Abstract
Cough is one of the common adverse effects in patients receiving angiotensin-converting enzyme inhibitors (ACEIs). This review presents the current evidence on incidence and mechanisms of cough associated with ACEIs use, and proposes a practical approach for managing the same for optimal cardiovascular (CV) risk reduction. The incidence of dry cough in patients receiving ACEIs vary among individual ACEIs, and is the lowest with perindopril. Cough is thought to originate from multiple mechanisms, bradykinin theory is the most commonly appealed hypothesis. The strategies for optimal management could be temporarily discontinuation of ACEI upon a reported incidence of cough and reintroduction after its remission. However, studies have reported disappearance of cough despite continuing treatment. Another important approach could be adding calcium channel blockers to ACEIs. Switching to alternative drugs such as angiotensin receptor blockers should be suggested in case intolerable symptoms recur and after exclusion of all other possible causes of cough.
Keywords: Angiotensin-converting enzyme inhibitors, Cough, Current evidence, Cardiovascular risk reduction
1. Introduction
Cough is a common cause for consultation that often becomes a challenge for attending physicians. It is frequently associated with use of angiotensin-converting enzyme inhibitors (ACEIs); however, other drugs such as acetylsalicylic acid and nonsteroidal anti-inflammatory agents, beta-blockers including cardioselective beta-blockers, cholinergic agonists, inhaled agents, vindesine, histamine liberators, etc. can induce bronchospasm and thereby cause cough.1 Cough management guidelines recommend a comprehensive medication history of the patient, particularly use of ACEIs for appropriate diagnosis.2 The present review summarizes the currently available evidence regarding association of ACEIs use and incidence of cough and provides a practical approach for managing this highly debatable condition in order to achieve optimal cardiovascular (CV) risk reduction.
2. ACEIs in CV risk reduction: current evidence and clinical practice guidelines
The renin angiotensin aldosterone system (RAAS) plays a critical role in the pathophysiology of cardiovascular diseases (CVDs) such as hypertension and vascular disease. Angiotensin II (ang II) is the principal effector peptide of the RAAS that plays critical role in blood pressure homeostasis. Its actions are mediated via binding to the ang II type 1 (AT1) receptor, which are expressed in a variety of organ systems including the heart, kidney, blood vessels, adrenal glands, and CV control centers in the brain. Activation of AT1 mediates a range of processes, including vasoconstriction, aldosterone and vasopressin release, sodium and water retention, and sympathetic activation and therefore elevated ang II levels are known to cause hypertension.3 The cardioprotective actions of ACEIs underlie both the blockage of conversion of ang I to ang II, thereby reducing ang II levels, and inhibition of bradykinin degradation. Bradykinin, through its B2 receptor, stimulates endothelial release of a number of vasodilators, such as nitric oxide, prostacyclin, and endothelium-derived hyperpolarizing factor (EDHF) producing vascular protective actions.4 In clinical practice, ACEIs were first used for treatment of hypertension over 30 years ago and have been a cornerstone in the management of CVD for decades. Angiotensin converting enzyme inhibitors have shown consistent CV protection, mediated by improved survival and reduced risk of major CV events, across a wide array of patients with vascular diseases including hypertension, stable coronary artery disease, myocardial infarction (MI), and heart failure (HF).5, 6, 7 They have been demonstrated to prevent stroke and exert cardioprotective and nephro-protective effects in patients with diabetes as well.8 As a result of such CV benefits, most clinical practice guidelines, including the European Society of Cardiology (ESC), American Heart Association (AHA) and American College of Cardiology (ACC) recommend ACEIs as first-line treatment for management of coronary and atherosclerotic vascular diseases, hypertension, HF, and MI.9, 10, 11, 12, 13 Further, the guidelines from Hypertension Canada and those from the European Society of Cardiology and the European Association for the Study of Diabetes (ESC-EASD) also recommend to prefer ACEIs over angiotensin receptor blockers (ARBs), suggesting that the ARBs should be used in patients with intolerance to ACEIs.14 Moreover, the large accessibility and relatively better safety profile of newer ACEIs have further improved overall outcomes.
3. Magnitude of cough
Hypotension, hyperkalemia, dizziness, and headache and a persistent dry cough are some of the common side effects of ACEIs.15 This cough is characterized by a tickling sensation in the throat that quickly wanes after discontinuation of ACEIs. Reportedly, the incidence of dry cough in patients treated with ACEIs were approximately 1.5–11%.16, 17, 18, 19, 20, 21 In fact, not all ACEI trials included cough as an endpoint, and these studies have been limited by smaller sample sizes and lack of long-term follow-up with a low number of events, which, in turn, has resulted in marked differences in reported incidences.16, 17, 18, 19, 20, 21 Moreover, the incidence of cough varies among individual ACEIs, and only a few ACEIs have real-world clinical practice data to support findings from randomized trials. In this context, perindopril is an ACEI for which extensive evidence from both randomized trials and real-world data are available. In a series of studies performed in real clinical practice, such as PAINT, PIANIST, PROOF, PETRA, the incidence of cough was reported to be very low (ranged from <0.001 to 0.8%)22, 23, 24, 25 even with the use of the maximum dose of perindopril (Table 1). Moreover, in a large pooled analysis of 27,492 patients randomized to receive perindopril, the incidence of ACEI-induced cough was 3.9%, and only 3.1% of patients discontinued treatment due to cough.19 Similarly, the data from Indian studies (STRONG, MONOCOMB, PROTECT) demonstrated the cough incidence to be 1.5%–4% that corroborated with the incidence reported in global perindopril studies.17,18,21
Table 1.
Summary of incidence of cough with perindopril in clinical studies.
| Name of study/Author | Type of study | Perindopril | Cough Incidence (%) |
|---|---|---|---|
| PIANIST23 | Observational | Perindopril, 10 mg | 0.8 |
| PAINT24 | Observational | Perindopril, 5 and 10 mg | <0.1 |
| PETRA25 | Observational | Perindopril, 5 mg and 10 mg | 0.04 |
| GREEK cohort60 | Observational | Perindopril, 5 mg and 10 mg | 0.001 |
| PROOF22 | Observational | Perindopril, 5 mg and 10 mg | |
| Nedogoda SV et al.61 | Randomized | Perindopril 5 mg | No incidence was reported (0) |
| Mourad JJ et al62 | Randomized | Perindopril, 5 mg and 10 mg | 1.1 |
| PROTECT I63 | Observational | Perindopril 4 mg, 8 mg | 4.3 |
| Sandeep Bansal et al.21 | Perindopril, N = 1250 | Monotherapy: 3.6 | |
| Combination:1.8 and 4.3 | |||
| STRONG17 | Perindopril, N = 427 | 3.2 | |
| MV Padma et al.18 | Perindopril, N = 298 | 4 |
PIANIST, Perindopril-Indapamide plus AmlodipiNe in high rISk hyperTensive patients; PAINT, Perindopril-Amlodipine plus Indapamide combination for controlled hypertension Non-intervention Trial; PETRA, The Antihypertensive Efficacy of the Triple Fixed Combination of Perindopril, Indapamide, and Amlodipine; PROOF, Combined Therapy of Arterial Hypertension With a Triple Fixed-Dose Combination of Amlodipine/Indapamide/ Perindopril Arginine in Real Clinical Practice; PROTECT, Effectiveness of PeRindOpril in the management of hyperTension: idEntification of patient and physiCian determinants of response to Treatment; STRONG, SafeTy & efficacy analysis of coveRsyl amlodipine in uncOntrolled and Newly diaGnosed hypertension.
4. Mechanism of ACEI-induced cough
Although the exact mechanism of ACEI-induced cough remains unclear, several theories have been postulated for cough development. Degradation of bradykinin and substance P by ACE and their subsequent accumulation in upper and lower respiratory tracts by ACEIs is the most widely accepted theory. Bradykinin induces sensitization of airway sensory nerves via rapidly adapting stretch receptors and C-fiber receptors that releases neurokinin A and substance P. This causes airway smooth muscle to constrict leading to bronchoconstriction and cough.26 However, the reason why cough does not occur in all patients receiving ACEIs still warrants further research. Other proposed mechanisms include bronchial hyperreactivity (BHR), asthma history, chronic heart failure (CHF), increased sensitivity of bradykinin-dependent airway sensory nerve fibers, bradykinin receptor gene polymorphism increased cough reflex sensitivity, aminopeptidase P (APP) enzyme deficiency in the breakdown of bradykinin, and mechanisms that include ACE insertion/deletion polymorphism. Few studies27,28 reported relations of ACEI-induced cough and asthma and BHRwhereas, other studies29, 30, 31 reported contrasting suggestion that previous asthma or BHR history does not pose a risk for developing ACE-I-induced cough. Studies have reported the relation of ACEI-induced cough and bradykinin β2 receptor polymorphism in population of East Asian origin.32,33 However, there are inconsistent results showing correlation between ACE-I-induced cough and ACE insertion/deletion polymorphisms and bradykinin receptor gene polymorphism have been reported.26 Common findings from studies evaluating these hypotheses indicate the involvement of two or more mechanisms in the development of ACEI-induced secondary cough.26
The association between HF and ACEI-induced cough also remains matter of further investigation due to contradicting reports. In their study, Ravid et al reported that the incidence of ACEI-induced cough was higher in HF patients compared to in those with hypertension.34 Given that HF itself can cause coughing, the true incidence of ACEI-induced cough is difficult to estimate in these patients, and rather ACEI treatment could cure the HF-associated cough may be via improvement in ventilation/perfusion imbalance, alveolar-capillary gas transfer, pulmonary congestion.15
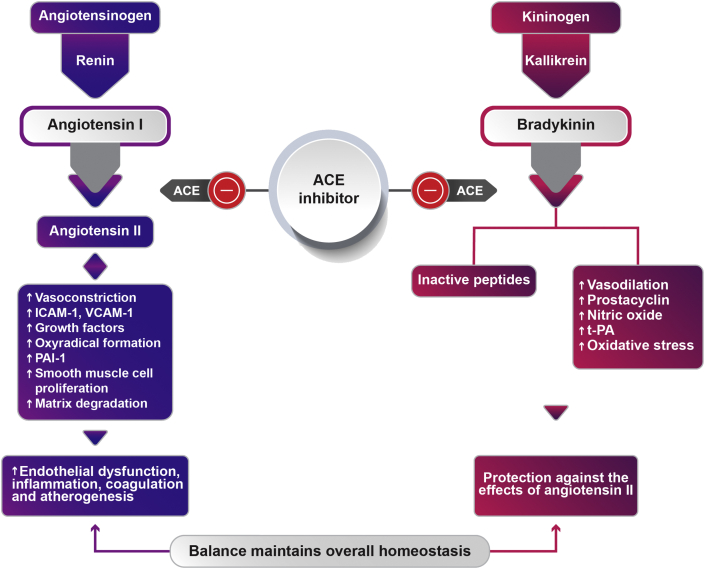
Despite bradykinin theory being the most commonly appealed hypothesis, two observations deduced from some studies challenge it. First, in a randomized double-blind study that used a de–challenge and re-challenge method to attribute cough, a 2-fold higher incidence of cough was seen in patients undergoing enalapril therapy (22%) compared with perindopril treatment (11%).35 Further, a retrospective study also found a 3-fold increase in cough incidence with enalapril (7%) vs. perindopril (2.2%) in patients with arterial hypertension. Lower incidences of cough observed in these studies are important in light of the fact that despite having highest bradykinin/angiotensin selectivity, perindopril has the lowest incidence of cough challenging the bradykinin theory for cough development. The second important observation was made in a study by Ceconi C et al, where, the bradykinin levels were found to be lower in patients with coronary artery disease (CAD) compared to healthy controls at baseline (12.4 ± 6.0 vs 18.3 ± 3.5 pg/mL). Notably, in patients treated with perindopril, these levels were found to increase and were normalized to that observed in healthy controls at 1 year (17.7 ± 6.3 pg/mL) indicating that increased bradykinin levels would instead decrease the incidence of cough.36 This could be explained by the critical role played by ang II and bradykinin balance in maintaining homeostasis. The Michaelis–Menten constant for bradykinin inactivation and ang II formation indicates that ACE inhibition leads to lower ang II and perpetually higher bradykinin levels, which helps maintain homeostasis. As shown in Fig. 1, bradykinin offsets the harmful effects of ang II by mediating vasodilation and vascular protective effects. As explained earlier, among ACEIs, this bradykinin potentiation is particularly pronounced for perindopril, which could, instead, underlie the CV benefits offered by the drug.
Fig. 1.
Role of ACEI in maintaining overall homeostasis in the body. ACE: Angiotensinconvertingenzyme; ICAM-1: Intercellularadhesionmolecule1; VCAM-1: Vascular cell adhesion protein 1; PAI-1: Plasminogen activator inhibitor-1-; t-Pa: Tissueplasminogenactivator.
5. Are angiotensin receptor inhibitors the alternative to ACEIs?
The reported low incidence of cough in patients on ARBs is attributed to the lack of effect on bradykinin. However, at the same time, the favorable pleiotropic effects (anti atherosclerotic properties and antithrombotic effects) of ACEIs are attributed to ACEI-induced increase in bradykinin along with the decrease in ang II levels.
There is an inconclusive debate for selecting RAAS inhibitors, ACEI or ARBs based on the available evidence. In a meta-analysis evaluating treatment benefits of RAAS inhibitors conducted in 158,998 patients with hypertension, RAAS inhibition resulted in a significant 5% reduction in all-cause mortality (HR: 0.95, 95% CI: 0.91–1.00, p = 0.032).37 Moreover, the observed treatment benefit was entirely from the class of ACEIs, with a significant 10% reduction in all-cause mortality (HR: 0.90, 95% CI: 0.84–0.97, p = 0.004), while ARB treatment did not provide any mortality reduction (HR: 0.99, 95% CI: 0.94–1.04, p = 0.683). There was also significant reduction in CV mortality with the ACEIs (HR: 0.88, 95%CI: 0.77–1.00, p = 0.051) but no effect was observed with the ARBs (HR: 0.96, 95% CI:0.90–1.01, p = 0.14) in the same dataset of patients with hypertension.37 Moreover, among the included ACEIs studies, the principal mortality benefits were shown in the HYVET, ASCOT-BPLA, and ADVANCE, all of which are the perindopril trials (HR: 0.87, 95% CI: 0.81–0.93, p < 0.001).37 Another systematic review of 26 studies that included 61,264 patients with diabetes and normoalbuminuria showed that ACEIs reduced the risk of new-onset microalbuminuria, macroalbuminuria or both (RR: 0.71, 95% CI: 0.56–0.89), and mortality risk (RR: 0.84, 95% CI: 0.73–0.97) when compared to placebo. In addition, it also provided similar benefits in patients with or without hypertension (p = 0.74).38 Lesser reduction in the new microalbuminuria, macroalbuminuria or both (RR: 0.90, 95% CI: 0.68–1.19), and increased risk of mortality (RR: 1.12, 95% CI: 0.88–1.41) were observed with ARBs compared to placebo.38 However, a meta-analysis of 106 studies with 254,301 patients with no history of HF in placebo-controlled, active-controlled, head-to-head randomized trials suggested an equal efficacy and safety of ARBs and ACEIs.39 Similar findings were also reported in a network meta-analysis of randomized trials that included patients at high CV risk without HF, ARBs were similar to ACEIs in reducing the risk for composite outcomes of CV death, MI and stroke (RR: 0.92, 95% CI: 0.78–1.08).40 Although the data for head-to-head comparison of ACEI and ARB is scarce, cumulative availability of evidence suggests that both are equally effective in reducing outcomes in patients with hypertension or in patients at high risk of CV events.41 Therefore, the ACC/AHA, the ESC/ESH, the NICE, and the Hypertension Canada guidelines recommend ACEIs or ARBs in equivalence and as first line for treating hypertension.11, 12, 13,42 However, the ESC-EASD14 guideline, the ACCF/AHA guideline on HF43 and the ESC guideline on chronic coronary syndrome44 recommend ACEIs as first line treatment and ARBs only if the patient is intolerant to ACEI for HF due to large experiences and evidence with ACEI underlie these recommendations.
The difference in effects has been attributed to the different modes of action of ACEIs versus ARBs, primarily because of the pleiotropic effects and beneficial role of the bradykinin pathway with ACEIs.45,46 In fact, there only two ACEIs, perindopril and ramipril, which have shown benefits in terms of decrease in CV events in patients who are at high risk of developing coronary heart disease (CHD) with normal left ventricular (LV) function.47,48 However when both are compared, the perindopril studies included patients who are treated in a manner reflective of current day medical practice as opposed to ramipril studies which were completed before the era of optimal medical therapy. Moreover, perindopril seems to be the only ACEI with evidence for morbidity and mortality benefits in HF patients with preserved EF,49 and the findings from several clinical studies also show CV benefits in patients with AMI and in those with or at high risk for CHD without HF.36
The hypertension management guidelines by the Hypertension Canada, and those of ESC-EASD, particularly for patients at a high CV risk, such as those with diabetes mellitus appraise the fact that ARBs lack the CV-protective effects of ACEIs and therefore recommend ACEIs as the preferred RAAS inhibitors for high-risk patients.11,14
6. Measures towards better management of ACEI-induced cough
6.1. Continue or discontinue ACEI treatment?
Although the intensity of cough is usually mild to moderate, it can be occasionally severe enough and require discontinuation of treatment. In a study by Reisin and Schneeweiss, for a significant number of patients (27.4%), the cough spontaneously disappeared after 2–8 months of therapy despite continued and unaltered administration of ACEIs and without any therapy aimed for its suppression.50 All patients were followed up for 13 months after disappearance of cough, and no recurrences were observed. In another study by the same authors, >50% of patients developed complete resolution of symptoms despite continuing with treatment.51 Similar findings were reported in a case–control study, wherein the odds of developing cough (ratio) were 1.6 (95% CI: 0.9–2.7) for long-term users (>6 months), 2.0 (95% CI: 1.1–3.8) for patients who had been on ACEI treatment for 2–6 months, and 4.8 (95% CI: 1.7–13.3) for those just starting treatment (<2 months). More recently, in 2015, Atsuhisa Sato and Seiichi Fukuda noted a reduced incidence of cough reported with continuous use of ACEIs.52 Nevertheless, additional studies focused particularly on continuation of ACEIs and monitoring the development of cough in patients belonging to different categories of CV risk are warranted before the strategy is widely recommended.
Another measure could be temporarily discontinuing ACEI treatment when an incidence of cough is reported and reintroduction after remission of cough, but owing to lack of evidence, this strategy has been a matter of debate. Recently, this strategy was demonstrated to effectively reduce the incidence of cough,52 and we believe that it is worth exploring in Indian patients as well. Moreover, cough being a class effect for ACEI, it may not be reasonable to immediately switch from one ACEI to another in case of cough leading to discontinuation of drug.
6.2. Do concomitant medications help to reduce incidence of cough?
Clinical studies have indicated that addition of calcium channel blockers to ACEIs reduce cough reflex by two mechanisms. First, by inhibiting prostaglandin synthesis and second by inhibiting Ca++-dependent release of glutamate which play an important role in the central transmission of the cough reflex.53 This finding was further supported in a study by Franova et al that reported lower incidence of cough with concomitant calcium channel blockers or diuretics compared to ACEIs monotherapy.54., 55 As per ACCP evidence-based clinical practice guidelines, in patients for whom the cessation of ACEI therapy is not an option, medications including that with sodium cromoglycate, theophylline, sulindac, indomethacin, amlodipine, nifedipine, ferrous sulfate, and picotamide to suppress cough should be attempted.56
6.3. Is prior adverse event information necessary for patients?
The incidence of cough depends on the way and the extent of emphasis with which cough as an adverse reaction is explained to patients. In a previous study, when cough as an adverse event of ACEIs was emphasized to patients, >25% of patients, including those receiving calcium antagonists, reported the incidence of cough. Surprisingly, when patients were not informed of cough as a side effect of ACEIs, none of the patients, including those receiving ACEIs, developed cough.52,57 In large-scale trials of hypertension, the adverse reaction of cough had been reported in the placebo group as well.52 Taken together, ACEI use is associated with cough, but every incidence of cough is not always associated with ACEI use. Hence, it is suggested that cough as an adverse event should not be over emphasized to patients.
6.4. Does selection of type of ACEI reduce incidence of cough?
As discussed earlier, the incidence of cough varies based on the individual ACEI used. ACEIs are categorized into three groups based on the presence of a sulfhydryl, carboxyl, or phosphoryl group, but the clinical relevance of this structural difference remains unclear.58 However, in an Indian ADR monitoring study, phosphoryl group-containing ACEIs (fosinopril) were associated with a higher incidence of cough compared with carboxyl group-containing ACEIs (enalapril, lisinopril, and ramipril).59 It is reasonable to use ACEIs that induce cough less frequently. In this context, perindopril has been associated with a relatively low incidence of cough and has extensive evidence supporting its cardiovascular benefits and tolerability.
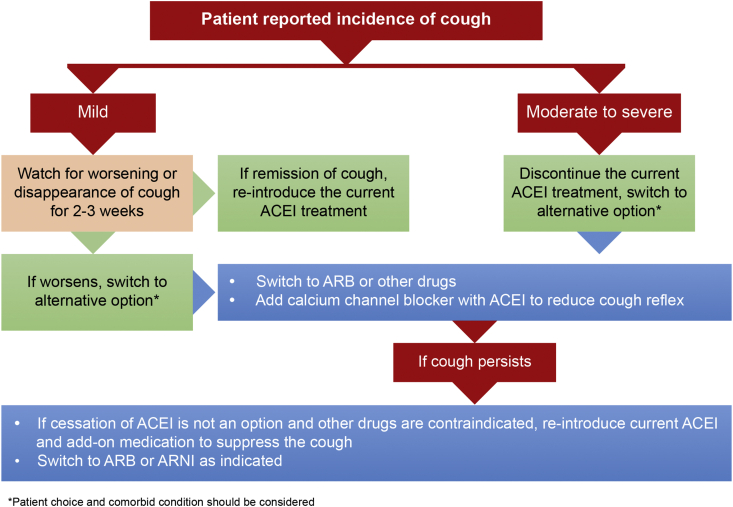
Overall, management of ACEI-induced cough can be done as per algorithm given as Fig. 2.
Fig. 2.
Algorithm for management of ACEI-induced cough. ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin II receptor blocker; ARNI:Angiotensin receptor neprilysin inhibitor.
7. Conclusion
Given the high prevalence of CVDs in India, there is a greater need to improve treatment adherence to ACEIs, which is highly relevant considering the lower-than-expected incidence of cough with certain ACEIs (e.g., perindopril). Cough as an adverse event of ACEIs should not be overemphasized, and even if cough occurs, patients with mild-to-moderate cough should be counseled to continue treatment considering the probability of natural disappearance of cough. Switching to alternative drugs should be suggested in case of intolerable symptoms that recur (after re-challenge with ACEIs) and after exclusion of all other possible causes of cough, particularly bronchial disease and pulmonary edema.
Source(s) of support
Nil.
Conflicts of interest
Nishita Shah is an employee of Serdia Pharmaceuticals (India) Pvt Ltd. All other authors have no conflict of interest to declare.
Acknowledgement
Authors would like to thank CBCC Global Research for providing medical writing assistance which was supported by Serdia Pharmaceuticals (India) Pvt Ltd.
References
- 1.Cottin V., Cordier J. Iatrogenic drug-induced bronchospasm, cough, and bronchiolitis. Etiologic and physiopathologic aspects. Rev Mal Respir. 1996;13:339–360. [PubMed] [Google Scholar]
- 2.Irwin R.S., French C.L., Chang A.B. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153:196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benigni A., Cassis P., Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphey L., Vaughan D., Brown N. Contribution of bradykinin to the cardioprotective effects of ACE inhibitors. Eur Heart J Suppl. 2003;5:A37–A41. [Google Scholar]
- 5.Pfeffer M.A., Braunwald E., Moye L.A. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. Ann Intern Med. 1991;115:67. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 7.Investigators TPT Angiotensin-converting–enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J., Zhang W., Zhang X. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA internal medicine. 2014;174:773–785. doi: 10.1001/jamainternmed.2014.348. [DOI] [PubMed] [Google Scholar]
- 9.Montalescot G., Sechtem U., Achenbach S., Task Force Members 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 10.Fihn S.D., Blankenship J.C., Alexander K.P. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines, a. J Thorac Cardiovasc Surg. 2015;149:e5–e23. doi: 10.1016/j.jtcvs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Nerenberg K.A., Zarnke K.B., Leung A.A. Hypertension Canada's 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506–525. doi: 10.1016/j.cjca.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Whelton P., Carey R., Aronow W., Jr C. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 13.NICE Hypertension in adults: diagnosis and management NICE guideline [NG136] 2019. available from: https://www.nice.org.uk/guidance/ng136/resources/hypertension-in-adults-diagnosis-and-management-pdf-66141722710213.
- 14.Cosentino F., Grant P.J., Aboyans V. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 15.Sica D.A. Angiotensin-converting enzyme inhibitors side effects? physiologic and non-physiologic considerations. J Clin Hypertens. 2004;6:410–416. doi: 10.1111/j.1524-6175.2004.02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyskida K., Jura-Szołtys E., Smertka M., Owczarek A., Chudek J. Factors that favor the occurrence of cough in patients treated with ramipril – a pharmacoepidemiological study. Med Sci Mon Int Med J Exp Clin Res. 2012;18:PI21–PI28. doi: 10.12659/MSM.883336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahl V.K., Jadhav U.M., Thacker H.P. Management of hypertension with the fixed combination of perindopril and amlodipine in daily clinical practice: results from the STRONG prospective, observational, multicenter study. Am J Cardiovasc Drugs. 2009;9:135–142. doi: 10.1007/BF03256570. [DOI] [PubMed] [Google Scholar]
- 18.Mv P., Kaul S. Incidence of recurrent stroke in primary care during preventive treatment based on perindopril with or without indapamide. Neurol India. 2007;55:141–144. doi: 10.4103/0028-3886.32785. [DOI] [PubMed] [Google Scholar]
- 19.Brugts J.J., Arima H., Remme W. The incidence and clinical predictors of ACE-inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol. 2014;176:718–723. doi: 10.1016/j.ijcard.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 20.Shankar P.K., Vidyasagar S., Adiga S. Efficacy and tolerability of trandolapril in mild to moderate hypertension - a double blind comparative clinical trial with enalapril in Indian population. Indian J Physiol Pharmacol. 2006;50:421–426. [PubMed] [Google Scholar]
- 21.Bansal S., Chauhan D., Ramesh D., Barmare S., Chakraborty S. Blood pressure control and acceptability of Perindopril and its fixed dose combinations with Amlodipine or Indapamide, in younger patients with hypertension. Indian Heart J. 2014;66:635–639. doi: 10.1016/j.ihj.2014.10.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobalava Z.D., Troitskaya E., Tolkacheva V. Combined therapy of arterial hypertension with triple fixed-dose combination of amlodipine/indapamide/perindopril arginine in real clinical practice: the organization and the main results of the DOKAZATEL'STVO (Proof) study. Kardiologiia. 2018;58:21–30. [PubMed] [Google Scholar]
- 23.Tóth K. PIANIST Investigators: antihypertensive efficacy of triple combination perindopril/indapamide plus amlodipine in high-risk hypertensives: results of the PIANIST study (Perindopril-Indapamide plus AmlodipiNe in high rISk hyperTensive patients) Am J Cardiovasc Drugs. 2014;14:137–145. doi: 10.1007/s40256-014-0067-2. [DOI] [PubMed] [Google Scholar]
- 24.Páll D., Szántó I., Szabó Z. Triple combination therapy in hypertension: the antihypertensive efficacy of treatment with perindopril, amlodipine, and indapamide SR. Clin Drug Invest. 2014;34:701–708. doi: 10.1007/s40261-014-0223-0. [DOI] [PubMed] [Google Scholar]
- 25.Ábrahám G., Dézsi C.A. The antihypertensive efficacy of the triple fixed combination of perindopril, indapamide, and amlodipine: the results of the PETRA study. Adv Ther. 2017;34:1753–1763. doi: 10.1007/s12325-017-0572-1. [DOI] [PubMed] [Google Scholar]
- 26.Yılmaz İ. Angiotensin-converting enzyme inhibitors induce cough. Turkish thoracic journal. 2019;20:36–42. doi: 10.5152/TurkThoracJ.2018.18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman J., Casanova J.E., Riendl P., Schiuder D.P. Bronchial hyperreactivity and cough due to angiotensin-converting enzyme inhibitors. Chest. 1989;95:544–548. doi: 10.1378/chest.95.3.544. [DOI] [PubMed] [Google Scholar]
- 28.Bucknall C.E., Neilly J.B., Carter R., Stevenson R.D., Semple P.F. Bronchial hyperreactivity in patients who cough after receiving angiotensin converting enzyme inhibitors. British medicaljournal (Clin Res Ed). 1988;296:86–88. doi: 10.1136/bmj.296.6615.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman J., Schmitt S., Barnard J., Busse W. Angiotensin-converting enzyme inhibitors in patients with bronchial responsiveness and asthma. Chest. 1992;101:922–925. doi: 10.1378/chest.101.4.922. [DOI] [PubMed] [Google Scholar]
- 30.Wood R. Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril. A controlled retrospective cohort study. Br J Clin Pharmacol. 1995;39:265–270. doi: 10.1111/j.1365-2125.1995.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sala H., Abad J., Juanmiquel L. Captopril and bronchial reactivity. Postgrad Med. 1986;62:76–77. [PubMed] [Google Scholar]
- 32.Grilo A., Sáez-Rosas M.P., Santos-Morano J. Identification of genetic factors associated with susceptibility to angiotensin-converting enzyme inhibitors-induced cough. Pharmacogenetics Genom. 2011;21:10–17. doi: 10.1097/FPC.0b013e328341041c. [DOI] [PubMed] [Google Scholar]
- 33.Mas S., Gassò P., Álvarez S. Pharmacogenetic predictors of angiotensin-converting enzyme inhibitor-induced cough: the role of ACE, ABO, and BDKRB2 genes. Pharmacogenetics Genom. 2011;21:531–538. doi: 10.1097/FPC.0b013e328348c6db. [DOI] [PubMed] [Google Scholar]
- 34.Ravid D., Lishner M., Lang R., Ravid M. Angiotensin-converting enzyme inhibitors and cough: a prospective evaluation in hypertension and in congestive heart failure. J Clin Pharmacol. 1994;34:1116–1120. doi: 10.1002/j.1552-4604.1994.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 35.Tumanan-Mendoza B.A., Dans A.L., Villacin L.L. Dechallenge and rechallenge method showed different incidences of cough among four ACE-Is. J Clin Epidemiol. 2007;60:547–553. doi: 10.1016/j.jclinepi.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Dinicolantonio J.J., Lavie C.J., O'keefe J.H. Not all angiotensin-converting enzyme inhibitors are equal: focus on ramipril and perindopril. PGM (Postgrad Med) 2013;125:154–168. doi: 10.3810/pgm.2013.07.2687. [DOI] [PubMed] [Google Scholar]
- 37.van Vark L.C., Bertrand M., Akkerhuis K.M. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158 998 patients. Eur Heart J. 2012;33:2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv J., Perkovic V., Foote C.V., Craig M.E., Craig J.C., Strippoli G.F. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev. 2012;12:CD004136. doi: 10.1002/14651858.CD004136.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bangalore S., Fakheri R., Toklu B., Ogedegbe G., Weintraub H., Messerli F.H. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc. 2016;91:51–60. doi: 10.1016/j.mayocp.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Ricci F., Di Castelnuovo A., Savarese G., Perrone Filardi P., De Caterina R. ACE-inhibitors versus angiotensin receptor blockers for prevention of events in cardiovascular patients without heart failure: a network meta-analysis. Int J Cardiol. 2016;217:128–134. doi: 10.1016/j.ijcard.2016.04.132. [DOI] [PubMed] [Google Scholar]
- 41.Franz H. Messerli, sripal Bangalore, chirag bavishi and stefano F. Rimoldi. Angiotensin-converting enzyme inhibitors in hypertension to use or not to use? J Am Coll Cardiol. 2018;71:1474–1482. doi: 10.1016/j.jacc.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 42.Williams B., Mancia G., Spiering W. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of Cardiology (ESC) and the European society of hypertension (ESH) Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 43.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Knuuti J., Wijns W., Saraste A. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 45.Brugts J.J., den Uil C.A., Danser A.H.J., Boersma E. The renin-angiotensin-system: approaches to guide angiotensin-converting-enzyme inhibition in patients with coronary artery disease. Cardiology. 2009;112:303–312. doi: 10.1159/000159124. [DOI] [PubMed] [Google Scholar]
- 46.Brugts J.J., Isaacs A., De Maat M.P. A pharmacogenetic analysis of determinants of hypertension and blood pressure response to angiotensin-converting enzyme inhibitor therapy in patients with vascular disease and healthy individuals. J Hypertens. 2011;29:509–519. doi: 10.1097/HJH.0b013e328341d117. [DOI] [PubMed] [Google Scholar]
- 47.Fox K.M., Bertrand M., Ferrari R. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 48.Yusuf S., Sleight P., Pogue Jf, Bosch J., Davies R., Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 49.Cleland J.G., Tendera M., Adamus J., Freemantle N., Polonski L., Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 50.Reisin L., Schneeweiss A. Spontaneous disappearance of cough induced by angiotensin-converting enzyme inhibitors (captopril or enalapril) Am J Cardiol. 1992;70:398–399. doi: 10.1016/0002-9149(92)90630-h. [DOI] [PubMed] [Google Scholar]
- 51.Reisin L., Schneeweiss A. Complete spontaneous remission of cough induced by ACE inhibitors during chronic therapy in hypertensive patients. J Hum Hypertens. 1992;6:333–335. [PubMed] [Google Scholar]
- 52.Sato A., Fukuda S. A prospective study of frequency and characteristics of cough during ACE inhibitor treatment. Clin Exp Hypertens. 2015;37:563–568. doi: 10.3109/10641963.2015.1026040. [DOI] [PubMed] [Google Scholar]
- 53.Fogari R., Zoppi A., Mugellini A., Preti P., Banderali A., Salvetti A. Effects of amlodipine, nifedipine GITS, and indomethacin on angiotensin-converting enzyme inhibitor-induced cough: a randomized, placebo-controlled, double-masked, crossover study. Curr Ther Res. 1999;60:121–128. [Google Scholar]
- 54.Fraňová S., Nosál’Ová G., Antošová M., Nosáľ S. Enalapril and diltiazem co-administration and respiratory side effects of enalapril. Physiol Res. 2005;54:515–520. [PubMed] [Google Scholar]
- 55.Franova S. The influence of inhaled furosemide on adverse effects of ACE-inhibitors in airways. Bratisl Lek Listy. 2001;102:309–313. [PubMed] [Google Scholar]
- 56.Dicpinigaitis P.V. Angiotensin-converting enzyme inhibitor-induced cough. Chest. 2006;129:169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 57.Goto N., Shirahase M., Hatta H. Influence of type of questionnaire on the prevalence of coughing in patients taking angiotensin converting enzyme inhibitors (ACEI) Jpn J Clin Pharmacol Therapeut. 1996;27:725–730. [Google Scholar]
- 58.Sebastián G.Z., Roberto P. Cough and angioedema in patients receiving angiotensin-converting enzyme inhibitors. Are they always attributable to medication? Rev Argent Cardiol. 2011;79:157–163. [Google Scholar]
- 59.Sangole N.V., Dadkar V.N. Adverse drug reaction monitoring with angiotensin converting enzyme inhibitors: a prospective, randomized, open-label, comparative study. Indian J Pharmacol. 2010;42:27–31. doi: 10.4103/0253-7613.62408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsioufis K., Douma S., Kallistratos M.S., Manolis A.J. Effectiveness and adherence to treatment with perindopril/indapamide/amlodipine single-pill combination in a Greek population with hypertension. Clin Drug Invest. 2019;39:385–393. doi: 10.1007/s40261-019-00761-0. [DOI] [PubMed] [Google Scholar]
- 61.Nedogoda S.V., Stojanov V.J. Single-pill combination of perindopril/indapamide/amlodipine in patients with uncontrolled hypertension: a randomized controlled trial. Cardiol therapy. 2017;6:91–104. doi: 10.1007/s40119-017-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mourad J.-J., Amodeo C., de Champvallins M., Brzozowska-Villatte R., Asmar R. Blood pressure-lowering efficacy and safety of perindopril/indapamide/amlodipine single-pill combination in patients with uncontrolled essential hypertension: a multicenter, randomized, double-blind, controlled trial. J Hypertens. 2017;35:1481–1495. doi: 10.1097/HJH.0000000000001359. [DOI] [PubMed] [Google Scholar]
- 63.Sampalis J.S., Psaradellis E., Stutz M., Rickard J., Rampakakis E. Post Hoc Analysis of the CONFIDENCE II, PROTECT I, SHAKE THE HABIT I and SHAKE THE HABIT II observational studies in mild to moderate hypertensive patients treated with perindopril and atorvastatin concomitantly. Drugs R. 2018;18:283–293. doi: 10.1007/s40268-018-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]