Highlights
-
•
Lower age of non-metastatic patients developed metastasis during follow-up.
-
•
Higher levels of IL-8 and MMP-9 were observed in these patients.
-
•
IL-8 and age together improved metastasis prognostic ability.
Keywords: Cancer metastasis, Lung cancer, Serum biomarkers, Metastasis prognosis
Abstract
At the diagnostic stage, metastasis detection is around 75% in the lung cancer patients. Major clinical challenge faced by medical oncologists is the unpredictable metastasis development in non-metastatic patients. The literature regarding the biomarkers/factors prognosticating metastasis in non-metastatic patients during follow-up is very limited. In this pilot study, the levels of serum biomarkers (IL-8, VEGF, MMP-2, MMP-9) were measured at diagnosis stage of non-metastatic lung cancer patients and these observations were evaluated for metastasis development after follow-up of median 29.2 months. After follow-up, ∼40% of these patients developed metastasis. The average age of non-metastatic patients which later developed metastasis, was found to be lower than the patients continued to be non-metastatic. These patients also showed higher levels of IL-8 and MMP-9 than the patients which did not develop metastasis. Analysis of Receiver Operating Characteristic Curves, Youden's Index and positive likelihood ratio values showed better diagnostic ability for IL-8 and MMP-9, which improved when both markers used together. Moreover, patients with age ≤60 years showed higher prognostic ability of metastasis development, which was significantly enhanced when patient age was analysed with IL-8. These results suggest potential of serum analytes (IL-8, MMP-9) and/or patient age in prognosticating the metastasis development in non-metastatic patients.
Introduction
Lung cancer is the leading cause of death and accounts for 18.4% of cancer-related deaths all over the world [1]. Around 85% of the lung cancer patients are non-small cell lung cancer (NSCLC), which includes subtypes of adenocarcinoma and squamous cell carcinoma [2]. Furthermore, in India, majority of the NSCLC patients (82%) are diagnosed at advanced stages with disease that has spread either to the mediastinal lymph nodes (stage III) or distant sites (stage IV). In contrast, only ∼18 % patients are diagnosed at early stage (I–IIIA) [3] based on imaging and sputum cytology. The 5-year survival for these patients was not yet improved since they are at high risk for metastasis [4,5], which is difficult to prognosticate at the early stage [6,7]. Prognosis of metastasis development in non-metastatic patients holds immense value in clinical management of these patients not just for adjuvant therapy but also for enabling better follow-up schedule.
In breast and liver cancer patients, variables (like levels of serum receptors, growth factors, cytokeratins, age/smoking history and stage of tumour) were used to prognosticate the risk of disease recurrence and metastasis [8,9]. In lung cancer, metastatic and non-metastatic patients have been differentiated based on gene mutations (EGFR, KRAS, HER2, ERCC1, RRM1 and BRCA) [10,[11], [12], [13], [14], [15]]. These studies were performed using lung tumour tissues collected invasively, which show high variability in the levels of these biomarkers due to tumour heterogeneity. However, the liquid biopsy has received significant attention because of their clinical advantages [14,16,17] of easier sample collection and better sample homogeneity. Type of markers evaluated in liquid biopsy were circulating tumour cells and tumour derived nucleic acids (circulating tumour DNA/free DNA, methylated DNA, micro RNA) [10,18].However, the presence of circulating tumor cells and tumour derived nucleic acids at the early stage of lung cancer is too low for reliable diagnostic applications [17]. Furthermore, most of these studies evaluated differences between early/late stages of lung cancer and healthy controls without analysing the prognostic value for the development of metastasis. The question that has not been clearly addressed in the literature pertains to the identification of markers that might prognosticate metastasis development in non-metastatic or early stage lung cancer patients.
In our previous study [18], we have studied the levels of serum biomarkers (VEGF, IL-8, MMP-2, MMP-9) for differential diagnosis of chronic obstructive pulmonary disease (COPD) (n=38), lung cancer (n=45) and age matched healthy controls (n=28). We have also showed that IL-8 being associated with systemic inflammation could differentiate COPD and lung cancer patients, but VEGF involved in carcinogenesis was found to be better diagnostic marker for metastatic and non-metastatic lung cancer patients [18]. In present study, the non-metastatic patients (n=25) were further followed-up (median period of 29.2 months) for metastasis development. The patients were segregated into two groups at the time of follow-up (i) patients who continued to be non-metastatic and (ii) patients with metastasis development. They were retrospectively analysed for the levels of serum biomarkers (VEGF, IL-8, MMP-2, MMP-9)/age at the time of non-metastatic diagnosis. Diagnostic test parameters [area under curve (AUC), Youden's Index (YI) and nomogram analysis] for serum analytes (IL-8, MMP-9) and/or age were analyzed for prognosticating metastasis development in non-metastatic patients.
Materials and methods
Patients and serum collection
Data of serum levels of biomarkers (VEGF, MMP2, IL-8 and MMP9) and age of patients were utilized from our previously published study [18]. Briefly, 25 patients with non-metastatic lung cancer were recruited for the study and serum samples were collected at the time of diagnosis in accordance with the approved experimental protocols/guidelines of Institutional Ethical Committees at Tata Memorial Hospital, Mumbai (IRB/1111) and informed consent was obtained from all participants and/or their legal guardian/s. Serum levels of biomarkers (VEGF, MMP2, IL-8 and MMP9) were estimated using ELISA (Quantikine ELISA, R&D Systems, USA) [18]. All patients were non-small cell lung cancer. Details of patients have been provided in the Supplementary Table 1. After completion of the treatment, the non-metastatic patients were followed-up for every 4-6 months. At the first follow-up all patients had undergone clinical examination and chest skiagram. In cases where metastasis was suspected, contrast enhanced computed tomography (CECT) thorax was done. Subsequently, based on the clinical presentation selected patients underwent either PET/CECT/fluoride PET/MRI-brain/bone scan or cytological analysis. The follow-up time considered in this study was either date of metastasis appearance or latest follow-up at the time of data analysis. The median follow-up was 29.2 months. The time of diagnosis was considered as zero time point in this study. Out of 25 patients, one patient was not available for follow-up, 10 patients were diagnosed with metastasis during follow-up and 14 patients did not develop metastasis. Out of 10 patients who developed metastasis, 8 patients developed bone or brain metastasis. Serum levels of VEGF, MMP2, IL-8 and MMP9 evaluated by ELISA in non-metastatic patients in the previous study [18] were used to categorise the patients into: the patients, who developed metastasis and second, who remained non-metastatic during follow-up (Supplementary Fig. S1). For MMP-9, 16 non-metastatic patients were used and categorised as 11 non-metastatic and 5 metastatic after follow-up. Age of the patients was also considered as one of the variables along with serum analytes to evaluate diagnostic ability of these variables to prognosticate metastasis development.
Statistical analysis
Statistical analysis was done using Origin Pro 8.0 software. Wherever required, values of analytes were represented as mean ± SEM with lower and upper 95% confidence intervals of mean. To evaluate the diagnostic ability of these analytes, initially AUC with 95% CI were determined from receiver operating characteristics (ROC) curves [sensitivity versus (1-specificity)] for the selected patient groups/analytes. This kind of calculation was required as our data has more discrete values on continuous rating scale. By considering either upper or lower 95% CI of mean as cut-off values (depending on the upward/downward trend of the particular analyte) the YI values were calculated. Nomogram analysis [19,20] was also performed to obtain positive and negative likelihood ratio based on pre- and post-probability odds to prognosticate the metastatic development in non-metastatic patients. Open access online diagnostic test calculator (version 2010042101 by A. Schwartz) was used to obtain nomograms [21].
Results
Levels of serum analytes in metastatic patients developed after follow-up in non-metastatic patients
The levels of serum analytes were re-analyzed (from the values of non-metastatic patients at the stage of diagnosis) for categorization of metastatic and non-metastatic patients after follow-up (Table 1). For these categories of patients, age as a variable (at the stage of diagnosis) was also considered. The mean age of non-metastatic patients, which developed metastasis during follow-up was found to be lower (52.8 ±3 years; 95% CI of mean=45.8-60) than patients who did not develop metastasis (59.2 ±2.9 years; 95% CI of mean= 52.8-65.6).The upper 95 % CI of mean of age for metastasis development was found to be 60 years. Patients which developed metastasis also showed higher level of IL-8 (17.6±1.3 pg/ml; 95% CI of mean=14.5-20.6; p<0.02)) and MMP-9 (1513.5±382.5 ng/ml; 95% CI of mean= 451.4-2575.7) than the patients which did not develop metastasis during follow-up (IL-8: 13.5±0.9 pg/ml, 95% CI of mean= 11.5-15.4; MMP-9: 959.6±154.6 ng/ml, 95% CI of mean= 614.9-1304) (Table 1 and Fig. 1). However, VEGF and MMP-2 levels did not show any significant variation in two categories of patients.
Table 1.
Mean ± SEM of age and analytes with their respective 95% confidence intervals mentioned in parentheses.
| Groups | Mean ± SEM (95% CI of mean) |
||||
|---|---|---|---|---|---|
| Age (in years) | VEGF (pg/ml) | IL-8 (pg/ml) | MMP9 (ng/ml) | MMP2 (ng/ml) | |
| Non- metastatic (n=14) | 59.2±2.9 (52.8-65.6) | 299.4±57.6 (174.8-423.9) | 13.5±0.9 (11.5-15.4) | 959.6±154.6 (614.9-1304) (n=11) | 254.5±14.5 (223.2-285.9) |
| Metastatic (n=10) | 52.8±3 (45.8-60) | 284.8±55.8 (158.5-411.1) | 17.6±1.3 (14.5-20.6) | 1513.5±382.5 (451.4-2575.7) (n=5) | 251.4±12.1 (223.9-278.9) |
Fig. 1.
Distribution of non-metastatic and metastatic patients serum cytokine levels and age were shown in box-whisker plots: (a) VEGF; (b) MMP2; (c) IL-8; (d) MMP9 and (e) age.
ROC curves of age and serum analytes for metastasis development in non-metastatic patients
The AUC values for individual variables i.e. VEGF, MMP2, IL-8, MMP9 and age for metastasis development in non-metastatic patients were found to be 0.49 (95% CI of mean: 0.24-0.73), 0.48 (95% CI of mean: 0.23-0.73), 0.76 (95% CI of mean: 0.56-0.96), 0.72 (95% CI of mean: 0.46-0.99) and 0.70 (95% CI of mean: 0.46-0.91), respectively (Fig. 2a–e). These results suggested that out of these variables IL-8, MMP9 and age showed better AUC values (≥0.7) for prognosis of metastasis development in non-metastatic patients.
Fig. 2.
Receiver Operating Curves of different analytes and age with corresponding AUC values (95% CI) to determine the diagnostic ability of the markers to prognosticate metastasis in non-metastatic patients after follow-up (a) VEGF; (b) MMP2;(c) IL-8;(d) MMP9 and (e) age.
Ability of serum analytes and age in prognosticating metastasis development in non-metastatic patients
After obtaining AUC values from ROC curves, the threshold cut off values were selected based on 95 % CI of mean, which was used to evaluate their diagnostic ability using YI and nomogram analysis. The cut off values considered for IL-8, VEGF, MMP-2, MMP-9 and age were ≥14.5 pg/ml, ≥174.8 pg/ml, ≤285.9 ng/ml, ≥1304 ng/ml and ≤60 years, respectively (Table 2). Out of these measured variables, age, IL-8 and MMP-9 showed better YI values(95% CI of mean) i.e. 0.39 (0.04-0.74), 0.51 (0.17-0.86) and 0.33(-0.18-0.83), respectively (Table 2). Other analytes (VEGF and MMP-2) showed significantly lower YI values. The combination of potential variables (i.e. IL-8, MMP-9 and age) were further analyzed for better prognosis of metastasis development in non-metastatic patients. When IL-8 and MMP-9 were considered together YI value (95% CI of mean) was found to be 0.42 (-0.07-0.9). YI value was substantially increased to 0.66 (95% CI of mean: 0.35-0.97), when age and IL-8 were considered together. Other combinations (i.e. MMP-9 and age; MMP-9, IL-8 and age) did not show significant YI values (data not shown).
Table 2.
Cut-off values of variables analysed with respective diagnostic test parameters.
| Parameters | Age (in years) | VEGF (pg/ml) | MMP-2 (ng/ml) | IL-8 (pg/ml) | MMP9 (ng/ml) | IL-8 AND MMP9 | AGE AND IL-8 |
|---|---|---|---|---|---|---|---|
| Cut-off values | ≤ 60 | ≥174.8 | ≤ 285.9 | ≥14.5 | ≥ 1304 | IL-8 ≥14.5 pg/ml and MMP9 ≥ 1304 ng/ml | IL-8 ≥ 14.5 pg/ml and age ≤ 60 years |
| Youden's Index (95% CI) | 0.39 (0.04-0.74) | -0.07 (-0.48-0.33) | 0.04 (-0.22-0.3) | 0.51 (0.17-0.86) | 0.33 (-0.18-0.83) | 0.42 (-0.07-0.9) | 0.66 (0.35-0.97) |
| Positive likelihood ratio (95% CI) | 1.58 (0.96-2.60) | 0.88 (0.41-1.89) | 1.05 (0.78-1.41) | 2.8 (1.16-6.78) | 2.2 (0.66-7.31) | 3.3 (0.78-14) | 5.6 (1.5-21) |
| Negative likelihood ratio (95% CI) | 0.23 (0.03-1.65) | 1.17 (0.49-2.77) | 0.70 (0.07-6.70) | 0.28 (0.08-1.01) | 0.55 (0.18-1.71) | 0.49 (0.16-1.48) | 0.23 (0.07-0.82) |
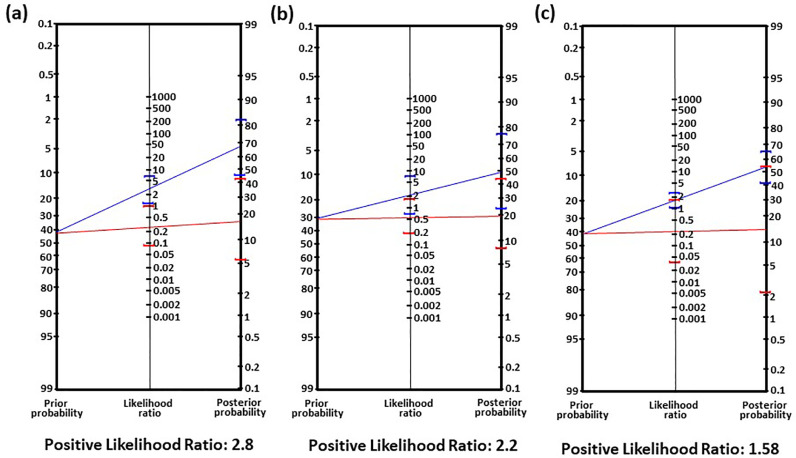
The positive likelihood ratios obtained from nomograms are in agreement with YI values. These ratios were found to be 2.4, 2.8 and 2.2 for age, IL-8 and MMP-9, respectively. These values were lower for VEGF and MMP-2 (Table 2; Fig. 3a–c). Furthermore, when combination of (i) IL-8 and MMP9 and (ii) IL-8 and age were used as criteria, the positive likelihood ratio was found to be 3.3 and 5.6, respectively (Table 2; Fig. 4a-b). These results suggested that when both variables (i) IL-8 and MMP-9, and (ii) IL-8 and age when used together, there is better probability of prognosticating metastatic development in non-metastatic patients. Out of these combinations, age and IL-8 together showed better set of variables to prognosticate metastasis development in non-metastatic patients.
Fig. 3.
Nomograms of analytes and age to determine the diagnostic ability of the markers to prognosticate metastasis in non-metastatic patients after follow-up (a) IL-8, (b) MMP9 and (c) age.
Fig. 4.
Nomograms considering the cut-off values of (a) IL-8 and MMP9 and (b) IL-8 and age to determine the diagnostic ability of the markers to prognosticate metastasis in non-metastatic patients after follow-up.
4. Discussion
In this study, the variables (age and levels of serum VEGF, MMP2, IL-8 and MMP9) in non-metastatic patients reported in our previous report [18] were re-analyzed after median follow-up period of 29.2 months. After follow-up, the patients were categorized into metastatic/non-metastatic patients and variables were used to prognosticate metastasis development in non-metastasis patients (Table 1). In our analysis, prognosis was done by considering non-metastatic as controls and metastatic patients as diseased condition. It was observed that levels of serum VEGF and MMP2 in metastatic patients did not show significant difference when compared with non-metastatic patients. On the contrary, VEGF was significantly higher in metastatic than non-metastatic lung cancer patients in our previous study [18]. VEGF being a major angiogenic factor seems to be up-regulated during the process of metastasis but not contributing in the prognosis of metastasis development at non-metastatic stage of disease. MMP2 was neither contributing in differential diagnosis of disease as reported previously [18] nor in prognosis as observed in the present study. Our previous in vitro results showed involvement of MMP-2 and MMP-9 in conditioned medium induced epithelial-to-mesenchymal transition changes and invasiveness in human lung adenocarcinoma cells (A549) [22]. This provided us basis to evaluate these analytes in serum samples of metastatic and non-metastatic lung cancer patients. MMP-2 and MMP-9 are gelatinases known to be involved in extracellular matrix disassembly, increased cell proliferation, invasion/migration and angiogenesis in various cancer types including lung cancer [23]. However, in our study, serum MMP-9 showed its prognostic ability for metastasis development in non-metastatic patients but not MMP-2. These observations may be associated with differential role of these MMPs in the process of initiation/progression of metastasis. Such hypothesis gets supported by literature suggesting a distinct role of MMP-9 in tumor angiogenesis regulating the bioavailability of VEGF through acting as an angiogenic switch [24]. Moreover, MMP-9 is known to be critical for the formation of the metastatic niche, which is most likely linked with its ability to support angiogenesis through release of VEGF [25]. These thoughts also get supported by our previous study which showed increased level of serum VEGF in metastatic lung cancer patients than non-metastatic subjects [18].
IL-8 values were significantly (p<0.02) higher in patients who developed metastasis during follow-up. This observation also gets supported by studies which showed IL-8 secretion in A549 cells associated with their metastatic features [22,26] and its higher level in NSCLC metastatic patients [27,28]. AUC value of IL-8 further confirms the ability of this biomarker in prognosticating the metastasis development.
It is observed that the metastatic patients have lower age but higher MMP9 values compared to the non-metastatic patients during follow-up. Further the MMP9 values in serum samples was shown to be associated with advanced stage of NSCLC [29,30]. In agreement with these studies, our study also showed a higher number of metastatic patients when MMP9 levels were higher at the time of diagnosis when the disease was non-metastatic. The levels of these biomarkers were not affected significantly with the age of the patient (data not shown). In our observation, the AUC value of MMP9 (0.72) has also indicated for its diagnostic ability of metastasis development. Furthermore, when other diagnostic test parameters (like YI and nomogram analysis) were applied with cut-off value of ≥1304 ng/ml, it showed promising results but the observation needs to be verified in higher number of samples. When both IL-8 (positive likelihood ratio 2.8) and MMP9 (positive likelihood ratio 2.2) cut-offs were used together, a higher positive likelihood ratio (3.3) was observed suggesting better ability of these biomarkers to prognosticate the metastasis development.
It was earlier reported that patients at younger age (60-70 years) are at higher risk for brain metastasis in NSCLC [31,32]. This is in agreement with our results showing that the patients with lower age are more likely to get metastasis (AUC value of 0.70) than the higher age patients. In our study about 40 % patients were noted to have developed brain metastasis. It is unclear why young patients have a higher brain metastasis risk [33]. In seed and soil hypothesis of brain metastasis, Fidler et al. suggested that better angiogenic microenvironment of brain in young patients may contribute to a higher incidence of brain metastasis [34]. Moreover, young patients may have better performance status associated with longer survival, which may contribute in higher metastasis incidence [33]. Similar to brain metastasis, in our study young patients also showed higher risk of bone metastasis. Possible mechanism of bone metastasis suggested to be is due to higher bone marrow flow along with circulating tumor cells in skeletal system in younger patients [35,36]. Our study gets supported by another recent lung cancer study which revealed that younger age was associated with the higher risk of bone metastasis. One year increase in age was associated with a 3% reduction in bone metastasis risk [37]. In our study, when age of ≤60 years was considered as criteria for YI and nomogram analysis, metastasis development was predominantly noted in patients with lower age. When age (positive likelihood ratio=1.56) and IL-8 (positive likelihood ratio=2.8) were used together the positive likelihood ratio increased significantly to 5.6 suggesting better ability of these variables in prognosticating metastasis development in non-metastatic patients when these two variables were used together.
The limited sample size in this study is associated with lower (∼17%) detection of early stage lung cancer patients [3]. With these limitations, we attempted to prognosticate metastasis development in non-metastatic patients based on serum biomarkers. Needless to mention that compared to molecular biomarkers and tumor biopsy-based assays, cytokine-based serum biomarkers provide patient friendly, economic and convenient prognostic technique for clinical implementation.
5. Conclusion
In summary, our study showed that the combination of age and IL-8 could be used as a prognostic marker for development of metastasis in early stage NSCLC. MMP9 along with IL-8 may also be a good prognostic marker. However, for clinical translation of age as a variable in combination with these serum-based biomarkers for metastasis prognosis, a multi-centric study with bigger patient cohort is required.
Declaration of Competing Interest
None
Acknowledgments
Funding
This work was supported by Bhabha Atomic Research Centre, Department of Atomic Energy, Government of India.
Acknowledgment
Sejal Patwardhan would like to acknowledge Department of Atomic Energy Graduate Fellowship Scheme for financial support.
Author contribution
Murali Mohan Sagar Balla conducted the experiments, analysed the results, designed the study and wrote the manuscript, Sejal Patwardhan conducted the experiments and helped in editing the manuscript, Pooja Kamal Melwani helped in analysis of data, writing and editing the manuscript, Pallavi Purwar, Amit Kumar, C.S. Pramesh and Siddharth Laskar helped in editing the manuscript and designing the study. Badri Narain Pandey analysed the results, designed the study and wrote the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100933.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Knight S.B.S.B., Crosbie P.A.P.A., Balata H., Chudziak J., Hussell T., Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7 doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh N., Aggarwal A.N., Gupta D., Behera D., Jindal S.K. Unchanging clinico-epidemiological profile of lung cancer in north India over three decades. Cancer Epidemiol. 2010;34:101–104. doi: 10.1016/j.canep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Burotto M., Thomas A., Subramaniam D., Giaccone G., Rajan A. Biomarkers in early-stage non-small-cell lung cancer: current concepts and future directions. J. Thorac. Oncol. 2014;9:1609–1617. doi: 10.1097/JTO.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Statistics Review, 1975-2012 - Previous Version - SEER cancer statistics review, (n.d.). https://seer.cancer.gov/archive/csr/1975_2012/ (accessed October 13, 2019).
- 6.Croswell J.M., Baker S.G., Marcus P.M., Clapp J.D., Kramer B.S. Cumulative incidence of false-positive test results in lung cancer screening: a randomized trial. Ann. Intern. Med. 2010;152:505–512. doi: 10.7326/0003-4819-152-8-201004200-00007. [DOI] [PubMed] [Google Scholar]
- 7.De Koning H.J., Meza R., Plevritis S.K., Ten Haaf K., Munshi V.N., Jeon J., Erdogan S.A., Kong C.Y., Han S.S., Van Rosmalen J., Choi S.E., Pinsky P.F., De Gonzalez A.B., Berg C.D., Black W.C., Tammemagi M.C., Hazelton W.D., Feuer E.J., McMahon P.M. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive services task force. Ann. Intern. Med. 2014;160:311–320. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millar E.K.A., Graham P.H., O'Toole S.A., McNeil C.M., Browne L., Morey A.L., Eggleton S., Beretov J., Theocharous C., Capp A., Nasser E., Kearsley J.H., Delaney G., Papadatos G., Fox C., Sutherland R.L. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J. Clin. Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 9.Colleoni M., O'Neill A., Goldhirsch A., Gelber R.D., Bonetti M., Thürlimann B., Price K.N., Castiglione-Gertsch M., Coates A.S., Lindtner J., Collins J., Senn H.J., Cavalli F., Forbes J., Gudgeon A., Simoncini E., Cortes-Funes H., Veronesi A., Fey M., Rudenstam C.M. Identifying breast cancer patients at high risk for bone metastases. J. Clin. Oncol. 2000;18:3925–3935. doi: 10.1200/JCO.2000.18.23.3925. [DOI] [PubMed] [Google Scholar]
- 10.Balla M.S, Melwani P., Kumar A., Pandey B. Biomarkers in chronic obstructive pulmonary disease patients for prediction of lung cancer development. J. Radiat. Cancer Res. 2018;9:165–176. doi: 10.4103/jrcr.jrcr_28_18. [DOI] [Google Scholar]
- 11.Graziano S.L., Gu L., Wang X., Tatum A.H., Vollmer R.T., Strauss G.M., Kratzke R., Dudek A.Z., Vokes E.E., Green M.R. Prognostic significance of mucin and p53 expression in stage IB non-small cell lung cancer: a laboratory companion study to CALGB 9633. J. Thorac. Oncol. 2010;5:810–817. doi: 10.1097/jto.0b013e3181d89f95. http://www.ncbi.nlm.nih.gov/pubmed/20521348 (accessed October 13, 2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonobe M., Kobayashi M., Ishikawa M., Kikuchi R., Nakayama E., Takahashi T., Menju T., Takenaka K., Miyahara R., Huang C.L., Okubo K., Bando T., Date H. Impact of KRAS and EGFR gene mutations on recurrence and survival in patients with surgically resected lung adenocarcinomas. Ann. Surg. Oncol. 2012;19 doi: 10.1245/s10434-011-1799-8. [DOI] [PubMed] [Google Scholar]
- 13.Takenaka M., Hanagiri T., Shinohara S., Kuwata T., Chikaishi Y., Oka S., Shigematsu Y., Nagata Y., Shimokawa H., Nakagawa M., Uramoto H., So T., Tanaka F. The prognostic significance of HER2 overexpression in non-small cell lung cancer. Anticancer Res. 2011;31:4631–4636. http://www.ncbi.nlm.nih.gov/pubmed/22199341 (accessed October 13, 2019) [PubMed] [Google Scholar]
- 14.Oellerich M., Christenson R.H., Beck J., Walson P.D. Plasma EGFR mutation testing in non-small cell lung cancer: a value proposition. Clin. Chim. Acta. 2019;495:481–486. doi: 10.1016/j.cca.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Olaussen K.A., Dunant A., Fouret P., Brambilla E., André F., Haddad V., Taranchon E., Filipits M., Pirker R., Popper H.H., Stahel R., Sabatier L., Pignon J.P., Tursz T., Le Chevalier T., Soria J.C. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 16.Crowley E., Di Nicolantonio F., Loupakis F., Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013;10:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 17.Wan J.C.M., Massie C., Garcia-Corbacho J., Mouliere F., Brenton J.D., Caldas C., Pacey S., Baird R., Rosenfeld N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 18.Balla M.M.S., Desai S., Purwar P., Kumar A., Bhandarkar P., Shejul Y.K., Pramesh C.S., Laskar S., Pandey B.N. Differential diagnosis of lung cancer, its metastasis and chronic obstructive pulmonary disease based on serum Vegf, Il-8 and MMP-9. Sci. Rep. 2016;6:36065. doi: 10.1038/srep36065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delpech Y., Bashour S.I., Lousquy R., Rouzier R., Hess K., Coutant C., Barranger E., Esteva F.J., Ueno N.T., Pusztai L., Ibrahim N.K. Clinical nomogram to predict bone-only metastasis in patients with early breast carcinoma. Br. J. Cancer. 2015;113:1003–1009. doi: 10.1038/bjc.2015.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagan T.J. Letter: Nomogram for Bayes theorem., N. Engl. J. Med. 1975;293:257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 21.Diagnostic Test Calculator, (n.d.). http://araw.mede.uic.edu/cgi-bin/testcalc.pl (accessed October 13, 2019).
- 22.Desai S., Laskar S., Pandey B.N. Autocrine IL-8 and VEGF mediate epithelial-mesenchymal transition and invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer cells. Cell Signal. 2013;25:1780–1791. doi: 10.1016/j.cellsig.2013.05.025. S0898-6568(13)00156-3 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Merchant N., Nagaraju G.P., Rajitha B., Lammata S., Jella K.K., S.Buchwald Z., S.Lakka S., Ali A.N. Matrix metalloproteinases: their functional role in lung cancer. Carcinogenesis. 2017;38:766–780. doi: 10.1093/carcin/bgx063. [DOI] [PubMed] [Google Scholar]
- 24.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergers G., Brekken R., McMahon G., Vu T.H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai S., Kumar A., Laskar S., Pandey B.N. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine. 2013;61:54–62. doi: 10.1016/j.cyto.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Cheng D., Kong H., Li Y. Prognostic values of VEGF and IL-8 in malignant pleural effusion in patients with lung cancer. Biomarkers. 2013;18:386–390. doi: 10.3109/1354750X.2013.797499. [DOI] [PubMed] [Google Scholar]
- 28.Millar H.J., Nemeth J.A., McCabe F.L., Pikounis B., Wickstrom E. Circulating human interleukin-8 as an indicator of cancer progression in a nude rat orthotopic human non-small cell lung carcinoma model. Cancer Epidemiol. Biomarkers Prev. 2008;17:2180–2187. doi: 10.1158/1055-9965.EPI-07-2915. [DOI] [PubMed] [Google Scholar]
- 29.He W., Zhang H., Wang Y., Zhou Y., Luo Y., Cui Y., Jiang N., Jiang W., Wang H., Xu D., Li S., Wang Z., Chen Y., Sun Y., Zhang Y., Tseng H.-R., Zou X., Wang L., Ke Z. CTHRC1 induces non-small cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9. BMC Cancer. 2018;18:400. doi: 10.1186/s12885-018-4317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Badrawy M.K., Yousef A.M., Shaalan D., Elsamanoudy A.Z. Matrix metalloproteinase-9 expression in lung cancer patients and its relation to serum mmp-9 activity, pathologic type, and prognosis. J. Bronchol. Interv. Pulmonol. 2014;21:327–334. doi: 10.1097/LBR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 31.An N., Jing W., Wang H., Li J., Liu Y., Yu J., Zhu H. Risk factors for brain metastases in patients with non–small-cell lung cancer. Cancer Med. 2018;7:6357–6364. doi: 10.1002/cam4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceresoli G.L., Reni M., Chiesa G., Carretta A., Schipani S., Passoni P., Bolognesi A., Zannini P., Villa E. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer. 2002;95:605–612. doi: 10.1002/cncr.10687. [DOI] [PubMed] [Google Scholar]
- 33.Ji Z., Bi N., Wang J., Hui Z., Xiao Z., Feng Q., Zhou Z., Chen D., Lv J., Liang J., Fan C., Liu L., Wang L. Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:330–337. doi: 10.1016/j.ijrobp.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Fidler I.J., Yano S., Zhang R.-D., Fujimaki T., Bucana C.D. The seed and soil hypothesis: vascularisation and brain metastases. Lancet. Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. http://www.ncbi.nlm.nih.gov/pubmed/11905606 (accessed October 8, 2019) [DOI] [PubMed] [Google Scholar]
- 35.Batson Oscar V. The function of the vertebral veins and their role in the spread of metastasis. Ann. Surg. 1940;112:138–149. doi: 10.1097/00000658-194007000-00016. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1387927/pdf/annsurg00494-0147.pdf (accessed October 12, 2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahn D., Weiner G.J., Ben-Haim S., Ponto L.L., Madsen M.T., Bushnell D.L., Watkins G.L., Argenyi E.A., Hichwa R.D. Positron emission tomographic measurement of bone marrow blood flow to the pelvis and lumbar vertebrae in young normal adults. Blood. 1994;83:958–963. http://www.ncbi.nlm.nih.gov/pubmed/8111065 (accessed October 12, 2019) [PubMed] [Google Scholar]
- 37.da Silva G.T., Bergmann A., Thuler L.C.S. Incidence and risk factors for bone metastasis in Non-Small Cell Lung Cancer. Asian Pacific J. Cancer Prev. 2019;20:45–51. doi: 10.31557/APJCP.2019.20.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.