Abstract
Background
Knowledge about the association between frailty and self-reported health among patients undergoing heart valve surgery remains sparse. Thus, the objectives were to I) describe changes in self-reported health at different time points according to frailty status, and to II) investigate the association between frailty status at discharge and poor self-reported health four weeks after discharge among patients undergoing heart valve surgery.
Methods
In a prospective cohort study, consecutive patients undergoing heart valve surgery, including transapical/transaortic valve procedures were included. Frailty was measured using the Fried score, and self-reported health using the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the EuroQoL-5 Dimensions 5-Levels Health Status Questionnaire (EQ-5D-5L).
To investigate the association between frailty and self-reported health, multivariable logistic regression models were used. Analyses were adjusted for sex, age, surgical risk evaluation (EuroScore) and procedure and presented as odds ratios (OR) with 95% confidence intervals (CI).
Results
Frailty was assessed at discharge in 288 patients (median age 71, 69% men); 51 patients (18%) were frail. In the multivariable analyses, frailty at discharge remained significantly associated with poor self-reported health at four weeks, OR (95% CI): EQ-5D-5L Index 3.38 (1.51–7.52), VAS 2.41 (1.13–5.14), and KCCQ 2.84 (1.35–5.97).
Conclusion
Frailty is present at discharge in 18% of patients undergoing heart valve surgery, and being frail is associated with poor self-reported health at four weeks of follow-up. This supports a clinical need to address the unique risk of frail patients among heart valve teams broadly, and not only to measure frailty as a marker of operative risk.
Keywords: Heart valves, Cardiac surgery, Frailty, Health-related quality of life
1. Introduction
Frailty reflects a multidimensional state of decreased physiological reserves, a vulnerability towards pathological stressors, and a loss of adaptive capacity [1], [2], [3]. Elements of frailty commonly include lowered activity level, loss of muscle strength, unintentional weight loss, and self-reported exhaustion [4], [2]. These elements represent an overall marker of the functional and physical condition of the patient [4], [5], and combined; frailty status describes a patient’s physiological reserves and resistance to stressors [2].
Among patients with severe heart valve disease, the 2017-European guidelines on the management of valvular heart disease recommend assessment of frailty status before surgery as a strategy for stratifying patients at risk of poor outcomes [6]. Similarly, there is a growing body of evidence demonstrating that preoperative frailty is associated with mortality, morbidity, and functional decline after aortic valve replacement, mainly investigated in patients undergoing transcatheter aortic valve replacement (TAVR) [7], [8], [9].
Regardless of the choice of procedure (e.g., TAVR procedure or open heart valve surgery), frail patients are known to have worse self-reported health compared with non-frail patients [10], [11]. Nevertheless, evidence demonstrating whether frail patients perceive similar improvements in self-reported health status post-surgery as non-frail patients remain sparse and current studies fails to include patients who require surgical treatment [10], [11]. Although transcatheter approaches are increasingly used to treat various valvular heart diseases, surgical approaches remain the standard of care for a large group of patients; accordingly, this warrants further investigation of the prognosis and recovery trajectory of frail patients after discharge in the setting of surgical treatment of valvular heart disease.
Thus, in a population of patients undergoing heart valve surgery the objectives were to I) describe changes in self-reported health at different time points according to frailty status, and to II) investigate the association between frailty status at discharge and poor self-reported health four weeks after discharge.
2. Methods
2.1. Study design
A prospective cohort study investigating the association between frailty status and self-reported health among patients undergoing heart valve surgery.
The study is a pre-defined sub-study of the findings of the Individualised Follow-up after Valve Surgery (INVOLVE) study [12]. The INVOLVE study was a prospective cohort study comparing a composite endpoint of the first event of unplanned cardiac hospital readmissions or all-cause mortality in patients undergoing early, individualised, and intensified follow-up after heart valve surgery with a propensity-matched historical control group [12]. In the current sub-study, the association between frailty status and self-reported health following heart valve surgery among the intervention group of INVOLVE was investigated. Furthermore, additional data on frailty assessment at three different time points was performed in a consecutively selected sub-population (n = 120).
2.2. Participants and setting
Consecutive patients undergoing heart valve surgery (conventional trans-sternal surgery (replacement or repair) or transapical or transaortic TAVR) were enrolled in the intervention from November 2016 – November 2017, at Odense University Hospital, Denmark. The sub-population was included from May 2017 to November 2017. Patients were included based on their surgical procedure codes [13]: Aortic (KFCA, KFMA, KFMC, KFMD), Mitral (KFKB, KFKC, KFKD, KDKW) and Tricuspid (KFGC, KFGE).
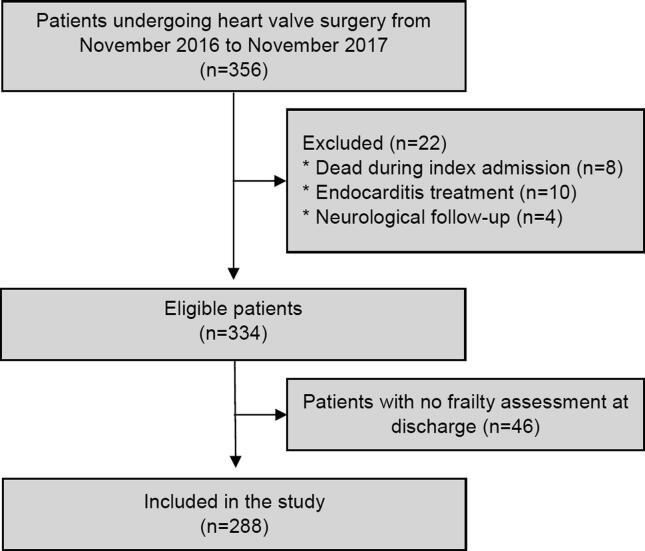
Patients with disabling perioperative stroke and patients undergoing surgery due to infective endocarditis were not eligible for inclusion (Fig. 1). Patients undergoing transfemoral TAVR were not included in the current study.
Fig. 1.
Study Flowchart.
2.3. Data collection
2.3.1. Demographic and clinical data
Demographics and clinical characteristics were obtained from hospital records and the Western Denmark Heart Registry [14]. Living status, body mass index (BMI), alcohol consumption, smoking status and length of stay were obtained from the electronic medical records, while comorbidity, the type of surgery, and EuroScore II (surgical risk evaluation) were obtained from WDHR. The EuroScore II is a logistic surgical risk evaluation calculated before surgery including age, sex, renal impairment, extracardiac arteriopathy, poor mobility, chronic lung disease, active endocarditis, critical pre-operative state, angina status, recent myocardial infarction, pulmonary hypertension, urgency and weight of the procedure [15].
2.3.2. Frailty assessment
Frailty status was assessed at discharge with a modified version of the frailty phenotype by Fried [4] including; unintentional weight loss, feelings of exhaustion, weakness (grip strength), gait speed, and independence in Activities of Daily Living (ADL) measured with the Katz Index [16]. Cut-off scores, as defined by Fried, were used for gait speed and grip strength [4].
The overall frailty status of the patient was assessed based on the above domains. Patients with problems in ≥ 3 domains were considered as frail [4].
Further, in the sub-population of 120 consecutively included patients, frailty was assessed preoperatively, at discharge and four weeks after discharge.
2.3.3. Self-reported health
The following patient-reported outcome measurements were included: The EuroQoL-5 Dimensions 5-Levels Health Status Questionnaire (EQ-5D-5L), and the Kansas City Cardiomyopathy Questionnaire (KCCQ), 12-item [17], [18]. The measurements were handed out on the day before surgery (baseline), at discharge and four weeks after discharge. Due to two weeks recall, KCCQ was only handed out at baseline and four weeks, whereas EQ-5D-5L was handed out at all time points.
The EQ-5D-5L assesses generic health with an Index Score and a Visual Analogue Scale, VAS, and has proven high validity and performance among patients undergoing heart valve surgery [17], [19]. Higher scores indicate better health. Minimal clinically important differences (MCID) has been reported to be 0.10/0.125 (index score) and 8.61 (VAS) [20], [19].
The KCCQ is a disease-specific questionnaire assessing four domains, and combined into an overall summary score (0–100). Higher scores indicate better health and low symptom burden [18]. The original KCCQ is validated among patients with aortic stenosis [21]. A MCID of 5 points for the KCCQ has previously been detected [18].
Supplementary Table S1 illustrates the measurement of frailty status and self-reported health at different time points.
2.4. Outcomes
Poor self-reported health measured with the EQ-5D-5L was defined as the composite of: 1) a score within the worst quartile of scores, and/or, 2) a decrease in scores from baseline to four weeks after discharge of more than the MCID.
Poor self-reported health with the KCCQ was defined as a composite of: 1) a score < 45 measured with the overall summary score (KCCQ-OS) score, and/or 2) a decrease of ≥ 10 points in the KCCQ-OS score from baseline to four weeks after discharge [22].
2.5. Statistical analyses
Baseline characteristics are presented as numbers and proportions, and median with 25th to 75th percentiles (interquartile range, IQR), as appropriate. Due to non-normally of data, the Mann-Whitney U test was used to compare continuous data, whereas categorical variables were compared using the χ2 test.
Differences in proportions of patients having problems within the different elements of the frailty test were investigated among non-frail and frail patients using the χ2 test and plotted as a radar chart. The proportion of patients being frail before surgery, at discharge and four weeks after discharge were investigated in the sub-population.
Due to the skewed distribution of scores of self-reported health, these were presented as median and IQR, and non-parametric tests were applied to analyse these data. Differences in median scores of the EQ-5D-5L preoperatively, at discharge, and four weeks after discharge among non-frail and frail patients were tested with the Wilcoxon signed-rank test (paired), similar as the differences in scores of the KCCQ preoperatively and four weeks after discharge. Changes in mean scores of both EQ-5D-5L and KCCQ at different time point were analysed with the paired-samples t-test.
Univariable and multivariable logistic regression models were used to assess the association between being frail (vs non-frail) and poor self-reported health. Each instrument was adjusted for sex, age, EuroScore II, and TAVR procedure (as this was expected to influence frailty status) in the multivariable regression models. Results are presented as odds ratios (OR) with 95% confidence intervals (CI).
The interaction between the patients undergoing surgical and transapical procedures on poor self-reported health was assessed separately in post-hoc analyses.
A two-sided value of p < 0.05 was considered as statistically significant. The statistical analyses were performed using STATA 14.2 (StataCorp, College Station, TX, USA) and SPSS 24 (IBM Corp. Armonk, NY).
2.6. Ethics approval
The investigation conformed with the principles outlined in the Declaration of Helsinki. The study was approved by the Danish Data Protection Agency (18/19152). All patients received oral and written information and provided written consent.
3. Results
3.1. Study population
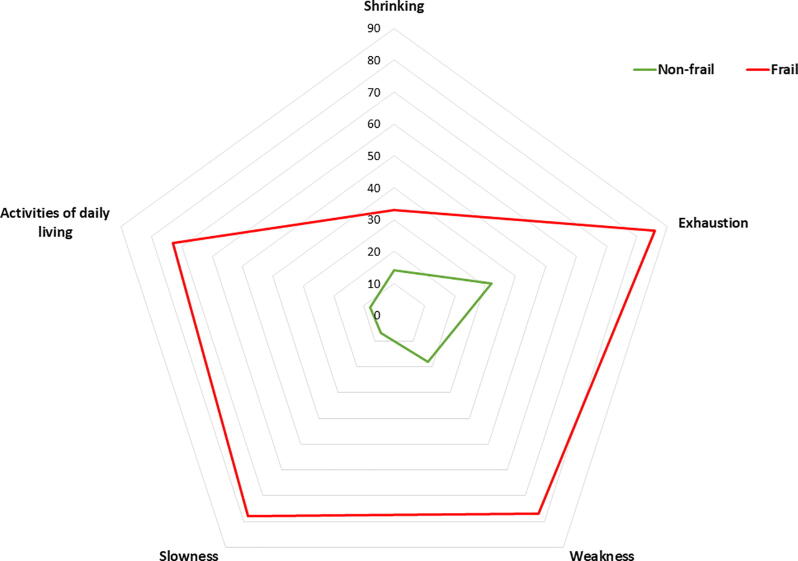
Among 334 patients enrolled in the prospective study, 288 patients (86%) had a frailty assessment at discharge (median age 71, 69% men, 64% were diagnosed with aortic stenosis) and were included in the current study (Fig. 1). Of those, 51 (18%) were frail, and among patients above the age of 70, n = 41 (24%) were frail. Compared to non-frail patients, frail patients were more likely to be women (53% vs 26%, p < 0.001), were older (median 78 years vs 70 years, p < 0.001), and living alone (43% vs 22%, p < 0.001) (Table 1). In addition, several clinical and procedural differences were found among non-frail and frail patients, including a higher proportion of frail patients having a EuroScore II ≥ 2 (71% vs 44%, p < 0.001) and an estimated glomerular filtration rate ≤ 60 (49% vs 22%, p < 0.001) (Table 1). The proportion of patients being frail was highest at discharge and was significantly reduced at follow-up one month after discharge (Supplementary Fig. S1). Also, among the frail patients, the proportion of patients experiencing problems within each area included; shrinking (33%), exhaustion (86%), weakness (77%), slowness (78%), and ADL (73%) (Fig. 2, Supplementary Table S2).
Table 1.
Demographic and clinical characteristics.
| Non-frail (n = 237) | Frail (n = 51) | p* | |
|---|---|---|---|
| Characteristics | |||
| Sex, female, n (%) | 62 (26) | 27 (53) | <0.001 |
| Age, median (IQR) | 70 (63;75) | 78 (72;83) | <0.001 |
| Living alone, n (%) | 52 (22) | 22 (43) | 0.002 |
| Patient characteristics | |||
| Reduced pulmonary functiona, n (%) | 85 (36) | 24 (47) | 0.135 |
| EuroScore II ≥ 4, n (%) | 50 (21) | 23 (45) | <0.001 |
| Estimated glomerular filtration rate ml/min.b ≤ 60, n (%) | 53 (22) | 25 (49) | <0.001 |
| Prior cardiac surgery, n (%) | 24 (11) | 11 (22) | 0.030 |
| Permanent pacemaker, n (%) | 11 (5) | <5 | 0.720 |
| Ejection fraction ≤ 50%, n (%) | 73 (31) | 19 (37) | 0.370 |
| NYHA class ≥ 2, n (%) | 223 (94) | 50 (98) | 0.484 |
| Body Mass Index, median (IQR) | 26 (23;29) | 26 (22;28) | 0.064 |
| Type of valve procedure, n (%) | |||
| Aortic valve, surgical replacement or repair | 186 (79) | 31 (61) | 0.008 |
| TAVRf (transaortic/ transapical) | 11 (5) | 11 (22) | <0.001 |
| Mitral valve, surgical replacement or repair | 39 (17) | 9 (18) | 0.836 |
| Concomitant CABG + aortic valve surgery | 39 (17) | 9 (18) | 0.836 |
| Concomitant CABG + mitral valve surgery | 6 (3) | <5 | 0.584 |
| Post-operatively | |||
| Re-operation, n (%) | 14 (6) | 9 (18) | 0.010 |
| Length of stay, intensive care unit, median (IQR) | 1 (1;1) | 1 (1;2) | <0.001 |
| Length of stay, median (IQR) | 9 (7;11) | 12 (8;19) | <0.001 |
P < 0.05 is considered as statistically significant.
Forced expiratory volume,% ≤80% of predicted value and/or a history of chronic obstructive pulmonary disease.
Estimated by the Cockcroft-Gault Equation.
Fig. 2.
The proportion of patients with problems within the specific domains of the Fried Frailty Scale. The figure plots the proportion of problems within the specific areas among non-frail and frail patients with the use of a radar plot.
3.2. Differences and changes in self-reported health among frail and non-frail patients
Self-reported health measured with the EQ-5D-5L Index score, the EQ-5D-5L VAS score, and the KCCQ were significantly better among non-frail and frail patients before surgery, at discharge, and four weeks after discharge (Table 2).
Table 2.
Differences in scores of self-reported health status among non-frail and frail patients.
| Median (IQR) | Non-frail (n = 237) | Frail (n = 51) | p |
|---|---|---|---|
| Preoperative | |||
| EQ-5D-5L | |||
| Index score | 0.79 (0.71–0.86) | 0.70 (0.63–0.81) | <0.001 |
| VAS score | 70 (50–80) | 61 (41–74) | 0.002 |
| KCCQ | |||
| Summary score | 64.6 (47.9–78.1) | 58.9 (41.4–68.0) | 0.018 |
| Discharge | |||
| EQ-5D-5L | |||
| Index score | 0.75 (0.67–0.81) | 0.65 (0.57–0.73) | <0.001 |
| VAS score | 70 (50–80) | 50 (40–61.25) | <0.001 |
| Four weeks after discharge | |||
| EQ-5D-5L | |||
| Index score | 0.80 (0.75–0.86) | 0.73 (0.66–0.79) | <0.001 |
| VAS score | 80 (66–87) | 70 (60–75) | <0.001 |
| KCCQ | |||
| Summary score | 77.6 (62.5–87.5) | 61.2 (49.5–75.0) | <0.001 |
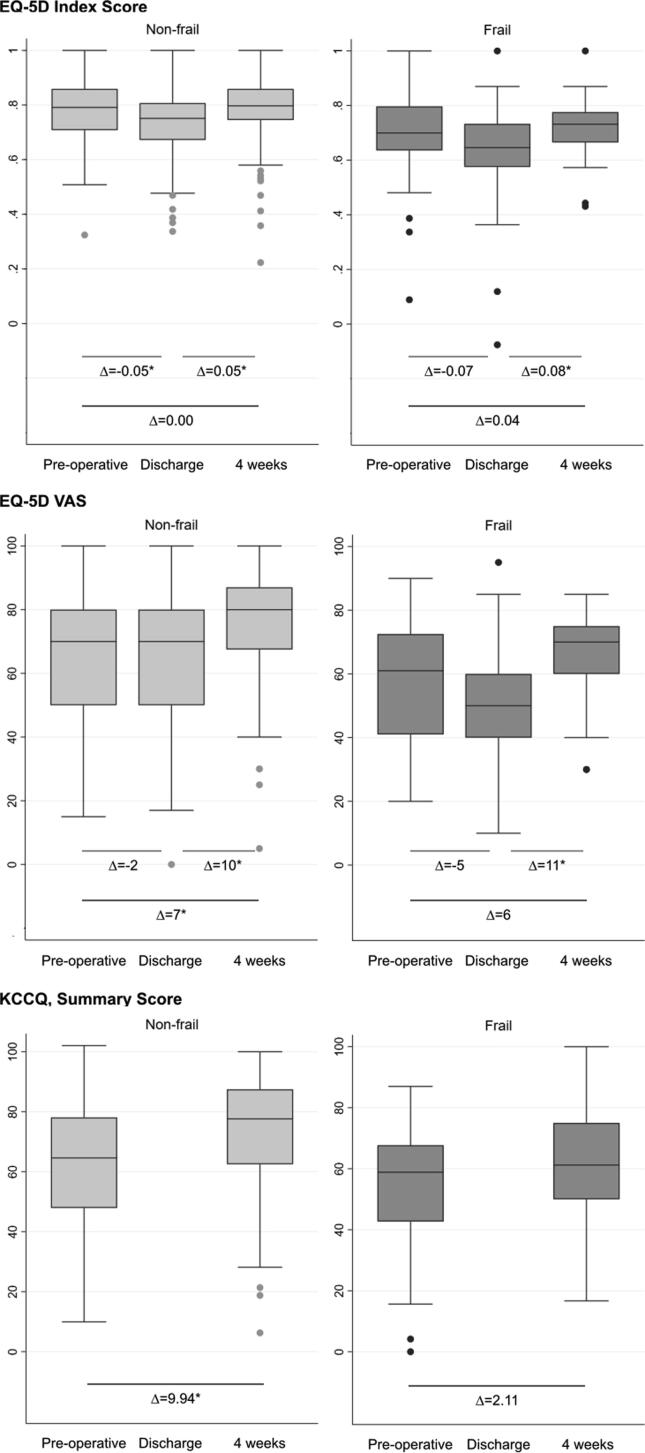
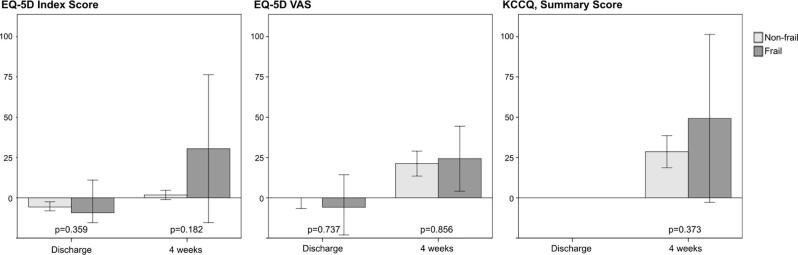
Non-frail patients experienced a significant reduction in the EQ-5D-5L Index scores between the preoperative assessment and discharge (EQ-5D-5L Index Δ-0.05, 95% CI −0.07 – −0.03) and a significant increase between discharge and four weeks post-discharge on both the EQ-5D-5L Index score and VAS (EQ-5D-5L Index score Δ0.05, 95% CI 0.03–0.07, VAS Δ10, 95% CI 7–13). Although statistically significant, the changes only reached a clinically important difference for the EQ-5D-5L VAS. Scores of the EQ-5D-5L VAS and the KCCQ significantly increased from the preoperative assessment to four weeks after discharge (VAS Δ7, 95% CI 4–10 and KCCQ Δ9.94, 95% CI 6.86–13.01), reaching a clinically important difference for the KCCQ (Fig. 3, Supplementary Table S3). Contrary, among frail patients, a statistically significant improvement from discharge to four weeks after discharge was shown on the EQ-5D-5L Index and the EQ-5D-5L VAS (Index Δ0.08, 95% CI 0.02–0.13, VAS Δ11, 95% CI 5–18), but not on the KCCQ (Fig. 3, Supplementary Table S3). The scores of the EQ-5D-5D VAS reached a clinically important difference. Relative changes (%) in scores among frail and non-frail patients from baseline to discharge and four weeks after discharge did not significantly differ (Fig. 4).
Fig. 3.
Changes in scores of the EQ-5D-5L and the KCCQ among non-frail and frail patients. The figure illustrates changes in self-reported health among non-frail and frail patients, including mean change in scores on the different time points. * P < 0.05 is considered as statistically significant.
Fig. 4.
Relative changes, percentage, in scores at different time point among non-frail and frail patients. The figure illustrates relative changes (%) in self-reported health status from baseline to discharge and four weeks after discharge among non-frail and frail patients. Self-reported health was only measured at two time points on the KCCQ and thus, not illustrated from baseline to discharge. * P < 0.05 is considered as statistically significant.
3.3. Association between frailty and poor self-reported health four weeks after discharge
In the logistic regression models, being frail vs non-frail was significantly associated with poor self-reported health in the adjusted analyses (OR (95% CI)) on the EQ-5D-5L Index (3.38 (1.51–7.52)), the EQ-5D-5L VAS (2.41 (1.13–5.14)), and the KCCQ (2.84 (1.35–5.97)). The analyses were adjusted for sex, age, EuroScore and TAVR procedure. Crude and adjusted analyses are presented in Table 3.
Table 3.
The association between frailty at discharge and poor self-reported health status four weeks after discharge.
| Crude OR (CI) | Adjusted OR (CI) | |
|---|---|---|
| EQ-5D-5L index, poor outcomes | ||
| Frail vs non-frail | 2.46 (1.20–5.05) | 3.38 (1.51–7.52) |
| EQ-5D-5L VAS, poor outcomes | ||
| Frail vs non-frail | 2.14 (1.07–4.27) | 2.41 (1.13–5.14) |
| KCCQ, poor outcomes | ||
| Frail vs non-frail | 2.14 (1.10–4.16) | 2.84 (1.35–5.97) |
Adjusted for sex, age, EuroScore and TAVR procedure.
The results of the post-hoc analyses revealed no statistically significant interactions between the surgical and the transapical patients on poor self-reported health (p-value of the interactions; EQ-5D 5L Index 0.364, VAS 0.873, KCCQ 0.236).
4. Discussion
In this prospective study, we investigated the association between frailty and poor self-reported health in a population of patients undergoing heart valve surgery. Nearly one out of five was frail at discharge. Main problem areas among the frail population were exhaustion, weakness, and slowness. In addition, frail patients reported significantly lower scores of health and frailty was independently associated with worse self-reported health on all measures.
Several studies have demonstrated the proportion of patients being frail when undergoing heart valve surgery, but tend to focus on preoperative frailty assessments [7], [11], [23]. Previously, Afilalo et al. have demonstrated how 25% of patients above 70 years undergoing surgical aortic valve replacement are frail measured with the Fried frailty score at different time points (during admission and in outpatient clinics) [24]. This is comparable with 24% of patients above 70 years being frail in our study. To our knowledge, though, the current study is the first study to investigate frailty status at discharge in a consecutive population of patients following heart valve surgery. Although only measured in a sub-population of 120 patients, we have demonstrated how the proportion of patients being frail at the time of discharge is higher than before surgery – and reduces again after four weeks. As this highlights how frailty is a dynamic state, it might be an essential measure of the post-procedural pathway and the knowledge of the surgical procedure on physical changes can guide clinicians on expected outcomes. Thus, although not previously investigated, post-surgical measurements of frailty might be a useful marker of the clinical pathway.
Notably, while one-third of the frail patients were experiencing problems within the overall area “shrinking”, this was also the area where fewest frail patients had problems. Due to the metabolic stress of the surgery, more patients were expected to experience problems within this area; however, problems with exhaustion, weakness, and slowness dominated the frail population. Previous studies have demonstrated how the most effective way to improve the status of frail patients is exercise and physical rehabilitation, as it improves balance (and prevent falls), increase gait speed and improve overall performance in ADL functions [25], [26]. Currently, the PERFORM-TAVR trial is testing an intervention consisting of a home-based exercise programme in combination with nutritional supplements on physical performance [27]. Similarly, the FOCUS-recommendations suggest interventions to include physical activity, nutritional interventions or a combination, to prevent, delay or reverse frailty [28]. In general, though, there is a need for high-quality studies investigating interventions with the potential to affect frailty [28]. Consequently, to reverse and change outcomes among frail patients, interventions should be aimed at the specific problem areas.
While the risk of morbidity and mortality increases with age and frailty status, potential gains in health status are important measures to include in the overall assessment of a patient. We demonstrated the importance of such assessment by demonstrating an association between frailty at discharge and the risk of poor self-reported health four weeks after discharge. When measuring health status with a disease-specific measurement (KCCQ), frail patients do not show the same increase in scores as non-frail patients from before surgery to four weeks, why an effort in improving these outcomes should be performed. Contrary, on the EQ-5D-5L VAS scale, both non-frail and frail patients experienced an improvement of more than the clinical minimal importance difference measured from discharge to four weeks after. These findings correspond to previous studies, although improvements were demonstrated based on a longer follow-up period [10], [23]. Surprisingly, the findings combined suggest that overall improvements in generic self-reported health remain similar, despite frail patients having lower scores. We expect that the surgical procedure influence patients being frail to a greater extent than non-frail patients, due to the vulnerability towards stressors, and frail patients might, therefore, experience more symptoms related to the disease and the surgery. This might explain the lack of improvement in disease-specific self-reported health. Accordingly, follow-up among frail patients should be targeted the disease-specific symptoms.
Finally, although a clear “gold standard” when measuring frailty is missing, and some studies have highlighted how the “eyeball test” might supplement objective measurement among patients with aortic stenosis [29], [30], a more systematic approach is needed to support the recovery trajectories of the patients [31]. Besides risk stratification before surgery, the current study showed how a frailty assessment at discharge could stratify patients at higher risks of poor outcomes when going home. Also, we acknowledge how the assessment of frailty may include more than physical elements, as characteristics such as cognition and social support are known to influence outcomes [32]. Future studies are encouraged to include a broader frailty assessment when investigating outcomes among patients with valvular heart disease.
4.1. Strength and limitations
The main strength of the study is the use of prospective data on frailty status and self-reported health and the investigation of frailty status at discharge – also among a surgical population. Combined, this adds to the current knowledge of the clinical pathway following heart valve surgery. Similarly, a strength of the study is the inclusion of both generic and disease-specific measurements of self-reported health. As these instruments seem to detect different trajectories, the inclusion of both strengthens the findings.
In total, 46 patients (14%) did not receive a frailty assessment due to early discharge without the involvement of research personnel. These patients may potentially have influenced the results, but due to similar baseline characteristics (median age 71, 69% men, 73% underwent surgical aorta valve replacement, 8% TAVR-procedure), this is unlikely. Patients receiving an alternative access TAVR-procedure were included as they are expected to have a clinical pathway following the procedure similar to those of patients undergoing a surgical procedure. We recognise, though, how a higher proportion of these patients might be frail, but as interaction analyses revealed no significant interactions between the surgical and the transapical groups, we have kept this group in the study. Frailty assessment at all time points among the total population and not only a consecutive sub-population would have strengthened the results. Also, the present study had no power to determine whether frailty status at discharge is of prognostic importance.
In general, the response rates of the questionnaires were high (77–92%) [33], and missing data were assumed to be missing at random, why the regression analyses were performed as a complete case analysis. Moreover, although the sample size is small, leading to a conservative estimate of the results, the change in median scores on the EQ-5D-5L did not reach a clinically important difference. Thus, the overall results are not expected to be different in a larger population.
In conclusion, frailty at discharge occurs in approximately one out of five patients while being frail is associated with poor self-reported health. However, frailty assessment in patients after heart valve surgery is scarce. Future research is needed to study integration of a standardised measurement of frailty to improve outcomes after heart valve surgery.
4.2. Implications for practice
The study underlined the association between being frail at discharge and worse self-reported health after four weeks. This knowledge supports a clinical need to address the unique risk of frail surgical patients among heart valve teams broadly, and not only to measure frailty as a marker of operative risk. A complex care plan of frail patients should be incorporated in the discharge planning, and frail surgical valve patients potentially require closer follow-up and targeted interventions to improve overall outcomes, frailty status and self-reported health. Also, to prevent further deterioration in physical capacity, an exercise plan, early in-hospital rehabilitation, and referral for rehabilitation following discharge should gain increased focus [34], [35]. Despite the prognostic and therapeutic relevance, frailty is not routinely assessed among patients undergoing open heart valve surgery and the current study highlight how ongoing efforts must focus on improving both the measurement and the treatment of this health state – to improve the overall health outcome of the valve patient.
Author contribution
BBO, JD, and JEM designed the study. BBO conducted the analyses in collaboration with JD, JR and JEM. BBO drew up the first draft of the manuscript, tables and figures. All authors contributed to the interpretation of data, revisions to the manuscript and approved the final version to be published.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thank you to the patients for participating in the study and to Siri Rosenkilde and Louise Stougaard for helping with the frailty assessments in the sub-study. Also, we thank the following foundations for their contributions: Helsefonden, The Odense University Hospital PhD Foundation, Ove William Buhl Olesen Foundation, and Kurt Bøn.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100671.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Xue Q.-L. The frailty syndrome: definition and natural history. Clin. Geriatr. Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan D.B. Models, definitions, and criteria for frailty. Conn's handbook of models for human aging. Elsevier. 2018:35–44. [Google Scholar]
- 3.Afilalo Jonathan. Conceptual models of frailty: The sarcopenia phenotype. Can. J. Cardiol. 2016;32(9):1051–1055. doi: 10.1016/j.cjca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., McBurnie M.A. Frailty in older adults: evidence for a phenotype. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 5.Sacha J., Sacha M., Sobon J., Borysiuk Z., Feusette P. Is it time to begin a public campaign concerning frailty and pre-frailty? A review article. Frontiers Physiol. 2017;8:484. doi: 10.3389/fphys.2017.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner Helmut, Falk Volkmar, Bax Jeroen J, De Bonis Michele, Hamm Christian, Holm Per Johan, Iung Bernard, Lancellotti Patrizio, Lansac Emmanuel, Rodriguez Muñoz Daniel, Rosenhek Raphael, Sjögren Johan, Tornos Mas Pilar, Vahanian Alec, Walther Thomas, Wendler Olaf, Windecker Stephan, Zamorano Jose Luis, Roffi Marco, Alfieri Ottavio, Agewall Stefan, Ahlsson Anders, Barbato Emanuele, Bueno Héctor, Collet Jean-Philippe, Coman Ioan Mircea, Czerny Martin, Delgado Victoria, Fitzsimons Donna, Folliguet Thierry, Gaemperli Oliver, Habib Gilbert, Harringer Wolfgang, Haude Michael, Hindricks Gerhard, Katus Hugo A, Knuuti Juhani, Kolh Philippe, Leclercq Christophe, McDonagh Theresa A, Piepoli Massimo Francesco, Pierard Luc A, Ponikowski Piotr, Rosano Giuseppe M C, Ruschitzka Frank, Shlyakhto Evgeny, Simpson Iain A, Sousa-Uva Miguel, Stepinska Janina, Tarantini Giuseppe, Tchétché Didier, Aboyans Victor, Windecker Stephan, Aboyans Victor, Agewall Stefan, Barbato Emanuele, Bueno Héctor, Coca Antonio, Collet Jean-Philippe, Coman Ioan Mircea, Dean Veronica, Delgado Victoria, Fitzsimons Donna, Gaemperli Oliver, Hindricks Gerhard, Iung Bernard, Jüni Peter, Katus Hugo A, Knuuti Juhani, Lancellotti Patrizio, Leclercq Christophe, McDonagh Theresa, Piepoli Massimo Francesco, Ponikowski Piotr, Richter Dimitrios J, Roffi Marco, Shlyakhto Evgeny, Simpson Iain A, Zamorano Jose Luis, Kzhdryan Hovhannes K, Mascherbauer Julia, Samadov Fuad, Shumavets Vadim, Camp Guy Van, Lončar Daniela, Lovric Daniel, Georgiou Georgios M, Linhartova Katerina, Ihlemann Nikolaj, Abdelhamid Magdy, Pern Teele, Turpeinen Anu, Srbinovska-Kostovska Elizabeta, Cohen Ariel, Bakhutashvili Zviad, Ince Hüseyin, Vavuranakis Manolis, Temesvári András, Gudnason Thorarinn, Mylotte Darren, Kuperstein Rafael, Indolfi Ciro, Pya Yury, Bajraktari Gani, Kerimkulova Alina, Rudzitis Ainars, Mizariene Vaida, Lebrun Frédéric, Demarco Daniela Cassar, Oukerraj Latifa, Bouma Berto J, Steigen Terje Kristian, Komar Monika, De Moura Branco Luisa Maria, Popescu Bogdan A, Uspenskiy Vladimir, Foscoli Marina, Jovovic Ljiljana, Simkova Iveta, Bunc Matjaz, de Prada José Antonio Vázquez, Stagmo Martin, Kaufmann Beat Andreas, Mahdhaoui Abdallah, Bozkurt Engin, Nesukay Elena, Brecker Stephen J D. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 7.Afilalo J., Lauck S., Kim D.H., Lefevre T., Piazza N., Lachapelle K. Frailty in older adults undergoing aortic valve replacement: The FRAILTY-AVR study. J. Am. Coll. Cardiol. 2017;70(6):689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Skaar Elisabeth, Eide Leslie Sofia Pareja, Norekvål Tone Merete, Ranhoff Anette Hylen, Nordrehaug Jan Erik, Forman Daniel Edward, Schoenenberger Andreas W, Hufthammer Karl Ove, Kuiper Karel Kier-Jan, Bleie Øyvind, Packer Erik Jerome Stene, Langørgen Jørund, Haaverstad Rune, Schaufel Margrethe Aase. A novel geriatric assessment frailty score predicts 2-year mortality after transcatheter aortic valve implantation. Eur. Heart J. Qual Care Clin. Outcomes. 2019;5(2):153–160. doi: 10.1093/ehjqcco/qcy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundi Harun, Popma Jeffrey J, Reynolds Matthew R, Strom Jordan B, Pinto Duane S, Valsdottir Linda R, Shen Changyu, Choi Eunhee, Yeh Robert W. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur. Heart J. 2019;40(27):2231–2239. doi: 10.1093/eurheartj/ehz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotajarvi Brian R., Schafer Marissa J., Atkinson Elizabeth J., Traynor Megan M., Bruce Charles J., Greason Kevin L., Suri Rakesh M., Miller Jordan D., LeBrasseur Nathan K. The impact of frailty on patient-centered outcomes following aortic valve replacement. J. Gerontology Ser. A, Biol. Sci. Med. Sci. 2017;72(7):917–921. doi: 10.1093/gerona/glx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry Linda, Halpin Linda, Barnett Scott D., Pritchard Grace, Sarin Eric, Speir Alan M. Frailty in the Cardiac Surgical Patient: Comparison of Frailty Tools and Associated Outcomes. The Annals of Thoracic Surgery. 2019;108(1):16–22. doi: 10.1016/j.athoracsur.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Borregaard Britt, Dahl Jordi Sanchez, Riber Lars Peter Schødt, Ekholm Ola, Sibilitz Kirstine Lærum, Weiss Marc, Sørensen Jan, Berg Selina Kikkenborg, Møller Jacob Eifer. Effect of early, individualised and intensified follow-up after open heart valve surgery on unplanned cardiac hospital readmissions and all-cause mortality. Int. J. Cardiol. 2019;289:30–36. doi: 10.1016/j.ijcard.2019.02.056. [DOI] [PubMed] [Google Scholar]
- 13.Committee NM-S. NOMESCO classification of surgical procedures. Copenhagen; 2011.
- 14.Schmidt M., Maeng M., Jakobsen C.J., Madsen M., Thuesen L., Nielsen P.H. Existing data sources for clinical epidemiology: The Western Denmark Heart Registry. Clin. Epidemiol. 2010;2:137–144. doi: 10.2147/clep.s10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nashef S.A.M., Roques F., Sharples L.D., Nilsson J., Smith C., Goldstone A.R., Lockowandt U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012;41(4):734–745. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 16.Katz S., Ford A.B., Moskowitz R.W., Jackson B.A., Jaffe M.W. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 17.Rabin R., de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 18.Spertus John A., Jones Philip G. Development and Validation of a short version of the kansas city cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469–476. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes Charlotte, Briffa Norman. Patient-Reported Outcome Measures (PROMS) in patients undergoing heart valve surgery: why should we measure them and which instruments should we use? Open Heart. 2016;3(1):e000315. doi: 10.1136/openhrt-2015-000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Poyu, Lin Keh-Chung, Liing Rong-Jiuan, Wu Ching-Yi, Chen Chia-Ling, Chang Ku-Chou. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Qual Life Res. 2016;25(6):1585–1596. doi: 10.1007/s11136-015-1196-z. [DOI] [PubMed] [Google Scholar]
- 21.Arnold Suzanne V., Spertus John A., Lei Yang, Allen Keith B., Chhatriwalla Adnan K., Leon Martin B., Smith Craig R., Reynolds Matthew R., Webb John G., Svensson Lars G., Cohen David J. Use of the Kansas City cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 22.Arnold Suzanne V., Spertus John A., Lei Yang, Green Philip, Kirtane Ajay J., Kapadia Samir, Thourani Vinod H., Herrmann Howard C., Beohar Nirat, Zajarias Alan, Mack Michael J., Leon Martin B., Cohen David J. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6(5):591–597. doi: 10.1161/CIRCOUTCOMES.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miguelena-Hycka Javier, Lopez-Menendez Jose, Prada Pablo-Cesar, Rodriguez-Roda Jorge, Martin Miren, Vigil-Escalera Carlota, Hernandez-Vaquero Daniel, Miguelena Jose M., Silva Jacobo, Gonzalez-Colaço Magali. Influence of preoperative frailty on health-related quality of life after cardiac surgery. Ann. Thoracic Surg. 2019;108(1):23–29. doi: 10.1016/j.athoracsur.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Afilalo Jonathan, Lauck Sandra, Kim Dae Hyun, Lefèvre Thierry, Piazza Nicolo, Lachapelle Kevin, Martucci Giuseppe, Lamy Andre, Labinaz Marino, Peterson Mark, Arora Rakesh, Noiseux Nicolas, Rassi Andrew, Palacios Igor, Généreux Philippe, Lindman Brian, Asgar Anita, Kim Caroline, Trnkus Amanda, Morais Jose, Langlois Yves, Rudski Lawrence, Popma Jeffrey, Webb John, Perrault Louis. Frailty assessment in older adults undergoing transcatheter or surgical aortic valve replacement: the frailty-avr study. J. Am. Coll. Cardiol. 2016;67(13):8. doi: 10.1016/S0735-1097(16)30009-2. [DOI] [Google Scholar]
- 25.Macera Caroline A., Cavanaugh Alyson, Bellettiere John. State of the art review: physical activity and older adults. Am. J. Lifestyle Med. 2017;11(1):42–57. doi: 10.1177/1559827615571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Yixiong, Zhang Yuqun, Du Shizheng, Wang Qiuling, Xia Haozhi, Sun Rong. Exercise interventions for improving physical function, daily living activities and quality of life in community-dwelling frail older adults: A systematic review and meta-analysis of randomized controlled trials. Geriatric Nursing. 2020;41(3):261–273. doi: 10.1016/j.gerinurse.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 27.J. Afilalo, https://clinicaltrials.gov/ct2/show/NCT03522454.
- 28.Marcucci Maura, Damanti Sarah, Germini Federico, Apostolo Joao, Bobrowicz-Campos Elzbieta, Gwyther Holly, Holland Carol, Kurpas Donata, Bujnowska-Fedak Maria, Szwamel Katarzyna, Santana Silvina, Nobili Alessandro, D’Avanzo Barbara, Cano Antonio. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1) doi: 10.1186/s12916-019-1434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauck Sandra B, Achtem Leslie, Borregaard Britt, Baumbusch Jennifer, Afilalo Jonathan, Wood David A, Forman Jacqueline, Cheung Anson, Ye Jian, Webb John G. Can you see frailty? An exploratory study of the use of a patient photograph in the transcatheter aortic valve implantation programme. Eur. J. Cardiovascular Nursing : J. Working Group on Cardiovascular Nursing of the Eur. Soc. Cardiology. 2020 doi: 10.1177/1474515120953739. 147451512095373. [DOI] [PubMed] [Google Scholar]
- 30.Green Philip, Chung Christine J., Oberweis Brandon S., George Isaac, Vahl Torsten, Harjai Kishore, Liao Ming, Jaquez Luz, Hawkey Marian, Khalique Omar, Hahn Rebecca T., Williams Mathew R., Kirtane Ajay J., Leon Martin B., Kodali Susheel K., Nazif Tamim M. The “eyeball test” for risk assessment in aortic stenosis: characterizing subjective frailty using objective measures. Structural Heart. 2019;3(1):44–52. doi: 10.1080/24748706.2018.1524610. [DOI] [Google Scholar]
- 31.Yanagawa Bobby, Graham Michelle M., Afilalo Jonathan, Hassan Ansar, Arora Rakesh C. Frailty as a risk predictor in cardiac surgery: Beyond the eyeball test. The J. Thoracic Cardiovascular Surg. 2019;157(5):1905–1909. doi: 10.1016/j.jtcvs.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 32.Dent Elsa, Martin Finbarr C, Bergman Howard, Woo Jean, Romero-Ortuno Roman, Walston Jeremy D. Management of frailty: opportunities, challenges, and future directions. The Lancet. 2019;394(10206):1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 33.Borregaard Britt, Pedersen Susanne S., Berg Selina Kikkenborg, Dahl Jordi, Ekholm Ola, Sibilitz Kirstine, Zwisler Ann Dorthe Olsen, Lauck Sandra B., Kyte Derek, Calvert Melanie, Riber Lars Peter Schødt, Møller Jacob Eifer. What to expect after open heart valve surgery? Changes in health-related quality of life. Qual Life Res. 2020;29(5):1247–1258. doi: 10.1007/s11136-019-02400-9. [DOI] [PubMed] [Google Scholar]
- 34.Tamuleviciute-Prasciene Egle, Drulyte Kristina, Jurenaite Greta, Kubilius Raimondas, Bjarnason-Wehrens Birna. Frailty and exercise training: how to provide best care after cardiac surgery or intervention for elder patients with valvular heart disease. Biomed Res. Int. 2018;2018:1–36. doi: 10.1155/2018/9849475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez Pedro, Pinto Ronei Silveira, Radaelli Regis, Rech Anderson, Grazioli Rafael, Izquierdo Mikel, Cadore Eduardo Lusa. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin. Exp. Res. 2018;30(8):889–899. doi: 10.1007/s40520-017-0863-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.