Abstract
Well-differentiated (WDL) and dedifferentiated liposarcomas (DL) of the pharynx, larynx and oral cavity are rare, often mimicking benign lipomatous neoplasms or non-lipogenic mesenchymal tumors. Cases of WDL/DL arising in the upper aerodigestive tract, exclusive of the cervical esophagus, were reviewed. Morphologic features, ancillary studies, including fluorescence in situ hybridization (FISH) studies for CPM/MDM2, and clinical data was catalogued. Eight WDL/DL (4 WDL, 4 DL); were identified in patients ranging from 32 to 77 years (median 52.5 years; 6 males, 2 females) with sites of origin including hypopharynx (5 cases), larynx (2 cases) and oral cavity (1 case). Six of the 8 cases were received for expert consultation, and the remaining 2 cases were initially misdiagnosed as benign lymphangiomatous or fibroepithelial polyps. Morphologically, 4 tumors had areas mimicking various non-lipomatous soft tissue tumors including nodular fasciitis, mammary-type myofibroblastoma, low-grade myofibroblastic sarcoma and undifferentiated pleomorphic sarcoma, 2 cases simulated benign hypopharyngeal polyps, and 1 lesion was notable for a dense lymphoplasmacytic infiltrate suggestive of hematolymphoid neoplasm or IgG4-related sclerosing disease. FISH showed amplification of CPM/MDM2 (8/8 cases). All cases (4/4) with longer than 1-year of follow-up recurred (45–118 months) with 1 tumor showing progression to DL. WDL/DL presenting in the upper aerodigestive tract are rare and diagnostically challenging. Awareness of the morphologic spectrum of WDL/DL coupled with appropriate use of MDM2 FISH is essential for accurate classification and management, as these tumors appear to have a high risk for local recurrence and eventual dedifferentiation in these anatomical locations.
Keywords: Liposarcoma, Larynx, Hypopharynx, Upper aerodigestive tract, MDM2
Introduction
Atypical lipomatous tumor/well-differentiated liposarcoma (WDL) and dedifferentiated liposarcoma (DL), the most commonly encountered sarcomas in adulthood, usually arise in the extremities and retroperitoneum. When these tumors occur in the head and neck region, they typically involve the soft tissue of the neck rather than submucosal sites. Prompted by the recent recognition that the majority of giant fibrovascular polyps of the esophagus are WDL/DL [1], we wondered whether liposarcomas arising at other upper aerodigestive tract sites might also present similar diagnostic challenges, especially cases without a well-defined adipocytic component. Herein we detail our experience with a cohort of 8 WDL/DL arising in upper aerodigestive locations, highlighting unusual morphologic features to raise awareness of these rare neoplasms and aid in their appropriate classification and management.
Methods
The Institutional Review Board of the Mayo Clinic approved this study. The consultation and institutional anatomic pathology archives of our hospital were searched for cases of WDL and DL arising in the upper aerodigestive tract, exclusive of the cervical esophagus, from January 1, 1992 to November 30, 2019. Clinicopathologic information, including patient age and sex, tumor size, and anatomic location was collected. The available H&E slides were reviewed for morphologic patterns, as well as quantitation of the adipocytic component (< 10%, minimal; 10 to 25%, focal). Immunostains and fluorescence in situ hybridization studies (FISH) for MDM2 or CPM, a diagnostic surrogate for MDM2 [2], were catalogued. Treatment data and clinical follow-up information, when available, was acquired through the institutional electronic medical records or the submitting pathologist.
Results
A total of 8 cases of WDL/DL (4 WDL; 4 DL) were identified in 6 males and 2 females (32 to 77 years; median 52.5 years) with sites of origin including hypopharynx (5 cases), larynx (2 cases) and oral cavity (1 case) and sizes ranging from 2.8 to 6 cm (n = 4) (Table 1). Presenting symptoms included dysphagia, foreign sensation, choking and difficulty breathing.
Table 1.
Clinicopathologic features of cohort
| Case | Dx | Age/sex | Site | Initial diagnosis | Expert consultation requested | IHC performed (number) | Intralesional fat | Morphologic ddx | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | WDL | 32/F | Left lateral pharyngeal wall/pyriform sinus | Lymphangiomatous polyp | No | No | Minimal | Lymphangiomatous polyp | Recurrence (93 mo) |
| 2 | DL | 41/M | Supraglottis | DL | Yes | Yes (9) | Minimal | Undifferentiated pleomorphic sarcoma | NA |
| 3 | WDL+ | 42/M | Hyopharynx | Fibroepithelial polyp | No | No | Focal | Fibroepithelial polyp | Recurrence (118 mo) |
| 4 | WDL | 50/M | Buccal mass | WDL | Yes | Yes (6) | Minimal | Lymphoma, IgG4-related disease or fibroinflammatory proliferation | NA |
| 5 | DL | 55/M | Pyriform sinus | Low-grade myofibroblastic sarcoma | Yes | Yes (8) | Mimimal | Myofibroblastic sarcoma, perineurioma | Recurrence (45 mo) |
| 6 | WDL | 66/F | Epiglottis | WDL | Yes | Yes (5) | Minimal | Mammary-type myofibroblastoma | Recurrence (96 mo) |
| 7 | DL | 71/M | Pyriform sinus | DL | Yes | Yes (18) | Focal | Nodular fasciitis | NA |
| 8 | DL | 77/M | Hypopharynx | DL | Yes | Yes (16) | Minimal | Nodular fasciitis | NED (4 mo) |
Dx diagnosis, Ddx differential diagnosis, F female, M male, WDL well-differentiated liposarcoma, DL dedifferentiated liposarcoma, NA not available, NED no evidence of disease, mo months
+Recurrence harbored areas of dedifferentiation
The majority of cases in our cohort (n = 6) were consultation cases that were sent for second opinion either at the time of primary diagnosis or after recurrence. Diagnoses and differential diagnoses from the submitting pathologists included: “reactive,” “bland spindle cell proliferation,” “fibroblastic/myofibroblastic proliferation,” “smooth muscle lesion,” IgG4-related sclerosing disease, nodular fasciitis, inflammatory myofibroblastic tumor, low-grade myofibroblastic sarcoma, and “malignant spindle cell neoplasm.” The final 2 cases were originally diagnosed as benign lymphangiomatous and fibroepithelial polyps, respectively, and only correctly classified upon review of the recurrences when the patient presented for care at our institution.
Histologic examination of all cases showed a component of mature adipose tissue and varying numbers of atypical spindled or multinucleated cells with hyperchromatic and smudgy nuclei, as seen in well-differentiated liposarcoma (Fig. 1). However, the adipocytic component was always a minor component of the mass with areas of mature fat ranging from focal (n = 2) to minimal (n = 6). Furthermore, a wide spectrum of other morphologic patterns was appreciated.
Fig. 1.
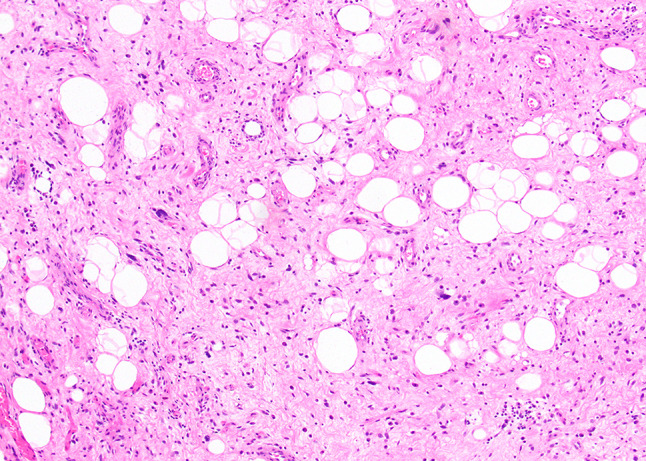
All cases showed at least a small adipocytic component composed of mature fat and atypical hyperchromatic stromal cells within areas of fibrosis
Histologic examination of 2 cases (Cases 1 and 3) revealed a polypoid submucosal proliferation of spindled cells with features reminiscent of esophageal lesions previously termed ‘giant fibrovascular polyps. ’ The spindled cell population was deposited within a minimally to focally fibrofatty background admixed with ectatic lymphatic and vascular spaces (Fig. 2). Only subtle cytologic atypia and subtle hyperchromasia were present in the lesional cells, and mitotic figures were difficult to identify.
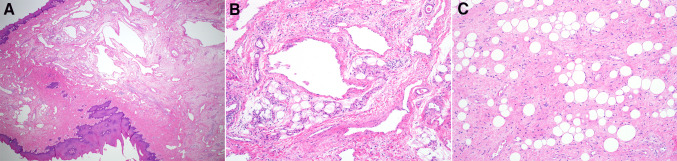
Fig. 2.
Case 1 showed an exophytic and polypoid mass composed of ectactic vascular spaces (a, b). The surrounding stroma contained spindle cells with only minimal atypia and areas of mature fat (c)
Case 2 presented as a supraglottic mass which was superficially biopsied at first. The initial biopsy showed a spindle cell proliferation with myxoid changes in the background. Spindled cells with hyperchromatic nuclei were also seen. The significance of the atypia was uncertain at this time and the differential diagnosis of a mesenchymal neoplasm versus reactive changes was considered. A second, deeper biopsy was then performed which showed spindled cells with marked nuclear pleomorphism and brisk mitotic activity. Amplification of the MDM2 gene was detected by FISH, which in conjunction with the morphologic findings, supported the diagnosis of dedifferentiated liposarcoma. In retrospect, the spindle cells seen in the earlier biopsy were also present in the deeper biopsy and considered to be part of the same tumor.
Case 4 harbored features suggestive of an inflammatory process or hematolymphoid neoplasm. In addition to a small focus of mature fat, microscopic examination revealed a densely hyalinized stroma with a prominent nodular lymphoplasmacytic infiltrate (Fig. 3). Admixed spindled cells with mild cytologic atypia and rare enlarged multinucleated cells with hyperchromatic nuclei could be appreciated with careful examination, and a focus of metaplastic bone was noted. A battery of hematolymphoid markers including CD3, CD20, IgG, IgG4 and kappa and lambda studies failed to show evidence of lymphoma, plasma cell neoplasm or increased numbers of IgG4-positive cells.
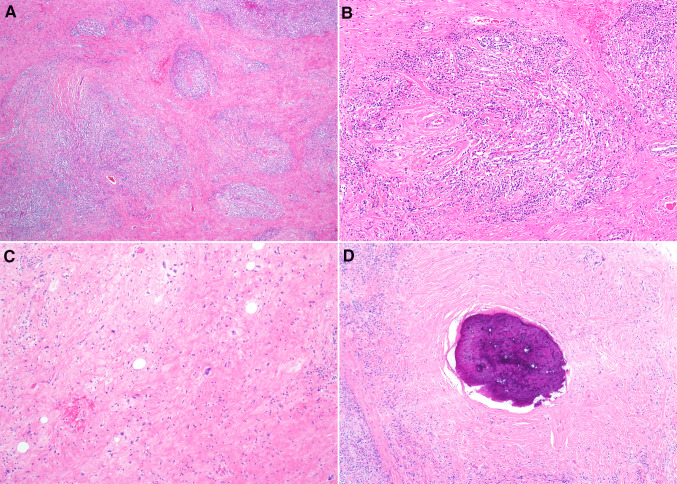
Fig. 3.
Histologic sections of Case 4 showed a prominent lymphoplasmacytic infiltrate (a). The inflammatory cells were deposited in a hyalinized background with thick bands of collagen (b). Rare atypical hyperchromatic stromal cells were noted (c), and a focus of metaplastic bone was also appreciated (d)
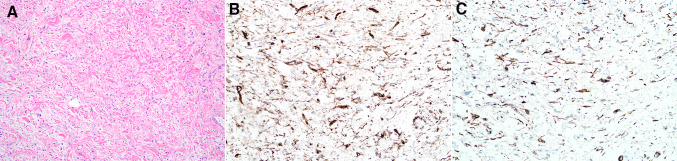
The dominant histologic feature in the remaining 4 cases (Cases 5–8) was a spindle cell proliferation with atypia varying from mild to severe, although the mitotic rate was generally low, ranging from 1 to 5 mitotic figures/10 high power fields. A single case harbored markedly pleomorphic epithelioid cells and multinucleated cells, while perineurial-like nodules (Case 5) and meningothelial-like whorls (Case 7) were noted in a single case each (Fig. 4). The spindle cell component in Cases 7 and 8 was focally arranged in a loose storiform pattern, raising the possibility of myofibroblastic lesions such as nodular fasciitis (Fig. 5a). Case 7 showed patchy staining for actin and STAT6 (Fig. 5b), and the spindle cells of Case 8 were focally immunoreactive for actin, calponin and desmin. Examination of the recurrence of Case 6 revealed small foci of spindle cells with minimal atypia admixed with variably sized bundles of ropy collagen reminiscent of mammary-type myofibroblastoma, and these spindle cells showed staining for CD34 and desmin (Fig. 6),
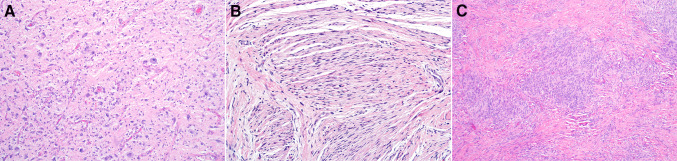
Fig. 4.
Other morphologic patterns included markedly atypical epithelioid and spindled cells (a), perineurial-like areas (b), and meningothelial-like whorls (c)
Fig. 5.
Case 6 had areas containing a loose arrangement of spindled cells resembling nodular fasciitis (a), and focal nuclear expression of STAT6 (b) was also noted in this case
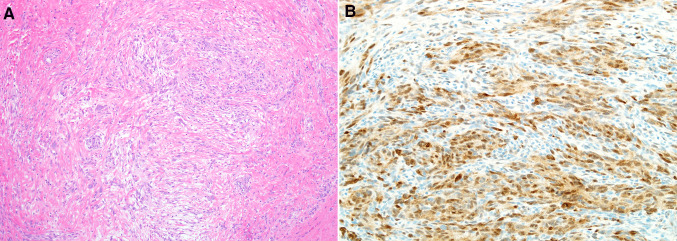
Fig. 6.
The recurrent tumor from Case 5 showed a paucicellular proliferation of relatively bland spindled cells admixed with dense ropey collagen bundles, suggestive of mammary-type myofibroblastoma (a). The tumor cells also demonstrated staining for CD34 (b) with focal desmin expression (c)
FISH studies performed either on the primary tumor or recurrence material was positive for CPM (1/1 case) or MDM2 (7/7 cases) amplification in all cases. Florescence in situ hybridization for USP6 performed on Case 8 was negative.
Follow-up was available on 5 patients (4 to 212 months, median 96 months), and all 4 tumors (3 WDL, 1 DL) with longer than 1-year of follow-up recurred (at 45, 93, 96 and 118 months). Progression to dedifferentiated liposarcoma was observed in a single tumor (Case 3) during recurrence at 118 months. No metastases have been reported to date.
Discussion
Atypical lipomatous tumor/well-differentiated liposarcoma is an adipocytic neoplasm composed of mature adipose tissue and irregular widened fibrous septa containing diagnostic atypical hyperchromatic stromal cells (and less commonly lipoblasts). Occasionally, the degree of cytologic atypia in these tumors may be mild and/or the characteristic atypical hyperchromatic stromal cells sparse. However, given the frequency of this tumor within deep somatic soft tissue or at deep central sites such as the retroperitoneum or mediastinum, pathologists have learned to recognize these pitfalls and utilize MDM2 studies when unequivocal cytologic atypia is absent. Dedifferentiated liposarcoma, on the other hand, is a biphasic malignancy with a predilection for the retroperitoneum, composed either of areas of well-differentiated liposarcoma juxtaposed to (usually) non-lipogenic spindle cell sarcoma, or consisting only of non-lipogenic spindle cell sarcoma in the recurrence of a previous WDL. In fact, the vast majority of pleomorphic sarcomas in the retroperitoneum are now felt to represent dedifferentiated liposarcomas [3]. Consequently, even when tumors lack an adipocytic component or harbor heterologous elements, pathologists are able to appropriately classify these sarcomas given their classic clinical presentation. Our group recently reported a series of WDL/DL of the esophagus and found that the majority was misclassified as benign polyps, and we suspected that these tumors may be similarly misdiagnosed at other upper aerodigestive tract sites given their morphologic diversity [1].
We identified a total of 8 cases of WDL/DL in our institutional and consultation archives in 6 males and 2 females ranging in age from 32 to 77 years with sites including the hypopharynx, lateral pharyngeal wall/pyriform sinus, supraglottis, epiglottis and buccal region. Six of the 8 cases were sent for expert consultation, and the remaining 2 cases were initially misdiagnosed as benign polyps. Furthermore, 5 of the 6 cases sent for expert consultation underwent an extensive immunohistochemical/molecular work-up before a diagnosis of WDL/DL was rendered. Cases of WDL/DL presenting in the oral cavity, larynx and pharynx are rare and most often harbor a well-differentiated lipomatous component [4–44]. Even though a subset of these tumors may be initially misdiagnosed as benign lipomas or fibrolipomas, pathologists often can recognize them as adipocytic neoplasms. Conversely, cases in our cohort had minimal amounts of mature fat, resulting in diagnostic difficulty. Based on the morphologic features cataloged in our series, the most common morphologic mimics included benign polyps, inflammatory processes and non-lipogenic mesenchymal spindle cell neoplasms.
Two cases in our series were originally felt to represent simply fibroepithelial or lymphangiomatous polyps and were only correctly diagnosed after the patients experienced recurrence. Morphologically, these tumors were composed of a variably cellular proliferation of spindled cells with minimal cytologic atypia deposited in a fibrofatty background. The majority of lymphangiomatous polyps are reported to occur in the tonsil. Microscopically, lymphangiomatous polyps are composed of a combination of lymphatic channels, fibrous and/or adipose stroma, and lymphoid tissue [45]. Fibroepithelial or lymphangiomatous polyps in adults in the larynx and hypopharynx may represent well-differentiated liposarcoma in some cases, and caution should be exercised before rendering these diagnoses. Careful morphologic examination with attention to cytologic atypia in the form of atypical hyperchromatic stromal cells, as well as MDM2 studies, will help to confirm or exclude a diagnosis of well-differentiated liposarcoma in this setting.
It is well recognized that retroperitoneal well-differentiated liposarcomas may contain a significant inflammatory component (so-called inflammatory variant) [46]. One case in our series consisted largely of densely hyalinized fibroconnective tissue containing a prominent lymphoplasmacytic infiltrate which largely obscured the scattered enlarged, hyperchromatic stromal cells of WDL, simulating some type of hematolymphoid process, such as lymphoma, plasma cell neoplasm, or IgG4-related sclerosing disease. As hematolymphoid neoplasms and IgG4-relating sclerosing disease outnumber WDL/DL in the head and neck, pathologists must maintain high degree of suspicion for the latter diagnosis in this anatomic location. Helpful morphologic clues to the diagnosis of inflammatory WDL in the upper aerodigestive tract include the presence of fat in unusual locations, as well as the presence of a spindled cell population with cytologic atypia, and these findings should prompt MDM2 studies.
Dedifferentiated liposarcomas are notorious for their morphologic complexity. Not only are these malignancies capable of harboring heterologous elements, the degree of atypia in the dedifferentiated areas may be minimal, potentially mimicking reactive processes or benign neoplasms. As these tumors may contain a population of low-grade spindle cells arranged in fascicles with some combination of actin and/or desmin staining, the morphologic findings overlap with numerous myofibroblastic lesions such as reactive proliferations, nodular fasciitis or low-grade myofibroblastic sarcoma. The presence of cytologic atypia would exclude the first two possibilities. Furthermore, cases of nodular fasciitis arising in the larynx and hypopharynx are rare, and confirmation with USP6 gene rearrangement should be pursued [47–49]. Low-grade myofibroblastic sarcoma, as well as undifferentiated pleomorphic sarcoma, may be impossible to differentiate from DL by histologic examination alone. Before making a diagnosis of these two entities, the pathologist should search diligently for areas of well-differentiated liposarcoma or correlate with imaging findings which may show areas of fat. When a well-differentiated lipomatous component is not identified, the presence of MDM2 expression by immunohistochemistry or MDM2 amplification will help support the diagnosis of DL. Finally, a myoepithelial neoplasm can be excluded by absence of biphasic appearance, ductal elements and/or keratin/S100-protein staining.
Interestingly, one case in our study showed expression of STAT6, a marker which has been shown to be a sensitive and relatively specific surrogate for the characteristic NAB2-STAT6 fusion that drives the pathogenesis of solitary fibrous tumors [50–53]. While the pleura is the most common anatomic site for this tumor, solitary fibrous tumors may arise in the head and neck region [54]. Furthermore, solitary fibrous tumors occasionally harbor areas of mature fat [55–58]. Doyle and colleagues found that STAT6 immunoreactivity may rarely be seen in dedifferentiated liposarcoma, likely reflecting the close proximity of the STAT6 gene to genes frequently amplified in WDL/DL (e.g., MDM2, CDK4) [53]. Pathologists should be aware of this pitfall when interpreting STAT6 staining in spindle cell lesions with lipomatous differentiation in the upper aerodigestive tract.
All patients (4 of 4) with significant follow-up experienced local recurrence, similar to the behavior of WDL/DL at other deep central sites, and a single case in our cohort also progressed to DL on recurrence. Although there is evidence that dedifferentiated liposarcomas have a better prognosis than other high grade pleomorphic sarcomas, these tumors still have high rates of recurrence if not completely excised [54, 59, 60]. These findings underscore the need for accurate classification of these tumors, complete tumor resection, and close follow-up of these patients.
In conclusion, well-differentiated and dedifferentiated liposarcomas arising in the upper aerodigestive tract are rare and diagnostically challenging, often resulting in expert consultation, extensive immunohistochemical/molecular workup and/or incorrect diagnosis. Additionally, the morphologic spectrum of DL is broad, further complicating appropriate classification. Consequently, pathologists should be aware that these tumors arise at sites such as the oral cavity, larynx and hypopharynx. Careful morphologic examination and appropriate use of MDM2 studies should aid in correct diagnosis and clinical management.
Author Contributions
TG helped with data acquisition and manuscript preparation. DS-W helped with manuscript preparation. MR helped with project design, data acquisition and manuscript preparation. AR helped with data acquisition and manuscript preparation. RG helped with manuscript preparation. AF helped with manuscript preparation. KF helped with project design, data acquisition and manuscript preparation. WDL/DL presenting in the upper aerodigestive tract are rare and diagnostically challenging. Awareness of the morphologic spectrum of WDL/DL coupled with appropriate use of MDM2 FISH is essential for accurate classification and management, as these tumors appear to have a high risk for local recurrence and eventual dedifferentiation in these anatomical locations.
Funding
No funding obtained.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The Institutional Review Board of the Mayo Clinic approved this study.
References
- 1.Graham RP, Yasir S, Fritchie KJ, Reid MD, Greipp PT, Folpe AL. Polypoid fibroadipose tumors of the esophagus: ‘giant fibrovascular polyp’ or liposarcoma? A clinicopathological and molecular cytogenetic study of 13 cases. Mod Pathol. 2018;31(2):337–42. doi: 10.1038/modpathol.2017.140. [DOI] [PubMed] [Google Scholar]
- 2.Erickson-Johnson MR, Seys AR, Roth CW, King AA, Hulshizer RL, Wang X, et al. Carboxypeptidase M: a biomarker for the discrimination of well-differentiated liposarcoma from lipoma. Mod Pathol. 2009;22(12):1541–7. doi: 10.1038/modpathol.2009.149. [DOI] [PubMed] [Google Scholar]
- 3.Coindre JM, Mariani O, Chibon F, Mairal A, De Saint Aubain Somerhausen N, Favre-Guillevin E, et al. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol. 2003;16(3):256–62. doi: 10.1097/01.MP.0000056983.78547.77. [DOI] [PubMed] [Google Scholar]
- 4.Tobey DN, Wheelis RF, Yarington CT., Jr. Electron microscopy in the diagnosis of liposarcoma and fibrosarcoma of the larynx. Ann Otol Rhinol Laryngol. 1979;88(Pt 1):867–71. doi: 10.1177/000348947908800623. [DOI] [PubMed] [Google Scholar]
- 5.Meis JM, Mackay B, Goepfert H. Liposarcoma of the larynx. Case report and literature review. Arch Otolaryngol Head Neck Surg. 1986;112(12):1289–92. doi: 10.1001/archotol.1986.03780120053010. [DOI] [PubMed] [Google Scholar]
- 6.Nofal F, Thomas M. Liposarcoma in the pharynx. J Laryngol Otol. 1989;103(11):1080–2. doi: 10.1017/S0022215100111053. [DOI] [PubMed] [Google Scholar]
- 7.Wenig BM, Weiss SW, Gnepp DR. Laryngeal and hypopharyngeal liposarcoma. A clinicopathologic study of 10 cases with a comparison to soft-tissue counterparts. Am J Surg Pathol. 1990;14(2):134–41. doi: 10.1097/00000478-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 8.McCulloch TM, Makielski KH, McNutt MA. Head and neck liposarcoma. A histopathologic reevaluation of reported cases. Arch Otolaryngol Head Neck Surg. 1992;118(10):1045–9. doi: 10.1001/archotol.1992.01880100035010. [DOI] [PubMed] [Google Scholar]
- 9.Esclamado RM, Disher MJ, Ditto JL, Rontal E, McClatchey KD. Laryngeal liposarcoma. Arch Otolaryngol Head Neck Surg. 1994;120(4):422–6. doi: 10.1001/archotol.1994.01880280050009. [DOI] [PubMed] [Google Scholar]
- 10.Brauchle RW, Farhood AI, Pereira KD. Well-differentiated liposarcoma of the epiglottis. J Laryngol Otol. 2001;115(7):593–5. doi: 10.1258/0022215011908342. [DOI] [PubMed] [Google Scholar]
- 11.McQueen C, Montgomery E, Dufour B, Olney MS, Illei PB. Giant hypopharyngeal atypical lipomatous tumor. Adv Anat Pathol. 2010;17(1):38–41. doi: 10.1097/PAP.0b013e3181bb6b35. [DOI] [PubMed] [Google Scholar]
- 12.Takano K, Kondoh A, Matsumiya H, Himi T. A well-differentiated liposarcoma of the hypopharynx. Otolaryngol Head Neck Surg. 2011;144(3):479–80. doi: 10.1177/0194599810391727. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Yang LH, Liu TT, Wang J, Li H, Yu G, et al. Liposarcoma of the larynx: report of a case and review of literature. Int J Clin Exp Pathol. 2015;8(1):1068–72. [PMC free article] [PubMed] [Google Scholar]
- 14.Kodiyan J, Rudman JR, Rosow DE, Thomas GR. Lipoma and liposarcoma of the larynx: case reports and literature review. Am J Otolaryngol. 2015;36(4):611–5. doi: 10.1016/j.amjoto.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Eyermann C, Raguin T, Hemar P, Debry C. Well-differentiated, pedunculated liposarcoma of the hypopharynx. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1):63–5. doi: 10.1016/j.anorl.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Sun J, Wei S, Wang D, Brandwein M. Well-differentiated laryngeal/hypopharyngeal liposarcoma in the MDM2 era report of three cases and literature review. Head Neck Pathol. 2017;11(2):146–51. doi: 10.1007/s12105-016-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krausen AS, Gall AM, Garza R, Spector GJ, Ansel DG. Liposarcoma of the larynx: a multicentric or a metastatic malignancy. Laryngoscope. 1977;87(7):1116–24. doi: 10.1288/00005537-197707000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Baden E, Newman R. Liposarcoma of the oropharyngeal region. Review of the literature and report of two cases. Oral Surg Oral Med Oral Pathol. 1977;44(6):889–902. doi: 10.1016/0030-4220(77)90033-0. [DOI] [PubMed] [Google Scholar]
- 19.Mandell DL, Brandwein MS, Woo P, Som PM, Biller HF, Urken ML. Upper aerodigestive tract liposarcoma: report on four cases and literature review. Laryngoscope. 1999;109(8):1245–52. doi: 10.1097/00005537-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Fanburg-Smith JC, Furlong MA, Childers EL. Liposarcoma of the oral and salivary gland region: a clinicopathologic study of 18 cases with emphasis on specific sites, morphologic subtypes, and clinical outcome. Mod Pathol. 2002;15(10):1020–31. doi: 10.1097/01.MP.0000027625.79334.F5. [DOI] [PubMed] [Google Scholar]
- 21.Powitzky R, Powitzky ES, Garcia R. Liposarcoma of the larynx. Ann Otol Rhinol Laryngol. 2007;116(6):418–24. doi: 10.1177/000348940711600605. [DOI] [PubMed] [Google Scholar]
- 22.Muddaiah A, Zaffarullah W, Tewary A. Recurrent well-differentiated liposarcoma of the larynx: a case report and review of literature. Eur Arch Otorhinolaryngol. 2010;267(7):1163–5. doi: 10.1007/s00405-010-1247-6. [DOI] [PubMed] [Google Scholar]
- 23.Pajaniappane A, Farzan J, Green DM, De M. Well-differentiated liposarcoma of the epiglottis. J Laryngol Otol. 2014;128(3):296–8. doi: 10.1017/S0022215114000085. [DOI] [PubMed] [Google Scholar]
- 24.Miller D, Goodman M, Weber A, Goldstein A. Primary liposarcoma of the larynx. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1975;80(5):444–7. [PubMed] [Google Scholar]
- 25.Hurtado JF, Lopez JJ, Aranda FI, Talavera J. Primary liposarcoma of the larynx. Case report and literature review. Ann Otol Rhinol Laryngol. 1994;103(4 Pt 1):315–8. doi: 10.1177/000348949410300409. [DOI] [PubMed] [Google Scholar]
- 26.Velek JP. Liposarcoma of the larynx. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1976;82(5):569–70. [PubMed] [Google Scholar]
- 27.Kapur TR. Recurrent lipomata of the larynx and the pharynx with late malignant change. J Laryngol Otol. 1968;82(8):761–8. doi: 10.1017/S0022215100069425. [DOI] [PubMed] [Google Scholar]
- 28.Allsbrook WC, Jr, Harmon JD, Chongchitnant N, Erwin S. Liposarcoma of the larynx. Arch Pathol Lab Med. 1985;109(3):294–6. [PubMed] [Google Scholar]
- 29.McCormick D, Mentzel T, Beham A, Fletcher CD. Dedifferentiated liposarcoma. Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18(12):1213–23. doi: 10.1097/00000478-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Makeieff M, Pelliccia P, Poizat F, Arnaud S, Rat F, Cupissol D, et al. Laryngeal dedifferentiated liposarcoma. Eur Arch Otorhinolaryngol. 2010;267(6):991–4. doi: 10.1007/s00405-010-1234-y. [DOI] [PubMed] [Google Scholar]
- 31.Riva G, Sensini M, Corvino A, Vittone F, Garzaro M, Pecorari G. Rare Giant Pedunculated Liposarcoma of the Hypopharynx: Case Report and Review of Literature. J Gastrointest Cancer. 2016;47(4):449–53. doi: 10.1007/s12029-015-9767-3. [DOI] [PubMed] [Google Scholar]
- 32.Farkas AB, House LK, Khan M, Saad AG, Parker E, Joyner D. Liposarcoma of the glottis: a report of an unusual diagnosis in an unusual location. Radiol Case Rep. 2018;13(3):631–4. doi: 10.1016/j.radcr.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allon I, Aballo S, Dayan D, Vered M. Lipomatous tumors of the oral mucosa: histomorphological, histochemical and immunohistochemical features. Acta Histochem. 2011;113(8):803–9. doi: 10.1016/j.acthis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Sotirovic J, Vukomanovic-Djurdjevic B, Baletic N, Pavicevic L, Bijelic D, Peric A. Recurrent lipomatous tumor of hypopharynx: case report and literature review. Acta Clin Croat. 2014;53(3):365–8. [PubMed] [Google Scholar]
- 35.Nascimento AF, McMenamin ME, Fletcher CD. Liposarcomas/atypical lipomatous tumors of the oral cavity: a clinicopathologic study of 23 cases. Ann Diagn Pathol. 2002;6(2):83–93. doi: 10.1053/adpa.2002.32375. [DOI] [PubMed] [Google Scholar]
- 36.Piperi E, Tosios KI, Nikitakis NG, Kyriakopoulos VF, Tzerbos F, Koutlas IG, et al. Well-differentiated liposarcoma/atypical lipomatous tumor of the oral cavity: report of three cases and review of the literature. Head Neck Pathol. 2012;6(3):354–63. doi: 10.1007/s12105-011-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J, Yu H, Wang L, Wang X, Shen G. Primary oral and maxillofacial liposarcoma: a clinicopathological and immunohistochemical study of eleven cases. Arch Med Sci. 2012;8(2):316–23. doi: 10.5114/aoms.2012.28560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YB, Leem DH, Baek JA, Ko SO. Atypical lipomatous tumor/well-differentiated liposarcoma of the gingiva: a case report and review of literature. J Oral Maxillofac Surg. 2014;72(2):431–9. doi: 10.1016/j.joms.2013.06.222. [DOI] [PubMed] [Google Scholar]
- 39.Nili F, Baghai F, Aghai A, Etebarian A. Well-differentiated liposarcoma of the floor of the mouth: report of a rare case and review of the literature. J Oral Maxillofac Pathol. 2016;20(2):312–5. doi: 10.4103/0973-029X.185984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikitakis NG, Lopes MA, Pazoki AE, Ord RA, Sauk JJ. MDM2+/CDK4+/p53+ oral liposarcoma: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(2):194–201. doi: 10.1067/moe.2001.116815. [DOI] [PubMed] [Google Scholar]
- 41.Laco J, Mentzel T, Hornychova H, Kohout A, Jirousek Z, Ryska A. Atypical lipomatous tumors of the tongue: report of six cases. Virchows Arch. 2009;455(4):383–8. doi: 10.1007/s00428-009-0835-6. [DOI] [PubMed] [Google Scholar]
- 42.Kacker A, Taskin M. Atypical intramuscular lipoma of the tongue. J Laryngol Otol. 1996;110(2):189–91. doi: 10.1017/s0022215100133146. [DOI] [PubMed] [Google Scholar]
- 43.Kamikaidou N, Kirita T, Mishima K, Sugimura M. Liposarcoma of the cheek: report of a case. J Oral Maxillofac Surg. 1998;56(5):662–5. doi: 10.1016/s0278-2391(98)90469-4. [DOI] [PubMed] [Google Scholar]
- 44.Nimura F, Nakasone T, Matsumoto H, Maruyama T, Matayoshi A, Maruyama N, et al. Dedifferentiated liposarcoma of the oral floor: a case study and literature review of 50 cases of head and neck neoplasm. Oncol Lett. 2018;15(5):7681–8. doi: 10.3892/ol.2018.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kardon DE, Wenig BM, Heffner DK, Thompson LD. Tonsillar lymphangiomatous polyps: a clinicopathologic series of 26 cases. Mod Pathol. 2000;13(10):1128–33. doi: 10.1038/modpathol.3880208. [DOI] [PubMed] [Google Scholar]
- 46.Kraus MD, Guillou L, Fletcher CD. Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma. Am J Surg Pathol. 1997;21(5):518–27. doi: 10.1097/00000478-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Svrakic M, Bent JP, 3rd, Adler E. Neonatal nodular fasciitis of the larynx. Int J Pediatr Otorhinolaryngol. 2009;73(7):1007–9. doi: 10.1016/j.ijporl.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa T, Sugimoto T, Komiyama S, Yamamoto T, Uemura T. Giant tumor formed by nodular fasciitis of the pharynx: a case report. Auris Nasus Larynx. 1994;21(3):196–9. doi: 10.1016/S0385-8146(12)80145-3. [DOI] [PubMed] [Google Scholar]
- 49.Xie S, Liu W, Xiang Y, Dai Y, Ren J. A huge nodular fasciitis in parapharygneal space in a 7-year-old girl: a case report and review of literature. Int J Clin Exp Pathol. 2014;7(12):9023–7. [PMC free article] [PubMed] [Google Scholar]
- 50.Koelsche C, Schweizer L, Renner M, Warth A, Jones DT, Sahm F, et al. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology. 2014;65(5):613–22. doi: 10.1111/his.12431. [DOI] [PubMed] [Google Scholar]
- 51.Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125(5):651–8. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 52.Demicco EG, Harms PW, Patel RM, Smith SC, Ingram D, Torres K, et al. Extensive survey of STAT6 expression in a large series of mesenchymal tumors. Am J Clin Pathol. 2015;143(5):672–82. doi: 10.1309/AJCPN25NJTOUNPNF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27(3):390–5. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]
- 54.Fletcher CDBJ, Hogendoorn PCW, Mertens F. World Health Organization classification of tumours. WHO classification of tumours of soft tissue and bone. Lyon: International Agency for Research on Cancer; 2013. pp. 33–18. [Google Scholar]
- 55.Chen Y, Wang F, Han A. Fat-forming solitary fibrous tumor of the kidney: a case report and literature review. Int J Clin Exp Pathol. 2015;8(7):8632–5. [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen GP, Dickersin GR, Provenzal JM, Rosenberg AE. Lipomatous hemangiopericytoma. A histologic, ultrastructural and immunohistochemical study of a unique variant of hemangiopericytoma. Am J Surg Pathol. 1995;19(7):748–56. doi: 10.1097/00000478-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Guillou L, Gebhard S, Coindre JM. Lipomatous hemangiopericytoma: a fat-containing variant of solitary fibrous tumor? Clinicopathologic, immunohistochemical, and ultrastructural analysis of a series in favor of a unifying concept. Hum Pathol. 2000;31(9):1108–15. doi: 10.1053/hupa.2000.9777. [DOI] [PubMed] [Google Scholar]
- 58.Folpe AL, Devaney K, Weiss SW. Lipomatous hemangiopericytoma: a rare variant of hemangiopericytoma that may be confused with liposarcoma. Am J Surg Pathol. 1999;23(10):1201–7. doi: 10.1097/00000478-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Lucas DR, Nascimento AG, Sanjay BK, Rock MG. Well-differentiated liposarcoma. The Mayo Clinic experience with 58 cases. Am J Clin Pathol. 1994;102(5):677–83. doi: 10.1093/ajcp/102.5.677. [DOI] [PubMed] [Google Scholar]
- 60.Weiss SW, Rao VK. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow-up study of 92 cases with analysis of the incidence of “dedifferentiation". Am J Surg Pathol. 1992;16(11):1051–8. doi: 10.1097/00000478-199211000-00003. [DOI] [PubMed] [Google Scholar]