) as reported by Hellquist et al. [11]. This suggests a possible role for considering ‘‘elective management’’ of the neck in AdCC-HGT. However, in the present case, although the lymph nodes were uninvolved, the patient had multiple lung nodules suggestive of distant metastasis.
The diagnosis of HGT in AdCC requires two distinct carcinomatous components: conventional AdCC of any growth pattern, and high grade carcinoma with a loss of the histologic features characteristic of AdCC (e.g. biphasic ductal and myoepithelial differentiation). The high grade carcinoma is usually either a poorly differentiated adenocarcinoma or less often, an undifferentiated carcinoma. Thus, diagnosing AdCC-HGT is a challenge on FNAC. The cytological features have been documented in three cases [12–14]. Malhotra et al. considered a broad range of differential diagnoses (adenocarcinoma-nos, undifferentiated carcinoma, salivary duct carcinoma, basaloid adenocarcinoma and carcinoma ex-pleomorphic adenoma). However, on review the aspirate smears revealed small groups of basaloid cells of conventional AdCC and thus they emphasized on a thorough analysis of FNA smears as preoperative diagnosis of AdCC would be helpful in deciding the extent of surgery and would also promote thorough sectioning and sampling of excised tumour [12]. The other two cases were diagnosed as poorly differentiated carcinoma by Bhardwaj et al. and adenoid cystic carcinoma by Ly et al. on FNAC [13, 14]. The present case was diagnosed as basaloid neoplasm with differentials of AdCC and basal cell adenocarcinoma on FNAC. On histology, the conventional AdCC and the HG carcinoma components could be separate or a transitional zone can be recognised. HGT has to be distinguished from hybrid tumours and collision tumours. Hybrid tumours are composed of two distinct tumours that can be identified as defined tumour categories in the same topographic area forming a single tumour mass. The hybrid tumours demonstrate identical origin/histogenesis. For example, a hybrid tumour composed of basal cell adenoma and adenoid cystic carcinoma. However, a collision tumour comprises of two histologically distinct tumours of clearly different origins from two separate neoplastic clones of different cell types developing close to each other. Majority of collision tumours reported in literature are combinations of adenocarcinoma and sarcoma or lymphoma [15]. The presence of a transitional zone is helpful in distinguishing AdCC-HGT from a hybrid tumours and collision tumours. The distinction of AdCC-HGT from grade III conventional AdCC could be difficult at times. Seethala et al. enumerated the comparative histologic and immunophenotypic features of both these components and also proposed the criteria for diagnosis of AdCC-HGT [4]. The poorly differentiated carcinoma component in the present case was arranged in solid confluent nests in a desmoplastic stroma and composed of cells with large pleomorphic nuclei (at least 2–3 times the size of grades I–II AdCC nuclei), exhibited loss of abluminal cell layer as highlighted by p63 and was diffusely and strongly positive for p53, thus fulfilling five of six major criteria established by Seethala et al. However, micropapillae and squamoid areas were not seen. The high grade component in the reported cases showed significantly high Ki67 labeling index and almost half of the reported cases demonstrated overexpression of p53 and Cyclin-D1 in [3, 4, 10, 16, 17]. Her-2 immunopositivity has been described in only four cases [14, 16, 17]. In the present case, both the high grade components showed a Ki-67 labeling index of 90%, overexpressed p53 and were negative for Her-2. Cases with high grade transformation showing sarcomatoid features are rare and only three cases have been reported till date [3, 5, 6]. In all three cases the sarcomatoid component was the sole high grade component and exhibited myoepithelial differentiation in the form of strong and diffuse S100 positivity in two cases and focal positivity in the third case [3, 5, 6, 18, 19]. In the reported cases of AdCC with HGT only five cases have been described showing expression of myoepithelial markers in the high grade component. This includes the three cases with sarcomatoid differentiation [3, 5, 6], out of which one was called as myoepithelial carcinoma [6]. Remaining two cases showed solid nests with comedo necrosis as the dedifferentiated component [18, 19], out of which one was reported as myoepithelial carcinoma [19].
As described in literature, there was loss of myoepithelial differentiation in the form of p63 negativity in both the high grade carcinomatous component and sarcomatoid component in the present case. However, an immunostain for S-100 and CD117 showed diffuse strong positivity in high grade carcinomatous component as mentioned in other reported cases in literature [8] while S100 and CD117 was negative in most of the tumor cells in the sarcomatoid component. The sarcomatoid component was also negative for cytokeratin (AE1/AE3) and desmin, while it was strongly positive for vimentin. EMA showed weak patchy expression in few cells. Variable expression of these markers has been reported in the prior cases with sarcomatoid dedifferentiation (Table 2). SOX10 protein, which is a transcription factor for the differentiation of neural crest cells, was recently found to be expressed in salivary gland tumours arising from myoepithelial, acinar and intercalated duct cells such as acinic cell carcinomas, adenoid cystic carcinomas, epithelial-myoepithelial carcinomas, myoepithelial carcinomas, pleomorphic adenomas and is not expressed in tumors arising from striated and excretory ducts like salivary duct carcinomas, mucoepidermoid carcinomas, squamous cell carcinomas, oncocytic carcinomas/oncocytomas and Warthin tumor [20]. AdCCs show consistent expression of SOX10 and hence it is considered as a potential marker for the diagnosis of these tumours [20]. Immunohistochemsitry with SOX10 in our case displayed positivity in the low grade and high grade carcinomatous component, while there was loss of expression of the marker in the sarcomatoid component. Therefore, the sarcomatoid component showed loss of expression of both epithelial and myoepithelial markers and also showed non expression of the marker suggestive of cell of origin (SOX10) and thus was identified as a true sarcomatoid dedifferentiation in our case. We believe that the distinct carcinomatous and sarcomatoid morphologic components of the high grade transformation in this case actually represents a mesenchymal dedifferentiation of the neoplastic epithelial cells. However, the possibility of collision of separate malignant processes is a subject of debate. Although c-Kit (CD117) is overexpressed in AdCCs, activating mutations are rare as in our case which did not show c-Kit mutation suggesting that Imatinib may not be effective in the treatment of these tumours [21]. Recently a recurrent translocation, t (6;9)(q21-24;p13-23), was recognized in AdCC which is associated with fusion of the MYB-NFIB genes [22]. However, since MYB rearrangements are described in literature in about less than half of AdCCs and MYB FISH probe is associated with technical problems, MYB FISH is not as clinically useful as it was supposed to be [23]. Moreover, MYB antibody available is highly sensitive and shows expression in 46% to 61% of translocation negative tumours [20]. Studies have shown strong MYB expression in most of MYB positive AdCC while only focal weak positivity was seen in other salivary gland tumors. Hence MYB could be a useful marker for differentiation of AdCCs from other salivary gland tumours [20]. MYBL1-NFIB fusions as a result of t (8;9) translocations have been identified recently in a subset of MYB-NFIB negative cases [24]. Whole genome sequencing of AdCCs has shown wide mutational diversity and low rates of somatic mutations involving different pathways, including fibroblast growth factor, insulin-like growth factor, Pl3K and NOTCH signalling and MYB-MYC signalling pathways [25]. MYB/MYBL1 activations due to gene fusion or other mechanisms are found in > 80% of AdCCs and may also be useful potential therapeutic targets [8]. Furthermore, AdCCs which are negative for MYB or MYBL1 fusions show mutations in the NOTCH signalling pathway [25]. High grade tumours with solid morphology and loss of myoepithelial cells were associated with activation of Notch signaling by gain-of-function mutation in NOTCH1 or loss-of-function aberrations in SPEN which is a negative NOTCH signaling regulator. Hence activation of Notch signaling was found to be associated with an aggressive phenotype [25]. Therefore, this opens up the possibility of targeting these tumors with NOTCH1 inhibitors, as proved by a recent study [26]. The NOTCH pathway or MYB/MYBL1 activation was not interrogated in the present case since the targeted therapy for these pathways is still investigational.
In conclusion, we present the first report of AdCC with high-grade transformation to poorly differentiated carcinoma and dedifferentiation to sarcoma. Additional cases need to be examined to understand the clinico-pathological behaviour and underlying molecular pathogenesis. We emphasize on thorough sampling of all initially diagnosed adenoid cystic carcinomas and use of broad panel of immunohistochemistry to rule out presence of high-grade transformation since it is a highly aggressive disease with poor prognosis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petersson F. High-grade transformation (“dedifferentiation”)—malignant progression of salivary gland neoplasms, including carcinoma ex pleomorphic adenoma. Pathol Case Rev. 2015;20(1):27–37. [Google Scholar]
- 2.Dahlin DC, Beabout JW. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28(2):461–466. doi: 10.1002/1097-0142(197108)28:2<461::aid-cncr2820280227>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Cheuk W, Chan JK, Ngan RK. Dedifferentiation in adenoid cystic carcinoma of salivary gland: an uncommon complication associated with an accelerated clinical course. Am J Surg Pathol. 1999;23(4):465–472. doi: 10.1097/00000478-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Seethala RR, Hunt JL, Baloch ZW, LiVolsi VA, Leon BE. Adenoid cystic carcinoma with high-grade transformation. Am J Surg Pathol. 2007;31(11):1683–1694. doi: 10.1097/PAS.0b013e3180dc928c. [DOI] [PubMed] [Google Scholar]
- 5.Panarelli JF, Zoumalan CI, Mukkamala K, Maher EA, Iacob C, Della Rocca DA. Dedifferentiated adenoid cystic carcinoma of the lacrimal gland. Ophthalmic Plast Reconstr Surg. 2011;27(5):e119–e121. doi: 10.1097/IOP.0b013e318201cb90. [DOI] [PubMed] [Google Scholar]
- 6.Tando S, Nagao T, Kayano K, Fushiki S, Itoh K. High-grade transformation/dedifferentiation of an adenoid cystic carcinoma of the minor salivary gland to myoepithelial carcinoma. Pathol Int. 2018;68(2):133–138. doi: 10.1111/pin.12624. [DOI] [PubMed] [Google Scholar]
- 7.Sung M-W, Kim KH, Kim J-W, Min Y-G, Seong W-J, Roh J-L, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Neck Surg. 2003;129(11):1193. doi: 10.1001/archotol.129.11.1193. [DOI] [PubMed] [Google Scholar]
- 8.El-Naggar AK, Chan JKC, Grandis JR, Takashi TPJS. WHO classification of head and neck tumours. 4. Lyon: WHO, IARC; 2017. pp. 164–5. [Google Scholar]
- 9.Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54(6):1062–1069. doi: 10.1002/1097-0142(19840915)54:6<1062::aid-cncr2820540622>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Costa AF, Altemani A, Vékony H, Bloemena E, Fresno F, Suárez C, et al. Genetic profile of adenoid cystic carcinomas (ACC) with high-grade transformation versus solid type. Cell Oncol. 2011;34(4):369–379. doi: 10.1007/s13402-011-0037-5. [DOI] [PubMed] [Google Scholar]
- 11.Hellquist H, Skálová A, Barnes L, Cardesa A, Thompson LDR, Triantafyllou A, et al. Cervical lymph node metastasis in high-grade transformation of head and neck adenoid cystic carcinoma: a collective international review. Adv Ther. 2016;33(3):357–368. doi: 10.1007/s12325-016-0298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preet K, Ae M, Ae VA, Pandey R. High grade transformation in adenoid cystic carcinoma of the parotid: report of a case with cytologic, histologic and immunohistochemical study. Head Neck Pathol. 2009;3(4):310–314. doi: 10.1007/s12105-009-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ly CK, Cheng HM, Vermeulen T. High grade transformation in a case of adenoid cystic carcinoma associated with Epstein-Barr virus expression. Pathology. 2013;45(7):693–695. doi: 10.1097/PAT.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 14.Bhardwaj M, Gupta P. Dedifferentiated adenoid cystic carcinoma ex pleomorphic adenoma of the parotid. J Cancer Res Ther. 2018;14(3):706. doi: 10.4103/0973-1482.179522. [DOI] [PubMed] [Google Scholar]
- 15.Seifert G, Donath K. Hybrid tumours of salivary glands. Definition and classification of five rare cases. Eur J Cancer B Oral Oncol. 1996;32B(4):251–259. doi: 10.1016/0964-1955(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 16.Nagao T, Gaffey TA, Serizawa H, Sugano I, Ishida Y, Yamazaki K, et al. Dedifferentiated adenoid cystic carcinoma: a clinicopathologic study of 6 cases. Mod Pathol. 2003;16(12):1265–1272. doi: 10.1097/01.MP.0000097366.88165.08. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Ueda Y, Sakurai A, Ishikawa Y, Kaji S, Nojima T, et al. Adenoid cystic carcinoma of the maxillary sinus with gradual histologic transformation to high-grade adenocarcinoma: a comparative report with dedifferentiated carcinoma. Virchows Arch. 2006;448(2):204–208. doi: 10.1007/s00428-005-0054-8. [DOI] [PubMed] [Google Scholar]
- 18.Terasaki M, Tokutomi T, Maruiwa H, Sugita Y, Harada H, Shigemori M. High-grade adenoid cystic carcinoma originating from the lacrimal gland. Brain Tumor Pathol. 2000;17(3):159–163. doi: 10.1007/BF02484288. [DOI] [PubMed] [Google Scholar]
- 19.Argyris PP, Pambuccian SE, Cayci Z, Singh C, Tosios KI, Koutlas IG. Lacrimal gland adenoid cystic carcinoma with high-grade transformation to myoepithelial carcinoma: report of a case and review of literature. Head Neck Pathol. 2013;7(1):85–92. doi: 10.1007/s12105-012-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu S, Schuerch C, Hunt J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med. 2015;139(1):55–66. doi: 10.5858/arpa.2014-0167-RA. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer MR, Talmi Y, Catane R, Symon Z, Yosepovitch A, Levitt M. A phase II study of Imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007;43(1):33–36. doi: 10.1016/j.oraloncology.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106(44):18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith CC, Schmitt AC, Little JL, Magliocca KR. New developments in salivary gland pathology: clinically useful ancillary testing and new potentially targetable molecular alterations. Arch Pathol Lab Med. 2017;141(3):381–395. doi: 10.5858/arpa.2016-0259-SA. [DOI] [PubMed] [Google Scholar]
- 24.Skálová A, Stenman G, Simpson RHW, Hellquist H, Slouka D, Svoboda T, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018;42(2):e11–e27. doi: 10.1097/PAS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 25.Wysocki PT, Izumchenko E, Meir J, Ha PK, Sidransky D, Brait M. Adenoid cystic carcinoma: emerging role of translocations and gene fusions. Oncotarget. 2016;7(40):66239–66254. doi: 10.18632/oncotarget.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoeck A, Lejnine S, Truong A, Pan L, Wang H, Zang C, et al. Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov. 2014;4(10):1154–1167. doi: 10.1158/2159-8290.CD-13-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moles MAG, Avila IR, Archilla AR. Dedifferentiation occurring in adenoid cystic carcinoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 1999;88(2):177–180. doi: 10.1016/s1079-2104(99)70114-9. [DOI] [PubMed] [Google Scholar]
- 28.Chau Y, Hongyo T, Aozasa K, Chan JK. Dedifferentiation of adenoid cystic carcinoma: report of a case implicating p53 gene mutation. Hum Pathol. 2001;32(12):1403–1407. doi: 10.1053/hupa.2001.28966. [DOI] [PubMed] [Google Scholar]
- 29.Ide F, Mishima K, Saito I. Small foci of high-grade carcinoma cells in adenoid cystic carcinoma represent an incipient phase of dedifferentiation. Histopathology. 2003;43(6):604–606. doi: 10.1111/j.1365-2559.2003.01682.x. [DOI] [PubMed] [Google Scholar]
- 30.Brackrock S, Krüll A, Röser K, Schwarz R, Riethdorf L, Alberti W. Neutron therapy, prognostic factors and dedifferentiation of adenoid cystic carcinomas (ACC) of salivary glands. Anticancer Res. 2019;25(2B):1321–1326. [PubMed] [Google Scholar]
- 31.Handra-Luca A, Planchard D, Fouret P. Docetaxel-cisplatin-radiotherapy in adenoid cystic carcinoma with high-grade transformation. Oral Oncol. 2009;45(11):e208–e209. doi: 10.1016/j.oraloncology.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Bonfitto VL, Demasi AP, Costa AF, Bonfitto JF, Araujo VC, Altemani A. High-grade transformation of adenoid cystic carcinomas: a study of the expression of GLUT1 glucose transporter and of mitochondrial antigen. J Clin Pathol. 2010;63(7):615–619. doi: 10.1136/jcp.2010.075390. [DOI] [PubMed] [Google Scholar]
- 33.Boland JM, McPhail ED, García JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25(4):529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 34.Bavle R, D′Mello S, Makarla S, Hosthor S. Dedifferentiation in adenoid cystic carcinoma. J Oral Maxillofac Pathol. 2013;17(3):474. doi: 10.4103/0973-029X.125225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayar H, Sarıoğlu S, Bakariş S, Yıldırım İ, Öztarakçı H. High-grade transformation of adenoid cystic carcinoma delineated with a fibrous rim: a case report. Balkan Med J. 2013;30:333–336. doi: 10.5152/balkanmedj.2013.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusafuka K, Miki T, Nakajima T. Salivary adenoid cystic carcinoma with an early phase of high-grade transformation: case report with an immunohistochemical analysis. Diagn Pathol. 2013;8(1):113. doi: 10.1186/1746-1596-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noda Y, Hori Y, Kishino M, Satou S, Ogawa Y, Harada H, et al. A case of dedifferentiated adenoid cystic carcinoma in submandibular gland: morphological and immunohistochemical features. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(3):e140. [Google Scholar]