A preceding peanut sensitization amplifies allergic asthma accompanied by enhanced Th2/Th17 response in PBMC‐engrafted humanized mice. This murine model could be instrumental to further elucidate the mechanisms of interplay between different primary sensitizations, which is also suitable for investigating existing drugs, novel pharmacologic and immunologic intervention for treatment of allergic diseases.

Keywords: asthma, IgE, mouse model, peanut allergy, Th2/Th17
Summary
Food allergy is related to increasing risk of the development of allergic asthma, but the precise interplay between sensitization to different allergens in different compartments of the body is not fully understood. The aim of this study was to develop a novel humanized murine model of mixed food and respiratory allergy that recapitulates the human anaphylactic response and to more clearly understand the impact of food allergies on asthma. Immunodeficient mice transferred with peripheral blood mononuclear cells (PBMCs) from donors with peanut and house dust mite (HDM) allergy were exposed and challenged to peanut. Between peanut exposure and challenge, mice were intranasally treated to HDM. Allergic parameters were analyzed. Allergen‐specific immunoglobulin (Ig)E in sera could only be measured in mice treated with peripheral blood mononuclear cells (PBMCs) plus allergen. A preceding peanut exposure increased IgE levels, histamine release, bronchial hyper‐responsiveness and lung inflammation. Recruitment of inflammatory cells to the airways was aggravated associated with an enhanced T helper type 2 (Th2)/Th17 cytokine secretion when the two allergies were present. A preceding peanut exposure amplifies allergic asthma in this humanized model, which may contribute to the understanding of underlying immunological mechanism of polysensitization occurring in allergic individuals and evaluation of therapeutic interventions.
Introduction
In the past four decades, atopic disorders including food allergy and allergic asthma have dramatically increased. Individuals who are allergic are more sensitive to various allergens, and may have systematic symptoms in multiple organs. Rhino‐conjunctivitis and/or asthma in allergic patients are not only induced by house dust mite (HDM), but also by food allergy to peanuts [1, 2]. Food allergy has been identified as an independent risk factor for life‐threatening asthma. Moreover, it is one of the first manifestations of the so‐called ‘atopic march’, as many children with food allergy develop allergic asthma later in life [3, 4]. However, the underlying immunological mechanism of polysensitization still needs to be elucidated.
Peanuts contribute to the majority of fatal or near‐fatal food‐induced, anaphylactic reactions. For example, peanut allergy provokes characteristic gastrointestinal responses such as vomiting, diarrhea and abdominal pain. Skin (angioedema, eczema, urticaria et al.) or respiratory (cough, wheezing, stridor et al.) allergic symptoms may also occur in peanut allergy [5, 6]. Although a link of peanut allergy to asthmatic reactions has been established by previous epidemiological and clinical studies, the precise interplay between events involving gut and lung inflammation remains unclear. It has been suggested that asthma accompanying food allergy is a risk factor for the occurrence of anaphylaxis, but how asthma accounts for this systemic response has not been fully understood [7, 8]. Significant progress has been made in studies using animal models to elucidate the immunological process of sensitization and allergy, but such animal models are mainly used to investigate a single sensitization and associated clinical phenotype [9, 10]. Moreover, both immunoglobulin (Ig)E and IgG1 elicit allergic reactions in murine models (not humanized), whereas in human anaphylaxis IgG appears to play a smaller role [11, 12].
To avoid this bias, a unique humanized mouse model of human IgE‐mediated anaphylaxis in response to peanut and HDM was established using non‐obese diabetic‐severe combined immunodeficiency (NOD‐SCID) interleukin (IL)‐2Rγnull (NSG) immune‐deficient mice reconstituted with allergic human blood mononuclear cells to study the possible interplay between food and respiratory allergy. Cellular and humoral immune responses to peanut and HDM, including T helper type 2 (Th2)/Th17‐related cytokines, as well as the subsequent clinical manifestations were investigated.
Methods
Blood samples/donors
Heparinized blood was obtained from donors with allergy to both peanuts and HDM. Specific sensitization was documented by positive skin prick test responses and detection of allergen‐specific IgE in the sera of donors (ImmunoCAP‐specific IgE blood test; Phadia AB, Uppsala, Sweden). Approval for the study was obtained from the Institutional Review Board of the West China Hospital of Sichuan University. Informed consent was obtained from all subjects before the study.
Mice
NSG mice (aged 6–8 weeks) were housed under specific pathogen‐free conditions in microisolator cages in the animal center of the State Key Laboratory of Biotherapy of China, Sichuan University. All food and water were autoclaved. Only female mice were used, as they are more susceptible to anaphylaxis than male mice [13]. The study protocol was approved by the Animal Care and Use Committee of West China Hospital.
Crude peanut extract (CPE) preparation
Protein extracts from roasted unsalted peanuts (Arachis hypogaea; Hampton Farms, Severn, NC, USA) were prepared according to the method described previously. Briefly, 25 g of ground peanuts were homogenized in 250 ml of 20 mmol/l Tris buffer (pH 7.2), which was centrifuged to remove residual traces of fat and insoluble particles after 2 h at 23°C [14]. Concentrations of protein were detected using Bradford analysis with bovine serum albumin (BSA) as a standard. Different molecular weights of peanut allergen protein in CPE preparations were confirmed using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS‐PAGE).
Reconstitution of mice with peripheral blood mononuclear cells (PBMCs) and allergen challenge
Approximately 3 × 107 blood mononuclear cells isolated from heparinized blood mixed with 100 μg of CPE were administered to each NSG mouse intraperitoneally (i.p.), as previously described [15]. To co‐expose humanized mice to peanut and HDM, mice were first administered once a week at weeks 0–3 with 100 μg of CPE through i.p. injections, followed by intranasal administration of 100 μg HDM (Greer Laboratories, Lenoir, NC, USA) at weeks 4–6. Mice were then challenged at weeks 7–8 by intragastric gavage with 300 μg of CPE, after being fasted for 8 h to maximize absorption of peanut antigen across the gastric mucosa. For single allergen‐exposed controls, one group of humanized mice was exposed to CPE but not to HDM, and the other group was only exposed to HDM; phosphate‐buffered saline (PBS) was used instead of the related allergen. For sham allergen‐exposed controls, both CPE and HDM were replaced by PBS. Four allergic donors were included into the study and PBMCs of one donor were used for reconstitution for one group.
Assessment of mice
Serum levels of human IgG, mouse IgG, total human IgE and total mouse IgE were detected by a commercial enzyme‐linked immunosorbent assay (ELISA) kit (Abcam, Cambridge, MA, USA). Levels of allergen‐specific human IgE were also measured by ELISA (Biopanda, Dundonald, UK), according to the manufacturer’s instructions. Plasma histamine was examined 30 min after challenge and analyzed with an enzyme immunoassay kit (Abnova, Walnut, CA, USA), as described by the manufacturer. Symptoms of anaphylaxis were evaluated with a mouse‐defined anaphylaxis scale 30 min after challenge (Table 1). Three independent investigators performed scoring in a blind manner [15].
Table 1.
Anaphylaxis score a
| Score | Symptom |
|---|---|
| 0 | No signs of shock |
| 1 | Itching/ruffling fur |
| 2 | Puffiness around eyes/mouth |
| Diarrhea, pilar erecti, decreased activity | |
| Decreased respiratory rate | |
| 3 | Wheezing/labored breathing |
| Cyanosis | |
| 4 | No activity (after prodding) |
| 5 | Death |
Severity of the anaphylactic response was scored 30 min after.
challenge using a scoring system from 0 to 5 in all mice.
Airway hyper‐reactivity (AHR) was measured by exposing awake mice to increasing concentrations of aerosolized methacholine via ultrasonic nebulization 24 h after CPE challenge using a whole‐body plethysmograph (Buxco Electronics, Troy, NY, USA) [16]. Pressure fluctuations were quantified by algorithm for PenH (enhanced pause).
After reconstitution and allergen challenge, mice were euthanized with intraperitoneal 3% sodium pentobarbital. The right lung was lavaged three times with 0·5 ml of PBS, with a recovery rate of 95%. The supernatant of bronchoalveolar lavage fluid (BALF) was collected and stored at 80°C for further analyses. The pellet was resuspended in PBS, and cells of different categories were counted using cytospins stained with May–Grunwald–Giemsa by classification of 300 cells using standard morphological criteria [17]. The levels of the human cytokines IL‐4, IL‐5 and IL‐17 in BALF were detected by specific ELISA using Eli‐pairs (Biotest, Buc, France).
The left lung was fixed with 4% formalin, embedded in paraffin and subsequently cut into 4‐µm sections. The sections were stained with hematoxylin and eosin or periodic acid–Schiff. The degree of inflammation and mucus‐producing goblet cells were semiquantified as previously described with a microscope (BX40; Olympus, Hamburg, Germany) [18]. Quantification of mucus‐containing cells was performed by counting the positive cells per mm basement membrane of the bronchi.
Statistical analysis
All data were presented as medians and ranges, unless otherwise stated. Intergroup comparisons were evaluated using Student’s t‐test. For symptom scores, differences among groups were analyzed by Kruskal–Wallis test. The value of P < 0·05 was considered significant.
Results
Prior CPE exposure enhanced immunoglobulin production and Th2/Th17 response
We first investigated whether exposure to CPE via gastrointestinal mucosa primed exposure to HDM via the lung. Dual allergen‐exposed mice were developed using NSG mice reconstituted with allergic PBMCs after both CPE and HDM exposure (Fig. 1a). Neither mouse IgE nor mouse IgG could be detected in NSG mice (data not shown). As expected, NSG mice reconstituted with PBMCs from patients with allergy expressed human IgG after reconstitution (Supporting information, Fig. S1). In contrast, only the mice after reconstitution and CPE exposure expressed peanut‐specific IgE (Fig. 1c), and those after reconstitution and HDM exposure expressed HDM‐specific IgE (Fig. 1d). Moreover, humanized mice exposed to both CPE and HDM had a higher level of total IgE (Fig. 1b) and peanut‐specific IgE (Fig. 1c) compared with single allergen‐exposed mice. Release of HDM‐specific IgE increased significantly in HDM‐only exposed‐mice, while no further increase in HDM‐specific IgE was observed in dual allergen‐exposed mice (Fig. 1d. Cytokine production was analyzed in BALF supernatants. Increasing levels of IL‐4 and IL‐5 were detected in dual allergen‐exposed mice compared to single allergen‐exposed mice (Fig. 2,a,b). Similarly, IL‐17 levels were also higher in dual allergen‐exposed mice compared to controls (Fig. 2c). HDM‐only‐exposed mice showed a similar increasing trend in the level of cytokines compared with CPE‐only‐exposed mice (Fig. 2).
Fig. 1.
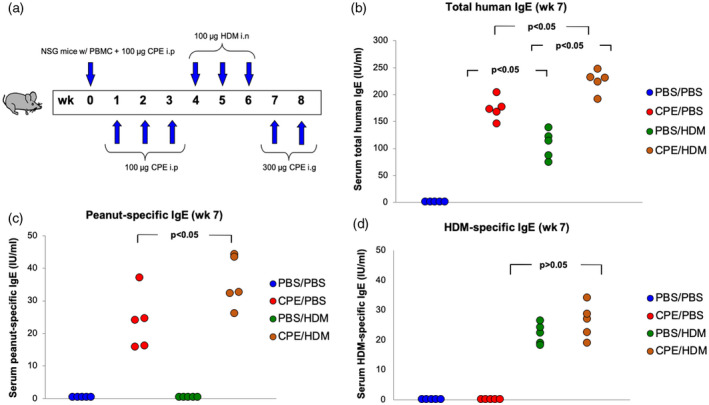
Production of human total and allergen‐specific immunoglobulin (Ig)E in human peripheral blood mononuclear cells (PBMC)‐engrafted non‐obese diabetic/severe combined immunodeficiency (NOD‐SCID) interleukin (IL)‐2Rγnull (NSG) mice. NSG mice were first administrated once weekly at weeks 0–3 with 100 μg of crude peanut extract (CPE) through intraperitoneal injections followed by intranasal administration of 100 μg house dust mite (HDM) at weeks 4–6. Mice were then challenged at weeks 7–8 through intragastric gavage with 300 μg of CPE. For single allergen‐exposed controls, one group of humanized mice were exposed to CPE but not to HDM, and the other group were only exposed to HDM; phosphate‐buffered saline (PBS) was used instead of the related allergen. For sham allergen‐exposed controls, both CPE and HDM were replaced by PBS (a). Human total (b) and allergen‐specific IgE (c,d) were determined. Data from three independent experiments with five mice per group. Each point represents a mean value from three independent experiments.
Fig. 2.
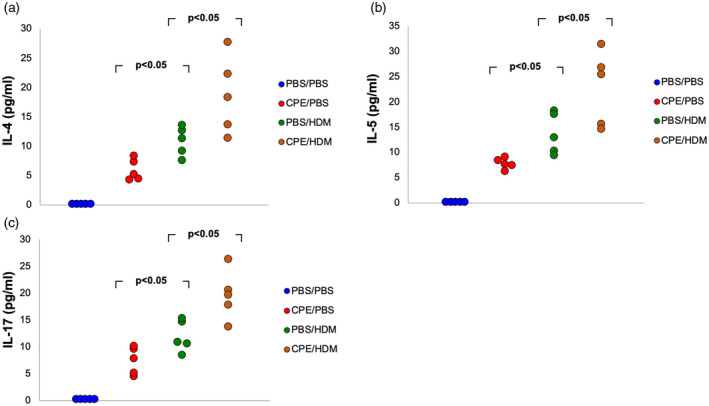
Allergen‐specific cytokine production in bronchoalveolar lavage fluid (BALF) supernatants. Eight weeks after peripheral blood mononuclear cells (PBMC) engraftment, allergen exposure and challenge, production of interleukin (IL)‐4 (a), IL‐5 (b) and IL‐17 (c) in BALF supernatants were analyzed. Data from three independent experiments with five mice per group. Each point represents a mean value from three independent experiments.
Prior CPE exposure promoted the development of lung inflammation and AHR
Either in single allergen‐exposed or dual allergen‐exposed mice, recruitment of neutrophils, eosinophils and lymphocytes to the bronchoalveolar compartment was clearly observed (data not shown), while a significantly higher level of eosinophils and lymphocytes was seen in dual allergen‐exposed mice compared to single allergen‐exposed mice (Fig. 3a,b). A moderate but significant increase of inflammatory cell influx was observed in the lungs of dual allergen‐exposed mice and a slight increase in mucus secretion in lungs was present compared with single allergen‐exposed mice (Fig. 4a, Supporting information, Table S1). To investigate whether both CPE and HDM exposure led to higher level of airway hyper‐reactivity, AHR to methacholine was determined. As expected, dual allergen‐exposed mice displayed significantly greater AHR than mice exposed to only one allergen (Fig. 4b).
Fig. 3.
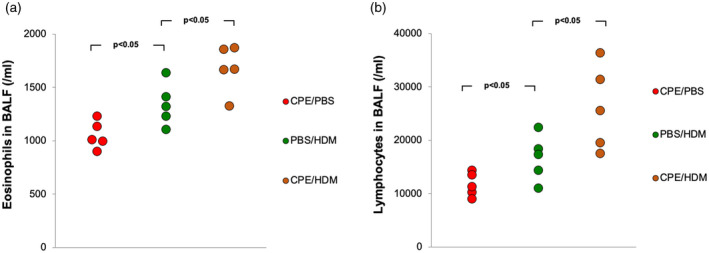
Crude peanut extract (CPE)‐induced food allergy influences pulmonary inflammation in house dust mite (HDM)‐induced asthma model. Eight weeks after peripheral blood mononuclear cells (PBMC) engraftment, allergen exposure and challenge, eosinophils (a) and lymphocytes (b) the count in bronchoalveolar lavage fluid (BALF). Data from three independent experiments with five mice per group. Each point represents a mean value from three independent experiments.
Fig. 4.
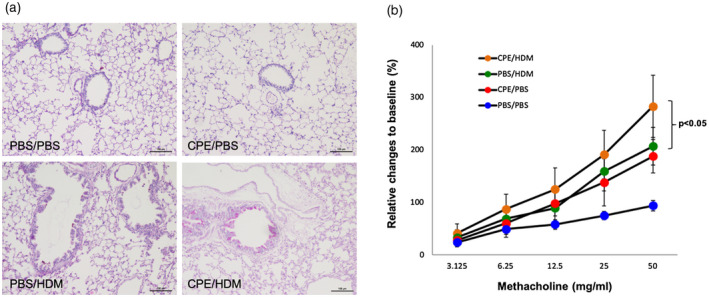
Prior crude peanut extract (CPE) exposure promoted the development of lung inflammation and airway hyper‐reactivity (AHR). Lung slides shown at ×100 were stained with periodic acid–Schiff reagent to determine peribronchial inflammatory infiltrates and mucus production (a). Seven weeks after peripheral blood mononuclear cells (PBMC) engraftment, allergen exposure and challenge, airway resistance in response to methacholine was measured. Shown are means ± standard error of the mean (s.e.m.) of the relative change to baseline value of three independent experiments with at least three mice per group (b).
Prior CPE exposure increased histamine release and severity of systemic anaphylactic reactions
Plasma histamine level was determined as a major mediator of systemic anaphylactic reactions. Dual allergen‐exposed mice had a higher level of histamine compared with single allergen‐exposed mice, while the histamine level in CPE‐only‐exposed mice was slightly increased compared with HDM‐only‐exposed mice (Fig. 5a). Anaphylactic symptom scores were determined 30 min after challenge. As a result, mice reconstituted with mononuclear cells from allergic patients showed itching/ruffling of fur, puffiness around the eyes and snout, pilar erecti, decreased ambulation and respiratory rate after allergen exposure and challenge, indicating a clinical phenotype related to allergic response. Such clinical characteristics were not present in the humanized mice receiving sham allergen exposure. Strikingly, dual allergen‐exposed mice displayed a more severe allergic phenotype compared with single allergen‐exposed mice, as defined by clinical phenotype and a higher anaphylaxis score. However, the anaphylaxis score was significantly lower for HDM‐only‐exposed mice compared with CPE‐only‐exposed mice (Fig. 5b).
Fig. 5.
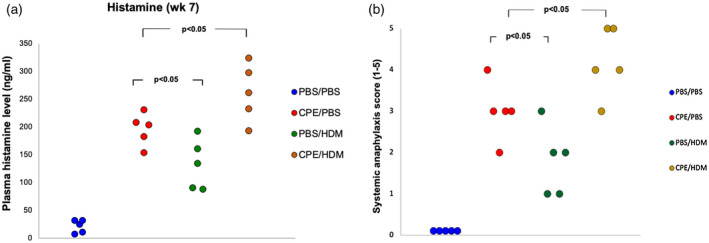
Prior crude peanut extract (CPE) exposure increased histamine release and severity of systemic anaphylactic reactions. Plasma histamine levels were measured 30 min after peanut challenge at week 7 (a). Anaphylaxis score (0–5) was determined 30 min after challenge at week 8 (b). Data from three independent experiments with five mice per group. Each point represents a mean value from three independent experiments. Scoring was performed in a blind manner by three independent investigators.
Discussion
Developing a relevant animal model for high‐risk human subjects of anaphylaxis remains a huge challenge. However, there is a tendency that antigen‐induced anaphylaxis is primarily mediated via IgG in mice, and animals may also have immunological tolerance to ingested antigens. As mice do not express specific human therapeutic targets, and investigations related to novel drugs in humans are limited by ethical constraints [19, 20], development of a humanized murine model is crucial for investigating such novel strategies in vivo. In the present study, a humanized allergen‐sensitive NSG mouse model was established and characterized in which exposure to peanut was followed by exposure to HDM, and a robust allergic response mimicking allergic cascade in human was reproduced. The main hallmarks for both peanut allergy and HDM‐allergic asthma were successfully induced in the same model. Peanut exposure was effective, as reflected by the increase in human total and peanut‐specific IgE in serum [21, 22]. The phenotype of the mouse model was defined with an anaphylaxis score, histamine levels and clinical phenotype [15]. To induce airway allergy, humanized mice were treated to HDM between CPE exposure and challenge. Allergic asthma in mice previously exposed to CPE was more obvious with higher total IgE levels compared to other groups. Furthermore, increased airway hyper‐reactivity to methacholine, eosinophilic and lymphocytic lung inflammation, and increased peribronchial infiltrates were observed in dual allergen‐exposed mice compared to single allergen‐exposed mice. Moreover, dual allergen‐exposed mice had higher levels of Th2 and Th17 cytokines in response to HDM compared to HDM‐only‐exposed mice. In addition, dual allergen‐exposed mice displayed a more severe allergic phenotype when peanut was systemically administrated.
Our study suggests that a primary exposure to one allergen primed the immune system to develop an intense response to an unrelated allergen administered subsequently, implying that the sensitization to multiple allergens observed in atopic subjects may be attributed to a synergic interaction between the immune responses initiated by each allergen, rather than being an independent event occurring in response to multiple exposures on a common genetic background [23, 24]. These results are consistent with epidemiological data showing that food allergy is associated with an increased risk to develop allergic airway disease [25, 26].
It has been demonstrated in previous studies that patients with allergies were characterized by allergen‐specific Th2‐mediated responses, and that resolution of allergy coincided with a shift to a Th1 response [27, 28]. Recent studies have shown that the Th1/Th2 dichotomy in allergy may expand to other T cell effector subsets, including Th17 cells characterized by the hallmark production of IL‐17. In humans, emerging evidence has supported that IL‐17 expression was increasing in moderate to severe asthma [29, 30, 31]. However, it is uncertain whether this up‐regulation is associated with granulocytic airway inflammation. In the present study, preceding peanut exposure increased Th2 and Th17 cytokine production in response to HDM in humanized mice reconstituted with PBMCs from allergic donors, and this translated into aggravated lung inflammation and AHR. Therefore, our data support a potential role of IL‐17 in asthma and food allergy. Future studies on therapeutic strategies targeted on the IL‐17 axis would further define the functional importance of IL‐17 in atopic disorders.
In conclusion, we developed a human PBMC‐engrafted murine model of both peanut allergy and asthma that could be instrumental to further elucidate the mechanisms of interplay between different primary sensitizations, which is also suitable for investigating existing drugs, novel pharmacological and immunological intervention for treatment of allergic diseases. However, the underlying mechanisms remain to be deciphered.
Disclosures
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, including employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Author contributions
B. W., D. L. and J. H. conceived and designed the study. designed the experiments. B. W., J. H. and Y. L. performed the experiments. D. D. L., Q. L. and Y. L. analyzed the data and performed the statistical analysis. D. D. L., Q. L. and J. H. contributed reagents/materials/analysis tools. B. W. and D. D. L. contributed to the writing of the manuscript. All authors read and approved the final manuscript. All authors contributed to subjects’ recruitment, data collection, statistical analyses, paper drafting, critically revising and gave final approval of the version to be published.
Supporting information
Fig. S1. Production of human total IgG in NOD‐scid IL2Rgammanull mice after reconstitution with PBMCs. Shown are means ± SEMs of 3 independent experiments with 5 mice per group.
Table S1. The positive cells per mm basement membrane of the bronchi (means ± SEMs).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81701586 and 81800042). The funders had no role in study design, data collection or analyses, decision to publish and manuscript preparation.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Fanta CH. Asthma. N Engl J Med 2009; 360:1002–14. [DOI] [PubMed] [Google Scholar]
- 2. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy 2014; 69:17–27. [DOI] [PubMed] [Google Scholar]
- 3. Bohle B. T lymphocytes and food allergy. Mol Nutr Food Res 2004; 48:424–33. [DOI] [PubMed] [Google Scholar]
- 4. Malmberg LP, Saarinen KM, Pelkonen AS, Savilahti E, Mäkelä MJ. Cow’s milk allergy as a predictor of bronchial hyperresponsiveness and airway inflammation at school age. Clin Exp Allergy 2010; 40:1491–7. [DOI] [PubMed] [Google Scholar]
- 5. Liu AH, Jaramillo R, Sicherer SH et al National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 2010; 126:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simons FE, Ardusso LR, Bilo MB et al World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J 2011; 4:13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J, Visness CM, Sampson HA. Food allergen sensitization in inner‐city children with asthma. J Allergy Clin Immunol 2005; 115:1076–80. [DOI] [PubMed] [Google Scholar]
- 8. Koplin JJ, Martin PE, Allen KJ. An update on epidemiology of anaphylaxis in children and adults. Curr Opin Allergy Clin Immunol 2011; 11:492–6. [DOI] [PubMed] [Google Scholar]
- 9. Hammad H, Plantinga M, Deswarte K et al Inflammatory dendritic cells – not basophils – are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 2010; 207:2097–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tourdot S, Airouche S, Berjont N et al Evaluation of therapeutic sublingual vaccines in a murine model of chronic house dust mite allergic airway inflammation. Clin Exp Allergy 2011; 41:1784–92. [DOI] [PubMed] [Google Scholar]
- 11. Van Gramberg JL, de Veer MJ, O’Hehir RE, Meeusen EN, Bischof RJ. Use of animal models to investigate major allergens associated with food allergy. J Allergy 2013; 2013:635695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Heijden J, Geissler J, van Mirre E et al A novel splice variant of FcgammaRIIa: a risk factor for anaphylaxis in patients with hypogammaglobulinemia. J Allergy Clin Immunol 2013; 131:1408–16. [DOI] [PubMed] [Google Scholar]
- 13. Hox V, Desai A, Bandara G, Gilfillan AM, Metcalfe DD, Olivera A. Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J Allergy Clin Immunol 2015; 135:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koppelman SJ, Hefle SL, Taylor SL, de Jong GA. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: a comparative in vitro study and partial characterization of digestion‐resistant peptides. Mol Nutr Food Res 2010; 54:1711–21. [DOI] [PubMed] [Google Scholar]
- 15. Pagovich OE, Wang B, Chiuchiolo MJ et al Anti‐hIgE gene therapy of peanut‐induced anaphylaxis in a humanized murine model of peanut allergy. J Allergy Clin Immunol 2016; 138:1652–62. [DOI] [PubMed] [Google Scholar]
- 16. Bellinghausen I, Reuter S, Martin H et al Enhanced production of CCL18 by tolerogenic dendritic cells is associated with inhibition of allergic airway reactivity. J Allergy Clin Immunol 2012; 130:1384–93. [DOI] [PubMed] [Google Scholar]
- 17. van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods 2004; 288:111–21. [DOI] [PubMed] [Google Scholar]
- 18. Utsch L, Logiantara A, van Ree R, van Rijt LS. Experimental food allergy to peanut enhances the immune response to house dust mite in the airways of mice. Clin Exp Allergy 2017; 47:121–8. [DOI] [PubMed] [Google Scholar]
- 19. Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol 2007; 7:118–30. [DOI] [PubMed] [Google Scholar]
- 20. Van Gramberg JL, de Veer MJ, O’Hehir RE, Meeusen EN, Bischof RJ. Use of animal models to investigate major allergens associated with food allergy. J Allergy 2013; 2013:635695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol 2014; 14:247–59. [DOI] [PubMed] [Google Scholar]
- 22. Weigmann B, Schughart N, Wiebe C et al Allergen‐induced IgE‐dependent gut inflammation in a human PBMC‐engrafted murine model of allergy. J Allergy Clin Immunol 2012; 129:1126–35. [DOI] [PubMed] [Google Scholar]
- 23. Di Genova G, Savelyeva N, Suchacki A, Thirdborough SM, Stevenson FK. Bystander stimulation of activated CD4+ T cells of unrelated specificity following a booster vaccination with tetanus toxoid. Eur J Immunol 2010; 40:976–85. [DOI] [PubMed] [Google Scholar]
- 24. Bangs SC, Baban D, Cattan HJ, Li CK, McMichael AJ, Xu XN. Human CD4+ memory T cells are preferential targets for bystander activation and apoptosis. J Immunol 2009; 182:1962–71. [DOI] [PubMed] [Google Scholar]
- 25. Tariq SM, Matthews SM, Hakim EA, Arshad SH. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr Allergy Immunol 2000; 11:162–7. [DOI] [PubMed] [Google Scholar]
- 26. Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol 2001; 108:720–5. [DOI] [PubMed] [Google Scholar]
- 27. Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin‐10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol 1996; 97:1288–96. [DOI] [PubMed] [Google Scholar]
- 28. Rissoan MC, Soumelis V, Kadowaki N et al Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999; 283:1183–6. [DOI] [PubMed] [Google Scholar]
- 29. Lv H, Bing L, Qian X‐J, Huang J‐A, Qiu T‐F. Serum IL‐17 & eotaxin levels in asthmatic patients with allergic rhinitis. Pak J Med Sci 2016; 32:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh M, Agarwal A, Chatterjee B, Chauhan A, Das R, Paul N. Correlation of cutaneous sensitivity and cytokine response in children with asthma. Lung India 2017; 34:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA 2007; 104:15817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Production of human total IgG in NOD‐scid IL2Rgammanull mice after reconstitution with PBMCs. Shown are means ± SEMs of 3 independent experiments with 5 mice per group.
Table S1. The positive cells per mm basement membrane of the bronchi (means ± SEMs).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


