Abstract
Background
Bone metastasis can induce multiple types of bone diseases which reduce the non-small-cell lung cancer (NSCLC) patient’s quality-of-life. Due to the difficulty of finding bone metastases and lack of effective early diagnosis, it is easy to miss the best treatment. Therefore, the study of serum tumor biomarkers is of great significance in the diagnosis of NSCLC bone metastasis.
Methods
The qRT-PCR assay was performed to assess has_circ_0060937 expression in 100 NSCLC patients. Furthermore, the small interfering RNAs si-has_circ_0060937 or si-NC were transfected into NSCLC bone metastasis cells. CCK8 assay was exercised to detect cell proliferation, and cell invasion assays were used to detect cell invasion in NSCLC bone metastasis cells.
Results
In this study, we firstly found that the expression of has_circ_0060937 in boneless metastasis NSCLC tissues and bone metastasis NSCLC tissues was significantly increased compared to normal tissues, and the expression of has_circ_0060937 was highest in bone metastasis. Expression of serum has_circ_0060937 in bone metastasis group from Grade I to Grade III NSCLC was drastically higher than boneless metastasis group or healthy group. In the Grade I to Grade III bone metastasis group, the expression of serum has_circ_0060937 gradually boosted with the increase of bone metastasis grades. Additionally, knockdown of has_circ_0060937 inhibited cell proliferation and cell invasion in NSCLC bone metastasis cell line.
Conclusion
The results suggestthat has_circ_0060937 is closely associated with bone metastasis in NSCLC, and the circRNAs we inspected may be a potential biomarker of bone metastasis in NSCLC.
Keywords: circRNA, has_circ_0060937, bone metastasis, NSCLC
Introduction
Non-small-cell lung cancer (NSCLC) is the predominant form of lung cancer and one of cancer in the world with the highest incidence and mortality.1–3 Distant metastasis, especially bone metastasis, is the main cause of death in patients. Moreover, bone metastasis can induce the multiple types of bone diseases to reduce the NSCLC patient’s quality-of-life.4,5 Due to the difficulty of finding bone metastases and lack of effective early diagnosis, it is easy to miss the best treatment. The diagnosis of serum tumor biomarkers plays a significant role in the clinical diagnosis and treatment of cancer.6,7 Therefore, the study of serum tumor biomarkers is of great significance in the diagnosis of NSCLC bone metastasis.
CircRNAs, endogenous non-coding RNAs (ncRNAs), have a covalently closed-loop structure. Various circRNAs have been found to be dysregulated in cancer, and they are tightly linked to tumorigenesis, tumor development, and tumor metastasis. For example, circRNA circ_0020710 drives tumor progression and immune evasion by regulating the miR-370-3p/CXCL12 axis in melanoma.8 CircRNA hsa_circRNA_101996 increases cervical cancer proliferation and invasion through activating TPX2 expression by restraining miR-8075.9 CircRNA hsa_circRNA_100290 serves as a ceRNA for miR-378a to regulate oral squamous cell carcinoma cell growth via Glucose transporter-1 (GLUT1) and glycolysis.10 In addition, circRNA has been found to play a vital role in tumor diagnosis. For example, circRNA hsa_circ_0067582 is reduced in human gastric cancer and exhibits its potential diagnostic values.11 Both hsa_circ_0067582 and hsa_circ_0005758 may be potential indicators for GC diagnosis.12
In this study, we identified a novel circRNA derived from the CYP24A1, termed has_circ_0060937, was remarkably increased in NSCLC tissues compared with normal tissues. Moreover, expression of has_circ_0060937 in serum was increased in NSCLC patient with bone metastasis compared with NSCLC patient with boneless metastasis or healthy people. Our results revealed that has_circ_0060937 may play a significant role in bone metastasis and they may function as potential indicators for diagnosis of NSCLC bone metastasis.
Patients and Methods
Patients and Samples
One hundred samples with NSCLC were randomly gathered from NSCLC patients who had not received preoperative chemotherapy. This study was approved by the Ethics Committee of the First People’s Hospital of Wenling, and was conducted in accordance with the Declaration of Helsinki. Each patient signed informed consent.
qRT-PCR
Serum of NSCLC patients were treated according to standard protocols as in a previously study.13 RNA in serum was extracted, cDNA was synthesized by using the SuperScript™ III First-Strand Synthesis System (Thermo Fisher Scientific, Inc., Waltham, MA). Gene expression was analyzed with iQ SYBR Green (BIO-RAD, USA) on the CFX96 system (BIO-RAD, USA). The sequences of the PCR primers were as follows: 5’-GTATGCTGCTGTCACAGAGCTCC-3’ and 5’-GCTCTTGTGCAGCTCGACTGG-3’ for has_circ_0060937; 5’-CTCGCTTCGGCAGCACA-3’ and 5’-AACGCTTCACGAATTTGCGT-3’ for U6 snRNA, as normalized control.
Bioinformatics Analysis
The microarray data of circRNA profiles in NSCLC samples and normal lung samples were obtained in NCBI GEO datasets (GSE112214). After applying log 2 transformation, GEO2R was exercised to analyze normalized microarray data.
Cell Culture
NSCLC bone metastasis cells were cultured in DMEM (Invitrogen, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified 5% CO2 incubator.
The small interfering RNAs si-has_circ_0060937 or si-NC were transfected into NSCLC bone metastasis cells. Bone metastasis cells were seeded in 6-well plates until confluent, then transfected with Lipofectamine 2000 (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. The siRNA against has_circ_0060937 (si-has_circ_0060937: 5’-GCCTCGTGTTGTATGAGAA-3’).
Cell Counting Kit-8 (CCK-8) Assay
Five thousand cells were seeded into 96-well plates. After 0, 24, 48, 72, or 96 hours, the cell proliferation was detected with CCK-8 solution according to the manufacturer’s instructions. Then, the absorbance was examined at 490 nm.
Cell Invasion Assays
105 cells were seeded into the upper chamber (8.0 μm; Costar) with a porous membrane with Matrigel solution (BD, Franklin Lake, NJ) in serum-free DMEM medium, while the lower chamber was filled with full medium. After 24 hours, the invasion on the lower membrane surface was assessed by staining with Hoechst 33342, and invasive cells were calculated.
Statistical Methods
Student’s t-test (two-tailed), one-way ANOVA, and variance were used to analyze data with GraphPad Prism 7 (GraphPad, La Jolla, CA, USA) and SPSS 26.0 software (IBM, Chicago, IL, USA). P<0.05 was considered as statistically significance.
Results
Has_circ_0060937 Were Increased in NSCLC by Using the Bioinformatics Analysis
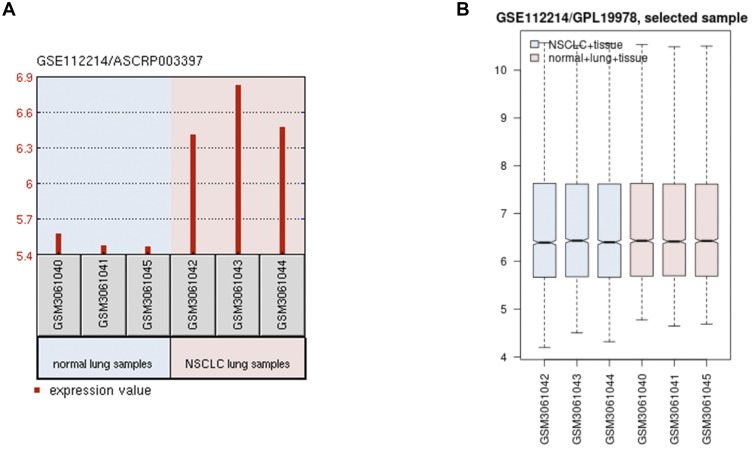
Initially, we found that has_circ_0060937 was upregulated on NSCLC by analyzing microarray data in the GEO dataset (GSE112214) (Figure 1A), and box plots pointed out normalized intensities from the cancerous and adjacent normal tissues (Figure 1B). Therefore, has_circ_0060937 was chosen for further study.
Figure 1.
Has_circ_0060937 were increased in NSCLC by using the bioinformatics analysis. (A) Has_circ_0060937 was upregulated on NSCLC by analyzing microarray data in the GEO dataset (GSE112214). (B) Box plots pointed out normalized intensities from the cancerous and adjacent normal tissues.
Has_circ_0060937 Were Highly Expressed in NSCLC and Bone Metastasis of NSCLC
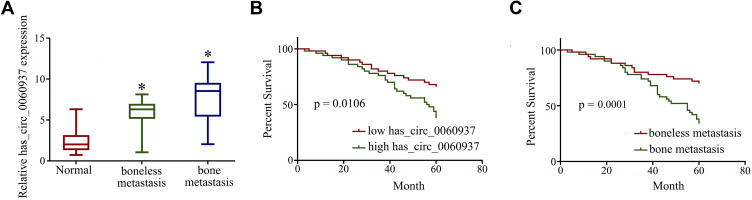
After qRT-PCR detection, we found that the expression of has_circ_0060937 in boneless metastasis NSCLC tissues and bone metastasis NSCLC tissues were significantly increased compared to normal tissues (Figure 2A). Notably, the expression of has_circ_0060937 in bone metastasis NSCLC tissues were higher than boneless metastasis NSCLC tissues, suggested overexpression of has_circ_0060937 was associated with bone metastasis. The relationship between the expression of has_circ_0060937 in boneless metastasis (n=50) and bone metastasis (n=50) from NSCLC and the clinicopathological factors were presented in Table 1. The data revealed the expression of has_circ_0060937 had no correlation with the age, gender, degree of differentiation, and lymph node metastasis, and there was a significant difference between has_circ_0060937 and Duke stage or the tumor size. In addition, Log-rank (Mantel-Cox) test showed overexpression of has_circ_0060937 NSCLC patients were associated with lower survival rate (Figure 2B) and survival rate was the lowest among NSCLC patients with bone metastases (Figure 2C). Thus, overexpression of has_circ_0060937 may play a role in tumorigenesis and bone metastasis.
Figure 2.
Has_circ_0060937 were highly expressed in NSCLC and bone metastasis of NSCLC. (A) The expression of has_circ_0060937 in boneless metastasis NSCLC tissues and bone metastasis NSCLC tissues. (B) Overexpression of has_circ_0060937 NSCLC patients were associated with lower survival rate. (C) Survival rate was the lowest among NSCLC patients with bone metastases. *P<0.05.
Table 1.
Relationship Between the Expression of Has_circ_0060937 and Clinicopathological Factors of 100 Cases with NSCLC
| Pathological Parameters | n | Has_circ_0060937 Expression | P-value | |
|---|---|---|---|---|
| Low | High | |||
| Age | 0.721 | |||
| >65 | 51 | 24 | 27 | |
| ≤65 | 49 | 21 | 28 | |
| Gender | 0.853 | |||
| Male | 53 | 26 | 27 | |
| Female | 47 | 21 | 26 | |
| Degree of differentiation | 0.812 | |||
| High | 35 | 17 | 18 | |
| Middle | 31 | 14 | 17 | |
| Low | 34 | 16 | 18 | |
| Lymph node metastasis | 0.523 | |||
| No | 42 | 19 | 23 | |
| Yes | 58 | 25 | 33 | |
| Duke stage | < 0.05 | |||
| A | 26 | 8 | 18 | |
| B | 21 | 5 | 16 | |
| C | 38 | 13 | 15 | |
| D | 15 | 5 | 10 | |
| Tumor size (cm) | < 0.05 | |||
| >2 | 54 | 16 | 38 | |
| ≤2 | 46 | 32 | 14 | |
| Bone metastasis | < 0.05 | |||
| No | 50 | 24 | 26 | |
| Yes | 50 | 16 | 34 | |
Correlation Between the Levels of Serum Has_circ_0060937 and Bone Metastasis in Different Grades of NSCLC
Among 100 NSCLC patients, there were 50 cases in the bone metastasis group, and 50 cases in the boneless metastasis group. As Table 2 shows, the data indicated there was no significant difference between expression of has_circ_0060937 in serum and age, gender, and primary focus of NSCLC. In addition, there was a significant difference between expression of has_circ_0060937 in serum and bone metastasis of NSCLC. Furtherly, expression of serum has_circ_0060937 in the bone metastasis group from Grade I to Grade III NSCLC were drastically higher than the boneless metastasis group or healthy group (Table 3, P<0.05). Notably, there was no significant difference in expression of serum has_circ_0060937 in boneless metastasis with the healthy group (P>0.05). Then, elevated levels of serum has_circ_0060937 predicted the new bone metastasis in patients with NSCLC. In the Grade I to Grade III bone metastasis group, the expression of serum has_circ_0060937 gradually boosted with the increase of bone metastasis grades (Table 4), suggesting has_circ_0060937 could serve as a diagnostic biomarker for NSCLC patients with bone metastasis.
Table 2.
Comparison of Clinical Data and the Expression of Serum Has_circ_0060937 of 100 Patients with NSCLC .
| Factor | n | Has_circ_0060937 | P |
|---|---|---|---|
| Age | 0.721 | ||
| >65 | 53 | 6.75±0.96 | |
| ≤65 | 47 | 6.03±1.53 | |
| Gender | 0.824 | ||
| Male | 51 | 6.19±1.43 | |
| Female | 49 | 6.34±1.56 | |
| Primary focus | 0.772 | ||
| >2 | 54 | 6.57±0.62 | |
| ≤2 | 46 | 6.23±1.02 | |
| Transfer Situation | <0.05 | ||
| Bone metastasis | 50 | 7.62±1.79* | |
| Boneless metastasis | 50 | 5.85±1.22 |
Note: *P<0.05.
Table 3.
Comparison of the Expression of Serum Has_circ_0060937 in Each Group
| Group | n | Has_circ_0060937 | P-value |
|---|---|---|---|
| Healthy | 50 | 5.57±1.27 | – |
| Boneless metastasis | 50 | 5.73±1.53 | 0.856 |
| Bone metastasis | |||
| Grader I | 27 | 6.21±1.02 | 0.005 |
| Grader II | 13 | 6.75±0.96 | 0.003 |
| Grander II | 10 | 7.21±1.18 | 0.001 |
Table 4.
ROC Curve Area (AUC) and Correlation of Serum Has_circ_0060937 in Diagnosis of Bone Metastasis in Bone Metastasis Group
| Bone Metastasis | n | AUC | Cut-off Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Grade I | 27 | 0.791 | 4.0283 | 66.7 | 88.9 |
| Grade II | 13 | 0.852 | 2.9452 | 92.3 | 69.2 |
| Grade III | 10 | 0.950 | 2.0507 | 100 | 80 |
Knockdown of Has_circ_0060937 Inhibited Proliferation and Invasion of NSCLC Bone Metastasis Cell Line
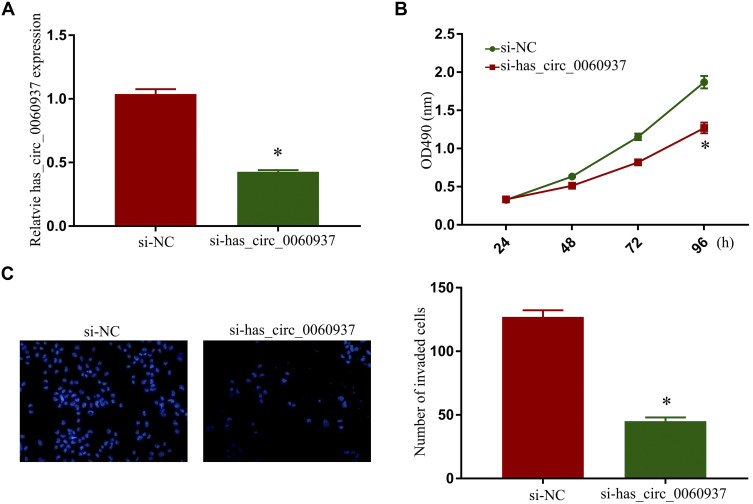
To further explore the correlation between has_circ_0060937 expression and bone metastasis in NSCLC, we isolated bone metastasis cells from NSCLC patients’ cancer tissues and successfully knocked down has_circ_0060937 expression (Figure 3A). In addition, knockdown of has_circ_0060937 inhibited cell proliferation (Figure 3B) and cell invasion (Figure 3C) in the NSCLC bone metastasis cell line.
Figure 3.
Knockdown of has_circ_0060937 inhibited proliferation and invasion of NSCLC bone metastasis cell line. (A) Knockdown of has_circ_0060937 in bone metastasis cell line were successfully constructed. Knockdown of has_circ_0060937 inhibited cell proliferation (B) and cell invasion (C) of the NSCLC bone metastasis cell line. *P<0.05.
Discussion
CircRNAs, nonpolyadenylated without 3′ terminal, are abundant in eukaryotes and involved in many diseases, including different cancers. Actually, many studies have found that many circRNAs are dysregulated in cancers. For example, circular RNA-9119 promotes the proliferation of cervical cancer cells by sponging miR-126/MDM4.14 CircNRIP1 promotes migration and invasion in cervical cancer by sponging miR-629-3p and regulating the PTP4A1/ERK1/2 pathway.15 Circular RNA SMARCA5 may serve as a tumor suppressor in non-small cell lung cancer.16 Some of the latest studies have shown that non-coding RNAs, especially circRNAs, play vital roles in the pathology and progression of NSCLC. For example, circRNA_101237 promotes NSCLC progression via the miRNA-490-3p/MAPK1 axis.17 CircRNA BIRC6 promotes NSCLC cell progression by sponging microRNA-145.18 Circ_0072088 promotes the development of NSCLC via the miR7/NOVA2 axis.19 Notably, a lot of studies revealed circRNA can function as an excellent biomarker. For instance, circRNA_104075 may serve as a diagnostic marker in hepatocellular carcinoma.20 Hsa_circRNA_102958 may serve as a diagnostic marker for gastric cancer.21 Hsa_Circ_0091579 may serve as a diagnostic and prognostic marker for hepatocellular carcinoma.22
In this study, we firstly found has_circ_0060937 was upregulated on NSCLC by analyzing microarray data in the GEO dataset. Furtherly, the expression of has_circ_0060937 in boneless metastasis NSCLC tissues and bone metastasis NSCLC tissues were significantly increased compared to normal tissues, and the expression of has_circ_0060937 was highest in bone metastasis. Additionally, the clinicopathological factors revealed the expression of has_circ_0060937 had no correlation with the age, gender, degree of differentiation and lymph node metastasis, and there was a significant difference between has_circ_0060937 and Duke stage or the tumor size. Overexpression of has_circ_0060937 NSCLC patients were associated with lower survival rate.
Due to the difficulty of finding bone metastases and lack of effective early diagnosis, it is easy to miss the best treatment. The diagnosis of serum tumor biomarkers plays a significant role in the clinical diagnosis and treatment of cancer. Therefore, the study of serum tumor biomarkers is of great significance in the diagnosis of NSCLC bone metastasis. In this study, it was found that expression of serum has_circ_0060937 in the bone metastasis group from Grade I to Grade III NSCLC were drastically higher than the boneless metastasis group or healthy group. In the Grade I to Grade III bone metastasis group, the expression of serum has_circ_0060937 gradually boosted with the increase of bone metastasis grades, suggested has_circ_0060937 could serve as a diagnostic biomarker for NSCLC patients with bone metastasis. To further confirm the results of clinical research, cell experiments were performed in this study, and the results indicated knockdown of has_circ_0060937 inhibited cell proliferation and cell invasion NSCLC bone metastasis cell line.
Conclusively, our results suggested that has_circ_0060937 are closely associated with bone metastasis in NSCLC, and the circRNAs we inspected may be a potential biomarker of bone metastasis in NSCLC.
Disclosure
The authors declare no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Cai Z, Liu Q. Understanding the global cancer statistics 2018: implications for cancer control. Sci China Life Sci. 2019. doi: 10.1007/s11427-019-9816-1 [DOI] [PubMed] [Google Scholar]
- 3.Mathur P, Sathishkumar K, Chaturvedi M, et al. Cancer statistics, 2020: report From National Cancer Registry Programme, India. JCO Glob Oncol. 2020;6:1063–1075. doi: 10.1200/GO.20.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang W, Haque W, Verma V, Butler B, Teh BS. Stereotactic radiosurgery for brain metastases from newly diagnosed small cell lung cancer: practice patterns and outcomes. Acta Oncol. 2019;58(4):491–498. doi: 10.1080/0284186X.2018.1562207 [DOI] [PubMed] [Google Scholar]
- 5.Imakita T, Matsumoto H, Hirano K, Morisawa T, Sakurai A, Kataoka Y. Impact on prognosis of rebiopsy in advanced non-small cell lung cancer patients after epidermal growth factor receptor-tyrosine kinase inhibitor treatment: a systematic review. BMC Cancer. 2019;19(1):105. doi: 10.1186/s12885-019-5309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatzakis KD, Froudarakis ME, Bouros D, Tzanakis N, Karkavitsas N, Siafakas NM. Prognostic value of serum tumor markers in patients with lung cancer. Respiration. 2002;69(1):25–29. doi: 10.1159/000049366 [DOI] [PubMed] [Google Scholar]
- 7.Daamen LA, Groot VP, Heerkens HD, Intven MPW, van Santvoort HC, Molenaar IQ. Systematic review on the role of serum tumor markers in the detection of recurrent pancreatic cancer. HPB (Oxford). 2018;20(4):297–304. doi: 10.1016/j.hpb.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 8.Wei CY, Zhu MX, Lu NH, et al. Circular RNA circ_0020710 drives tumor progression and immune evasion by regulating the miR-370-3p/CXCL12 axis in melanoma. Mol Cancer. 2020;19(1):84. doi: 10.1186/s12943-020-01191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song T, Xu A, Zhang Z, et al. CircRNA hsa_circRNA_101996 increases cervical cancer proliferation and invasion through activating TPX2 expression by restraining miR-8075. J Cell Physiol. 2019;234(8):14296–14305. doi: 10.1002/jcp.28128 [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Yu J, Tian H, et al. Circle RNA hsa_circRNA_100290 serves as a ceRNA for miR-378a to regulate oral squamous cell carcinoma cells growth via glucose transporter-1 (GLUT1) and glycolysis. J Cell Physiol. 2019;234(11):19130–19140. doi: 10.1002/jcp.28692 [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Ding H, Yang L, et al. Reduced expression of circRNA hsa_circ_0067582 in human gastric cancer and its potential diagnostic values. J Clin Lab Anal. 2020;34(3):e23080. doi: 10.1002/jcla.23080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu R, Shao Y, Tao X, Ye G, Xiao B, Guo J. Clinical significances of hsa_circ_0067582 and hsa_circ_0005758 in gastric cancer tissues. J Clin Lab Anal. 2019;33(9):e22984. doi: 10.1002/jcla.22984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye T, Changyu S, Limeng Z, Yuan P. Clinical significance of miRNA - 106a in non-small cell lung cancer patients who received cisplatin combined with gemcitabine chemotherapy. Cancer Biol Med. 2018;15(2):157–164. doi: 10.20892/j.issn.2095-3941.2017.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian Y, Xu Z, Fu J. CircularRNA-9119 promotes the proliferation of cervical cancer cells by sponging miR-126/MDM4. Mol Cell Biochem. 2020;470(1–2):53–62. doi: 10.1007/s11010-020-03745-3 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Ma N, Zhang Y, et al. Circular RNA circNRIP1 promotes migration and invasion in cervical cancer by sponging miR-629-3p and regulating the PTP4A1/ERK1/2 pathway. Cell Death Dis. 2020;11(5):399. doi: 10.1038/s41419-020-2607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong S. Circular RNA SMARCA5 may serve as a tumor suppressor in non-small cell lung cancer. J Clin Lab Anal. 2020;34(5):e23195. doi: 10.1002/jcla.23195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZY, Gao XH, Ma MY, Zhao CL, Zhang YL, Guo SS. CircRNA_101237 promotes NSCLC progression via the miRNA-490-3p/MAPK1 axis. Sci Rep. 2020;10(1):9024. doi: 10.1038/s41598-020-65920-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, Zhao M, Zhao L, Li P, Duan Y, Li G. CircRNA BIRC6 promotes non-small cell lung cancer cell progression by sponging microRNA-145. Cell Oncol (Dordr). 2020;43(3):477–488. doi: 10.1007/s13402-020-00503-x [DOI] [PubMed] [Google Scholar]
- 19.Tan Z, Cao F, Jia B, Xia L. Circ_0072088 promotes the development of non-small cell lung cancer via the miR-377-5p/NOVA2 axis. Thorac Cancer. 2020;11(8):2224–2236. doi: 10.1111/1759-7714.13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Xu Y, Qian Z, et al. circRNA_104075 stimulates YAP-dependent tumorigenesis through the regulation of HNF4a and may serve as a diagnostic marker in hepatocellular carcinoma. Cell Death Dis. 2018;9(11):1091. doi: 10.1038/s41419-018-1132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Wei W, Xu H, et al. Circular RNA hsa_circRNA_102958 may serve as a diagnostic marker for gastric cancer. Cancer Biomark. 2020;27(2):139–145. doi: 10.3233/CBM-182029 [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Zhang C, Lin J, Wang H. Circular RNA Hsa_Circ_0091579 serves as a diagnostic and prognostic marker for hepatocellular carcinoma. Cell Physiol Biochem. 2018;51(1):290–300. doi: 10.1159/000495230 [DOI] [PubMed] [Google Scholar]