Highlights
-
•
The incidence of IE during lockdown was 11.1 IE cases per 100,000 PY.
-
•
No reduction in the incidence of IE during the lockdown compared to preceding years.
-
•
No difference in the incidence of IE pre- versus post-lockdown in 2020.
Keywords: Infective endocarditis, COVID-19, Pandemic, Epidemiology
Abstract
Background
The incidence of infective endocarditis (IE) has increased in recent decades. Societal lockdown including reorganization of the healthcare system during the COVID-19 pandemic may influence the incidence of IE. This study sets out to investigate the incidence of IE during the Danish national lockdown.
Methods
In this nationwide cohort study, patients admitted with IE in either one of two periods A) A combined period of 1 January to 7 May for 2018 and 2019, or B) 1 January to 6 May 2020, were identified using Danish nationwide registries. Weekly incidence rates of IE admissions for the 2018/2019-period and 2020-period were computed and incidence rate ratios (IRR) for 2020-incidence vs 2018/2019-incidence were calculated using Poisson regression analysis.
Results
In total, 208 (67.3% men, median age 74.1 years) and 429 (64.1% men, median age 72.7 years) patients were admitted with IE in 2020 and 2018/2019, respectively. No significant difference in incidence rates were found comparing the 2020-period and 2018/2019-period (IRR: 0.96 (95% CI: 0.82–1.14). The overall incidence rate pre-lockdown (week 1–10: 1 January to 11 March 2020) was 14.2 IE cases per 100,000 person years (95% CI: 12.0–16.9) as compared with 11.4 IE cases per 100,000 person years (95% CI: 9.1–14.1) during lockdown (week 11–18: 12 March to 6 May 2020) corresponding to an IRR of 0.80 (95% CI: 0.60–1.06) and thus no significant difference pre- versus post-lockdown.
Conclusion
In this nationwide cohort study, no significant difference in the incidence of IE admissions during the national lockdown due to the COVID-19 pandemic was found.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the novel acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has entailed a dramatic impact on global health pushing health care systems to the limit of breakdown. COVID-19 may cause variable symptoms from mild upper respiratory disease to severe lower respiratory disease potentially complicated by acute respiratory distress syndrome, sepsis, and multi-organ failure [1], [2]. Due to high infection pressures and risk of severe outcomes, international efforts including social distancing, societal lockdown, and reorganization of healthcare systems optimized for handling COVID-19 have been imposed to prevent spread of the virus. In Denmark, the first confirmed COVID-19 positive case was identified on 27 February 2020 and national lockdown was announced on 11 March 2020 [3].
Infective endocarditis (IE) is associated with high morbidity and mortality caused by mainly gram positive cocci [4], [5], [6], [7], [8]. Recent studies have reported an increasing incidence of IE during the past two decades [9], [10], [11], [12]. First, reorganization of the healthcare system during lockdown includes postponing elective procedures entailing fewer hospitalizations and secondary presumably fewer hospital-acquired bacteremia and thus potentially a declined incidence of IE. Second, increased focus on sanitation and societal lockdown are preventive measures against COVID-19 but presumably also against other infectious diseases even though mode of transmission differs. A decline in influenza rates during societal lockdowns have been reported [13]. Finally, in fear of COVID-19 infection or overloading the health care system, some patients may have refrained from consulting a physician despite a display of symptoms. Thus, one could hypothesize a decline in the incidence and/or diagnosis of IE during lockdown. During the COVID-19 pandemic plural studies have shown a decline within admissions with acute coronary syndrome, whether the same pattern is seen for IE is yet unknown [14], [15], [16].
In order to enlighten the impact of the COVID-19 pandemic on IE, we set out to investigate the incidence of IE during the Danish national lockdown.
2. Methods
2.1. Data sources
All Danish citizens are assigned a permanent unique identification number allowing accurate linkage of nationwide administrative registries at an individual level. For this study, data from the following nationwide administrative registries were obtained: 1) The Danish National Patient Registry, which holds information on all hospital admissions and outpatient contacts according to the International Classification of Diseases (ICD) 8th and 10th revision and surgical procedures according to the NOMESCO Classification of Surgical Procedures (NCSP) [17]. 2) The Danish National Prescription Registry, which contains information until 29 February 2020 on dispensing date, strength, and quantity on all claimed drug prescriptions in Denmark [18]. 3) The Danish Civil Registration System, which holds data on birth date, sex, and vital status (i.e. whether a person is alive and resident in Denmark, disappeared [persons whose residence is unknown to Danish authorities], emigrated, or dead - along with the date of these events) [19]. The Danish registries are validated and of high quality as described in detail previously [17], [18], [19], [20], [21].
2.2. Danish health care system during the COVID-19 pandemic
The Danish health care system, funded by taxes, provides equal access to healthcare services and welfare benefits for all residents regardless of socioeconomic or insurance status. In Denmark, the first incidence of COVID-19 was confirmed on 27 February 2020, the Danish government implemented national lockdown on 11 March 2020, and the borders were closed on 18 March 2020. The Danish healthcare system was reorganized and optimized for handling COVID-19; outpatient visits were cancelled or converted to telemedicine, elective procedures and dental appointments were postponed, and furthermore, emergency rooms, test centers, and intensive care units were adjusted to adapt to COVID-19 patients. On 6 April 2020, the Danish Government announced the first step of controlled reopening of the Danish society.
2.3. Study population
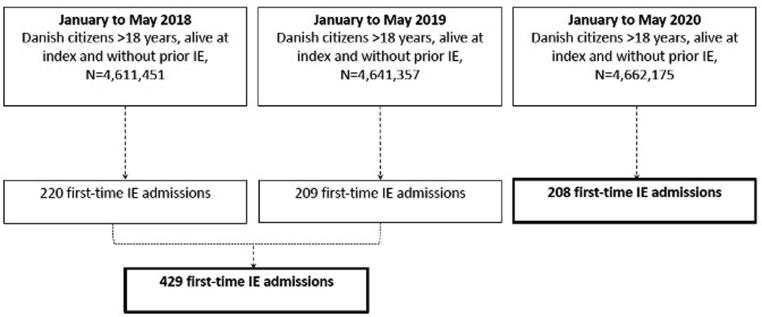
The study cohort comprised all Danish citizens in either one of two periods: 1) A combined period of 1 January 2018 to 7 May 2018 and 1 January 2019 to 7 May 2019, or 2) 1 January 2020 to 6 May 2020. Due to leap year in 2020, the study dates differ between the study periods, thus, the total follow-up time was maximum 126 days for each period. A combined period from 2018 and 2019 were used in order to get reliable information about the historic incidence of IE in similar times of the year and thereby reduce the risk of random variation. Patients admitted with IE prior to the abovementioned study periods were excluded. Fig. 1 depicts a flowchart of the patient selection.
Fig. 1.
Flowchart. The figure shows the selection process for the study population.
2.4. Covariates
Demographic data (age and sex) were obtained from the Danish Civil Registration System. Comorbidities were assessed from the National Patient Registry as in-hospital and out-patient diagnoses any time prior to IE admission. The following comorbidities were assessed: stroke, atrial fibrillation, peripheral vascular disease, heart failure, chronic renal failure, dialysis, acute renal failure, diabetes, chronic obstructive pulmonary disease, liver disease, rheumatologic disease, malignancy, presence of a cardiac implantable electronic device (defined as an implantation of a pacemaker or implantable cardioverter), and presence of a prosthetic heart valve (Supplementary Table 1 presents ICD-8, ICD-10, and NCSP codes). Concomitant pharmacotherapy was assessed from the Danish National Prescription Registry as a filled prescription within one year prior to IE admission (Supplementary Table 1 presents ATC codes). Hypertension was defined using claimed antihypertensive drug prescriptions as described previously [22], [23].
2.5. Outcomes
The primary outcome was first-time admission with IE. IE was defined from the following ICD-10 codes: I33 (acute and sub-acute endocarditis), I38 (endocarditis, valve unspecified), and I398 (endocarditis unspecified). Only patients hospitalized ≥ 14 days or those who died earlier were included. Patients were considered hospitalized during transfers between departments and hospitals if a period of discharge was less than 24 h. Using these criteria, the diagnosis of IE in the Danish National Patient Registry has previously been validated with a positive predictive value of 90% [24]. Persons included in the study were followed from date of entry until occurrence of the outcome of interest, death, or end of study (7 May 2018, 7 May 2019, or 6 May 2020, respectively), whichever came first. Concomitant COVID-19 infection was defined from the following ICD-10 codes: B342A, B972, and B972A.
2.6. Statistics
Descriptive characteristics for patients admitted to hospital with IE were compared between the two study periods: A) 1 January to 7 May 2018/2019, versus B) 1 January to 6 May 2020. Characteristics were reported by use of frequencies and percentages for categorical variables and medians with 25th-75th percentiles for continuous variables. The number of IE admissions was compared between study periods. For the combined study period of 2018 and 2019, the average number of IE admissions were calculated for comparison with the number of IE admissions in the 2020 study period and differences between the two periods were analyzed. Weekly incidence rates were computed as the number of IE admissions per 100,000 person years. Thus, for the computation of incidence rates the study cohort was split in bands of weeks (7 days) from index date (January 1). Incidence rate ratios with 95% confidence intervals were computed using a Poisson regression analysis to estimate the relative incidence of IE between the two study periods for every week (week 1–18).
Further, we investigated the incidence of IE before and after the lockdown (lock down announcement on 11 March 2020) and two additional study periods were compared: A) 13 March to 7 May 2018 and 13 March to 7 May 2019, and B) 12 March to 6 May 2020. Baseline characteristics were compared between cohorts in the two periods and differences in IE admissions were assessed. Moreover, the incidence of IE post-lockdown (12 March to 6 May 2020) was compared with the pre-lockdown period of 2020 (1 January to 11 March 2020) using Poisson regression analysis. Data management and statistical analyses were performed with SAS statistical software (SAS 9.4, SAS Institute, Cary, North Carolina, USA) and R (version 3.6.1, The R Foundation) [12]. The level of statistical significance was set at 5%.
3. Results
In total, 4,611,451, 4,641,357, and 4,662,175 Danish citizens were identified at 1 January 2018, 1 January 2019, and 1 January 2020, respectively. In the 2018/2019-period, 429 patients were admitted with first-time IE while 208 patients were admitted in the 2020-period. Table 1 depicts baseline characteristics for patients with IE in the two study periods and overall no differences in sex, age, or comorbidities were found.
Table 1.
Baseline characteristics for patients admitted with IE.
| 1 January to 7 May 2018/2019 N = 429 | 1 January to 6 May 2020 N = 208 | |
|---|---|---|
| Demographics | ||
| Age (years), median (IQR) | 72.7 (62.9–80.2) | 74.1 (63.8–80.6) |
| Male, N (%) | 275 (64.1%) | 140 (67.3%) |
| Comorbidities, N (%) | ||
| Stroke | 49 (11.4%) | 19 (9.1%) |
| Atrial flutter/fibrillation | 103 (24.0%) | 57 (27.4%) |
| Peripheral vascular disease | 27 (6.3%) | 20 (9.6%) |
| Chronic heart Failure | 64 (14.9%) | 36 (17.3%) |
| Chronic renal failure | 69 (16.1%) | 29 (13.9%) |
| Dialysis | 40 (9.3%) | 20 (9.6%) |
| Diabetes | 106 (24.7%) | 41 (19.7%) |
| COPD | 46 (10.7%) | 27 (13.0%) |
| Liver disease | 27 (6.3%) | 15 (7.2%) |
| Rheumatologic disease | 35 (8.2%) | 15 (7.2%) |
| Malignancy | 95 (22.1%) | 53 (25.5%) |
| CIED | 80 (18.6%) | 34 (16.3%) |
| Prosthetic heart valve | 88 (20.5%) | 45 (21.6%) |
| Hypertension | 219 (51.0%) | 119 (57.2%) |
| Pharmacotherapy, N (%) | ||
| Beta blockers | 169 (39.4%) | 92 (44.2%) |
| Calcium channel blockers | 99 (23.1%) | 63 (30.3%) |
| RASi | 182 (42.4%) | 95 (45.7%) |
| Loop-diuretics | 158 (36.8%) | 87 (41.8%) |
| Statins | 190 (44.3%) | 92 (44.2%) |
| ASA | 109 (25.4%) | 38 (18.3%) |
| ADPi | 52 (12.1%) | 26 (12.5%) |
| OAC | 147 (34.3%) | 81 (38.9%) |
IE: infective endocarditis, IQR: interquartile range, COPD: chronic obstructive pulmonary disease, CIED: cardiac implantable electronic device, RASi: renin angiotensin system inhibitor, ASA: aspirin, ADPi: adenosine-di-phosphate inhibitor, OAC: oral anticoagulant therapy
3.1. Comparison of IE admissions; 2020 versus 2018/2019
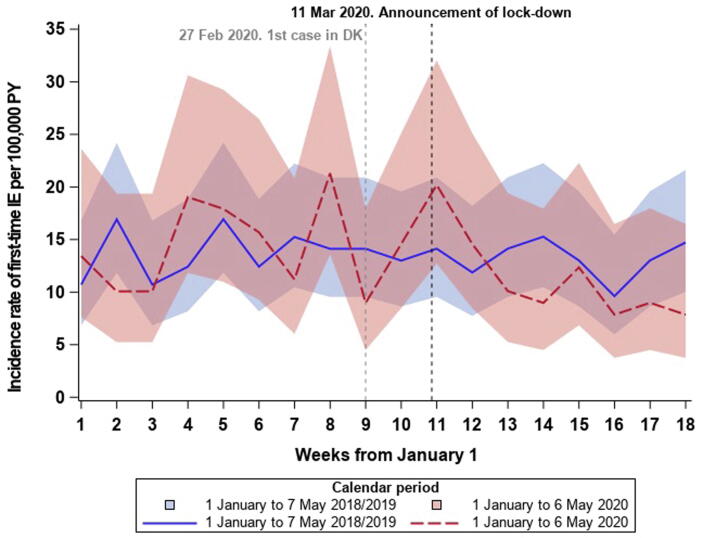
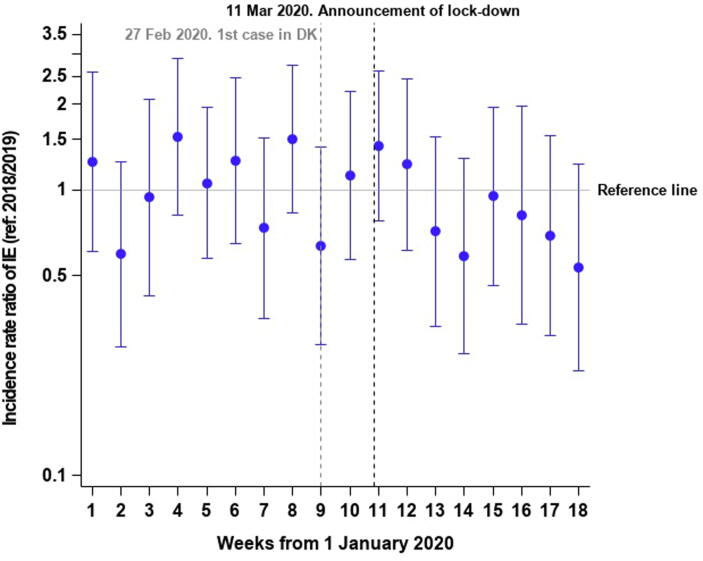
Comparing the average number of IE admissions in the 2018/2019-period with the 2020-period, we identified a 3.0% decline. Fig. 2 depicts incidence rates with 95% CI bands per week in 2020 as compared with 2018/2019 and a slight though non-significant decline was seen after lockdown in 2020 when compared with the average in 2018/2019. The overall incidence rate in week 1–18 was 13.5 IE cases per 100,000 PY (95% CI: 12.3–14.8) in 2018/2019 and 13.0 IE cases per 100,000 PY (95% CI: 11.3–14.8) in 2020. In regression analysis no significant differences were seen between 2018/2019 and 2020 (IRR: 0.96 (95% CI: 0.82–1.14), Fig. 3 depicts the incidence rate ratios per week in 2020 compared with 2018/2019). Supplementary figure 1 depicts the weekly number of IE admissions in the two study periods.
Fig. 2.
Incidence rates of IE admissions with 95% CI bands in 2020 versus 2018/2019. The figure shows the incidence rates in 2020 as compared with 2018/2019. IE: Infective endocarditis.
Fig. 3.
Incidence rate ratios of IE admissions in 2020 versus 2018/2019. The figure shows the incidence rate ratios per week (1–18) in 2020 as compared with week 1–18 in 2018/2019. IE: Infective endocarditis.
3.2. Lockdown period in 2020
From 13 March to 7 May 2018/2019 an average of 93.5 patients were admitted with IE whereas 81.0 patients were admitted from 12 March to 6 May 2020, corresponding to a 13.4% decline. Table 2 depicts baseline characteristics for patients with IE in the two study periods before and after announcement of lockdown and overall no differences in sex, age, or comorbidities were found. From week 1–10 2020 (1 January to 11 March), the incidence rate was 14.2 IE cases per 100,000 PY (95% CI 12.0–16.9) in 2020 as compared with 13.7 IE cases per 100,000 PY (95% CI 12.0–15.5) in the corresponding period in 2018/2019. The overall incidence during lockdown (week 11–18: 12 March to 6 May 2020) was 11.4 IE cases per 100,000 PY (95% CI 9.1–14.1) whereas it was 13.2 IE cases per 100,000 PY (95% CI 11.5–15.3) in the corresponding period in 2018/2019. The IRR was 0.80 (95% CI: 0.60–1.06) for week 11–18 2020 as compared with week 1–10 2020. Hence, no significant differences pre- versus post-lockdown was found. In total, less than four patients admitted with IE during lockdown had concomitant COVID-19 infection. Because of the low number of patients, no further information can be given on these cases due to the rules on anonymity by Statistics Denmark.
Table 2.
Baseline characteristics for patients admitted with IE.
| 1 January to 11 March 2018/2019 N = 242 | 13 March to 7 May 2018/2019 N = 187 | 1 January to 11 March 2020 N = 127 | 12 March to 6 May 2020 N = 81 | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), median (IQR) | 71.1 (61.0–79.5) | 74.4 (65.5–81.8) | 74.6 (62.7–80.6) | 73.8 (63.9–80.8) |
| Male, N (%) | 152 (62.8%) | 123 (65.8%) | 85 (66.9) | 55 (67.9%) |
| Comorbidities, N (%) | ||||
| Stroke | 28 (11.6%) | 21 (11.2%) | 15 (11.8%) | 4 (4.9%) |
| Atrial flutter/fibrillation | 52 (21.5%) | 51 (27.3%) | 35 (27.6%) | 22 (27.2%) |
| Peripheral vascular disease | 16 (6.6%) | 11 (5.9%) | 11 (8.7%) | 9 (11.1%) |
| Chronic heart failure | 35 (14.5%) | 29 (15.5%) | 25 (19.7%) | 11 (13.6%) |
| Chronic renal failure | 39 (16.1%) | 30 (16.0%) | 21 (16.5%) | 8 (9.9%) |
| Dialysis | 24 (9.9%) | 16 (8.6%) | 16 (12.6%) | 4 (4.9%) |
| Diabetes | 59 (24.4%) | 47 (25.1%) | 26 (20.5%) | 15 (18.5%) |
| COPD | 27 (11.2%) | 19 (10.2%) | 13 (10.2%) | 14 (17.3%) |
| Liver disease | 15 (6.2%) | 12 (6.4%) | 11 (8.7%) | 4 (4.9%) |
| Rheumatologic disease | 21 (8.7%) | 14 (7.5%) | 5 (3.9%) | 10 (12.3%) |
| Malignancy | 44 (18.2%) | 51 (27.3%) | 32 (25.2%) | 21 (25.9%) |
| CIED | 42 (17.4%) | 38 (20.4%) | 19 (15.0%) | 15 (18.5%) |
| Prosthetic heart valve | 54 (22.3%) | 34 (18.2%) | 25 (19.7%) | 20 (24.7%) |
| Hypertension | 122 (50.4%) | 97 (51.9%) | 74 (58.3%) | 45 (55.6%) |
| Pharmacotherapy, N (%) | ||||
| Beta blockers | 91 (37.6%) | 78 (41.7%) | 60 (47.2%) | 32 (39.5%) |
| Calcium channel blockers | 56 (23.1%) | 43 (23.0%) | 37 (29.1%) | 26 (32.1%) |
| RASi | 104 (43.0%) | 78 (41.7%) | 56 (44.1%) | 39 (48.1%) |
| Loop-diuretics | 87 (36.0%) | 71 (38.0%) | 56 (44.1%) | 31 (38.3%) |
| Statins | 103 (42.6%) | 87 (46.5%) | 60 (47.2%) | 32 (39.5%) |
| ASA | 63 (26.0%) | 46 (24.6%) | 23 (18.1%) | 15 (18.5%) |
| ADPi | 28 (11.6%) | 24 (12.8%) | 18 (14.2%) | 8 (9.9%) |
| OAC | 74 (30.6%) | 73 (39.9%) | 48 (37.8%) | 33 (40.7%) |
IE: infective endocarditis, IQR: interquartile range, COPD: chronic obstructive pulmonary disease, CIED: cardiac implantable electronic device, RASi: renin angiotensin system inhibitor, ASA: aspirin, ADPi: adenosine-di-phosphate inhibitor, OAC: oral anticoagulant therapy
4. Discussion
In this Danish nationwide cohort study, we investigated the incidence of IE during the Danish national lockdown. The main finding of this study was that there was no significant reduction in the incidence of IE during the national lockdown as compared with the corresponding period in 2018/2019.
In recent studies, the annual incidence of IE have been found to range from 3 to 7 per 100,000 person-years [9], [6] Patients with prior IE, prosthetic heart valve, or a cyanotic congenial heart disease are considered in high risk of IE, whereas patients with heart valve disorder, cardiac implantable electronic devices, or hypertrophic cardiomyopathy are considered in moderate risk of IE [5], [25], [26]. Correspondingly, a number of diseases and conditions has been associated with increased susceptibility to and severe course of COVID-19; in Denmark, it concerns elderly (>65 years), persons with chronic diseases including cardiovascular disease, immune-compromised patients, and children with sequalae of prematurity [27]. Thus, there is an overlap of risk factors among patients in risk of IE and COVID-19. In Denmark, emphasis has been placed on recommendations for persons in increased risk of COVID-19, including intensified hygienic precautions. Cutaneous hygiene is the general advice for those in moderate- and high-risk of IE[5], consequently, increased awareness in the general population and among vulnerable persons may potentially ameliorate the effort of precautions and one may have expected a decline in the admission rate of IE.
Elective surgical and dental procedures were cancelled or postponed during lockdown. It is well known that dental procedures are at-risk procedures for those in high-risk of IE, and antibiotic prophylaxis is recommended, whereas antibiotic prophylaxis routinely is not recommended for respiratory tract-, gastrointestinal-, urogenital-, skin-, or soft tissue procedures [5]. Therefore, if there were room for improvement concerning various procedures, a decline in the incidence of IE during lockdown could be expected. Fewer elective procedures would cause fewer hospitalizations and potentially fewer hospital-acquired bacteremia and hypothetically a declined incidence of IE. Thus, the unaltered incidence of IE from 2018/2019 to 2020 substantiate the current clinical guidelines concerning antibiotic prophylaxis for those at high-risk of IE. On the other hand, one could speculate that prolonged postponement of routine dental check-ups could deteriorate dental state and increase the risk of IE – presumably, a longer lockdown period would be needed in order to clarify this hypothesis.
Moreover, in fear of COVID-19 infection or overloading the health care system, some patients may have refrained from consulting a physician despite having symptoms, but fortunately this seems not to be the case concerning IE. However, a trend towards a decline in the lockdown period could be interpreted from our results and the wide confidence intervals could suggest a type II-error.
Reorganization of the healthcare system including postponing elective cardiac surgeries may impact patients with IE, as reported in a recent case report by Hussain et al. on an IE case complicated by COVID-19 and severe hemorrhages [28]. Further studies are needed in order to enlighten the course of concomitant IE and COVID-19 infection. Lockdown has proven to be an effective strategy in slowing down the COVID-19 pandemic with significant reduction in infection and death rates [29]. However, our study does not provide evidence of a significant effect of lockdown on the incidence of IE.
4.1. Strengths and limitations
The main strength of this study is the completeness of data in a large nationwide cohort. The Danish healthcare registries provide a unique opportunity for the investigation of a national lockdown during the COVID-19 pandemic. The accuracy of the data relies upon the coding in nationwide administrative registries, though these have been validated previously [30], [24]. The diagnosis of IE has previously been validated with high positive predictive values [24]. However, the findings in this study should be viewed in the context of a number of limitations. Due to the relatively low incidence of IE, a type 2 error (false negative finding) cannot be ruled out. Further, we were not able to differentiate between the location of IE (i.e. prosthetic valve, native valve, or cardiac implantable electronic devices). In addition, data on microbiology, use of antibiotic therapy, echocardiographic findings, and other imaging modalities (e.g. PET-CT) in patients with IE were not available. The observational nature of this study precludes the assessment of cause-effect relationships, and the influence of potential confounders and thus residual confounding cannot be omitted.
5. Conclusion
In this nationwide cohort study, no significant difference in the incidence of IE admissions during the national lockdown due to the COVID-19 pandemic was found as compared to similar periods in the preceding years and to the pre-lockdown period of 2020.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
EHB: None declared
ELF: Previous research funding from Janssen and Janssen and Bristol-Myers
JHB: None declared
JKP: None declared
ADJ: None declared
FK: None declared
MS: None declared
MP: None declared
KK: None declared
GHG: Research grants from Bayer, Bristol-Myers Squibb, Pfizer, AstraZeneca and Boehring Ingelheim
CTP: Consultant fees and research funding from Bayer and Biotronic
LK: None declared
LØ: None declared
Acknowledgments
None
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100675.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jain V., Yuan J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Brown T.S., Der Nigoghossian C., Zidar D.A., Haythe J., Brodie D., Beckman J.A., Kirtane A.J., Stone G.W., Krumholz H.M., Parikh S.A. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. Journal of the American College of Cardiology. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Statens Serum institut, Første dansker testet positiv for COVID-19, (2020) 27–28. https://www.ssi.dk/aktuelt/nyheder/2020/02_27_foerste-tilfaelde-af-ny-coronavirus-i-dk.
- 4.Østergaard L., Bruun N.E., Voldstedlund M., Arpi M., Andersen C.Ø., Schønheyder H.C., Lemming L., Rosenvinge F., Valeur N., Søgaard P., Andersen P.S., Skov R., Chen M., Iversen K., Gill S., Lauridsen T.K., Dahl A., Oestergaard L.B., Povlsen J.A., Moser C., Bundgaard H., Køber L., Fosbøl E.L. Prevalence of infective endocarditis in patients with positive blood cultures: a Danish nationwide study. Eur. Heart J. 2019;40(39):3237–3244. doi: 10.1093/eurheartj/ehz327. [DOI] [PubMed] [Google Scholar]
- 5.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.-P., Del Zotti F., Dulgheru R., El Khoury G., Erba P.A., Iung B., Miro J.M., Mulder B.J., Plonska-Gosciniak E., Price S., Roos-Hesselink J., Snygg-Martin U., Thuny F., Tornos Mas P., Vilacosta I., Zamorano J.L. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 6.Baddour L.M., Wilson W.R., Bayer A.S., Fowler V.G., Jr, Tleyjeh I.M., Rybak M.J., Barsic B., Lockhart P.B., Gewitz M.H., Levison M.E., Bolger A.F., Steckelberg J.M., Baltimore R.S., Fink A.M., O’Gara P., Taubert K.A. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132(15):1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 7.Havers-Borgersen E., Fosbøl E.L., Rørth R., Kragholm K., Kristensen S.L., Bundgaard H., Bruun N.E., Østergaard L., Aslam M., Valeur N., Gislason G.H., Torp-Pedersen C., Køber L., Butt J.H. Nursing Home Admission and Initiation of Domiciliary Care Following Infective Endocarditis. gh. 2019;14(1):41. doi: 10.1016/j.gheart.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Havers-Borgersen E., Butt J.H., Østergaard L., Bundgaard H., Smerup M., Bruun N.E., Gislason G.H., Torp-Pedersen C., Køber L., Fosbøl E.L. Recurrent infective endocarditis versus first-time infective endocarditis after heart valve surgery. Clin Res Cardiol. 2020;109(11):1342–1351. doi: 10.1007/s00392-020-01628-7. [DOI] [PubMed] [Google Scholar]
- 9.Erichsen P., Gislason G.H., Bruun N.E. The increasing incidence of infective endocarditis in Denmark, 1994–2011. European Journal of Internal Medicine. 2016;35:95–99. doi: 10.1016/j.ejim.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Olmos C., Vilacosta I., Fernández-Pérez C., Bernal J.L., Ferrera C., García-Arribas D., Pérez-García C.N., San Román J.A., Maroto L., Macaya C., Elola F.J. The Evolving Nature of Infective Endocarditis in Spain. Journal of the American College of Cardiology. 2017;70(22):2795–2804. doi: 10.1016/j.jacc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Pant S., Patel N.J., Deshmukh A., Golwala H., Patel N., Badheka A., Hirsch G.A., Mehta J.L. Trends in Infective Endocarditis Incidence, Microbiology, and Valve Replacement in the United States From 2000 to 2011. Journal of the American College of Cardiology. 2015;65(19):2070–2076. doi: 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- 12.and M.H.T. Mark J Dayer, Simon Jones, Bernard Prendergast, Larry M. Baddour, Peter B Lockhart, Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time series analysis, Lancet. (2015) 1219–1228. [DOI] [PMC free article] [PubMed]
- 13.WHO, Influenza Update N ° 191, (2013) 1–7.
- 14.M.D. et al. Ovidio De Filippo, Reduced Rate of Hospital Admissions for ACS during Covid-19 Outbreak in Northern Italy, N. Engl. J. Med. (2020) 1–3. [DOI] [PMC free article] [PubMed]
- 15.Metzler Bernhard, Siostrzonek Peter, Binder Ronald K, Bauer Axel, Reinstadler Sebastian Johannes. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur. Heart J. 2020;41(19):1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia Santiago, Albaghdadi Mazen S., Meraj Perwaiz M., Schmidt Christian, Garberich Ross, Jaffer Farouc A., Dixon Simon, Rade Jeffrey J., Tannenbaum Mark, Chambers Jenny, Huang Paul P., Henry Timothy D. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. Journal of the American College of Cardiology. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynge Elsebeth, Sandegaard Jakob Lynge, Rebolj Matejka. The Danish National Patient Register. Scand J Public Health. 2011;39(7_suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 18.Wallach Kildemoes Helle, Toft Sørensen Henrik, Hallas Jesper. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7_suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen Carsten Bøcker. The Danish Civil Registration System. Scand J Public Health. 2011;39(7_suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 20.Baadsgaard Mikkel, Quitzau Jarl. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7_suppl):103–105. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 21.Jensen Vibeke M., Rasmussen Astrid W. Danish education registers. Scand J Public Health. 2011;39(7_suppl):91–94. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 22.Schramm Tina Ken, Gislason Gunnar H., Køber Lars, Rasmussen Søren, Rasmussen Jeppe N., Abildstrøm Steen Z., Hansen Morten Lock, Folke Fredrik, Buch Pernille, Madsen Mette, Vaag Allan, Torp-Pedersen Christian. Diabetes Patients Requiring Glucose-Lowering Therapy and Nondiabetics With a Prior Myocardial Infarction Carry the Same Cardiovascular Risk: A Population Study of 3.3 Million People. Circulation. 2008;117(15):1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 23.Olesen J.B., Lip G.Y.H., Hansen M.L., Hansen P.R., Tolstrup J.S., Lindhardsen J., Selmer C., Ahlehoff O., Olsen A.-M.S., Gislason G.H., Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342(jan31 1):d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Østergaard L., Adelborg K., Sundbøll J., Pedersen L., Fosbøl E.L., Schmidt M. Positive predictive value of infective endocarditis in the Danish National Patient Registry: a validation study. BMJ Open. 2018;6 doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Østergaard Lauge, Valeur Nana, Ihlemann Nikolaj, Bundgaard Henning, Gislason Gunnar, Torp-Pedersen Christian, Bruun Niels Eske, Søndergaard Lars, Køber Lars, Fosbøl Emil Loldrup. Incidence of infective endocarditis among patients considered at high risk. Eur. Heart J. 2018;39(7):623–629. doi: 10.1093/eurheartj/ehx682. [DOI] [PubMed] [Google Scholar]
- 26.Østergaard Lauge, Valeur Nana, Wang Andrew, Bundgaard Henning, Aslam Mohsin, Gislason Gunnar, Torp-Pedersen Christian, Bruun Niels Eske, Søndergaard Lars, Køber Lars, Fosbøl Emil Loldrup. Incidence of infective endocarditis in patients considered at moderate risk. Eur. Heart J. 2019;40(17):1355–1361. doi: 10.1093/eurheartj/ehy629. [DOI] [PubMed] [Google Scholar]
- 27.Sundhedsstyrelsen, Personer med øget risiko ved COVID-19, (2020) 48.
- 28.Hussain Azhar, Roberts Neil, Oo Aung. Prosthetic aortic valve endocarditis complicated by COVID‐19 and hemorrhage. J Card Surg. 2020;35(6):1348–1350. doi: 10.1111/jocs.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samit Ghosal M.M., Bhattacharyya Rahul. Impact of complete lockdown on total infection and death rates: A hierarchical cluster analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020 doi: 10.1016/j.dsx.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundbøll Jens, Adelborg Kasper, Munch Troels, Frøslev Trine, Sørensen Henrik Toft, Bøtker Hans Erik, Schmidt Morten. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.