The current study suggests that a high pretreatment neutrophil to lymphocyte ratio and presence of low muscle mass (sarcopenia) are potential risk factors for the development of hyperprogressive disease and shorter survival upon treatment with Pembrolizumab.

Keywords: hyperprogressive disease, neutrophil : lymphocyte ratio, sarcopenia
Summary
The aim of this multi‐center retrospective study was to evaluate the incidence of hyperprogressive disease (HPD) after second‐line treatment with pembrolizumab in patients (n = 167) with metastatic non‐small‐cell lung cancer (NSCLC) whose tumors expressed programmed cell death ligand 1 (PD‐L1) in ≥ 1% and to search for hematological and imaging biomarkers associated with its development. Prior to chemotherapy, neutrophil : lymphocyte ratio (NLR1) and platelet : lymphocyte ratio (PLR1), and prior to immunotherapy, NLR2 and PLR2 were retrospectively analyzed. The psoas major muscle area (PMMA) was calculated at the L3 position on computed tomography before chemotherapy (PMMA1) and before immunotherapy (PMMA2) (n = 112). Patients with ∆PMMA (1‐PMMA2/PMMA1) × 100 ≥ 10% were considered to have sarcopenia (low muscle mass). After treatment with pembrolizumab on the first computerized tomography (CT) scan evaluation, patients were subdivided as follows as: hyperprogressors (HPs), progressors (Ps), non‐progressors (NPs) and pseudoprogressors (PPs). HPs had significantly higher ∆PMMA levels, NLR2 and PLR2 than the other patients. Moreover, in multinomial logistic regression analysis, higher levels of ∆PMMA were associated with a decreased likelihood of being a P [odds ratio (OR) = 0·81; 95% confidence interval (CI) = 0·65–0·99; P = 0·047] or an NP (OR = 0·76; 95% CI = 0·62–0·94; P = 0·012) versus an HP. Higher NLRs tended to decrease the likelihood of being a P versus an HP (OR = 0·66; 95% CI = 0·42–1·06; P = 0·09) and significantly decreased the likelihood of being an NP versus an HP (OR = 0·44; 95% CI = 0·28–0·69; P < 0·0001). Our data suggest that a high pre‐immunotherapy NLR2 and the presence of sarcopenia are potential risk factors for the development of HPD.
Introduction
Lung cancer is the leading cause of cancer death worldwide [1], with non‐small‐cell lung cancer (NSCLC) accounting for approximately 85% of lung cancers. Recently, immunotherapy has presented a breakthrough in oncology, especially due to its promise to treat a broad range of advanced cancer types, including NSCLC [2]. Human immune checkpoint inhibitor antibodies inhibit the programmed cell death (PD‐1) receptor or its ligand PD‐L1 and thus restore an efficient anti‐tumor T cell response.
Despite advances in the therapeutic landscape of advanced NSCLC without targetable oncogenic driver alterations, a minority of patients benefit from immunotherapy as a single agent, whereas the vast majority are inevitably candidates for chemotherapy [3, 4]. In the Phase II/III KEYNOTE‐010 study, pembrolizumab significantly prolonged overall survival (OS) over docetaxel as a second‐line therapy in advanced NSCLC [5]. Despite these advances in treatment and the increased knowledge of the molecular pathways, there are still challenges in the identification of patients who are most likely to benefit and those who will not [6, 7]. Unfortunately, there are no baseline clinical or tumor characteristics that can clearly discern patients who may benefit from immune checkpoint inhibitors (ICIs), and the minority of patients experience durable responses and benefits from immunotherapy [8, 9, 10].
Over‐expression of PD‐L1 is an important and widely explored predictive biomarker for the response to anti‐PD‐1/PD‐L1 antibodies [11]. Previous studies have demonstrated that the tumor microenvironment, involving neutrophils, platelets, macrophages and regulatory T cells, plays an essential regulatory role in cancer progression, metastasis and outcome [12, 13]. The neutrophil : lymphocyte ratio (NLR) and platelet : lymphocyte ratio (PLR), which are easily and commonly measured in clinical practice, were shown to be strong prognostic markers associated with worse OS in several tumor types, including NSCLC, in the pre‐immunotherapy era [14]. Similar findings suggest that high NLR and PLR may predict a poor response to ICIs and poor outcome in patients with NSCLC [15, 16, 17]. Body mass index (calculated as weight in kilograms divided by height in meters squared) is the most common measure of body size in cancer patients, and its associations with survival have shown controversial results. This is thought to be due to the inability of this method to discriminate between muscle and adipose tissue, which have different impacts on survival [18]. Thus, measurement of sarcopenia (low muscle mass) via computed tomography (CT) scan at the L3 position was introduced in the clinic. Recent studies proposed that the development of sarcopenia, measured by the change in the psoas major muscle area (PMMA) at the L3 position, is a negative indicator of ICI response [19, 20]. In addition to poor responses, immunotherapy was also associated with rapid disease progression, i.e. hyperprogressive disease (HPD), in patients with different types of cancer, such as recurrent and metastatic head and neck cancer, renal cell cancer, melanoma and lung cancer [21], with incidences ranging between 4 and 29% [22]. Unfortunately, there are no current biomarkers that predict the development of this life‐threatening condition. To complicate this further, in some patients treated with ICIs, enlarged tumor size or the appearance of a new lesion followed by tumor regression without clinical deterioration has been observed. This phenomenon is described as pseudoprogression, and histopathological biopsies have shown that lymphocyte infiltration is the main cause [23].
The purpose of this retrospective study was to evaluate the incidence of HPD after treatment with pembrolizumab as a second‐line treatment in metastatic NSCLC patients and to search for indicators that are associated with the development of HPD.
Materials and methods
Patient selection
In this retrospective cohort study, we reviewed the cases of 167 patients from five centers in Bulgaria with metastatic NSCLC treated with pembrolizumab after progression upon first‐line platinum‐based chemotherapy between April 2017 and February 2020. The procedure was approved by the scientific research ethics committee at the Hospital ‘Nadezhda’ in Sofia. The eligibility criteria were as follows: (1) age ≥ 18 years, (2) histologically confirmed diagnosis of NSCLC in the metastatic stage, (3) wild‐type epidermal growth factor receptor/anaplastic lymphoma kinase, (4) Eastern Cooperative Oncology Group–performance status (ECOG‐PS) < 2, (5) disease progression after receiving one prior platinum‐based systemic therapy for metastatic disease with measurable disease lesions, (6) available blood cell count and blood samples and (7) available CT scans. Immunotherapy was administered at least 3 weeks after the previous treatment. Patients were excluded if they had brain metastases (as corticosteroid use may compromise therapy), autoimmune disease, symptomatic interstitial lung disease, systemic immunosuppression or prior treatment with immune‐stimulatory anti‐tumor agents, including checkpoint inhibitors. Patients did not show any clinical or computed tomography signs of active infection. Tumor PD‐L1 status was assessed. Pembrolizumab was initially administered via 2 mg/kg intravenous (i.v.) injection over 60 min every 3 weeks and later via 200 mg i.v. injection (flat dose) every 3 weeks. No formal consent was required for this type of study.
Data collection
Data collected included demographics, PD‐L1 status, metastatic sites, description of first‐line treatment, date of progression as determined by radiology reports and date of death or last follow‐up. Peripheral blood samples were collected from patients included into the study on the day of first‐line chemotherapy administration at baseline and the day of first immunotherapy infusion. Of interest were the following hematological and biochemistry parameters: absolute neutrophil count (ANC), absolute lymphocyte count (ALC) and absolute platelet count (APC), which enable calculation of the NLR (ANC/ALC) and PLR (APC/ALC). NLR1 and PLR1 were calculated before the first cycle of chemotherapy, and NLR2 and PLR2 were calculated before the first pembrolizumab infusion. ΔNLR (NLR2–NLR1) and ΔPLR (PLR2–PLR1) were calculated. An NLR > 5 was considered high, in accordance with earlier reports [24, 25, 26]. The median value (174) of the PLR was used to group cases into two categories of low (≤ median) and high (> median) PLRs.
Tumor PD‐L1 protein expression was analyzed by partial or complete circumferential immunohistochemical staining of tumor cells in archived biopsy samples of tumors, and the cut‐off for positivity was 1%. The Dako/Agilent 22c3 assay was used as a diagnostic tool to evaluate PD‐L1 status. PD‐L1 was estimated as the percentage of positive PD‐L1 tumor cells over total tumor cells in the sample [27]. In addition, we subdivided the positive group into expression categories: expression in ≥ 50%, expression in 25–49% and expression in 1–24%.
Measurement of PMMA
The PMMA was calculated at the L3 position by computed tomography, and was calculated before chemotherapy and before pembrolizumab infusion. We were able to measure ∆PMMA in only 112 patients of the whole cohort, as the rest were staged only with a CT scan of the thorax and upper abdomen, and the area of the patient’s psoas major muscle at the L3 position was not available. The PMMA was measured in the region of interest by tracing an outline using the image viewer software dicom. The following formula was used: % change of PMMA = (1 – PMMA before pembrolizumab/PMMA before chemotherapy) × 100 (Fig. 1).
Fig. 1.

Calculation of the rate of change of the psoas major muscle area (PMMA). The total PMMA was calculated as the sum of both right and left PMMA at the L3 position. In the case presented before chemotherapy, the left and right PMMA were 5·74 and 7·16 cm2, respectively; before immunotherapy, the left and right PMMA were 4·98 and 6·12 cm2, respectively. The change rate of PMMA was calculated as follows: (1 – 11·1/12·9) × 100 = 13·9%.
Patients with a change in PMMA ≥ 10% were considered to have sarcopenia [19].
End‐points
The tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (recist version 1.1), and clinical tumor response was assessed every 8–12 weeks or at clinical deterioration. Hyperprogression was defined if at least three of the following existed: (1) time to treatment failure < 3 months; (2) increase ≥ 50% in the sum of target lesion major diameters between baseline and first radiological evaluation; (3) appearance of at least two new lesions in an organ already involved between baseline and first radiological evaluation; (4) spread of the disease to a new organ between baseline and first radiological evaluation; and (5) clinical deterioration with ECOG PS ≥ 2 during the first 3 months of treatment. Criteria 1 and 5 were mandatory. Pseudoprogression was defined as initial progression followed either by partial response or stable disease lasting at least 6 months. OS was defined as the interval between diagnosis of the disease and death or the date of the last follow‐up evaluation.
Statistical design and analysis
Data were managed and analyzed using spss software version 23. The demographic characteristics were expressed as frequencies and percentages for categorical variables and as medians and means with standard deviations for quantitative variables. The Mann–Whitney U‐test, Spearman’s correlation and χ2 tests were used to compare and evaluate the correlations between the biomarkers and the clinicopathological characteristics of the patients, such as age, sex, NLR and PLR. To assess the correlations between test results, rho values were interpreted as follows: < 0·39, weak correlation; 0·40–0·59, moderate correlation; 0·60–0·79, strong correlation; and ≥ 0·80, very strong correlation. The Kruskal–Wallis one‐way analysis of variance (anova) was used to compare the levels of hematological biomarkers, ΔPMMA and response to pembrolizumab at the first CT scan. The Wilcoxon and McNemar tests were used to compare quantitative and categorical biomarker values and their derivations. The diagnostic accuracy of biomarkers was determined by obtaining the largest possible area under the curve (AUC) in receiver operating characteristic curve (ROC) analysis. AUC values ≥ 0·9 were considered ‘excellent’, ≥ 0·80 were considered ‘good’, ≥ 0·7 were considered ‘fair’ and < 0·70 were considered ‘poor’. Survival curves according to the response on the first CT scan were estimated using the Kaplan–Meier method, and differences were assessed using the log‐rank test. We also performed multinomial logistic regression to estimate the effects of hematological biomarkers and ∆PMMA on the response to treatment. Two‐tailed P‐values < 0·05 were considered significant.
Results
Baseline characteristics
This study included 167 patients who received anti‐PD‐1 treatment with pembrolizumab after failure of first‐line chemotherapy. The clinical characteristics of the patients and relationships with response on the first CT scan are summarized in Table 1. The mean age was 60·2 ± 6·8 years; most of the patients were men (64%), and almost all patients exhibited an ECOG PS of 1 (98%). The lung was the most common metastatic site (73%), followed by pleural effusion (59%) and bone (40%). All patients were eligible for the examination of tumor PD‐L1 expression, of which 13 patients (7·8%) had expression in more than 50%, 80 patients (47·9%) had expression in 25–49% and 74 patients (44·3%) had expression in 1–24%. Of all the clinicopathological characteristics of the patients, only NLR2, PLR2 and the presence of sarcopenia were significantly related to the response on the first CT scan (Table 1). The median value of NLR2 was 4·8.
Table 1.
Relationship between baseline clinicopathological characteristics of patients and response on the first computed tomography scan – hyperprogressors (HPs), progressors (Ps) and non‐progressors (NPs)
| HPs | Ps | NPs | P‐value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 8 (7·5%) | 16 (15%) | 83 (77·6%) | 0·35 |
| Female | 8 (14·5%) | 8 (14·5%) | 39 (70·9%) | |
| ECOG PS | 0·70 | |||
| 0 | 0 (0%) | 1 (25%) | 3 (75%) | |
| 1 | 16 (10·1%) | 23 (14·6%) | 119 (75·3%) | |
| Lung metastasis | 0·97 | |||
| No | 4 (10·8%) | 5 (13·5%) | 28 (77·7%) | |
| Yes | 12 (9·8%) | 18 (14·8%) | 92 (75·4%) | |
| Liver metastasis | 0·35 | |||
| No | 10 (8·4%) | 16 (13·4%) | 93 (78·2%) | |
| Yes | 6 (15%) | 7 (17·5%) | 27 (67·5%) | |
| Pleural effusion | 0·85 | |||
| No | 5 (8·3%) | 9 (15%) | 46 (76·7%) | |
| Yes | 11 (11·1%) | 14 (14·1%) | 74(74·7%) | |
| Bone metastasis | 0·92 | |||
| No | 10 (10·9%) | 13 (14·1%) | 69 (75%) | |
| Yes | 6 (9%) | 10 (14·9%) | 51 (76·1) | |
| Number of metastatic lesions | 0·45 | |||
| < 2 | 7 (7·5%) | 14 (15·1%) | 72 (77·4%) | |
| ≥ 2 | 9 (13·8%) | 9 (13·8%) | 48 (72·7%) | |
| PD‐L1 expression | 0·71 | |||
| 1–24% | 8 (11·6%) | 12 (17·4%) | 49 (71%) | |
| 25–49% | 6 (7·5%) | 10 (12·5%) | 64 (80%) | |
| > 50% | 2 (15·4%) | 2 (15·4%) | 9 (69·2%) | |
| NLR2* | <0·0001 | |||
| ≤ 5 | 1 (1·2%) | 10 (11·8%) | 74 (87·1%) | |
| > 5 | 15 (19·5%) | 14 (18·2%) | 48 (62·3%) | |
| PLR2** | 0·004 | |||
| ≤ Median | 3 (3·6%) | 9 (10·8%) | 71 (85·5%) | |
| > Median | 13 (16·5%) | 15 (19%) | 51 (64·6%) | |
| Age (years) | 0·71 | |||
| < 65 | 9 (8·5%) | 16 (15·1%) | 81 (76·4%) | |
| ≥ 65 | 7 (12·5%) | 8 (14·3%) | 41 (73·2%) | |
| Sarcopenia*** | <0·0001 | |||
| No | 1 (1·3%) | 11 (14·3%) | 65 (84·4%) | |
| Yes | 15 (50%) | 7 (23·3%) | 8 (26·7%) |
Neutrophil : lymphocyte ratio 2 (NLR2) (before pembrolizumab infusion);
platelet : lymphocyte ratio 2 (before pembrolizumab infusion) (PLR2);
low muscle mass, measured by the change in the psoas major muscle area≥ 10% at the L3 position (before chemotherapy and before pembrolizumab infusion).
ECOG PS = Eastern Cooperative Oncology Group Performance Status; HP = hyperprogressor; NP = non‐progressor; P = progressor; PD‐L1 = programmed cell death 1.
Hematological biomarkers and their relationship to response on the first CT scan
On the first CT scan after chemotherapy treatment (four cycles), 15 (8·9%) patients showed progressive disease. After treatment with pembrolizumab on the first CT scan evaluation (8–12 weeks), these 15 patients were subdivided as follows: eight hyperprogressors (HPs), one pseudoprogressor (PP) and six non‐progressors (NPs). These 15 patients had significantly higher NLR1 and PLR1 than patients without progressive disease (7·49 ± 2·8 versus 4·31 ± 2·45; 283·3 ± 96·5 versus 207 ± 102·6, respectively). Twelve patients had an NLR > 5, and at least nine (∆PMMA was not available for three) had ∆PMMA ≥ 10%.
On the first CT scan after immunotherapy treatment, 45 (26·9%) patients showed progressive disease and at least 25 (∆PMMA was not available for seven) had ∆PMMA ≥ 10%. Of these, 16 patients (9·6%) were classified as HPs, five (2·9%) were classified as PPs and the remaining 24 (14%) were classified as Ps. Patients with pseudoprogression did not show clinical deterioration and received further treatment with immunotherapy for another 8 weeks, when the control CT scan showed a partial response for three patients and stable disease for two patients; the response lasted for at least 6 months. Of all HPs, 15 (93%) had an NLR > 5. HPs had higher mean values of NLR2, PLR2 and ∆NLR, but not higher ∆PLR values, than Ps or NPs (Table 2). There was no significant difference in hematological parameters between HPs and PPs, Ps and NPs, Ps and PPs or NPs and PPs, except for in NLR2, for which NPs had significantly lower values than PPs (Table 2).
Table 2.
Comparison between the neutrophil : lymphocyte ratio (NLR), platelet : lymphocyte ratio (PLR), their derivations and the response on the first computed tomography scan: hyperprogressors (HPs), pseudoprogressors (PPs), progressors (Ps) and non‐progressors (NPs); means with standard deviations are shown. Adjusted P‐values were used
| NLR2 | P | PLR2 | P | ∆NLR | P | ∆PLR | P | |
|---|---|---|---|---|---|---|---|---|
| HPs versus Ps | 9·01 ± 2·66 versus 5·31 ± 3·26 | 0·006 | 368·29 ± 193·8 versus 218·53 ± 108·01 | 0·044 | 1·29 ± 3·02 versus 0·09 ± 2·3 | 0·04 | 65·67 ± 185·31 versus 24·15 ± 99·82 | 0·44 |
| HPs versus PPs | 9·01 ± 2·66 versus 8·39 ± 1·32 | 0·91 | 368·29 ± 193·8 versus 303·09 ± 115·69 | 0·72 | 1·29 ± 3·02 versus 2·28 ± 1·5 | 0·37 | 65·67 ± 185·31 versus 17·53 ± 59·75 | 0·78 |
| HPs versus NPs | 9·01 ± 2·66 versus 4·35 ± 3·01 | <0·0001 | 368·29 ± 193·8 versus 192·67 ± 109·34 | <0·0001 | 1·29 ± 3·02 versus 0·45 ± 2·01 | 0·017 | 65·67 ± 185·31 versus ‐9·99 ± 85·88 | <0·0001 |
| Ps versus NPs | 5·31 ± 3·26 versus 4·35 ± 3·01 | 0·29 | 218·53 ± 108·01 versus 192·67 ± 109·34 | 0·36 | 0·09 ± 2·3 versus 0·45 ± 2·01 | 0·28 | 24·15 ± 99·82 versus ‐9·99 ± 85·88 | 0·36 |
| Ps versus PPs | 5·31 ± 3·26 versus 8·39 ± 1·32 | 0·25 | 218·53 ± 108·01 versus 303·09 ± 115·69 | 0·97 | 0·09 ± 2·3 versus 2·28 ± 1·5 | 0·041 | 24·15 ± 99·82 versus 17·53 ± 59·75 | 0·98 |
| NPs versus PPs | 4·35 ± 3·01 versus 8·39 ± 1·32 | 0·040 | 192·67 ± 109·34 versus 303·09 ± 115·69 | 0·31 | 0·45 ± 2·01 versus 2·28 ± 1·5 | 0·10 | ‐9·99 ± 85·88 versus 17·53 ± 59·75 | 0·30 |
ROC analysis was performed to explore the potential predictive roles of NLR2, PLR2, ΔNLR and ΔPLR as non‐invasive biomarkers for the discrimination between patients with or without HPD (Table 3). At the optimal cut‐off values for NLR2, the biomarker could significantly and easily distinguish between patients with or without HPD [AUC = 0·85, 95% confidence interval (CI) = 0·75–0·95, P < 0·001], with a sensitivity of 87·5% and a specificity of 68·9%. PLR2 also allowed significant but fair discrimination between patients with and without HPD (AUC = 0·79, 95% CI = 0·66–0·92, P < 0·001), with a sensitivity of 75·0% and a specificity of 64·1% (Fig. 2a,b). ΔNLR could also discriminate between patients with and without HPD, but did so poorly (Table 3). The Wilcoxon test showed that the ALC and APC did not change significantly following chemotherapy. Nevertheless, the ANC significantly differed between the first cycle of chemotherapy and the first pembrolizumab infusion. The McNemar test showed that the proportion of patients with an NLR > 5 and a high PLR did not change significantly with chemotherapy treatment.
Table 3.
Receiver operating curve (ROC) curve analysis was performed using the neutrophil : lymphocyte ratio (NLR), platelet : lymphocyte ratio (PLR) and their derivations to differentiate between patients with and without hyperprogressive disease; the diagnostic accuracy of biomarkers was determined by obtaining the largest possible area under the curve (AUC) in ROC analysis
| Biomarker | AUC 95% CI | P‐value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| NLR2 | 0·85 (0·75–0·95) | < 0·001 | 87·5 | 68·9 |
| PLR2 | 0·79 (0·66–0·92) | < 0·001 | 75·0 | 64·1 |
| ΔNLR | 0·68 (0·54–0·83) | 0·016 | 62·5 | 61·3 |
| ΔPLR | 0·62 (0·44–0·79) | 0·13 | 56·3 | 60·7 |
Fig. 2.
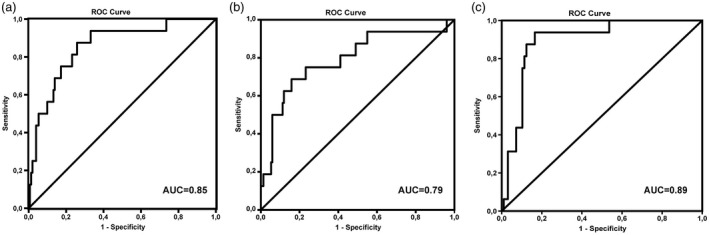
Receiver operating curve (ROC) curve analysis using the neutrophil : lymphocyte ratio (NLR), the platelet : lymphocyte ratio (PLR) and psoas major muscle area (∆PMMA) to differentiate between patients with and without hyperprogressive disease. The diagnostic accuracy of biomarkers was determined by obtaining the largest possible area under the curve (AUC) in ROC analysis. (a) NLR2 AUC = 0·85; (b) PLR2 AUC = 0·79; (c) ∆PMMA AUC = 0·89.
A significantly strong correlation was detected between NLR1 and PLR1 (rho = 0·763) and NLR2 and PLR2 (rho = 0·785), and a moderate correlation was detected between ΔNLR and ΔPLR (rho = 0·465).
Sarcopenia and its relationship to response on the first CT scan
Thirty‐four patients developed sarcopenia during chemotherapy treatment (30·3%). After treatment with pembrolizumab following the first CT scan evaluation, these patients were subdivided as follows: 15 were HPs, four were PPs, seven were Ps and eight were NPs. There was a significant relationship between the presence of sarcopenia and the response on the first CT scan (Table 1).
The Kruskal–Wallis anova showed that there were significant differences in ∆PMMA only between HPs and Ps (16·2 ± 4·8 versus 8·3 ± 8·1, P = 0·009) and NPs and HPs (5·8 ± 13·8 versus 16·2 ± 4·8, P < 0·0001). The Mann–Whitney U‐test showed that patients with sarcopenia had significantly higher NLR2 and PLR2 values than patients without sarcopenia (7·9 ± 3·2 versus 3·6 ± 2·3 and 315·9 ± 157·9 versus 168·7 ± 93·8, respectively; both P < 0·0001). ROC analysis revealed that the cut‐off value of ΔPMMA ≥ 10% could distinguish between patients with or without HPD, with an AUC = 0·89 (95% CI = 0·82–0·96, P < 0·0001), a sensitivity of 93·8% and a specificity of 79·2% (Fig. 1c). After adjustment for age, sex, PD‐L1 expression, number of metastatic sites, NLR, PLR and their derivations, higher levels of ∆PMMA were associated with a decreased likelihood of being a P versus an HP [odds ratio (OR) = 0.81, 95% CI = 0.65–0.99, P = 0.047] and being an NP versus an HP (OR = 0.76, 95% CI = 0.62–0.94, P = 0.012).
A significant but weak correlation was detected between ∆PMMA and NLR2 (rho = 0·365), ∆PMMA and PLR2 (rho = 0·279), and ∆PMMA and age (rho = 0·292).
Association between hematological biomarkers and response on the first CT scan
In univariate analysis, higher levels of NLR2 and PLR2 were associated with a decreased likelihood of being a P versus an HP (OR = 0·67, 95% CI = 0·53–0·86, P = 0·001, OR = 0·993; 95% CI = 0·98–0·99, P = 0·006, respectively). On univariate analysis, higher levels of NLR2, PLR2 and ∆PLR were associated with a decreased likelihood of being an NP versus an HP (OR = 0·61, 95% CI = 0·48–0·76, P < 0·0001; OR = 0·991, 95% CI = 0·98–0·99, P < 0·0001; OR = 0·994, 95% CI = 0·98–0·99, P = 0·008, respectively). After adjustment for age, sex, PD‐L1 expression and number of metastatic sites, higher levels of NLR2 were associated with a decreased likelihood of being an NP versus an HP (OR = 0·44, 95% CI = 0·28–0·69, P < 0·0001) and showed a trend for being associated with a decreased likelihood of being a P versus an HP (OR = 0·66, 95% CI = 0·42–1·06, P = 0·09) (Table 4).
Table 4.
Associations between hematological biomarkers and response to pembrolizumab on the first computed tomography scan: hyperprogressors (HPs), progressors (Ps) and non‐progressors (NPs)
| Multinomial outcome | Predictor | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Ps versus HPs | NLR2 | 0·67 | 0·53–0·86 | 0·001 | 0·66 | 0·42–1·06 | 0·09 |
| NPs versus HPs | NLR2 | 0·61 | 0·48–0·76 | < 0·0001 | 0·44 | 0·28–0·69 | < 0·0001 |
| Ps versus HPs | PLR2 | 0·993 | 0·98–0·99 | 0·006 | 0·992 | 0·98–1·002 | 0·12 |
| NPs versus HPs | PLR2 | 0·991 | 0·98–0·99 | < 0·0001 | 0·999 | 0·99–1·008 | 0·82 |
| Ps versus HPs | ∆NLR | 0·74 | 0·54–1·02 | 0·066 | 1·10 | 0·67–1·79 | 0·69 |
| NPs versus HPs | ∆NLR | 0·79 | 0·61–1·02 | 0·070 | 0·69 | 0·38–1·24 | 0·22 |
| Ps versus HPs | ∆PLR | 0·997 | 0·99–1·002 | 0·29 | 1·005 | 0·99–1·015 | 0·36 |
| NPs versus HPs | ∆PLR | 0·994 | 0·98–0·99 | 0·008 | 0·994 | 0·98–1·003 | 0·16 |
Adjusted for age, sex, PD‐L1 expression and number of metastatic sites.
NLR = neutrophil : lymphocyte ratio; PLR = platelet : lymphocyte ratio; CI = confidence interval; OR = odds ratio; PD‐L1 = programmed cell death 1.
Overall survival of HPs, PPs, Ps, NPs and patients with sarcopenia
HPs at the first CT evaluation had a significantly shorter mean OS (9·83 months, 95% CI = 8·44–11·22) than PPs (19·18 months, 95% CI = 14·13–24·22) (log‐rank test P = 0·001); Ps (17·32 months, 95% CI = 15·67–18·98) (log‐rank test P < 0·001); and NPs (29·79 months, 95%, CI 26·87–32·71) (log‐rank test P < 0·001) (Fig. 3a). Patients with sarcopenia had a significantly shorter mean OS (13·5 months, 95% CI = 11·7–15·2) than patients without sarcopenia (31·5 months, 95% CI = 27·6–35·8) (log‐rank test P < 0·001) (Fig. 3b).
Fig. 3.
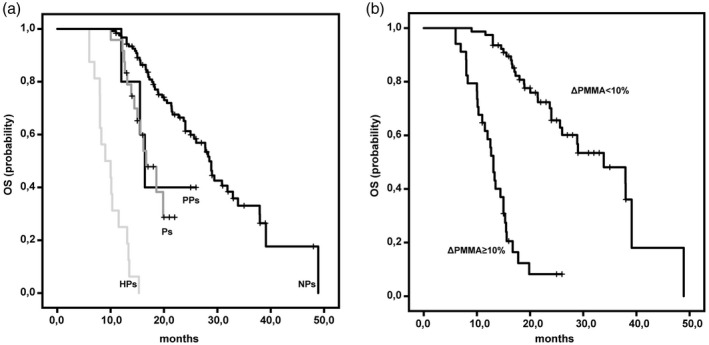
Kaplan–Meier estimates of overall survival (OS) in hyperprogressors (HPs), pseudoprogressors (PPs), progressors (Ps), non‐progressors (NPs) and patients with sarcopenia. (a) HPs had a significantly shorter mean OS [9·83 months; 95%, confidence interval (CI) = 8·44–11·22] than PPs (19·18 months; 95%, CI = 14·13–24·22) (log‐rank test P = 0·001), Ps (17·32 months; 95%, CI = 15·67–18·98) (log‐rank test P < 0·001) and NPs (29·79 months; 95%, CI = 26·87–32·71) (log‐rank test P < 0·001). (b) Patients with psoas major muscle area (∆PMMA) ≥ 10% had a significantly shorter mean OS (13·5 months, 95%, CI = 11·7–15·2) than patients with ∆PMMA < 10% (31·5 months, 95%, CI = 27·6–35·8) (log‐rank test P < 0·001).
Discussion
The current study found that the incidence of HPD after treatment with pembrolizumab as a second‐line therapy was 9·6%. Higher NLRs and ∆PMMA before treatment with pembrolizumab were associated with a higher risk of HPD development. Our results suggest for the first time, to our knowledge, that some patients who do not respond early to platinum‐based chemotherapy are at higher risk for the development of HPD upon immunotherapy.
ICIs may promote tumor growth kinetics in certain patients and lead to the development of HPD, with an incidence ranging from 4 to 29% in different studies [21, 28] There is no uniform definition for HPD, even though some authors still doubt its existence because it is an ad‐hoc observation, and may reflect the natural history of disease [29, 30]. The majority of researchers rely upon the rate of target lesion growth to define HPD [21, 31, 32]. As this underestimates the rate of development of metastasis, we included the involvement of new lesions as a part of the definition of HPD in our study. In conjunction with others, our results suggest that a dramatic increase in tumor growth induced by ICIs is restricted to approximately 10% of patients [21, 28]. All evidence for HPD is retrospective in nature, and different studies could not reveal common genomic or clinicopathological parameters [30]. Similarly, we could not find any association between HPD and other reported risk factors for its development, such as age, sex, PD‐L1 status or number of metastatic sites. HPD has been reported in patients receiving chemotherapy or targeted therapy, although this phenomenon had limited survival impact when treated with targeted therapy [30]. HPD is not specific or unique for immunotherapy, but is more likely to occur upon ICI treatment [21].
Although how and why HPD occurs is still unclear, our results shed some light on this problem. Our results show that the NLR is an important risk factor for the development of HPD. It is well known that high baseline NLR and PLR values and their derivations are significantly linked with worse OS and PFS in patients with NSCLC treated with ICIs [6, 17]. Although there is no clear explanation for this phenomenon, neutrophils and platelets may promote tumor progression as well as metastases by exercising a direct effect on tumor cells or by indirectly affecting other components of the tumor microenvironment [33, 34]. This effect is achieved through the secretion and release of various chemokines and cytokines, including transforming growth factor‐beta, vascular endothelial growth factor, IL‐6, IL‐8, matrix metalloproteinases and the formation of neutrophil extracellular traps [34]. Neutrophils are considered the most important inflammatory cell population in the tumors of NSCLC patients and promote metastasis, thus potentially compromising the anti‐tumor immune response [35]. Recent studies have reported that blood neutrophils, identified by the NLR, were directly linked with the number of intratumoral neutrophil populations, which may have the potential to compromise the anti‐tumor immune response [36]. Lower counts of lymphocytes usually reflect an impairment of cell‐mediated immunity. It has been shown that increased infiltration of lymphocytes in the tumor region is associated with better responsiveness to treatment and prognosis in patients with solid tumors [37]. To further complicate the results, it has been shown that activation of tumor lymphocytes can trigger local inflammation and matrix and metabolism modifications that could lead to tumor escape [38]. Moreover, in murine models, it has been shown that HPD is associated with vast infiltration of primary myeloid cells that express high levels of activation and inhibitory receptors (mostly precursors of neutrophils and macrophages) into the tumor microenvironment [39].
Consistent with others, our research finds a positive relationship between sarcopenia and the NLR [40, 41]. Cancer‐associated cachexia is a well‐known negative prognostic marker, with an incidence of up to 40% in the cancer population [42]. Although there are differences in the definitions used for cachexia and sarcopenia they are often indistinguishable in clinical practice, and cachexia and its key feature inflammation can lead to sarcopenia [43]. It was shown that sarcopenia measured via ∆PMMA with a CT scan at the L3 position is much more reliable than body mass index, and is widely used with a variety of cut‐off values [19, 44, 45]. A recent report showed that patients receiving immunotherapy may be particularly susceptible to cancer‐associated cachexia [46]. This may, at least in part, explain why immunotherapy may induce rapid tumor growth in some patients with pre‐existing sarcopenia; up‐regulation of stress hormone production together with pre‐existing systemic immunosuppression and the presence of high inflammatory markers, such as NLR, accelerate tumor growth and thus ultimately lead to HPD.
Several inherent limitations were identified in our study. First, our study was retrospective and had a relatively small sample size; therefore, there is potential for bias. Moreover, the predictive values of the NLR and sarcopenia were not compared to those of some genetic predictive markers, such as EGFR, MDM2 and DNMT3A. Similarly, tumor mutational burden was not available. Secondly, in our study, only 112 patients (67%) were available for evaluation of the ∆PMMA. Thirdly, the majority of patients were PS 1, which precluded adequate statistical analysis. ECOG PS is known to be associated with muscle mass, and poor PS usually corresponds with sarcopenic state. Fourthly, there is a lack of a validation cohort, and a limited number of clinicopathological variables were presented in our study. Finally, due to the vague and unclear definitions of HPD in the literature, our definition for this phenomenon might be criticized.
Despite these limitations, our results suggest for the first time that patients with a high NLR and sarcopenia before immunotherapy are at higher risk for hyperprogression and shorter overall survival. This may be helpful to clinicians in their choice of treatment, especially for patients who progress rapidly on platinum‐based chemotherapy and who show high NLRs and sarcopenia. Perhaps a combination of chemotherapy and immunotherapy or new molecules in clinical trials could be used for these patients. Drugs that are capable of transforming neutrophils into a functional state with anti‐tumor activity are needed to improve patient outcomes.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
None declared.
References
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomark Prev 2016; 25:16–27. [DOI] [PubMed] [Google Scholar]
- 2. Nixon NA, Blais N, Ernst S et al Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol 2018; 25:e373–e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prabhash K. Treatment of advanced nonsmall cell lung cancer: first line, maintenance and second line – Indian consensus statement update. S Asian J Cancer 2019; 8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu YL, Planchard D, Lu S et al Pan‐Asian adapted clinical practice guidelines for the management of patients with metastatic non‐small‐cell lung cancer: a CSCO–ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol 2019; 30:171–210. [DOI] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet 2016; 387:1540–50. [DOI] [PubMed] [Google Scholar]
- 6. Jiang T, Bai Y, Zhou F et al Clinical value of neutrophil‐to‐lymphocyte ratio in patients with non‐small‐cell lung cancer treated with PD‐1/PD‐L1 inhibitors. Lung Cancer 2019; 130:76–83. [DOI] [PubMed] [Google Scholar]
- 7. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vokes EE, Ready N, Felip E et al Nivolumab versus docetaxel in previously treated advanced non‐small‐cell lung cancer (CheckMate 017 and CheckMate 057): 3‐year update and outcomes in patients with liver metastases. Ann Oncol 2018; 29:959–65. [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Rodriguez‐Abreu D, Robinson AG et al Updated analysis of KEYNOTE‐024: pembrolizumab versus platinum‐based chemotherapy for advanced non‐small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37:537–46. [DOI] [PubMed] [Google Scholar]
- 10. Peters S, Reck M, Smit EF, Mok T, Hellmann MD. How to make the best use of immunotherapy as first‐line treatment of advanced/metastatic non‐small‐cell lung cancer. Ann Oncol 2019; 30:884–96. [DOI] [PubMed] [Google Scholar]
- 11. Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD‐1/PD‐L1 checkpoint blockade immunotherapy. Cancer Treat Rev 2015; 41:868–76. [DOI] [PubMed] [Google Scholar]
- 12. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014; 15:e493–e503. [DOI] [PubMed] [Google Scholar]
- 13. Tao H, Mimura Y, Aoe K et al Prognostic potential of FOXP3 expression in non‐small cell lung cancer cells combined with tumor‐infiltrating regulatory T cells. Lung Cancer 2012; 75:95–101. [DOI] [PubMed] [Google Scholar]
- 14. Templeton AJ, McNamara MG, Seruga B et al Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst 2014; 106:dju124. [DOI] [PubMed] [Google Scholar]
- 15. Petrova MP, Eneva MI, Arabadjiev JI et al Neutrophil to lymphocyte ratio as a potential predictive marker for treatment with pembrolizumab as a second line treatment in patients with non‐small cell lung cancer. Biosci Trends 2020; 14:48–55. [DOI] [PubMed] [Google Scholar]
- 16. Diem S, Schmid S, Krapf M et al Neutrophil‐to‐Lymphocyte ratio (NLR) and Platelet‐to‐Lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111:176–81. [DOI] [PubMed] [Google Scholar]
- 17. Russo A, Franchina T, Ricciardi GRR et al Baseline neutrophilia, derived neutrophil‐to‐lymphocyte ratio (dNLR), platelet‐to‐lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or Docetaxel. J Cell Physiol 2018; 233:6337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cespedes Feliciano EM, Kroenke CH, Caan BJ. The obesity paradox in cancer: how important is muscle? Annu Rev Nutr 2018; 38:357–79. [DOI] [PubMed] [Google Scholar]
- 19. Nishioka N, Uchino J, Hirai S et al Association of sarcopenia with and efficacy of anti‐PD‐1/PD‐L1 therapy in non‐small‐cell lung cancer. J Clin Med 2019; 8:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiroyama T, Nagatomo I, Koyama S et al Impact of sarcopenia in patients with advanced non‐small cell lung cancer treated with PD‐1 inhibitors: A preliminary retrospective study. Sci Rep 2019; 9:2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrara R, Mezquita L, Texier M et al Hyperprogressive disease in patients with advanced non‐small cell lung cancer treated with PD‐1/PD‐L1 inhibitors or with single‐agent chemotherapy. JAMA Oncol 2018; 4:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tachihara M, Nishimura Y. Who will suffer from hyperprogressive disease in patients with advanced non‐small cell lung cancer treated with PD‐1/PD‐L1 inhibitors. J Thorac Dis 2019; 11:S1289–S1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015; 33:3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park W, Kwon D, Saravia D et al Developing a predictive model for clinical outcomes of advanced non‐small cell lung cancer patients treated with nivolumab. Clin Lung Cancer 2018;19:280–8.e4. [DOI] [PubMed] [Google Scholar]
- 25. Bagley SJ, Kothari S, Aggarwal C et al Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer 2017; 106:1–7. [DOI] [PubMed] [Google Scholar]
- 26. Cedres S, Torrejon D, Martinez A et al Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non‐small cell lung cancer. Clin Transl Oncol 2012; 14:864–9. [DOI] [PubMed] [Google Scholar]
- 27. Thunnissen E, de Langen AJ, Smit EF. PD‐L1 IHC in NSCLC with a global and methodological perspective. Lung Cancer 2017; 113:102–5. [DOI] [PubMed] [Google Scholar]
- 28. Champiat S, Dercle L, Ammari S et al Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti‐PD‐1/PD‐L1. Clin Cancer Res 2017; 23:1920–8. [DOI] [PubMed] [Google Scholar]
- 29. Frelaut M, Le Tourneau C, Borcoman E. Hyperprogression under Immunotherapy. Int J Mol Sci 2019; 20:2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adashek JJ, Subbiah IM, Matos I et al Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer 2020; 6:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saada‐Bouzid E, Defaucheux C, Karabajakian A et al Hyperprogression during anti‐PD‐1/PD‐L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017; 28:1605–11. [DOI] [PubMed] [Google Scholar]
- 32. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23:4242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res 2002; 22:913–22. [DOI] [PubMed] [Google Scholar]
- 34. Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev 2016; 273:312–28. [DOI] [PubMed] [Google Scholar]
- 35. Kargl J, Busch SE, Yang GH et al Neutrophils dominate the immune cell composition in non‐small cell lung cancer. Nat Commun 2017; 8:14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol 2013; 23:141–8. [DOI] [PubMed] [Google Scholar]
- 37. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: a systematic review with meta‐analysis. Br J Cancer 2011; 105:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer‐related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009; 30:1073–81. [DOI] [PubMed] [Google Scholar]
- 39. Knorr DA, Ravetch JV. Immunotherapy and hyperprogression: unwanted outcomes, unclear mechanism. Clin Cancer Res 2019; 25:904–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Go SI, Park MJ, Song HN et al Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 2016; 24:2075–84. [DOI] [PubMed] [Google Scholar]
- 41. Feliciano EMC, Kroenke CH, Meyerhardt JA et al Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol 2017; 3:e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vigano A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez‐Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med 2000; 160:861–8. [DOI] [PubMed] [Google Scholar]
- 43. Rolland Y, Abellan van Kan G, Gillette‐Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care 2011; 14:15–21. [DOI] [PubMed] [Google Scholar]
- 44. Pecorelli N, Carrara G, De Cobelli F et al Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surgery 2016; 103:434–42. [DOI] [PubMed] [Google Scholar]
- 45. Nakamura R, Inage Y, Tobita R et al Sarcopenia in resected NSCLC: effect on postoperative outcomes. J Thoracic Oncol 2018; 13:895–903. [DOI] [PubMed] [Google Scholar]
- 46. Flint TR, Janowitz T, Connell CM et al Tumor‐induced IL‐6 reprograms host metabolism to suppress anti‐tumor immunity. Cell Metab 2016; 24:672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


