Abstract
COVID-19 patients have presented with a wide range of neurological disorders, among which stroke is the most devastating. We have reviewed current studies, case series, and case reports with a focus on COVID-19 patients complicated with stroke, and presented the current understanding of stroke in this patient population. As evidenced by increased D-dimer, fibrinogen, factor VIII and von Willebrand factor, SARS-CoV-2 infection induces coagulopathy, disrupts endothelial function, and promotes hypercoagulative state. Collectively, it predisposes patients to cerebrovascular events. Additionally, due to the unprecedented strain on the healthcare system, stroke care has been inevitably compromised. The underlying mechanism between COVID-19 and stroke warrants further study, so does the development of an effective therapeutic or preventive intervention.
Abbreviations: COVID-19, Coronavirus disease 2019; ACE2, Angiotensin-converting enzyme 2; aPL, Antiphospholipid; aPTT, Activated partial thromboplastin time; CPR, C-reactive protein; CVD, Cerebrovascular disease; DIC, Disseminated intravascular coagulation; ECMO, Extracorporeal membrane oxygenation; ICH, Intracranial hemorrhage; IL-6, Interleukin-6; MERS, Middle East Respiratory Syndrome; NIHSS, National Institutes of Health Stroke Scale; PT, Prothrombin time; rt-PCR, Reverse transcription polymerase chain reaction; SARS-CoV-1, Severe acute respiratory syndrome coronavirus 1; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TNF-alpha, Tumor necrosis factor-alpha; vWF, Von Willebrand Factor
Keywords: COVID-19, SARS-CoV-2, Stroke, Cerebrovascular diseases
1. Introduction
Despite being a severe acute respiratory syndrome, coronavirus disease 2019 (COVID-19) can present in various ways not restricted to pulmonary symptoms. A wide range of symptoms has been reported from asymptomatic disease to kidney injuries, cardiac damages, and neurological manifestations. Neurological symptoms, including headache, dizziness, cranial nerves damage such as anosmia, confusion, cerebrovascular diseases, and encephalopathies, can be the initial presentation of COVID-19 or concur with respiratory symptoms.1, 2, 3 Neurological involvement has been observed in up to 36% of COVID-19 patients.4, 5 In severe cases, cerebrovascular diseases are among the most prevalent comorbidities and are presented as an independent risk factor for poor prognosis.6, 7 Preliminary evidence indicates that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection could cause ischemic stroke through hypercoagulative state, endothelial injury, and cardiogenic embolism.8 Stroke has previously been observed in severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East Respiratory Syndrome (MERS) patients.9, 10 Currently, numerous cases of COVID-19 patients complicated with stroke have been reported; some patients had stroke symptoms as the initial presentation.11, 12 This has raised the recognition of SARS-CoV-2 infection manifesting as a cerebrovascular incident. As SARS-CoV-2 swept through the world, the COVID-19 pandemic has called increasing attention to neurological providers as the novel coronavirus infection causes a higher rate of stroke compared to the regular population. We hereby conducted a literature review and presented current studies, case series, and understanding of COVID-19 and stroke.
2. Cohort studies, case series, and case reports of stroke complicated COVID-19 infection
We have conducted a search of literature through PubMed and Google Scholar with the following keywords “SARS-CoV-2”, “COVID-19”, “stroke,” and “neurological symptoms.” Publications of cohort studies, case series, and case reports of stroke (both ischemic and hemorrhagic stroke) complicated with COVID-19 infections have been included from January of 2020 to July of 2020. Studies that were not focused on stoke and Covid-19 infection have been excluded. A total of sixteen studies with authors, journals, publication dates, characteristics, and outcomes are listed (Table 1 ).
Table 1.
Summary of literature with a focus on stroke in COVID-19 patients.
| Authors | Journal | Publication Date | Study type | Number of Stroke Patients | Age (Median) | Sex | Characteristics | Outcome |
|---|---|---|---|---|---|---|---|---|
| Yaghi et al. 1 | Stroke | Jul-20 | Cohort study | 32 | 62.5[52.0–69.25] | 23 Male | 0.9% (32/3556) hospitalized COVID-19 infection identified with stroke | 75.0% (24/32) deceased/critically ill |
| 9 Female | Median time from COVID-19 Symptoms to stoke 10 days [5–16.5] | |||||||
| 43.8% (14) admitted for stroke; 56.2% (18) admitted for COVID-19 related symptoms | ||||||||
| 21.9% (7) cardioembolic; 6.3% (2) Large Vessel disease | ||||||||
| 65.6% (21) cryptogenic, 6.3% (2) other types of stroke | ||||||||
| Median D-dimer 3913 ng/mL (2549–10000); median CRP 101.1 ng/mL (38.8–214.3) | ||||||||
| Morassi et al. 56 | Journal of Neurology | Apr-20 | Case series | 6 | 69[57–82] | 5 Male | Four ischemic strokes; two hemorrhagic strokes Five males with severe COVID-19; one female with moderate COVID-19; Increased LDH, abnormal blood clotting tests in four patients | Death (83%) 5 (severe COVID-19 all died); severe neurological deficits (mRS:4) |
| 1 female | ||||||||
| Klok et al. 18 | Thrombosis Research | Apr-20 | Cohort study | 3 | n/aa | n/a | Focused on thrombotic complications in critically ill COVID-19 patients. Reported 3 ischemic strokes. | n/a |
| Mao et al. 5 | JAMA Neurology | 20-Apr | Cohort study | 6 | n/a | n/a | Study focused on neurological manifestations of COVID-19 patients; five ischemic stroke, one hemorrhagic stroke was reported; Five severe covid-19 cases, one non-severe COVID-19 case; Median time from COVID-19 symptoms to stroke 9 days (1 to 18) | One hemorrhagic stroke died; others unknown |
| Helms et al. 20 | The New England Journal of Medicine | Jun-20 | Case series | 3 | n/a | n/a | Observation case series with a focus on neurological features. Reported three patients with ischemic stroke. | n/a |
| Helms et al. 33 | Intensive care Med | Apr-20 | Cohort study | 4 | n/a | n/a | Focused on high thrombosis risks in COVID-19 patients, reported four stroke patients, no details regarding age and outcome etc. | n/a |
| Li et al.4 | SSRN Electronic Journal | Jul-20 | Retrospective observational study | 13 | 72 [32–91] | 7 Male | Eleven acute ischemic strokes, 1 CVSTb, 1 cerebral hemorrhage | 38.5% (5) death |
| 6 Female | 11 severe COVID-19; 2 non-severe COVID-19 | |||||||
| Median time from COVID symptoms to stroke 9 days (0 to 28) | ||||||||
| Authors | Journal | Publication date | Study type | Number of Stroke Patient | Age (median) | Sex | Characteristics | Outcome |
| Merkler et al.57 | JAMA Neurology | 20-Jul | Retrospective observational study | 31 | 69 (interquartile range, 66–78) | 18 Male 13 Female | All presented with ischemic stroke; 8 patients presented with stroke initially. | n/a |
| Avula et al.11 | Brain, Behavior, and Immunity | Apr-20 | case report | 4 | 81.5 [73–88] | 1 Male 3 Female | All patients presented with acute ischemic stroke with COVID-19; Of patients who had D-dimer and CRPc tested, they are all increased; D-dimer CRP levels both elevated when data available | 3 died; 1 discharged to rehab |
| Valderrama et al.53 | Stroke | Jul-20 | Case report | 1 | 52 | Male | Patient presented with covid-19 symptoms, developed stroke on day 7 of COVID-19 symptoms; Angiography revealed partially occlusive left terminal internal carotid artery thrombus; Mechanical thrombectomy was performed; D-dimer and CRP were both high. | Discharged |
| Guillan et al.58 | Thrombosis Research | Sep-20 | Case report | 1 | 67 | Male | Simultaneous presentation of ischemic stroke and mild Covid-19; Cerebral infracts in multiple arterial territories; High D-dimer and CRP. | Favorable without new clinical events |
| Vu et al.59 | Emergency Radiology | Mar-20 | Case report | 1 | 30 | Male | Presented with dysarthria, right hemiparesis, right facial droop; CT showed acute left basal ganglia hemorrhage (hemorrhagic stroke); Asymptomatic COVID-19 (CT neck revealed nodules in both upper lobes of the lung, which led to covid-19 diagnosis eventually) | n/a |
| Fu et al.60 | BMC Neurology | Jun-20 | Case report | 2 | 45 | Male | Six days after mild covid-19 symptom (fever), patient developed stroke symptoms including dysarthria, weakness of left limbs, facial droop. CT revealed right corona radiata infarction; D-dimer and CRP were both high. | Recovered, discharged |
| 50 | Male | Patient presented with left side weakness after 9 days of fever; Confirmed with COVID-19 with rt-PCRd; CT showed right basal ganglia infarction; D-dimer and CRP both high. | Discharged with residual neurological deficits | |||||
| Gunasekeran et al.61 | QJM | May-20 | Case report | 1 | 40 | Female | Seven days after intubation, patient showed sluggish pupils and absent corneal responses. CT revealed a large middle cerebral artery territory infarct with extensive mass effect, including midline shift and downward herniation. Patient had diabetes insipidus which was deemed the cause of the massive stroke. | Deceased |
| Authors | Journal | Publication date | Study type | Number of Stroke Patient | Age (median) | Sex | Characteristics | Outcome |
| Oxley et al.62 | The New England Journal of Medicine | Apr-20 | Case Report | 5 | 39 [33–49] | 4 Male 1 Female | Moderate to mild COVID-19 infection; all had large vessel disease; mean NIHSS upon admission was 19; | 3 discharged home or rehab, 1 in ICU, 1 in stroke unit |
| Beyrouti et al.63 | J neurol Neurosurg Psychiatry | May-20 | Case report | 6 | 68.5 [53–85] | 5 Male 1 Female | Five severe and one moderate COVID-19 infection; CT or MRI confirmed ischemic stroke; CRP all elevated; antiphospholipid antibodies were all detected. | 1 died; others unknown |
| Sharifi-Razavi et al.64 | New Microbes and New Infections | Mar-20 | Case report | 1 | 79 | Male | Three days after COVID-19 symptoms (fever, cough), patient presented with loss of consciousness, hemorrhagic stroke confirmed with CT, rt-PCR confirmed Covid-19. | unknown |
| TUNÇ et al.65 | Journal of clinical Neuroscience | 20-May | case report | 4 | 69.5 [45–77] | 2 Male 2 Female | Median 1.5 days (1–4) stroke presentation after COVID-19 symptoms; COVID-19 non-severe; two patients had large vessel disease; two patients had small vessel disease. | 2 discharged well; 2 bedridden, all survived. |
| Goldberg et al.66 | AJNR Am J Neuroradiol | May-20 | case report | 1 | 64 | Male | Sixteen days after onset of COVID-19 symptoms, patient woke up with hemiparesis. CT confirmed right middle cerebral artery and bilateral anterior cerebral artery territories acute ischemic infarction; high D-dimer and antiphospholipid antibodies. | n/a |
| Hughes et al.12 | European Journal of Case Report in Internal Medicine | 20-Apr | case report | 59 | Male | Presented with right hemiparesis, dysphasia 4 days after COVID-19 confirmation, was diagnosed with cerebral venous sinus thrombosis. | Recovered | |
| Carroll et al.67 | Neurocrit care | Jun-20 | Case report | 2 | 66 [62–72] | Male | Two severe COVID-19 cases under intubation were absent of brainstem reflexes; CT revealed multifocal hemorrhages and severe diffuse cerebral edema. | Deceased |
| Fara et al.68 | Journal of Thrombosis and Haemostasis | May-20 | Case report | 3 | 33 | Female | Patient with no medical history presented with left hemiplegia and left facial hypoesthesia; MRI revealed acute infarction in the right middle cerebral artery territory. Treated with anticoagulation. | Near-complete resolution of thrombosis. |
| 77 | Female | Patient with history of hypertension and hyperlipidemia presented with sudden onset of aphasia, left hemiparesis. Found to have non-occlusive thrombosis of the distal right common carotid artery. (coughing led to confirmation with COVID-19, no fever, no oxygenation supplementation required). | Thrombosis had completely resolved | |||||
| 55 | Male | Patient with history of diabetes presented with left hemiparesis. CTA showed thrombosis of right common carotid artery. Conventional angiography showed the thrombosis as non-occlusive, and he was treated with anticoagulation. Low-grade fever at presentation and required supplemental oxygenation, but did not develop significant respiratory distress. | n/a | |||||
| Zahid et al.17 | Journal of Stroke | Jun-20 | Case report | 1 | 38 | Male | Patient had severe Covid-19, was intubated and later on put on ECMOe support with continuous heparin infusion. Patient developed encephalopathic and head CT revealed left sub-insular parenchymal hemorrhage. | Obtained overall substantial clinical improvement. |
n/a: not available (data not provided in the original publications);
CVST: Cerebral venous sinus thrombosis;
CRP: C-reactive protein;
rt-PCR: reverse transcription polymerase chain reaction;
ECMO: extracorporeal membrane oxygenation.
3. Stroke presentation in COVID-19 patients
During the pandemic, a decreased volume of stroke emergencies has been observed by independent institutions across the globe, which has persisted even after the number of confirmed COVID-19 cases decreased.13, 14 Siegler et al. reported a 38% fall in newly diagnosed stroke patients and 25% fewer consultations via stroke telemedicine at the local institution.13 This phenomenon has been attributed to the possibility that the fear of contracting SARS-CoV-2 while being exposed to a healthcare facility has deterred patients with mild symptoms from seeking medical attention and treatment.1, 14, 15 Although further study is warranted to attest to this hypothesis. Additionally, among patients treated for ischemic stroke during the pandemic, Meza et al. reported that there was no difference in the number of patients received intravenous thrombolysis compared to those received endovascular therapy. However, the onset-to-door time of stroke patients during the emergency-state lockdown had increased while being compared to non-emergency state.14
In a systemic review conducted in May of 2020, Ghannam et al. reported that 48.8% of neurological involvement in Covid-19 patients were cerebrovascular incidents, which consisted of 87.5% ischemic stroke, 5% cerebral venous thrombosis, 5% intraparenchymal hemorrhage, and 2.5% subarachnoid hemorrhage.3 The majority of ischemic stroke subtype was large vessel occlusion, which consisted of 77% within a total pooled sample of 35 ischemic stroke cases. However, in a retrospective cohort study examining COVID-19 and stroke from a New York Healthcare system conducted in July of 2020, Yaghi et al. reported that large vessel disease consisted of only 6.2% out of total 32 ischemic stroke cases, and the majority of stroke subtypes was cryptogenic. Intracranial hemorrhages (ICH) have been reported in Covid-19 patients as well. Dogra et al. identified 33 COVID-19 patients afflicted with ICH, of which 75.8% was receiving anticoagulation either for therapeutic or prophylactic purpose.16 In severe cases requiring extracorporeal membrane oxygenation (ECMO) support with continuous anticoagulation, intraparenchymal hemorrhage with a poor prognosis has been observed.17 Nonetheless, macrothrombosis, microangiopathy, venous thrombosis, and hemorrhagic stroke have all been observed in patients infected with SARS-CoV-2. The incidence of ischemic stroke among COVID-19 patients is reported to be at 1.6% by Klok et al. and 2.5% by Lodigiani et al. in studies from two different institutes.18, 19 The disparity of incidence can be attributed to the small number of samples, timing of the study, and study methods. Of note, both studies targeted COVID-19 patients required treatment in the ICU, which had automatically excluded mild to moderate COVID-19 patients. Therefore, the actual incidence of stroke in COVID-19 patients could be higher.20 This calls for further investigation as SARS-CoV-2 poses a continuing threat to the neurological well-being of COVID-19 patients.
In general, levels of d-dimer, interleukin-6 (IL-6), C-reactive protein (CRP), fibrinogen, and platelet were increased in COVID-19 patients.21 Stroke patients with COVID-19 were found to be generally younger with higher admission National Institutes of Health Stroke Scale (NIHSS) score than their counterparts without COVID-19.1 This could be attributed to the hypercoagulative state that predisposes COVID-19 patients to thromboembolic incidents.
Cerebrovascular disease (CVD) as a prognostic factor in COVID-19 patients have been systemically reviewed and analyzed in different studies. Prananta et al. 22 reported that CVD was associated with mortality in COVID-19 patients. The risk ratio (RR) was 2.38 [1.92,2.96], which means COVID-19 patients with cerebrovascular events have poor outcomes. However, the association between cerebrovascular disease and severe COVID-19 was borderline significant (RR1.88[1.00,3.51]). This is in stark contrast with the finding from Aggarwal et al., who had reported an increased odd of association between CVD and severe COVID-19 infection and no statistical significance in the association between CVD and mortality in severe COVID-19 patients.23 The disparity of the conclusions between the two studies could be caused by the fact that both studies pooled data from selected published literature, in which the number of studies was limited, and the sample size was small. Furthermore, severe COVID-19 patients tend to develop multiorgan impairment, which could potentially confound the association between CVD and severity of COVID-19. For stroke patients of mild to moderate COVID-19 patients, although single institute studies and case reports have reported optimistic outcomes,4 systemic reviews are still lacking in this patient group.
4. Mechanism of stroke in COVID-19 patients
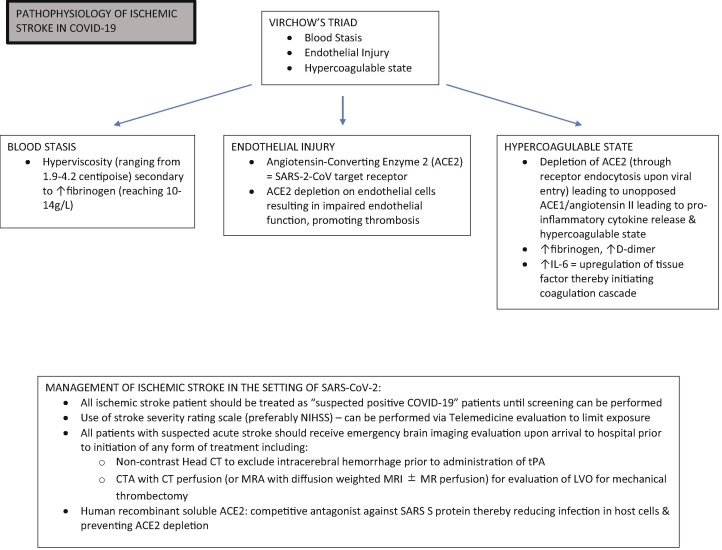
The genome sequence of SARS-CoV-2 shares 89.1% similarity with a group of SARS-like coronaviruses.24 Unsurprisingly, similar to SARS-CoV-1, SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) receptor with viral surface spike (S) proteins and gains entry to host cells.25, 26 ACE2 is widely expressed in human body,27, 28 specifically the neurons, glial cells, endothelial cells, and arterial smooth muscles in the central nervous system.25, 29, 30 This renders them vulnerable targets to SARS-CoV-2. Although the neurotropism of SARS-CoV-2 is still disputable, the affinity of SARS-CoV-2 S protein to ACE2 receptor is 10- to 20-fold higher than that found in SARS-CoV-1,31 which potentially explains many of the neurological manifestations such as anosmia in COVID-19 infection. The cause of stroke, however, is plausibly multifactorial. Coagulopathy and hypercoagulability as a result of systemic response to SARS-CoV-2 infection, endothelial injury caused by direct viral invasion, and venous stasis due to immobilization are all implicated in CVD in COVID-19 patients (Fig. 1 ).
Fig. 1.
Pathophysiology and Management of Ischemic Stroke in COVID-19.
Coagulopathy attributes to thrombotic events and has been widely observed in COVID-19 patients regardless of severity. More than 95% of severe COVID-19 patients have elevated levels of D-dimer and fibrinogen.32, 33 However, classical coagulation markers, prothrombin time (PT), and activated partial thromboplastin time (aPTT) are normal or only slightly prolonged and do not reflect the procoagulant state, which is distinct from disseminated intravascular coagulation (DIC). In fact, severe COVID-19 patients rarely progress to an overt DIC.34 Additionally, antiphospholipid antibodies (aPL) were detected in some severe cases, which is associated with thrombosis.33, 35
Hypercoagulability as well has been reported in both severe and mild COVID-19 patients. Clot waveform analysis (CWA) demonstrated hypercoagulability that precedes or coincides with severe illness.36 Hypercoagulability, along with a systemic inflammatory response to the viral infection, could lead to macro- and microthrombi formation, ultimately eliciting cerebrovascular incidents.37, 38 A thromboelastography study of the coagulation profile in critical COVID-19 patients yielded consistency with a hypercoagulative state. It’s been postulated that substantially increased factor VIII is associated with COVID-19 related hypercoagulability.37 Activation of the complement pathway, inflammatory cytokines, as well as cytoplasmic microparticles originated from platelets or lymphocytes could also induce hypercoagulative state.39, 40, 41
Hyperviscosity is another risk factor for thrombosis. This has been demonstrated in a case series of critically ill COVID-19 patients. Fifteen patients had their plasma viscosity assessed with traditional capillary viscometry, which was ranging from 1.9 to 4.2 centipoise (normal range 1.4–1.8 centipoise).42 Although it is commonly seen in monoclonal gammopathies, hyperviscosity can be caused by substantially increased fibrinogen, which is consistent with observations in COVID-19 patients. Furthermore, SARS-CoV-2 rapidly activates immune response through the angiotensin 2 pathway and induces an imbalanced systemic inflammatory cascade, followed by a cytokine storm with high IL-6 and tumor necrosis factor-alpha (TNF- α).43, 44 Apart from direct impairment of organ function, a cytokine storm contributes to hyperviscosity as well. In return, hyperviscosity impairs endothelium and promote hypercoagulative state.45
Endothelial impairment is evidenced by elevated von Willebrand factor (vWF) and soluble P-selectin in COVID-19 patients as well. Specifically, the elevation of vWF is associated with the severity of the disease.46 Overexpression of ACE2 in neuronal cells and endothelia prevents the development of ischemic stroke; this protective function is counteracted by the depletion of ACE2 as it serves as the target of SARS-CoV-2 spike protein.47, 48, 49 Autopsy from SARS-CoV-1 have revealed vasculitis of venules in brain tissue. This could be equally true in COVID-19 patients.50 Through direct viral invasion and inflammatory response, SARS-CoV-2 facilitates endotheliitis, leads to microvascular dysfunction and a procoagulant state, and further increases risks of thrombotic events. Moreover, endothelial dysfunction compounded with anticoagulant treatment and preexisting comorbidities could lead to hemorrhagic stroke and hemorrhagic transformation of ischemic stroke in COVID-19 patients.16
5. Management of stoke amid COVID-19 pandemic
Response to stroke code amid the pandemic is challenging due to the potential exposure to COVID-19, requirement of isolation, and relative shortage of healthcare resources. A preplanned protocol is strongly recommended for institutes of different levels (Fig. 1). If patients are unable to provide essential information because of altered mental status, aphasia, or few witnesses, screening for COVID-19 could be difficult due to limited history of illness. In this case, stroke patients should be treated as potentially infected, and full personal protective equipment should be implemented. Stroke team members could have been redesignated to meet the increasing need for care of COVID-19, which forces the stroke team to operate with less staff. These all pose a threat to the delivery of healthcare to stroke patients with or without COVID-19. There is a high mortality rate in acute ischemic stroke in spite of timely treatment achieving revascularization. In one series, the mortality rate reaches 31%, while a pooled analysis revealed a dire 50% in COVID-19 patients complicated with acute ischemic stroke.51 Nevertheless, no efforts should be spared in adhering to published stroke care guidelines. Telemedicine is superior at maximizing social distancing and physical isolation, and it should be made widely available. The NIHSS can be efficiently conducted via telemedicine.52 It also shortens the time from symptoms onset to initial evaluation.
COVID-19 patients with ischemic stroke should undergo a full diagnostic work-up, including brain imaging, vascular imaging, cardiac evaluation. Coagulation profile should be ordered, such as D-dimer, fibrinogen, cytokines, and CRP.53 In hospitalized COVID-19 patients with high levels of D-dimer indicating hypercoagulative state, anticoagulation therapy can be implemented to mitigate thrombotic complications, including ischemic stroke.1 Intravenous recombinant tissue plasminogen activator (rt-PA) is recommended for selected patients following guidelines,54 although the hypercoagulative profile predisposes patients to a higher rate of mortality or disability.55 Mechanical thrombectomy is recommended for large vessel occlusion within six hours of symptom onset. The challenge lies in the transition from triage to angiographic suite while being compliant with the isolation policy.
Exogenous ACE2 with human recombinant soluble ACE2 is a novel treatment for COVID-19 patients with stroke. It competes with spike protein on SARS-CoV-2 and inhibit SARS-CoV-2 infections of the blood vessel, impedes viral related ACE2 depletion on endothelium and preserve its protective function. Collectively, exogenous ACE2 could prevent or reverse direct impairment of endothelial function enacted by SARS-CoV-2.48 Other treatments targeting the renin-angiotensin system are also under investigation as a promising preventive treatment for stroke in COVID-19 patients.
6. Summary
COVID-19 patients have presented with a wide range of neurological disorders, among which stroke is the most devastating. SARS-CoV-2 infection induces coagulopathy, disrupts endothelial function, and promotes hypercoagulative state. Severe COVID-19 infection renders patients bedridden, and in certain cases requiring instrumental support. Collectively, it predisposes patients to cerebrovascular events. Due to the unprecedented strain on the healthcare system, stroke care has been inevitably compromised from initial encounter, treatment, to rehabilitation. The understanding of the underlying mechanism between COVID-19 and stroke warrants further study, so does the development of an effective therapeutic or preventive intervention.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported in part by National Institute of Health (NIH-R01-NS-067435 awarded to JHH) and Helen Vosburg McCrillus Plummer and Robert Edward Lee Plummer, Jr. Chair fund (to JHH).
References
- 1.Yaghi S, Ishida K, Torres J, et al. SARS2-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020:STROKEAHA120030335. doi:10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed]
- 2.Leadership A.S.C. Temporary emergency guidance to US stroke centers during the coronavirus disease 2019 (COVID-19) pandemic: on behalf of the american heart association/american stroke association stroke council leadership. Stroke. 2020;51(6):1910–1912. doi: 10.1161/STROKEAHA.120.030023. [DOI] [PubMed] [Google Scholar]
- 3.Ghannam M., Alshaer Q., Al-Chalabi M., Zakarna L., Robertson J., Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic. Review. 2020 doi: 10.1007/s00415-020-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Wang M, Zhou Y, et al. Acute Cerebrovascular Disease Following COVID-19: A Single Center, Retrospective, Observational Study. Ssrn Electron J. 2020. doi:10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed]
- 5.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B.o., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020. doi:10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed]
- 8.Markus Hugh S, Brainin Michael. COVID-19 and stroke—A global world stroke organization perspective. Int J Stroke. 2020;15(4):361–364. doi: 10.1177/1747493020923472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Jee-Eun, Heo Jae-Hyeok, Kim Hye-ok. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(3):227. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umapathi T., Kor Ai Ching, Venketasubramanian N. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avula Akshay, Nalleballe Krishna, Narula Naureen. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Int Med. 2020;7(5) doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegler J.E., Heslin M.E., Thau L., Smith A., Jovin T.G. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020;29(8):104953. doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tejada Meza H., Lambea Gil Á., Sancho Saldaña A. Ischaemic stroke in the time of coronavirus disease 2019. Eur J Neurol. 2020;27(9):1788–1792. doi: 10.1111/ene.14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia Santiago, Albaghdadi Mazen S., Meraj Perwaiz M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the united states during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogra Siddhant, Jain Rajan, Cao Meng. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahid Muhammad J., Baig Anam, Galvez-Jimenez Nestor, Martinez Nydia. Hemorrhagic stroke in setting of severe COVID-19 infection requiring extracorporeal membrane oxygenation (ECMO) J Stroke Cerebrovasc Dis. 2020;29(9):105016. doi: 10.1016/j.jstrokecerebrovasdis.2020.105016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodigiani Corrado, Iapichino Giacomo, Carenzo Luca. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helms Julie, Kremer Stéphane, Merdji Hamid. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors Jean M., Levy Jerrold H. Thromboinflammation and the hypercoagulability of COVID‐19. J Thromb Haemost. 2020;18(7):1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pranata Raymond, Huang Ian, Lim Michael Anthonius. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29(8):104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal Gaurav, Lippi Giuseppe, Michael Henry Brandon. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): A pooled analysis of published literature. Int J Stroke. 2020;15(4):385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Zhiqiang, Kang Huicong, Li Shiyong, Zhao Xu. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Roujian, Zhao Xiang, Li Juan. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann Markus, Kleine-Weber Hannah, Schroeder Simon. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Wang K, Yu J, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. Biorxiv. 2020:2020.04.07.030650. doi:10.1101/2020.04.07.030650. [DOI] [PMC free article] [PubMed]
- 28.Zubair Adeel S., McAlpine Lindsay S., Gardin Tova, Farhadian Shelli, Kuruvilla Deena E., Spudich Serena. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alenina Natalia, Bader Michael. ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem Res. 2019;44(6):1323–1329. doi: 10.1007/s11064-018-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baig Abdul Mannan, Khaleeq Areeba, Ali Usman, Syeda Hira. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 31.Wrapp Daniel, Wang Nianshuang, Corbett Kizzmekia S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Ning, Bai Huan, Chen Xing, Gong Jiale, Li Dengju, Sun Ziyong. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helms Julie, Tacquard Charles, Severac François, Leonard-Lorant Ian, Ohana Mickaël, Delabranche Xavier, Merdji Hamid, Clere-Jehl Raphaël, Schenck Malika, Fagot Gandet Florence, Fafi-Kremer Samira, Castelain Vincent, Schneider Francis, Grunebaum Lélia. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan Wei-jie, Ni Zheng-yi, Hu Yu. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Yan, Xiao Meng, Zhang Shulan. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan C.W., Low J.G.H., Wong W.H., Chua Y.Y., Goh S.L., Ng H.J. Critically ill COVID -19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am J Hematol. 2020;95(7):E156–E158. doi: 10.1002/ajh.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panigada Mauro, Bottino Nicola, Tagliabue Paola, Grasselli Giacomo, Novembrino Cristina. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emert Roger, Shah Payal, Zampella John G. COVID-19 and hypercoagulability in the outpatient setting. Thromb Res. 2020;192:122–123. doi: 10.1016/j.thromres.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez-Garza Eduardo, Jerjes-Sanchez Carlos, Navarrete Aline, Joya-Harrison Jorge, Rodriguez David. Venous thromboembolism: thrombosis, inflammation, and immunothrombosis for clinicians. J Thromb Thrombolysis. 2017;44(3):377–385. doi: 10.1007/s11239-017-1528-7. [DOI] [PubMed] [Google Scholar]
- 40.Bucciarelli Paolo, Martinelli Ida, Artoni Andrea, Passamonti Serena M. Circulating microparticles and risk of venous thromboembolism. Thromb Res. 2012;129(5):591–597. doi: 10.1016/j.thromres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Magro Cynthia, Mulvey J. Justin, Berlin David, Nuovo Gerard. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maier Cheryl L, Truong Alexander D, Auld Sara C, Polly Derek M, Tanksley Christin-Lauren, Duncan Alexander. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano Toshio, Murakami Masaaki. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gertz Morie A., Kyle Robert A. Hyperviscosity syndrome. J Intensive Care Med. 1995;10(3):128–141. doi: 10.1177/088506669501000304. [DOI] [PubMed] [Google Scholar]
- 46.Goshua George, Pine Alexander B, Meizlish Matthew L. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Ji, Xiao Xiang, Chen Shuzhen, Zhang Cheng, Chen Jianying, Yi Dan. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 2013;61(3):681–689. doi: 10.1161/HYPERTENSIONAHA.111.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hess David C., Eldahshan Wael, Rutkowski Elizabeth. COVID-19-related stroke. Transl Stroke Res. 2020;11(3):322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi Ji-Young, Lee Hye-Kyung, Park Jung Hyun. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem Biophys Res Commun. 2020;528(3):413–419. doi: 10.1016/j.bbrc.2020.05.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding Yanqing, Wang Huijun, Shen Hong. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweid A., Hammoud B., Bekelis K. EXPRESS: brain ischemic and hemorrhagic complications of COVID-19. Int J Stroke. 2020;174749302093718 doi: 10.1177/1747493020937189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alasheev Andrey M., Andreev Aleksey Y., Gonysheva Yuliya V. A comparison of remote and bedside assessment of the national institute of health stroke scale in acute stroke patients. Eur Neurol. 2017;77(5-6):267–271. doi: 10.1159/000469706. [DOI] [PubMed] [Google Scholar]
- 53.Valderrama Eduard Valdes, Humbert Kelley, Lord Aaron, Frontera Jennifer, Yaghi Shadi. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020;51(7) doi: 10.1161/STROKEAHA.120.030153. [DOI] [PubMed] [Google Scholar]
- 54.Powers William J., Rabinstein Alejandro A., Ackerson Teri. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2019;50(12) doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 55.Qureshi Adnan I, Abd-Allah Foad, Al-Senani Fahmi. Management of acute ischemic stroke in patients with COVID-19 infection: report of an international panel. Int J Stroke. 2020;15(5):540–554. doi: 10.1177/1747493020923234. [DOI] [PubMed] [Google Scholar]
- 56.Morassi Mauro, Bagatto Daniele, Cobelli Milena. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merkler Alexander E., Parikh Neal S., Mir Saad. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1366. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guillan Marta, Villacieros-Alvarez Javier, Bellido Sara. Unusual simultaneous cerebral infarcts in multiple arterial territories in a COVID-19 patient. Thrombr Res. 2020;193:107–109. doi: 10.1016/j.thromres.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vu David, Ruggiero Maryanne, Choi Woo Sung. Three unsuspected CT diagnoses of COVID-19. Emerg Radiol. 2020;27(3):229–232. doi: 10.1007/s10140-020-01775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu B, Chen Y, Li P. The 2019 novel coronavirus disease with secondary ischemic stroke: two cases report. 2020. doi:10.21203/rs.3.rs-20943/v1. [DOI] [PMC free article] [PubMed]
- 61.Gunasekaran K, Amoah K, Rajasurya V, Buscher MG. Stroke in a young COVID -19 patient. Qjm Mon J Assoc Physicians. 2020:hcaa177-. doi:10.1093/qjmed/hcaa177. [DOI] [PMC free article] [PubMed]
- 62.Oxley Thomas J., Mocco J., Majidi Shahram. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beyrouti Rahma, Adams Matthew E, Benjamin Laura. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharifi-Razavi A., Karimi N., Rouhani N. COVID 19 and intra cerebral hemorrhage: causative or coincidental. New Microbes New Infect. 2020;35 doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tunç Abdulkadir, Ünlübaş Yonca, Alemdar Murat, Akyüz Enes. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. 2020;77:227–229. doi: 10.1016/j.jocn.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldberg MF, Goldberg MF, Cerejo R, Tayal AH. Cerebrovascular Disease in COVID-19. Ajnr Am J Neuroradiol. 2020. doi:10.3174/ajnr.a6588. [DOI] [PMC free article] [PubMed]
- 67.Carroll E., Lewis A. Catastrophic intracranial hemorrhage in two critically Ill patients with COVID-19. Neurocrit Care. 2020;1–5 doi: 10.1007/s12028-020-00993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fara Michael G., Stein Laura K., Skliut Maryna, Morgello Susan, Fifi Johanna T., Dhamoon Mandip S. Macrothrombosis and stroke in patients with mild Covid‐19 infection. J Thromb Haemost. 2020;18(8):2031–2033. doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]