Alternative pathway complement activation in ANCA‐associated vasculitis is a key feature in the pathogenesis. Our findings reinforce the finding that complement C5a and C3a are regulated during phases of disease activity. Meta‐analysis revealed significant changes in MAC, C5a, factor B, and borderline changes in C3a.

Keywords: ANCA, biomarker, complement, meta‐analysis, vasculitis
Summary
We compared the common pathway components C3a, C5a and membrane attack complex (MAC), also known as C5b‐9, and the alternative pathway components factor B and properdin in patients with ANCA‐associated vasculitis (AAV) and healthy controls, and conducted a meta‐analysis of the available clinical evidence for the role of complement activation in the pathogenesis of AAV. Complement components were evaluated in 59 patients with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis and 36 healthy volunteers. In 28 patients, testing was repeated in remission. Next, we performed a meta‐analysis by searching databases to identify studies comparing complement levels in AAV patients and controls. A random‐effects model was used for statistical analyses. The median concentrations of MAC, C5a, C3a and factor B were higher in active AAV patients (P < 0·001). Achievement of remission was associated with reductions in C3a (P = 0·005), C5a (P = 0·035) and factor B levels (P = 0·045), whereas MAC and properdin levels did not change. In active AAV, there were no effects of ANCA specificity, disease phenotype, previous immunosuppression or disease severity on complement levels. A total of 1122 articles were screened, and five studies, including this report, were entered into the meta‐analysis. Plasma MAC, C5a and factor B in patients with active AAV were increased compared to patients in remission (excluding factor B) and controls. Changes in C3a were of borderline significance. Our findings and the results of the meta‐analysis support activation of the complement system predominantly via the alternative pathway in AAV patients.
Introduction
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) belongs to a group of systemic vasculitides characterized by necrotizing inflammation of small‐ to medium‐sized blood vessels. Anti‐neutrophil cytoplasmic antibodies (ANCA) in the circulation are present in a majority of patients with generalized disease. ANCA target neutrophil cytoplasmic constituents, in particular proteinase 3 (PR3) and myeloperoxidase (MPO), cause activation of neutrophils that release inflammatory cytokines, reactive oxygen species and lytic enzymes and induce the excessive formation of neutrophil extracellular traps (NETs) [1]. These mechanisms underlie, at least in part, the pathogenicity of ANCA and their involvement in the development of inflammation and injury of endothelial vascular cells. Disease manifestations are usually characterized by a pauci‐immune phenomenon as the biopsy finding with little, if any, immunoglobulin and complement deposition in the vessel walls. In general, complement C3 and C4 levels measured in the blood are normal [2].
However, recent experimental and clinical evidence suggests that activation of the alternative complement pathway is crucial in the pathogenesis of AAV [3, 4]. In 2007, Xiao et al. published the first study that highlighted the role of the complement system in the development of AAV [5]. In an animal model of pauci‐immune crescentic glomerulonephritis induced by a single injection of anti‐MPO immunoglobulin (Ig)G, the complement activation pathways were studied using mice with knock‐out of the common pathway component C5, classic and lectin binding pathway component C4, and alternative pathway component factor B. C5‐ and factor B‐deficient mice were found to be resistant to the development of crescentic glomerulonephritis, whereas deficiency of C4 that is required for activation of C3 convertase and C5 convertase via both classic and lectin pathways did not prevent the disease. Conversely, Hilhorst et al. found C4d in the majority of renal biopsies, also suggesting classical pathway activation [6]. Incubation of MPO‐ANCA or PR3‐ANCA, unlike IgG from healthy controls, with human neutrophils was associated with the release of factors that activated complement [7, 8]. These findings suggested that ANCA can cause an amplification loop between neutrophils and complement; that is, ANCA‐induced activation of neutrophils leading to the generation of C5a which, in turn, enhances neutrophil recruitment and priming and amplifies the inflammatory response [3, 9].
The role of the complement system in the development of AAV was further substantiated by clinical studies that analyzed various complement components in patients during different states of disease activity and healthy controls [10, 11, 12, 13]. However, these studies were performed with a relatively small number of patients. Therefore, we aimed to compare the common pathway components C3a, C5a and membrane attack complex (MAC), also known as C5b‐9, and the alternate pathway components factor B and properdin in patients with AAV and healthy controls, and to conduct a meta‐analysis of the available clinical evidence for the role of complement activation in the pathogenesis of AAV.
Materials and methods
Original study
For inclusion into the study, we selected patients with newly diagnosed or relapsing AAV, who had a Birmingham Vasculitis Activity Score version 3 (BVAS version 3) of ≥ 3 that was calculated at the time of complement testing. Diagnosis of granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA) was established according to the American College of Rheumatology criteria [14], EMA algorithm [15] and the Chapel Hill Conference Consensus definition [16]. Healthy control subjects were recruited at Tareev Clinic of Internal Disease (Moscow) and Vladimir Regional Clinical Hospital. All subjects provided written informed consent approved by the local ethics committee of the Sechenov University. The study was conducted in compliance with the Declaration of Helsinki Principles.
Plasma concentrations of human complement components were determined by enzyme‐linked immunosorbent assay, including MAC (HK328‐02; Hycult Biotech, Uden, the Netherlands), C5a (HK349‐01; Hycult Biotech), C3a (HK354‐01; Hycult Biotech), factor B (EF7001‐1; Assaypro, St Charles, MO, USA) and properdin (factor P, SEA783Hu; Cloud‐Clone Corporation, Katy, TX, USA). All the complement components were assayed according to the manufacturer’s instructions. Blood samples were collected into tubes containing ethylenediamine tetraacetic acid (EDTA) and cooled immediately to 4°C. Plasma was separated within 20 min after blood collection (for 15 min at 4°C), transferred to fresh polypropylene tubes and stored at −70°C. Repeated freeze–thaw cycles were avoided. Upper reference limits (the 97·5th percentile) for each complement component were defined from the values in the control group after log‐transformation of the primary data.
Renal biopsies were evaluated according to a standardized protocol [17]. ANCA‐associated glomerulonephritis class was established according to the Berden classification [18]. The percentage of glomeruli with crescents and global sclerosis was calculated as the percentage of the total number of glomeruli in a biopsy. Interstitial fibrosis and tubular atrophy are given as the percentage of the tubulointerstitial compartment affected. Deposition of IgA, IgG, IgM, C3, kappa and lambda light chains was scored semiquantitatively by immunofluorescence staining. Remission of AAV was defined as BVAS version 3 equal to 0.
Meta‐analysis
Literature search and study selection
We performed a PubMed, EMBASE and SCOPUS search to identify eligible articles. A forward search of the retrieved articles was performed and Google Scholar was also assessed to screen for non‐indexed publications. The last search was performed on 12 December 2019. The search terms included: ‘ANCA vasculitis’ OR ‘ANCA‐associated vasculitis’ OR ‘antineutrophil cytoplasmic antibody vasculitis’ OR ‘AAV’ AND complement. Publications were screened first by title, secondly by abstract and finally by full text, based on our eligibility criteria.
Inclusion and exclusion criteria
We included cross‐sectional or longitudinal studies, which compared plasma levels of complement factors in patients with AAV and healthy controls. We excluded studies that measured complement levels in the urine, other body fluids or biopsy specimens. The exclusion criteria also included review articles, case reports and animal experiments.
Data extraction and outcome
Data extraction was carried out as recommended by the Cochrane Handbook and included authors, year of publication, study design, participants, demographic characteristics and measurement of serum complements.
Quality assessment
This meta‐analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement (Supporting information, Table S1). Risk of bias of individual studies at the outcome level was assessed by the Newcastle–Ottawa scale. The scoring was performed independently by two researches (L. J. M. and S. J. I.) (Supporting information, Table S2).
Statistical analysis and evaluation of heterogeneity and publication bias
In the meta‐analysis, the standard mean difference (s.m.d.) method and corresponding 95% confidence intervals (CIs) were used to compare complement levels [mean ± standard deviation (s.d.)]. Random‐effect models were used because of the heterogeneity of the included studies. We assessed the heterogeneity of the studies by the Cochran Q‐test, and a P‐value of < 0·1 was considered significant. The inconsistency across the studies was also measured by the I 2 metric as a measure of the percentage of total variation across the studies because of the heterogeneity. I 2 values of < 25, 25–75 and > 75% were considered to represent low, moderate and high levels of heterogeneity, respectively. Publication bias of each article was estimated by inspecting the funnel plot. All analyses were conducted using RevMan version 5.3.
Results
Original study
Demographic and clinical characteristics
Fifty‐nine patients with newly diagnosed or relapsing GPA (n = 41) or MPA (n = 18) were enrolled into this prospective study (Table 1). All patients presented with active vasculitis with a median BVAS of 16·5. Thirty‐five patients had newly diagnosed AAV. Twenty‐three patients were studied within 4–6 weeks after initiation of immunosuppressive therapy (up to two to three infusions of cyclophosphamide with high‐dose glucocorticoids) which was started before referral to our clinic, whereas 12 patients were not treated with any immunosuppressive agents at the time of testing. Twenty‐four patients had relapsing AAV and continued maintenance immunosuppressive therapy at the time of testing. Six ANCA‐negative patients had biopsy‐proven localized GPA.
Table 1.
Demography and clinical features of 59 patients with ANCA‐associated vasculitis
| Parameters | Values |
|---|---|
| Females, n (%) | 37 (62·7) |
| Average age (mean ± s.d.), years | 52·5 ± 14·3 |
| Diagnosis, n (%) | |
| GPA | 41 (69·5) |
| MPA | 18 (30·5) |
| Newly diagnosed AAV, n (%) | 35 (59·3) |
| Visceral disease, n (%) | |
| Pulmonary | 29 (49·1) |
| Renal | 37 (62·7) |
| ENT | 38 (64·4) |
| Eyes | 14 (23·7) |
| ANCA, n (%) | |
| PR3 | 36 (61·0) |
| MPO | 13 (22·0) |
| Unspecified | 4 (6·8) |
| Negative | 6 (10·2) |
| Previous immunosuppression, n (%) | 47 (79·7) |
| Glucocorticoids* | 47 |
| Immunosuppressive agents** | 12 |
| Laboratory parameters, medians (95% CI) | |
| Serum creatinine, μmol/l | 105·2 (95·7, 200·7) |
| ESR, mm/h | 55·0 (38·0, 64·0) |
| C‐reactive protein, mg/l | 10·2 (9·8, 36·7) |
| Median BVAS (95% CI) | 16·5 (13·6, 16·9) |
GPA = granulomatosis with polyangiitis; MPA = microscopic polyangiitis; PR3 = proteinase 3; MPO = myeloperoxidase; ESR = erythrocyte sedimentation rate; BVAS = Birmingham Vasculitis Activity Index; ANCA = anti‐neutrophil cytoplasmic antibody; AAV = ANCA‐associated vasculitis; s.d. = standard deviation; CI = confidence interval.
Median dose of prednisone was 12 (13; 22) mg.
Immunosuppressive medications included azathioprine (8), methotrexate (2) and mycophenolate mofetil (2).
In 28 patients, testing was repeated in remission within 3–38 months (median 16 months) after the initiation of immunosuppressive treatment with rituximab (n = 4), cyclophosphamide (n = 20), methotrexate (n = 3) or mycophenolate mofetil (n = 1). Thirty‐six healthy volunteers (nine males and 27 females, average age 55·7 ± 12·4 years) comprised the control group.
MAC, C5a, C3a and factors B and P (properdin) in patients with active AAV
In patients with active AAV, the median concentrations of MAC, C5a, C3a and factor B were higher than in the control group (P < 0·001 for all comparisons; Table 2). The median properdin level was lower in patients with AAV. However, the difference between the two groups did not reach statistical significance. Concentrations of MAC, C5a, C3a and factor B exceeded the upper reference levels in 50 (84·7%), 15 (25·4%), 49 (83·1%) and eight (13·6%) patients, respectively, whereas the properdin level was decreased in 13 (22·0%) patients.
Table 2.
Complement component levels in patients with serial measurements (active and remission), and during disease activity compared to healthy controls
| Active AAV versus controls | Active AAV versus remission | |||
|---|---|---|---|---|
| Active AAV (n = 59) | Control (n = 36) | Active AAV (n = 28) | Remission (n = 28) | |
| C5a, ng/ml | 22·9 (14·4; 33·0) | 3·0 (0·4; 6·7)*** | 21·6 (21·7; 46·0) | 18·8 (14·6; 25·7)* |
| C3a, ng/ml | 21436·0 (11395·0; 21436·0) | 1224·5 (798·5; 1947·7)*** | 21436·0 (17102·0; 20971·3) | 11811·0 (11003·0; 16292·6)* |
| MAC, mAU/ml | 24646·0 (15342·0; 46681·0) | 3305·5 (2780·2; 3777·5)*** | 29286·0 (23448·3; 43048·5) | 20057·4 (19709·3; 41477·7) |
| Factor B, µg/ml | 1586·0 (1175·0; 2145·0) | 1013·5 (770·7; 1548·5)*** | 1804·0 (1514·6; 2438·0) | 1480·0 (1124·3; 1658·2)* |
| Properdin, µg/ml | 402·0 (360·0; 447·0) | 416·0 (400·2; 437·0) | 388·0 (372·0; 417·2) | 402·0 (381·9; 423·5) |
P < 0.05,
P < 0.001. Changes in complement component levels were studied in 28 patients who have undergone repeated testing following remission induction therapy. Using data from the control group, the upper reference levels of the complement components were defined as following: MAC = 11271·1 mAU/ml; C5a = 33·1 ng/ml (three outliers were excluded); C3a = 8955·3 ng/ml; factor B = 2864·1 µg/ml. The lower reference level of properdin was defined as a cut‐off of 357·8 µg/ml.
AAV = anti‐neutrophil cytoplasmic antibody‐associated vasculitis; MAC = membrane attack complex.
In 28 patients with AAV, we measured complement components levels both before and following remission induction. Achievement of remission was associated with a significant reduction in C3a from 21 436·0 to 11 811·0 ng/ml (P = 0·005), a decrease in C5a from 21·6 to 18·8 ng/ml (P = 0·035) and factor B levels from 1804·0 to 1480·0 µg/ml (P = 0·045). MAC and properdin levels did not change following remission induction therapy (Table 2).
Normalization of elevated complement component levels was achieved in five of eight patients (62·5%) for C5a, 11 of 25 patients (44·0%) for C3a, six of 24 patients (25·0%) for MAC and all six patients (100%) for factor B. Properdin levels in all 28 patients were normal both before and following remission induction treatment. Of note, in a few patients, there was an increase in complement component levels following immunosuppressive treatment, including C3a in two patients, MAC in three patients, and factor B in one patient.
Complement levels according to ANCA serotype, disease phenotype, new diagnosis or relapsing disease or treatment‐naive versus treated patients
There was no difference in MAC, C5a, C3a, factor B and properdin levels between PR3‐ANCA positive and MPO‐ANCA‐positive patients, patients with GPA and MPA, patients with newly diagnosed AAV or relapsing disease and treatment‐naive patients and those already receiving immunosuppression (Supporting information, Tables S3–S6).
We also evaluated the activation of the complement system in patients with ‘non‐severe’, ‘severe PR3‐ANCA‐positive’ and ‘severe MPO‐ANCA‐positive’ AAV, representing the predominantly granulomatous, mixed granulomatous–vasculitic and predominantly vasculitic patterns of AAV [19]. Non‐severe AAV (usually PR3‐ANCA‐positive or sometimes ANCA‐negative) was defined as granulomatosis (ENT‐disease, lung nodules/masses, retro‐orbital tumour and/or pachymeningitis) without life/organ‐threatening disease, whereas ‘severe’ vasculitis‐related manifestations included glomerulonephritis, alveolar haemorrhage, mononeuritis multiplex and scleritis. Median levels of all studied complement components were similar in these groups of patients (Supporting information, Table S7).
Furthermore, patients were distributed into two groups depending on the severity of disease, which was defined as the presence of at least one major BVAS item and a total BVAS > 6. Median BVAS scores in patients with severe and non‐severe AAV were 15 (13;16) and eight (6;9), respectively. Complement component levels did not differ between patients with severe and non‐severe vasculitis (Supporting information, Table S8).
Correlation between levels of various complement components and clinical and pathological parameters in patients with active AAV
In 59 patients with active AAV, C5a levels had significant positive correlations with C3a (r = 0·371, P = 0·004), MAC (r = 0·355, P = 0·006) and factor B (r = 0·281, P = 0·031). However, there was no correlation between various complement components and BVAS, daily proteinuria, serum creatinine, C‐reactive protein or erythrocyte sedimentation rate (ESR) (Supporting information, Table S9).
In 13 patients with kidney biopsies, factor B level correlated with C3 deposition in the glomeruli (r = 0·648, P = 0·023), whereas all other correlations between complement components and histological parameters did not reach statistical significance (Supporting information, Table S10).
Meta‐analysis
Study selection and characteristics
A total of 1122 articles were identified using electronic and manual research (Supporting information, Fig. S1). After reviewing titles and abstracts, 19 studies were selected for full‐text reading. Of these, 14 were excluded (one study was not available for the raw data) to finally include four eligible articles by 12 December 2019 [11, 13, 20]. Among these, Gou et al. measured the levels of complement pathway factors twice at different time‐points, to which we refer as ‘Gou, sequential’. In addition to this, we added the data from our current study. The respective characteristics of the included studies are described in detail in Supporting information, Table S11. The study quality assessed by the Newcastle–Ottawa scale scored 8 in three studies and 6 in one study [range = 1 (very poor) to 9 (very high), Supporting information, Table S2].
Meta‐analysis of complement levels
The meta‐analysis on MAC levels examined 178 patients with active AAV, 148 patients with AAV in remission and 100 healthy controls. Plasma MAC levels were significantly higher in the active AAV group compared to patients in remission (s.m.d. = 1·58, 95% CI = 0·22–2·93) and healthy controls (s.m.d. = 1·50, 95% CI = 0·84–2·17) (Fig. 1).
Fig. 1.

Forest plot of random‐effects meta‐analysis of membrane attack complex (MAC levels). 1.1.1 = Anti‐neutrophil cytoplasmic antibody‐associated vasculitis (AAV) patients in active state versus in remission; 1.1.2 = active AAV patients versus healthy controls; 1.1.3 = AAV patients in remission versus healthy controls. Squares are proportional to study weight.
C5a levels were measured in 220 patients with active AAV, 192 patients with AAV in remission and 124 healthy controls. Plasma C5a levels were significantly higher in the active AAV group compared to patients in remission (s.m.d. = 0·80, 95% CI = 0·33–1·27) and healthy controls (s.m.d. = 1·18, 95% CI = 0·56–1·80) (Fig. 2). The remitted group showed higher plasma C5a levels when compared to controls (s.m.d. = 1.19, 95% CI = 0.27–2.12).
Fig. 2.
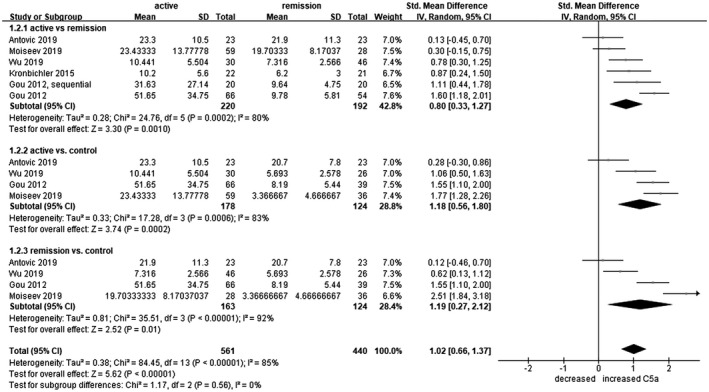
Forest plot of random‐effects meta‐analysis of C5a levels. 1.2.1. Anti‐neutrophil cytoplasmic antibody‐associated vasculitis (AAV) patients in active state versus in remission; 1.2.2 = active AAV patients versus healthy controls; 1.2.3 = AAV patients in remission versus healthy controls. Squares are proportional to study weight.
The meta‐analysis on C3a levels examined 223 patients with active AAV, 192 patients with AAV in remission and 124 healthy controls. Plasma C3a levels had a marginal tendency to be higher in the active AAV group compared to patients in remission (s.m.d. = 1·35, 95% CI = 0·20–2·51) and healthy controls (s.m.d. = 1·87, 95% CI = 0·08–3·66), and also in the remitted AAV groups compared to healthy controls (s.m.d. = 0·73, 95% CI = 0·04–1·41). Significance of C3a regulation was, in general, borderline (Fig. 3).
Fig. 3.
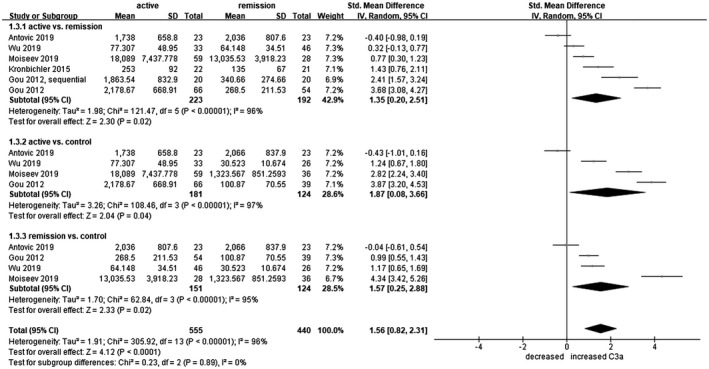
Forest plot of random‐effects meta‐analysis of C3a levels. 1.3.1 = Anti‐neutrophil cytoplasmic antibody‐associated vasculitis (AAV) patients in active state versus in remission; 1.3.2 = active AAV patients versus healthy controls; 1.3.3 = AAV patients in remission versus healthy controls. Squares are proportional to study weight.
Properdin levels were examined and compared in 158 active patients, 128 remitted patients and 97 healthy controls, but did not show the difference in any of the comparisons (Supporting information, Fig. S2).
Plasma C4d levels were measured in 98 active AAV patients, 90 remitted AAV patients and 58 healthy controls. The levels were higher in the active AAV group compared to healthy controls (s.m.d. = 1·64, 95% CI = 0·34–2·94), and also elevated in AAV patients in remission compared to controls (s.m.d. = 1·11, 95% CI = 0·31–1·92). C4d levels were not different between AAV patients in different disease activity states (Supporting information, Fig. S3).
Plasma factor B levels were examined in 125 patients with active AAV, 82 patients with AAV in remission and 75 healthy controls. The levels were higher in the active AAV group compared to controls (s.m.d. = 0·83, 95% CI = 0·53–1·12) and also elevated in AAV patients in remission compared to controls (s.m.d. = 0·87, 95% CI = 0·39–1·34) (Fig. 4). Plasma Bb levels were investigated in 119 patients with active AAV, 120 patients with AAV in remission and 65 healthy controls. None of the comparisons differed significantly (Supporting information, Fig. S4).
Fig. 4.
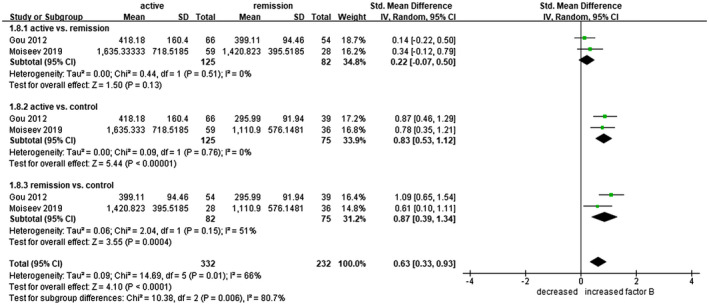
Forest plot of random‐effects meta‐analysis of factor B levels. 1.8.1 = Anti‐neutrophil cytoplasmic antibody‐associated vasculitis (AAV) patients in active state versus in remission; 1.8.2 = active AAV patients versus healthy controls; 1.8.3 = AAV patients in remission versus healthy controls. Squares are proportional to study weight.
Assessment of heterogeneity and publication bias
We assessed statistical heterogeneity between the included studies. As the I2 test showed a value of > 50%, indicating substantial heterogeneity, we used random‐effect models for meta‐analyses. The funnel plot showed near symmetry (Supporting information, Fig. S5).
Discussion
All three complement activation pathways converge at the generation of C3 convertase that cleaves C3 into anaphylatoxin C3a and opsonin C3b [21]. The association of C3b with the C3 convertase results in the formation of a C5 convertase cleaving C5 into anaphylatoxins C5a and C5b. This cleavage triggers the terminal complement cascade leading to the assembly of MAC (C5b‐9). In our and previous studies, the terminal pathway components levels, C5a and MAC, were elevated in patients with active AAV compared to healthy controls. This finding indicates that complement activation occurs in the course of systemic vasculitis. In our patients, effective remission induction treatment was associated with a decrease in C5a levels, whereas MAC levels did not change. Nevertheless, the meta‐analysis showed that both C5a and MAC levels in patients with remission of AAV were lower compared to patients with active disease. Remission induction therapy also resulted in a decrease in C3a levels. Activation of complement in AAV occurred irrespective of ANCA serotype, severity of disease or previous immunosuppression, as we detected no difference between C5a and MAC levels in the groups of patients with MPO‐ANCA or PR3‐ANCA vasculitis, active MPA or GPA, severe or non‐severe vasculitis, newly diagnosed or relapsing disease, predominant granulomatous or vasculitic or mixed disease, immunosuppressive‐naive or previously immunosuppressed patients. C5a and MAC levels did not correlate with BVAS, proteinuria, serum creatinine or laboratory markers of inflammation and were increased only in a proportion of patients with active AAV. Of note, C5a concentrations exceeded the upper reference level only in a quarter of patients who showed clinical signs of active AAV. Therefore, analysis of both complement components alone does not allow for distinction between active disease and remission.
We also measured factor B and properdin levels that are components of the alternative complement pathways. Factor B can be cleaved by factor D into Ba and Bb and is necessary for the formation of the alternative pathway C3 convertase (C3bBb), whereas properdin increases the half‐life of the convertase activity and promotes constant cleavage of C3 into C3a and C3b. Median factor B levels were elevated in patients with active AAV compared to healthy controls and decreased after remission. However, we detected no difference between median properdin levels in AAV patients and healthy volunteers. These data were confirmed by our meta‐analysis.
Deposition of Bb in glomeruli of patients with AAV correlated with the proportion of crescents, the extent of interstitial infiltrates, interstitial fibrosis and tubular atrophy, and inversely with the proportion of normal glomeruli [22]. Moreover, plasma Bb concentrations correlated with common pathway components levels (C3a, C5a and MAC), clinical and laboratory signs of vasculitis activity (BVAS, ESR) and the proportion of total and cellular crescents in kidney biopsies [11]. These findings indicate that circulating Bb levels may reflect both systemic and renal disease activity of AAV. In another study that analyzed 187 renal biopsy samples from patients with AAV, properdin staining was associated with the proportion of cellular crescents and the presence of properdin correlated with the level of proteinuria [6]. In our study, factor B or properdin levels did not correlate with proteinuria, serum creatinine, BVAS or laboratory markers of inflammation (ESR, C‐reactive protein). In a small group of patients with kidney biopsies, factor B levels correlated with C3 deposition in the glomeruli, whereas all other correlations between complement components and histological parameters were not significant.
A crucial role of complement activation in AAV pathogenesis makes targeting complement components an attractive therapeutic strategy. Currently, two C5a inhibitors are in clinical development for AAV: avacopan, an oral C5a receptor (C5aR) inhibitor, and IFX‐1, a monoclonal antibody to C5a [23]. Efficacy, safety and steroid‐sparing effects of avacopan in patients with GPA/MPA were shown in two Phase II trials (CLEAR and CLASSIC) [23, 24], whereas IFX‐1 has entered Phase II development. The positive results of the Phase III trial of avacopan (ADVOCATE) in AAV patients were recently announced, although not yet published. C5a also interacts with C5L2 receptors that compete with C5aR for binding of anaphylotoxins, and therefore may have anti‐inflammatory effects. Knock‐out of C5L2 in mice resulted in a more severe MPO‐ANCA‐associated glomerulonephritis [5]. Conversely, C5L2 was up‐regulated in glomeruli in patients with ANCA‐associated glomerulonephritis [10].
A major limitation of our study is the relatively small sample of patients, particularly studied sequentially both prior to and after the achievement of remission. However, our findings are in accordance with previous studies and were reinforced by the meta‐analysis of all available data on the activation of the complement system in AAV.
In summary, our findings and the results of the meta‐analysis provided additional evidence for the activation of the complement system via the alternative pathway in AAV. Signs of complement activation were found only in a proportion of patients with active AAV, whereas achievement of clinical remission following immunosuppressive therapy did not always lead to normalization of various complement component levels. Therefore, we cannot conclude that any plasma complement component alone may be useful as a biomarker of the disease activity or a guide for treatment decisions. However, studies of the complement system in AAV and other autoimmune diseases pave the way to new drugs development and can turn the dream of steroid‐free regimens into a reality. In addition, investigations of key signalling pathways and molecules (i.e. macrophage migration inhibitory factor, sphingosine‐1‐phosphate, high mobility group box 1) of C5a‐mediated neutrophil priming and activation by ANCA may provide new insights into the complex AAV pathogenesis.
Disclosures
The authors declare no conflicts of interest.
Supporting information
Table S1. PRISMA 2009 Checklist.
Table S2. The Newcastle‐Ottawa Scale (NOS).
Table S3. Complement component levels in patients with active MPO‐ANCA‐ and PR3‐ANCA positive patients.
Table S4. Complement component levels in patients with active MPA and GPA.
Table S5. Complement component levels in patients with newly diagnosed AAV and relapsing disease.
Table S6. Complement component levels in patients who received any immunosuppressive agents or had no history of immunosuppressive therapy.
Table S7. Complement component levels in patients with predominantly granulomatous, mixed granulomatous‐vasculitic and predominantly vasculitic disease.
Table S8. Complement component levels in patients with non‐severe and severe AAV.
Table S9. Correlations (r) between various complement components and serum creatinine, 24‐hour proteinuria and parameters of activity in 59 patients AAV.
Table S10. Correlations (r) between various complement components and histological parameters in 13 patients with kidney biopsy.
Table S11. Characteristics of selected studies.
Fig. S1. The process of articles search and selection.
Fig. S2. Forest plot of random‐effects meta‐analysis of properdin levels in 1) AAV patients in active state versus in remission; 2) active AAV patients versus healthy controls; 3) AAV patients in remission versus healthy controls. Squares are proportional to study weight. AAV ‐ ANCA‐associated vasculitis.
Fig. S3. Forest plot of random‐effects meta‐analysis of C4d levels in 1) AAV patients in active state versus in remission; 2) active AAV patients versus healthy controls; 3) AAV patients in remission versus healthy controls. Squares are proportional to study weight. AAV ‐ ANCA‐associated vasculitis.
Fig. S4. Forest plot of random‐effects meta‐analysis of factor Bb levels in 1) AAV patients in active state versus in remission; 2) active AAV patients versus healthy controls; 3) AAV patients in remission versus healthy controls. Squares are proportional to study weight. AAV ‐ ANCA‐associated vasculitis.
Fig. S5. Funnel plots of meta‐analyses for serum levels of (a) C5‐9b, (b) C5a, (c) C3a, (d) Properdin, (e) C4d, (f) Bb, and (g) factor B levels.
Acknowledgements
This work was in part supported by the Russian Academic Excellence Project 5‐100 and by an unrestricted grant to Andreas Kronbichler from the Austrian Society of Rheumatology.
Contributor Information
A. Zykova, Email: shinji@yuhs.ac, Email: ansezy@gmail.com.
J. I. Shin, Email: shinji@yuhs.ac.
Data availability statement
data available on request.
References
- 1. Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA‐associated vasculitis. Nat Rev Rheumatol 2019; 15:91–101. [DOI] [PubMed] [Google Scholar]
- 2. Deshayes S, Aouba A, Khoy K et al Hypocomplementemia is associated with worse renal survival in ANCA‐positive granulomatosis with polyangiitis and microscopic polyangiitis. PLOS ONE 2018; 13:e0195680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen M, Jayne DRW, Zhao MH. Complement in ANCA‐associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol 2017; 13:359–67. [DOI] [PubMed] [Google Scholar]
- 4. Quintana LF, Kronbichler A, Blasco M et al ANCA associated vasculitis: the journey to complement‐targeted therapies. Mol Immunol 2019; 112:394–8. [DOI] [PubMed] [Google Scholar]
- 5. Xiao H, Dairaghi DJ, Powers JP, et al C5a receptor (CD88) blockade protects against MPO‐ANCA GN. J Am Soc Nephrol 2014; 25:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hilhorst M, van Paassen P, van Rie H et al Complement in ANCA‐associated glomerulonephritis. Nephrol Dial Transplant 2017; 32:1302–13. [DOI] [PubMed] [Google Scholar]
- 7. Schreiber A, Xiao H, Jennette JC et al C5a receptor mediates neutrophil activation and ANCA‐induced glomerulonephritis. J Am Soc Nephrol 2009; 20:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiao H, Schreiber A, Heeringa P et al Alternative complement pathway in the pathogenesis of disease mediated by antineutrophil cytoplasmic autoantibodies. Am J Pathol 2007; 170:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brilland B, Garnier AS, Chevailler A et al Complement alternative pathway in ANCA‐associated vasculitis: two decades from bench to bedside. Autoimmun Rev 2020; 19:102424. [DOI] [PubMed] [Google Scholar]
- 10. Yuan J, Gou SJ, Huang J et al C5a and its receptors in human anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. Arthritis Res Ther 2012; 14:R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gou SJ, Yuan J, Chen M, Yu F, Zhao MH. Circulating complement activation in patients with anti‐neutrophil cytoplasmic antibody‐associated vasculitis. Kidney Int 2013; 83:129–37. [DOI] [PubMed] [Google Scholar]
- 12. Kronbichler A, Kerschbaum J, Gründlinger G et al Evaluation and validation of biomarkers in granulomatosis with polyangiitis and microscopic polyangiitis. Nephrol Dial Transplant 2016; 31:930–6. [DOI] [PubMed] [Google Scholar]
- 13. Antovic A, Mobarrez F, Manojlovic M et al Microparticles expressing myeloperoxidase and complement C3a and C5a as markers of renal involvement in antineutrophil cytoplasmic antibody‐associated vasculitis. J Rheumatol 2020; 47:714–21. [DOI] [PubMed] [Google Scholar]
- 14. Leavitt RY, Fauci AS, Bloch DA et al The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum 1990; 33:1101–7. [DOI] [PubMed] [Google Scholar]
- 15. Watts R, Lane S, Hanslik T et al Development and validation of a consensus methodology for the classification of the ANCA‐associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007; 66:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jennette JC, Falk RJ, Bacon PA et al 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65:1–11. [DOI] [PubMed] [Google Scholar]
- 17. Bajema IM, Hagen EC, Hansen BE et al The renal histopathology in systemic vasculitis: an international survey study of inter‐ and intra‐observer agreement. Nephrol Dial Transplant 1996; 11:1989–95. [DOI] [PubMed] [Google Scholar]
- 18. Berden AE, Ferrario F, Hagen EC et al Histopathologic classification of ANCA‐associated glomerulonephritis. J Am Soc Nephrol 2010; 21:1628–36. [DOI] [PubMed] [Google Scholar]
- 19. Mahr A, Specks U, Jayne D. Subclassifying ANCA‐associated vasculitis: a unifying view of disease spectrum. Rheumatology 2019; 58:1707–9. [DOI] [PubMed] [Google Scholar]
- 20. Wu EY, McInnis EA, Boyer‐Suavet S et al Measuring circulating complement activation products in myeloperoxidase‐ and proteinase 3‐antineutrophil cytoplasmic antibody‐associated Vasculitis. Arthritis Rheumatol 2019; 71:1894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ling M, Murali M. Analysis of the complement system in the clinical immunology laboratory. Clin Lab Med 2019; 39:579–90. [DOI] [PubMed] [Google Scholar]
- 22. Gou SJ, Yuan J, Wang C et al Alternative complement pathway activation products in urine and kidneys of patients with ANCA‐associated GN. Clin J Am Soc Nephrol 2013; 8:1884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jayne D. Complement inhibition in ANCA vasculitis. Nephrol Ther 2019; 15:409–12. [DOI] [PubMed] [Google Scholar]
- 24. Jayne DRW, Bruchfeld AN, Harper L et al Randomized trial of C5a receptor inhibitor avacopan in ANCA‐associated vasculitis. J Am Soc Nephrol 2017; 28:2756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PRISMA 2009 Checklist.
Table S2. The Newcastle‐Ottawa Scale (NOS).
Table S3. Complement component levels in patients with active MPO‐ANCA‐ and PR3‐ANCA positive patients.
Table S4. Complement component levels in patients with active MPA and GPA.
Table S5. Complement component levels in patients with newly diagnosed AAV and relapsing disease.
Table S6. Complement component levels in patients who received any immunosuppressive agents or had no history of immunosuppressive therapy.
Table S7. Complement component levels in patients with predominantly granulomatous, mixed granulomatous‐vasculitic and predominantly vasculitic disease.
Table S8. Complement component levels in patients with non‐severe and severe AAV.
Table S9. Correlations (r) between various complement components and serum creatinine, 24‐hour proteinuria and parameters of activity in 59 patients AAV.
Table S10. Correlations (r) between various complement components and histological parameters in 13 patients with kidney biopsy.
Table S11. Characteristics of selected studies.
Fig. S1. The process of articles search and selection.
Fig. S2. Forest plot of random‐effects meta‐analysis of properdin levels in 1) AAV patients in active state versus in remission; 2) active AAV patients versus healthy controls; 3) AAV patients in remission versus healthy controls. Squares are proportional to study weight. AAV ‐ ANCA‐associated vasculitis.
Fig. S3. Forest plot of random‐effects meta‐analysis of C4d levels in 1) AAV patients in active state versus in remission; 2) active AAV patients versus healthy controls; 3) AAV patients in remission versus healthy controls. Squares are proportional to study weight. AAV ‐ ANCA‐associated vasculitis.
Fig. S4. Forest plot of random‐effects meta‐analysis of factor Bb levels in 1) AAV patients in active state versus in remission; 2) active AAV patients versus healthy controls; 3) AAV patients in remission versus healthy controls. Squares are proportional to study weight. AAV ‐ ANCA‐associated vasculitis.
Fig. S5. Funnel plots of meta‐analyses for serum levels of (a) C5‐9b, (b) C5a, (c) C3a, (d) Properdin, (e) C4d, (f) Bb, and (g) factor B levels.
Data Availability Statement
data available on request.


