Abstract
Warthin tumor is one of the most common benign salivary gland tumors. Overt lymphoma is known to occur in the lymphoid stroma of Warthin tumor. In situ follicular neoplasia is difficult to identify in routine histologic examination of lymphoid tissue and has not been reported in association with Warthin tumor. Our objective is to determine the prevalence of overt malignant lymphoma and in situ follicular neoplasia in Warthin tumor. We conducted a retrospective histological evaluation of 89 sequential Warthin tumor cases with available slides and blocks from the years 2010–2019. Of these, 84 cases were subjected to immunohistochemical testing, while 5 cases had been previously worked up for the suspicion of lymphoma. We identified two additional cases of lymphoid neoplasia associated with Warthin tumor including small lymphocytic lymphoma/chronic lymphocytic leukemia (n = 1) and in situ follicular neoplasia (n = 1) in addition to previously reported case of follicular lymphoma included in this study. The prevalence rate of first-time detected lymphoid neoplasia in Warthin tumor is 3.4%. The prevalence rate of overt lymphoma is 2.2%, while the prevalence of in situ follicular neoplasia is 1.1%. We propose histologic criteria to identify small lymphocytic lymphoma and follicular lymphoma in Warthin tumor. These include a monotonous interfollicular expansion of small lymphocytes and germinal centers composed of a monotonous population of lymphocytes without polarity or tingible body macrophages respectively. It is very important for pathologists to perform a diligent morphological examination and perform immunohistochemistry in suspected cases to identify subtle involvement of Warthin tumor by lymphoma. In patients with involvement of Warthin tumor by in situ follicular neoplasia, concurrent lymphoma in the same tissue and other sites should be considered. Patients without overt lymphoma elsewhere likely have a low risk of progression to follicular lymphoma. The low prevalence of in situ follicular neoplasia in Warthin tumor, combined with the low rate of clinical progression to lymphoma, make routine screening of Warthin tumor for in situ follicular neoplasia unnecessary.
Keywords: Parotid gland, Warthin tumor, Lymph node, Lymphoma, Chronic lymphocytic lymphoma/small lymphocytic lymphoma, In situ follicular neoplasia, Follicular lymphoma
Introduction
Warthin tumor, historically known as cyst-adenolymphoma of parotid, was initially subclassified based on the ratio of epithelial tumor component to lymphoid stroma, specific epithelial differentiation, and reaction pattern of the lymphoid stroma [1]. It is well recognized that carcinoma can arise in the epithelial component of Warthin tumor [2–6]. It has also been speculated that long term antigenic stimulation could be the potential pathogenesis behind the clonal proliferation of a lymphomatous cell population in Warthin tumor [7]. To date, thirty-four cases have been reported in literature in which lymphoma was detected for the first time in Warthin tumor. The majority were non-Hodgkin B-cell lymphoma with follicular lymphoma predominating [8]. In view of those published cases, first time lymphoma diagnosis in association with Warthin tumor may be more than a co-incidence. Of the types of lymphoma reported to occur in Warthin tumor, small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL) and follicular lymphoma (FL) may be most likely to go unnoticed by pathologists because of their mimicry of the interfollicular and follicular components of Warthin tumor respectively.
In situ follicular neoplasia (ISFN), initially described as “in situ localization of follicular lymphoma” is considered a precursor clonal process with potential to develop FL [9]. ISFN has been reported up to 2.3–3.2% of otherwise reactive lymph nodes [10, 11]. The clinical risk to progression to overt lymphoma is less than 5% [12, 13]. In a study of 34 patients with ISFN, 6 patients had prior or concurrent FL and 5 patients had composite lymphoma with ISFN [12]. Some patients with FL require watchful waiting or further treatment including radiotherapy or chemotherapy [14].
Hence, a thorough and diligent histologic evaluation of the lymphoid stroma of Warthin tumor is necessary to identify subtle involvement by lymphoma. This study reports the frequency of first-time detection of lymphoid neoplasia in Warthin tumors. The study also describes the morphologic features that should heighten concern for subtle lymphoma and a judicious immunohistochemical approach to confirm the diagnosis in this setting.
Materials and Methods
The study was approved by the institutional review board. Eighty-nine cases of Warthin tumor diagnosed at Methodist University Hospital, Memphis from January 2010through December 2019 with available blocks and slides were selected and evaluated by histological examination. Tumors with biphasic morphology on routine hematoxylin and eosin (H&E) stained slides were included in the study. Prior to this study, 5/89 cases (5.6%) were evaluated by immunohistochemistry (IHC) because of histological suspicion for lymphoma. Of these, one case of FL published previously [8] was included in this study. Cases with monotonous interfollicular small lymphocytes were subjected to extended IHC evaluation including CD20, CD3, CD5, CD43, CD10, BCL6, BCL2 and cyclin D1. To detect ISFN, BCL-2 immunohistochemical stains were performed on selected paraffin embedded tissue blocks of the remaining eighty-four cases. Further workup with CD10 and BCL6 were performed in cases with BCL-2 positive germinal centers (GC). For calculation of the confidence interval, the Clopper–Pearson method was used. Clinical histories and follow up were obtained from the hospital’s electronic medical record. Immunohistochemical studies using paraffin embedded tissue sections were performed on fully automated IHC Stainer, Leica BOND-III and Ventana Benchmark Ultra as described previously.
Results
The patients ranged from 24 to 95 years of age (mean age 61.6, ± 11.1). 66.3% were males (n = 59) and 33.7% females (n = 30) with mean age 60.6 ± 10.4 and 63.6 ± 12.2 respectively. Of the eighty-four cases of Warthin tumor that were subjected to IHC evaluation in this study, two of them showed involvement by lymphoid neoplasia. These two cases are described in detail below.
Case 1
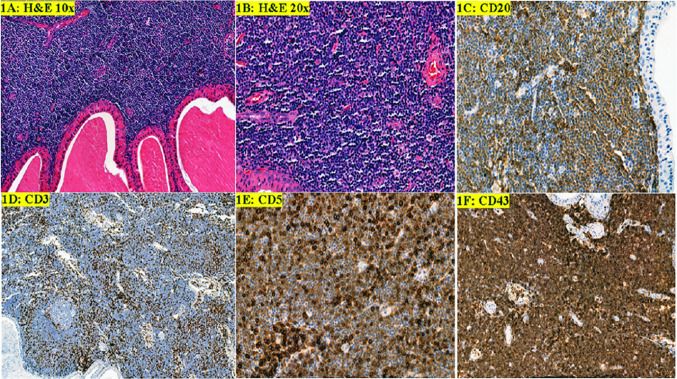
A 60-year-old male with history of smoking (one pack a day) presented with a gradually increasing right side neck mass for 18 months. On examination he had a mass inferior to right ear lobule. The patient denied any fever, night sweats, or weight loss (B symptoms). A complete blood count at the time of surgery showed a normal leukocyte count. Fine needle aspiration of the right neck mass performed at an outside facility showed Warthin Tumor. Right superficial parotidectomy was performed. The specimen was received in formalin and showed a 4.6 × 4 × 2.7 cm tan fleshy mass. Histopathologic evaluation showed cystic structures lined by oncocytic tall columnar cells with eosinophilic cytoplasm and a basal layer of cuboidal cells. The basal cells had round to oval nuclei and small conspicuous nucleoli. The cystic lumens contained eosinophilic secretions. These findings were diagnostic of Warthin tumor (Fig. 1a). The sub-epithelial lymphoid stroma showed expansion of monotonous population of small lymphocytes with scattered pale areas that appeared to represent proliferation centers. The lymphocytes had round nuclei with clumped chromatin. Mitotic activity was rare to absent (Fig. 1b). By immunohistochemistry, most of the small lymphocytes were B-cells with dim expression of CD20, aberrant expression of CD5 and CD43. The B-cells were negative for CD10 and cyclin D1. Scattered CD10 + , BCL6 + , BCL2- reactive GC were also seen. Few intermixed T-cells were highlighted by positive CD3 and strong CD5 staining. (Fig. 1c–f) These findings were diagnostic of SLL/CLL.
Fig. 1.
Warthin tumor and small lymphocytic lymphoma/chronic lymphocytic lymphoma. a, b Warthin tumor bi-layered epithelium and monotonous small lymphocytes with vague proliferation centers (H&E, 10 × and 20 ×). c Dim CD20 staining of neoplastic B-cells with some strong positive normal B-cells. d CD3 highlights scattered benign T-cells. e Aberrant weak staining of CD5 of neoplastic B-cells. f Aberrant CD43 expression of neoplastic B-cells
Four years later, patient was admitted due to septic shock secondary to Methicillin-resistant Staphylococcus aureus pneumonia. During that admission, he had persistent absolute lymphocytosis even after clinical improvement and normalization of the white blood cell count. Computed tomography (CT) of the abdomen and pelvis showed splenomegaly, however no lymphadenopathy was seen. Although imaging and laboratory studies were concerning for involvement by a lymphoproliferative disorder, no further laboratory studies or clinical follow-up was available.
Case 2
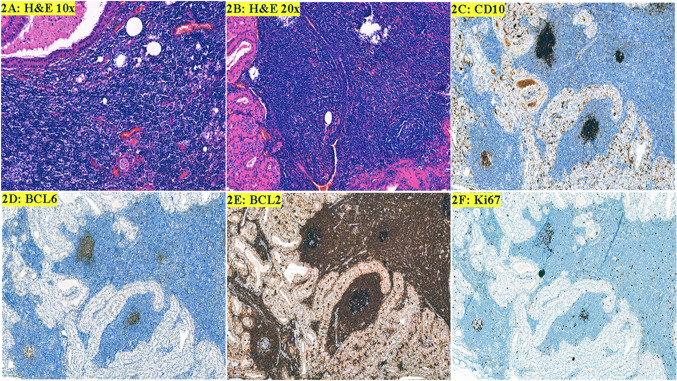
A 74-year-old male presented with an enlarging left parotid mass. CT scan of neck at outside facility showed bilateral parotid mass; right side being smaller than left. The patient denied any history of smoking, fever, night sweats, or weight loss (B symptoms). A complete blood count was within normal limits. Fine needle aspiration of left parotid mass done at outside facility revealed Warthin tumor. Left superficial parotidectomy was performed. The specimen was received in formalin and showed firm tan-yellow cut surface. Microscopic examination showed an intra-parotid lymph node with altered architecture. The majority of the lymph node was replaced by bi-layered oncocytic epithelium forming cystic spaces containing eosinophilic secretions (Fig. 2a). The background lymphoid stroma showed scattered follicles of uniform size. Most of the follicles were composed of centrocytes with variable numbers of large centroblasts and scattered tingible body macrophages. Upon retrospective close scrutiny, a few of the follicles were composed entirely of small cleaved lymphocytes (Fig. 2b). Immunohistochemistry showed bright CD10 expression in some of the GCs (Fig. 2c). A uniform BCL6 staining pattern was noted in the GCs (Fig. 2d). Inspection showed strongly BCL2 positive small B-lymphocytes in some GCs. The intensity of stain was brighter than adjacent mantle zones and T-cells. Some of the involved GCs contained only a few BCL2 positive B-cells, whereas others were more extensively replaced by BCL2 positive B-cells (Fig. 2e). Ki-67 showed loss of polarity in ISFN GCs (Fig. 2f). Although the morphology was not outwardly suspicious, the IHC findings support involvement of Warthin tumor by ISFN. The right parotid mass is sub-centimeter and asymptomatic. The patient is being observed by a primary care physician at an outside hospital facility for which clinical follow-up is not available.
Fig. 2.
Warthin tumor and In situ follicular neoplasia. a Warthin tumor bi-layered epithelium and lymphoid stroma (H&E, 10 ×). b The follicles are composed of small cleaved lymphocytes (H&E, 20 ×). c Intense CD10 staining in GCs involved by ISFN with weaker staining of a GC that contains more reactive B-cells on the bottom left. Neoplastic cells are confined to GCs. d BCL6 expression is uniform in the GCs. e Numerous BCL2 positive cells in the GCs with intense CD10 expression and partial involvment of the GC with weaker CD10 expression. f Ki67 shows loss of polarity in ISFN
Including the previously published case and the above described cases, we found the incidence of first-time detection of lymphoid neoplasia in Warthin tumor to be 3.4% (3/89 cases, confidence interval 0.007–0.095, confidence level 0.95). The prevalence rate of overt lymphoma is 2.2% (2/89 cases), while the prevalence of ISFN is 1.1% (1/89 cases).
Discussion
Warthin tumor is a benign salivary gland tumor found exclusively in parotid gland and intra parotid lymph nodes [15]. Warthin tumor has been reported in submandibular gland [43], however, the parotid gland tail is in proximity of the posterior submandibular space that may lead to an incorrect radiological impression that tumor is arising from the submandibular salivary gland rather than the parotid gland [44]. The parotid gland is the only salivary gland that contains lymph nodes. Hence, lymphoma in association with Warthin tumor is not unusual. Cases of Hodgkin lymphoma and non-Hodgkin lymphoma have been reported in association with Warthin tumor and FL is the most common type. Table 1 summarizes the frequency of various lymphoma reported in association with Warthin tumor including two cases from our current study [8]. The current study found that the prevalence of overt lymphoma in Warthin tumor is 2.2% (n = 2/89) based on a single institution database including SLL/CLL (n = 1) and FL (n = 1). The first case in this study showed a monotonous interfollicular expansion of small lymphocytes, which is an important morphological clue to consider further IHC evaluation to rule out lymphoma. In such cases we suggest an IHC panel including CD20, CD3, CD5, CD43, CD10, BCL6, BCL2 and cyclin D1. In case 1, the interfollicular B-cells co-expressed CD20, CD5, and CD43, without cyclin D1 expression supporting the diagnosis of SLL/CLL. Table 2 summarizes the clinical characteristics of patients with lymphoma reported in association with Warthin tumor.
Table 1.
Frequencies of malignant lymphoma associated with Warthin tumor
| Hodgkin lymphoma (n = 6, 16.7%) | Frequency (n) |
|---|---|
| CHL, mixed cellularity | 2 (17, 18) |
| CHL, lymphocyte rich | 1 (19) |
| CHL (Not distinguished) | 2 (20, 21) |
| NLPHL | 1 (22) |
| Non-Hodgkin lymphoma (n = 30, 83.3%) | |
| Follicular lymphoma | 14 (1, 7, 23–31) |
| In situ follicular neoplasia | 1 |
| DLBCL | 5 (26, 32–35) |
| SLL/CLL | 4 (36–38) |
| Mantle cell lymphoma | 2 (1, 39) |
| MALT-type lymphoma | 1 (40) |
| Peripheral T cell lymphoma | 1 (41) |
| T cell-lymphoblastic lymphoma | 1 (42) |
| Unclassified | 1 (24) |
CHL classical Hodgkin lymphoma; NLPHL nodular lymphocyte predominant Hodgkin lymphoma; DLBCL diffuse large B cell lymphoma; SLL/CLL small cell lymphoma/chronic lymphocytic leukemia
Table 2.
Characteristics of malignant lymphoma associated with Warthin tumor
| Study and Year | Age | Gender | Site | Lymphoma type | Stage |
|---|---|---|---|---|---|
| Hodgkin lymphoma | |||||
| Melato et al. (1986) [18] | 69 | M | Right parotid | CHL, mixed cellularity | NA |
| Badve et al. (1993) [21] | 76 | M | Left parotid mass for 9 months | CHL, not distinguished | II |
| Cozzolino et al. (2009) [20] | 60 | M | Right parotid | CHL, not distinguished | IA |
| Ye-qing Liu et al. (2013) [19] | 78 | M | Left parotid mass for years, increasing in size for 4 months | CHL, lymphocyte rich | IVB |
| Napoli et al. (2015) [22] | 73 | M | Left cervical mass for 2 months | NLPHL | IIA |
| Jun et al. (2018) [17] | 59 | M | Right neck mass | CHL, mixed cellularity | 1A |
| Non-Hodgkin lymphoma | |||||
| Colby et al. (1979) [24] | NA | NA | Parotid | Follicular lymphoma/grade 1 | NA |
| Seifert et al. (1980) [1] | 83 | M | Parotid | Follicular lymphoma | NA |
| Miller et al. (1982) [29] | 49 | M | Angle of mandible | Follicular lymphoma/grade 1 | IA |
| Banik et al. (1984) [23] | 75 | M | Left parotid | Follicular lymphoma/ grade 2 | NA |
| 76 | M | Right parotid | Follicular lymphoma/grade 2 | IIIA | |
| Hall et al. (1985) [27] | 64 | M | Right parotid | Follicular lymphoma/grade 2 | IA |
| Griesser et al. (1986) [26] | 64 | F | Palate | Follicular lymphoma/ grade 2 | NA |
| Medeiros et al. (1990) [28] | 71 | M | Left parotid | Follicular lymphoma/grade 2 | IIIA |
| Giardini et al. (1990) [25] | 57 | M | Right and left parotid | Follicular lymphomagrade 1 | IIA |
| Shikhani et al. (1993) [31] | 56 | M | Right Parotid | Follicular lymphoma | IA |
| Park et al. (2000) [7] | 68 | F | Right peri-parotid LN | Follicular lymphoma/grade 1 | IA |
| 55 | M | Right parotid | Follicular lymphoma/grade 1 | IIA | |
| Romero et al. (2016) [30] | 82 | F | Right Parotid | Follicular lymphoma/low grade | IV |
| Alnoor et al. (2018) [8] | 69 | M | Right neck swelling for 3 months, increasing for 2 weeks | Follicular lymphoma/grade 1–2 | IV |
| Reiner et al. (1979) [35] | 56 | M | Right parotid | DLBCL | NA |
| Griesser et al. (1986) [26] | 82 | M | Left submandibular swelling, rapidly growing for 2 weeks | DLBCL | NA |
| Gorai et al. (2007) [33] | 102 | M | left neck mass for 1 year | DLBCL | NA |
| Ozkok et al. (2012) [34] | 60 | M | Swelling in left side of Jaw for 2 months | DLBCL | IIIA |
| Chu et al. (2015) [32] | 83 | M | Left cheek mass for 10 years, rapidly enlarging for 6 months | EBV positive DLBCL | IIIA |
| Bunker et al. (1988) [36] | 63 | F | Left parotid mass for 4–5 years, recently enlarging | SLL/CLL | NA |
| Saxena et al. (2005) [38] | 60 | M | Left parotid mass for 4 years | SLL/CLL | IVA |
| Jawad et al. (2018) [37] | 80 | M | Right parotid mass for 2 years, recently ulcerated overlying skin | SLL/CLL | NA |
| Seifert et al. (1980) [1] | 71 | M | Parotid | Mantle cell lymphoma | NA |
| Arcega et al. (2015) [39] | 70 | M | left neck mass for 1 year | Mantle cell lymphoma | IVA |
| Marioni et al. (2004) [40] | 61 | F | 20 years history of right parotid swelling | MALT-type lymphoma | IA |
| Pescarmona et al. (2005) [41] | 66 | M | Right cervical lymphadenopathy | Nodal peripheral T-cell lymphoma, NOS | IV |
| Giaslakiotis et al. (2009) [42] | 81 | M | Enlarging right parotid mass for 3 weeks | T-lymphoblastic lymphoma | IVB |
| Colby et al. (1979) [24] | 52 | M | Parotid | Lymphoma, unclassified | NA |
Considering the evidence presented here and the cases reported in the literature, the possibility of involvement of Warthin tumor by occult small lymphocytic lymphoma or FL is not uncommon and is likely underrecognized by surgical pathologists. Morphologic features such as interfollicular expansion of small lymphocytes, or germinal centers composed of a monotonous population of lymphocytes without tingible body macrophages, should raise the suspicion for lymphoproliferative disorder and must be evaluated by IHC to rule out lymphoma.
We also report the first case of first-time detection of ISFN in association with Warthin tumor. The involved GC were small and only histologically recognizable as abnormal in retrospect. The GC were similar in size to reactive GC, had closely packed small centrocytes without tingible body macrophages. The ISFN germinal center B-cells had more intense CD10 expression than the reactive GC B-cells and had intense BCL2 expression as well. A prior study suggested the inclusion of BCL2, CD10, and cyclin D1 IHC markers in the diagnostic panel. This approach can reveal isolated scattered follicles in case of ISFN that are colonized by B-cells overexpressing BCL2 and CD10, or by B cells co-expressing CD5 and cyclin D1, in the case of in situ mantle cell neoplasia (ISMN) [16]. Retrospective analysis of lymph node resection done for non-lymphoid malignancies had reported 2.3–3.2% prevalence of ISFN [10, 11]. ISFN has a low rate of progression to FL in published literature [12, 13]. Such patients should undergo meticulous staging workup to exclude the possible existence of overt lymphoma at other sites. Carbone et al. have suggested a “wait and see” policy in patients without evidence of overt lymphoma [16].
In summary, the prevalence of overt lymphoma in Warthin tumor is 2.2%. This finding suggests that occult lymphomas like small lymphocytic lymphoma and follicular lymphoma are underrecognized by surgical pathologists. Diligent histologic evaluation of Warthin tumor is of utmost significance and a limited IHC panel should be considered to evaluate interfollicular expansions of small lymphocytes or monotonous germinal centers. The low prevalence of ISFN in Warthin tumor, combined with the low rate of clinical progression to lymphoma make routine screening of Warthin tumor for ISFN unnecessary.
Compliance with Ethical Standards
Conflict of interest
All authors have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland: analysis of 275 cases. Virchows Arch A Pathol Anat Histol. 1980;388(1):13–38. doi: 10.1007/BF00430674. [DOI] [PubMed] [Google Scholar]
- 2.Bengoechea O, Sanchez F, Larrinaga B, Martinez-Penuela JM. Oncocytic adenocarcinoma arising in Warthin's tumor. Pathol Res Pract. 1989;185(6):907–911. doi: 10.1016/s0344-0338(89)80296-1. [DOI] [PubMed] [Google Scholar]
- 3.Williamson JD, Simmons BH, el-Naggar A, Medeiros LJ. Mucoepidermoid carcinoma involving Warthin tumor: a report of five cases and review of the literature. Am J Clin Pathol. 2000;114(4):564–570. doi: 10.1309/GUT1-F58A-V4WV-0D8P. [DOI] [PubMed] [Google Scholar]
- 4.Yaranal PJ, Umashankar T. Squamous Cell Carcinoma Arising in Warthin's Tumour: A Case Report. J Clin Diagn Res. 2013;7(1):163–165. doi: 10.7860/JCDR/2012/4683.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolka W, Markowski J, Piotrowska-Seweryn A, Palen P, Dobrosz Z, Dziubdziela W. Mucoepidermoid carcinoma in Warthin tumor of the parotid gland. Arch Med Sci. 2015;11(3):691–695. doi: 10.5114/aoms.2015.52379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu C, Song Z, Xiao Z, Lin Q, Dong X. Mucoepidermoid carcinoma arising in Warthin's tumor of the parotid gland: Clinicopathological characteristics and immunophenotypes. Sci Rep. 2016;6:30149. doi: 10.1038/srep30149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CK, Manning JT, Jr, Battifora H, Medeiros LJ. Follicle center lymphoma and Warthin tumor involving the same anatomic site: Report of two cases and review of the literature. Am J Clin Pathol. 2000;113(1):113–119. doi: 10.1309/MJH0-RQGX-U128-VFC6. [DOI] [PubMed] [Google Scholar]
- 8.Alnoor F, Gandhi JS, Stein MK, Gradowski JF. Follicular lymphoma diagnosed in Warthin Tumor: a case report and review of the literature. Head Neck Pathol. 2019. [DOI] [PMC free article] [PubMed]
- 9.Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002;99(9):3376–3382. doi: 10.1182/blood.v99.9.3376. [DOI] [PubMed] [Google Scholar]
- 10.Henopp T, Quintanilla-Martinez L, Fend F, Adam P. Prevalence of follicular lymphoma in situ in consecutively analysed reactive lymph nodes. Histopathology. 2011;59(1):139–142. doi: 10.1111/j.1365-2559.2011.03897.x. [DOI] [PubMed] [Google Scholar]
- 11.Carbone A, Tibiletti MG, Canzonieri V, Rossi D, Perin T, Bernasconi B, et al. In situ follicular lymphoma associated with nonlymphoid malignancies. Leuk Lymphoma. 2012;53(4):603–608. doi: 10.3109/10428194.2011.624229. [DOI] [PubMed] [Google Scholar]
- 12.Jegalian AG, Eberle FC, Pack SD, Mirvis M, Raffeld M, Pittaluga S, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011;118(11):2976–2984. doi: 10.1182/blood-2011-05-355255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermudez G, de Villambrosia GS, Martinez-Lopez A, Batlle A, Revert-Arce JB, Cereceda CL, et al. Incidental and isolated follicular lymphoma in situ and mantle cell lymphoma in situ lack clinical significance. Am J Surg Pathol. 2016;40(7):943–949. doi: 10.1097/PAS.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 14.Bargetzi M, Baumann R, Cogliatti S, Dietrich PY, Duchosal M, Goede J, et al. Diagnosis and treatment of follicular lymphoma: an update. Swiss Med Wkly. 2018;148:w14635. doi: 10.4414/smw.2018.14635. [DOI] [PubMed] [Google Scholar]
- 15.Eveson JW, Cawson RA. Warthin's tumor (cystadenolymphoma) of salivary glands: a clinicopathologic investigation of 278 cases. Oral Surg Oral Med Oral Pathol. 1986;61(3):256–262. doi: 10.1016/0030-4220(86)90371-3. [DOI] [PubMed] [Google Scholar]
- 16.Carbone A, Santoro A. How I treat: diagnosing and managing "in situ" lymphoma. Blood. 2011;117(15):3954–3960. doi: 10.1182/blood-2010-10-299628. [DOI] [PubMed] [Google Scholar]
- 17.Jun L, Ming Z. Classical Hodgkin lymphoma arising from heterotopic Warthin's tumor in the cervical lymph node: a case report. Oncol Lett. 2018;16(1):619–622. doi: 10.3892/ol.2018.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melato M, Falconieri G, Fanin R, Baccarani M. Hodgkin's disease occurring in a Warthin's tumor: first case report. Pathol Res Pract. 1986;181(5):615–620. doi: 10.1016/S0344-0338(86)80158-3. [DOI] [PubMed] [Google Scholar]
- 19.Liu YQ, Tang QL, Wang LL, Liu QY, Fan S, Li HG. Concomitant lymphocyte-rich classical Hodgkin's lymphoma and Warthin's tumor. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):e117–e120. doi: 10.1016/j.oooo.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Cozzolino I, Zeppa P, Cuccuru A, Picardi M, Vetrani A, Palombini L. Collision Hodgkin lymphoma and Warthin tumour: report of a case and review of the literature. Oral Surg. 2009;2:188–192. [Google Scholar]
- 21.Badve S, Evans G, Mady S, Coppen M, Sloane J. A case of Warthin's tumour with coexistent Hodgkin's disease. Histopathology. 1993;22(3):280–281. doi: 10.1111/j.1365-2559.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 22.Di Napoli A, Mallel G, Bartolazzi A, Cavalieri E, Becelli R, Cippitelli C, et al. Nodular lymphocyte-predominant Hodgkin lymphoma in a Warthin tumor of the parotid gland: a case report and literature review. Int J Surg Pathol. 2015;23(5):419–423. doi: 10.1177/1066896915582263. [DOI] [PubMed] [Google Scholar]
- 23.Banik S, Howell JS, Wright DH. Non-Hodgkin's lymphoma arising in adenolymphoma–a report of two cases. J Pathol. 1985;146(3):167–177. doi: 10.1002/path.1711460303. [DOI] [PubMed] [Google Scholar]
- 24.Colby TV, Dorfman RF. Malignant lymphomas involving the salivary glands. Pathol Annu. 1979;14(Pt 2):307–324. [PubMed] [Google Scholar]
- 25.Giardini R, Mastore M. Follicular non Hodgkin's lymphoma in adenolymphoma: report of a case. Tumori. 1990;76(2):212–215. doi: 10.1177/030089169007600212. [DOI] [PubMed] [Google Scholar]
- 26.Griesser GH, Hansmann ML, Bogman MJ, Pielsticker K, Lennert K. Germinal center derived malignant lymphoma in cystadenolymphoma. Virchows Arch A Pathol Anat Histopathol. 1986;408(5):491–496. doi: 10.1007/BF00705302. [DOI] [PubMed] [Google Scholar]
- 27.Hall G, Tesluk H, Baron S. Lymphoma arising in an adenolymphoma. Hum Pathol. 1985;16(4):424–427. doi: 10.1016/s0046-8177(85)80238-0. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros LJ, Rizzi R, Lardelli P, Jaffe ES. Malignant lymphoma involving a Warthin's tumor: a case with immunophenotypic and gene rearrangement analysis. Hum Pathol. 1990;21(9):974–977. doi: 10.1016/0046-8177(90)90182-5. [DOI] [PubMed] [Google Scholar]
- 29.Miller R, Yanagihara ET, Dubrow AA, Lukes RJ. Malignant lymphoma in the Warthin's tumor. Rep Case. Cancer. 1982;50(12):2948–2950. doi: 10.1002/1097-0142(19821215)50:12<2948::aid-cncr2820501240>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 30.Romero M, Gonzalez-Fontal GR, Duarte M, Saavedra C, Henao-Martinez AF. Small clonal B-cell population in the bone marrow as a possible tool in the diagnosis of occult primary parotid lymphoma. Colomb Med. 2016;47(1):59–62. [PMC free article] [PubMed] [Google Scholar]
- 31.Shikhani AH, Shikhani LT, Kuhajda FP, Allam CK. Warthin's tumor-associated neoplasms: report of two cases and review of the literature. Ear Nose Throat J. 1993;72(4):264–269. [PubMed] [Google Scholar]
- 32.Chu CY, Pan SC, Chang KC. EBV-positive diffuse large B-cell lymphoma of the elderly involving Warthin tumor. Pathol Int. 2015;65(12):677–679. doi: 10.1111/pin.12325. [DOI] [PubMed] [Google Scholar]
- 33.Gorai S, Numata T, Kawada S, Nakano M, Tamaru J, Kobayashi T. Malignant lymphoma arising from heterotopic Warthin's tumor in the neck: case report and review of the literature. Tohoku J Exp Med. 2007;212(2):199–205. doi: 10.1620/tjem.212.199. [DOI] [PubMed] [Google Scholar]
- 34.Ozkok G, Tasli F, Ozsan N, Ozturk R, Postaci H. Diffuse large B-cell lymphoma arising in Warthin's tumor: case study and review of the literature. Korean J Pathol. 2013;47(6):579–582. doi: 10.4132/KoreanJPathol.2013.47.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiner M, Goldhirsch A, Luscieti PR, Pedrinis E, Kaplan E, Cavalli F. Warthin's tumor with Sjogren's syndrome and non-Hodgkin's lymphoma. Ear Nose Throat J. 1979;58(8):345–350. [PubMed] [Google Scholar]
- 36.Bunker ML, Locker J. Warthin's tumor with malignant lymphoma: DNA analysis of paraffin-embedded tissue. Am J Clin Pathol. 1989;91(3):341–344. doi: 10.1093/ajcp/91.3.341. [DOI] [PubMed] [Google Scholar]
- 37.Jawad H, McCarthy P, O'Leary G, Heffron CC. Presentation of Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma in a Warthin Tumor: Case Report and Literature Review. Int J Surg Pathol. 2018;26(3):256–260. doi: 10.1177/1066896917734371. [DOI] [PubMed] [Google Scholar]
- 38.Saxena A, Memauri B, Hasegawa W. Initial diagnosis of small lymphocytic lymphoma in parotidectomy for Warthin tumour, a rare collision tumour. J Clin Pathol. 2005;58(3):331–333. doi: 10.1136/jcp.2004.019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arcega RS, Feinstein AJ, Bhuta S, Blackwell KE, Rao NP, Pullarkat ST. An unusual initial presentation of mantle cell lymphoma arising from the lymphoid stroma of warthin tumor. Diagn Pathol. 2015;10:209. doi: 10.1186/s13000-015-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marioni G, Marchese-Ragona R, Marino F, Poletti A, Ottaviano G, de Filippis C, et al. MALT-type lymphoma and Warthin's tumour presenting in the same parotid gland. Acta Otolaryngol. 2004;124(3):318–323. doi: 10.1080/00016480310015263. [DOI] [PubMed] [Google Scholar]
- 41.Pescarmona E, Perez M, Faraggiana T, Granati L, Baroni CD. Nodal peripheral T-cell lymphoma associated with Warthin's tumour. Histopathology. 2005;47(2):221–222. doi: 10.1111/j.1365-2559.2005.02079.x. [DOI] [PubMed] [Google Scholar]
- 42.Gialakiotis K, Androulaki A, Panagoulias G, Kyrtsonis MC, Lazaris AC, Kanakis DN, et al. T cell lymphoblastic lymphoma in parotidectomy for Warthin's tumor: case report and review of the literature. Int J Hematol. 2009;89(3):359–364. doi: 10.1007/s12185-009-0271-z. [DOI] [PubMed] [Google Scholar]
- 43.Iwai T, Baba J, Murata S, Mitsudo K, Maegawa J, Nagahama K, et al. Warthin tumor arising from the minor salivary gland. Journal of Craniofacial Surgery. 2012;23(5):e374–e376. doi: 10.1097/SCS.0b013e318254359f. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton BE, Salzman KL, Wiggins RH, 3rd, Harnsberger HR. Earring lesions of the parotid tail. AJNR Am J Neuroradiol. 2003;24(9):1757–1764. [PMC free article] [PubMed] [Google Scholar]