Abstract
Background
While the pro-inflammatory and pro-coagulant effects of cigarette smoking have been well described, the effect of smokeless tobacco (ST) on inflammatory and coagulation markers is still not clear. The study aimed to evaluate impact of smokeless tobacco use on systemic markers of inflammation [(TLC), neutrophil-lymphocyte ratio (NLR) (ESR), interleukin (IL) IL-1β, IL-6 and tumor necrosis factor alpha (TNFα)] and hypercoagulable state [fibrinogen and d-dimer] leading to increased cardiovascular risk in ST users as compared to non-users.
Methods
150 healthy young adults using oral tobacco products for at least 1 year were included in the case group and 50 age-matched non-consumers as controls. Subjects with any known chronic illness or comorbidity were excluded from the study. Blood samples were tested for TLC, NLR, ESR, IL-1β, IL-6, TNFα, fibrinogen and d-dimer. Statistical analysis was done using SPSS 17.0 software.
Results
The baseline clinical and cardio-metabolic characteristics were comparable between the two groups. ST users had significantly elevated serum IL-6 [59.29 ± 124.69 pg/mL (n = 149) vs 8.21 ± 27.27 pg/mL (n = 47), p-value = 0.005], TNFα [77.18 ± 236.10 pg/mL (n = 149) vs 8.32 ± 9.36 pg/mL (n = 47), p-value = 0.041], fibrinogen [310.53 ± 129.05 mg/dL (n = 143) vs 282.82 ± 65.23 mg/dL (n = 42), p-value = 0.045] and d-dimer [0.28 ± 0.42 mg/L (n = 144) vs 0.17 ± 0.09 mg/L (n = 45), p-value = 0.043] levels as compared to non-users. Serum TLC, NLR, ESR and IL-1β remained unchanged in ST users and were similar to that of controls.
Conclusions
Chronic use of ST is associated with systemic inflammation and coagulation, which may increase the risk of athero-thrombotic cardiovascular events among ST users.
Keywords: Smokeless tobacco(ST), Inflammatory, Coagulation
1. Introduction
Tobacco is a commonly used recreational agent by people from different strata of the society. It is either smoked or used in its smokeless form. While cigarette smoking has been well-recognized as a cardiovascular risk factor, the effect of smokeless tobacco (ST) in this regard is still not clear. Studies that have assessed the association of ST with cardiovascular disease have shown mixed results.1 Considerable inter-regional differences in the ST products have been postulated to be responsible for such variability. The manufacturing process, chemical composition and the consumption technique of ST products differ from region to region.
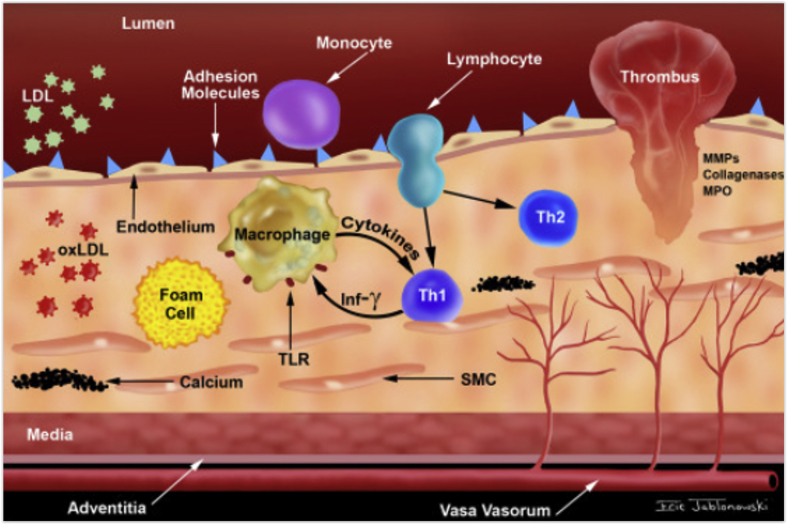
It is well known that inflammation and coagulation are important mechanisms underlying the pathophysiology of cardiovascular disease (Fig. 1). While the pro-inflammatory and pro-coagulant effects of cigarette smoking (CS), with its associated cardiovascular risk have been well studied,2, 3, 4, 5 the effect of ST on biomarkers of systemic inflammation and coagulation have been addressed in very few small scale studies. Moreover, majority of these studies have been carried out in the western countries, and have involved ST products that are considerably different from those used in the Asian-Pacific region. Also, other cardiovascular risk factors like obesity, hypertension, diabetes, dyslipidemia and metabolic syndrome which can act as confounding factors have not been judiciously adjusted.
Fig. 1.
Role of inflammation in the pathogenesis of atherosclerosis (Adapted from Raggi P et al. Atherosclerosis. 2018 Sep 1; 276:98–108.).
As per the Global Adult Tobacco Survey (GATS-2), 2016–17, the population of adult ST users in India, itself, was around 199 million.6 While social welfare programs have focused on the risk of malignancy and oral cavity disease caused by these ST products, their possible cardiovascular risk, if any, has not received much attention. In this study, we evaluated the effect of chronic ST use on systemic inflammatory and thrombotic markers of cardiovascular risk among young Indian ST users, who were otherwise healthy and free from any disease or co-morbidity.
2. Methods
2.1. Study design and population
It was a single-site cross-sectional study conducted in GIPMER, New Delhi, India. Participants were recruited by opportunistic screening of young healthy people attending the hospital for various reasons. Hospital staff and other personnel working within the hospital premises were also included in the study. Cases were defined as participants 20–40 years age, who were using oral tobacco products at least two portions/day for >5 days a week for at least 1 year. Details were taken for the daily amount/frequency and total duration of tobacco consumption. Controls were defined as healthy, age-matched adults not using tobacco in any form. Subjects who smoked tobacco or had regular exposure to passive tobacco smoke were excluded from both the case and control groups. Participants were also excluded if they had any diagnosed atherosclerotic cardiovascular disease, chronic inflammatory disease, hypertension (HTN), diabetes mellitus (DM), chronic kidney disease (CKD), chronic liver disease (CLD) or any active infection/recent infection in the last 7 days.
2.2. Patient physical examination, anthropometric data and sampling
Patient physical examination, anthropometric data and blood sampling were done in resting state remote from tobacco consumption. Heart rate (HR), systolic blood pressure (SBP) and diastolic blood pressure (DBP), height, weight, body mass index (BMI) and waist circumference were recorded. Peripheral venous blood sample was taken after complete informed written consent and tested for cardio-metabolic markers like random blood sugar level (RBS), glycated haemoglobin (HbA1c), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) and triglycerides (TG). Inflammatory markers like total leucocyte count (TLC), neutrophil-lymphocyte ratio (NLR), erythrocyte sedimentation rate (ESR), interleukin IL-1β, IL-6 and tumor necrosis factor alpha (TNFα); and coagulation markers like serum fibrinogen and d-dimer were measured. TLC and NLR were measured using the electrical impedance principle in Transasia Sysmex system followed by peripheral smear examination. ESR was measured by the Westergren method. IL-1β, IL-6 and TNFα were assessed using ELISA technique in the Diaclone system, while fibrinogen was assessed by the Clauss method in ELITE system. D-dimer was measured by calorimetric method in Roche system. The study was conducted after approval from the institutional ethics committee.
2.3. Objectives
The primary objective of the study was to evaluate the impact of chronic oral tobacco use on traditional systemic markers of inflammation (TLC, NLR, ESR, IL-1β, IL-6 and TNFα) and coagulation (fibrinogen, d-dimer) leading to increased cardiovascular risk in ST users as compared to non-users.
2.4. Statistical analysis
Data was entered in MS–Excel and analysed using SPSS 17.0 software. Categorical variables were expressed as percentages or proportions and continuous variables as mean ± SD. Categorical variables were compared using Chi–Square test, and continuous variables including the mean TLC, NLR, ESR, IL-1β, IL-6, TNFα, fibrinogen and d-dimer using unpaired t-test. Co-relation with total duration/daily amount of tobacco intake was done using Spearman's correlation analysis. Cases were divided into 3 different subgroups based on total duration and daily amount of tobacco intake. One way ANOVA test was used to compare the mean level of inflammatory and coagulation markers amongst the subgroups, with post-hoc test for inter-group comparison. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Baseline demographic, clinical and cardio-metabolic characteristics
A total of 150 cases and 50 controls were recruited during the period January 2018–July 2019. All the participants were males. The ST products used were gutkha (n = 76, 50.6%), khaini (n = 46, 30.6%) and zarda (n = 28, 18.6%). All the three are chewable forms of ST. There was no significant difference in the age, socio-economic status, HR, SBP, DBP, waist circumference, BMI, RBS, HbA1c, TC, LDL, HDL and TG between cases and controls. Baseline characteristics are shown in Table 1.
Table 1.
Baseline clinical, cardio-metabolic characteristics of study population with comparison of inflammatory and coagulation markers.
| Case group (n = 150) Mean ± SD (%) | Control group (n = 50) Mean ± SD (%) | p-value | |
|---|---|---|---|
| Age (years) | 31.29 ± 6.42 | 29.64 ± 5.91 | 0.111 |
| Socio-economic class: | |||
| Upper middle | 29 (19.3%) | 11 (22.0%) | 0.918 |
| Lower middle | 105 (70.0%) | 34 (68.0%) | |
| Upper lower | 16 (10.7%) | 5 (10.0%) | |
| Heart rate (HR) (beats per minute) | 78.96 ± 7.61 | 77.28 ± 4.89 | 0.145 |
| SBP (mm Hg) | 116.23 ± 12.03 | 115.16 ± 13.59 | 0.600 |
| DBP (mm Hg) | 74.61 ± 9.02 | 75.88 ± 8.21 | 0.380 |
| Waist circumference (cm) | 83.49 ± 8.48 | 82.76 ± 9.09 | 0.302 |
| BMI (kg/m2) | 23.82 ± 3.57 | 23.92 ± 3.42 | 0.860 |
| RBS (mg/dL) | 97.19 ± 22.30 | 97.28 ± 18.81 | 0.979 |
| HbA1c (g/dL) | 5.17 ± 0.44 (n = 144) | 5.28 ± 0.46 (n = 47) | 0.118 |
| Total cholesterol (mg/dL) | 167.09 ± 39.41 (n = 149) | 169.46 ± 38.56 | 0.712 |
| LDL (mg/dL) | 84.01 ± 32.83 (n = 149) | 91.30 ± 29.33 | 0.165 |
| HDL (mg/dL) | 42.19 ± 8.54 (n = 149) | 41.46 ± 7.71 | 0.594 |
| Triglycerides (mg/dL) | 200.62 ± 125.14 (n = 149) | 173.00 ± 71.89 | 0.058 |
| Comparison of inflammatory and coagulation markers: | |||
| TLC (cells/mm3) | 7409.52 ± 1612.08 (n = 147) | 7524.00 ± 1476.97 (n = 50) | 0.658 |
| NLR | 1.86 ± 0.75 (n = 147) | 1.90 ± 0.84 (n = 50) | 0.746 |
| ESR (mm/hr) | 11.53 ± 7.70 (n = 147) | 13.16 ± 8.57 (n = 50) | 0.658 |
| IL-1β (pg/mL) | 10.34 ± 41.52 (n = 149) | 10.04 ± 24.16 (n = 47) | 0.961 |
| IL-6 (pg/mL) | 59.29 ± 124.69 (n = 149) | 8.21 ± 27.27 (n = 47) | 0.005 |
| TNFα (pg/mL) | 77.18 ± 236.10 (n = 149) | 8.32 ± 9.36 (n = 47) | 0.041 |
| Fibrinogen (mg/dL) | 310.53 ± 129.05 (n = 143) | 282.82 ± 65.23 (n = 42) | 0.045 |
| d-dimer (mg/L) | 0.28 ± 0.42 (n = 144) | 0.17 ± 0.09 (n = 45) | 0.043 |
(modified Kuppuswamy socio-economic class, updated 2019, SBP – systolic blood pressure, DBP - diastolic blood pressure, BMI – body mass index, RBS – random blood sugar, HbA1c – glycated haemoglobin, LDL – low density lipoprotein, HDL – high density lipoprotein, TLC – total leucocyte count, NLR – neutrophil lymphocyte ratio, ESR – erythrocyte sedimentation rate, IL – interleukin, TNF – tumor necrosis factor).
p-value < 0.05 : significant.
3.2. Inflammatory and coagulation markers
Significant difference was found in IL-6, TNFα, fibrinogen and d-dimer between cases and controls, with cases having higher levels of these inflammatory and coagulation markers. There was no significant difference in TLC, NLR, ESR and IL-1β between cases and controls. Results are shown in Table 1.
3.3. Co-relation and subgroup analysis (based on duration and daily amount of tobacco use)
The mean duration of tobacco intake was 9.13 ± 6.91 (SD) years with a mean amount of 12.45 ± 13.46 (SD) grams per day. A positive correlation was found between TNFα and fibrinogen with both, the total duration and daily amount of tobacco intake. Such co-relation was not observed with the other inflammatory and coagulation markers studied. Results are shown in Table 2.
Table 2.
Co-relation of inflammatory and coagulation markers with duration and daily amount of tobacco use.
| Duration of tobacco use |
Daily amount |
|||
|---|---|---|---|---|
| Co-relation co-efficient | p-value | Co-relation co-efficient | p-value | |
| TLC | −0.240 | 0.003 | −0.092 | 0.265 |
| NLR | −0.119 | 0.151 | 0.000 | 0.996 |
| ESR | 0.102 | 0.219 | 0.069 | 0.408 |
| IL-1β | 0.002 | 0.982 | −0.009 | 0.914 |
| IL-6 | −0.035 | 0.671 | 0.117 | 0.154 |
| TNFα | 0.175 | 0.036 | 0.267 | 0.001 |
| Fibrinogen | 0.212 | 0.017 | 0.163 | 0.048 |
| d-dimer | −0.029 | 0.729 | −0.067 | 0.418 |
(TLC – total leucocyte count, NLR – neutrophil lymphocyte ratio, ESR – erythrocyte sedimentation rate, IL – interleukin, TNF – tumor necrosis factor).
p-value < 0.05 : significant.
Results of subgroup analysis are summarized in Supplementary Tables S1 and S2. NLR was significantly more in subjects with duration of tobacco use 5–10 years, while TNFα was significantly greater in cases consuming >7.5 g of tobacco per day.
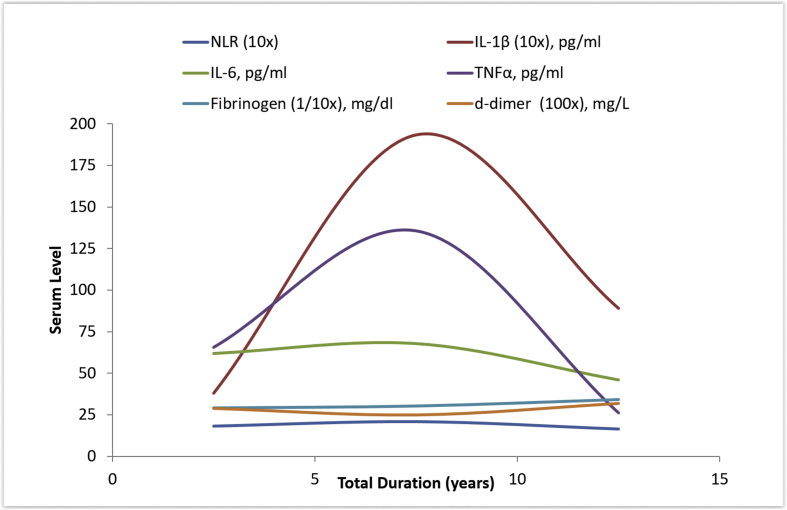
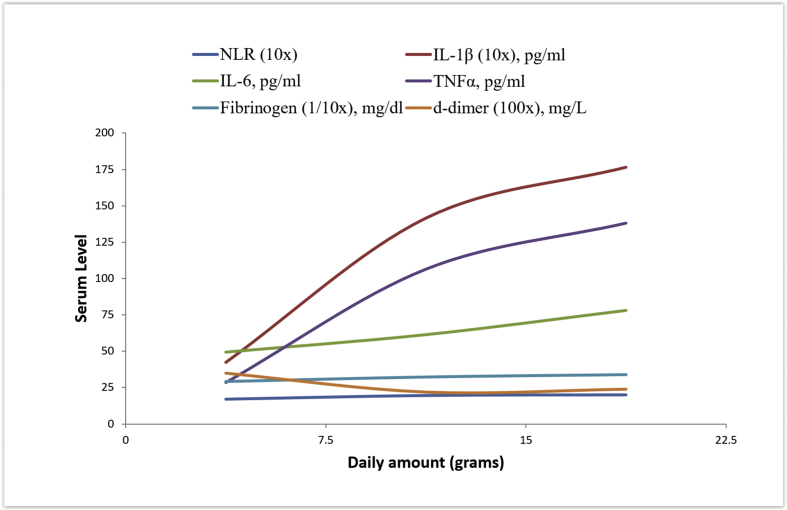
Although the difference was statistically insignificant, the levels of NLR, IL-1β, IL-6 and TNFα were higher among cases with duration of tobacco use between 5 and 10 years as compared to those with duration <5 and >10 years. This could suggest an inflammatory response that increases with the duration of tobacco exposure and reaches its peak between 5 and 10 years, following which it tends to decline; raising the possibility of a late adaptive response to these products with prolonged exposure beyond 10 years. Such a pattern was not noted for TLC, ESR, fibrinogen and d-dimer. Whether this exposure time frame of 5–10 years corresponds to the period of highest cardiovascular risk in this population can be an interesting question. On the other hand, the mean NLR, IL-1β, IL-6, TNFα and fibrinogen increased progressively among subgroups with greater amount of daily tobacco intake. No decline in these markers was noted with increase in the amount of tobacco consumed. Trend of inflammatory and thrombotic markers with duration and daily amount of tobacco use is depicted in Fig. 2, Fig. 3.
Fig. 2.
Trend of inflammatory and thrombotic markers with duration of tobacco use.
Fig. 3.
Trend of inflammatory and thrombotic markers with daily amount of tobacco use.
4. Discussion
The present study shows that chronic use of oral tobacco by healthy adults may be associated with subclinical systemic inflammation and pro-thrombotic state. Participants of the study used chewable forms of oral tobacco; unlike snuff, that is predominantly used in the western countries. ST users were found to have elevated serum IL-6, TNFα, fibrinogen and d-dimer as compared to non-tobacco users. Levels of IL-1β, TLC, NLR and ESR remained unchanged. There are very few studies on this topic in the literature. In an American study on moist snuff users, levels of IL-1β, IL-6 and TNFα were found to be below detection limits.7 Similarly, in a study on naswar users in Pakistan, IL-6 levels were not significantly elevated among ST users as compared to non-tobacco users.8 In contrast, this study on users of oral chewable tobacco showed raised serum IL-6 levels. Besides a considerable difference in the ST products studied, differences in the mean duration and dose of tobacco exposure among the cases may have also contributed to this finding. While some Indian studies have looked at the gingival crevicular fluid IL-1β levels among tobacco chewers suffering from periodontitis,9,10 this is the first study from the Asia–Pacific region to address systemic IL-1β levels among ST users. Also, the association of serum TNFα with ST use has been previously addressed only in some in-vitro and animal studies.11,12
Current literature on the association of serum fibrinogen with ST has been biased by the western studies that have predominantly included snuff users. No significant difference was found in the serum fibrinogen levels between ST and non-ST users in these studies.13,14 In fact, a recent study suggested serum fibrinogen as a marker to differentiate ST users from tobacco smokers, as smokers were found to have elevated fibrinogen levels, while the serum fibrinogen was similar in ST users and non-tobacco users.15 The present study showed raised serum fibrinogen and d-dimer levels among oral tobacco users as compared to non-tobacco users. This finding reiterates the variability in the systemic response across the spectrum of ST products. While snuff users in the western countries did not show any thrombotic tendency, Indian ST users consuming oral tobacco in the chewable form were susceptible to their pro-coagulant effects. With increasing duration and amount of tobacco use, serum fibrinogen levels showed an absolute increase.
The study had few merits. It was adequately sized and adjusted for majority of the confounding factors judiciously. There were also some limitations. Firstly, it was a single-site cross-sectional study. Second, it recruited only male participants. Majority of the studies on ST till date have exclusively recruited males. Also, the effect of menstruation on the studied biochemical parameters makes blood sampling and result interpretation tricky among females.
In conclusion, smokeless tobacco is not devoid of consequences on the cardiovascular health of users. Subclinical systemic inflammation and the resultant activation of the coagulation cascade can predispose this population to athero-thrombotic cardiovascular events. Public awareness programs should incorporate the possible cardiovascular risk of these products and encourage their abstinence. Further large scale, multi-centre studies that also incorporate clinical end-points may be worthwhile.
Source of funding
None.
Conflict of interest
All authors have none to declare.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2020.08.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rostron B.L., Chang J.T., Anic G.M., Tanwar M., Chang C.M., Corey C.G. Smokeless tobacco use and circulatory disease risk: a systematic review and meta-analysis. Open heart. 2018 Nov 1;5(2) doi: 10.1136/openhrt-2018-000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiels M.S., Katki H.A., Freedman N.D. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. 2014 Oct 1;106(11):dju294. doi: 10.1093/jnci/dju294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sultana S., Afsar N., Jawad M., Hazari M.A. Effects of cigarette smoking on erythrocyte sedimentation rate, platelet count, total and differential leucocyte counts in adult male smokers. Ann Med Physiol. 2019 Mar 31;3(1) [Google Scholar]
- 4.Chao F.C., Tullis J.L., Alper C.A., Glynn R.J., Silbert J.E. Alteration in plasma proteins and platelet functions with aging and cigarette smoking in healthy men. Thromb Haemostasis. 1982 Dec;48(3):259–264. [PubMed] [Google Scholar]
- 5.Blann A.D., Steele C., McCollum C.N. The influence of smoking and of oral and transdermal nicotine on blood pressure, and haematology and coagulation indices. Thromb Haemostasis. 1997 Jun;78(1):1093–1096. [PubMed] [Google Scholar]
- 6.Sharma A., Dixit P., Goswami B. Different forms of tobacco consumption among minors in India: a comparative analysis of global adult tobacco surveys 1 and 2, India. Soc Dev Issues. 2019 Nov 1;41(3):12–27. [Google Scholar]
- 7.Nordskog B.K., Brown B.G., Marano K.M., Campell L.R., Jones B.A., Borgerding M.F. Study of cardiovascular disease biomarkers among tobacco consumers, part 2: biomarkers of biological effect. Inhal Toxicol. 2015 Feb 23;27(3):157–166. doi: 10.3109/08958378.2015.1013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajid F., Bano S. Pro inflammatory interleukins and thyroid function in naswar (dipping tobacco) users: a case control study. BMC Endocr Disord. 2016 Dec;16(1):47. doi: 10.1186/s12902-016-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob P.S., Nath S., Patel R.P. Evaluation of interleukin-1β and 8 in gutka chewers with periodontitis among a rural Indian population. J Periodontal & Implant Sci. 2014 Jun 1;44(3):126–133. doi: 10.5051/jpis.2014.44.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey V., Salam S.A., Moda A., Agarwal P., Nath S., Pulikkotil S.J. Effect of the use of snuff on the levels of interleukin-1 β and interleukin-8 in the gingival crevicular fluid of periodontitis patients. Dent Res J. 2015 Sep;12(5):461. doi: 10.4103/1735-3327.166222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seyedroudbari S.A., Khan M.M. In vitro effects of smokeless tobacco extract on tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) production, and on lymphocyte proliferation. Toxicon. 1998 May 8;36(4):631–637. doi: 10.1016/s0041-0101(97)00092-5. [DOI] [PubMed] [Google Scholar]
- 12.Malovichko M.V., Zeller I., Krivokhizhina T.V. Systemic toxicity of smokeless tobacco products in mice. Nicotine Tob Res. 2017 Oct 7;21(1):101–110. doi: 10.1093/ntr/ntx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliasson M., Asplund K., Evrin P.E., Lundblad D. Relationship of cigarette smoking and snuff dipping to plasma fibrinogen, fibrinolytic variables and serum insulin. The Northern Sweden MONICA Study. Atherosclerosis. 1995 Feb 1;113(1):41–53. doi: 10.1016/0021-9150(94)05425-i. [DOI] [PubMed] [Google Scholar]
- 14.Eliasson M., Lundblad D., Hägg E. Cardiovascular risk factors in young snuff-users and cigarette smokers. J Intern Med. 1991 Jul;230(1):17–22. doi: 10.1111/j.1365-2796.1991.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 15.Sgambato J.A., Jones B.A., Caraway J.W., Prasad G.L. Inflammatory profile analysis reveals differences in cytokine expression between smokers, moist snuff users, and dual users compared to non-tobacco consumers. Cytokine. 2018 Jul 1;107:43–51. doi: 10.1016/j.cyto.2017.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.