M1 macrophages were more frequent in TAK and atherosclerotic patients compared to heart transplant donors. The expression of CD206 is higher than the expression of CD86 in the aorta from TAK patients. T cells were associated with histological disease activity and with prednisone use in TAK.
Keywords: B cells, innate immunity, macrophages, NK cells, T cells, Takayasu arteritis
Summary
Takayasu arteritis (TAK) is a large‐vessel granulomatous vasculitis; the inflammatory infiltration in arteries comprises macrophages, multi‐nucleated giant cells, CD4+ and CD8+ T cells, γδ T cells, natural killer (NK) cells and neutrophils. However, it is unknown which subtype of macrophages predominates. This study aims to evaluate macrophages subpopulations in the aorta in TAK. Immunohistochemistry was performed in the aorta from TAK patients (n = 22), patients with atherosclerotic disease (n = 9) and heart transplant donors (n = 8) using the markers CD68, CD86, CD206, CD3, CD20 and CD56. Active disease was observed in 54·5% of patients and active histological lesions were found in 40·9%. TAK patients presented atherosclerotic lesions in 27·3% of cases. The frequency of macrophages, M1 macrophages, T, B and NK cells was higher in the aorta from TAK and atherosclerotic patients compared to heart transplant donors. In TAK, macrophages and T cells were the most abundant cells in the aorta, and the expression of CD206 was higher than CD86 (P = 0·0007). No associations were found between the expression of cell markers and active disease or with atherosclerotic lesions. In TAK patients, histological disease activity led to higher T cell counts than chronic fibrotic lesions (P = 0.030), whereas prednisone use was associated with lower T cell counts (P = 0·035). In conclusion, M1 macrophages were more frequent in TAK and atherosclerotic patients compared to heart transplant donors, while M2 macrophages dominated M1 macrophages in TAK. T cells were associated with histological disease activity and with prednisone use in TAK.
Introduction
Takayasu arteritis (TAK) is a systemic and granulomatous vasculitis that affects large vessels, usually in individuals younger than 40 years [1]. The inflammatory process in the arteries of TAK patients leads to concentric vessel wall thickening, and eventually evolves to segmental stenosis, occlusions, dilation or aneurysm formation [2, 3]. Adventitia and vasa vasorum are the primary sites where inflammatory lesions begin, and extend to all layers of vessel walls. Although TAK is pan‐arteritis, affected arteries in TAK patients can have non‐affected areas, so‐called ‘skip lesions’ [3].
The inflammatory infiltrates in TAK comprise dendritic cells, macrophages, multi‐nucleated giant cells, neutrophils, γδ T cells, natural killer (NK) cells, B cells and CD4+ and CD8+ T cells [4, 5, 6]. The granulomatous inflammation in large‐vessel vasculitis may start with the arrival of interferon (IFN)‐γ‐producing T helper type 1 (Th1) CD4+ T cells [7]. In‐vitro studies have shown that stimulation of peripheral blood mononuclear cells from TAK patients not only drives the production of Th1 cytokines such as interleukin (IL)‐12 and IFN‐γ, but also the production of IL‐6 and IL‐17A [8]. Moreover, IFN‐γ, IL‐6 and IL‐17A are all present in arteries from TAK patients [8, 9]. These findings indicate that both Th1 and Th17 cells play a role in the pathogenesis of TAK [10].
Macrophages are heterogeneous innate immune cells present in all organs and tissues either as resident cells or as migrating inflammatory cells derived from blood monocytes during inflammation or infectious states [11]. Macrophages play an important role in host defense against infectious agents and cancer cells, but they are also involved in tissue homeostasis by promoting the resolution of inflammatory processes and tissue repair [12]. Macrophages differentiate into different subpopulations, depending on the features of the cytokines produced by other inflammatory cells in the environment [13]. Once differentiated, each macrophage subpopulation has a different pattern of gene expression and protein secretion, especially cytokines and chemokines. These subpopulations include the M1 and M2 macrophages that usually mirror Th1 and Th2 responses. M2 macrophages can further differentiate into the following subtypes: M2a, M2b, M2c and M2d [14, 15].
Although TAK is a granulomatous vasculitis with a central role of macrophages in the pathophysiology, it is as yet unknown which macrophage phenotypes are predominant in arteries from TAK patients. Gaining insight into these subpopulations could help in defining new biomarkers and the development of new treatment strategies.
Therefore, this study aims to evaluate M1 and M2 macrophage subpopulations in the aorta from TAK patients. Patients with atherosclerotic disease and heart transplant donors comprised the control groups. We also investigated associations between macrophage subpopulations and clinical and histology disease activity in TAK patients, as well as with other cell types present in the inflamed vessel wall, such as T, B and NK cells.
Patients and methods
Patients and controls
This study had a cross‐sectional design. The inclusion criteria in the TAK group were the fulfillment of the American College of Rheumatology classification criteria or the Ishikawa diagnosis criteria for TAK modified by Sharma [16, 17] and availability of aorta specimens after aortic surgery or autopsy. The control groups comprised patients who underwent aortic surgery for complicated atherosclerotic disease and from heart transplant donors. Atherosclerotic lesions in the aorta from the study’s participants were defined as previously described [18]. The Institutional Review Board approved the research protocol on 17 December 2015, and this study complied with the Declaration of Helsinki (Comitê de Ética em Pesquisa da UNIFESP‐EPM, no. 1210/2015).
Disease‐related variables
Information concerning demographics, disease features and therapy was retrieved from medical records. Disease activity at the time of surgical intervention was ascertained by Kerr’s criteria and by ITAS2010 (Indian Takayasu Clinical Activity Score) [19, 20], and was considered by the ITAS2010 if the patient scored ≥ 2 [20]. In TAK patients, disease activity based on histology was ascertained by a pathologist, as previously described [2, 3]. The active histological disease was considered if mononuclear inflammatory infiltrates were detected in arterial layers such as media and adventitia with or without multi‐nucleated giant cells, edema and intimal hyperplasia. Conversely, the absence of histological disease activity in TAK was considered in the presence of prominent medial fibrosis, loss of smooth muscle cells and vascularization within arterial wall layers without inflammatory mononuclear infiltration in the aorta [3]. Arteries from TAK patients were also assessed for concomitant atherosclerotic lesions.
Immunohistochemistry
Paraffin‐embedded formalin‐fixed arterial specimens were processed for immunohistochemistry. Six 4‐μm sections were deparaffinized at 75–80°C for 30 min, and slides where washed in consecutive baths with alcohol, distilled water and Dako buffer for 5 min each. All slides then underwent antigen retrieval using Dako target antigen retrieval solution at the PT Link device (Agilent/Dako, Santa Clara, CA, USA) with two baths of 20 min each at 90°C followed by cooling of the slides at 65°C. After this step, all slides were washed with EnVisionTMFlex buffer (Agilent/Dako) for 5 min. The slides were then placed in the Dako Autostainer 48TM (Agilent/Dako) for automated immunohistochemistry. Endogenous peroxidase was blocked with a 10‐vol concentration H2O2 solution for 10 min. The following primary antibodies were incubated for 30 min: mouse anti‐human antibodies anti‐CD3 (ref. no. ab699) as T cell, anti‐CD20 (ref. no. ab9475) as B cell and anti‐CD56 (ref. no. ab8233) as NK cell markers, while anti‐CD86 (ref. no. ab196564) and anti‐CD206 (ref no. ab117644) were used as M1 and M2 macrophage markers, respectively (Abcam, Cambridge, UK). Mouse anti‐human anti‐CD68 (ref. no. IS613) antibodies were the pan‐macrophage markers (Agilent/Dako). Antibody binding was detected with secondary rabbit anti‐mouse antibodies labeled with horseradish peroxidase (HRP) (ref. no. ab97046; Abcam, Cambridge, UK). The peroxidase activity was developed with 3′‐diaminobenzidine tetrachloride (DAB) for 10 min. Afterwards, the nuclei were counterstained with hematoxylin for 5 s.
Quantification of cellular markers
All slides were scanned and digitally stored using the Nanozoomer Digital Pathology Scanner (NDP Scan U10074‐01; Hamamatsu Photonics K.K., Hamamatsu, Japan), and the Aperio ImageScope Viewer software (Leica Biosystems, Wetzlar, Germany) was used for quantification of the expression of each marker in arteries. A semiquantitative score was used for quantification as follows: 0 (absence of expression), 1 (≤ 1%), 2 (2–20%), 3 (20–50%) and 4 (> 50%) according to the frequency of positive cells in ×10 magnification fields [21] throughout the whole artery by two raters (A.W.S.S. and J.P.S.) who were blinded for the patient data. The mean score of each rater was calculated by summing the quantification of each cell marker in all fields of the aorta and by dividing for the total number of fields. Then, the mean score from both raters was calculated and used for statistical analysis. Seven aorta specimens presenting the best tissue integrity and clear distinction between layers throughout the arteries were chosen for the quantification of macrophage markers in each layer (i.e. intima, media and adventitia).
Statistical analysis
Data were analyzed by the IBM spss Statistics for Windows version 21.0, and graphs were built using GraphPad Prism software version 5.0. The median and interquartile range (IQR) were used to present continuous variables, while categorical variables were presented as the percentage and absolute number. Comparisons between two groups for continuous variables were performed by the Mann–Whitney U‐test, while for comparisons among three groups, the Kruskal–Wallis test was used. The Mann–Whitney U‐test was used as a post‐hoc test; the χ2 or Fisher’s exact tests analyzed categorical variables. Spearman’s correlation coefficient analyzed correlations. The significance level considered was 5% (P < 0·05).
Results
Features of patients with Takayasu arteritis
The study groups comprised TAK patients (n = 22), patients with atherosclerotic disease in the aorta (n = 9) and heart transplant donors (n = 8). TAK patients were younger than those with atherosclerotic disease and heart transplant donors [26·0 (19·0–31·8) versus 69·0 (61·5–75·0) versus 64·5 (50·0–70·5) years; P < 0·0001]. The frequency of females was also higher in TAK patients than in patients with atherosclerotic disease but not between TAK and heart transplant donors (72·7 versus 22·2 versus 42·9%; P = 0·028). All enrolled TA patients had aortic aneurysms, and the thoracic aorta was the most frequent artery studied in all groups (86·4 versus 77·8 versus 100·0%), followed by the abdominal aorta.
Twelve (54·5%) TAK patients had active disease based on the clinical evaluation by Kerr’s criteria, while nine (40·9%) patients were considered to present active disease based on ITAS2010. Twelve (54·5%) of all TAK patients underwent vascular/heart surgery at disease presentation due to aortic root dilation and aortic insufficiency. The median time since the diagnosis of TAK was 6·5 (1·0–48·0) months when aorta specimens were obtained. Twelve (54·5%) TAK patients were not receiving glucocorticoid or immunosuppressive therapy at the time of the vascular procedure (Supporting information, Table S1).
Nine TAK patients (40·9%) presented active histological lesions in the aorta. Seven (31·8%) of TAK patients had both clinical and histological disease activity, whereas 20·0% of TAK patients considered in remission had active inflammation in the aorta. Six TAK patients (27·3%) presented concomitant atherosclerotic disease in aorta specimens. Nine (40·9%) TAK patients received prednisone therapy at a median daily dose of 17·5 mg (5·0–20·0 mg), and three patients (13·6%) received conventional synthetic disease‐modifying anti‐rheumatic agents (csDMARDs) or biological bDMARDs (i.e. two patients on methotrexate, one on mycophenolate mofetil and one on tocilizumab) (Supporting information, Table S1).
Immunohistochemistry in the aorta
The markers CD68, CD86, CD3, CD20 and CD56 had significantly increased expression in the aorta from TAK patients and patients with atherosclerotic disease in comparison to heart transplant donors. The only expression of M2 macrophages was similar in all groups (Table 1). CD68, CD86, CD206, CD3, CD20 and CD56 are all expressed in the aorta of an active TAK patient (Fig. 1). Figure 2 illustrates the expression of CD68 in multi‐nucleated giant cells and the surroundings of vasa vasorum in the aorta from a TAK patient. The expression of CD68, CD86, CD206, CD3, CD20 and CD56 is shown in the aorta from patients with atherosclerotic disease and in heart transplant donors (Supporting information, Figs S1 and S2).
Table 1.
The expression of cell markers in the aorta from patients in all groups
| Variables | TAK (n = 22) | AD (n = 9) | HTD (n = 8) | P | TAK versus AD | TAK versus HTD | AD versus HTD |
|---|---|---|---|---|---|---|---|
| P | P | P | |||||
| CD68 | 2·120 (1·937–2·662) | 1·771 (1·465–2·229) | 0·807 (0·351–1·538) | 0·001* | 0·164 | < 0·0001* | 0·007* |
| CD86 | 0·581 (0·366–0·949) | 0·631 (0·476–1·086) | 0·257 (0·178–0·380) | 0·003* | 0·354 | 0·005* | 0·001* |
| CD206 | 1·671 (0·667–2·229) | 1·364 (1·279–1·729) | 1·278 (0·324–1·468) | 0·273 | 0·543 | 0·121 | 0·338 |
| CD3 | 0·943 (0·562–1·749) | 0·930 (0·634–1·177) | 0·462 (0·153–0·784) | 0·033* | 0·728 | 0·017* | 0·021* |
| CD20 | 0·176 (0·077–0·687) | 0·242 (0·202–0·299) | 0·047 (0·025–0·100) | 0·004* | 0·896 | 0·002* | 0·004* |
| CD56 | 0·305 (0·139–0·637) | 0·283 (0·134–0·454) | 0·072 (0·002–0·268) | 0·016* | 0·500 | 0·007* | 0·021* |
AD = atherosclerotic disease; HTD = heart transplant donors; n = number of patients; TAK = Takayasu arteritis. Data are presented as median and interquartile range. Comparisons among groups were performed by the Kruskal–Wallis test and the Mann–Whitney U‐test as a post‐hoc test.
Flags significant results.
Fig. 1.
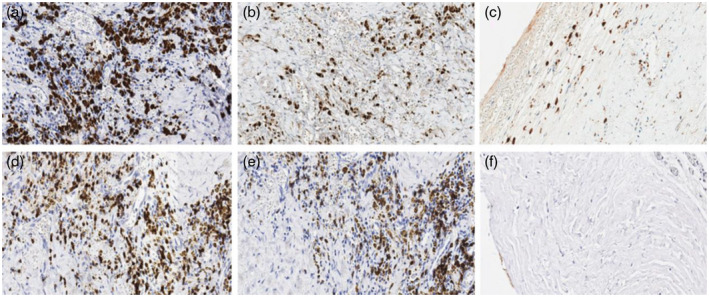
Immunohistochemistry in the aorta from a Takayasu arteritis (TAK) patient. Representative images from a TAK patient with staining at ×20 magnification for the pan‐macrophage marker CD68 (a), CD86 for M1 macrophages (b), CD206 for M2 macrophages (c), CD3 for T cells (d), CD20 for B cells (e) and CD56 for natural killer (NK) cells (f). The images shown in (a,b,d,e) were taken in the media layer of the aorta, and the images shown in (c,f) were taken at the boundaries between the intima and the media layer. Cell nuclei were stained in blue by hematoxylin, and each slide was stained with a brown specific marker [horseradish peroxidase (HRP)‐conjugated secondary antibodies].
Fig. 2.
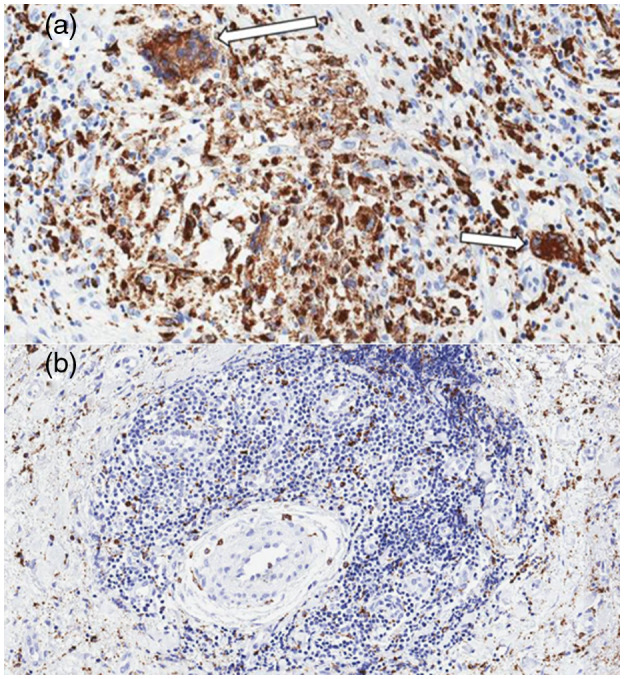
Active inflammatory infiltration in the aorta from a Takayasu arteritis (TAK) patient. Representative images in the aorta from a TAK patient with staining at ×20 magnification for the pan‐macrophage marker CD68 showing multi‐nucleated giant cells (white arrows) in the media layer (a) and the adventitial inflammatory infiltrate surrounding the vasa vasorum (b).
Macrophages are the most abundant cells in arteries of TAK patients compared to T cells (P = 0·0008), B cells (P < 0·0001) and NK cells (P < 0·0001). Conversely, T cells were more frequent than B cells (P = 0·0002) and NK cells (P = 0·0001). No significant differences were observed between B and NK cells in TAK patients (P = 0·404) (Fig. 3). M2 macrophages were more frequent in the aorta from TAK patients compared to M1 macrophages (Fig. 4). The M1/M2 ratio in the aorta from TAK patients was 0·420 (IQR = 0·289–0·756).
Fig. 3.
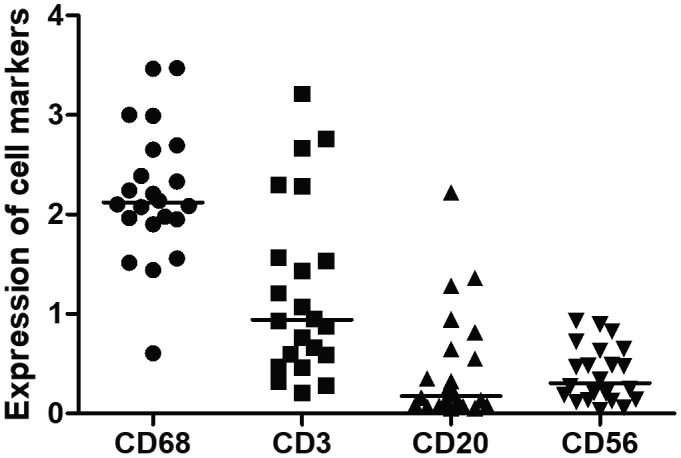
Expression of cell markers in the aorta from Takayasu arteritis (TAK) patients. Macrophages are the most frequent cells in the inflammatory infiltration in the aorta from TAK patients, followed by T cells. The median and interquartile range (IQR) expressions of CD68, CD3, CD20 and CD56 were 2·121 (1·938–2·663) versus 0·943 (0·562–1·749) versus 0·176 (0·077–0·687) versus 0·305 (0·139–0·637) P < 0·0001, respectively. The crossbar represents the median expression of cell markers. Data are presented as median and IQR and analysis was performed by the Kruskal–Wallis test and the Mann–Whitney U‐test.
Fig. 4.
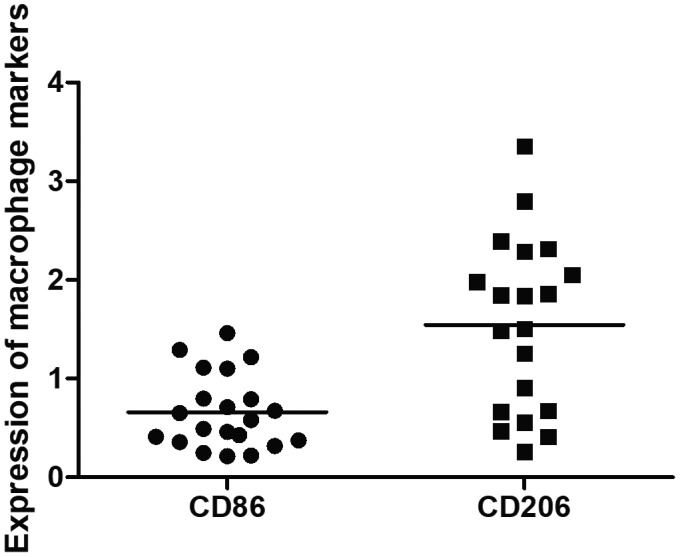
M1 and M2 macrophage markers in the aorta from Takayasu arteritis (TAK) patients. CD206 versus CD86: 1·672 (0·667–2·230) versus 0·581 (0·366–0·949); P = 0·0007. The crossbar represents the mean expression of macrophage markers. Data were presented as median and interquartile range and the analysis was performed by the Mann–Whitney U‐test.
Correlations between the expression of cell markers in the aorta from TAK patients are described in Table 2 and Supproting information, Fig S3. The expression of the pan‐macrophage marker (CD68) was significantly correlated with the expression of CD3 and of CD20, but not with CD56. CD68 was more strongly correlated with the M2 marker (CD206) than with the M1 marker (CD86). However, the expression of the M1 macrophage marker (CD86) was correlated with CD3 and CD20, while no other correlations were found with CD206 expression, including CD3, CD20 and CD86.
Table 2.
Correlation analysis between the expression of cell markers in the aorta from TAK patients
| Macrophage markers | Cell markers of lymphocytes and NK cells | ||||
|---|---|---|---|---|---|
| CD86 | CD206 | CD3 | CD20 | CD56 | |
| CD68 | Rho = 0·429 | Rho = 0·798 | Rho = 0·617 | Rho = 0·493 | Rho = −0·126 |
| P = 0·053 | P < 0·0001* | P = 0·002* | P = 0·020* | P = 0·577 | |
| CD86 | – | Rho = 0·044 | Rho = 0·621 | Rho = 0·586 | Rho = 0·047 |
| P = 0·855 | P = 0·003* | P = 0·005* | P = 0·841 | ||
| CD206 | Rho = 0·044 | – | Rho = 0·397 | Rho = 0·177 | Rho = −0·111 |
| P = 0·855 | P = 0·083 | P = 0·454 | P = 0·640 | ||
Correlation analysis were performed by Spearman’s correlation coefficient.
Flags significant results.
TAK = Takayasu arteritis; NK = natural killer.
The distribution of macrophages was analyzed in the three layers of the aorta from seven TAK patients. Although the median expression of all macrophage markers was higher in the adventitia compared to the media and the intima, these differences were not statistically significant (Fig. 5).
Fig. 5.
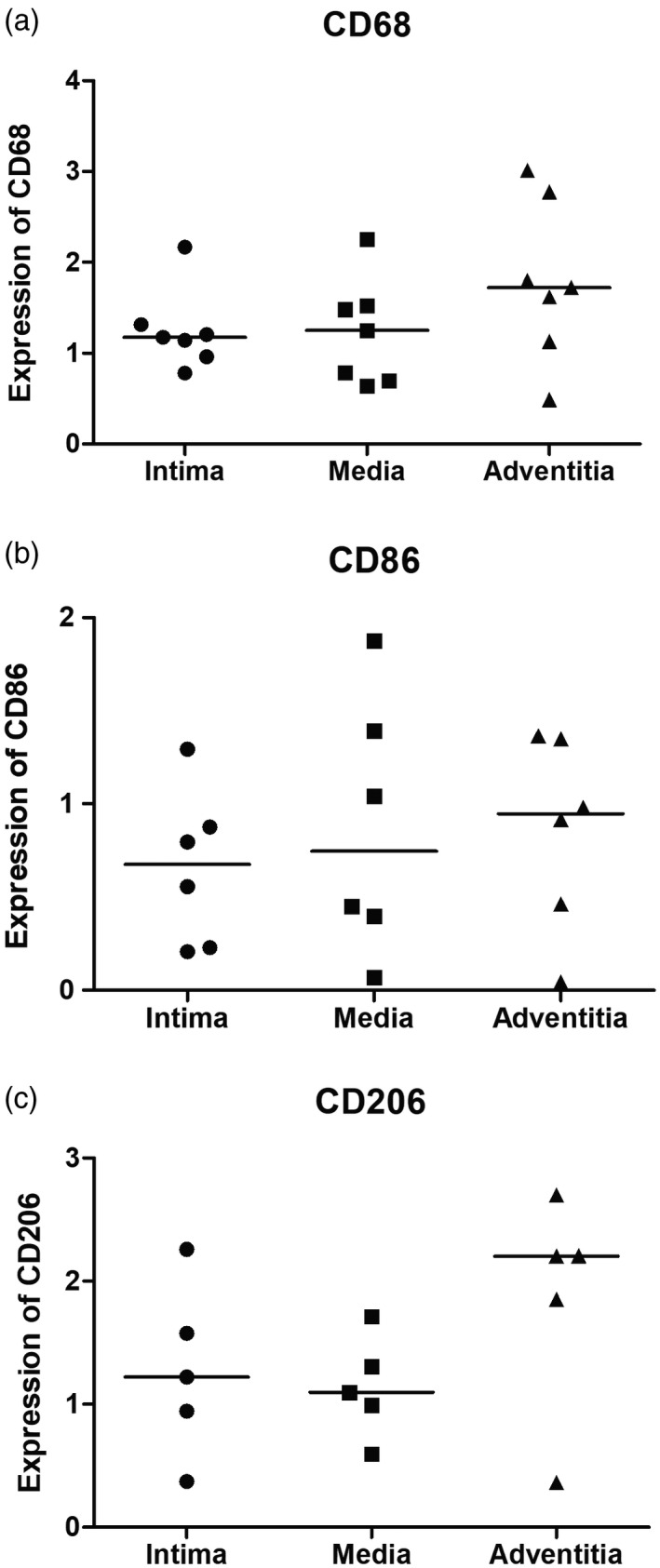
Expression of macrophage markers in the three layers of the aorta from Takayasu arteritis (TAK) patients. No significant differences were observed for the expression of macrophages markers in three layers of the aorta, as follows: CD68 [intima: 1·174 (0·963–1·316) versus media: 1·253 (0·697–1·522) versus adventitia: 1·720 (1·129–2·776); P = 0·328], CD86 [intima: 0·676 (0·223–0·980) versus media: 0·746 (0·316–1·513) versus adventitia: 0·947 (0·358–1·353); P = 0·700] and CD206 [intima: 1·222 (0·658–1·918) versus media: 1·095 (0·793–1·510) versus adventitia: 2·204 (1·107–2·454); P = 0·326]. The crossbar represents the median expression of macrophage markers. Data were presented as median and interquartile range and the analysis was performed by the Kruskal–Wallis test.
Disease‐related variables and cellular infiltration in the aorta of TAK patients
The expression of each cell marker was analyzed regarding disease activity based on clinical assessment, histological disease activity, concomitant atherosclerotic lesions in the aorta and prednisone use. There were no significant differences between TAK patients presenting with active disease and those considered in remission using Kerr’s criteria and the definition of disease activity by the ITAS2010 (Supporting information, Table S2). Nevertheless, when histological disease activity was analyzed, the T cell marker CD3 was more highly expressed in TAK patients with active inflammatory infiltrate compared to those presenting chronic fibrotic lesions [1·540 (0·808–2·483) versus 0·764 (0·392–1·255); P = 0·030]. The expression of macrophage markers in the aorta was similar between TAK patients with and without histological disease activity (Supporting information, Table S3).
The impact of atherosclerotic lesions on cellular infiltration in the artery evaluated in TAK patients was also analyzed. A tendency for a higher frequency of M2 macrophages was observed in TAK patients with atherosclerotic lesions compared to those without atherosclerosis [1·922 (1·845–2·146) versus 1·081 (0·536–2·116); P = 0·070]. No other differences regarding the pan‐macrophage marker CD68, M1 macrophages, T cells, B cells and NK cells were found between TAK patients with and without atherosclerotic lesions in the aorta (Supporting information, Table S4). The majority of TAK patients included in this study were not treated with csDMARDs or bDMARDs. Thus, current prednisone use was the only variable assessed related to therapy. The expression of the T cell marker CD3 was significantly lower in TAK patients who used prednisone compared to patients without prednisone [0·470 (0·303–1·306) versus 1·211 (0·714–2·483); P = 0·035]. The expression of macrophage markers, B cell or NK cell markers was similar in the aorta from TAK patients using prednisone or not (Supporting information, Table S5).
No significant correlations were found between prednisone daily dose and the expression of CD68 (rho = 0·026; P = 0·951), CD86 (rho = −0·039; P = 0·927), CD206 (rho = 0·013; P = 0·976), CD3 (rho = 0·196; P = 0·642), CD20 (rho = 0·143; P = 0·735) or CD56 (rho = 0·665; P = 0·072) in the aorta from TAK patients.
Discussion
In this study, TAK patients and patients with atherosclerotic disease had a higher expression of macrophage markers, especially markers of M1 macrophages, as well as T, B and NK cell markers in the aorta compared to heart transplant donors, whereas M2 macrophages were found to be equally present in TAK and control groups. Macrophages are the most frequent cell type in the aorta from TAK patients, followed by T, B and NK cells. Conversely, the expression of the macrophage M2 marker CD206 is higher than the M1 marker CD86 in the aorta from TAK patients. Macrophages and their subpopulations were found in all layers of the aorta from TAK patients. Active disease based on histology findings and prednisone use had a positive and a negative association with CD3 expression in the aorta, respectively. No macrophage marker had significant associations with histological or clinical parameters in TAK patients.
Due to its inflammatory nature, patients with atherosclerosis [22] were included in this study as a positive control group, while heart transplant donors were included as negative controls, i.e. healthy individuals with a lower chance of presenting any disease of the aorta. We also decided to assess only aorta specimens from TAK patients and from control groups to avoid the influence of vascular‐bed specific phenotypes, as vascular responses to the same stimulus may vary in different vascular trees [23]. As expected, expression of all but one cell marker was higher in the aorta from both TAK patients and patients with atherosclerotic disease. Only expression of the M2 marker CD206 was similar in all groups. This finding may reflect the predominance of M2 macrophages in the aorta from healthy individuals, which may be either tissue‐resident macrophages or the response to stressful stimuli elsewhere in individuals with brain death due to other causes.
In this study, macrophages and T cells were the most frequent cells in the aorta from TAK patients. This observation highlights the importance of these two cell types in the pathogenesis of TAK, in which chronic granulomatous inflammation of the artery is a hallmark of the disease [6].
In TAK, the prevailing pathogenic model is that CD4+ T cells are stimulated by dendritic cells in the adventitia towards Th1 and Th17 responses, with the production of cytokines that lead to activation of macrophages [6, 7, 8]. In line with the Th1 response in the pathophysiology of TAK, M1 macrophages were more frequent in the aorta from TAK patients compared to heart transplant donors, as it is well known that M1 and M2 macrophages phenotypes mirror the Th1 and Th2 responses [24], respectively. However, IL‐17A induces a heterogeneous profile of macrophages with M1/M2 features, also known as atypical M2‐like macrophages that have increased expression of inducible nitric oxide synthase (iNOS) and CD206 in mouse studies [25, 26]. Thus, the higher expression of CD206 than CD86 in the aorta from TAK patients may not only be related to M2a or M2c macrophages, the subtypes that mostly express CD206 [27], but a reflex of the effect of Th17 response on macrophages leading to the atypical M2‐like macrophages in the aorta from TAK patients. Further studies are necessary to clarify this issue.
In line with our results, the investigation of macrophage markers in systemic vasculitides such as giant cell arteritis (GCA), immunoglobulin (Ig)A vasculitis and in granulomatosis with polyangiitis (GPA) demonstrated predominantly M2 macrophages in affected tissues, despite significant differences in the pathophysiology of these diseases [28, 29, 30, 31, 32, 33]. In GPA patients, the granulomatous inflammation of the upper airways is associated with a higher frequency of M2 macrophages and Th2 CD4+ T cells compared to M1 macrophages and Th1 CD4+ cells [29]. In addition, soluble CD163, a scavenger receptor used as an M2 marker, served as a surrogate marker of disease activity in GCA and glomerulonephritis in anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis [32, 33]. This evidence highlights the role of M2 macrophages in the pathophysiology of systemic vasculitides.
In TAK, the inflammatory process is believed to start in the adventitia and to evolve to all arterial layers as the disease progresses [3]. Thus, we assessed the expression of macrophage markers in the three layers of the arterial wall (i.e. intima, media and adventitia) to evaluate whether macrophages would cluster in a predominant layer of the aorta in TAK. Nonetheless, macrophages markers were found equally in all layers, and this observation may indicate that the disease duration of TAK patients evaluated in this study may have been much longer than we expected, as macrophages had already migrated from the adventitia to other layers. Conversely, a recent study showed that inflammatory mononuclear cells aggregate in the adventitia of the aorta from TAK patients, especially surrounding the vasa vasorum [34].
The thoracic aorta is a vascular site frequently affected by atherosclerosis in TAK [35], and we found concomitant atherosclerotic lesions in the aorta in one‐third of our TAK patients. However, the presence of atherosclerotic lesions in the aorta from TAK patients did not yield significant differences in the expression of cell markers; only a tendency for higher expression of CD206 was found in those with atherosclerotic lesions. The significance of these findings is uncertain. Previous evidence in atherosclerotic disease has shown that M2 macrophages are associated with plaque stability, while M1 macrophages are associated with the progression of atherosclerotic lesions and the development of ischemic cardiovascular events [36].
In this study, there was no association between disease activity based on clinical assessment of TAK patients and the presence of active inflammatory infiltration in the aorta, and no cell markers were increased in TAK patients presenting with clinically active disease. This dissociation between systemic and arterial inflammation is well known in TAK patients who continue to present arterial inflammation and may develop new angiographic lesions even after the remission state has been achieved [19, 37]. Alternatively, for TAK patients presenting signs and symptoms of active disease, the absence of inflammation in the aorta may be due to an active inflammatory process in arteries other than the aorta. Beyond the analysis between the expression of cell markers in arteries from TAK patients and overt signs of disease activity, analysis of correlations with systemic arterial remodeling would also disclose interesting findings. The angiographic score is a validated tool developed to assess the degree of arterial involvement, including stenosis and dilatation in large‐vessel vasculitis, and may be used as a surrogate marker of systemic remodeling for future studies assessing arterial inflammation in TAK [38].
The higher expression of CD3 in the aorta from TAK patients presenting active histological lesions indicates that T cells are important players in the pathogenesis of TAK, as these cells activate macrophages, induce the formation of giant cells and sustain granulomatous inflammation in arterial walls [6, 7]. Indeed, a previous study has shown that CD4+ and CD8+ T cells comprise approximately 30% of cells found in the inflammatory infiltrate in arteries from TAK patients [39]. Conversely, prednisone use had a significant impact on the expression of CD3+ cells in the aorta from TAK patients. The induction of T cell apoptosis is one of the glucocorticoid immunoregulatory properties that may be involved in decreasing T cells in the aorta from TAK patients [40]. In GCA, prednisone use led to the suppression of Th17 CD4+ T cells in temporal arteries, with the persistence of Th1 CD4+ T cells [41]. This issue is still controversial in TAK as the in‐vitro production of Th1 cytokines is inhibited by prednisone use, whereas serum levels of Th17 cytokines are decreased in TAK patients on prednisone [8, 42]. We could not find significant differences in the expression of other cell markers, including macrophage markers, regarding histological disease activity or prednisone use in the aorta from TAK patients.
The expression of the B cell marker CD20 was relatively low in the aorta from TAK patients. This finding is the opposite of that reported for large‐vessel GCA, that massive B cell infiltration in the aorta, especially in the adventitia, outnumbered T cells and led to tertiary lymphoid organ formation in the artery [43]. However, this issue is still controversial, as another study observed higher expression of CD20 and CD138 in the aorta from TAK patients compared to temporal arteries from GCA patients [34]. B cell disturbances are not yet as well characterized in TAK as they are in GCA patients [21]. The findings of circulating autoantibodies and the increased number of newly formed plasmablasts in peripheral blood from TAK patients presenting active disease indicate a role of B cells in the pathophysiology of TAK, but this issue needs to be further explored [44, 45].
The limitations of this study include the relatively low number of aorta specimens evaluated as well as the lower frequency of females and the higher median age in the control groups compared to TAK patients. These differences may be due to the epidemiological profile of TAK, a disease that affects mainly young females, while older males are affected more frequently by atherosclerotic disease. Moreover, the higher median age in the heart transplant donors’ group may be associated with an increased number of co‐morbidities and even subclinical atherosclerotic disease, and these features would increase the chance of finding more inflammatory cells in the aorta.
M1 macrophages were more frequent in the aorta from TAK and atherosclerotic disease patients compared to heart transplant donors. Conversely, the expression of CD206 was higher than CD86 in the aorta from TAK patients, and this finding may indicate the presence of either M2 macrophages or the atypical M2‐like macrophages induced by the Th17 response. Macrophages are found in all layers of the aorta in TAK. Histological disease activity and prednisone use are both of influence in the expression of CD3 but not on macrophage markers. Clinical disease activity and concomitant atherosclerotic disease had no impact on the expression of macrophage markers in the aorta from TAK patients.
Disclosures
The authors declare no conflicts of interest related to this study.
Supporting information
Figure S1. Expression of cell markers in the aorta from a patient with atherosclerotic disease.
Figure S2. Expression of cell markers in the aorta from a heart transplant donor.
Figure S3. Significant correlations between cell markers in the aorta from TAK patients.
Table S1. Disease features of patients with Takayasu arteritis.
Table S2. The expression of cell markers in the aorta of TAK patients and disease activity.
Table S3. The expression of cell markers in the aorta of TAK patients and histologic disease activity.
Table S4. Atherosclerotic lesions in the aorta from TAK patients and the expression of cell markers.
Table S5. The expression of cell markers in the aorta of TAK patients and prednisone use.
Acknowledgements
The authors thank Dr Mabel de Moura Barros Zamorano (Pathologist from Instituto ‘Dante Pazzanese’ de Cardiologia, São Paulo, Brazil), Dr Vera Demarchi Aiello (pathologist from InCor, São Paulo, Brazil), Francisco Dênis Batista Veiga (Pathology Division, InCor, São Paulo, Brazil), Diego Cardoso dos Santos (Pathology Department UNIFESP‐EPM, São Paulo, Brazil) and all members of the Department of Clinical Laboratory and Pathology from Hospital Israelita Albert Einstein, São Paulo, Brazil.
References
- 1. Jennette JC, Falk RJ, Bacon PA et al 2012 Revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013; 65:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Vaideeswar P, Deshpande JR. Pathology of Takayasu arteritis: a brief review. Ann Pediatr Cardiol 2013; 6:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotchi M. Pathological studies on Takayasu arteritis. Heart Vessels Suppl 1992; 7:11–7. [DOI] [PubMed] [Google Scholar]
- 4. Seko Y. Takayasu arteritis: insights into immunopathology. Jpn Heart J 2000; 41:15–26. [DOI] [PubMed] [Google Scholar]
- 5. Inder SJ, Bobryshev YV, Cherian SM, Wang AY, Lord RS, Masuda K, Yutani C. Immunophenotypic analysis of the aortic wall in Takayasu’s arteritis: involvement of lymphocytes, dendritic cells and granulocytes in immuno‐inflammatory reactions. Cardiovasc Surg 2000; 8:141–8. [DOI] [PubMed] [Google Scholar]
- 6. Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev 2011; 11:61–7. [DOI] [PubMed] [Google Scholar]
- 7. Weyand CM, Goronzy JJ. Medium‐ and large‐vessel vasculitis. N Engl J Med 2003; 349:160–9. [DOI] [PubMed] [Google Scholar]
- 8. Saadoun D, Garrido M, Comarmond C et al Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol 2015; 67:1353–60. [DOI] [PubMed] [Google Scholar]
- 9. Kong X, Sun Y, Ma L et al The critical role of IL‐6 in the pathogenesis of Takayasu arteritis. Clin Exp Rheumatol 2016; 34:S21–7. [PubMed] [Google Scholar]
- 10. Savioli B, Abdulahad WH, Brouwer E, Kallenberg CGM, de Souza AWS. Are cytokines and chemokines suitable biomarkers for Takayasu arteritis? Autoimmun Rev 2017; 16:1071–8. [DOI] [PubMed] [Google Scholar]
- 11. Davies LC, Jenkins SJ, Allen JE, Taylor P. Tissue‐resident macrophages. Nat Immunol 2013; 14:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229:176–85. [DOI] [PubMed] [Google Scholar]
- 15. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arend WP, Michel BA, Bloch DA et al The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990; 33:1129–34. [DOI] [PubMed] [Google Scholar]
- 17. Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol 1996; 54:S141–7. [DOI] [PubMed] [Google Scholar]
- 18. Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 2009; 122:S3–S14. [DOI] [PubMed] [Google Scholar]
- 19. Kerr GS, Hallahan CW, Giordano J et al Takayasu arteritis. Ann Intern Med 1994; 120:919–29. [DOI] [PubMed] [Google Scholar]
- 20. Misra R, Danda D, Rajappa SM et al Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology 2013; 52:1795–801. [DOI] [PubMed] [Google Scholar]
- 21. van der Geest KS, Abdulahad WH, Chalan P et al Disturbed B cell homeostasis in newly diagnosed giant cell arteritis and polymyalgia rheumatica. Arthritis Rheumatol 2014; 66:1927–38. [DOI] [PubMed] [Google Scholar]
- 22. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–95. [DOI] [PubMed] [Google Scholar]
- 23. Rosenberg RD, Aird WC. Vascular‐bed‐specific hemostasis and hypercoagulable states. N Engl J Med 1999; 340:1555–64. [DOI] [PubMed] [Google Scholar]
- 24. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23:549–55. [DOI] [PubMed] [Google Scholar]
- 25. Nishikawa K, Seo N, Torii M et al Interleukin‐17 induces an atypical M2‐like macrophage subpopulation that regulates intestinal inflammation. PLOS ONE 2014; 9:e108494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakai K, He YY, Nishiyama F et al IL‐17A induces heterogeneous macrophages, and it does not alter the effects of lipopolysaccharides on macrophage activation in the skin of mice. Sci Rep 2017; 7:12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shapouri‐Moghaddam A, Mohammadian S, Vazini H et al Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018; 233:6425–40. [DOI] [PubMed] [Google Scholar]
- 28. Zhao L, David MZ, Hyjek E, Chang A, Meehan SM. M2 macrophage infiltrates in the early stages of ANCA‐associated pauci‐immune necrotizing GN. Clin J Am Soc Nephrol 2015; 10:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Souza AWS, van Timmeren M, Sanders JS et al M2 macrophage is the predominant phenotype in airways inflammatory lesions in patients with granulomatosis with polyangiitis. Arthritis Res Ther 2017; 19:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim J, Choi SE, Lee KH, Jeong HJ, Shin JI, Lim BJ. Tubulointerstitial infiltration of M2 macrophages in Henoch–Schönlein purpura nephritis indicates the presence of glomerular crescents and bad clinical parameters. Biomed Res Int 2019; 2019:8579619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albano‐Aluquin S, Malysz J, Kidacki M, Ratnam M, Olsen NJ. An immunohistopathologic study to profile the folate receptor beta macrophage and vascular immune microenvironment in giant cell arteritis. J Vis Exp 2019;144 10.3791/58713. [DOI] [PubMed] [Google Scholar]
- 32. O’Reilly VP, Wong L, Kennedy C et al Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 2016; 27:2906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Sleen Y, Sandovici M, Abdulahad WH et al Markers of angiogenesis and macrophage products for predicting disease course and monitoring vascular inflammation in giant cell arteritis. Rheumatology 2019; 58:1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurata A, Saito A, Hashimoto H et al Difference in immunohistochemical characteristics between Takayasu arteritis and giant cell arteritis: it may be better to distinguish them in the same age. Mod Rheumatol 2019; 29:992–1001. [DOI] [PubMed] [Google Scholar]
- 35. Seyahi E, Ucgul A, Cebi Olgun D et al Aortic and coronary calcifications in Takayasu arteritis. Semin Arthritis Rheum 2013; 43:96–104. [DOI] [PubMed] [Google Scholar]
- 36. De Gaetano M, Crean D, Barry M, Belton O. M1‐ and M2‐type macrophage responses are predictive of adverse outcomes in human atherosclerosis. Front Immunol 2016; 7:275 10.3389/fimmu.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keser G, Aksu K, Direskeneli H. Discrepancies between vascular and systemic inflammation in large vessel vasculitis: an important problem revisited. Rheumatology 2018; 57:784–90. [DOI] [PubMed] [Google Scholar]
- 38. Tombetti E, Godi C, Ambrosi A et al Novel angiographic scores for evaluation of large vessel vasculitis. Sci Rep 2018; 8:15979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seko Y, Minota S, Kawasaki A et al Perforin‐secreting killer cell infiltration and expression of a 65‐kD heat‐shock protein in aortic tissue of patients with Takayasu’s arteritis. J Clin Invest 1994; 93:750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci 2006; 63:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T‐cell responses in giant cell arteritis. Circulation 2010; 121:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Savioli B, Salu BR, de Brito MV, Vilela Oliva ML, de Souza AWS. Silent arterial inflammation during the apparent remission state of Takayasu’s arteritis. What do cytokines tell us? Clin Exp Rheumatol 2018;36:33–9. [PubMed] [Google Scholar]
- 43. Graver JC, Boots AMH, Haacke EA, Diepstra A, Brouwer E, Sandovici M. Massive B‐cell infiltration and organization into artery tertiary lymphoid organs in the aorta of large vessel giant cell arteritis. Front Immunol 2019; 10:83 10.3389/fimmu.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silva de Souza AW. Autoantibodies in systemic vasculitis. Front Immunol 2015; 6:184 10.3389/fimmu.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoyer BF, Mumtaz IM, Loddenkemper K et al Takayasu arteritis is characterised by disturbances of B cell homeostasis and responds to B cell depletion therapy with rituximab. Ann Rheum Dis 2012; 71:75–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of cell markers in the aorta from a patient with atherosclerotic disease.
Figure S2. Expression of cell markers in the aorta from a heart transplant donor.
Figure S3. Significant correlations between cell markers in the aorta from TAK patients.
Table S1. Disease features of patients with Takayasu arteritis.
Table S2. The expression of cell markers in the aorta of TAK patients and disease activity.
Table S3. The expression of cell markers in the aorta of TAK patients and histologic disease activity.
Table S4. Atherosclerotic lesions in the aorta from TAK patients and the expression of cell markers.
Table S5. The expression of cell markers in the aorta of TAK patients and prednisone use.


