Abstract
Patient: Female, 37-year-old
Final Diagnosis: Ventricular arrhythmia
Symptoms: Arrhythmia
Medication: Azithromycin
Clinical Procedure: ECMO insertion
Specialty: Cardiac surgery • Cardiology • Critical Care Medicine
Objective:
Rare disease
Background:
Azithromycin is a commonly prescribed antibiotic due to several advantages, including the broad range of indications, spectrum of activity, favorable drug interaction profile, and convenience of dosing. Although azithromycin carries a black-box warning for QTc prolongation and ventricular arrhythmias, these are considered rare adverse effects.
Case Report:
We present the case of a 37-year-old woman who received azithromycin (500 mg) for follicular tonsillitis and was admitted for worsening of symptoms. On the same day of admission to a secondary hospital, she became unresponsive and had cardiac arrest, for which cardiopulmonary resuscitation (CPR) was performed for 26 min. As per the input from the secondary hospital, she had multiple ventricular tachycardia (VT) and ventricular fibrillation, and needed to be transferred to a tertiary care hospital for further management. Veno-arterial extracorporeal membrane oxygenation (ECMO) support was inserted to support her hemodynamics, and serial ECGs showed significant QT interval prolongation up to 600 msec. The QT prolongation resolved over 10 days and she was successfully weaned-off ECMO.
Conclusions:
Although azithromycin has a relatively safe profile, it is also associated with life-threatening cardiac arrhythmias that may require surgical intervention to stabilize the patient hemodynamically.
MeSH Keywords: Arrhythmias, Cardiac; Azithromycin; Ventricular Fibrillation
Background
Azithromycin is a macrolide antibiotic that works by inhibiting RNA-dependent protein synthesis by binding to the 50S ribosomal subunit, resulting in blockage of transpeptidation. It is commonly prescribed due to a number of advantages, including broad range of indications, spectrum of activity, favorable drug interaction profile, and convenience of dosing. However, azithromycin carries a black-box warning for rare QTc prolongation and ventricular arrhythmias, including torsades de pointes [1]. The warning was mainly based on a retrospective cohort study that demonstrated a 5-day course of azithromycin was associated with an increased risk of cardiovascular death (hazard ratio=2.88; 95% confidence interval [CI], 1.79 to 4.63) P<0.001. In addition, it was associated with increased risk of death from any cause (HR=1.85; 95% CI, 1.25 to 2.75) P=0.002 [2].
The mechanism by which macrolides prolong the QT interval is through a blockade of the rapid component, IKr, of the delayed rectifier potassium current IK, which is encoded by the human ether-a-go-go-related gene 1 (hERG1). The IKr is important in regulation of the outward flow of potassium ions and stimulating ventricular repolarization. Inhibition of hERG can lead to intracellular accumulation of potassium and ventricular repolarization and results in QT prolongation and TdP [3]. Moreover, azithromycin has unique pharmacological properties, with rapid and high serum and tissues concentrations and an average half-life between 2 and 4 days. In addition, a single 500 mg dose of azithromycin has been demonstrated to have excellent tissue concentration on day 4 [4]. The high and prolonged tissue concentrations may be attributed to the drug efficacy. However, as it remains in the system for several days, this might explain the persistent QT prolongation in some reported cases [4]. Published evidence varies with respect to the time to onset of azithromycin-induced QT prolongation and time to offset (Table 1) [5–10]. The prolongation of QT can begin as soon as a few hours, while it takes several days to resolve.
Table 1.
Reported onset and offset of QT prolongation induced by azithromycin.
| Case | Administration route | Time to QT prolongation onset | Time to offset |
|---|---|---|---|
| Samarendra et al. 2001 [5] | Oral | 3 days | Day 4 after discontinuation |
| Kezerashvili et al. 2007 [6] | Oral | 7 days | Same day of discontinuing |
| Huang et al. 2007 [7] | Not reported | 4 hours | One day |
| Del Rosario et al. 2010 [8] | Not reported | Unknown | Patient died on day 3 before QT interval normalizes |
| Yazdan-Ashoori et al. 2012 [9] | Intravenous | 2 days | Not reported |
| Winton and Twilla, 2013 [10] | Not reported | 3 days | Not reported but patient improved and left ICU after 12 days |
In this case report, we present possible azithromycin-induced cardiac arrhythmias that mandated extracorporeal membrane oxygenation (ECMO) insertion.
Case Report
We present the case of 37-year-old woman with a history significant for previous syncopal attacks in 2013, 2014, and 2016. She was seen in various private hospitals and was diagnosed with sinus tachycardia, with no other significant electrocardiogram (ECG) abnormalities. She was offered beta blocker for symptomatic relief as needed.
The patient presented to a secondary hospital’s Emergency Department multiple times complaining of sore throat, cough, nausea and vomiting. She was initially diagnosed with viral pharyngitis. The patient did not feel well and came back to the hospital the next day and was given azithromycin, with no clear documentation of the duration. However, according to the patient, she received 2 capsules of 250 mg (500 mg orally) for 1 day. The symptoms did not improve and she was admitted for follicular tonsillitis. On the same day of admission, she became unresponsive and had cardiac arrest, for which cardiopulmonary resuscitation (CPR) was carried for 26 min with intubation. The ECG rhythm was showing ventricular fibrillation and asystole during CPR cycles. She received 2 shocks and was started on intravenous amiodarone. On the next day, she was extubated and her vital signs were stable. One day later, she again developed ventricular fibrillation and was electrically cardioverted. In the ICU, she received clindamycin, ceftriaxone, and amiodarone infusion. She had 3 to 4 episodes of non-sustained ventricular tachycardia and sustained VT, managed with electrical shock.
The echocardiogram showed a dilated left ventricle with severe LV dysfunction with ejection fraction (EF) of less than 20%, severe mitral regurgitation, and moderate tricuspid regurgitation. She had another unstable ventricular tachycardia and underwent cardiac catheterization, which showed no significant coronary artery disease, and intra-aortic balloon pump was inserted. After a few minutes, she had cardiac arrest and required 4 shocks and was electively intubated. She was transferred to our tertiary care hospital for further management and possible left ventricular assist device (LVAD) insertion or heart transplantation.
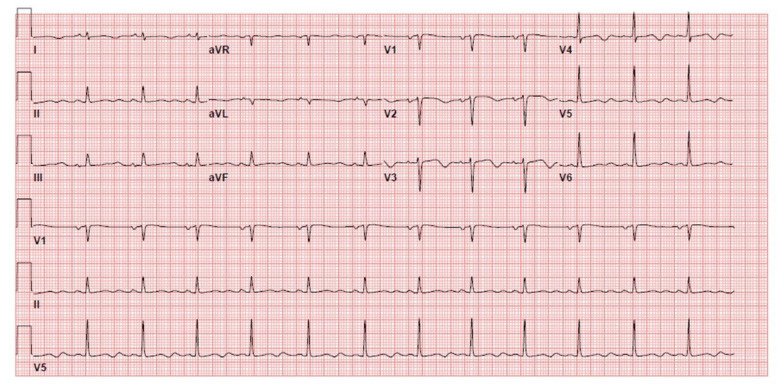
During transfer, she again had repeated episodes of ventricular fibrillation and torsades de pointes (TdP). She was shocked in the ambulance and reverted back to sinus rhythm. Her initial echocardiogram upon presentation showed reduced left ventricular systolic function with ejection fraction around 10%, so the decision was to insert ECMO as a bridge to recovery. A veno-arterial ECMO was inserted in the Cardiac Surgery Intensive Care Unit (CS-ICU) at bedside, and good flow was achieved. The serial ECGs from the referral hospital showed significant QT interval prolongation up to 600 msec at different times, as shown in Figure 1. She continued to have a prolonged QT interval up to day 5 of admission (Figure 2). The cardiac electrophysiology team was consulted and they diagnosed her as having possible prolong QT syndrome, most likely type 3. The ICU team deferential diagnosis was acute viral myocarditis. Tests for genetic disorders were negative.
Figure 1.
A 12-lead ECG showing the QT interval prolongation up to 600 msec on the second day of hospital admission.
Figure 2.

QTc interval values since admission to the tertiary care hospital (small size 11 compared to Figure1 legend 12).
During her stay in the CS-ICU, she required several antihypertensive medications and vasodilators to control blood pressure. Her medications are illustrated in Table 2. She was gradually improving, until extubation on the third day of admission to the CS-ICU. Later, after 12 days on ECMO, the left ventricular systolic function significantly improved, with EF of 50% to 55%. As a result, she was weaned off ECMO. After removal of ECMO, she recovered very well and was transferred to the general patient floor, where she was maintained on captopril 25 mg orally 3 times daily, metoprolol tartrate 50 mg orally twice daily, and amiodarone 200 mg orally daily. On day 17, she underwent subcutaneous implantable cardioverter defibrillator (ICD) insertion (model name: EMBLEM MRI S-ICD).
Table 2.
Medications received by the patient during CS-ICU stay*.
| Medications | Duration |
|---|---|
| Antiarrhythmic medications | |
| Amiodarone intravenous continuous infusion for a total of 10 g as loading dose | Day 1–Day 12 |
| Amiodarone 200 mg orally daily | Day 13–Day 17 |
| Anticoagulation therapy | |
| Heparin intravenous continuous infusion (hospital ECMO** protocol) | Day 1–Day 12 |
| Antihypertensive and vasodilator medications | |
| Nitroglycerin 200–150 mcg/min. intravenous continuous infusion | Day 1–Day 6 |
| Hydralazine 10–20 mg intravenous q 6h*** | Day 1–Day 13 |
| Nitroprusside 0.3–2 mcg/kg/min. intravenous continuous infusion | Day 2–Day 4 |
| Amlodipine 10 mg orally daily | Day 2–Day 13 |
| Metoprolol tartrate 12.5–50 mg orally twice daily | Day 3–Day 17 |
| Captopril 25 mg orally 3 times daily | Day 5–Day 14 |
| Antibiotics | |
| Vancomycin 1 g intravenous q 12h (adjusted according to patient’s renal function) | Day 2–Day 8 |
| Ceftazidime 1 g intravenous q12h (adjusted according to patient’s renal function) | Day 2–Day 8 |
CS-ICU=cardiac surgery intensive care unit,
ECMO=extracorporeal membrane oxygenation support,
hr=hour.
She was discharged on day 18 on the following medications: captopril 25 mg orally 3 times daily, metoprolol tartrate 50 mg orally twice daily, and amiodarone 200 mg orally daily.
Discussion
The cause of this storming and recurrent ventricular fibrillation and cardiac arrest in our patient was most likely multifactorial. The patient received azithromycin, which has an FDA warning for rare QTc prolongation and ventricular arrhythmias, including torsades de pointes, although other causes, including viral myocarditis, cannot be ruled out. The calculated Naranjo algorithm (ADR Probability Scale) score for this adverse drug reaction in this patient was 4, which indicates a possible causation (Table 3) [11]. Tests for genetic disorders for our patient were found to be significant for the CACNB2 gene and MYH6 gene variations. The patient was heterozygous in the CACNB2 gene for a sequence variant designated c.479G<C, which is predicated to result in the amino acid substitution p.Ser160Thr. This variant has been reported in a 15-year-old boy with sudden cardiac death during physical activity [12] and an individual with early repolarization syndrome [13]. Also, the patient was heterozygous in the MYH6 gene for a sequence variant designated c.35C<T, which is predicted to result in the amino acid substitution p.Ala12Val. This variant has been reported in an individual with hypertrophic cardiomyopathy [14]. At this time, the clinical significance of both variants is uncertain due to the absence of conclusive functional and genetic evidence.
Table 3.
Adverse drug reaction probability scale [11].
| Question | Yes | No | Do not know | Score |
|---|---|---|---|---|
| 1. Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | +1 |
| 2. Did the adverse event appear after the suspected drug was administered? | +2 | −1 | 0 | +2 |
| 3. Did the adverse event improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | +1 |
| 4. Did the adverse event reappear when the drug was readministered? | +2 | −1 | 0 | 0 |
| 5. Are there alternative causes that could on their own have caused the reaction? | −1 | +2 | 0 | −1 |
| 6. Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 |
| 7. Was the drug detected in blood or other fluids in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 0 |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | +1 |
| Total score: 4 |
Clinicians must be aware of certain risk factors that might pre-dispose patients to develop this condition, including pre-existing QT interval prolongation, electrolyte disturbances, and using other medications known to induce QT prolongation. In a narrative review published in 2013 that included 12 case reports, the traditional risk factors independent of azithromycin administration were female sex (n=6), older age (n=4), cardiovascular disease (n=6), acute medical condition (n=11), medications associated with QTc interval prolongation (n=6), hypokalemia (n=4), and bradycardia (n=3). All subjects had at least 2 risk factors in addition to receiving azithromycin [15].
In this case report, our patient was previously healthy with no significant past medical history, apart from syncopal attacks. To the best of our knowledge, this is one of the rare reported cases where azithromycin-induce QT interval prolongation progressed to TdP in a young and healthy individual and the patient’s life was saved through interventional ECMO placement.
Conclusions
Azithromycin is one of the old and effective antibiotics in both outpatient and inpatient setting. Despite its relatively safe drug profile, clinicians have to assess patients carefully prior to starting azithromycin to optimize patient safety.
References:
- 1.US Food and Drug Administration Azithromycin (Zithromax or Zmax): Drug safety communication - risk of potentially fatal heart rhythms (December 2013) http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm343350.htm?source=govdelivery.
- 2.Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–90. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein EJ, Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: Pointes of interest. Clin Infect Dis. 2006;43(12):1603–11. doi: 10.1086/508873. [DOI] [PubMed] [Google Scholar]
- 4.Lalak NJ, Morris DL. Azithromycin clinical pharmacokinetics. Clin Pharmacokinet. 1993;25(5):370–74. doi: 10.2165/00003088-199325050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Samarendra P, Kumari S, Evans SJ, et al. QT prolongation associated with azithromycin/amiodarone combination. Pacing Clin Electrophysiol. 2001;24(10):1572–74. doi: 10.1046/j.1460-9592.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- 6.Kezerashvili A, Khattak H, Barsky A, et al. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J Intervent Cardiac Electrophysiol. 2007;18(3):243–46. doi: 10.1007/s10840-007-9124-y. [DOI] [PubMed] [Google Scholar]
- 7.Huang BH, Wu CH, Hsia CP, et al. Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol. 2007;30(12):1579–82. doi: 10.1111/j.1540-8159.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 8.Del Rosario ME, Weachter R, Flaker GC. Drug-induced QT prolongation and sudden death. Mo Med. 2010;107(1):53–58. [PMC free article] [PubMed] [Google Scholar]
- 9.Yazdan-Ashoori P, Digby G, Baranchuk A. Failure to treat Torsades de pointes. Cardiol Res. 2012;3(1):34–36. doi: 10.4021/cr139w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winton JC, Twilla JD. Sudden cardiac arrest in a patient on chronic metha-done after the addition of azithromycin. Am J Med Sci. 2013;345(2):160–62. doi: 10.1097/MAJ.0b013e318266e7af. [DOI] [PubMed] [Google Scholar]
- 11.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen SL, Hertz CL, Ferrero-Miliani L, et al. Genetic investigation of 100 heart genes in sudden unexplained death victims in a forensic setting. Eur J Hum Genet. 2016;24(12):1797–802. doi: 10.1038/ejhg.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burashnikov E, Pfeiffer R, Barajas-Martinez H, et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7(12):1872–82. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, Fan C, Tian L, et al. The clinical features, outcomes and genetic characteristics of hypertrophic cardiomyopathy patients with severe right ventricular hypertrophy. PLoS One. 2017;12(3):e0174118. doi: 10.1371/journal.pone.0174118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancox JC, Hasnain M, Vieweg WV, et al. Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: A narrative review based on the study of case reports. Ther Adv Infect Dis. 2013;1(5):155–65. doi: 10.1177/2049936113501816. [DOI] [PMC free article] [PubMed] [Google Scholar]