Abstract
Background
Surgical aortic valve replacement (SAVR) is the conventional treatment for patients with severe aortic valve stenosis at low surgical risk. Transcatheter aortic valve implantation (TAVI) is a less invasive procedure. We conducted a health technology assessment (HTA) of TAVI for patients with severe aortic valve stenosis at low surgical risk, which included an evaluation of effectiveness, safety, cost-effectiveness, the budget impact of publicly funding TAVI, and patient preferences and values.
Methods
We used the 2016 Health Quality Ontario HTA on TAVI2 as a source of eligible studies and performed a systematic literature search for studies published since the 2016 review. Eligible primary studies identified both through the 2016 HTA and through our complementary literature search were used in a de novo analysis. We assessed the risk of bias of each included study using the Cochrane risk-of-bias tool and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria.
An applicable, previously conducted cost-effectiveness analysis was available, so we did not conduct a primary economic evaluation. We analyzed the budget impact of publicly funding TAVI in people at low surgical risk in Ontario. We also performed a literature survey of the quantitative evidence of preferences and values of patients for TAVI. The Canadian Agency for Drugs and Technologies in Health (CADTH) conducted a review to evaluate the qualitative literature on patient and provider preferences and values for TAVI. To contextualize the potential value of TAVI, we spoke with people with severe aortic valve stenosis.
Results
We identified two randomized controlled trials that compared TAVI (transfemoral route) and SAVR in patients with severe aortic valve stenosis at low surgical risk. Both studies have an ongoing follow-up of 10 years, but 1-year and limited 2-year follow-up results are currently available. At 30 days, compared with SAVR, TAVI had a slightly lower risk of mortality (risk difference −0.8%, 95% confidence interval [CI] −1.5% to −0.1%, GRADE: Moderate) and disabling stroke (risk difference −0.8%, 95% CI −1.8% to −0.2%, GRADE: Moderate), and resulted in more patients with symptom improvement (risk difference 11.8%, 95% CI 8.2% to 15.5%, GRADE: High) and in a greater improvement in quality of life (GRADE: High). At 1 year, TAVI and SAVR were similar with regard to mortality (GRADE: Low), although TAVI may result in a slightly lower risk of disabling stroke (GRADE: Moderate). Both TAVI and SAVR resulted in a similar improvement in symptoms and quality of life at 1 year (GRADE: Moderate). Compared with SAVR, TAVI had a higher risk of some complications and a lower risk of others.
Device-related costs for TAVI (about $25,000) are higher than for SAVR (about $6,000). A published cost-effectiveness analysis conducted from an Ontario Ministry of Health perspective showed TAVI to be more expensive and, on average, slightly more effective (i.e., it was associated with more quality-adjusted life-years [QALYs]) than SAVR. Compared with SAVR, the incremental cost-effectiveness ratios (ICERs) were $27,196 per QALY and $59,641 per QALY for balloon-expandable and self-expanding TAVI, respectively. Balloon-expandable TAVI was less costly (by $2,330 on average) and slightly more effective (by 0.02 QALY on average) than self-expanding TAVI. Among the three interventions, balloon-expandable TAVI had the highest probability of being cost-effective. It was the preferred option in 53% and 59% of model iterations, at willingness-to-pay values of $50,000 and $100,000 per QALY, respectively. Self-expanding TAVI was preferred in less than 10% of iterations. The budget impact of publicly funding TAVI in Ontario is estimated to be an additional $5 to $8 million each year for the next 5 years. The budget impact could be significantly reduced with reductions in the device price.
We did not find any quantitative or qualitative evidence on patient preferences and values specific to the low-risk surgical group. Among a mixed or generally high-risk and population, people typically preferred the less invasive nature and the faster recovery time of TAVI compared with SAVR, and people were satisfied with the TAVI procedure. Patients with severe aortic valve stenosis at low surgical risk and their caregivers perceived that TAVI minimized pain and recovery time. Most patients who had TAVI returned to their usual activities more quickly than they would have if they had had SAVR. Our direct patient and caregiver consultations indicated a preference for TAVI over SAVR.
Conclusions
Both TAVI (transfemoral route) and SAVR resulted in improved patient symptoms and quality of life during the 1 year of follow-up. The TAVI procedure is less invasive and resulted in greater symptom improvement and quality of life than SAVR 30 days after surgery. The TAVI procedure also resulted in a small improvement in mortality and disabling stroke at 30 days. At 1 year, TAVI and SAVR were similar with regard to mortality, although TAVI may result in a slightly lower risk of disabling stroke. According to the study authors, longer follow-up is needed to better understand how long TAVI valves last and to draw definitive conclusions on the long-term outcomes of TAVI compared with SAVR beyond 1 year.
The TAVI procedure might be cost-effective for patients at low surgical risk; however, there is some uncertainty in this result. We estimated that the additional cost to provide public funding for TAVI in people with severe aortic valve stenosis at low surgical risk would range from about $5 million to $8 million over the next 5 years.
Among a mixed or generally high-risk population, people typically preferred the less invasive nature and the faster recovery time of TAVI compared with SAVR.
OBJECTIVE
This health technology assessment (HTA) evaluates the clinical effectiveness, safety, and cost-effectiveness of transcatheter aortic valve implantation (TAVI) for adults with severe aortic valve stenosis who are at low surgical risk. It also evaluates the budget impact of publicly funding TAVI and the experiences, preferences, and values of people with severe aortic valve stenosis at low surgical risk.
BACKGROUND
Health Condition
The aortic valve is located between the aorta and the left ventricle of the heart.2 It opens to allow blood to flow from the left ventricle into the aorta when the heart contracts and closes to prevent blood from flowing backward into the heart when the heart relaxes.2
Aortic valve stenosis occurs when the valve narrows, obstructing blood flow from the heart into the aorta. The most common cause in men older than 65 years and women older than 75 years of age is degenerative calcification: a buildup of calcium deposits on the valve over time, causing it to narrow.3–5 In younger patients, the most common cause is congenital bicuspid aortic valve (an inherited condition in which the aortic valve has two leaves instead of the usual three).6 Narrowing of the aortic valve causes the heart to work harder and is usually progressive, eventually leading to left ventricular hypertrophy (thickening of the walls of the left ventricle) and heart failure.3 Symptoms of aortic valve stenosis include chest pain, shortness of breath, fainting spells, and fatigue that decrease people's quality of life and affect their activities of daily living.2
Clinical Need and Target Population
The prevalence of moderate to severe aortic valve stenosis increases with age: it is estimated to affect 0.02% of people 18 to 44 years of age and 2% of people older than 65 years.4 One study reported that the prevalence of severe aortic valve stenosis in people older than 75 years was 3.4%.7
Research on a cohort of patients who underwent surgery in the United States indicates about 80% of patients with severe aortic valve stenosis are at low surgical risk.8
Severe aortic valve stenosis is associated with a poor prognosis: without aortic valve replacement, a person's estimated life expectancy is less than 5 years, and more than half of patients will die within 2 to 3 years of the onset of symptoms.3 Medications can ease the symptoms, but percutaneous or surgical replacement of the valve is the only way to treat aortic valve stenosis.3
Current Treatment Options
Surgical aortic valve replacement (SAVR) is the conventional way to treat severe aortic valve stenosis in patients at low surgical risk3 and results in good treatment outcomes.9
During the procedure, the damaged aortic valve is removed and replaced with an artificial valve, which can be either mechanical or bioprosthetic.10 The procedure is an open-heart surgery that requires cardiopulmonary bypass (using a heart-lung machine) and is performed with the patient receiving general anesthesia,3 although a less invasive incision can be used.3 Patients undergoing SAVR who require revascularization may be considered for SAVR combined with a coronary artery bypass graft (CABG).10
Each patient's surgical risk is assessed by a multidisciplinary heart team and might be informed by the Society of Thoracic Surgeons (STS) risk score,11 which considers the presence of comorbidities to predict mortality 30 days after the surgery.12 The STS risk score has been validated in standard surgical-risk populations. In general, a risk score of 8% or more is considered to be high risk, a score of 4% to 8% is considered intermediate risk, and a score below 4% is considered low surgical risk.12 However, other comorbidities that are not represented in the STS score also need to be taken into account when assessing surgical risk, including frailty, porcelain aorta (an ascending aorta that is heavily calcified), and severe liver disease.11,12
Health Technology Under Review
The TAVI procedure involves placing a collapsed, bioprosthetic aortic valve inside the existing valve through a catheter, without the need for open-heart surgery.3 When the new valve is expanded, it pushes the narrowed valve outward and takes over control of blood flow from the left ventricle to the aorta.6
The TAVI procedure can be done with the patient receiving local or general anesthesia if the catheter is inserted using the transfemoral route, or receiving general anesthesia if using other routes.11 The transfemoral route is the most common: inserting the catheter via a puncture site in the common femoral artery (a large artery in the thigh).6 Other routes—such as the transaortic route (via an incision in the chest) or the subclavian route (via an artery that sits below the collarbone)—are alternatives when the femoral artery cannot be used because of size, calcification, or tortuosity.3,13 The narrowed native aortic valve can be expanded before or after TAVI using a procedure called balloon valvuloplasty, also known as balloon dilation.14
Balloon-expandable and self-expanding bioprosthetic valves are currently available in Canada. The Sapien valve is a first-generation balloon-expandable valve. Since its release, the second-generation Sapien XT and third-generation Sapien 3 balloon-expandable valves have also been developed. The Sapien valves consist of bovine pericardium tissue mounted on a stent frame. The CoreValve is a first-generation self-expanding valve; the Evolut R and Evolut PRO valves are its second- and third-generation valves. These valves consist of porcine pericardium tissue mounted on a self-expanding stent frame. The self-expanding Acurate Neo valve is also available: a porcine pericardium valve mounted on a self-expanding nitinol frame; currently no published data on the Acurate Neo valve cover the population that is the focus of this report.
The TAVI procedure is performed by clinicians and teams with specific training and experience in complex endovascular cardiac procedures.3 If coronary revascularization is necessary, percutaneous coronary intervention can be performed, either before or occasionally at the same time as the TAVI procedure.
In November 2016, given the finding that mortality with TAVI was not higher than with SAVR, and given that both treatments improved patients' quality of life during the first year after surgery, the Ontario Health Technology Advisory Committee recommended public funding for TAVI in patients with severe, symptomatic, degenerative aortic valve stenosis who were not candidates for SAVR or who had an estimated risk of mortality of 8% or greater within 30 days of surgery.15 The committee also recommended that TAVI be offered in select hospitals, as determined by the Cardiac Care Network of Ontario (now CorHealth Ontario).15 Additionally, Ontario Health (Quality) has recommended public funding of TAVI in patients at intermediate surgical risk.16 Since these reports, studies evaluating TAVI in patients at low surgical risk have been published.17,18
Regulatory Information
Balloon-expandable (Sapien XT and Sapien 3) and self-expanding (CoreValve, Evolut R, Evolut PRO, Acurate Neo) TAVI valves are approved by Health Canada as Class IV devices.19
According to information received from Health Canada (personal communication, October 11, 2019), none of the TAVI valves identified is approved for use in patients with severe aortic valve stenosis at low surgical risk.
Health Canada approved the use of these valves in patients with severe, symptomatic, aortic valve stenosis who are at high or greater surgical risk or who are inoperable. The Acurate Neo valve is further restricted to patients 75 years of age or older and to the transfemoral route of implantation. The Sapien 3 valve was also approved for use in patients with severe, symptomatic, calcific aortic valve stenosis who are judged by a heart team to be at intermediate risk for open-heart surgery (personal communication with Health Canada, October 11, 2019).
Ontario Context
Both the TAVI and the SAVR procedures are conducted at 11 sites in Ontario. The annual number of SAVR and TAVI procedures (all cases in which the procedure was started) is provided in Table 1.
Table 1:
Number of SAVR and TAVI Procedures Performed in Ontario, 2011/12 to 2017/18
| Procedure | 2012/13 | 2013/14 | 2014/15 | 2015/16 | 2016/17 | 2017/18 | 2018/19 |
|---|---|---|---|---|---|---|---|
| SAVR only | 1,691 | 1,666 | 1,628 | 1,710 | 1,728 | 1,801 | 1,872 |
| SAVR + CABG | 1,149 | 1,094 | 1,136 | 1,165 | 1,247 | 1,136 | 1,157 |
| TAVI | 341a | 486a | 645 | 744 | 861 | 962 | 1,369 |
Abbreviations: CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Data could be incomplete; mandatory TAVI data collection started in November 2013.
Source: CorHealth Ontario Cardiac Registry. Data retrieved July 2019.
At the time of writing this report, there is no formal public funding for TAVI in patients at intermediate or low surgical risk in Ontario.
Expert Consultation
We engaged with experts in the specialty area of aortic valve stenosis to help inform our understanding of aspects of the health technology and the condition and to contextualize the evidence. We also consulted with methodologists in order to confirm that we used the appropriate methodology in our analyses.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD 42020145232), available at https://www.crd.york.ac.uk/PROSPERO.
CLINICAL EVIDENCE
Research Question
What are the clinical effectiveness and safety of transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) for adults with severe aortic valve stenosis who are at low surgical risk?
Methods
Given that Health Quality Ontario published a health technology assessment (HTA) in 2016 evaluating TAVI in patients with severe aortic valve stenosis at different surgical levels,2 we used this 2016 HTA as a source of eligible studies in patients at low surgical risk published until its literature search date. The 2016 HTA included individual studies published between January 1, 2011, and September 30, 2015. We searched the literature for additional studies published after the end of its search date. Eligible primary studies identified both through the 2016 HTA20 and through our complementary literature search were used in a de novo analysis. Given that good quality randomized controlled trials (RCTs) are available for the topic, we decided to restrict our review to RCTs and synthesized reports of RCTs, i.e., HTAs, systematic reviews and meta-analyses.
Clinical Literature Search
We performed a clinical literature search on July 9, 2019, to retrieve studies published from January 1, 2015, until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Health Technology Assessment database, and the National Health Service Economic Evaluation Database (NHS EED).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. Methodological filters were used to limit retrieval to systematic reviews, meta-analyses, HTAs, and RCTs. The final search strategy was peer-reviewed using the PRESS Checklist.21
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of HTA agency websites as well as clinical trial and systematic review registries. Appendix 1 outlines our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
Randomized controlled trials
Health technology assessments, systematic reviews, and meta-analyses of RCTs if they included the most recent RCTs in patients at low surgical risk
Identified both through the 2016 Health Quality Ontario HTA2 and our literature search
English language full-text publications
Exclusion Criteria
Health technology assessments and systematic reviews if they did not include the most recent RCTs in patients at low surgical risk, non-randomized studies, noncomparative studies, editorials, commentaries, case reports, conference abstracts, and letters
Animal and in vitro studies
Participants
Inclusion Criteria
Adults with severe aortic valve stenosis and low surgical risk Surgical risk is defined by the study site's multidisciplinary heart team informed by the Society of Thoracic Surgeons (STS) score and assessment of comorbidities. An STS score below 4% is generally considered low risk
Exclusion Criteria
Studies that included a mixed population of patients with various surgical risks, i.e., low, intermediate, or high, without providing results specific to the low-risk population
Patients with a pre-existing mechanical or bioprosthetic aortic valve
Patients with a bicuspid aortic valve
Intervention
Transcatheter aortic valve implantation (any type of valve [e.g., self-expanding, balloon expandable], using any implantation route)
Comparator
Inclusion Criteria
Surgical aortic valve replacement with a bioprosthetic valve (either open-heart or less invasive surgery)
Exclusion Criteria
Surgical aortic valve replacement with a mechanical valve
Outcome Measures
Effectiveness
Composite end point (all-cause mortality, stroke, or rehospitalization at 1 year18; all-cause mortality or disabling stroke at 2 years17)
All-cause mortality
Stroke and transient ischemic attack
Aortic valve reintervention
Rehospitalization
Change in New York Heart Association (NYHA) scores
Quality of life
6-minute walk test
Valve hemodynamics (aortic valve area and aortic valve gradient)
Length of hospital stay for TAVI and SAVR procedures
Safety
Procedural complications
Life-threatening or disabling bleeding
Major vascular complications
Acute kidney injury
New-onset atrial fibrillation
Myocardial infarction
New permanent pacemaker implantation
New left bundle branch block
Moderate-to-severe paravalvular aortic regurgitation
Valve thrombosis
Leaflet thickening and leaflet mobility restriction
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence22 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search.
If there were questions regarding whether the surgical risk of the study population matches that in our population of interest, we sought confirmation from the study authors and clinical experts.
Data Extraction
We extracted relevant data from individual studies on study design and characteristics, risk-of-bias items, results, and PICOT (population, intervention, comparator, outcomes, and timing). Baseline characteristics of patients included in the studies, including those based on the PROGRESS-Plus23 categories (place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status, social capital) were extracted if available.
We contacted study authors to provide clarification as needed.
Data Presentation and Statistical Analysis
We presented the study results as reported in the studies identified. The PARTNER 3 study18 reported the results of dichotomous outcomes as the number and percentage of patients who experienced an event based on Kaplan-Meier estimates; hazard ratios (HRs) based on Cox proportional hazards analyses were also provided. For continuous variables, either the mean and standard deviation (SD) or the median and interquartile range (IQR) were reported. The Evolut LRT study17 reported the percentage of patients who experienced an event for dichotomous variables and mean and SD for continuous variables.
We used the absolute risk difference (risk difference) between TAVI and SAVR as the main measure of effect. If the effect was not provided in the studies, we calculated it with use of the information reported. When the number of events reported in the studies was small (i.e., less than 20), we used the exact method to calculate the risk difference, as it does not rely on the approximation to the normal distribution and is therefore more suitable for rare event data.24 Otherwise the normal approximation method was used. The results of the as-treated population, i.e., patients who were randomized and in whom the TAVI or SAVR procedure was attempted, were used, as this population was used in the primary analyses of both studies.
We stratified study results according to PROGRESS-Plus categories,23 concomitant revascularization, and other comorbidities when data were available. For procedure-related outcomes, we based our main results and conclusions on the 30-day follow-up point (life-threatening or major/disabling bleeding, acute kidney injury, atrial fibrillation, new permanent pacemaker implantation, and major vascular complications). For other outcomes, we based our main results and conclusions on the entire study follow-up available.
The PARTNER 3 study18 used the frequentist approach in their analyses. The Evolut LRT researchers17 used Bayesian statistical methods in their analyses, but because it used noninformative prior distributions, we assumed that the numerical results would be the same as those obtained using a frequentist approach, and this justified pooling the results of the two included studies when meta-analysis was considered appropriate.
For dichotomous outcomes, we performed meta-analyses for 30-day outcomes when appropriate using the number of events reported in the studies to calculate the risk difference between TAVI and SAVR. When the number of events reported in the studies was small (less than 20), we performed the meta-analysis by using the exact method, as it does not use the normal distribution approximation and is therefore more suitable for rare event data24; otherwise the Mantel-Hansel method was used. We assessed statistical heterogeneity using the I2 statistic25 and by examining the forest plots. We used a fixed- or a random-effects model depending on the extent of the heterogeneity in each meta-analysis. Meta-analyses were performed using Review Manager (RevMan [Computer program] Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and R version 3.5.126 using the packages “meta,” “exactmeta,” and “gplots.”
The Evolut LRT study17 identified through our systematic literature search is based on an interim analysis prespecified to occur when 850 (58%) patients reached 1 year of follow-up. The outcomes of patients who had not reached 1 and 2 years of follow-up were estimated using imputation methods.17 Given the large degree of imputation in the Evolut LRT study,17 we decided not to perform a meta-analysis of the results of the two studies at 1 year given methodological heterogeneity, unless the actual number of patients who experienced the events and those available at a given follow-up time were provided. If meta-analysis was inappropriate because of clinical, methodological, or statistical heterogeneity, we provided a narrative summary of results.
Critical Appraisal of Evidence
We assessed risk of bias of RCTs using the Cochrane risk of bias tool.27
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.28 The body of evidence was assessed in consideration of the following: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence.
Results
Clinical Literature Search
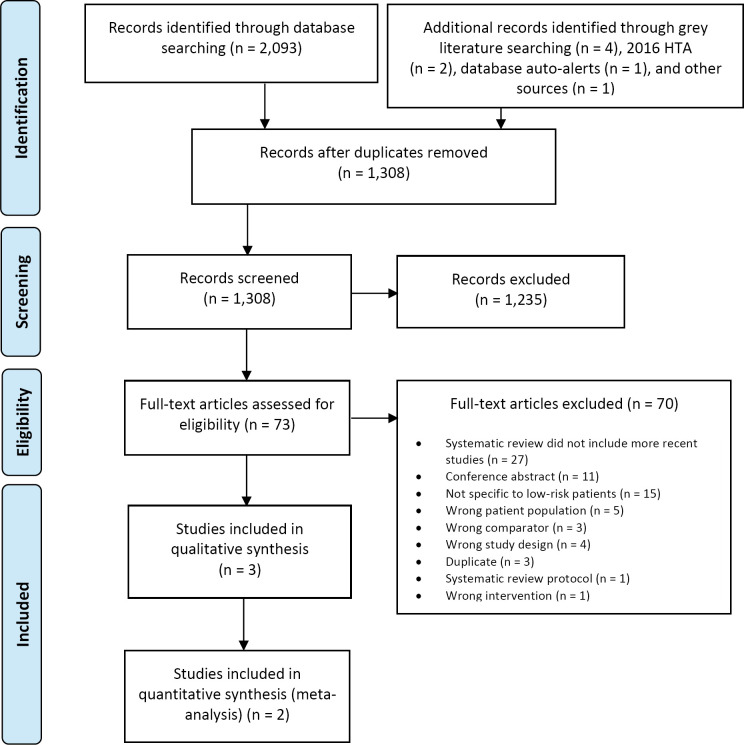
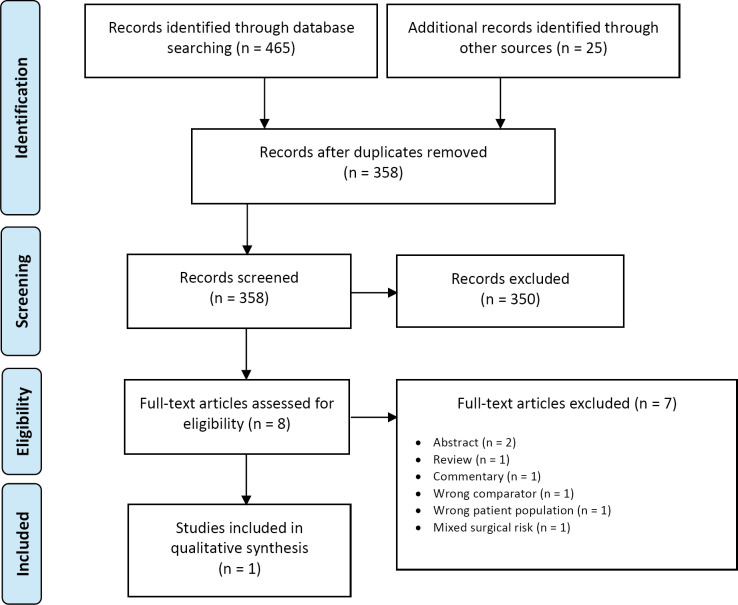
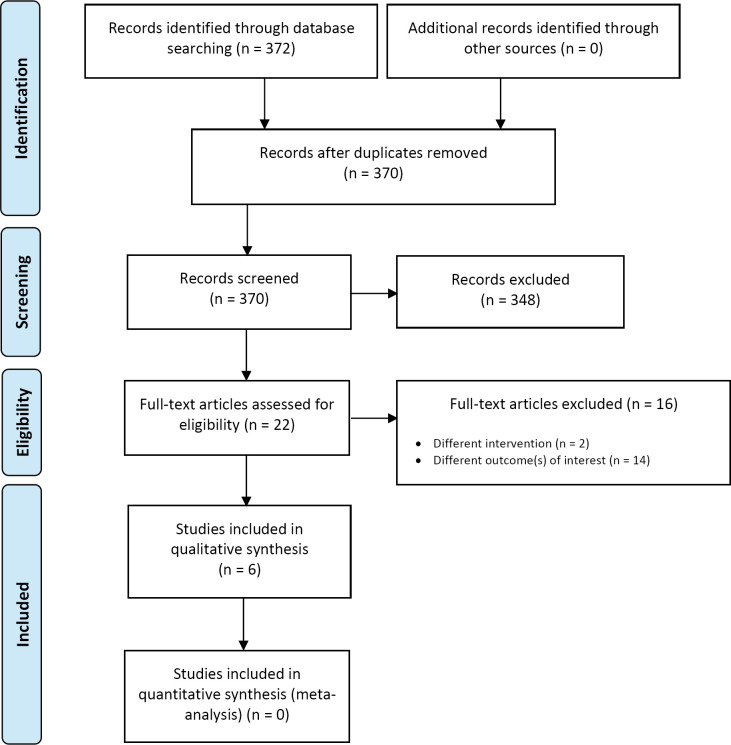
The database search of the clinical literature yielded 2,093 citations published between January 1, 2015, and July 9, 2019. We identified four additional publications from searching the grey literature, two from the 2016 HTA,2 one from database auto-alerts, and one from other sources. Three publications (2 RCTs and 1 additional publication of one of the RCTs) met our inclusion criteria.17,18,29 Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Abbreviations: HTA, health technology assessment; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses. Source: Adapted from Moher et al.30
Studies Identified
The 2016 Health Quality Ontario HTA2 identified two RCTs comparing TAVI and SAVR that included patients at low surgical risk.31,32 However, these two studies were considered ineligible for our report because their population included patients with different surgical risk levels and because results specific to the low-risk population were not provided.31,32 Two systematic reviews that included the most recent RCTs in patients at low surgical risk were excluded because their results also could not be directly applied to our report.33,34
Two RCTs comparing TAVI and SAVR for patients at low surgical risk were identified and were included in our report, the PARTNER 318 and the Evolut LRT17 studies. A second publication of the PARTNER 3 study that reported on the results of quality of life was also identified and included in this report.29 Additional information on these two RCTs was also available in the summary of safety and effectiveness data provided on the Food and Drug Administration website.35,36
Characteristics of Included Studies
In the PARTNER 3 study,18 patients 65 years of age and older with severe, calcific aortic valve stenosis at low surgical risk were randomized to undergo either TAVI (SAPIEN 3) via the transfemoral route or SAVR (any commercially available bioprosthetic valve) between March 2016 and October 2017. It was a multicentre study with sites in the United States, Canada, and Japan. Only patients who were suitable for transfemoral TAVI were eligible for the study.18 Patients with a bicuspid aortic valve, a previous mechanical or bioprosthetic valve in any position, severe aortic regurgitation, or anatomical features that increase the risk of complications with either TAVI or SAVR were excluded.18 Surgical risk was determined according to a clinical and anatomical assessment that included the STS score (< 4%) and agreement between the heart team and the trial review committee.18 Balloon dilation before and after TAVI could be performed at the investigator's discretion.18 The primary objective was to test the noninferiority of TAVI versus SAVR for the composite end point of all-cause mortality, stroke, or rehospitalization at 1 year.18 A superiority test was prespecified if the noninferiority criteria were satisfied.18
From the 1,520 patients screened in the PARTNER 3 study,18 1,000 were randomized, 503 in the TAVI group and 497 in the SAVR group. Reasons for exclusion before the randomization are shown in Table 2. The 10-year follow-up is ongoing for this study; however, the first-year results have been published.18
Table 2:
Reasons for Withdrawal
| Author, Year N (TAVI/SAVR) | Patients Not Randomized, N (%) | Procedure Not Attempted, n (%) | Procedure Attempted but Valve Not Implanted, n (%) | Withdrawal After Implantation Procedure, n (%) |
|---|---|---|---|---|
| Mack et al, 201918 1,000 (503/497) PARTNER 3 |
520 (34.2) Reasons:
|
TAVI: 7 (1.4) SAVR: 43 (8.7) Reasons: Ineligible TAVI: 1 (0.2) SAVR: 8 (1.6) Consent withdrawn TAVI: 6 (1.2) SAVR: 35 (7.0)a |
TAVI: 1 (0.2)—converted to SAVR SAVR: 1 (0.2)—procedure aborted |
Patient withdrawal or loss to follow-up TAVI: 1 (0.2) SAVR: 16 (3.5) |
| Popma et al, 201917 1,468 (734/734) Evolut LRT |
255 (14.7) Reasons:
|
TAVI: 13 (1.8) SAVR: 53 (7.2) Reasons: Ineligible TAVI: 1 (0.1) SAVR: 4 (0.5) Consent withdrawn TAVI: 8 (1.1) SAVR: 33 (4.5) Withdrawn by physician TAVI: 1 (0.1) SAVR: 12 (1.6) Death TAVI: 0 SAVR: 1 (0.1) Lost to follow-up TAVI: 0 SAVR: 2 (0.3) Pending procedure TAVI: 2 (0.3) SAVR: 1 (0.1) |
TAVI: 4 (0.5)—underwent surgery SAVR: 2 (0.3)—underwent TAVI (1), valve not implanted (1) |
Patient withdrawal or loss to follow-up TAVI: 9 (1.2) SAVR: 27 (3.7) |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Decided not to undergo surgery or to undergo surgery at a nontrial hospital.
Aortic dimensions outside of the sizing guidelines (n = 60), predicted risk of mortality was outside of the protocol criteria (n = 6), bicuspid aortic valve (n = 138), prohibitive left ventricular outflow tract calcification (n = 18), anatomy unsuited for TAVI access (n = 1), other reasons (n = 2). Patients could have had multiple reasons for disapproval.
In the Evolut LRT study,17 patients with severe aortic valve stenosis at low surgical risk were randomized to undergo either TAVI or SAVR between March 2016 and November 2018. It was a multicentre study with sites in the United States, Canada, Europe, Australia, New Zealand, and Japan. The randomization was stratified by the need for coronary artery revascularization.17 Patients with a bicuspid aortic valve and a previous mechanical or bioprosthetic valve in any position were excluded.17 The study's Screening Committee verified the patients' eligibility for the study before the randomization was performed. Low surgical risk was determined by the site's heart team informed by the STS score (< 3%).17 Three generations of the TAVI valve were used in the study (CoreValve, Evolut R, and Evolut PRO). The choice of bioprosthetic valve used in the SAVR group was left to the investigator's discretion.17 The primary objective was to test the noninferiority of TAVI versus SAVR for the composite end point of all-cause mortality or disabling stroke at 2 years.17
From the 1,723 patients screened, 1,468 patients were randomized in the Evolut LRT study,17 734 in the TAVI group and 734 in the SAVR group. Reasons for exclusion before randomization are shown in Table 2. The 10-year follow-up is ongoing for this study; however, the 1-year results for all outcomes and 2-year results for the main end point and its components have been published. The publication is based on an interim analysis that was performed when 784 (53%) and 137 (9.3%) patients reached 1 and 2 years of follow-up, respectively.
Outcomes of patients who had not reached these follow-up points were estimated using imputation methods (Appendix 2).
Computed Tomography Sub-Study
Both RCTs included a sub-study to evaluate the occurrence of leaflet thickening and leaflet reduced mobility in a subset of patients randomized to either TAVI or SAVR. The PARTNER 3 included 200 patients who were randomized to each group (TAVI and SAVR) in sites where multi-phasic, electrocardiogram (ECG)-gated CT technology was available.37 The CT scans were performed at 30 days and 1 year. Patients with conditions requiring or expected to require anticoagulants after the valve implant procedure were excluded from the sub-study.37
The rationale for this sub-study was the concern with recent reports of leaflet thickening and reduced leaflet mobility with TAVI identified by multi-phasic, ECG-gated CT in some patients in the absence of clinical symptoms and echocardiographic abnormalities.37
The goals of the CT sub-study were to establish the prevalence of the imaging abnormality and its relationship with patient, procedural and pharmacology factors, and clinical events.37 According to the study protocol, the CT sub-study analysis did not have sufficient statistical power to detect a difference between TAVI and SAVR.37 Details about the Evolut LRT CT sub study could not be identified.
Baseline Patient Characteristics
The mean age of patients included in the studies was 73 years and most were men (69% in the PARTNER 318 and 65% in the Evolut LRT17 studies). The mean STS score was 1.9% in both studies, and approximately 27% of the patients had NYHA class III or IV symptoms. The mean left ventricular ejection fraction was 66% in the PARTNER 3 study18 and 62% in the Evolut LRT study(Appendix 3).17
Patient Withdrawal
Among 1,520 patients screened, 1,000 were randomized to either TAVI (n = 503) or SAVR (n = 497) in the PARTNER 3 study18 and the procedure was attempted in 950 (95%) patients, 496 with TAVI and 454 with SAVR—this is referred to as the as-treated population. The authors reported that characteristics of patients in the as-treated population were similar to those who were randomized but excluded from the as-treated population.18
In the Evolut LRT study,17 among 1,723 patients screened, 1,468 were randomized to either TAVI (n = 734) or SAVR (n = 734), and the assigned procedure was attempted in 1,403 (96%) patients, 722 and 681 in each group, respectively. Three patients from the SAVR group crossed over to the TAVI group; therefore, the as-treated population included 725 patients in the TAVI group and 678 patients in the SAVR group.17 According to the authors, the baseline characteristics of patients who underwent SAVR were similar to characteristics of patients who were randomized to but did not undergo SAVR.17
Additional information and reasons for study withdrawal are provided in Table 2.
Risk of Bias in Included Studies
Both studies used appropriate methods of randomization and allocation concealment, and we found no issues with the completeness of outcome data or selective reporting.17,18
Although patients and study personnel were not blinded to the treatment assigned, this was not considered a risk of bias because all components of the primary end point and key secondary end points were adjudicated by a clinical events committee in the PARTNER 318 study, and an independent academic clinical events committee adjudicated all end points in the Evolut LRT17 study. Most other end points were considered objective and might not have been substantially affected by the lack of blinding.
A limitation of the Evolut LRT17 study is the fact that published study results correspond to a prespecified interim analysis performed when 784 (56%) and 137 (9.2%) patients had reached 1 and 2 years of follow-up, respectively. Therefore approximately 50% and 90% of patients hadn't reached 1 and 2 years of follow-up, respectively. A prespecified statistical model based on patients' last known clinical status was used to impute the outcome of patients who hadn't reached full follow-up. However, given the substantial proportion of patients for which outcome data were unavailable at 1 and 2 years, there is a potential for the missing data to affect the effect estimate. Given the difficulty of ascertaining the extent to which this would bias the effect estimate, we judged conservatively that this would not substantially increase the risk of bias at 1 year of follow-up. At 2 years, given the larger extent of the imputation, we considered the results to be at high risk of bias (See Appendix 2) (see Appendix 2).
Aortic Valve Implantation Procedure
In the TAVI group of the PARTNER 3 study,18 the transfemoral implantation route was used for all patients as per the study protocol. Predilation and postdilation were performed in 286 (57.8%) and 103 (20.9%) patients, respectively.18 General anesthetic was given to 165 (33.3%) patients, conscious sedation to 323 (65.1%) patients, and one (0.2%) patient needed to convert to general anesthesia during the procedure.18 The newest (third) generation SAPIEN 3 valve was used in all patients.18 Embolic protection was not permitted in this study. Thirty-eight (7.7%) patients had a concomitant procedure: coronary revascularization was performed in 32 (6.5%) patients, 1 (0.2%) patient had to be converted to SAVR and received an aortic root enlargement, and 5 (1.0%) patients received a pacemaker or implantable cardioverter-defibrillator.18
In the SAVR group of the PARTNER 3 study,18 full sternotomy was performed in 336 (74.2%) patients whereas a less invasive incision was used in 110 (24.3%). The SAVR procedure had to be interrupted in one patient owing to a heavily calcified aorta—the patient was withdrawn from the study and underwent a TAVI procedure.18 Concomitant procedures were performed in 120 (26.4%) patients. The most common procedures included CABG (n = 58 [12.8%]), left atrial appendage closure (n = 43 [9.5%]), surgical treatment of atrial fibrillation (n = 22 [4.8%]), and aortic root enlargement (n = 21 [4.6%]).18
In the TAVI group of the Evolut LRT study,17 the transfemoral route was used in most patients (99.0%), whereas the transaortic and subclavian approaches were used in 0.4% and 0.6%, respectively. General anesthesia was used for 418 (56.9%) patients.17 The newer (second and third) generation Evolut R and Evolut PRO valves were used in 537 (74.1%) and 162 (22.3%) patients, respectively, whereas the older generation CoreValve was used in 26 (3.6%) patients.17 Predilation and postdilation were performed in 249 (34.9%) and 227 (31.3%) patients, respectively.17 The valve was repositioned in 270 (37.3%) patients. Conversion to surgery was required for 4 (0.6%) patients.17 Concomitant or staged coronary intervention was performed in 50 (6.9%) patients. An embolic protection device was used in in 9 (1.2%) patients.17
In the SAVR group of the Evolut LRT study,17 among 678 patients in whom SAVR was attempted, one received a TAVI valve and in one the valve implantation could not be performed. Concomitant procedures were performed in 178 (26.3%) patients.17 The most common concomitant procedures were CABG (n = 92, 13.6%), left atrial appendage closure (n = 42, 6.2%), and surgical treatment of atrial fibrillation (n = 24, 3.5%).17
Composite End Point
The main outcome in the PARTNER 318 study was the composite end point of all-cause mortality, stroke, or rehospitalization at 1 year. It occurred in 42 (8.5%) patients in the TAVI group and 68 (15.1%) in the SAVR group (HR 0.54; 95% confidence interval [CI]: 0.37 to 0.79; risk difference −7%, 95% CI −11% to −2%).18 The study's prespecified noninferiority and superiority criteria were met.18 There were no differences in the effect of TAVI compared with SAVR according to age, sex, STS score, left ventricular ejection fraction, NYHA functional class, or Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score.18 We rated the certainty of evidence as high (Table 10 and Appendix 6).
Table 10:
Effectiveness of TAVI and SAVR
| Outcome (Time Frame) | Risk Difference (95% CI) TAVI vs. SAVR | GRADE Reason for Downgrading |
|---|---|---|
| Composite end point of all-cause mortality, stroke, and rehospitalization (1 y) | −7.0% (−11.0% to −2%)18 | ⊕⊕⊕⊕ High |
| Composite end point of all-cause mortality or disabling stroke (2 y) | −1.4% (95% BCrI −4.9% to 2.1%)17 | ⊕⊕⊕ Moderate Large degree of imputation |
| All-cause mortality (30 d) | Pooled: −0.8°% (−1.5°% to −0.1%) | ⊕⊕⊕ Moderate Serious imprecisiona |
| All-cause mortality (1 y) |
−1.5°% (−3.5% to 1.0%)18 −0.6°% (95% BCrI −2.6% to 1.3%)17 |
⊕⊕ Low Very serious imprecisiona |
| All-cause mortality (2 y) | 0.0 (−3.2% to 3.2%)17 | ⊕ Very low Very serious imprecision and large degree of imputation |
| Any stroke (30 d) | −1.8% (−3.8% to 0.6%)18 0 (95% BCrI −1.9% to 1.9%)17 |
⊕⊕ Low Very serious imprecisiona |
| Any stroke (1 y) | −1.9% (−4.1% to 0.7%)18 −0.2% (95% BCrI −2.4% to 1.9%)17 |
⊕⊕ Low Very serious imprecisiona |
| Disabling stroke (30 d) | Pooled: −0.8% (−1.8% to −0.2%) | ⊕⊕⊕ Moderate Serious imprecisiona |
| Disabling stroke (1 y) | −0.7% (−1.9% to 0.7%)18 −1.6% (95% BCrI −3.1% to −0.3%)17 |
⊕⊕⊕ Moderate Serious imprecisiona |
| Disabling stroke (2 y) | −2.3% (−4.8% to −0.4%)17 | ⊕⊕ Low Serious imprecisiona and large degree of imputation |
| Nondisabling stroke (30 d) | −1.4% (−3.1% to 0.06%)18 1.2% (95% BCrI −0.3% to 2.9%)17 |
⊕⊕ Low Very serious imprecisiona |
| Nondisabling stroke (1 year) | −1.2% (−3.2% to 1.1%)18 1.1% (95% BCrI −0.6% to 2.9%)17 |
⊕⊕ Low Very serious imprecisiona |
| Transient ischemic attack (30 d) | Pooled: −0.4% (−1.1% to 0.3%) | ⊕⊕ Low Very serious imprecisiona |
| Transient ischemic attack (1 y) | −1.1% (−1.9% to 1.5%)18 −0.2% (95% BCrI −1.6% to 1.3%)17 |
⊕⊕ Low Very serious imprecisiona |
| Symptoms (% patients in NYHA class I at 30 d) | Pooled: 11.8% (8.2% to 15.5%) | ⊕⊕⊕⊕ High |
| Symptoms (% patients in NYHA class I at 1 y) | Pooled: −2.0% (−5.7% to 1.8%) | ⊕⊕⊕ Moderate Serious imprecision |
| Quality of life (KCCQ overall score at 30 d) | Mean difference in change from baseline (95% CI) 16.1 (14.2 to 18.0)18 10.9 (95% BCrI 8.6 to 13.2)17 |
⊕⊕⊕⊕ High |
| Quality of life (KCCQ overall score at 1 y) | Mean difference in change from baseline with TAVI vs. SAVR (95% CI) 1.8 (0.2 to 3.5)b 18 1.3 (95% BCrI −1.2 to 3.8)17 |
⊕⊕⊕ Moderate Serious imprecision |
| 6-min walk test (30 d) | Mean difference in change from baseline with TAVI vs. SAVR (95% CI) 33.7 (19.9 to 47.4)18 |
⊕⊕⊕⊕ High |
| 6-min walk test (1 y) | Mean difference in change from baseline with TAVI vs. SAVR (95% CI) −1.4 (−15.2 to 12.5)18 |
⊕⊕⊕ Moderate Serious imprecision |
| Length of hospital stay (index procedure) | Median difference (95% CI), days: −4.0 (−4.0 to −3.0)18 Mean difference, days: 3.617 |
⊕⊕⊕⊕ High |
| Aortic valve reinterventions (30 d) | No events reported18 0.0 (95% BCrI −0.8% to 0.7%)17 |
⊕⊕ Low Very serious imprecisiona |
| Aortic valve reintervention (1 y) | 0.1% (−1.1% to 1.5%)18 0.0% (95% BCrI −1.0% to 0.9%)17 |
⊕⊕ Low Very serious imprecisiona |
| Rehospitalization (30 d) | Valve-, procedure-, heart-failure-related −3.0% (−6.0% to −0.2%)18 Heart failure-related −1.3% (95% BCrI −2.8% to 0.1%)17 |
⊕⊕⊕ Moderate Serious imprecision |
| Rehospitalization (1 y) | Valve-, procedure-, heart failure-related −3.5% (−7.2% to 0.1%)18 Heart failure-related −3.4% (95% BCrI −5.9% to −1.0%)17 |
⊕⊕⊕ Moderate Serious imprecision |
| Valve hemodynamics (30 d) | Aortic valve area (cm2) Mean difference (95% CI) −0.1 (−0.1 to −0.0)18 Mean difference (95% BCrI) 0.2 (0.1 to 0.2)17 Mean aortic valve gradient (mm Hg) Mean difference (95% CI) 1.5 (0.9 to 2.0)18 Mean difference (95% BCrI) −2.1 (−2.5 to −1.7)17 |
⊕⊕ Low Serious inconsistency and imprecision |
| Valve hemodynamics (1 y) | Aortic valve area (cm2) Mean difference (95% CI) −0.1 (−0.1 to 0.0)18 Mean difference (95% BCrI) 0.3 (0.2 to 0.4)17 Mean aortic valve gradient (mm Hg) Mean difference (95% CI) 2.0 (1.3 to 2.7)18 Mean difference (95% BCrI) −2.1 (−2.5 to −1.7)17 |
⊕⊕ Low Serious inconsistency and imprecision |
Abbreviations: BCrI, Bayesian credible interval; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; TAVI, transaortic valve implantation.
Fragility of estimates was considered when few events were reported and an increase of few events in either group could change direction of point estimate or render results not statistically significant. Evidence was downgraded once if results of at least one study were statistically significant owing to fragility of estimates and twice if results were both not statistically significant and few events were reported for a given outcome.
Threshold of clinical importance was not reached and no statistically significant difference between groups was observed.
The main outcome in the Evolut LRT17 study was the composite end point of all-cause mortality or disabling stroke at 2 years. It occurred in 5.3% of patients in the TAVI group and 6.7% of patients in the SAVR group (risk difference −1.4%, 95% Bayesian credible interval [BCrI] −4.9 to 2.1).17 The TAVI procedure was considered noninferior to SAVR because the prespecified criterion was met. There were no differences in the effect of TAVI compared with SAVR according to age, sex, need for revascularization, or comorbidities such as diabetes and peripheral arterial disease.17 We rated the certainty of evidence as moderate, downgrading owing to risk of bias (large degree of imputation) (Table 10 and Appendix 6).
All-Cause Mortality
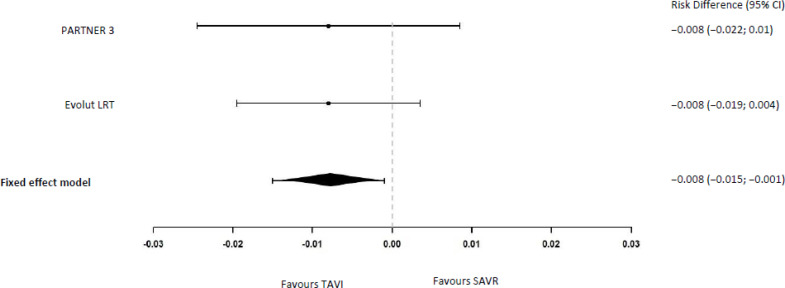
In the PARTNER 3 study,18 two (0.4%) and five (1.1%) patients died in the TAVI and SAVR groups, respectively, at 30 days (risk difference −0.8%, 95% CI −2.2% to 1.0%). In the Evolut LRT study,17 mortality occurred in 0.5% and 1.3% of patients in the TAVI and SAVR groups, respectively, at 30 days (risk difference −0.8%, 95% BCrI −1.9% to 0.2%). Pooling the two studies resulted in a risk difference of −0.8% ([95% CI −1.5% to −0.1%] Figure 2).
Figure 2: All-Cause Mortality at 30 Days.
Abbreviations: CI, confidence interval; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
aRisk differences and meta-analysis were calculated by the authors of this report using the exact method.24
At 1 year, 5 (1.0%) and 11 (2.5%) patients died in the TAVI and SAVR groups, respectively, in the PARTNER 3 study18 (risk difference −1.5%, 95% CI −3.5% to 1.0%). In the Evolut LRT study,17 2.4% and 3.0% of patients died at 1 year in the TAVI and SAVR groups, respectively (risk difference −0.6%, 95% BCrI −2.6% to 1.3%).
At 2 years, 4.5% of the patients in both the TAVI and SAVR groups died, as reported in the Evolut LRT17 study (not reported in the PARTNER 3 study18).
Additional information and causes of death are provided in Table 3.
Table 3:
All-Cause Mortality
| Author, Year N (TAVI/SAVR) | 30 Days | 1- to 2-Year Follow Up | Causes of Death | |
|---|---|---|---|---|
| TAVI | SAVR | |||
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimate,a n (%) TAVI: 2 (0.4) SAVR: 5 (1.1) HR (95% CI) 0.37 (0.07, 1.88) RD (95% CI)b −0.8%, (−2.2% to 1.0%) |
KM estimate,a n (%) 1 y TAVI: 5 (1.0) SAVR: 11 (2.5) HR (95% CI) 0.41 (0.14, 1.17) RD (95% CI)b −1.5%, (−3.5% to 1.0%) 2 y Not yet reported |
Within 30 d
After 30 d
|
Within 30 d
After 30 d
|
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
Percentage TAVI: 0.5 SAVR: 1.3 RD (95% BCrI) −0.8% (−1.9% to 0.2%) |
Percentage 1 y TAVI: 2.4 SAVR: 3.0 RD (95% BCrI) −0.6% (−2.6% to 1.3%) 2 y TAVI: 4.5 SAVR: 4.5 RD (95% BCrI) 0 (−3.2% to 3.2%) |
Within 30 d
After 30 d Information not provided |
Within 30 d
After 30 d Information not provided |
Abbreviations: BCrI, Bayesian credible interval; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; KM, Kaplan-Meier; HR, hazard ratio; N/A, not available; PEA, pulseless electrical activity; RD, risk difference; SAVR, surgical aortic valve implantation; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal number of patients who experienced event divided by total number of patients in treatment group at given time point.
Calculated by the authors of this report using the exact method.24
We rated the certainty of evidence as moderate at 30 days, low at 1 year, and very low at 2 years. The evidence was downgraded due to different degrees of imprecision at the different timepoints, and due to the additional risk of bias at 2 years given the large degree of imputation (Table 10 and Appendix 6).
Stroke and Transient Ischemic Attack
Any Stroke
In the PARTNER 318 study, stroke occurred in 3 (0.6%) and 11 (2.4%) patients in the TAVI and SAVR groups, respectively, at 30 days (risk difference −1.8%, 95% CI −3.8% to 0.6%). In the Evolut LRT study17 the frequency of stroke was similar in both groups at 30 days (3.4% in both groups, risk difference 0.00%, 95% BCrI −1.9% to 1.9%).
A meta-analysis was not feasible because of statistical heterogeneity between studies. One-year results and additional information are provided in Table 4.
Table 4:
Stroke and Transient Ischemic Attack
| Author, Year N (TAVI/SAVR) | Any Stroke | Disabling Stroke | Nondisabling Stroke | Transient Ischemic Attack |
|---|---|---|---|---|
| 30 Days | ||||
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimate,a n (%) TAVI: 3 (0.6) SAVR: 11 (2.4) HR (95% CI) 0.25 (0.07–0.88) RD (95% CI)b −1.8% (−3.8% to 0.6%) |
KM estimate,a n (%) TAVI: 0 SAVR: 2 (0.4) HR cannot be calculated RD (95% CI)b −0.4% (−1.5% to 0.7%) |
KM estimate,a n (%) TAVI: 3 (0.6) SAVR: 9 (2.0) HR (95% CI) 0.30 (0.08–1.12) RD (95% CI)b −1.4% (−3.1% to 0.06%) |
KM estimate,a n (%) TAVI: 0 SAVR: 3 (0.7) HR cannot be calculated RD (95% CI)b −0.7% (−1.8% to 0.7%) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 3.4% SAVR: 3.4% RD (95% BCrI) 0% (−1.9% to 1.9%) |
TAVI: 0.5% SAVR: 1.7% RD (95% BCrI) −1.2% (−2.4% to −0.2%) |
TAVI: 3.0% SAVR: 1.7% RD (95% BCrI) 1.2% (−0.3% to 2.9%) |
TAVI: 0.6% SAVR: 0.8% RD (95% BCrI) −0.2% (−1.2% to 0.7%) |
| 1–2 Years | ||||
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimatea, n (%) TAVI: 6 (1.2) SAVR: 14 (3.1) HR (95% CI) 0.38 (0.15–1.00) RD (95% CI)b −1.9% (−4.1% to 0.7%) |
KM estimatea, n (%) TAVI: 1 (0.2) SAVR: 4 (0.9) HR (95% CI) 0.22 (0.03–2.00) RD (95% CI)b −0.7% (−1.9% to 0.7%) |
KM estimatea, n (%) TAVI: 5 (1.0) SAVR: 10 (2.2) HR (95% CI) 0.45 (0.15–1.32) RD (95% CI)b −1.2% (−3.2% to 1.1%) |
KM estimatea, n (%) TAVI: 5 (1.0) SAVR: 5 (1.1) HR (95% CI) 0.89 (0.26–3.06) RD (95% CI)b −0.09% (−1.9% to 1.5%) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 4.1% SAVR: 4.3% RD (95% BCrI) −0.2% (−2.4% to 1.9%) |
1 y TAVI: 0.8% SAVR: 2.4% RD (95% BCrI) −1.6% (−3.1% to −0.3%) 2 y TAVI: 1.1 SAVR: 3.5 RD (95% BCrI) −2.3% (−4.8% to −0.4%) |
TAVI: 3.4% SAVR: 2.2% RD (95% CI)b 1.1% (−0.6% to 2.9%) |
TAVI: 1.7% SAVR: 1.8% RD (95% CI)b −0.1% (−1.7% to 1.6%) |
Abbreviations: BCrI, Bayesian credible interval; CI, confidence interval; HR, hazard ratio; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal number of patients who experienced the event divided by total number of patients in treatment group at the given time point.
Calculated by the authors of this report using the exact method.24
We rated the certainty of evidence as low at both 30 days and 1 year, downgrading due to very serious imprecision (Table 10 and Appendix 6).
Disabling Stroke
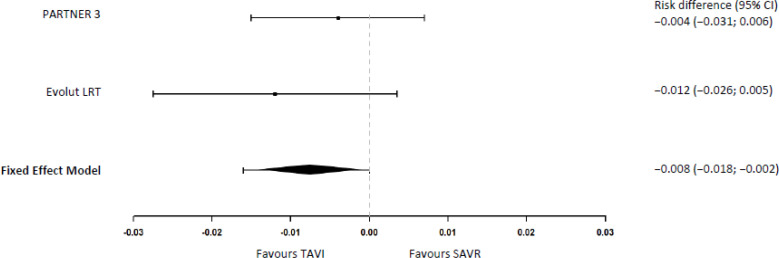
In the PARTNER 318 study, none of the patients in the TAVI group suffered a disabling stroke compared with 2 (0.4%) in the SAVR group at 30 days (risk difference −0.4%, 95% CI −1.5% to 0.7%). In the Evolut LRT study,17 0.5% of patients in the TAVI group and 1.7% in the SAVR group had a disabling stroke at 30 days (risk difference −1.2%, 95% BCrI −2.4% to −0.2%). Our meta-analysis yielded a disabling stroke risk reduction at 30 days of −0.8% ([95% CI −1.8% to −0.2%] Figure 3) with TAVI versus SAVR.
Figure 3: Disabling Stroke at 30 Days.
Abbreviations: CI, confidence interval; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
1Risk differences and meta-analysis were calculated by the authors of this report using the exact method.24
The PARTNER 3 study18 did not find a statistically significant difference between TAVI and SAVR at 1 year (2-year results not yet reported), but in the Evolut LRT study,17 there was a lower risk of disabling stroke with TAVI than with SAVR at 1 and 2 years (see Table 4).
We rated the certainty of evidence as moderate at both 30 days and 1 year, and low at 2 years, downgrading due to serious imprecision; the evidence was further downgraded at 2 years due to risk of bias given the large degree of imputation (Table 10 and Appendix 6).
Nondisabling Stroke
Nondisabling stroke at 30 days was reported in three (0.6%) patients treated with TAVI and nine (2.0%) patients treated with SAVR (risk difference −1.4%, 95% CI −3.1% to 0.06%) in the PARTNER 318 study. In the Evolut LRT17 study, at 30 days, 3.0% and 1.7% of patients in the TAVI and SAVR groups, respectively, experienced a nondisabling stroke (risk difference 1.2%, 95% BCrI −0.3% to 2.9%).
Meta-analysis was not feasible because of statistical heterogeneity between the studies. One-year results and additional information are provided in Table 4.
We rated the certainty of evidence as low at both 30 days and 1 year, downgrading due to very serious imprecision (Table 10 and Appendix 6).
Transient Ischemic Attack
None of the patients in the TAVI group of the PARTNER 318 study presented with a transient ischemic attack at 30 days, compared with three (0.7%) in the SAVR group (risk difference −0.7%, 95% CI −1.8% to 0.7%).
In the Evolut LRT17 study, transient ischemic attack occurred in 0.6% and 0.8% of patients in the TAVI and SAVR groups, respectively, at 30 days (risk difference −0.2%, 95% BCrI −1.2% to 0.7%).
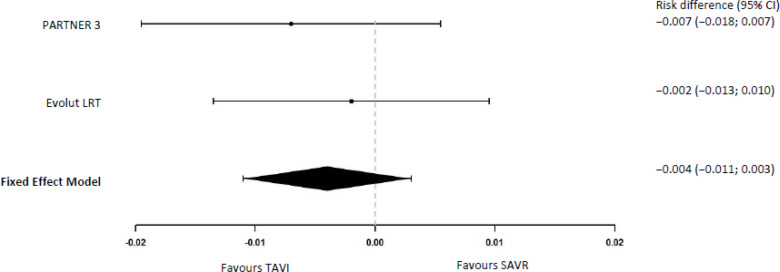
In our meta-analysis the difference in the risk of transient ischemic attack at 30 days was −0.4% (95% CI −1.1% to 0.3%) with TAVI versus SAVR (Figure 4).
Figure 4: Transient Ischemic Attack at 30 Days.
Abbreviations: CI, confidence interval; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
aRisk differences and meta-analysis were calculated by the authors of this report using the exact method.24
One-year results and additional information are provided in Table 4.
We rated the certainty of evidence as low at both 30 days and 1 year, downgrading due to very serious imprecision (Table 10 and Appendix 6).
Aortic Valve Reintervention
Aortic valve reinterventions included any intervention to repair, alter, or replace a previously implanted valve, such as balloon dilation, SAVR, valve-in-valve procedures, and percutaneous paravalvular leak closures.
No instances of aortic valve reintervention were reported at 30 days in either the TAVI or SAVR groups in the PARTNER 3 study.18 In the Evolut LRT study,17 there was a very low risk of aortic valve reintervention at 30 days, 0.4%, with both TAVI and SAVR (risk difference 0.00%, 95% BCrI −0.8% to 0.7%).
At 1 year in the PARTNER 3 study,18 three (0.6%) patients in the TAVI group and two (0.5%) in the SAVR group had an aortic valve reintervention (HR 1.33, 95% CI 0.22 to 7.95, risk difference 0.1%, 95% CI −1.1% to 1.5%). In the Evolut LRT study,17 0.7% and 0.6% of patients in the TAVI and SAVR groups, respectively, had an aortic valve reintervention at 1 year (risk difference 0.00%, 95% BCrI −1.0% to 0.9%).
The studies did not specify which types of reinterventions were performed.
We rated the certainty of evidence as low at both 30 days and 1 year, downgrading due to very serious imprecision (Table 10 and Appendix 6).
Rehospitalization
The PARTNER 3 study18 reported a slightly lower risk of valve-, procedure-, or heart failure-related hospitalizations with TAVI compared with SAVR at 30 days (17 [3.4%] vs. 29 [6.5%], respectively; HR 0.53, 95% CI 0.29 to 0.97; risk difference −3.0%, 95% CI −6.0% to −0.2%). At 1 year these hospitalizations occurred in 36 (7.3%) and 49 (11.0%) of patients in the TAVI and SAVR groups, respectively (HR 0.65, 95% CI 0.42 to 1.00); risk difference −3.5%, 95% CI −7.2% to 0.1%).18
In the Evolut LRT study,17 at 30 days 1.2% of patients in the TAVI group and 2.5% in the SAVR group were hospitalized for heart failure (risk difference −1.3%, BCrI −2.8% to 0.1%). At 1 year, 3.2% of the patients in the TAVI group and 6.5% in the SAVR group (risk difference −3.4% BCrI −5.9% to −1.0%) were hospitalized for heart failure.17
Study results could not be pooled, as the outcome definition differed between studies.
We rated the certainty of evidence as moderate at both 30 days and 1 year, downgrading due to serious imprecision (Table 10 and Appendix 6).
New York Heart Association Classification
The NYHA functional classification assesses how much the patient's physical activity is affected by their heart failure symptoms.38 The classification ranges from I to IV, with higher ratings representing more severe symptoms with physical activity (NYHA I: No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, dyspnea [shortness of breath], or palpitations; NYHA IV: Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest).38 Additional information appears in Appendix 4.38
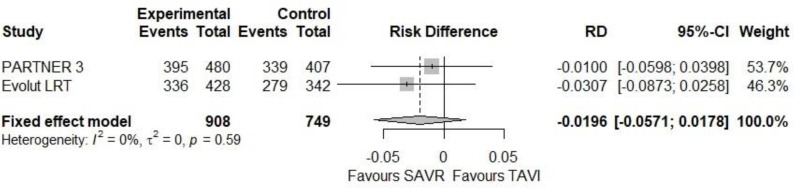
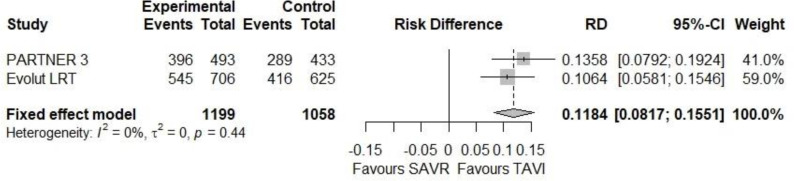
Both studies reported that symptoms of patients in both TAVI and SAVR groups improved at 30 days and 1 year compared with their level before the TAVI or SAVR procedure.17,18 At 30 days, more patients in the TAVI than in the SAVR group were in NYHA class I in both studies: 396 (80.3%) versus 289 (66.7%) in the PARTNER 3 study,18 and 545 (77.2%) versus 416 (66.6%) in the Evolut LRT study.17 In our meta-analysis, the absolute difference in the percentages of patients in NYHA class I between TAVI and SAVR was 11.8% at 30 days (95% CI 8.2%-15.5%, Figure 2). At 1 year, it was similar in the TAVI and SAVR groups (see Figure 3).
Additional information appears in Figures 5 and 6 and Appendix 5.
Figure 6: Patients in NYHA Class I at 1 year.
Abbreviations: CI, confidence interval; NYHA, New York Heart Association; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation
We rated the certainty of evidence as high at 30 days and moderate at 1 year, downgrading at 1 year due to serious imprecision (Table 10 and Appendix 6).
Figure 5: Patients in NYHA Class I at 30 Days.
Abbreviations: CI, confidence interval; NYHA, New York Heart Association; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Quality of Life
Quality of life was measured using the 36-question Short-Form Health Survey (SF-36) from the Medical Outcomes Study, the European Quality of Life in Five Dimensions instrument (EQ-5D), and the KCCQ in the two studies.17,18
Kansas City Cardiomyopathy Questionnaire
The KCCQ is a 23-item questionnaire that covers specific health domains pertaining to heart failure: physical limitation, symptoms, quality of life, social limitation, symptom stability, and self-efficacy.39 The first four domains are combined into an overall summary score. It is scored from 0 to 100; higher scores indicate a better quality of life.39 An increase of more than 10 points in the overall score of the KCCQ is considered clinically meaningful.40
The studies reported an improvement in the overall score of the KCCQ for both the TAVI and SAVR groups during the first year of follow-up compared with baseline. At 30 days, the improvement was greater in the TAVI than in the SAVR group. However, at 6 months17 and 1 year,17,18 both TAVI and SAVR had a similar level of improvement (i.e., the difference between groups was lower than the clinically meaningful threshold). Additional information appears in Table 5.
Table 5:
Kansas City Cardiomyopathy Questionnaire Overall Summary Score
| Author, Year Study | Baseline | 30 Days | 6 Months | 1 Year |
|---|---|---|---|---|
| Mack et al, 201918,29 PARTNER 3 |
Not reported | N = 950 (496 TAVI/454 SAVR) Mean change from baseline (95% CI) TAVI: 18.5 (16.9 to 20.1) SAVR: 2.5 (0.5 to 4.6) Mean difference in change from baseline (95% CI) 16.1 (14.2 to 18.0) |
N = 950 (496 TAVI/454 SAVR) Mean change from baseline (95% CI) TAVI: 20.2 (18.5 to 21.9) SAVR: 17.4 (15.5 to 19.3) Mean difference in change from baseline (95% CI) 2.6 (1.0 to 4.3) |
N = 950 (496 TAVI/454 SAVR) Mean change from baseline (95% CI) TAVI: 19.4 (17.7 to 21.1) SAVR: 17.4 (15.4 to 19.3) Mean difference in change from baseline (95% CI) 1.8 (0.2 to 3.5) |
| Popma et al, 201917 Evolut LRT |
N = 1,396 (722 TAVI/674 SAVR) Mean (SD) TAVI: 68.7 (21.8) SAVR: 69.3 (20.7) |
N = 1,351 (714 TAVI/637 SAVR) Mean (SD) TAVI: 88.7 (14.2) SAVR: 78.6 (18.9) Mean change from baseline (SD) TAVI: 20.0 (21.1) SAVR: 9.1 (22.3) Mean difference in change from baseline (95% BCrl) 10.9 (8.6 to 13.2) |
N = 1,180 (633 TAVI/547 SAVR) Mean (SD) TAVI: 90.3 (13.4) SAVR: 90.2 (13.8) Mean change from baseline (SD) TAVI: 21.6 (NR) SAVR: 20.9 (NR) Mean difference in change from baseline (95% BCrl) 0.7 (−1.0 to 3.8) |
N = 775 (428 TAVI/347 SAVR) Mean (SD) TAVI: 90.3 (12.7) SAVR: 90.8 (12.4) Mean change from baseline (SD) TAVI: 22.2 (20.3) SAVR: 20.9 (21.0) Mean difference in change from baseline (95% BCrI) 1.3 (−1.2 to 3.8) |
Abbreviations: BCrI, Bayesian credible interval, CI, confidence interval; NR, not reported; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
The results for the KCCQ physical and social limitations subscales followed a similar direction to what was reported for the overall score (see Appendix 5).29
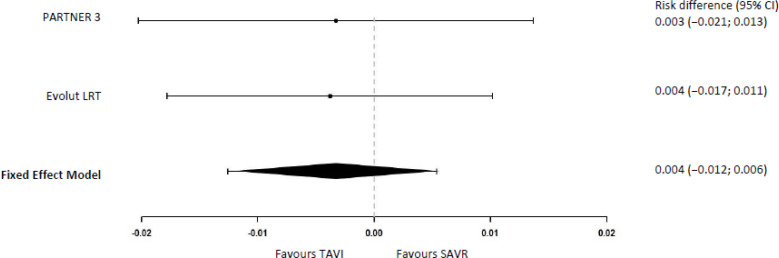
In a separate analysis of the PARTNER 3 study,29 the KCCQ overall score was combined with mortality in a categorical variable ranging from dead, worse (> 5-point decrease in the KCCQ overall score), no change (change in KCCQ overall score between −5 and < 5 points), small improvement (increase in KCCQ overall score between 5 and < 10 points), moderate improvement (increase between 10 and < 20 points), to large improvement (≥ 20-point increase). In this analysis, 306 (62.0%) patients in the TAVI group and 150 (33.4%) in the SAVR group had a moderate or large improvement at 30 days (risk difference 29.0%, 95% CI 22.0% to 35.0%). At 1 year, 310 (62.8%) patients in the TAVI group and 264 (58.8%) in the SAVR group presented with a moderate or large improvement (risk difference 4.0%, 95% CI −3.0% to 10.0%).
We rated the certainty of evidence as high at 30 days and moderate at 1 year, downgrading at 1 year due to serious imprecision (Table 10 and Appendix 6).
36-Question Short-Form Health Survey
The SF-36 questionnaire evaluates eight domains of health including the physical and mental summary scales.29 These components are scored such that the United States population mean is 50 (SD 10), and higher scores represent better health status.29 The minimum clinically important difference for the physical and mental summary scales is approximately 2 points.29
A separate publication of the PARTNER 3 study29 reported the results of the physical and mental summaries of the SF-36 questionnaire. These two domains improved in both the TAVI and SAVR groups during the first year of follow-up compared with baseline. At 30 days, the improvement was greater in the TAVI than in the SAVR group. However, at 6 months and 1 year, both TAVI and SAVR groups had a similar level of improvement (see Appendix 5). Results from the SF-36 were not reported for the Evolut LRT study.
EuroQol-5D
The EQ-5D measures the patient's generic health status by assessing five dimensions of general health using a 3-level scale, transformed into preference-based utility weights using validated population-sampling methods.29 The utilities range from 0 (death) to 1 (ideal health).
In the PARTNER 3 study,29 the TAVI group showed an improvement in the mean EQ-5D score at 30 days, 6 months, and 1 year compared with baseline. In the SAVR group, scores decreased slightly at 30 days compared with baseline, but improved at 6 months and 1 year. At 30 days, TAVI (0.06, 95% CI 0.05 to 0.07) had a greater improvement from baseline in the EQ-5D utilities compared with SAVR (-0.01, 95% CI −0.03 to 0.00), adjusted mean difference 0.07 (95% CI 0.06 to 0.09). However, the two groups had a similar improvement at 6 months and 1 year.29 The information was not provided in the Evolut LRT study (see Appendix 5).17
6-Minute Walk Test
The 6-minute walk test measures the distance a person is able to walk for a total of 6 minutes on a hard, flat surface with the goal of testing functional exercise capacity.41 The clinically important increase reported in studies of patients with heart failure or chronic obstructive pulmonary disease ranged from 43 to 70 meters.41
In the PARTNER 3 study,18 patients in the TAVI group increased the distance covered in the 6-minute walk test at 30 days over baseline (mean change 17.2 meters, standard error 4.6) whereas patients in the SAVR group were unable to walk as far as baseline (mean change −15.2 meters, standard error 6.3). At 1 year, both the TAVI and SAVR groups improved over baseline, and the degree of improvement was similar between groups (Table 6).18 Results of the 6-minute walk test were not reported in the Evolut LRT17 study.
Table 6:
6-Minute Walk Test
| Author, Year N (TAVI/SAVR) | Baseline | 30 Days | 1 Year |
|---|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
Mean distance, m TAVI: 331.0 SAVR: 329.4 |
Mean distance, m TAVI: 349.1 SAVR: 314.4 Mean change from baseline, m (standard error) TAVI: 17.2 (4.6) SAVR: −15.2 (6.3) Mean difference (TAVI vs. SAVR) in change from baseline, m (95% CI) 33.7 (19.9 to 47.4) |
Mean distance, m TAVI: 347.6 SAVR: 351.7 Mean change from baseline, m (standard error) TAVI: 15.4 (5.3) SAVR: 15.1 (5.9) Mean difference (TAVI vs. SAVR) in change from baseline, m (95% CI) −1.4 (−15.2 to 12.5) |
Abbreviations: CI, confidence interval; m, meters; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
We rated the certainty of evidence as high at 30 days and moderate at 1 year, downgrading at 1 year owing to serious imprecision (Table 10 and Appendix 6).
Valve Hemodynamics (Aortic Valve Area and Aortic Valve Gradient)
The aortic valve area and gradient improved in the TAVI and SAVR groups at 30 days and 1 year over baseline in both studies (see Appendix 5).17,18 Both studies reported a small difference between groups at 30 days and 1 year, favouring SAVR in the PARTNER 3 study18 and TAVI in the Evolut LRT study.17 It is unclear whether the difference between groups was clinically important.
We rated the certainty of evidence at 30 days and 1 year as low, downgrading because of serious inconsistency and imprecision (Table 10 and Appendix 6).
Length of Hospital Stay
The median length of stay for TAVI or SAVR procedures was 3.0 days (interquartile range [IQR] 2.0; 3.0) in the TAVI group and 7.0 days (IQR 6.0; 8.0) in the SAVR group in the PARTNER 3 study18; the difference of the medians was −4.0 days (95% CI −4.0 to −3.0, estimated using the bootstrap method). The median length of stay in the intensive care unit was 2.0 (IQR 0.0; 2.0) and 3.0 (2.0; 4.0) days with TAVI and SAVR, respectively, with a difference of −1.0 day (95% CI −2.0 to −1.0, estimated using the bootstrap method).18 In the Evolut LRT study,36 patients in the TAVI group had a mean length of stay of 2.6 days (SD 2.1) compared with 6.2 (SD 3.3) in the SAVR group.
We rated the certainty of evidence as high (Table 10 and Appendix 6).
Procedural Complications
Table 7 lists serious procedural complications reported in the PARTNER 318 study.
Table 7:
Procedural Complications
| Author, Year N (TAVI/SAVR) | Deathsa n (%) | ≥ 2 Transcatheter Valves n (%) | Annulus Rupture n (%) | Coronary Obstruction n (%) | Ventricular Perforation n (%) | Access Site Infections n (%) |
|---|---|---|---|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
During index hospitalization TAVI: 2 (0.4) SAVR: 4 (0.9) RD (95% CI)b −0.5% (−1.9% to 1.0%) |
TAVI: 1 (0.2) SAVR: N/A |
TAVI: 1 (0.2) SAVR: N/A |
TAVI: 1 (0.2) SAVR: 2 (0.4) RD (95% CI)b −0.2% (−1.2% to 0.7%) |
TAVI: 1 (0.2) SAVR: 2 (0.4) RD (95% CI)b −0.2% (−1.2% to 0.7%) |
TAVI: 2 (0.4) SAVR: 6 (1.3) RD (95% CI)b −0.9% (−2.6% to 0.7%) |
The Evolut LRT17 study did not provide results specifically for procedural complications. However the study reported that nine (1.2%) patients in the TAVI group needed two or more transcatheter aortic valves.17 Coronary obstruction occurred in seven (0.9%) patients in the TAVI group and three (0.4%) patients in the SAVR group (risk difference 0.5%, 95% BCrI −0.3% to 1.4%).17
We rated the certainty of evidence as low, downgrading due to very serious imprecision (Table 11 and Appendix 6).
Table 11:
Safety of TAVI and SAVR
| Outcome (Time Frame) | Risk Difference (95% CI) TAVI vs. SAVR | GRADE Reason for Downgrading |
|---|---|---|
| Procedural complications | Mortality −0.5% (−1.9 to 1.0)18 Coronary obstruction −0.2% (−1.2 to 0.7)18 Ventricular perforation −0.2% (−1.2 to 0.7)18 Access-site infections −0.9% (−2.6 to 0.7)18 |
⊕⊕ Low Very serious imprecisiona |
| Life-threatening bleeding (30 d) | −10.7% (−13.8% to −7.6%)18 −5.1% (95% BCrI −7.5% to −2.9%)17 |
⊕⊕⊕⊕ High |
| Major vascular complications (30 d) | Pooled: 0.7% (−0.6% to 1.9%) | ⊕⊕ Low Very serious imprecisiona |
| Acute kidney injury stages 2 or 3 | Pooled: −1.7% (−2.7% to −0.7%) | ⊕⊕⊕⊕ High |
| New-onset atrial fibrillation (30 d) | Pooled: −27.7% (−30.8% to −24.6%) | ⊕⊕⊕⊕ High |
| Myocardial infarction (30 d) | Pooled: −0.4% (−1.2% to 0.6%) | ⊕⊕ Low Very serious imprecisiona |
| Myocardial infarction (1 y) | −1.0% (−3.1% to 1.0%)18 0.1% (95% BCrI −1.3% to 1.5%)17 |
⊕⊕ Low Very serious imprecisiona |
| New pacemaker implantation (30 d) Self-expanding valve | 11.3% (95% BCrI 8.0% to 14.7%)17 | ⊕⊕⊕⊕ High |
| New pacemaker implantation (30 d) Balloon-expandable valve | 2.5% (−0.3% to 5.3%)18 | ⊕⊕⊕ Moderate Serious imprecision |
| New left bundle branch block | 13.7% (9.3% to 18.0%)18 | ⊕⊕⊕⊕ High |
| Moderate-to-severe paravalvular aortic regurgitation (30 d) | 0.8% (−0.08% to 2.0%)18 3.1% (95% BCrI 1.7% to 4.7%)17 |
⊕⊕⊕ Moderate Serious imprecision |
| Moderate-to-severe paravalvular aortic regurgitation (1 y) | 0.1% (−1.4% to 1.5%)18 3.1% (95% BCrI 1.0% to 5.4%)17 |
⊕⊕⊕ Moderate Serious imprecision |
| Moderate-to-severe paravalvular aortic regurgitation (2 y) | 5.8% (95% BCrI −0.7% to 12.1%)17 | ⊕⊕⊕ Moderate Serious imprecision |
| Asymptomatic valve thrombosis (1 y) | 0.8% (−0.2% to 1.8%)18 | ⊕⊕ Low Very serious imprecisiona |
| Valve thrombosis (1 y) | −0.1% (95% BCrI −0.9% to 0.5%)17 | ⊕⊕ Low Very serious imprecisiona |
Abbreviations: BCrI, Bayesian credible interval; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; SAVR, surgical aortic valve replacement; TAVI, transaortic valve implantation.
Fragility of estimates was considered when few events were reported and an increase of few events in either group could change direction of point estimate or render results not statistically significant. Evidence was downgraded once if results of at least one study were statistically significant owing to fragility of estimates and twice if results were both not statistically significant and few events were reported for a given outcome.
Life-Threatening and Disabling Bleeding
Both the PARTNER 318 and the Evolut LRT17 studies reported a lower risk of life-threatening or disabling bleeding with TAVI than with SAVR throughout the study follow-up (Table 8). We were unable perform a meta-analysis of results at 30 days owing to statistical heterogeneity between the studies.
Table 8:
Life-Threatening or Disabling Bleeding
| Author, Year N (TAVI/SAVR) | 30 Days n (%) | 1 Year n (%) |
|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimate,a n (%) TAVI: 6 (1.2) SAVR: 54 (11.9) HR (95% CI) 0.09 (0.04 to 0.22) RD (95% CI) −10.7% (−13.8% to −7.6%) |
KM estimate,a n (%) TAVI: 14 (2.8) SAVR: 58 (12.8) HR (95% CI) 0.20 (0.11 to 0.36) RD (95% CI) −10.0% (−13.4% to −6.6%) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 2.4% SAVR: 7.5% RD (95% BCrI) −5.1% (−7.5% to −2.9%) |
TAVI: 3.2% SAVR: 8.9% RD (95% BCrI) −5.7% (−8.4% to −3.1%) |
Abbreviations: BCrI, Bayesian credible interval; CI, confidence interval; HR, hazard ratio; KM, Kaplan-Meier; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal number of patients who experienced event divided by total number of patients in the treatment group at the given time point.
We rated the certainty of evidence as high (Table 11 and Appendix 6).
Major Vascular Complications
Major vascular complications were defined as the occurrence of any one of the following events: aortic dissection, aortic rupture, annulus rupture, left ventricular perforation, new apical aneurysm/pseudo-aneurysm, or distal embolization requiring surgery, among others.17,18
In the PARTNER 3 study,18 11 (2.2%) and 7 (1.5%) patients experienced major vascular complications in the TAVI and SAVR groups, respectively, at 30 days (HR 1.44, 95% CI 0.56–3.73; risk difference 0.7%, 95% CI −1.5% to 2.7%). In the Evolut LRT study,17 major vascular complications occurred in 3.8% and 3.2% of patients in the TAVI and SAVR groups, respectively, at 30 days (risk difference 0.6%, 95% BCrI −1.4% to 2.5%).
In our meta-analysis, the risk difference between TAVI and SAVR at 30 days was 0.7% (95% CI −0.6% to 1.9%; Figure 7).
Figure 7: Major Vascular Complications at 30 Days.
Abbreviations: CI, confidence interval; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
aRisk differences and meta-analysis were calculated by the authors of this report using the exact method.24
Additional information and 1-year follow-up results are provided in Appendix 5.
We rated the certainty of evidence as low, downgrading due to very serious imprecision (Table 11 and Appendix 6).
Acute Kidney Injury
Acute kidney injury stages 2 and 3 was reported in 2 (0.4%) and 8 (1.8%) patients at 30 days in the TAVI and SAVR groups, respectively, in the PARTNER 3 study18 (risk difference −1.4%, 95% CI −3.2% to 0.7%). In the Evolut LRT study17 acute kidney injury stages 2 and 3 occurred in 0.9% and 2.8% of patients, respectively, at 30 days (risk difference −1.8%, 95% BCrI −3.4% to −0.5%).
Our meta-analysis showed a 1.7% reduction in the risk of acute kidney injury stages 2 and 3 at 30 days with TAVI compared with SAVR (95% CI −2.7% to −0.7%; Figure 8).
Figure 8: Risk of Acute Kidney Injury Stages 2 and 3 at 30 Days.
Abbreviations: CI, confidence interval; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
aRisk differences and meta-analysis were calculated by the authors of this report using the exact method.24
Additional information and 1-year results are provided in Appendix 5.
We rated the certainty of evidence as high (Table 11 and Appendix 6).
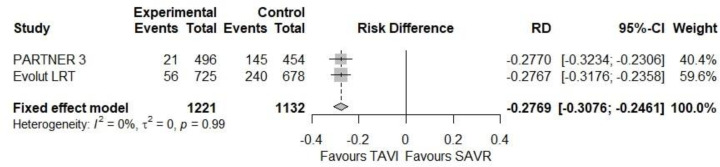
New-Onset Atrial Fibrillation
In the PARTNER 3 study,18 21 (5.0%) and 145 (39.5%) patients in the TAVI and SAVR groups, respectively, presented with new-onset atrial fibrillation within 30 days of the procedure (risk difference −27.7%, 95% CI −32.3% to −23.1%). In the Evolut LRT study,17 7.7% and 35.4% of patients in the TAVI and SAVR groups, respectively, presented with new-onset atrial fibrillation at 30 days (risk difference −27.7%, 95% BCrI −31.8% to −23.6%).
Our meta-analysis showed a 27.7% lower risk of new-onset atrial fibrillation at 30 days with TAVI than with SAVR (95% CI −30.8% to −24.6%; Figure 9).
Figure 9: Risk of New-Onset Atrial Fibrillation at 30 Days.
Abbreviations: CI, confidence interval; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
The results of the PARTNER 3 study18 indicated that atrial fibrillation could have been a transient event, as 94.1% of the 17 episodes of atrial fibrillation identified during hospitalization in the TAVI group and 91.6% of the 131 events in the SAVR group were not present on the 30-day ECG (ECG missing in 8 [6.1%] patients in the SAVR group). In the TAVI group, the most common treatments for atrial fibrillation during the index hospitalization were electrical cardioversion (n = 7 [41.2%]), new medication (n = 4 [23.5%]), medical cardioversion, and electrical ablation (n = 1 [5.9%] each).18 In the SAVR group, the most common treatments during the index hospitalization were new medication (n = 100 [76.3%]), electrical cardioversion (n = 11 [8.4%]), and medical cardioversion (n = 8 [6.1%]).18
Additional information and 1-year results are provided in Appendix 5.
We rated the certainty of evidence as high (Table 11 and Appendix 6).
Myocardial Infarction
In the PARTNER 3 study,18 5 (1.0%) and 6 (1.3%) patients experienced a myocardial infarction at 30 days in the TAVI and SAVR groups, respectively (risk reduction −0.3%, 95% CI −2.1% to 1.3%). In the Evolut LRT study,17 0.9% and 1.3% of the patients experienced the event at 30 days, respectively (risk difference −0.4%, 95% BCrI −1.5% to 0.7%).
In our meta-analysis the risk difference between TAVI and SAVR at 30 days was −0.4% (95% CI −1.2% to 0.6%; Figure 10).
Figure 10: Myocardial Infarction at 30 Days.
Abbreviations: CI, confidence interval; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
aRisk differences and meta-analysis were calculated by the authors of this report using the exact method.24
One-year results and additional information are listed in Table 9.
Table 9:
Myocardial Infarction
| Author, Year N (TAVI/SAVR) | 30 Days n (%) | 1 Year n (%) |
|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimate,a n (%) TAVI: 5 (1.0) SAVR: 6 (1.3) HR (95% CI) 0.76 (0.23 to 2.50) RD (95% CI)b −0.3% (−2.1% to 1.3%) |
KM estimate,a n (%) TAVI: 6 (1.2) SAVR: 10 (2.2) HR (95% CI) 0.54 (0.20 to 1.49) RD (95% CI)b −1.0% (−3.1% to 1.0%) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 0.9% SAVR: 1.3% RD (95% BCrI) −0.4% (−1.5% to 0.7%) |
TAVI: 1.7% SAVR: 1.6% RD (95% BCrI) 0.1% (−1.3% to 1.5%) |
Abbreviations: BCrI, Bayesian credible interval; CI, confidence interval; HR, hazard ratio; KM, Kaplan-Meier; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal number of patients who experienced event divided by total number of patients in treatment group at the given time point.
Calculated by the authors of this report using the exact method.24
We rated the certainty of evidence as low at both 30 days and 1 year, downgrading due to very serious imprecision (Table 11 and Appendix 6).
New Pacemaker Implantation
A total of 32 (6.5%) and 18 (4.0%) patients needed a new pacemaker implantation in the TAVI and SAVR groups, respectively, of the PARTNER 3 study18 (HR 1.66, 95% CI 0.93 to 2.96; risk difference 2.5%, 95% CI −0.3% to 5.3%) at 30 days. The PARTNER 3 study18 used a balloon-expandable TAVI valve.
In the Evolut LRT study,17 there was an increased risk of new pacemaker implantation with TAVI compared with SAVR: 17.4% and 6.1%, respectively, at 30 days (risk difference 11.3%, BCrI 8.0%-14.7%). The Evolut LRT study17 used a self-expanding TAVI valve.
We did not perform a meta-analysis of study results owing to statistical heterogeneity between studies.
Additional information and 1-year results are provided in Appendix 5.
We rated the certainty of evidence as high for the self-expanding TAVI valve and moderate for the balloon expandable TAVI valve, downgrading due to serious imprecision (Table 11 and Appendix 6).
New Left Bundle Branch Block
The PARTNER 3 study18 reported a higher risk of new left bundle branch block with TAVI than with SAVR: 106 (22.0%) and 35 (8.0%) at 30 days, respectively (HR, 3.17, 95% CI 2.13–4.72; risk difference 13.7%, 95% CI 9.3%-18.0%).
This outcome was not reported in the Evolut LRT study.17
We rated the certainty of evidence as high (Table 11 and Appendix 6).
Moderate-to-Severe Paravalvular Aortic Valve Regurgitation
In the PARTNER 3 study,18 4 (0.8%) of 487 patients in the TAVI group and none of the 421 patients in the SAVR group presented with moderate-to-severe paravalvular aortic regurgitation at 30 days (risk difference 0.8%, 95% CI −0.08% to 2.0%). At 1 year of follow-up, 3 (0.6%) of 466 patients in the TAVI group and 2 (0.5%) of 381 in the SAVR group had moderate-to-severe aortic valve regurgitation (risk difference 0.1%, 95% CI −1.4% to 1.5%).18
In the Evolut LRT study,17 the risk of moderate-to-severe paravalvular aortic valve regurgitation was higher with TAVI than with SAVR both at 30 days and 1 year. At 30 days, 24 (3.5%) of 703 patients in the TAVI group and 2 (0.4%) of 608 patients in the SAVR group had this regurgitation (risk difference 3.1%, 95% CI 1.7%-4.7%). At 1 year, 15 (3.6%) of 407 patients in the TAVI group and 2 (0.6%) of 326 patients in the SAVR group had moderate-to-severe regurgitation (risk difference 3.1%, 95% CI 1.0%-5.4%). At 2 years, 4 (5.7%) of 70 patients in the TAVI group and none of the 61 patients in the SAVR group had moderate aortic valve regurgitation (risk difference 5.8%, 95% BCrI −0.7% to 12.1%).17 None of the patients had severe paravalvular aortic regurgitation in the study at 2 years (Appendix 5).17
We did not perform a meta-analysis of study results given the statistical heterogeneity between studies.
We rated the certainty of evidence as moderate at 30 days, 1 and 2 years, downgrading due to serious imprecision (Table 11 and Appendix 6).
Valve Thrombosis
Valve thrombosis was defined as any thrombus attached to or near the implanted valve that occludes part of the blood flow, interferes with the valve function, or is sufficiently large to warrant treatment.18
In the PARTNER 3 study,18 at 30 days, one patient (0.2%) in the TAVI group and no patients in the SAVR group presented with asymptomatic valve thrombosis based on either echocardiography or computed tomography (CT) (risk difference 0.2%, 95% CI −0.4% to 0.8%). At 1 year, five (1.0%) patients in the TAVI group and one (0.2%) in the SAVR group experienced asymptomatic valve thrombosis (HR 4.47, 95% CI 0.52–38.24; risk difference 0.8%, 95% CI −0.2% to 1.8%).18 Onset varied between days 29 and 189 in the TAVI group and the event happened on day 197 in the SAVR group.18 The asymptomatic valve thrombosis was resolved with anticoagulants in two of five patients in the TAVI group; the anticoagulant treatment was ongoing in all five patients at the time of the study's publication.18 In the SAVR group, echocardiography had not yet been repeated at the time of the study's publication, so resolution of the event is unknown (anticoagulant therapy was ongoing).18 None of the episodes were associated with clinical events.18
In the Evolut LRT study,17 valve thrombosis was reported in 0.1% of the patients in the TAVI and SAVR groups (risk difference 0, 95% BCrI −0.4% to 0.4%) at 30 days. At 1 year, valve thrombosis occurred in 0.2% and 0.3% of the patients, respectively (risk difference −0.1%, 95% BCrI −0.9% to 0.5%).17
We rated the certainty of evidence as low, downgrading due to very serious imprecision (Table 11 and Appendix 6).
Computed Tomography Sub-study
The results of the CT sub-study for both trials have not been published in the peer-reviewed literature. The information summarized below is based on the U.S. Food and Drug Administration's summary of safety and effectiveness data reports.35,36
Leaflet Thickening
Presence of leaflet thickening at 30 days was observed using CT scan in 28 (15.2%) and 7 (4.3%) of patients in the TAVI and SAVR groups, respectively, in the PARTNER 3 study35 and 35 (17.8%) and 22 (12.4%), respectively in the Evolut LRT study.36
At 1 year, 41 (25.6%) and 24 (17.9%) of TAVI and SAVR patients, respectively, in the PARTNER 3 study35 and 33 (29.5%) and 23 (24.5%), respectively, in the Evolut LRT study36 presented with leaflet thickening on CT scan.
Leaflet Mobility Restriction
Leaflet mobility restriction at 30 days was observed in 25 (14.7%) and 5 (3.2%) patients in the TAVI and SAVR groups, respectively, in the PARTNER 3 study35 and 27 (15.4%) and 19 (11.0%) patients, respectively in the Evolut LRT study.36
At 1 year, leaflet mobility restriction was observed in 34 patients (22.4%) in the TAVI group and 21 patients (17.0%) in the SAVR group in the PARTNER 3 study.35 At 1 year, 32 patients (29.4%) in the TAVI group and 20 (22.5%) patients in the SAVR group had leaflet mobility restriction in the Evolut LRT study.36
Additional information about clinical events in patients with and without leaflet thickening and restriction is provided in Appendix 5.
Ongoing Studies
In addition to the ongoing follow-up of the PARTNER 318 and Evolut LRT17 studies, two RCTs (NOTION 242 and DEDICATE43) are currently under way. The NOTION 2 study42 included patients 75 years of age and younger with severe aortic valve stenosis at low surgical risk. Its estimated primary outcome completion date is June 2020, and the estimated study completion date is March 2029. The DEDICATE study43 included patients with severe aortic valve stenosis 70 to 85 years of age at low-to-intermediate surgical risk. Its estimated primary outcome and study completion date is December 2024.
Summary of Study Results
In patients with severe aortic valve stenosis at low surgical risk:
TAVI was superior to SAVR on the basis of a composite end point of all-cause mortality, stroke, or rehospitalization at 1 year (GRADE: High). It was also shown to be noninferior to SAVR on the basis of the composite end point of all-cause mortality or disabling stroke at 2 years (GRADE: Moderate)
TAVI resulted in a slightly lower risk of all-cause mortality at 30 days compared with SAVR (GRADE: Moderate). At 1 and 2 years, it is uncertain whether there is a difference between TAVI and SAVR with regards to all-cause mortality due to imprecision (GRADE: Low and Very low, respectively)
TAVI resulted in a slightly lower risk of disabling stroke at 30 days compared with SAVR on the basis of results from both studies (GRADE: Moderate). At 1 and 2 years one study reported a slightly lower risk of disabling stroke with TAVI than with SAVR17 (GRADE: Moderate and Low, respectively)
The effect of TAVI compared with SAVR on the risk of stroke, nondisabling stroke, and transient ischemic attack at 30 days and 1 year is uncertain due to imprecision (GRADE: Low)
The length of hospital stay was shorter for the TAVI than for the SAVR procedure (GRADE: High)
A very low risk (< 1%) of aortic valve reinterventions was reported in the studies at 30 days and 1 year for both TAVI and SAVR, and the risk was similar in both groups (GRADE: Low)
Both at 30 days and 1 year, TAVI might slightly reduce the risk of rehospitalization related to the valve, procedure, or heart failure compared with SAVR, but results were imprecise (GRADE: Moderate)
Patients in both the TAVI and SAVR groups experienced an improvement in symptoms and quality of life compared with before treatment. At 30 days, the degree of improvement for both outcomes was greater with TAVI than SAVR (GRADE: High). However, at 6 months and 1 year, both TAVI and SAVR presented a similar degree of improvement (GRADE: Moderate)
At 30 days patients in the TAVI group had an improvement in the 6-minute walk test compared with baseline whereas patients in the SAVR group worsened (GRADE: High). At 1 year, there was an improvement with both TAVI and SAVR, and the degree of improvement was similar between groups (GRADE: Moderate)
The use of TAVI resulted in a lower risk of life-threatening or disabling bleeding, new-onset atrial fibrillation, and a slightly lower risk of acute kidney injury stages 2 or 3 compared with SAVR at 30 days (GRADE: High)
Based on the results of one study,18 there was an increased risk of new left bundle branch block with TAVI compared with SAVR (GRADE: High); the event was not reported in the other study17
There was a higher risk of new pacemaker implantation with TAVI vs. SAVR in the study using a self-expanding valve17 (GRADE: High), whereas based on the study using a balloon expandable valve,18 TAVI may potentially pose a slightly higher risk of new pacemaker implantation than SAVR; however, results were imprecise (GRADE: Moderate)
There was a slightly higher risk of moderate-to-severe paravalvular regurgitation with TAVI than with SAVR at 30 days and 1 year according to one study.17 However, no difference between groups was observed in the other study18 (GRADE: Moderate)
The effect of TAVI compared with SAVR on the risk of major vascular complications at 30 days is uncertain owing to imprecision (GRADE: Low)
The risk of procedural complications was similar between the two groups; however, the very low number of events experienced in both groups makes it difficult to draw conclusions (GRADE: Low)
A numerically higher risk of asymptomatic valve thrombosis with TAVI versus SAVR was reported in one study.18 The other study showed no difference in the risk of valve thrombosis between groups.17 Both studies reported a numerically higher risk of leaflet thickening and reduced leaflet mobility with TAVI than with SAVR at 30 days and 1 year. However, this is based on very few events and the statistical significance of the difference was not reported. Moreover, the long-term clinical significance of these events is currently unknown
A summary of the study results is provided in Tables 10 and 11. Full GRADE assessment is available in Appendix 6.
Discussion
The conventional treatment of severe aortic valve stenosis in patients at low surgical risk3 is SAVR, and it results in good treatment outcomes.9
Our literature search identified two RCTs comparing TAVI and SAVR in patients with severe aortic valve stenosis at low surgical risk.17,18 In one study, TAVI showed a slight improvement on the composite end point of all-cause mortality, stroke, or rehospitalization compared with SAVR at 1 year.18 The other study showed that TAVI was noninferior to SAVR on the basis of the composite end point of all-cause mortality and disabling stroke at 2 years.17 When assessed separately, at 30 days, the risks of all-cause mortality and disabling stroke were slightly lower with TAVI than with SAVR. The number needed to treat with TAVI to avoid one of each of these events is 125 and 111, respectively. At 1 year, there might be no difference between TAVI and SAVR for all-cause mortality, but possibly a slightly lower risk of disabling stroke with TAVI.
There was a lower risk of life-threatening and disabling bleeding, of stages 2 or 3 acute kidney injury, and of new-onset atrial fibrillation with TAVI than with SAVR at 30 days, although the last could be transient in some patients.18
There was a higher risk of new pacemaker implantation with TAVI than with SAVR in the Evolut LRT study,17 which could be caused in part by the design of the TAVI valve used and its positioning in the outflow tract.44 In contrast, the PARTNER 3 study,18 which used a different TAVI valve, did not show a statistically significant difference between groups.
One study reported a higher risk of new-onset left bundle branch block with TAVI than with SAVR.18 According to the study authors, this event could be associated with reduced left ventricular systolic function; consequently, patients are being followed for 10 years to assess the issue.45
One study observed a higher risk of moderate-to-severe paravalvular regurgitation with TAVI than with SAVR,17 whereas the other study did not.18 Paravalvular regurgitation is defined as leakage of blood between the TAVI and the native valve,12 usually because of incomplete annular sealing.13 It is usually mild to moderate, but if necessary, can be treated through valve redilation, repositioning, or implantation of a second transcatheter valve, which can be associated with important risks.13 Moderate-to-severe paravalvular aortic regurgitation was associated with a higher mortality risk in one study.46
The risk of aortic valve reinterventions was very low in both the TAVI and SAVR groups during the first year of follow-up. Reinterventions consist of any intervention to repair, alter, or replace a previously implanted valve, such as balloon dilation, SAVR, valve-in-valve procedure, and percutaneous paravalvular leak closures.17,18 Nevertheless, these are considered serious clinical events and are associated with a higher risk than the initial procedure.9
One study reported a numerically higher risk of asymptomatic valve thrombosis with TAVI than with SAVR, which was treated with anticoagulants.18
Strengths and Limitations
Our systematic review identified two large RCTs comparing TAVI and SAVR in patients with severe aortic valve stenosis at low surgical risk using the most recent (second- and third-generation) TAVI valves.17,18 The publications available provided mainly 1-year results for studies planned to continue for 10 years.
The studies were powered to assess the noninferiority of TAVI compared with SAVR with respect to a composite outcome. Therefore, there could have been insufficient statistical power to detect a difference between groups for other outcomes. In one study, about half of the patients had not yet reached the 1-year follow-up timepoint, and approximately 90% had not yet reached 2 years of follow-up, and imputation methods were used to account for their outcomes.17
Both studies included a sub-study to evaluate the occurrence of bioprosthetic valve leaflet thickening and reduced mobility in a subset of the overall study population. The goal was to assess its prevalence and association with patient, procedural, and pharmacological factors. But according to the PARTNER 3 study,18 the sub-study was not powered to detect a difference in events between TAVI and SAVR.
Conclusions
The conventional treatment of severe aortic valve stenosis in patients at low surgical risk is SAVR,3 which results in good treatment outcomes.9
In patients with severe aortic valve stenosis at low surgical risk, the identified studies showed that both TAVI via the transfemoral route and SAVR resulted in improvement in patient symptoms and quality of life during the 1 year of follow-up currently available, with a low risk of adverse outcomes such as mortality, stroke, myocardial infarction, and reinterventions.
The TAVI procedure is less invasive and resulted in shorter hospital stays than SAVR. In the short term (30 days), more patients with severe aortic valve stenosis at low surgical risk had improved symptoms and had a greater improvement in quality of life with TAVI than with SAVR. The TAVI procedure also resulted in a small improvement in mortality and disabling stroke and a decreased risk of some adverse events such as bleeding, stages 2 or 3 kidney disease, and new-onset atrial fibrillation over SAVR at 30 days.
At 1 year, TAVI and SAVR had similar rates of mortality, myocardial infarction, and all-cause stroke, although TAVI may have a slightly lower risk of disabling stroke. Both TAVI and SAVR resulted in similar improvements in symptoms and quality of life at 1 year.
The use of TAVI resulted in an increased risk of some adverse events, such as need for implantation of a new pacemaker or new left bundle branch block. The use of TAVI also resulted in a slightly increased risk of moderate-to-severe paravalvular regurgitation than SAVR—longer follow-up is needed to understand the long-term clinical implications of these adverse events.17,18,45 The long-term clinical implications of the asymptomatic valve thrombosis and leaflet thickening events reported in the studies is currently unknown.
Studies were performed by highly experienced and trained staff and in carefully selected patients (15% to 34% of patients screened could not be randomized, mostly because they did not meet eligibility criteria). Results of these studies refer to patients with aortic valve stenosis at low surgical risk who were suitable for transfemoral TAVI implantation, with normal ventricular function, and a mean age of about 74 years. Patients with a previously implanted prosthetic aortic valve or with a bicuspid valve were excluded from the studies.17,18,47 Therefore it is difficult to generalize the evidence to patients with different characteristics.
Short-term results are available for the ongoing studies identified (i.e., 1 year of the planned 10 years of follow-up). According to study authors, longer follow-up is needed to better understand TAVI valve durability and to draw definitive conclusions on the long-term effects of TAVI compared with SAVR beyond 1 year in patients with severe aortic valve stenosis at low surgical risk.17,18
ECONOMIC EVIDENCE Research Question
What is the cost-effectiveness of transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) in adults with severe aortic valve stenosis who are at low surgical risk?
Methods
Similar to the clinical evidence review, we used the 2016 Health Quality Ontario health technology assessment (HTA) evaluating TAVI in patients with severe aortic valve stenosis at various surgical levels as a source of eligible studies in patients at low surgical risk published until its literature search date. The 2016 HTA included individual studies published between January 1, 2011, and September 30, 2015. We searched the literature for additional economic studies published after the search date. Eligible studies identified both through the 2016 HTA and through our complementary literature search were used in our results.
Economic Literature Search
We performed an economic literature search on July 12, 2019, to retrieve studies published from January 1, 2015, until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of HTA agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See the Clinical Evidence section for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published between January 1, 2011, and July 12, 2019
Cost-benefit analyses, cost-effectiveness analyses, or cost-utility analyses
Exclusion Criteria
Reviews, editorials, case reports, commentaries, and abstracts
Population
Adults with severe aortic valve stenosis and low surgical risk: Surgical risk is defined by the study site multidisciplinary heart team informed by the Society of Thoracic Surgeons (STS) risk score and assessment of comorbidities. An STS score lower than 4% is generally considered low risk
Intervention
Transcatheter aortic valve implantation (either self-expanding or balloon-expandable, using any implantation route)
Comparator
Surgical aortic valve replacement (either open-heart or less invasive surgery)
Outcome Measures
Costs
Health outcomes (e.g., quality-adjusted life-years [QALYs])
Incremental costs
Incremental effectiveness
Incremental cost-effectiveness ratio (ICER)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence22 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. The reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, ICERs) We contacted study authors to provide clarification as needed.
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of their clinical guidelines.48 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of studies we found to be directly applicable.
Results
Economic Literature Search
The economic literature search yielded 333 citations published between January 1, 2015, and July 12, 2019, after removing duplicates. We identified 11 additional studies from our 2016 TAVI HTA. In addition, we identified 13 studies published after our search date and 1 study49 that has been submitted for publication. We identified 1 study that met our inclusion criteria. Figure 11 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search. Figure 11 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 11: PRISMA Flow Diagram—Economic Search Strategy.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses. Source: Adapted from Moher et al, 2009.30
Overview of Included Economic Studies
We identified one study submitted for publication that compared the cost-effectiveness of TAVI versus SAVR in adults with severe aortic valve stenosis at low surgical risk.49 The study and its results are summarized in Table 12.
Table 12:
Results of Economic Literature Review—Summary
| Author, Country | Analytic Technique, Study Design, Perspective, Time Horizon | Population | Intervention(s) and Comparator(s) | Results | |||
|---|---|---|---|---|---|---|---|
| Health Outcomes | Costs (2019 CAD) | Cost-Effectiveness | Uncertainty | ||||
| Tam et al.,49 Canada |
Cost-utility analysis Markov model Canadian third-party payer (i.e., Ontario Ministry of Health) Lifetime horizon |
Adults with severe aortic valve stenosis at low surgical risk Age (mean): 73–74 y STS score (mean): 1.9% |
BE TAVI SE TAVI SAVR |
BE TAVI: 9.15 (± 3.23) QALYs SE TAVI: 9.13 (± 3.23) QALYs SAVR: (9.05 (± 3.20) QALYs Discount rate: 1.5% |
BE TAVI: $37,330 (± $4,724) SE TAVI: $39,660 (± $4,862) SAVR: $34,583 (± $6,730) Discount rate: 1.5% |
ICER (BE TAVI vs. SAVR): $27,196/QALY ICER (SE TAVI vs. SAVR): $59,641/QALY SE TAVI dominated by BE TAVI |
Probabilistic sensitivity analysisa BE TAVI probability of cost-effectiveness: 53% at WTP of $50,000/QALY 59% at WTP of $100,000/QALY SE TAVI probability of cost-effectivenessb: < 5% at WTP of $50,000/QALY < 10% at WTP of $100,000/QALY |
Abbreviations: BE, balloon-expandable; CAD, Canadian dollars; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years; SAVR, surgical aortic valve implantation; SE, self-expanding; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; WTP, willingness-to-pay.
Based on three-way comparison of BE TAVI, SE TAVI, and SAVR
The study, which compared balloon-expandable TAVI, self-expanding TAVI, and SAVR, conducted a cost- utility analysis using a Markov model. The perspective was the Canadian third-party payer; specifically, they used costs relevant to the Ontario this is the Ministry of Health. Most clinical inputs, including mortality and complications, were derived by conducting a network meta-analysis the two published clinical trials reported in the clinical review section of this HTA.17,18 Short-term (within 30 days) complications included perioperative death, nondisabling stroke, disabling stroke, major bleeding, major vascular complication, atrial fibrillation, new permanent pacemaker implantation, mild to moderate paravalvular leak, and rehospitalization. Longer-term (within 1 year) complications included death, disabling stroke, moderate to severe paravalvular leak, and rehospitalization. In the model, complications were associated with additional costs and disutilities (i.e., reductions in quality of life). Because few long-term clinical data are available, the authors assumed that complication rates occurring 1 year or longer after the procedure were the same for TAVI and SAVR and that mortality rates were equivalent to age- and gender-specific Canadian life tables. Baseline quality-of-life estimates were derived from the PARTNER 2 trial40 that compared SAVR and TAVI in patients at intermediate surgical risk. Quality-of-life decrements associated with complications were derived from the published literature.
The study included costs related to the valve, initial procedure, and complications. The cost of the TAVI valve was $25,000, which was substantially higher than that of the SAVR valve ($6,000). However, TAVI index hospitalization costs (excluding the valve) were generally lower than SAVR costs and thus offset some of the valve costs.
The results of the cost-effectiveness analysis showed that TAVI produced slightly more QALYs and was more expensive than SAVR. When compared with SAVR, balloon-expandable TAVI had an average ICER of $27,196/QALY and self-expanding TAVI had an average ICER of $59,641/QALY. Balloon-expandable TAVI was less costly (by $2,330 on average) and slightly more effective (by 0.02 QALY on average) than self-expanding TAVI. In probabilistic sensitivity analysis, balloon-expandable TAVI had the highest probability of being cost-effective; it was the preferred option in 53% and 58% of model iterations, at willingness-to-pay values of $50,000 and $100,000 per QALY, respectively. Self-expanding TAVI was preferred in less than 10% of iterations. The results were sensitive to the rates of SAVR complications. Using the complication rates from the Evolut low-risk trial17 increased the certainty that balloon-expandable TAVI was the most cost-effective alternative. Using the complication rates from the PARTNER 3 trial18 decreased the certainty that balloon-expandable TAVI was the most cost-effective alternative.
Applicability and Limitations of the Included Studies
Appendix 8 (Tables A17 and A18) provides the results of the quality appraisal checklist for economic evaluations applied to the only included study. The included study was deemed to be directly applicable to the research question. It was conducted using the perspective of a Canadian third-party payer (i.e., Ontario Ministry of Health). It also used probabilistic analyses and a discount rate of 1.5%, as recommended by the Canadian Agency for Drugs and Technologies in Health.50
The study had minor limitations. It included relevant costs and used Ontario sources (i.e., Ontario Health Insurance Plan billing codes, Canadian Institute for Health Information Patient Cost Estimator, St. Michael's Hospital) to estimate the costs and resource use associated with TAVI and SAVR. Health outcomes were based primarily on the PARTNER 318 and the Evolut LRT17 studies. These studies had a 1-year follow-up. The cost-utility studies used lifetime horizons, but made the conservative assumption that, after 1 year, mortality and complication rates were the same for people with TAVI and SAVR. However, comparative clinical outcomes beyond 2 years in low-risk patients have yet to be published. Given little research on people at low surgical risk at the time of publication, the studies used data from cohorts at intermediate surgical risk40 to inform health-state utility values for TAVI and SAVR.
Discussion
Our economic evidence review identified one study that assessed the cost-effectiveness of TAVI in people with severe aortic valve stenosis who were at low surgical risk. The study found that, on average, TAVI was more effective (i.e., produced more QALYs) than SAVR, but was also more expensive.
One additional cost-effectiveness analysis51 conducted in patients with “lower surgical risk” did not meet our specific inclusion criteria because the patient population had mixed surgical risk. The study assessed the cost-effectiveness of self-expanding TAVI versus SAVR using 60-month data from the NOTION trial, which was an “all-comers” trial conducted in Denmark and Sweden.31 About 81.8% of the NOTION trial's participants were considered to be at low surgical risk.31 Similar to the Ontario study,49 the NOTION cost-effectiveness analysis found that TAVI was slightly more effective than SAVR (additional 0.09 QALY).51 The study based on the NOTION trial found that TAVI was more expensive than SAVR (additional $65,000 Danish kroner [DKK] = $12,061 CAD, 1 DKK = 0.19 CAD September 27, 2019). While the direction of this result is the same as the Ontario study, which also found providing TAVI was more expensive than SAVR (additional $2,707-$5,077), the additional cost associated with providing TAVI in the NOTION trial TAVI was higher than the Ontario analysis. This could be due to differences in cost inputs (i.e., the SAVR valve in the NOTION study was approximately $3,000 CAD compared with $6,000 CAD in the Ontario study) and clinical event rates. The ICER in the analysis based on the NOTION trial was $696,264 DKK/QALY ($134,988 CAD/QALY, 1 DKK = 0.19 CAD September 27, 2019). The authors concluded that TAVI was cost-effective, despite some uncertainty in results. Based on the probabilistic sensitivity analysis results, 42% and 78% of model iterations showed TAVI was cost-effective at willingness-to-pay values of $375,489 DKK/QALY ($134,988 CAD/QALY, 1 DKK = 0.19 CAD September 27, 2019) and $1,126,467 DKK/QALY ($218,394 CAD/QALY, 1 DKK = 0.19 CAD September 27, 2019), respectively.
The ICER of the Ontario study indicates that TAVI might be cost-effective (ICER balloon-expandable TAVI vs. SAVR = $27,196/QALY; ICER self-expanding TAVI vs. SAVR = $59,641/QALY).49 However, there was uncertainty in these results. When considering all three interventions, balloon-expandable TAVI had a 53% and 59% probability of being cost-effective at willingness-to-pay values of $50,000/QALY and $100,000/QALY, respectively. Certainty was increased in a sensitivity analysis that used SAVR complication rates from the Evolut low-risk trial17. In this analysis, balloon-expandable TAVI had a 71.5% and 73.8% probability of being cost-effective at willingness-to-pay values of $50,000/QALY and $100,000/QALY, respectively.
The Ontario analysis demonstrated that balloon-expandable TAVI was slightly more effective (additional 0.02 QALY) and less costly (additional $2,300) than self-expanding TAVI. This can be explained by variation in the rates of clinical events between the TAVI valves in the clinical trials. Cost drivers likely include variation in rates of pacemaker implantation ($11,839/event), stroke ($3,644-$8,360/event) and variation in the length of TAVI procedure, which affected anesthesia costs in the economic model. As the authors suggest, in absence of direct head-to-head comparisons, this finding should be interpreted with caution.
Although the Ontario cost-effectiveness study was well conducted, its limitations should be considered when interpreting the findings. As the authors note, the trials that informed the clinical inputs were conducted in patients with a mean age of 74 years. There is a paucity of long-term data beyond one year on the effectiveness of TAVI, particularly in the low-risk population. While the model made a conservative assumption that the clinical event rates and mortality would be the same between the TAVI and SAVR arms, they were unable to capture long-term valve durability and its clinical consequences (e.g., need for reintervention). Valve durability in TAVI is a particular concern with younger patients. As the authors note, the trials were conducted in patients with a mean age of 74 years, so there is additional concern about generalizability of results to younger patients. Further, while the analysis provides an indication of the cost-effectiveness of TAVI in a controlled setting over a short time, the cost-effectiveness of TAVI in the Ontario population unknown. Last, at the time the economic model was developed we had no peer-reviewed quality-of-life data on the low surgical risk population. In the absence of such data, the authors used the results from the intermediate surgical risk trial. Recently, the US Food and Drug Administration published the Summary of Safety and Effectiveness Data for the two valves in patients at low surgical risk.35,36 In addition, quality-of-life data from the PARTNER 3 trial have been published.29 However, the quality-of-life data included in these reports show similar patterns to data on intermediate risk from the PARTNER 3 trial (i.e., initial improvement among TAVI patients at 30 days, followed by similar quality of life at 1 year). Therefore the cost-effectiveness estimates from the Ontario study, which were based on intermediate-risk quality-of-life data likely reflect the low-risk population.
The study by Tam et al49 was of good quality and applicable to the Ontario context. Based on current evidence from RCTs, this study demonstrates that TAVI could be cost-effective in people with severe aortic valve stenosis who are at low surgical risk. However, there is considerable uncertainty for this result.
Conclusions
Our review of the literature identified one published cost-effectiveness analysis that compared TAVI with SAVR in adults with severe aortic valve stenosis who were at low surgical risk. The study was directly applicable and conducted from the perspective of the Ontario Ministry of Health. The TAVI procedure might be cost-effective for patients at low surgical risk; however, there is some uncertainty in this result.
PRIMARY ECONOMIC EVALUATION
Our review of the literature identified a cost-effectiveness analysis that compared TAVI with SAVR in adults with severe aortic valve stenosis who were at low surgical risk. The study was directly applicable to our research question, had minor limitations, and used a Canadian third-party payer (i.e., Ontario Ministry of Health) perspective and costs derived from Ontario sources. Given this recent and relevant cost-effectiveness analysis, we decided not to conduct a primary economic evaluation.
BUDGET IMPACT ANALYSIS Research Question
What is the potential 5-year budget impact for the Ontario Ministry of Health of publicly funding transcatheter aortic valve implantation (TAVI) for adults with severe aortic valve stenosis who are at low surgical risk?
Methods
Analytic Framework
We estimated the 5-year budget impact of publicly funding TAVI in adults at low surgical risk using the cost difference between two scenarios: 1) current clinical practice without public funding for TAVI (the current scenario, where surgical aortic valve replacement [SAVR] is used) and 2) anticipated clinical practice with public funding for TAVI (the new scenario, where there is a mix of TAVI and SAVR). Figure 12 presents the budget impact model schematic.
Figure 12: Schematic Model of Budget Impact.
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transaortic valve implantation.
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results are affected by varying input parameters and model assumptions.
Key Assumptions
At present in Ontario, there is no formal public funding for TAVI in patients at low surgical risk
Patients currently receiving combined surgical aortic valve replacement (SAVR) and coronary artery bypass graft (CABG) for revascularization will be eligible to receive TAVI in our new scenario (given public funding in low-risk patients). This is consistent with data from the German Quality Assurance Programme on Aortic Valve Replacement, which has shown that volumes of both isolated SAVR and SAVR with CABG decreased as volumes of TAVI increased from 2008 to 201752
Society of Thoracic Surgeons (STS) scores are equivalent to the heart team's judgment
Due to the relatively short time horizon (5 years), we assume no revision surgeries would be required to correct a failed device
Human resource capacity and infrastructure (i.e., availability of catheterization labs and hybrid operating rooms) within Ontario cardiac centres are sufficient to expand the TAVI program to people with low surgical risk. Currently TAVI is offered in 11 centres in Ontario53
Target Population
The target population for this analysis is adults with severe aortic valve stenosis who are at low surgical risk.
Our method of estimating the target population is described below and in Table 13. We first obtained the number of people undergoing SAVR (isolated or in conjunction with CABG) in Ontario from the CorHealth Ontario Cardiac Registry from 2013/14 to 2018/19 (see Table 1 in the Clinical Evidence section). We assumed that all patients were adults. Using linear extrapolation, we estimated the number of people who would undergo SAVR over the next 5 years. We excluded patients who had a SAVR repair, and instead focused on the initial SAVR replacement.
Table 13:
People at Low Surgical Risk Eligible to Receive TAVI in Ontario, 2019/20 to 2023/24
| Year | |||||
|---|---|---|---|---|---|
| Population | 2019/20 | 2020/21 | 2022/23 | 2023/24 | 2024/25 |
| SAVR, all indicationsa (n) | 3,086 | 3,143 | 3,199 | 3,255 | 3,311 |
| SAVR, aortic stenosisb (n) | 2,006 | 2,043 | 2,079 | 2,116 | 2,152 |
| SAVR, aortic stenosis and low surgical riskc (n) | 1,609 | 1,638 | 1,667 | 1,696 | 1,725 |
| SAVR patients eligible or likely to receive TAVId (n) | 1,126 | 1,146 | 1,167 | 1,187 | 1,208 |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Projected from total SAVR volumes from 2013/14 to 2018/19 in Ontario; see Table 1 in the Clinical Evidence section.
65% of SAVR in patients with aortic stenosis, based on previous analysis in Ontario (2012/13–2017/18) (written communication; Harindra Wijeysundera, MD, August 14, 2019).
80.18% of SAVR in patients at low surgical risk.8
70% of SAVR eligible for TAVI (assumption).
Based on a previous analysis in Ontario (2012/13–2017/18) (written communication, Harindra Wijeysundera, MD, August 14, 2019), we estimated that approximately 65% of patients receiving SAVR have aortic stenosis. We excluded patients who had SAVR for other indications (e.g., aortic insufficiency and endocarditis), as they would not be eligible for TAVI.
Among patients who had undergone SAVR for aortic stenosis, we estimated the proportion that were at low surgical risk. We recognize that surgical risk is usually determined by a multidisciplinary heart team, and that STS score is only one of the factors that may be considered. However, in the absence of population data on the heart team's risk stratification, we used an STS score of less than 4% as a proxy. Based on the STS scores of 141,905 people who underwent SAVR in the United States, we assumed that 80.18% of people would be at low surgical risk.8 We examined both lower (60%) and higher (100%) percentages of patients at low surgical risk in our sensitivity analyses.
Among those at low surgical risk, we assumed some patients would continue to receive SAVR. The use of SAVR is likely preferred in younger patients (< 65 years), given uncertainty about the long-term durability of TAVI and evidence on diminished durability of tissue valves in younger patients.54 Based on a recent report by CorHealth Ontario on adult cardiac surgery, 32.2% of isolated SAVR procedures and 14.4% of SAVR with CABG procedures were younger than 65 years.53 As a weighted average, about 24% of all SAVR patients are younger than 65 years. The SAVR procedure could also be preferred in some patients with complex coronary artery disease requiring concomitant surgery or with bicuspid valves. Therefore, we assumed that about 30% of patients would continue to receive SAVR and 70% would be eligible for TAVI. It is difficult to determine an exact proportion of patients who would be eligible to receive TAVI, and this could change over time with additional long-term clinical evidence. Hence, we examined both lower (50%) and higher (90%) percentages of eligible patients in our sensitivity analyses.
Current Intervention Mix, Uptake of New Intervention, and Future Intervention Mix
We assumed that TAVI is not currently used in people at low surgical risk. Thus, in our current scenario, we assumed that all patients would continue to receive SAVR (Table 14).
Table 14:
People at Low Surgical Risk Eligible to Receive TAVI in Ontario, 2019/20 to 2024/25
| Year | |||||
|---|---|---|---|---|---|
| Procedure | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| Current Scenario: No Public Funding for TAVI | |||||
| SAVR, n | 1,126 | 1,146 | 1,167 | 1,187 | 1,208 |
| New Scenario: Public Funding for TAVI | |||||
| Uptake rate for TAVI, % | 50 | 61 | 73 | 84 | 95 |
| TAVI, n | 563 | 702 | 846 | 994 | 1,148 |
| SAVR, n | 563 | 444 | 321 | 193 | 60 |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
In our new scenario, in which TAVI is publicly funded for people at low surgical risk, we assumed that over time new patients could receive TAVI instead of SAVR (see Table 14). Currently there are 11 TAVI programs in Ontario.53 From 2013/14 to 2018/19, 5,067 TAVI procedures have been performed (see Table 1 in the Clinical Evidence section). Given that TAVI has already diffused into the system, we assumed that, a sufficient increase in public funding would prompt quick uptake to 50% in the first year. After this, we assumed a gradual linear uptake over the next 5 years, levelling out at 95%. We assumed that a small proportion of people (5%) would not receive TAVI owing to personal preference. We look at alternative uptake scenarios in our sensitivity analyses.
Resources and Costs
We obtained all costs used in our budget impact analyses from an Ontario-specific cost-effectiveness analysis by Tam et al. of TAVI in people at low surgical risk.49 This analysis, which we describe in detail in the Economic Evidence section of this HTA, was conducted using Ontario costs.
The economic analysis by Tam et al., compares balloon-expandable TAVI, self-expanding TAVI, and SAVR using clinical parameters obtained through a network meta-analysis of the PARTNER 3 and Evolut Low Risk Trials.49 The cohort of patients modelled were approximately 74 years of age. Key cost inputs included in the analyses are summarized in Table 15. The authors obtained device costs from the manufacturers and physician fees from the Ontario Schedule of Benefits.55 Complication costs were obtained from the Canadian Institute for Health Information patient cost estimator56 and the published literature. More detailed descriptions can be found in the original publication.49
Table 15:
Mean Cost Inputs Used in the Cost-Effectiveness Model
| Mean Costs (2019 CAD) | |||
|---|---|---|---|
| Resource | Balloon-Expandable TAVI | Self-Expanding TAVI | SAVR |
| Procedural Costs | |||
| Valvea | 25,000 | 25,000 | 6,000 |
| Index hospitalization stayb | 5,778 | 5,778 | 14,033 |
| Surgeon, surgical assistant, anesthesiologist feesb | 3,365 | 3,783 | 4,611 |
| Complicationsa | |||
| Atrial fibrillation | 4,544 | 4,544 | 4,544 |
| Bleeding | 2,376 | 2,376 | 2,376 |
| Disabling stroke | 8,360 | 8,360 | 8,360 |
| Nondisabling stroke | 3,644 | 3,644 | 3,644 |
| Permanent pacemaker implantation | 11,839 | 11,839 | 11,839 |
| Vascular | 11,305 | 11,305 | 11,305 |
| Rehospitalization (monthly) | 8,102 | 8,102 | 8,102 |
| Disabling stroke (monthly) | 3,260 | 3,260 | 3,260 |
The costs for our analysis were derived deterministically from the cost-effectiveness model by Tam et al.49 We used undiscounted annual costs of treatment with balloon-expandable TAVI, self-expanding TAVI, and SAVR (Table 16). For our reference case analysis, we used an average of balloon-expandable and self-expanding TAVI. In the sensitivity analyses, we calculated the budget impact assuming all balloon-expandable or self-expanding TAVI procedures. All costs are presented in 2019 Canadian dollars.
Table 16:
Mean Annual Per-Patient Costs for TAVI and SAVR
| Cost per Year Post-Implant, $a,b | ||||||
|---|---|---|---|---|---|---|
| Source | Intervention | Year 1c | Year 2d | Year 3d | Year 4d | Year 5d |
| Tam et al, 202049 | BE TAVI | 36,504 | 115 | 31 | 30 | 29 |
| SE TAVI | 37,782 | 230 | 163 | 157 | 152 | |
| Average TAVI | 37,143 | 172 | 97 | 94 | 90 | |
| SAVR | 28,833 | 637 | 495 | 479 | 462 |
Abbreviations: BE, balloon-expandable; SAVR, surgical aortic valve replacement; SE, self-expanding; TAVI, transcatheter aortic valve implantation.
Costs incorporate mortality.
We derived costs from model produced by Tam et al.49
Includes procedural (valve, index hospitalization, and fees for surgeon, surgical assistant, and anesthesiologist), short-term complication, and long-term complication costs.
Includes long-term complication costs.
Analysis
Reference Case Analysis
In the reference case analysis, we calculated the budget required to publicly fund TAVI in adults with severe aortic valve stenosis at low surgical risk in Ontario. We calculated the budget impact as the cost difference between the new scenario (public funding for TAVI) and the current scenario (no public funding for TAVI). We also calculated the budget impact broken down by cost type (i.e., device costs, professional fees, index hospitalization costs, and complications). Details of our method for calculating the budget impact are presented in Appendix 9.
Scenario Analyses
As a part of our analysis, we explored potential efficiencies related to the TAVI procedure. Our goal was to identify, if TAVI is publicly funded in patients with low surgical risk, ways in which the budget impact could be reduced to zero (i.e., such that costs of TAVI would become equivalent to costs of SAVR over 5 years).
The three key parameters we explored were the price of the TAVI valve, the length of stay in intensive care after the TAVI procedure, and the length of stay in the ward after the TAVI procedure. We varied these parameters in various combinations and examined when the budget impact would be equal to zero dollars.
We conducted additional scenario analyses, as summarized in Table 17. We examined a scenario with increased growth in our target population to account for an aging population (scenario 1). We also examined a scenario where funding for TAVI led to an expansion in the target population (scenario 2). In this scenario, we assumed if TAVI were to be publicly funded some people who previously had no intervention (e.g., people who chose not to have SAVR) would receive TAVI. We also included scenarios examining the variation in the proportion of SAVR patients who are at low surgical risk (scenarios 3 and 4), and variation in the proportion of SAVR patients eligible for TAVI (scenarios 5 and 6).
Table 17:
Scenario Analyses
| Scenario | Reference Case | Sensitivity Analysis |
|---|---|---|
| Target Population | ||
| Scenario 1: Increased population with severe aortic valve stenosis | Linear projection | 5% additional growth (Appendix 10, Table A23) |
| Scenario 2: Expansion of target population, TAVI uptake in patients currently getting no intervention | Only SAVR patients get TAVI | Additional 5% patients get TAVI (Appendix 10, Table A23) |
| Scenario 3: Increased proportion of SAVR patients at low surgical risk | 80% | 100% |
| Scenario 4: Decreased proportion of SAVR patients at low surgical risk | 80% | 60% |
| Scenario 5: Increased proportion of SAVR patients eligible for TAVI | 70% | 90% |
| Scenario 6: Decreased proportion of SAVR patients eligible for TAVI | 70% | 50% |
| Uptake | ||
| Scenario 7: Faster initial uptake | 50%-95% over 5 y | 75%-95% over 5 y (Appendix 10, Table A23) |
| Scenario 8: Gradual uptake | 50%-95% over 5 y | 20%-50% over 5 y (Appendix 10, Table A23) |
| Cost | ||
| Scenario 9: Cost from probabilistic analyses | Cost from deterministic analyses | Cost from probabilistic analyses (Appendix 10, Table A23) |
| Scenario 10: Cost using only BE TAVI | Average TAVI costs (Table 16) | BE TAVI costs (Table 16) |
| Scenario 11: Cost using only SE TAVI | Average TAVI costs (Table 16) | SE TAVI costs (Table 16) |
| Scenario 12: Varied SAVR procedural costs | Tables 15, 16 | Reduction of SAVR procedural costs by 10% (Appendix 10, Table A23) |
Abbreviations: BE, balloon-expandable; SAVR, surgical aortic valve replacement; SE, self-expanding; TAVI, transcatheter aortic valve implantation.
In addition, we explored variation in the uptake rates (scenarios 7 and 8) and costs (scenarios 9–12). In one cost scenario, we explored the effect of reducing SAVR costs. One clinical trial involving patients with aortic stenosis at low surgical risk reported that up to 24% of SAVR procedures used minimally invasive techniques.18 Using these techniques could reduce length of hospital stays and increase savings as compared with conventional SAVR.57–59 Hence we conducted a scenario analysis with a 10% reduction in SAVR procedural costs (scenario 12).
Results
Reference Case
The results of our budget impact analysis can be found in Table 18. In our new scenario, if TAVI (and SAVR) were publicly funded, we estimated that the total cost would be between $37 and $45 million yearly ($21-$43 million of this would be for TAVI). Given the current spending on SAVR, the annual budget impact of funding TAVI over the next 5 years is estimated to be an additional $5 and $8 million per year.
Table 18:
Budget Impact Analysis Results
| Budget Impact, $ Milliona,b | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Current Scenario | ||||||
| TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 |
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 |
| New Scenario | ||||||
| TAVI | 20.91 | 26.17 | 31.60 | 37.19 | 43.00 | 158.87 |
| SAVR | 16.23 | 13.16 | 9.81 | 6.26 | 2.50 | 47.96 |
| Total | 37.14 | 39.33 | 41.42 | 43.45 | 45.49 | 206.83 |
| Budget impact | 4.68 | 5.57 | 6.48 | 7.37 | 8.26 | 32.36 |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
In 2019 Canadian dollars.
Numbers might appear inexact owing to rounding.
A summary of cost breakdowns can be found in Appendix 10, Table A23. Although funding TAVI would result in a larger amount of money spent on valves as compared to our current scenario, the costs for procedural hospitalization, professional fees, and complications would likely be reduced.
Sensitivity Analysis
Efficiency Analysis
The results of our efficiency analysis are found in Table 19. We have highlighted reductions that would be needed in each of the parameters to achieve an overall budget impact of zero dollars. In our reference case the device price was $25,000 and the mean length of stay was 1.33 days in intensive care and 1.33 days in the ward. The budget impact was reduced to zero when device price was reduced by about 30% to $17,390. The budget impact could not be reduced to zero by reducing only the length of stay. However, when the length of stay is reduced to one day in the ward (i.e., 100% reduction in intensive care stay and 25% reduction in ward stay), the budget impact reaches zero when the device price is reduced by 21% to $19,750 (Table 19).
Table 19:
Efficiency Analysis Results, Joint Reduction in Parameters Needed to Achieve Budget Impact of Zero Dollars for TAVI
| Reduction in TAVI Device Price, % | Device Price, $ | Reduction in Length of ICU Stay, % | Length of ICU Stay, Days | Reduction in Length of Ward Stay, % | Length of Ward Stay, Days |
|---|---|---|---|---|---|
| 21 | 19,750 | 100 | 0 | 25 | 1 |
| 25 | 18,750 | 100 | 0 | 0 | 1.33 |
| 25 | 18,750 | 25 | 1 | 25 | 1 |
| 29 | 17,704 | 25 | 1 | 0 | 1.33 |
| 26 | 18,441 | 0 | 1.33 | 25 | 1 |
| 30 | 17,390 | 0 | 1.33 | 0 | 1.33 |
Abbreviations: ICU, intensive care unit; TAVI, transcatheter aortic valve implantation.
Other Sensitivity Analysis
The budget impact estimated in each scenario analysis can be found in Table 20. Full details can be found in Appendix 10, Table A24. We found the greatest increase in budget impact in the scenario where we assumed the introduction of TAVI would lead to an expansion of the target population (scenario 2) and the scenario where SAVR procedure costs were reduced by 10% (scenario 12). We found the greatest decrease in budget impact in the scenario with a gradual uptake rate from 20% to 50% over 5 years. The budget impact was also affected by the proportion of people at low surgical risk (scenarios 3 and 4) and by a lower proportion of people eligible for TAVI (scenarios 5 and 6).
Table 20:
Budget Impact, Scenario Analyses
| Budget Impact, $ Millionsa | ||||||
|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| Reference case | 4.68 | 5.57 | 6.48 | 7.37 | 8.26 | 32.36 |
| Scenario 1: Increased population with severe aortic valve stenosis | 5.16 | 6.45 | 7.87 | 9.38 | 10.98 | 39.83 |
| Scenario 2: Expansion of target population, TAVI uptake in patients getting no intervention | 8.40 | 9.38 | 10.37 | 11.34 | 12.30 | 51.80 |
| Scenario 3: Increased proportion of SAVR patients at low surgical risk | 5.83 | 6.95 | 8.08 | 9.20 | 10.30 | 40.36 |
| Scenario 4: Decreased proportion of SAVR patients at low surgical risk | 3.50 | 4.17 | 4.85 | 5.52 | 6.18 | 24.22 |
| Scenario 5: Increased proportion of SAVR patients eligible for TAVI | 6.02 | 7.17 | 8.33 | 9.48 | 10.62 | 41.61 |
| Scenario 6: Decreased proportion of SAVR patients eligible for TAVI | 3.34 | 3.98 | 4.63 | 5.27 | 5.90 | 23.12 |
| Scenario 7: Faster initial uptake | 7.02 | 7.23 | 7.48 | 7.73 | 7.98 | 37.43 |
| Scenario 8: Gradual uptake | 1.87 | 2.51 | 3.16 | 3.79 | 4.42 | 15.75 |
| Scenario 9: Cost from probabilistic analyses | 4.66 | 5.56 | 6.47 | 7.37 | 8.26 | 32.32 |
| Scenario 10: Cost using only BE TAVI | 4.32 | 5.09 | 5.86 | 6.61 | 7.33 | 29.21 |
| Scenario 11: Cost using only SE TAVI | 5.04 | 6.05 | 7.10 | 8.14 | 9.18 | 35.51 |
| Scenario 12: Reduced SAVR procedural costs | 6.07 | 7.30 | 8.57 | 9.82 | 11.09 | 42.84 |
Abbreviations: BE, balloon-expandable; SAVR, surgical aortic valve replacement; SE, self-expanding; TAVI, transcatheter aortic valve implantation.
Numbers might appear inexact owing to rounding.
Discussion
In our current scenario (no public funding allocated for TAVI patients who are at low surgical risk), we estimated that it would cost between $32 and $37 million yearly to fund SAVR in patients with aortic stenosis at low surgical risk. In our new scenario, if TAVI (and SAVR) were publicly funded, we estimated that the total cost would be between $37 and $45 million yearly ($21-$43 million of this would be for TAVI). Therefore, we estimated the budget impact to be an additional $5 to $8 million yearly to publicly fund TAVI for persons with aortic stenosis at low surgical risk.
We assumed that after 5 years, nearly all people suitable for TAVI in the population at low surgical risk would receive it. On the basis of data from the CorHealth Ontario Cardiac Registry, this analysis, and our previous analysis in patients at intermediate surgical risk,46,60 we estimate that after 5 years the number of TAVI procedures will exceed the number of SAVR procedures (SAVR alone or SAVR plus CABG). This is consistent with patterns seen in Germany, which has been at the forefront of TAVI adoption.52 It's important to note that we assume a substantial proportion of people will continue to get SAVR. This could include people currently receiving SAVR for other clinical indications (e.g., aortic insufficiency or endocarditis), younger than 65 years of age, and/or with complex coronary artery disease or bicuspid valves.
Similar to our previous budget impact analysis,46 we found that most of the budget impact could be attributed to device-related TAVI costs. While some of these costs were offset by cost reductions in the initial hospital stay, physician fees, and complications, TAVI on average costs an additional $8,000 per person compared with SAVR. We explored further potential for cost offsets by conducting an efficiency analysis, where we examined the reductions needed in TAVI length of stay and device price to achieve a budget impact of zero dollars. We found if the TAVI device price were reduced by 30% (to $17,390), over 5 years we could achieve a budget impact of zero. If length of stay were also reduced, the reduction in device price could be smaller. We derived the length of stay for TAVI and SAVR used in our reference case analysis from the PARTNER 3 trial as used in Tam et al.18,49 Evidence shows that minimally invasive TAVI approaches have led to even shorter lengths of stay.61–64 Further, analyses have shown that it's feasible to achieve cost reductions by introducing more minimally invasive or streamlined approaches.61,62 While we explore the effect of only device price and length of stay, there could be other factors that would lower the initial cost of the TAVI procedure.
There are also more minimally invasive alternatives to conventional SAVR, such as mini-sternotomy and mini-thoracotomy.65 Hence, we also explored the budget impact assuming reductions in the price of SAVR. We found that under a 10% percent reduction in procedural SAVR costs, the budget impact of TAVI increased to between $6 and $11 million annually. As methods of TAVI and SAVR evolve, and as new methods are introduced, the budget impact of funding TAVI could change.
Our analysis had several strengths. We used a recently published Ontario-specific cost-effectiveness analysis to inform our costing,49 based our volumes on an Ontario registry, and performed sensitivity analyses to explore our assumption.
However, our results should be interpreted with their limitations in mind. First, given the current infrastructure, we assumed there would be quick uptake of TAVI to 50% of eligible patients in the first year. Several factors could increase the uptake rate of TAVI in this population (e.g., system and infrastructure readiness) or reduce it (e.g., appropriate funding allocation or backlogs of people at high surgical risk, for whom TAVI is already funded). We explored these possibilities in scenario analyses, finding they have a large impact on the results. Second, it was challenging to estimate the proportion of patients at low surgical risk and number of patients who would be eligible or most likely to receive TAVI. While the determination of risk and eligibility considers the STS score and age, it is ultimately based on a heart team's decision, which incorporates several risk factors and comorbidities. As these proportions vary, the budget impact can range from an additional $3 million to $6 million annually to $6 million to $11 million annually. Third, given our relatively short time horizon, we did not account for potential revision surgeries. When TAVI is used in younger patients, there is a potential for increased number of revision surgeries. The durability of TAVI in the long term is still uncertain. As more evidence becomes available, TAVI durability should be incorporated in future analyses.
Conclusions
We estimate that the additional cost to provide public funding for TAVI in people with severe aortic valve stenosis at low surgical risk would range from about $5 to $8 million over the next 5 years.
PREFERENCES AND VALUES EVIDENCE
Objective
The objective of this analysis was to explore the underlying values, needs, preferences and priorities of people who have lived experience with aortic valve stenosis and those having experience with transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement (SAVR). The treatment focus was TAVI versus SAVR.
Background
Exploring patients' preferences and values provides unique information about people's experiences of a health condition and the health technologies or interventions used to diagnose, manage, or treat the health condition. Information includes the effect of the condition and its treatment on the person with the health condition, the family and other caregivers, and the person's personal environment. Engagement also provides insights into barriers or facilitators to patients accessing care for their health condition. Information shared from lived experience can also identify other important information that may not be reported in published literature (e.g., important outcomes or preferences for care).10,66,67 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
For this analysis, we examined in three ways the perspectives and values of those with lived experience with aortic valve stenosis and those who have experience with TAVI:
A review by Ontario Health of the published quantitative preferences evidence
A review by the Canadian Agency for Drugs and Technologies in Health (CADTH) of the published qualitative literature1
Direct engagement by Ontario Health of those with lived experience through interviews
Quantitative Evidence
Research Questions
What are patients' relative preferences for transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR)?
What is the relative importance of key attributes of TAVI and what trade-offs between attributes are patients willing to make?
How does TAVI affect patients' decisional conflict and psychological well-being?
How satisfied are patients with TAVI?
Methods
The quantitative preferences evaluation was conducted as a literature survey and used methods different from those of the clinical systematic review. The objective was to describe and understand patients' values and preferences regarding TAVI for aortic stenosis. Results are summarized narratively in text and in tables.
Quantitative Evidence Literature Search
We performed a targeted literature search of quantitative preferences on July 18, 2019, for studies published from January 1, 2002, until the search date. We used the Ovid interface to search MEDLINE.
The search was based on the population and intervention of the clinical search strategy, with a modified methodological search filter by Selva et al68 applied, which limited retrieval of studies to quantitative evidence of preferences and values. The final search strategy was peer reviewed using the PRESS Checklist.21
We created database auto-alerts in MEDLINE and monitored them for the duration of the assessment period. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies Inclusion Criteria
English-language full-text publications
Studies published from January 1, 2002, until July 18, 2019
-
Systematic reviews, meta-analyses, randomized controlled trials (RCTs), cohort studies, surveys
-
○
Studies of patients' preferences for TAVI
-
-
Utility measures: direct techniques (standard gamble, time trade-off, rating scales) or conjoint analysis (discrete choice experiment, contingent valuation and willingness-to-pay, probability trade-off)
-
-
Nonutility quantitative measures: direct choice techniques, decision aids, surveys, questionnaires
-
-
-
○
Exclusion Criteria
Studies where results for outcomes of interest cannot be extracted
Nonsystematic reviews, narrative reviews, abstracts, editorials, letters, case reports, commentaries, and qualitative studies
Animal and in vitro studies
Participants
Inclusion Criteria
Adults with aortic stenosis (any severity or surgical risk level)
Exclusion Criteria
People who do not have aortic stenosis (e.g., family members, caregivers, general public, health care providers)
Interventions
Inclusion Criteria
Transaortic valve implantation using a self-expanding or balloon-expandable bioprosthetic valve via any implantation route (any valve brand or model)
Exclusion Criteria
Other procedures or treatments for aortic stenosis
Comparator
Surgical aortic valve replacement (SAVR)
No comparator
Outcome Measures
Patient preferences for TAVI (i.e., attributes and trade-offs of TAVI)
Decisional conflict of patients to choose or undergo TAVI
Psychological well-being (e.g., anxiety, depression, distress, worry) of patients
Patient satisfaction
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence22 and then obtained the full text of studies that appeared eligible for the review according to the inclusion criteria. The reviewer then examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
We extracted relevant data on study characteristics using a data form to collect information about the following:
Source (e.g., citation information, contact details, study type)
Methods (e.g., study design, study duration, participant recruitment)
Outcomes (e.g., outcomes measured, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], time points at which outcomes were assessed)
Statistical Analysis
After determining that a meta-analysis to provide an overall statistical summary of the effect estimate was inappropriate for a broad summary of the evidence on quantitative preferences, we chose a descriptive approach using text or tables.
Critical Appraisal of Evidence
We did not critically appraise the included studies. The purpose of our literature survey is to gain a broad overview of the quantitative preferences of patients.
Results
Quantitative Evidence Literature Search
The quantitative preferences literature search yielded 370 citations published from January 1, 2002, until July 18, 2019, after removing duplicates. We identified six non-randomized studies69–74 that met our inclusion criteria. Figure 1330 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the quantitative preferences literature search.
Figure 13: PRISMA Flow Diagram—Quantitative Evidence of Preferences and Values Search Strategy.
Source: Adapted from Moher et al.30
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Characteristics of Included Studies
We found six studies on patient preferences and values for TAVI or TAVI compared with SAVR.69–74 Tables 21 and 22 show the characteristics of the included studies and their participant populations.
Table 21:
Characteristics of Included Quantitative Studies on Patient Preferences for TAVI
| Author, Year | Country | Study Design | Study Methods | Participants |
|---|---|---|---|---|
| Amonn et al, 201369 | Switzerland | Questionnaire | Validated HADS (tool comprising 14 questions that assesses anxiety [HADS-A] and depression [HADS-D]; every question is answered on a 4-point scale [0–3], resulting in an overall score of 0–21 [score 0–7 is negative, 8–10 suggests anxiety or depression, and ≥ 11 indicates probable anxiety or depression]) | High-risk patients (based on the EuroSCORE and STS risk calculator) referred for TAVI or SAVR were assigned to either TAVI or SAVR by interdisciplinary heart team |
| Dharmarajan et al, 201770 | United States | Questionnaire | Likert-type scale with choices of “strongly disagree,” “disagree,” “neither agree nor disagree,” “agree,” “strongly agree,” and “don't know” or “not applicable” | Patients from 9 established heart valve treatment centres in the United States Inclusion criteria: ≥ 19 years of age, referral to participating treatment centre for evaluation and treatment of aortic stenosis, completion of TAVI or SAVR or decision made to receive medical management in 18 months before study initiation, and ability to complete all survey questions online, in written form, or verbally |
| Marsh et al, 201971 | United States | Benefit-risk analysis | Online adapted swing weighting method (pairwise comparison of attributes) was used to elicit attribute trade-offs Survey data were used to estimate participants' weights for aortic stenosis treatment attributes Six attributes were examined: 1-month risk of mortality, 1-month risk of disabling nonfatal stroke, probability of having relief from aortic stenosis symptoms that affect daily life, 1-year risk of receiving new permanent pacemaker, 1-year risk of requirement for dialysis, years over which procedure has been proven to work |
Patients were self-reported as being diagnosed with aortic stenosis (either received treatment or were experiencing aortic stenosis-related physical activity limitations), recruited from advocacy organization databases Inclusion criteria: ≥ 19 years of age, self-reported diagnosis of aortic valve disease, able to read and understand English, willing and able to complete online survey, willing and able to provide (electronic) consent to participate in study, residence in United States, have been treated procedurally (TAVI or SAVR) for aortic stenosis in past 10 years Exclusion criteria: cognitive impairment, hearing difficulty, visual impairment, acute psychopathology, insufficient knowledge of English (which could interfere with patient's ability to provide written consent and complete web survey); are not experiencing at least one of symptoms of aortic stenosis, as described in inclusion criteria |
| Rosseel et al, 201972 | Denmark | Questionnaire | Questionnaire was designed by team of TAVI health care providers based on three components of “health” defined by WHO: physical, mental, and social well-being Pilot study was conducted to check validity of questionnaire |
All patients who underwent elective SAVR or TAVI between September 2015 and August 2016 were considered for inclusion; all patients were discussed by multidisciplinary heart team Questionnaire directed to patients and their nearest relative was sent to all patients, excluding those who had died by time questionnaire was mailed (September 2017) |
| van Mourik et al, 201873 | Netherlands | Questionnaire | Telephone questionnaire if patient was available, or contact person or primary care provider was contacted for follow-up information if patient was unavailable | Consecutive patients with symptomatic aortic stenosis who underwent TAVI between January 2012 and 2017; patients were assessed by multidisciplinary TAVI team |
| Wong et al, 201874 | Canada | Questionnaire | Telephone questionnaire at 3 and 30 d after discharge | All patients undergoing TAVI who were discharged from hospital during 6-mo study period |
Abbreviations: EuroSCORE, European System for Cardiac Operative Risk Evaluation; HADS, Hospital Anxiety and Depression Scale; SAVR, surgical aortic valve replacement; STS, Society for Thoracic Surgeons; TAVI, transcatheter aortic valve implantation WHO, World Health Organization.
Table 22:
Characteristics of Participants Within Included Studies
| Author, Year | N | Male, n (%) | Age, Years | Ethnicity, n (%) | STS Score (SD), % | NYHA Class, n (%) | Coronary Artery Disease, n (%) | LVEF, % |
|---|---|---|---|---|---|---|---|---|
| Amonn et al, 201369 | 144 (51 TAVI, 93 SAVR) |
TAVI: 26 (51) SAVR: 39 (42) P = .4 |
TAVI: mean 79.7 (SD 9.2) SAVR: mean 81.1 (SD 5.3) P = 1.0 |
NR | TAVI: 6.7 (3.8) SAVR: 4.4 (2.6) P < .001 |
TAVI Class I: 1 (2) Class II: 16 (31) Class III: 28 (55) Class IV: 6 (12) SAVR Class I: 6 (6) Class II: 44 (47) Class III: 36 (39) Class IV: 7 (8) P = .001 |
TAVI: 38 (75) SAVR: 53 (57) P = .047 |
TAVI: mean 49.6 (SD 17.2) SAVR: mean 58.1 (SD 10.3) P = .006 |
| Dharmarajan et al, 201770 | 407 (212 TAVI, 124 SAVR, 71 medical therapy) |
TAVI: 122 (58) SAVR: 81 (65) P = .06 |
TAVI: mean 80.7 (SD 8.6) SAVR: mean 73.2 (SD 9.5) P < .001 |
White TAVI: 205 (97) SAVR: 119 (96) P = .39 |
TAVI: 8.7 (4.5) SAVR: 3.4 (2.4) P < .001 |
TAVI Class III: 106 (50) Class IV: 64 (30) SAVR Class III: 39 (124) Class IV: 22 (18) Class III: P = .003 Class IV: P = .003 |
NR | TAVI: mean 53.9 (SD 12.5) SAVR: mean 56.2 (SD 10.7) P = .20 |
| Marsh et al, 201971 | 93 TAVI | 40 (43) | 19–39: 4 (4%) 40–59: 20 (22%) |
White 87 (94) |
NR | Class I: 53 (57) Class II: 25 (27) |
NR | NR |
| 60–74: 39 (42%) 75–89: 27 (29%) ≥ 90: 3 (3%) |
Class III: 15 (16) Class IV: 0 (0) |
|||||||
| Rosseel et al, 201972 | 429 (164 TAVI, 265 SAVR) |
TAVI: 87 (53) SAVR: 193 (73) P < .001 |
TAVI: Mean 80 (SD 8) SAVR: Mean 70 (SD 10) P < .001 |
NR | TAVI: 3.8 (2.4) SAVR: 3.1 (2.9) P < .001 |
NR | NR | TAVI: mean 48 (SD 13) SAVR: mean 50 (SD 12)P = .905 |
| van Mourik et al, 201873 | 809 TAVI (741 complete follow-up) | 326/741 (44) | Median: 81.9 (IQR 77.3–85.3) | NR | 5.49 (4.73) | Class I: 30/809 (4) Class II: 216/809 (27) Class III: 483/809 (60) Class IV: 80/809 (10) |
NR | Normal: 483/808 (60) Mildly impaired: 157/808 (19)Moderately to severely impaired: 168/808 (21) |
| Wong et al, 201874 | 77 TAVI | 31 (40) | Mean 81.6 (range 61–96) | NR | NR | NR | NR | NR |
Abbreviations: IQR, interquartile range; LVEF, left ventricular ejection fraction; N, number; NR, not reported; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; SD, standard deviation; STS, Society for Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Five of the studies69,70,72–74 examined patient preferences using questionnaires, while one study71 was a benefit-risk analysis. The number of participants who underwent TAVI within the studies ranged from 51 to 809 and represented people from Canada, the United States, Denmark, the Netherlands, and Switzerland.69,70,72–74
All studies included people with either unspecified surgical risk, or mixed or high surgical risk. In general, participants were typically of higher surgical risk. We found no studies that focused only on people at low surgical risk. Study population characteristics also varied between the studies, and the mean or median age of participants was generally about 80 years of age.
We found one systematic review that examined patient preferences and values for TAVI or SAVR, but it was excluded because the two included studies did not meet our inclusion criteria (one was a qualitative study on TAVI and the other was a quantitative preferences study on SAVR).75
Preferences for Transcatheter Aortic Valve Implantation
Marsh et al conducted a benefit-risk analysis of patient preferences for TAVI or SAVR.71 In a population with unspecified surgical risk levels, the authors found that people generally put greater value on attributes that favoured TAVI than on attributes favouring SAVR (Table 23). People placed greater value on TAVI on the basis of a lower 1-month mortality rate, reduced procedure invasiveness, and a faster time to return to normal quality of life offsetting the value placed on a longer period over which the procedure has been proven to work and the reduced risk for a pacemaker from SAVR.
Table 23:
Preferences for Transcatheter Aortic Valve Implantation
| Author, Year | N | Measurement Method | Results |
|---|---|---|---|
| Marsh et al, 201971 | 93 | Benefit-risk analysis | Maximum acceptable risk increase/minimum acceptable benefit reduction in exchange for reducing procedure invasiveness (from “invasive” to “minimally invasive”), for each attribute:
Percentage of patients who prefer TAVI to SAVR, for each attribute:
Minimum acceptable benefit/maximum acceptable risk that would make people indifferent between TAVI and SAVR, for each attribute:
|
Abbreviations: SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation
For attributes where TAVI performance is better than SAVR (1-month risk of mortality, 1-month risk of disabling nonfatal stroke, probability of relief from symptoms that affect daily life, and 1-year risk for dialysis), all patients preferred the improved performance and reduced invasiveness of TAVI. For attributes where SAVR performance is better than TAVI, 61% of people would be willing to accept the increased risk of needing a new permanent pacemaker, and 38% of people would be willing to accept the shorter time over which the procedure has been proven to work, for the reduced invasiveness of TAVI.
However, authors noted that there was substantial heterogeneity in patients' preferences, which was partly explained by age. Older participants (> 60 years of age) were less willing to tolerate the invasiveness of SAVR and instead preferred to accept greater potential procedure risks or reductions in benefit to avoid having to undergo an invasive procedure. Younger participants (< 60 years of age) might prefer to undergo a more invasive procedure that has been proven to work for 10 years or longer. No other correlations were found between patient characteristics and the maximum acceptable increase in risk or the maximum acceptable reduction in benefit.
Decisional Conflict
We did not find any studies that evaluated decisional conflict for TAVI.
Psychological Well-Being
Three studies examined patients' psychological well-being around the time of the TAVI procedure or during the follow-up recovery period (Table 24).69,72,74 Amonn et al used the Hospital Anxiety and Depression Scale (HADS), while the other two studies developed their own questionnaires for measuring psychological well-being.69
Table 24:
Results for Psychological Well-Being
| Author, Year | N | Measurement Method | Results |
|---|---|---|---|
| Amonn et al, 201369 | 144 (51 TAVI, 93 SAVR) |
HADS |
|
| Rosseel et al, 201972 | 429 (164 TAVI, 265 SAVR) |
Questionnaire |
|
| Wong et al, 201874 | 77 TAVI (77 at 3-d follow-up, 70 at 30-d follow-up |
Questionnaire |
|
Abbreviations: HADS, Hospital Anxiety and Depression Scale; HADS-A, Hospital Anxiety and Depression Scale, Anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale, Depression subscale; NR, not reported; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Ammon et al found no significant differences in anxiety and depression rates between TAVI and SAVR groups using the HADS.69 Rosseel et al found that people who underwent TAVI thought the procedure and recovery period was less mentally stressful compared with people who underwent SAVR.72 Wong et al found that about 20% of people who underwent TAVI experienced anxiety and depression at 3- or 30-day follow-up.74
Satisfaction
Four studies examined patient satisfaction through questionnaires (Table 25).69,70,72,73 Dharmarajan et al found that 97% of people either strongly agreed or agreed that TAVI or SAVR was the right decision for them.70 Amonn et al found that more people who had SAVR reaffirmed their decision to undergo SAVR, than people who had TAVI (94% vs. 87%, P value not reported).69 In another study more people who underwent TAVI would choose TAVI again, compared with people who underwent SAVR (89% vs. 64%; P value not reported).72 Van Mourik et al also found that a high number of people with or without symptomatic improvement (about 91% and 86%) would undergo TAVI again, but the difference between the two groups was not significant (P = .375).73
Table 25:
Satisfaction With Decision on Valve Replacement
| Author, Year | N | Measurement Method | Results |
|---|---|---|---|
| Amonn et al, 201369 | 144 (51 TAVI, 93 SAVR) |
Questionnaire |
|
| Dharmarajan et al, 201770 | 407 (212 TAVI, 124 SAVR, 71 medical therapy) |
Questionnaire |
|
| Rosseel et al, 201972 | 429 (164 TAVI, 265 SAVR) |
Questionnaire |
|
| van Mourik et al, 201873 | 507 TAVI | Questionnaire |
|
Abbreviations: NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Discussion
We found no studies that focused on the population at low surgical risk who had TAVI. All studies were of populations with unspecified risk levels or at primarily (or all) high-risk, and primarily populations of elderly people, limiting the generalizability of results to the population at low surgical risk. We were also unable to conduct subgroup analyses to explore the possible impact of different characteristics of TAVI (e.g., type of procedure or valve, patient characteristics) on the results given the lack of information provided within the studies and the limited number of studies.
Only the study by Marsh et al quantified patients' preferences for different attributes of TAVI and SAVR.71 The primary objective of most studies was not to examine patient preferences and values; therefore, study designs might be inadequate to detect differences between groups. Some studies also did not report levels of significance for differences found between groups for some outcomes.69,70,72,74
In addition, the use of varying questions and tools for measuring outcomes makes comparisons difficult between studies. Only one study69 measured anxiety and depression using a validated screening tool (HADS), and patient satisfaction was also inconsistently measured.
In general, we found that patients value the less invasive nature and faster recovery period of TAVI compared with SAVR. Patient preferences and treatment decision-making could also be influenced by factors such as age, with older people (> 60 years of age) possibly less willing to tolerate the invasiveness of SAVR than younger people. Younger people (< 60 years of age), who are more likely to be at low surgical risk, placed lower value on the invasiveness of a procedure, and higher value on the period over which the procedure has been proven to work, compared with older people. People were generally satisfied with their decision to undergo TAVI.
Conclusions
We did not find any quantitative evidence on patient preferences and values for TAVI within the low-risk surgical group. Among a generally high-risk and elderly population, people preferred the less invasive nature and the faster recovery time of TAVI compared with SAVR and people were satisfied with the TAVI procedure.
Qualitative Evidence
Ontario Health collaborated with the Canadian Agency for Drugs and Technologies in Health (CADTH) to conduct this health technology assessment (HTA). The Agency conducted a review of qualitative literature on perspectives on transaortic valve implantation (TAVI) among patients and health care providers.1 That review identified 13 relevant publications. Key findings from the evidence were:
“When deciding whether to undergo TAVI, patients reflected on the impact of their symptoms on their daily lives. Patients expected TAVI would afford them a longer life and a return to the activities that gave their lives meaning. They considered their prior health care experiences and those of their family members and friends, and how interventions had led to improved health. Although some chose to make the decision alone, many patients involved close family and adult children in their decision making. Trust in their physician helped patients feel confident in the decision-making process and their choice, and reduced worries about risk.
“Patients can encounter logistical challenges when accessing TAVI through the need to travel to specialist centres in urban areas. Patients described the importance of having a caregiver available for them at discharge and during the recovery period.
“Patients described experiencing a ‘new lease on life’ when they had rapid recovery and improvement in symptoms post-TAVI. But patients who had long hospital stays, and/or slow or no symptom improvement from TAVI struggled to reconcile their expectations with their actual experience. Figuring prominent amongst those whose expectations were not met was the way that, post-TAVI, the impact of comorbidities on their health and lives became clearer.
“From the limited information available on health care providers' perspectives, physicians appear to value TAVI for its short recovery time but expressed concerns about the use of TAVI for younger patients due to the lack of long-term data on the durability of valves.”1
Direct Patient Engagement
An HTA by Ontario Health (Quality) about TAVI for patients at intermediate surgical risk46 included findings from interviews with patients and caregivers about the impact of aortic stenosis and their preferences for TAVI or other surgical interventions as treatments. During these interviews, we noted that patients and families were unaware of the patient's surgical risk level and consequently concluded the cohort interviewed likely encompassed patients of all risk levels. We did not pursue direct patient engagement for this HTA because of the unlikelihood of finding patients who were aware they were low risk. Instead, we have summarized the results of the direct engagement evidence previously reported46 and reflected on any similarities and differences in these findings to those from the quantitative preference and qualitative evidence reviews (which were not available previously).
Methods
Engagement Plan
Full details of the engagement plan for the HTA on TAVI for treatment of aortic valve stenosis are found within that report.2 In brief, direct interviews via telephone were completed to examine the experiences of people with aortic valve stenosis and of their families and other caregivers.76
Qualitative interviews were conducted to explore the meaning of central themes in the experiences of people with aortic valve stenosis, as well as those of their families and caregivers.
Participant Outreach
We used an approach called purposive sampling,77–80 which involves actively contacting patients, families, and caregivers with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations and groups involved in offering the TAVI procedure or who were providing care for people with aortic valve stenosis to spread the word about this engagement activity and to make contact with patients, families, and caregivers, including those with experience of TAVI.
Inclusion Criteria
We sought to speak with people and their family or caregivers who had their aortic valve stenosis treated by TAVI or SAVR.
Exclusion Criteria
We did not set exclusion criteria.
Participants
We interviewed 13 persons older than 18 years of age who lived in various parts of Ontario. Participants differed in terms of their socioeconomic background and place of residence. All participants had lived experience of aortic valve stenosis, but they were unable to comment on their surgical risk level. Of the 13 interviewees, 10 were patients and 3 were caregivers. Of the 10 patients, 9 had undergone the TAVI procedure. All interviewees shared their values, preferences, and perspectives about aortic valve stenosis and its treatment.
Approach
At the beginning of the interview, we explained the role of Ontario Health, the purpose of the HTA, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants both verbally and in a printed letter of information (Appendix 11). We then obtained participants' verbal consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews.
Interviews lasted about 20 to 40 minutes. Interviews were semi-structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.81 Questions focused on how aortic valve stenosis affects the quality of life of people with aortic valve stenosis, their experiences with treatments to manage or treat aortic valve stenosis, and their perceptions of the benefits or limitations of TAVI. For family members and caregivers, questions focused on their perceptions of the impact of aortic valve stenosis, as well as the impact of the person's health condition and treatments on the family members and caregivers themselves. See Appendix 12 for our interview guide.
Data Extraction and Analysis
We used a modified version of a grounded-theory method to analyze interview transcripts and survey results. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.82,83 We used the qualitative data analysis software program NVivo to identify and interpret patterns in the data.
Results
The full results of the patient and caregiver interviews are reported in the HTA on TAVI for treatment of aortic valve stenosis.2 Key findings are reported below.
Impact of Aortic Valve Stenosis
Physical Effects
The symptoms of severe aortic stenosis can have a large impact on patients' quality of life. The qualitative literature reported that “Patients described their severe [aortic stenosis] as causing them to struggle with shortness of breath and fatigue, which reduced their ability to engage in social and physical activities and increased their dependence on others.”1 During direct interviews, people with severe aortic valve stenosis described similar situations: reporting shortness of breath, fatigue, and pain in their chest area. Participants said that these symptoms and the underlying condition slowed them down considerably:
I just rested a lot more; … when I got tired, I sat. And I found that … when I went out for walks, I couldn't walk very far, and I had to come back home, that kind of thing.
It was a heaviness, or sometimes it felt like a sharp pain, and then it was gone.
It has made me absolutely … slow down as far as doing things, … but what I used to do in an hour now takes me 4 hours, because I have to go slower.
I was not able to do everything that I normally would do, because I would have the shortness of breath on exertion particularly. I would … have to stop what I was doing and get my breathing back to normal and then continue on.
Some interviewees said that they depended on medications to manage their symptoms:
When I am outside and I'm working in the yard, I constantly draw on my nitro, the spray stuff. And I will use that nitro—I think I've used it three, four times already today. And in the wintertime when it was cold, … I couldn't even walk 80 feet to the end of my driveway without nitro … because the cold air really affects you.
Interviewed participants also mentioned increased dependence on their family or other people to get through their daily activities, similar to what was found in the qualitative literature:
He could not breathe or walk before his surgery. I had to take him to hospital. I had to go with him to help him all the time, could not leave him alone.
I have a husband, and he is a great help, because he does the shopping, he does the dishwasher, and he does all the washing. And without him, I think I would be in trouble, because it would be too much for me.
Psychological Effects
The physical burden of severe aortic stenosis could also affect the emotional and psychological well-being of patients. The qualitative literature reported that “living with severe [aortic stenosis] was challenging emotionally and mentally, and patients often described struggling with feelings of depression, loneliness, and worthlessness.”1 Interviewed participants also noted some psychological distress related to managing their condition:
Well, it was very stressful. You know, hard to breathe, and I didn't have any pain at all, but I couldn't get my breath, and that was the major issue that I had.
I couldn't do much of anything, and I'd barely walk. It sort of isolates you a little bit … because you can't contribute in any way to the everyday life pattern and things to do.
Surgical Aortic Valve Replacement
When asked about currently available treatments, all patients were aware of the “open-heart” SAVR procedure. They understood that it was an effective way of treating aortic valve stenosis, but considered it to be invasive, involving a great deal of pain and a long recovery period.
Treatment Process
Patients who had received SAVR perceived that it treated their condition effectively. Some patients considered SAVR to be the right option for them because of their medical history or other concurrent health conditions:
And [the doctor] felt that … I had low platelet counts and was bleeding easily. … So if I started to bleed, he wouldn't be able to stop the bleeding. … That's why he said we were going to have “to open you up” to do this.
Patients noted that hospital clinicians managed their pain levels well after this invasive procedure:
But [clinicians] manage [pain] very well when you're down in the [intensive care unit] after surgery. They managed the pain levels very well.
Patients and caregivers mentioned the physical and psychological aftermath of open-heart surgery. They described pain, effects on mood, and a difficult and long recovery period as some of the challenges after surgery.
There was definitely the terrifying aftermath to that open-heart surgery; … you have tubes everywhere. You have a large incision. You have a lot of pain. When he was immediately out of surgery, he was frozen like Frosty the Snowman. [W]hen the cooling subsided, Grumpy emerged.
Quite a bit of pain in the chest area, having your chest cracked open.
Recovery and Length of Hospital Stay
Patients noted that SAVR involved a long hospital stay and a longer recovery:
It was a difficult recovery; … it would be more like a couple of months to maybe even 6 months.
Barriers
Financial Barriers
Patients and caregivers noted that the costs involved with travel were an additional burden and a potential barrier to receiving SAVR. Surgery was associated with a longer hospital stay for patients and higher accommodation costs for caregivers. People living in northern Ontario or in remote areas were especially affected by these costs. They noted that the Northern Health Travel Grant did not cover all travel and other associated costs:
Yes, there's cost involved there, for accommodation and meals, and you need family with you at the time.
We're from … the other side of the province. So we had the cost of travel. We had the cost of renting a place to stay, both when he was in the hospital and then also for his postrecovery. He needed to stay 2 more weeks for postsurgical checkups here before we were free to go back. … So it was a month in a rental place. Altogether … our estimated our costs were between $5,000 and $6,000, and only $1,000 of that is covered on [the] travel grant. So we were out of pocket quite a bit.
Psychological Barriers
Because the procedure was invasive, patients expressed anxiety and worry when describing the possible outcomes of SAVR. A few patients chose to forgo SAVR and were waiting for their condition to get worse, so that they could qualify for TAVI:
I didn't want open-heart surgery because I had obvious reasons … like losing my best friend, my skiing buddy, to open-heart surgery. So I … did some research and found out that the TAVI procedure was available.
Patients and caregivers also spoke about reasons SAVR was not the right option for them, including age, and concurrent health conditions:
She could not stand up with a pillow on her chest. She has trouble just getting out of a chair. We have a stand-up chair for her to get to her feet here at the house. But if they were to do the open-heart procedure, she would [spend] the first year and a half having to hold a pillow to her every time she moved. She can't do it.
Limitations
A few patients indicated that they had had a valve rupture several years after their SAVR procedure and had to have another procedure to replace the ruptured valve:
I had valve replacement 11 years before my TAVI. The valve split on the side and was spraying blood on my lungs. I went to the emergency … my valve was split … and I was given TAVI.
Transcatheter Aortic Valve Implantation
The qualitative literature reported that many patients had positive expectations of the TAVI procedure and that it would help to relieve some of the symptoms of severe aortic stenosis. Patients “hoped that they would live longer as a result of undergoing TAVI, but also hoped that they would have a ‘new lease on life.’ This ‘new lease’ was described by patients as being an improvement in their symptoms, offering the ability to increase their independence and resume their daily and social activities.”1
Treatment Process
Interviewed patients said the TAVI procedure was explained very well to them. The qualitative literature indicated that patients' trust in their health care provider made the treatment decision easier; “Patients often expressed trust in their physicians' recommendation for TAVI and their skills as experts when sharing that they were not worried about undergoing TAVI.”1 Interviewed participants understood that the valve was to be inserted by a catheter, and that the entry point could be either through the chest or near the groin:
And he brought in the actual catheter that they would use, … an example. And he had vials containing … examples of the valves. And he went into quite a lot of detail.
He explained to me [the catheter went] up through the groin—sometimes I guess they go in under your arm. In my case … the doctor… said we're going to do the groin route with you.
Patients perceived the pain involved with TAVI to be much less than with SAVR. A few patients said, “There is no comparison” between the procedures, because in SAVR “they open your ribs wide open,” alluding to its invasiveness. Similar to the findings of the qualitative literature presented above, participants valued the less invasive TAVI procedure compared with SAVR.
Patients with TAVI experience who had had the implantation done through the chest or the groin reported less pain than with SAVR:
And I didn't have any pain afterwards at all. I didn't even know that I'd had incisions in my groin. I just didn't know it was there. It was amazing.
[It was] definitely less painful, [far] less painful, because … they usually go with the catheter in the groin. I couldn't have it; … my arteries were too small, so he put it up in the chest, and as I said, you just have one little cut where the catheter goes in. We had no patient(s) who had experienced TAVI via both the chest and groin.
Recovery and Length of Hospital Stay
Patients mentioned they thought that their length of hospital stay and hospital recovery period was probably shorter with TAVI than if they had SAVR because they perceived SAVR as being more invasive and needing a longer recovery. Participants indicated that they valued the shorter recovery time a TAVI procedure required, which is consistent with what was found in the qualitative literature presented above.
They had the telemetry on me all the time; they were monitoring the heart. [T]he second day … the surgeon … who did it came in [and said], we are watching the telemetry and there's just no problem at all, so there's no point in your staying here. I'm going to send you home tomorrow afternoon, and that was the third day.
Two days later I was discharged.
Your recuperating time is a lot less, because you don't have major surgery. I mean, … it's not having to have your breastbone opened … it's more invasive with open-heart [surgery], so [TAVI is] easier on the body than [open-heart surgery].
Anesthesia
Some patients reported that they were “put out completely,” referring to general anesthesia, while others mentioned that they did not even have “complete anesthesia.” All patients who had TAVI done through the chest indicated that they had general anesthesia.
Benefits
Interviewed participants reported that the TAVI procedure helped them address the problems they were having with aortic valve stenosis. They thought that it improved their quality of life more quickly than SAVR would:
I went into the hospital the day before, had the procedure in the afternoon. [By] the evening, I was sitting in bed having a sandwich.
I honestly can say that … I think I wouldn't even be alive if it wasn't for that valve, right now, and my quality of life is great.
Similar sentiments were expressed in the qualitative literature, as “those patients who had a quick recovery described TAVI as easy, and were pleasantly surprised by how fast they recovered from the procedure and how it immediately improved their [aortic stenosis] symptoms.”1 The symptoms of breathlessness in particular were reported to have greatly improved.
Barriers
Cost
Patients described the costs involved with the procedure as personal costs related to travel, accommodation, and parking. Patients who lived further TAVI centres, including in remote and northern parts of Ontario, were most affected by associated travel, accommodations and meal costs. However, given the shorter length of hospital stay, they perceived these costs to be much lower than what they would have incurred with SAVR:
My family wanted to be there when I had the surgery, so there was … overnight accommodation … and meals, and so on. And someone to help with the driving … It was basically … personal expenses
Access
Patients living further from TAVI centres also reported greater difficulty accessing the procedure. Access to TAVI was expressed as a challenge in the qualitative literature as well. Some patients “described the logistical challenges of TAVI. These involved travel to a specialized TAVI centre for assessment and the procedure, and those who lived far away from the centre expressed concern about the costs of accommodation, long-distance travel, and the need to find someone to accompany them.”1
Preferences and Values Evidence Discussion
People with severe aortic valve stenosis shared their experiences of the burden of their health condition on their daily life and their relationships. Interviewees were unable to comment on their surgical risk level, but all were able to share their perceptions about the TAVI and SAVR procedures. The quantitative and qualitative literature reinforced key findings of patient and caregiver perceptions surrounding the burden of severe aortic valve stenosis as well as some of the decision-making and expectations of the TAVI procedure reported in the HTAs on TAVI.2,46
Interviewees identified open-heart SAVR as the currently available treatment for people with aortic valve stenosis. Most thought that SAVR met their needs by improving their condition after the surgery. However, they mentioned that the pain and slow recovery period resulting from the invasive nature of SAVR made them dependent on their family and reduced their quality of life after the procedure.
People who had experienced TAVI indicated both through direct interview and in the qualitative literature that it generally resolved their medical condition and met their needs by minimizing their pain and their recovery period. Those interviewed reported that TAVI enhanced their quality of life by making it possible for them to get back to their usual activities more quickly than SAVR would. In addition to these findings, which were mostly consistent with the qualitative literature review, people living in northern and remote areas of the province reported lower out-of-pocket costs for travel, meal, and accommodation with TAVI. This was mostly because of the reduced length of hospital stay and fewer follow-up visits with TAVI than with SAVR.
Preferences and Values Evidence Conclusions
The previous HTA about TAVI for patients at intermediate surgical risk included findings about the impact of aortic stenosis and the preferences and benefits of TAVI from the perspective of patients and caregivers. Because patients and families were unaware of the patient's surgical risk, it is likely that these interviews encompassed patients of all risk levels. Given this fact, we did not pursue direct patient engagement for this HTA, but summarized the results previously reported.46 However, we were able to demonstrate great overlap and consistency between these results and the new quantitative and qualitative literature evidence presented.
We did not find any quantitative evidence on patient preferences and values for TAVI specific to the low-risk surgical group. Among a mixed or generally high-risk and elderly population, people typically preferred the less invasive nature and the faster recovery time of TAVI compared with SAVR and people were satisfied with the TAVI procedure.
Patients and caregivers perceived that TAVI minimized pain and recovery time involved with the procedure. Most patients returned to their usual activities more quickly than they would have if they had had SAVR. The direct patient and caregiver consultations indicated a preference for TAVI over SAVR.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
Both TAVI (transfemoral route) and SAVR resulted in improvement in patient symptoms and quality of life during the 1 year of follow-up. Short-term clinical outcomes (30 days) tended to favour TAVI over SAVR, though differences were small. Longer follow-up is needed to draw definitive conclusions on the long-term outcomes of TAVI compared with SAVR after 1 year.
The TAVI procedure might be cost-effective for patients at low surgical risk; however, there is some uncertainty in this result. We estimated that the additional cost to provide public funding for TAVI for people with severe aortic valve stenosis at low surgical risk would range from about $5 to $8 million over the next 5 years.
We did not find any quantitative evidence on patient preferences and values for TAVI within the low-risk surgical group. Among a mixed or generally high-risk and elderly population, people typically preferred the less invasive nature and the faster recovery time of TAVI compared with SAVR, and people were satisfied with the TAVI procedure.
The quantitative and qualitative evidence supported the findings of direct patient engagement reported in a previous HTA.2 Patients and caregivers perceived that TAVI minimized pain and recovery time involved with the procedure. Most patients returned to their usual activities more quickly than patients who had had SAVR. The direct patient and caregiver consultations indicated a preference for TAVI over SAVR.
Acknowledgments
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologists were Vania Costa and Myra Wang, the primary health economist was Lindsey Falk, the secondary health economist was Sean Tiggelaar, the patient and public partnership analyst was David Wells, and the medical librarians were Melissa Walter and Corinne Holubowich.
The medical editor was Elizabeth Betsch. Others involved in the development and production of this report were Paul Kolodziej, Claude Soulodre, Kara Cowan, Saleemeh Abdolzahraei, Sarah McDowell, Vivian Ng, Andrée Mitchell, Amy Lang, Nancy Sikich, Charles de Mestral, and Irfan Dhalla.
To support this report, the Canadian Agency for Drugs and Technologies in Health (CADTH) conducted a rapid qualitative review of patient preferences, published separately.1 From CADTH, the qualitative review authors were Andrea Smith and Charlene Argaez.
We would like to thank the following people and organizations for lending their expertise to the development of this report:
Christopher Feindel, Peter Munk Cardiac Centre, University Health Network
Laurie Lambert, Unité d'Evaluation Cardiovasculaire, Institut National d'Excellence en Santé et en Services Sociaux
Mark Osten, Peter Munk Cardiac Centre, University Health Network
Derrick Tam, Schulich Heart Centre, Sunnybrook Health Sciences Centre
James L. Velianou, Structural and Interventional Cardiology, Hamilton Health Sciences and McMaster University
Harindra Wijeysundera, Schulich Heart Centre, Sunnybrook Health Sciences Centre
Edwards Lifesciences (Canada) Inc.
Medtronic Canada
We are also grateful to CorHealth Ontario for lending their expertise to the development of this report and the Institut National d'Excellence en Santé et en Services Sociaux (INESSS) for their collaboration on this project.
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
ABBREVIATIONS
- BCrI
Bayesian credible interval
- CABG
Coronary artery bypass grafting
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CI
Confidence interval
- CT
Computed tomography
- DKK
Danish kroner
- EuroQol-5D
European Quality of Life questionnaire in five dimensions
- ECG
Electrocardiogram
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HADS
Hospital Anxiety and Depression Scale
- HR
Hazard ratio
- HTA
Health technology assessment
- ICER
Incremental cost-effectiveness ratio
- IQR
Interquartile range
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NYHA
New York Heart Association
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QALY
Quality-adjusted life-year
- RCT
Randomized controlled trial
- SAVR
Surgical aortic valve replacement
- SD
Standard deviation
- STS
Society of Thoracic Surgeons
- TAVI
Transcatheter aortic valve implantation
GLOSSARY
- Adverse event
An adverse event is an unexpected medical problem that happens during treatment for a health condition. Adverse events may be caused by something other than the treatment.
- Bootstrap method
The bootstrap method is a resampling technique used to estimate statistics on a population by sampling a dataset with replacement.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cohort model
In economic evaluations, a cohort model is used to simulate what happens to a homogeneous cohort (group) of patients after receiving a specific health care intervention. The proportion of the cohort who experiences certain health outcomes or events is estimated, along with the relevant costs and benefits. In contrast, a microsimulation model follows the course of individual patients.
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness analysis
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost-utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost-utility analysis
A cost-utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost-utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Discounting
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Ontario Health use an annual discount rate of 1.5% for both future costs and future benefits.
- Disutility
A disutility is a decrease in utility (i.e., a decrease in preference for a particular health outcome) typically resulting from a particular health condition (e.g., experiencing a symptom or complication).
- European Quality of Life Questionnaire in Five Dimensions (EQ-5D)
The EQ-5D is a generic health-related quality-of-life classification system widely used in clinical studies. In economic evaluations, it is used as an indirect method of obtaining health state preferences (i.e., utility values). The EQ-5D questionnaire consists of five questions relating to different domains of quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. For each domain, there are three response options: no problems, some problems, or severe problems. A newer instrument, the EQ-5D-5L, includes five response options for each domain. A scoring table is used to convert EQ-5D scores to utility values.
- Health-related quality of life
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Ministry of Health perspective
The perspective adopted in economic evaluations determines the types of costs and health benefits to include. Ontario Health develops health technology assessment reports from the perspective of the Ontario Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- Probabilistic sensitivity analysis (PSA)
A probabilistic sensitivity analysis (PSA) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost-utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Risk difference
Risk difference is the difference in the risk of an outcome occurring between one health care intervention and an alternative intervention.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Time horizon
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost- utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: July 9, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews − Cochrane Central Register of Controlled Trials <June 2019>, EBM Reviews −Cochrane Database of Systematic Reviews <2005 to July 3, 2019>, EBM Reviews − Health Technology Assessment <4th Quarter 2016>, EBM Reviews − NHS Economic Evaluation Database < 1st Quarter 2016 >, Embase <1980 to 2019 Week 27>, Ovid MEDLINE(R) ALL <1946 to July 08, 2019>
Search strategy:
————————————————————————-
-
1
Aortic valve/ (32771)
-
2
exp Aortic Valve Stenosis/ (42509)
-
3
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).ti,ab,kf. (51433)
-
4
(aortic valv∗ adj3 disease∗).ti,ab,kf. (9003)
-
5
or/1–4 (104397)
-
6
Heart Valve Prosthesis Implantation/ (32680)
-
7
Heart Valve Prosthesis/ (52181)
-
8
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).ti,ab,kf. (108999)
-
9
or/6–8 (160135)
-
10
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).ti,ab,kf. (476392)
-
11
9 and 10 (33771)
-
12
Transcatheter aortic valve implantation/ (22434)
-
13
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Evolut PRO∗ or Lotus Edge∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).ti,ab,kf. (22969)
-
14
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).ti,ab,kf. (232)
-
15
or/11–14 (39742)
-
16
5 and 15 (19805)
-
17
((low or lower or lesser or minor) adj3 risk∗).ti,ab,kf. (328258)
-
18
15 and 17 (1660)
-
19
16 or 18 (20413)
-
20
(Systematic Reviews or Meta Analysis).pt. (103092)
-
21
Systematic Review/ or Systematic Reviews as Topic/ or Meta-Analysis/ or exp Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (538873)
-
22
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).ti,ab,kf. (377691)
-
23
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab,kf. (389112)
-
24
(evidence adj (review∗ or overview∗ or synthes#s)).ti,ab,kf. (14481)
-
25
(review of reviews or overview of reviews).ti,ab,kf. (1314)
-
26
umbrella review∗.ti,ab,kf. (538)
-
27
GRADE Approach/ (171)
-
28
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).ti,ab,kf. (420217)
-
29
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (428359)
-
30
cochrane.ti,ab,kf. (185625)
-
31
(meta regress∗ or metaregress∗).ti,ab,kf. (17617)
-
32
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).ti,ab,kf. (23897)
-
33
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (62053)
-
34
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).ti,ab,kf. (49119)
-
35
Clinical Trials as Topic/ (293982)
-
36
controlled clinical trials as topic/ (14411)
-
37
exp Randomized Controlled Trials as Topic/ (298820)
-
38
controlled clinical trial.pt. (184129)
-
39
randomized controlled trial.pt. (959381)
-
40
Pragmatic Clinical Trial.pt. (2160)
-
41
Random Allocation/ (199297)
-
42
Single-Blind Method/ (80184)
-
43
Double-Blind Method/ (409805)
-
44
Placebos/ (324300)
-
45
trial.ti. (752244)
-
46
(random∗ or sham or placebo∗ or RCT∗1).ti,ab,kf. (3750561)
-
47
((singl∗ or doubl∗) adj (blind∗ or dumm∗ or mask∗)).ti,ab,kf. (631972)
-
48
((tripl∗ or trebl∗) adj (blind∗ or dumm∗ or mask∗)).ti,ab,kf. (3380)
-
49
or/20–48 (5506042)
-
50
19 and 49 (2941)
-
51
limit 50 to english language [Limit not valid in CDSR; records were retained] (2767)
-
52
limit 51 to yr=“2015 −Current” (1784)
-
53
52 use medall,cleed (672)
-
54
limit 19 to english language [Limit not valid in CDSR; records were retained] (19448)
-
55
limit 54 to yr=“2015 −Current” (11187)
-
56
55 use coch,cctr,clhta (392)
-
57
53 or 56 (1064)
-
58
aortic valve/ (32771)
-
59
exp aortic stenosis/ (47654)
-
60
aortic valve disease/ (1067)
-
61
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).tw,kw. (51901)
-
62
(aortic valv∗ adj3 disease∗).tw,kw. (8925)
-
63
or/58–62 (107738)
-
64
aorta valve replacement/ (18518)
-
65
exp aortic valve prosthesis/ (4074)
-
66
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).tw,kw,dv. (109464)
-
67
or/64–66 (115516)
-
68
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or transapical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).tw,kw,dv. (486144)
-
69
67 and 68 (31359)
-
70
transcatheter aortic valve implantation/ (22434)
-
71
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Evolut PRO∗ or Lotus Edge∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).tw,kw,dv. (24576)
-
72
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).tw,kw,dv. (233)
-
73
or/69–72 (37351)
-
74
63 and 73 (21040)
-
75
low risk patient/ (9449)
-
76
low risk population/ (8538)
-
77
((low or lower or lesser or minor) adj3 risk∗).tw,kw. (334902)
-
78
or/75–77 (339328)
-
79
73 and 78 (1771)
-
80
74 or 79 (21636)
-
81
Systematic review/ or “systematic review (topic)“/ or exp Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (532144)
-
82
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗ or systematic review∗).hw. (546491)
-
83
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).tw,kw. (392876)
-
84
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).tw,kw. (421353)
-
85
(evidence adj (review∗ or overview∗ or synthes#s)).tw,kw. (14868)
-
86
(review of reviews or overview of reviews).tw,kw. (1501)
-
87
umbrella review∗.tw,kw. (577)
-
88
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).tw,kw. (445748)
-
89
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (428359)
-
90
cochrane.tw,kw. (189413)
-
91
(meta regress∗ or metaregress∗).tw,kw. (18525)
-
92
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).tw,kw. (24806)
-
93
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (62053)
-
94
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).tw,kw. (66521)
-
95
“clinical trial (topic)”/ (102563)
-
96
“controlled clinical trial (topic)”/ (10203)
-
97
“randomized controlled trial (topic)”/ (163713)
-
98
randomization/ (182427)
-
99
Single Blind Procedure/ (35615)
-
100
Double Blind Procedure/ (159317)
-
101
placebo/ (323238)
-
102
trial.ti. (752244)
-
103
(random∗ or sham or placebo∗ or RCT∗1).tw,kw. (3811853)
-
104
((singl∗ or doubl∗) adj (blind∗ or dumm∗ or mask∗)).tw,kw. (661032)
-
105
((tripl∗ or trebl∗) adj (blind∗ or dumm∗ or mask∗)).tw,kw. (3914)
-
106
or/81–105 (5155577)
-
107
80 and 106 (3023)
-
108
limit 107 to english language [Limit not valid in CDSR; records were retained] (2846)
-
109
limit 108 to yr=“2015 −Current” (1887)
-
110
109 use emez (1029)
-
111
57 or 110 (2093)
-
112
111 use medall (672)
-
113
111 use coch (3)
-
114
111 use cctr (367)
-
115
111 use clhta (22)
-
116
111 use cleed (0)
-
117
111 use emez (1029)
-
118
remove duplicates from 111 (1363)
Economic Evidence Search
Search date: July 12, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews − Cochrane Central Register of Controlled Trials <June 2019>, EBM Reviews −Cochrane Database of Systematic Reviews <2005 to July 10, 2019>, EBM Reviews − Health Technology Assessment <4th Quarter 2016>, EBM Reviews − NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 27>, Ovid MEDLINE(R) ALL <1946 to July 10, 2019>
Search strategy:
————————————————————————-
-
1
Aortic valve/ (32780)
-
2
exp Aortic Valve Stenosis/ (42533)
-
3
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).ti,ab,kf. (51442)
-
4
(aortic valv∗ adj3 disease∗).ti,ab,kf. (9003)
-
5
or/1–4 (104430)
-
6
Heart Valve Prosthesis Implantation/ (32687)
-
7
Heart Valve Prosthesis/ (52191)
-
8
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).ti,ab,kf. (109010)
-
9
or/6–8 (160154)
-
10
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).ti,ab,kf. (476414)
-
11
9 and 10 (33780)
-
12
Transcatheter aortic valve implantation/ (22439)
-
13
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Evolut PRO∗ or Lotus Edge∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).ti,ab,kf. (22980)
-
14
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).ti,ab,kf. (232)
-
15
or/11–14 (39753)
-
16
5 and 15 (19819)
-
17
((low or lower or lesser or minor) adj3 risk∗).ti,ab,kf. (328288)
-
18
15 and 17 (1662)
-
19
16 or 18 (20427)
-
20
economics/ (252608)
-
21
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (827409)
-
22
economics.fs. (421271)
-
23
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (882678)
-
24
exp “costs and cost analysis“/ (577755)
-
25
(cost or costs or costing or costly).ti. (263021)
-
26
cost effective∗.ti,ab,kf. (323987)
-
27
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (212842)
-
28
models, economic/ (12705)
-
29
markov chains/ or monte carlo method/ (80263)
-
30
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (42088)
-
31
(markov or markow or monte carlo).ti,ab,kf. (128351)
-
32
quality-adjusted life years/ (39616)
-
33
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (73079)
-
34
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (119049)
-
35
or/20–34 (2535216)
-
36
19 and 35 (814)
-
37
Case Reports/ (2030830)
-
38
36 not 37 (811)
-
39
exp Animals/ not Humans/ (17181898)
-
40
38 not 39 (408)
-
41
limit 40 to english language [Limit not valid in CDSR; records were retained] (379)
-
42
limit 41 to yr=“2015 −Current” (209)
-
43
42 use medall,coch,cctr,clhta (165)
-
44
limit 19 to english language [Limit not valid in CDSR; records were retained] (19462)
-
45
limit 44 to yr=“2015 −Current” (11201)
-
46
45 use cleed (0)
-
47
43 or 46 (165)
-
48
Heart Valve Prosthesis Implantation/ (32687)
-
49
aortic valve/ (32780)
-
50
exp aortic stenosis/ (47678)
-
51
aortic valve disease/ (1067)
-
52
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).tw,kw. (51911)
-
53
(aortic valv∗ adj3 disease∗).tw,kw. (8924)
-
54
or/48–53 (127532)
-
55
aorta valve replacement/ (18518)
-
56
exp aortic valve prosthesis/ (4074)
-
57
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).tw,kw,dv. (109472)
-
58
or/55–57 (115524)
-
59
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).tw,kw,dv. (486168)
-
60
58 and 59 (31366)
-
61
transcatheter aortic valve implantation/ (22439)
-
62
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Evolut PRO∗ or Lotus Edge∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).tw,kw,dv. (24587)
-
63
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).tw,kw,dv. (233)
-
64
or/60–63 (37360)
-
65
54 and 64 (21958)
-
66
low risk patient/ (9449)
-
67
low risk population/ (8538)
-
68
((low or lower or lesser or minor) adj3 risk∗).tw,kw. (334938)
-
69
or/66–68 (339364)
-
70
64 and 69 (1773)
-
71
65 or 70 (22526)
-
72
Economics/ (252608)
-
73
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (128461)
-
74
Economic Aspect/ or exp Economic Evaluation/ (452848)
-
75
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (908380)
-
76
exp “Cost“/ (577755)
-
77
(cost or costs or costing or costly).ti. (263021)
-
78
cost effective∗.tw,kw. (336297)
-
79
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (223901)
-
80
Monte Carlo Method/ (63925)
-
81
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (45894)
-
82
(markov or markow or monte carlo).tw,kw. (133410)
-
83
Quality-Adjusted Life Years/ (39616)
-
84
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (76914)
-
85
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (139746)
-
86
or/72–85 (2172888)
-
87
71 and 86 (879)
-
88
Case Report/ (4305487)
-
89
87 not 88 (871)
-
90
(exp animal/ or nonhuman/) not exp human/ (10343947)
-
91
89 not 90 (867)
-
92
limit 91 to english language [Limit not valid in CDSR; records were retained] (830)
-
93
limit 92 to yr=“2015 −Current” (482)
-
94
93 use emez (300)
-
95
47 or 94 (465)
-
96
95 use medall (151)
-
97
95 use coch (0)
-
98
95 use cctr (13)
-
99
95 use clhta (1)
-
100
95 use cleed (0)
-
101
95 use emez (300)
-
102
remove duplicates from 95 (339)
Quantitative Evidence of Preferences and Values Search
Search date: July 18, 2019
Databases searched: Ovid MEDLINE
Search filter used: Quantitative preference evidence filter, modified from Selva et al.68
Database: Ovid MEDLINE(R) ALL <1946 to July 17, 2019>
Search Strategy:
-
1
Aortic valve/ (29693)
-
2
exp Aortic Valve Stenosis/ (39423)
-
3
((supravalvular or supra valvular or subvalvular or “sub valvular” or aorta or aortic or aortal) adj3 stenos?s).ti,ab,kf. (21168)
-
4
(aortic valv∗ adj3 disease∗).ti,ab,kf. (4035)
-
5
or/1–4 (67532)
-
6
Heart Valve Prosthesis Implantation/ (20618)
-
7
Heart Valve Prosthesis/ (33919)
-
8
(((aorta or aortic or aortal) adj4 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)) or avr).ti,ab,kf. (45090)
-
9
or/6–8 (75265)
-
10
(transcatheter∗ or trans-catheter∗ or transfemoral∗ or trans-femoral∗ or transapical∗ or trans-apical∗ or transarterial∗ or trans-arterial∗ or transcutaneous∗ or trans-cutaneous∗ or transsubclavian∗ or trans-subclavian∗ or transvascular∗ or trans-vascular∗ or transaxillar∗ or trans-axillar∗ or transluminal∗ or trans-luminal∗ or transcarotid∗ or trans-carotid∗ or transiliac∗ or trans-iliac∗ or transiliofemoral∗ or trans-iliofemoral∗ or percutaneous∗).ti,ab,kf. (185064)
-
11
9 and 10 (12793)
-
12
Transcatheter aortic valve implantation/ (3820)
-
13
(core-valve∗ or corevalve∗ or Edwards Sapien∗ or Sapien XT∗ or SapienXT∗ or Sapien 3∗ or Sapien3∗ or Evolut R∗ or Evolut PRO∗ or Lotus Edge∗ or Acurate Neo∗ or Symetis or TAVI or TAVR).ti,ab,kf. (7006)
-
14
((transaortic∗ or trans-aortic∗) adj2 valve adj2 (replac∗ or implant∗ or prosthe∗ or bioprosthe∗ or transplant∗ or insert∗ or surger∗)).ti,ab,kf. (74)
-
15
or/11–14 (13789)
-
16
5 and 15 (8854)
-
17
Attitude to Health/ (81835)
-
18
Health Knowledge, Attitudes, Practice/ (104072)
-
19
Patient Participation/ (24126)
-
20
exp Patient Satisfaction/ (84472)
-
21
Choice Behavior/ (31073)
-
22
(choice or choices or value∗ or valuation∗).ti. (191023)
-
23
(preference∗ or expectation∗ or attitude∗ or point of view).ti,ab. (388284)
-
24
((patient∗1 or user∗1 or men or women or personal) adj2 (participation or perspective∗ or perception∗ or misperception∗ or perceiv∗ or view∗ or value∗1 or acceptab∗ or satisfaction or dissatisfaction or satisfy∗ or dissatisfy∗ or satisfied or dissatisfied)).ti,ab. (128453)
-
25
health perception∗.ti,ab. (2548)
-
26
Stress, Psychological/ (114594)
-
27
(psycholog∗ or psychosocial or psycho social or emotion∗ or anxiet∗ or anxious∗ or worry∗ or worries or distress∗).ti,ab. (663827)
-
28
∗Decision Making/ (39554)
-
29
(patient∗1 or user∗1 or men or women or personal).ti. (2091060)
-
30
28 and 29 (5663)
-
31
(decision∗ and mak∗).ti. (26733)
-
32
(decision mak∗ or decisions mak∗).ti,ab. (128142)
-
33
31 or 32 (129612)
-
34
(patient∗1 or user∗1 or men or women or personal).ti,ab. (7182004)
-
35
33 and 34 (70223)
-
36
(discrete choice∗ or decision board∗ or decision analy∗ or decision-support or decision tool∗ or decision aid∗ or latent class∗ or decision∗ conflict∗ or decision∗ regret∗).ti,ab. (30868)
-
37
Decision Support Techniques/ (19010)
-
38
(health and utilit∗).ti. (1365)
-
39
(gamble∗ or prospect theory or health utilit∗ or utility value∗ or utility score∗ or utility estimate∗ or health state or feeling thermometer∗ or best-worst scaling or time trade-off or TTO or probability trade-off).ti,ab. (12245)
-
40
(preference based or preference score∗ or preference elicitation or multiattribute or multi attribute).ti,ab. (2569)
-
41
or/17–27,30,35–40 (1605925)
-
42
16 and 41 (439)
-
43
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congresses.pt. (3564342)
-
44
42 not 43 (393)
-
45
limit 44 to english language (375)
-
46
limit 45 to yr=“2002 −Current” (372)
Grey Literature Search
Performed: July 8–11, 2019
Websites searched:
HTA Database Canadian Repository, Alberta Health Evidence Reviews, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Universite de Quebec-Universite Laval, Health Technology Assessment Database, Epistemonikos, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Council of Australian Governments Health Technologies, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, Health Technology Wales, Oregon Health Authority Health Evidence Review Commission, Veterans Affairs Health Services Research and Development, Italian National Agency for Regional Health Services (AGENAS), Australian Safety and Efficacy Register of New Interventional Procedures −Surgical (ASERNIP-S), Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Ministry of Health Malaysia Health Technology Assessment Section, Swedish Agency for Health Technology Assessment and Assessment of Social Services, ClinicalTrials.gov, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used: Aort∗, transcatheter, TAVI, TAVR
Results from clinical search: (included in PRISMA): 4
Results from economic search: (included in PRISMA): 0
Ongoing systematic reviews (PROSPERO/EUnetHTA): 8
Ongoing clinical trials (ClinicalTrials.gov): 4
Appendix 2: Study Design and Characteristics
Table A1:
Study Design and Characteristics
| Author, Year N (TAVI/SAVR) Enrollment Period |
Study Design Statistical Analysis |
Patient Population | Intervention | Comparator | Outcomes |
|---|---|---|---|---|---|
| Mack et al, 201918 1,000 (503/497) 2016–2017 PARTNER 3 |
|
Exclusion
|
|
|
Primary Outcome: Noninferiority Composite of all-cause mortality, stroke, or rehospitalizationa at 1 yb Secondary Outcomes Effectiveness
Safety
|
| Popma et al, 201917 1,468 (734/734) 2016–2018 Evolut LRT |
|
|
Self-expanding TAVI inserted via transfemoral route (CoreValve, Evolut R, Evolut PRO valve) |
SAVR (bioprosthetic valve) |
Primary Safety and Effectiveness Outcome: Noninferiority
Secondary Outcomes Effectiveness
Safety
|
Abbreviations: AVA, aortic valve area; CT, computed tomography; ECG, electrocardiogram; EQ-5D, European Quality of Life in Five Dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire; ICU; intensive care unit; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SAE, serious adverse event; SAVR, surgical aortic valve replacement; SF-36, Medical Outcomes Study 36-question Short-Form Health Survey; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Any hospitalization related to procedure, valve, or heart failure.
End points adjusted for multiple comparisons at 30-day timepoint.
All patients underwent neurologic examinations at baseline and at 30 days. In case of suspected stroke after procedure, neurologic examinations including National Institutes of Health Stroke Scale assessment and modified Rankin scale at 90 days after event were performed.
Appendix 3: Baseline Patient Characteristics
Table A2:
Baseline Patient Characteristics
| Author, Year N (TAVI/SAVR) | Mean Age (SD; Range), y | Male Sex, n (%) | Ethnicity, n (%) | Mean STS Score, % (SD; Range) | NYHA Class, n (%) | Coronary Artery Disease, n (%) | Mean LVEF (SD), % |
|---|---|---|---|---|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
TAVI: 73.3 (5.8; 53–87) SAVR: 73.6 6.1; 50–85) |
TAVI: 335 (67.5) SAVR: 323 (71.1) |
Nonwhite or ethnic group TAVI: 38 (7.7) SAVR: 45 (9.9) |
TAVI: 1.9 (0.7; 0.6–3.8) SAVR: 1.9 (0.6; 0.5–3.9) |
< III-IV TAVI: 341 (68.8) SAVR: 346 (76.2) III-IV TAVI: 155 (31.2) SAVR: 108 (23.8) |
TAVI: 137/494 (27.7) SAVR: 127/454 (28.0) |
TAVI: 65.7 (9.0) SAVR: 66.2 (8.6) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 74.1 (5.8) SAVR: 73.6 (5.9) |
TAVI: 464 (64.0) SAVR: 449 (66.2) |
Not reported | TAVI: 1.9 (0.7) SAVR: 1.9 (0.7) |
I-II TAVI: 543 (74.9) SAVR: 485 (71.5) III-IV TAVI: 182 (25.1) SAVR: 193 (28.4) |
Not reported | TAVI: 61.7 (7.9) SAVR: 61.9 (7.7) |
Abbreviations: LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; SD, standard deviation; STS; Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Appendix 4: New York Heart Association Functional Classification
Under the New York Heart Association (NYHA) Functional Classification system, the heart failure class (I-IV) is defined according to the severity of the patient's symptoms and how much the patient is limited during physical activity, as follows:38
I: No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, or dyspnea (shortness of breath)
II: Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, or dyspnea
III: Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea
IV: Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases
Appendix 5: Study Results
Table A3:
NYHA Functional Class
| Author, Year N (TAVI/SAVR) | Baseline | 30 Days | 1 Year |
|---|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
N = 950 (496 TAVI/454 SAVR) NYHA I, n (%) TAVI: 2 (0.4) SAVR: 7 (1.5) NYHA II, n (%) TAVI: 339 (68.3) SAVR: 339 (74.7) NYHA III, n (%) TAVI: 152 (30.6) SAVR: 103 (22.7) NYHA IV, n (%) TAVI: 3 (0.6) SAVR: 5 (1.1) |
N = 926 (493 TAVI/433 SAVR) NYHA I, n (%) TAVI: 396 (80.3) SAVR: 289 (66.7) RD, % (95% CI) −13.4 (−19.2 to −7.9) NYHA II, n (%) TAVI: 339 (68.3) SAVR: 339 (74.7) NYHA III, n (%) TAVI: 91 (18.5) SAVR: 125 (28.9) NYHA IV, n (%) TAVI: 6 (1.2) SAVR: 19 (44) |
N = 887 (480 TAVI/407 SAVR) NYHA I, n (%) TAVI: 395 (82.3) SAVR: 339 (83.3) RD, % (95% CI) 1.0 (−4.0 to 6.0) NYHA II, n (%) TAVI: 339 (16.7) SAVR: 339 (74.7) NYHA III, n (%) TAVI: 80 (16.7) SAVR: 62 (15.2) NYHA IV, n (%) TAVI: 5 (1.0) SAVR: 6 (1.5) |
| Popma et al, 2019 1,403 (725/678) Evolut LRT |
N = 1,403 (725 TAVI/678 SAVR) NYHA I, n (%) AVI: 76 (10.5) SAVR: 63 (9.3) NYHA II, n (%) TAVI: 467 (64.4) SAVR: 422 (62.2) NYHA III, n (%) TAVI: 181 (25.0) SAVR: 190 (28.0) NYHA IV, n (%) TAVI: 1 (0.1) SAVR: 3 (0.4) |
N = 1,331 (706 TAVI/625 SAVR) NYHA I, n (%) TAVI: 545 (77.2) SAVR: 416 (66.6) NYHA II, n (%) TAVI: 149 (21.1) SAVR: 179 (28.6) NYHA III, n (%) TAVI: 12 (1.7) SAVR: 29 (4.6) NYHA IV, n (%) TAVI: 0 SAVR: 1 (0.2) |
N = 770 (428 TAVI/342 SAVR) Mean change from baseline (SD) TAVI: 0.9 (0.7) SAVR: 1.0 (0.7) Mean difference (95% BCrI) −0.1 (−0.2 to 0.0) NYHA I, n (%) TAVI: 336 (78.5) SAVR: 279 (81.6) NYHA II, n (%) TAVI: 84 (19.6) SAVR: 59 (17.3) NYHA III, n (%) TAVI: 7 (1.6) SAVR: 4 (1.2) NYHA IV, n (%) TAVI: 1 (0.2) SAVR: 0 |
Abbreviations: BCrI, Bayesian credible interval; NYHA, New York Heart Association; RD, risk difference; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
Table A4:
Quality of Life
| Author, Year N (TAVI/SAVR) | 30 Days Adjusteda Mean Difference TAVI vs. SAVR (95% CI) |
6 Months Adjusted a Mean Difference TAVI vs. SAVR (95% CI) |
1 Year n (%) |
|---|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KCCQ Physical Limitations 14.0 (12.0 to 15.9) KCCQ Social Limitations 23.9 (21.1 to 26.6) SF-36 Physical Summary 7.7 (6.8 to 8.6) SF-36 Mental Summary 4.1 (3.1 to 5.1) EQ-5D Utilities 0.07 (0.06 to 0.09) P < .001 for all comparisons |
KCCQ Physical Limitations 2.9 (1.1 to 4.8) P = .002 KCCQ Social Limitations 1.9 (−0.5 to 4.2) P = .12 SF-36 Physical Summary 0.6 (−0.3 to 1.6) P = .17 SF-36 Mental Summary 0 (−0.9 to 0.9) P = .996 EQ-5D Utilities 0.0 (−0.02 to 0.01) P = .774 |
KCCQ Physical Limitations 1.2 (−0.7 to 3.1) P = .22 KCCQ Social Limitations 2.5 (0.1 to 4.8) P = .04 SF-36 Physical Summary 0 (−1.0 to 1.0) P = .96 SF-36 Mental Summary 0.3 (−0.5 to 1.2) P = .45 EQ-5D Utilities 0.0 (−0.02 to 0.01) P = .766 |
Abbreviations: CI; confidence interval; EQ-5D, European Quality of Life in Five Dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire; SAVR, surgical aortic valve replacement; SF-36, Medical Outcomes Study 36-question Short-Form Health Survey; TAVI, transcatheter aortic valve implantation.
Analyses were adjusted for age, sex, baseline health status, and treatment assignment.
Table A5:
Aortic Valve Area and Aortic Valve Gradient
| Author, Year N (TAVI/SAVR) |
Baseline Mean (SD) |
30 Days n (%) |
1 Year n (%) |
|---|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
Aortic valve area (cm2) TAVI: 0.8 (SE 0.16) SAVR 0.8 (SE 0.15) |
Aortic valve area (cm2) TAVI: 1.7 (SE 0.02) SAVR 1.8 (SE 0.02) Mean difference (95% CI) |
Aortic valve area (cm2) TAVI: 1.7 (SE 0.02) SAVR 1.8 (SE 0.02) Mean difference (95% CI) |
| Mean aortic valve gradient (mm Hg) TAVI: 49.4 (SE 12.79) SAVR 48.3 (11.76) |
−0.1 (−0.1 to −0.0) Mean aortic valve gradient (mm Hg) TAVI: 12.8 (SE 0.20) SAVR 11.2 (SE 0.21) Mean difference (95% CI) 1.5 (0.9 to 2.0) |
−0.1 (−0.1 to 0.0) Mean aortic valve gradient (mm Hg) TAVI: 13.7 (SE 0.26) SAVR 11.6 (SE 0.25) Mean difference (95% CI) 2.0 (1.3 to 2.7) |
|
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
Not reported | N = 1,151 (610 TAVI/541 SAVR) Aortic valve area (cm2) TAVI: 2.2 (0.6) SAVR 2.0 (0.6) Mean difference (95% BCrI) 0.2 (0.1 to 0.2) N = 1,333 (699 TAVI/634 SAVR) Mean aortic valve gradient (mm Hg) TAVI: 8.4 (3.5) SAVR 10.5 (4.0) Mean difference (95% BCrI) −2.1 (−2.5 to −1.7) |
N = 634 (341 TAVI/293 SAVR) Aortic valve area (cm2) TAVI: 2.3 (0.7) SAVR 2.0 (0.6) Mean difference (95% BCrI) 0.3 (0.2 to 0.4) N = 748 (409 TAVI/339 SAVR) Mean aortic valve gradient (mm Hg) TAVI: 8.6 (3.7) SAVR 11.2 (4.9) Mean difference (95% BCrI) −2.1 (−2.5 to −1.7) |
Abbreviations: BCrI, Bayesian credible interval; SAVR, surgical aortic valve replacement; SD, standard deviation; SE, standard error; TAVI, transcatheter aortic valve implantation.
Table A6:
Major Vascular Complications
| Author, Year N (TAVI/SAVR) |
30 Days | 1 Year |
|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimatea, n (%) TAVI: 11 (2.2) SAVR: 7 (1.5) HR (95% CI) 1.44 (0.56–0.73) RD (95% CI)b 0.7% (−1.5% to 2.7%) |
KM estimatea, n (%) TAVI: 14 (2.8) SAVR: 7 (1.5) HR (95% CI) 1.83 (0.74–4.55) RD (95% CI) b 1.3% (−1.2% to 3.4%) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 3.8% SAVR: 3.2% RD (95% BCrI) 0.6% (−1.4% to 2.5%) |
TAVI: 3.8% SAVR: 3.5% RD (95% BCrI) 0.3% (−1.7% to 2.3%) |
Abbreviations: BCrI, Bayesian credible interval; CI; confidence interval; KM; Kaplan-Meier; HR hazard ratio; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal number of patients who experienced event divided by total number of patients in treatment group at given time point.
Calculated by the authors of this report using the exact method.24
Table A7:
Acute Kidney Injury Stage 2 to 3
| Author, Year N (TAVI/SAVR) |
30 Days | 1 Year |
|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimatea, n (%) TAVI: 2 (0.4) SAVR: 8 (1.8) HR (95% CI) 1.44 (0.56–0.73) RD (95% CI)b −1.4% (−3.2% to 0.7%) |
Not reported |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 0.9%b SAVR: 2.8%b RD (95% BCrI) −1.8 (−3.4 to −0.5) |
TAVI: 0.9%b SAVR: 2.8%b RD (95% BCrI) −1.8 (−3.4 to −0.5) |
Abbreviations: BCrI, Bayesian credible interval; CI; confidence interval; KM; Kaplan-Meier; RD, risk difference; HR hazard ratio; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal the number of patients who experienced the event divided by the total number of patients in the treatment group at the given time point.
Calculated by the authors of this report using the exact method.24
Table A8:
New-Onset Atrial Fibrillation
| Author, Year N (TAVI/SAVR) |
30 Days | 1 Year |
|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimate,a n (%)
HR (95% CI) 0.10 (0.06–0.16) RD (95% CI) −27.7% (−32.3% to −23.1%) |
KM estimate,a n (%)
HR (95% CI) 0.13 (0.09–0.20) RD (95% CI) −27.7% (−32.0% to −22.0%) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 7.7%b SAVR: 35.4%b RD (95% BCrI) −27.7 (−31.8 to −23.6) |
TAVI: 9.8%b SAVR: 38.3%b RD (95% BCrI) −28.5 (−32.8 to −24.1) |
Abbreviations: BCrI, Bayesian credible interval; CI; confidence interval; KM; Kaplan-Meier; RD, risk difference; HR hazard ratio; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at the specific time point and do not necessarily equal number of patients who experienced event divided by total number of patients in treatment group at given time point.
Calculated by means of Bayesian analyses.
Table A9:
New Pacemaker Implantation
| Author, Year N (TAVI/SAVR) |
30 Days n (%) |
1 Year n (%) |
|---|---|---|
| Mack et al, 201918 950 (496/454) PARTNER 3 |
KM estimate,a n (%)
HR (95% CI) 1.66 (0.93–2.96) RD (95% CI) 2.5% (−0.3% to 5.3%) |
KM estimate,a n (%)
HR (95% CI) 1.39 (0.83–2.33) RD (95% CI) 2.0% (−1.1% to 5.1%) |
| Popma et al, 201917 1,403 (725/678) Evolut LRT |
TAVI: 17.4%b SAVR: 6.1%b RD (95% BCrI) 11.3 (8.0 to 14.7) |
TAVI: 19.4%b SAVR: 6.7%b RD (95% BCrI) 12.6 (9.2 to 16.2) |
Abbreviations: BCrI, Bayesian credible interval; CI; confidence interval; KM; Kaplan-Meier; HR hazard ratio; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Percentages provided are Kaplan-Meier estimates at specific time point and do not necessarily equal number of patients who experienced event divided by total number of patients in treatment group at given time point.
Calculated by means of Bayesian analyses.
Table A10:
Paravalvular Aortic Regurgitation
| Author, Year N (TAVI/SAVR) |
30 Days n (%) |
1 Year n/Na (%) |
2 Years |
|---|---|---|---|
| Mack et al, 201918 PARTNER 3 |
Na / 487 TAVI/421 SAVR None/Trace TAVI: 343 (70.4) SAVR: 409 (97.1) Mild TAVI: 140 (28.7) SAVR: 12 (2.9) Moderate/Severe TAVI: 4 (0.8) SAVR: 0 RD (95% CI), 0.8% (−0.09% to 1.7%) |
Na / 466 TAVI/381 SAVR None/Trace TAVI: 326 (70.0) SAVR: 371 (97.4) Mild TAVI: 137 (29.4) SAVR: 8 (2.1) Moderate/Severe TAVI: 3 (0.6) SAVR: 2 (0.5) RD (95% CI), 0.1% (−0.91% to 1.15%) |
Not available |
| Popma et al, 201917 N = 1,403 (725/678) Evolut LRT |
Na TAVI/SAVR 703/608 None TAVI: 146 (20.8) SAVR: 544 (89.5) Trace TAVI: 280 (39.8) SAVR: 44 (7.2) Mild TAVI: 253 (36.0) SAVR: 18 (3.0) Moderate TAVI: 22 (3.1) SAVR: 1 (0.2) Severe TAVI: 2 (0.3) SAVR: 1 (0.2) |
Na TAVI/SAVR 407/326 None TAVI: 168 (41.3) SAVR: 299 (91.7) Trace TAVI: 86 (21.1) SAVR: 17 (5.2) Mild TAVI: 138 (33.9) SAVR: 8 (2.5) Moderate TAVI: 14 (3.4) SAVR: 2 (0.6) Severe TAVI: 1 (0.2) SAVR: 0 |
Na TAVI/SAVR 70/61 None TAVI: 39 (55.7) SAVR: 59 (96.7) Trace TAVI: 9 (12.9) SAVR: 1 (1.6) Mild TAVI: 18 (25.7) SAVR: 1 (1.6) Moderate TAVI: 4 (5.7) SAVR: 0 Severe TAVI: 0 SAVR: 0 |
Abbreviations: CI; confidence interval; RD, risk difference; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Number of patients with available echocardiograms.
Table A11:
Clinical Events at 1 Year According to Presence of Leaflet Thickening at 30 Days
| Author, Year N (TAVI/SAVR) |
All-Cause Mortality at 1 Year n (%) |
Any Stroke at 1 Year n (%) |
TIA at 1 Year n (%) |
All-Cause Mortality, Stroke, or TIA at 1 Year n (%) |
|---|---|---|---|---|
| PARTNER 335 With leaflet thickening at 30 d 35 (28/7) Without leaflet thickening at 30 d 311 (156/155) |
Presence of leaflet thickening at 30 d TAVI: 0 SAVR: 0 No leaflet thickening TAVI: 2 (1.3) SAVR: 2 (1.4) |
Presence of leaflet thickening at 30 d TAVI: 0 SAVR: 0 No leaflet thickening TAVI: 1 (0.7) SAVR: 0 |
Presence of leaflet thickening at 30 d TAVI: 1 (5.6) SAVR: 0 No leaflet thickening TAVI: 2 (1.3) SAVR: 0 |
Presence of leaflet thickening at 30 d TAVI: 1 (5.6) SAVR: 0 No leaflet thickening TAVI: 5 (3.3) SAVR: 2 (1.4) |
| Evolut LRT36 With leaflet thickening at 30 d 57 (35/22) Without leaflet thickening at 30 d 317 (162/155) |
Presence of leaflet thickening at 30 d TAVI: 0 SAVR: 1 (4.5) No leaflet thickening TAVI: 0 SAVR: 1 (0.9) |
Presence of leaflet thickening at 30 d TAVI: 1 (2.9) SAVR: 0 No leaflet thickening TAVI: 4 (2.5) SAVR: 3 (1.9) |
Presence of leaflet thickening at 30 d TAVI: 2 (5.7) SAVR: 0 No leaflet thickening TAVI: 3 (1.9) SAVR: 0 |
Presence of leaflet thickening at 30 d TAVI: 3 (8.6) SAVR: 1 (4.5) No leaflet thickening TAVI: 7 (4.3) SAVR: 4 (2.8) |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack.
Table A12:
Clinical Events at 1 Year According to Presence of Leaflet Mobility Restriction at 30 Days
| Author, Year N (TAVI/SAVR) |
All-Cause Mortality at 1 Year n (%) |
Any Stroke at 1 Year n (%) |
TIA at 1 year n (%) |
All-Cause Mortality, Stroke, or TIA at 1 Year n (%) |
|---|---|---|---|---|
| PARTNER 335 Reduced leaflet mobility at 30 d 30 (25/5) No leaflet mobility restriction at 30 d 294 (145/149) |
Reduced leaflet mobility at 30 d TAVI: 0 SAVR: 0 No leaflet mobility restriction at 30 d TAVI: 0 SAVR: 0 |
Reduced leaflet mobility at 30 d TAVI: 0 SAVR: 0 No leaflet mobility restriction at 30 d TAVI: 0 SAVR: 0 |
Reduced leaflet mobility at 30 d TAVI: 1 (6.3) SAVR: 0 No leaflet mobility restriction at 30 d TAVI: 1 (6.3)a SAVR: 0 |
Reduced leaflet mobility at 30 d TAVI: 1 (6.3) SAVR: 0 No leaflet mobility restriction at 30 d TAVI: 5 (3.6)a SAVR: 2 (1.4)a |
| Evolut LRT36 Reduced leaflet mobility at 30 d 46 (27/19) No leaflet mobility restriction at 30 d 347 (148/153) |
Reduced leaflet mobility at 30 d TAVI: 0 SAVR: 1 (5.3) No leaflet mobility restriction at 30 d TAVI: 0 SAVR: 1 (0.9) |
Reduced leaflet mobility at 30 d TAVI: 1 (3.7) SAVR: 0 No leaflet mobility restriction at 30 d TAVI: 4 (2.7) SAVR: 3 (2.0) |
Reduced leaflet mobility at 30 d TAVI: 2 (7.4) SAVR: 0 No leaflet mobility restriction at 30 d TAVI: 2 (1.4) SAVR: 0 |
Reduced leaflet mobility at 30 d TAVI: 3 (11.1) SAVR: 1 (5.3) No leaflet mobility restriction at 30 d TAVI: 6 (4.1) SAVR: 4 (2.8) |
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack.
Numbers as reported in the reference, we were not able to verify their accuracy.
Appendix 6: Critical Appraisal of Clinical Evidence
Table A13:
Risk of Biasa Among Randomized Controlled Trials (Cochrane Risk of Bias Tool)
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| Mack et al, 201918 PARTNER |
Low | Low | Lowb | Lowb | Low | Low | None |
| Popma et al, 201917 Evolut LRT |
Low | Low | Lowc | Lowc | Low (30 d) Uncleard (1 y) Highd (2 y) |
Low | None |
Possible risk of bias levels: low, high, and unclear.
Patients and study personnel were not blinded to treatment assigned. However, we considered risk of bias to be low, as all components of the primary end point and key secondary end points were adjudicated by clinical events committee (members were aware of treatment assignment). Committee members included cardiologists and cardiothoracic surgeons who were not involved in the study and had no conflicts of interest. Primary outcome: composite of all-cause mortality, stroke, or rehospitalization at 1 year. Key secondary end points: stroke, composite of death or stroke, new-onset atrial fibrillation, poor treatment outcome (composite of death or a low Kansas City Cardiomyopathy Questionnaire, overall summary score at 30 days, and length of index hospitalization.
Echocardiographic studies were assessed at independent core laboratory. An independent academic clinical-events committee adjudicated all end points, using standard definitions. End-point adjudication was blinded when feasible.
Current publication provides results of prespecified interim analysis performed when 784 (53%) and 137 (9.3%) patients had reached 1 and 2 years of follow-up, respectively. Although appropriate imputation methods were used for missing data, given the high proportion of patients for which outcome data were unavailable at 1 year, it is difficult to ascertain whether missing outcomes would result in relevant bias in the effect estimate. At 2 years, given extent of the imputation (91%), we considered results to be at high risk of bias.
Table A14:
GRADE Evidence Profile for Comparison of TAVI Versus SAVR (Effectiveness Outcomes at 30 Days)
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| All-Cause Mortality at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitations |
No serious limitations |
Serious limitations (−1)a |
Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Any Stroke at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitationsb |
No serious limitations |
Very serious limitations (−2)b |
Undetected | Not applicable | ⊕⊕ Low |
| Disabling Stroke at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitations |
No serious limitations |
Serious limitations (−1)a |
Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Nondisabling Stroke at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitations |
No serious limitations |
Very serious limitations (−2)b |
Undetected | Not applicable | ⊕⊕ Low |
| Transient Ischemic Attack at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitations |
No serious limitations |
Very serious limitations (−2)b |
Undetected | Not applicable | ⊕⊕ Low |
| NYHA Symptoms at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitations |
No serious limitations |
No serious limitations |
Undetected | Not applicable | ⊕⊕⊕⊕ High |
| Quality of Life (KCCQ Overall Score) at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitations |
No serious limitations |
No serious limitations |
Undetected | Not applicable | ⊕⊕⊕⊕ High |
| 6-Minute Walk Test at 30 Days | |||||||
| 1 RCT18 | No serious limitations |
Cannot be evaluated |
No serious limitations |
No serious limitations | Undetected | Not applicable | ⊕⊕⊕⊕ High |
| Aortic Valve Reintervention at 30 days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitations |
No serious limitations |
Very serious limitations (−2)b |
Undetected | Not applicable | ⊕⊕ Low |
| Rehospitalizations at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations |
No serious limitationsb |
No serious limitations |
Serious limitations (−1)c |
Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Valve Hemodynamics at 30 days | |||||||
| 2 RCTs17,18 | No serious limitations |
Serious limitations (−1)d |
No serious limitations |
Serious limitations (−1)e |
Undetected | Not applicable | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; RCT, randomized controlled trial; SAVR, surgical aortic valve replacement; TAVI, transaortic valve implantation.
Meta-analysis was adequately powered to detect difference between the two groups (optimal information size was met). However, we decided to downgrade owing to fragility given very few events in each group and to fragility of estimate (an increase of a few events in either group could change direction of point estimate or render results not statistically significant).
Studies had very low statistical power to detect a difference between groups. Evidence was downgraded by two levels given very few events in intervention and control groups of both studies and fragility of estimates (an increase of a few events in either group could change direction of point estimate or render results not statistically significant).
One study reported statistically significant difference between groups; however, the other study did not. We decided not to downgrade for inconsistency, but for imprecision, as we believed that inconsistency could have been caused by imprecision and by the very small number of events in each study.
Results were inconsistent between studies.
It is unclear whether small differences observed between groups are clinically important.
Table A15:
GRADE Evidence Profile for Comparison of TAVI Versus SAVR (Effectiveness Outcomes at 1 to 2 Years)
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Composite End Point of All-Cause Mortality, Stroke, or Rehospitalization at 1 Year—Noninferiority Hypothesis | |||||||
| 1 RCT18 | No serious limitations | Cannot be evaluated | No serious limitations | No serious limitations | Undetected | Not applicable | ⊕⊕⊕⊕ High |
| Composite End Point of All-Cause Mortality or disabling stroke at 2 years—Noninferiority hypothesis | |||||||
| 1 RCT17 | Serious limitations (−1)a |
Cannot be evaluated | No serious limitations | No serious limitationsb |
Undetected | Not applicable | ⊕⊕⊕ Moderate |
| All-cause mortality at 1 year | |||||||
| 2 RCTs17,18 | No serious limitationsc |
No serious limitations | No serious limitations | Very serious limitations (−2)d | Undetected | Not applicable | ⊕⊕ Low |
| All-cause mortality at 2 years | |||||||
| 1 RCT17 | Serious limitations (−1)a | Cannot be evaluated | No serious limitations | Very serious limitations (−2)d | Undetected | Not applicable | ⊕ Very Low |
| Any Stroke at 1 Year | |||||||
| 2 RCTs17,18 | No serious limitationsc | No serious limitations | No serious limitations | Very serious limitations (−2)d | Undetected | Not applicable | ⊕⊕ Low |
| Disabling Stroke at 1 Year | |||||||
| 2 RCTs17,18 | No serious limitationsc | No serious limitations | No serious limitations | Serious limitations (−1)e | Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Disabling Stroke at 2 Years | |||||||
| 1 RCT17 | Serious limitations (−1)a | Cannot be evaluated | No serious limitations | Serious limitations (−1)f | Undetected | Not applicable | ⊕⊕ Low |
| Nondisabling Stroke at 1 Year | |||||||
| 2 RCTs17,18 | No serious limitationsc | No serious limitations | No serious limitations | Very serious limitations (−2)d | Undetected | Not applicable | ⊕⊕ Low |
| Transient Ischemic Attack at 1 Year | |||||||
| 2 RCTs17,18 | No serious limitationsc | No serious limitations | No serious limitations | Very serious limitations (−2)d | Undetected | Not applicable | ⊕⊕ Low |
| NYHA Symptoms at 1 Year | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)g | Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Quality of Life (KCCQ Overall Score) at 1 Year | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)g | Undetected | Not applicable | ⊕⊕⊕ Moderate |
| 6-Minute Walk Test at 1 Year | |||||||
| 1 RCT18 | No serious limitations | Cannot be evaluated | No serious limitations | Serious limitations (−1)g | Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Aortic Valve Reintervention at 1 year | |||||||
| 2 RCTs17,18 | No serious limitationsc | No serious limitations | No serious limitations | Very serious limitations (−2)d | Undetected | Not applicable | ⊕⊕ Low |
| Rehospitalizations at 1 year | |||||||
| 2 RCTs17,18 | No serious limitationsc | No serious limitationsb | No serious limitations | Serious limitations (−1)e | Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Valve hemodynamics at 1 year | |||||||
| 2 RCTs17,18 | No serious limitations | Serious limitations (−1)h | No serious limitations | Serious limitations (−1)i | Undetected | Not applicable | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; RCT, randomized controlled trial; SAVR, surgical aortic valve replacement; TAVI, transaortic valve implantation.
Although appropriate imputation methods were used in this study, large extent of imputation used (i.e., approximately 90% of patients had not reached 2 years of follow-up at time of the analysis) could potentially increase risk of bias for this outcome.
Difference between TAVI and SAVR was not statistically significant; however, noninferiority criterion (main end point of this study) was satisfied (P < .0001 for noninferiority).
One study had a large degree of data imputation (i.e., approximately 45% of patients had not reached 1 year of follow-up at time of analysis. Although appropriate methods for imputation were used, this could potentially affect estimates obtained. Because we consequently cannot determine extent to which the risk of bias might be increased, we decided not to downgrade for risk of bias.
Study had very low statistical power to detect difference between groups. Evidence was downgraded by two levels given very few events in intervention and control groups of both studies and given fragility of estimates (an increase of a few events in either group could change direction of point estimate or render results not statistically significant).
One study reported a statistically significant result, but the other study did not.
Study result was statistically significant. However, we decided to downgrade owing to fragility given the very small number of events in each group (fragility of estimate).
Studies were not adequately powered to detect difference between groups.
Results were inconsistent between studies.
It is unclear whether small differences observed between groups are clinically important.
Table A16:
GRADE Evidence Profile for Comparison of TAVI Versus SAVR (Safety Outcomes)
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Procedural Complications | |||||||
| 1 RCT18 | No serious limitations | Cannot be evaluated | No serious limitations | Very serious limitations (−2)a | Undetected | Not applicable | ⊕⊕ Low |
| Life-Threatening Bleeding at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | Not applicable | ⊕⊕⊕⊕ High |
| Major Vascular Complications at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (−2)a | Undetected | Not applicable | ⊕⊕ Low |
| Acute Kidney Injury at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | Not applicable | ⊕⊕⊕⊕ High |
| New-Onset Atrial Fibrillation at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | Not applicable | ⊕⊕⊕⊕ High |
| Myocardial Infarction at 30 Days | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (−2)a | Undetected | Not applicable | ⊕⊕ Low |
| Myocardial Infarction at 1 Year | |||||||
| 2 RCTs17,18 | No serious limitations | No serious limitations | No serious limitations | Very serious limitations (−2)a | Undetected | Not applicable | ⊕⊕ Low |
| New Pacemaker Implantation at 30 Days, Self-Expanding Valve | |||||||
| 1 RCT17 | No serious limitations | Cannot be evaluated | No serious limitations | No serious limitations | Undetected | Not applicable | ⊕⊕⊕⊕ High |
| New Pacemaker Implantation at 30 Days, Balloon-Expandable Valve | |||||||
| 1 RCT18 | No serious limitations | Cannot be evaluated | No serious limitations | Serious limitations (−1)b |
Undetected | Not applicable | ⊕⊕⊕ Moderate |
| New Left Bundle Branch Block at 30 Days | |||||||
| 1 RCT18 | No serious limitations | Cannot be evaluated | No serious limitations | No serious limitations | Undetected | Not applicable | ⊕⊕⊕⊕ High |
| Moderate-to-Severe Paravalvular Aortic Valve Regurgitation at 30 Days and 1 Year | |||||||
| 2 RCTs17,18 | No serious limitations | Serious limitations (−1)c | No serious limitations | Serious limitations (−1)d | Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Moderate-to-Severe Paravalvular Aortic Valve Regurgitation at 2 Years | |||||||
| 1 RCT17 | No serious limitations | Cannot be evaluated | No serious limitations | Serious limitations (−1)e |
Undetected | Not applicable | ⊕⊕⊕ Moderate |
| Asymptomatic Valve Thrombosis at 1 Year | |||||||
| 1 RCT18 | No serious limitations | Cannot be evaluated | No serious limitations | Very serious limitations (−2)a | Undetected | Not applicable | ⊕⊕ Low |
| Valve Thrombosis at 1 Year | |||||||
| 1 RCT17 | No serious limitations | Cannot be evaluated | No serious limitations | Very serious limitations (−2)a | Undetected | Not applicable | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; N/A, not applicable; RCT, randomized controlled trial; SAVR, surgical aortic valve replacement; TAVI, transaortic valve implantation.
Studies identified had very low statistical power to detect a difference between groups. Evidence was downgraded by two levels given very few events in intervention and control groups.
Studies identified had very low statistical power to detect a difference between groups.
Possible inconsistency in magnitude of effect.
One study reported a statistically significant result, but the other one did not.
Study identified had low statistical power to detect a difference between groups.
Appendix 7: Selected Excluded Studies—Clinical Evidence
| Citation | Primary Reason for Exclusion |
|---|---|
| Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. Journal of the American College of Cardiology. 2015;65(20):2184–94. | Did not meet eligibility criteria for population (i.e., mixed surgical risk population without providing results specific to population at low surgical risk) |
| Nielsen HH, Klaaborg KE, Nissen H, Terp K, Mortensen PE, Kjeldsen BJ, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2012;8(3):383–9. | Did not meet eligibility criteria for population (i.e., mixed surgical risk population without providing results specific to population at low surgical risk) |
| Siontis GCM, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. European Heart Journal. 2019; 40(38):3143–3153. | Did not meet eligibility criteria for population (i.e., mixed surgical risk population without providing results specific to population at low surgical risk) |
| Ando T, Ashraf S, Villablanca P, Kuno T, Pahuja M, Shokr M, et al. Meta-Analysis of Effectiveness and Safety of Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement in Low-to-Intermediate Surgical Risk Cohort. Am J Cardiol. 2019;25:25. | Did not meet eligibility criteria for population (i.e., mixed surgical risk population without providing results specific to population at low surgical risk) |
Appendix 8: Results of Applicability and Limitation Checklists for Studies Included in Economic Literature Review
Table A17:
Applicability of Studies Evaluating Cost-Effectiveness of Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Low Surgical Risk
| Author, Country | Is the study population similar to the question?a | Are the interventions similar to the question?a | Is the health care system studied sufficiently similar to Ontario?a | Were the perspectives clearly stated? If yes, what were they?a | Are all direct effects included? Are all other effects included where they are material?a | Are all future costs and outcomes discounted? If yes, at what rate?a | Is the value of health effects expressed in terms of quality-adjusted life-years?a | Are costs and outcomes from other sectors fully and appropriately measured and valued?a | Overall judgmentb |
|---|---|---|---|---|---|---|---|---|---|
| Tam et al.,49 Canada | Yes | Yes | Yes (Ontario) | Yes (Canadian third-party payer, i.e., Ontario Ministry of Health) | Yes | Yes (1.5%) | Yes | Not applicable | Directly applicable |
Response options were “yes,” “partially,” “no,” “unclear,” and “not applicable.”
Overall judgment could be “directly applicable,” “partially applicable,” or “not applicable.”
Table A18:
Assessment of Limitations of Studies Evaluating the Cost-Effectiveness of TAVI Versus SAVR in Patients With Severe Aortic Stenosis at Low Surgical Risk
| Author, Country | Does the model structure adequately reflect the nature of the health condition under evaluation?a | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes?a | Are all important and relevant health outcomes included?a | Are the clinical inputsb obtained from the best available sources?a | Do the clinical inputsb match the estimates contained in the clinical sources?a | Are all important and relevant (direct) costs included in the analysis?a | Are the estimates of resource use obtained from the best available sources?a | Are the unit costs of resources obtained from the best available sources?a | Is an appropriate incremental analysis presented, or can it be calculated from the reported data?a | Are all important and uncertain parameters subjected to appropriate sensitivity analysis?a | Is there a potential conflict of interest?a | Overall judgmentc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tam et al., 49 Canada | Yes | Yes, lifetime | Yes | Partially (QOL obtained from population at intermediate risk) | Yes | Yes | Yes | Yes | Yes | Partially (PSA, one other SA) | No | Minor limitations |
Abbreviations: PSA, probabilistic sensitivity analysis; QOL, quality of life; SA, sensitivity analysis; SAVR, surgical aortic valve replacement; TAVI, transaortic valve implantation.
Response options were “yes,” “partially,” “no,” “unclear,” and “not applicable.”
Clinical inputs include relative treatment effects, natural history, and utilities.
Overall judgment could be “minor limitations,” “potentially serious limitations,” or “very serious limitations.”
Appendix 9: Budget Impact Analysis, Methods
Budget Impact Calculations
We calculated our budget impact as the difference in annual total costs between our new scenario (public funding for TAVI, thus a mix of TAVI and SAVR) and our current scenario (no public funding for TAVI, thus SAVR only).
We calculated the costs for each treatment group as follows. We calculated the annual costs for 2019/20 by multiplying the total volume of patients in 2019/20 (Appendix 10, Table A23) by the relevant first-year treatment costs (Table 16, Equation 1). We calculated annual costs for subsequent years using the ongoing costs of 2019/20 patients and costs of volumes of patients expected in respective years (Equations 2–5).
Note: The costs derived from the analysis by Tam and colleagues49 consider mortality, so calculations are based on the total number of people in the cohort; however, the number of patients who are expected to be alive over the analysis can be seen in Table A20.
| Equation 1 |
| Equation 2 |
| Equation 3 |
| Equation 4 |
| Equation 5 |
Table A20:
Number of Patients Considered in Budget Impact Analysis, Reference Case
| Scenario | Year | Patients/Year,a,b Total (Alivec), n | Total Patients, n | |||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||||
| Current scenario | TAVI | 2019/20 | 0 | — | — | — | — | 0 |
| 2020/21 | 0 | 0 | — | — | — | 0 | ||
| 2021/22 | 0 | 0 | 0 | — | — | 0 | ||
| 2022/23 | 0 | 0 | 0 | 0 | — | 0 | ||
| 2023/24 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| SAVR | 2019/20 | 1,126 (1,126) | — | — | — | — | 1,126 (1,126) | |
| 2020/21 | 1,146 (1,146) | 1,126 (1,088) | — | — | — | 2,272 (2,234) | ||
| 2021/22 | 1,167 (1,167) | 1,146 (1,108) | 1,126 (1,058) | — | — | 3,439 (3,333) | ||
| 2022/23 | 1,187 (1,187) | 1,167 (1,128) | 1,146 (1,077) | 1,126 (1,027) | — | 4,626 (4,419) | ||
| 2023/24 | 1,208 (1,208) | 1,187 (1,147) | 1,167 (1,097) | 1,146 (1,045) | 1,126 (993) | 5,834 (5,490) | ||
| New scenario | TAVI | 2019/20 | 563 (563) | — | — | — | — | 563 (563) |
| 2020/21 | 702 (702 | 563 (549) | — | — | — | 1,265 (1,250) | ||
| 2021/22 | 846 (846) | 702 (684) | 563 (534) | — | — | 2,111 (2,064) | ||
| 2022/23 | 994 (994) | 846 (824) | 702 (665) | 563 (518) | — | 3,105 (3,002) | ||
| 2023/24 | 1,148 (1,148) | 994 (969) | 846 (802) | 702 (646) | 563 (501) | 4,253 (4,065) | ||
| SAVR | 2019/20 | 563 (563) | — | — | — | — | 563 (563) | |
| 2020/21 | 444 (444) | 563 (544) | — | — | — | 1,007 (988) | ||
| 2021/22 | 321 (321) | 444 (429) | 563 (529) | — | — | 1,328 (1,279) | ||
| 2022/23 | 193 (193) | 321 (310) | 444 (417) | 563 (513) | — | 1,521 (1,434) | ||
| 2023/24 | 60 (60) | 193 (186) | 321 (302) | 444 (405) | 563 (496) | 1,581 (1,450) | ||
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers might appear inexact owing to rounding.
Year 1 represents all new patients; years 2 to 5 represent patients who have received TAVI or SAVR in a previous year.
People alive at the beginning of the year: incorporates average treatment-specific mortality rate derived from Tam et al.49
Table A21:
Scenario Analyses, Target Population, and Uptake Values
| Year | |||||||
|---|---|---|---|---|---|---|---|
| Scenario | Variable | 2019/20 | 2020/21 | 2022/23 | 2023/24 | 2024/25 | |
| Population | |||||||
| Reference case | SAVR for aortic stenosis, n | 2,006 | 2,043 | 2,079 | 2,116 | 2,152 | |
| Scenario 1: Increased population with severe aortic valve stenosis | SAVR for aortic stenosis, n | 2,106 | 2,247 | 2,391 | 2,539 | 2,690 | |
| Scenario 2: Expansion of target population, TAVI uptake in patients getting no intervention | SAVR for aortic stenosis + additional 5% eligible for TAVI, n | 2,006 + 100 | 2,043 + 102 | 2,079 + 104 | 2,116 + 106 | 2,152 + 108 | |
| Uptake (New Scenario) | |||||||
| Reference case | Uptake rate for TAVI, % | 50 | 61 | 73 | 84 | 95 | |
| TAVI, n | 563 | 702 | 846 | 994 | 1,148 | ||
| SAVR, n | 563 | 444 | 321 | 193 | 60 | ||
| Scenario 7: faster initial uptake | Uptake rate for TAVI, % | 75 | 80 | 85 | 90 | 95 | |
| TAVI, n | 845 | 917 | 992 | 1068 | 1148 | ||
| SAVR, n | 282 | 229 | 175 | 119 | 260 | ||
| Scenario 8: gradual uptake | Uptake rate for TAVI, % | 20 | 28 | 35 | 43 | 50 | |
| TAVI, n | 225 | 315 | 408 | 504 | 604 | ||
| SAVR, n | 901 | 831 | 759 | 683 | 604 | ||
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Table A22:
Scenario Analyses, Annual Per-Patient Costs for TAVI and SAVRa
| Cost per Year Post-Implant,b,c$ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Intervention | Year 1d | Year 2e | Year 3e | Year 4e | Year 5e | ||
| Reference Case: Cost From Deterministic Analysis | ||||||||
| Tam et al., 202049 | BE TAVI | 36,504 | 115 | 31 | 30 | 29 | ||
| SE TAVI | 37,782 | 230 | 163 | 157 | 152 | |||
| Average TAVI | 37,143 | 172 | 97 | 94 | 90 | |||
| SAVR | 28,833 | 637 | 495 | 479 | 462 | |||
| Scenario 9: Cost From Probabilistic Analysis | ||||||||
| Tam et al., 202049 | BE TAVI | 36,569 | 141 | 57 | 55 | 53 | ||
| SE TAVI | 37,655 | 230 | 161 | 156 | 150 | |||
| Average TAVI | 37,112 | 185 | 109 | 105 | 101 | |||
| SAVR | 28,802 | 614 | 469 | 453 | 435 | |||
| Scenario 12: Reduced SAVR Procedural Costs | ||||||||
| SAVR | 26,368 | 637 | 495 | 479 | 462 | |||
Abbreviations: BE, balloon-expandable; SAVR, surgical aortic valve replacement; SE, self-expanding; TAVI, transcatheter aortic valve implantation.
Subject to change after publication.
Costs incorporate mortality.
We derived costs from model produced by Tam et al.49
Includes procedural (valve, index hospitalization, and fees for surgeon, surgical assistant, and anesthesiologist), short-term complication, and long-term complication costs.
Includes long-term complication costs.
Appendix 10: Budget Impact Analysis, Results
Table A23:
Cost Breakdowns in Results of Budget Impact Analysis, Reference Case
| Budget Impact, $ Millionsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | ||
| Current scenario | TAVI | Valve | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Initial hospitalizationb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Professional feesc | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Complications | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Total | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| SAVR | Valve | 6.76 | 6.88 | 7.00 | 7.12 | 7.25 | 35.00 | |
| Initial hospitalizationb | 15.80 | 16.08 | 16.38 | 16.66 | 16.95 | 81.87 | ||
| Professional feesc | 5.19 | 5.28 | 5.38 | 5.47 | 5.57 | 26.90 | ||
| Complications | 4.72 | 5.52 | 6.18 | 6.82 | 7.46 | 30.69 | ||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | ||
| New scenario | TAVI | Valve | 14.08 | 17.55 | 21.15 | 24.85 | 28.69 | 106.32 |
| Initial hospitalizationb | 3.25 | 4.06 | 4.89 | 5.74 | 6.63 | 24.57 | ||
| Professional feesc | 2.00 | 2.49 | 3.00 | 3.53 | 4.08 | 15.10 | ||
| Complications | 1.58 | 2.07 | 2.56 | 3.06 | 3.60 | 12.87 | ||
| Total | 20.91 | 26.17 | 31.60 | 37.19 | 43.00 | 158.87 | ||
| SAVR | Valve | 3.38 | 2.66 | 1.93 | 1.16 | 0.36 | 9.49 | |
| Initial hospitalizationb | 7.90 | 6.23 | 4.50 | 2.71 | 0.85 | 22.19 | ||
| Professional feesc | 2.60 | 2.05 | 1.48 | 0.89 | 0.28 | 7.29 | ||
| Complications | 2.36 | 2.22 | 1.91 | 1.50 | 1.01 | 8.99 | ||
| Total | 16.23 | 13.16 | 9.81 | 6.26 | 2.50 | 47.96 | ||
| Budget impact | Valve | 10.70 | 13.34 | 16.08 | 18.89 | 21.80 | 80.80 | |
| Initial hospitalizationb | −4.65 | −5.79 | −6.98 | −8.21 | −9.47 | −35.11 | ||
| Professional feesc | −0.60 | −0.74 | −0.90 | −1.05 | −1.22 | −4.51 | ||
| Complications | −0.77 | −1.23 | −1.71 | −2.26 | −2.86 | −8.83 | ||
| Total | 4.68 | 5.57 | 6.48 | 7.37 | 8.26 | 32.36 | ||
Abbreviations: SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Numbers might appear inexact owing to rounding.
Includes cost of intensive care unit and ward stay after initial procedure.
Includes surgeon, surgical assistant, and anesthesiologist fees.
Table A24:
Budget Impact Analysis, Full Results, Scenario Analyses
| Budget Impact, $ Millionsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scenario Analysis | Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | ||
| Reference case | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| New scenario | TAVI | 20.91 | 26.17 | 31.60 | 37.19 | 43.00 | 158.87 | ||
| SAVR | 16.23 | 13.16 | 9.81 | 6.26 | 2.50 | 47.96 | |||
| Total | 37.14 | 39.33 | 41.42 | 43.45 | 45.49 | 206.83 | |||
| Budget impact | 4.68 | 5.57 | 6.48 | 7.37 | 8.26 | 32.36 | |||
| Scenario 1: increased population with severe aortic valve stenosis | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 35.78 | 38.97 | 42.08 | 45.28 | 48.56 | 210.67 | |||
| Total | 35.78 | 38.97 | 42.08 | 45.28 | 48.56 | 210.67 | |||
| New scenario | TAVI | 23.05 | 30.23 | 38.14 | 46.85 | 56.38 | 194.64 | ||
| SAVR | 17.89 | 15.19 | 11.81 | 7.81 | 3.16 | 55.86 | |||
| Total | 40.94 | 45.42 | 49.95 | 54.66 | 59.54 | 250.50 | |||
| Budget impact | 5.16 | 6.45 | 7.87 | 9.38 | 10.98 | 39.83 | |||
| Scenario 2: expansion of target population, TAVI uptake in patients getting no intervention | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| New scenario | TAVI | 24.64 | 29.98 | 35.49 | 41.16 | 47.04 | 178.30 | ||
| SAVR | 16.23 | 13.16 | 9.81 | 6.26 | 2.50 | 47.96 | |||
| Total | 40.87 | 43.14 | 45.30 | 47.41 | 49.54 | 226.26 | |||
| Budget impact | 8.40 | 9.38 | 10.37 | 11.34 | 12.30 | 51.80 | |||
| Scenario 3: increased proportion of SAVR patients who are at low surgical risk | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 40.48 | 42.13 | 43.56 | 45.01 | 46.42 | 217.59 | |||
| Total | 40.48 | 42.13 | 43.56 | 45.01 | 46.42 | 217.59 | |||
| New scenario | TAVI | 26.07 | 32.65 | 39.40 | 46.40 | 53.60 | 198.13 | ||
| SAVR | 20.24 | 16.42 | 12.24 | 7.80 | 3.11 | 59.82 | |||
| Total | 46.31 | 49.08 | 51.64 | 54.21 | 56.71 | 257.95 | |||
| Budget impact | 5.83 | 6.95 | 8.08 | 9.20 | 10.30 | 40.36 | |||
| Scenario 4: decreased proportion of SAVR patients who are at low surgical risk | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 24.31 | 25.28 | 26.13 | 27.02 | 27.86 | 130.60 | |||
| Total | 24.31 | 25.28 | 26.13 | 27.02 | 27.86 | 130.60 | |||
| New scenario | TAVI | 15.66 | 19.59 | 23.64 | 27.85 | 32.18 | 118.92 | ||
| SAVR | 12.15 | 9.85 | 7.34 | 4.68 | 1.87 | 35.90 | |||
| Total | 27.81 | 29.45 | 30.98 | 32.54 | 34.04 | 154.82 | |||
| Budget impact | 3.50 | 4.17 | 4.85 | 5.52 | 6.18 | 24.22 | |||
| Scenario 5: increased proportion of SAVR patients eligible for TAVI | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 41.75 | 43.42 | 44.90 | 46.41 | 47.87 | 224.35 | |||
| Total | 41.75 | 43.42 | 44.90 | 46.41 | 47.87 | 224.35 | |||
| New scenario | TAVI | 26.89 | 33.66 | 40.62 | 47.84 | 55.27 | 204.29 | ||
| SAVR | 20.87 | 16.93 | 12.62 | 8.05 | 3.21 | 61.68 | |||
| Total | 47.77 | 50.59 | 53.23 | 55.89 | 58.48 | 265.96 | |||
| Budget impact | 6.02 | 7.17 | 8.33 | 9.48 | 10.62 | 41.61 | |||
| Scenario 6: decreased proportion of SAVR patients eligible for TAVI | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 23.18 | 24.13 | 24.94 | 25.77 | 26.60 | 124.61 | |||
| Total | 23.18 | 24.13 | 24.94 | 25.77 | 26.60 | 124.61 | |||
| New scenario | TAVI | 14.93 | 18.70 | 22.56 | 26.57 | 30.72 | 113.48 | ||
| SAVR | 11.59 | 9.41 | 7.01 | 4.47 | 1.78 | 34.25 | |||
| Total | 26.52 | 28.11 | 29.56 | 31.04 | 32.50 | 147.73 | |||
| Budget impact | 3.34 | 3.98 | 4.63 | 5.27 | 5.90 | 23.12 | |||
| Scenario 7: faster initial uptake | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| New scenario | TAVI | 31.37 | 34.20 | 37.08 | 40.02 | 43.07 | 185.74 | ||
| SAVR | 8.12 | 6.79 | 5.33 | 3.78 | 2.14 | 26.16 | |||
| Total | 39.48 | 40.99 | 42.42 | 43.80 | 45.21 | 211.90 | |||
| Budget impact | 7.02 | 7.23 | 7.48 | 7.73 | 7.98 | 37.43 | |||
| Scenario 8: gradual uptake | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| New scenario | TAVI | 8.36 | 11.74 | 15.25 | 18.86 | 22.61 | 76.83 | ||
| SAVR | 25.97 | 24.53 | 22.85 | 21.00 | 19.04 | 113.39 | |||
| Total | 34.34 | 36.27 | 38.09 | 39.86 | 41.65 | 190.22 | |||
| Budget impact | 1.87 | 2.51 | 3.16 | 3.79 | 4.42 | 15.75 | |||
| Scenario 9: cost from probabilistic analyses | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| New scenario | TAVI | 20.89 | 26.15 | 31.59 | 37.19 | 43.00 | 158.82 | ||
| SAVR | 16.23 | 13.16 | 9.81 | 6.26 | 2.50 | 47.96 | |||
| Total | 37.13 | 39.32 | 41.41 | 43.44 | 45.49 | 206.78 | |||
| Budget impact | 4.66 | 5.56 | 6.47 | 7.37 | 8.26 | 32.32 | |||
| Scenario 10: cost using only BE TAVI | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| New scenario | TAVI | 20.55 | 25.69 | 30.98 | 36.43 | 42.07 | 155.72 | ||
| SAVR | 16.23 | 13.16 | 9.81 | 6.26 | 2.50 | 47.96 | |||
| Total | 36.78 | 38.85 | 40.80 | 42.68 | 44.57 | 203.68 | |||
| Budget impact | 4.32 | 5.09 | 5.86 | 6.61 | 7.33 | 29.21 | |||
| Scenario 11: cost using only SE TAVI | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| Total | 32.47 | 33.76 | 34.94 | 36.07 | 37.23 | 174.47 | |||
| New scenario | TAVI | 21.27 | 26.65 | 32.22 | 37.96 | 43.92 | 162.02 | ||
| SAVR | 16.23 | 13.16 | 9.81 | 6.26 | 2.50 | 47.96 | |||
| Total | 37.50 | 39.81 | 42.03 | 44.21 | 46.42 | 209.98 | |||
| Budget impact | 5.04 | 6.05 | 7.10 | 8.14 | 9.18 | 35.51 | |||
| Scenario 12: reduced SAVR procedural costs | Current scenario | TAVI | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| SAVR | 29.69 | 30.94 | 32.06 | 33.15 | 34.26 | 160.09 | |||
| Total | 29.69 | 30.94 | 32.06 | 33.15 | 34.26 | 160.09 | |||
| New scenario | TAVI | 20.91 | 26.17 | 31.60 | 37.19 | 43.00 | 158.87 | ||
| SAVR | 14.85 | 12.07 | 9.02 | 5.78 | 2.35 | 44.06 | |||
| Total | 35.76 | 38.24 | 40.63 | 42.97 | 45.34 | 202.93 | |||
| Budget impact | 6.07 | 7.30 | 8.57 | 9.82 | 11.09 | 42.84 | |||
Abbreviations: BE, balloon-expandable; SAVR, surgical aortic valve replacement; SE, self-expanding; TAVI, transcatheter aortic valve implantation.
Numbers might appear inexact owing to rounding.
Appendix 11: Letter of Information∗
Health Quality Ontario is now a part of Ontario Health.
Appendix 12: Interview Guide†
Health Quality Ontario is now a part of Ontario Health.
Author contributions
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologists were Vania Costa and Myra Wang, the primary health economist was Lindsey Falk, the secondary health economist was Sean Tiggelaar, the patient and public partnership analyst was David Wells, and the medical librarians were Melissa Walter and Corinne Holubowich.
Key Messages
What Is This Health Technology Assessment About?
The aortic valve is located between the left ventricle (the heart's lower left chamber) and the aorta (the main artery that distributes blood from the heart to the body). Aortic valve stenosis, or narrowing of the aortic valve, prevents the valve from opening completely and reduces blood flow from the heart. This causes the heart to work harder to pump blood to the body and can lead to symptoms such as chest pain, shortness of breath, passing out, and fatigue. Surgical aortic valve replacement (SAVR) is the usual treatment for people who have severe aortic valve stenosis and who are at low risk for surgery. With SAVR, surgeons replace the damaged valve with an artificial valve through a cut in the chest (open-heart surgery).
Transcatheter aortic valve implantation (TAVI) involves placing an artificial valve inside the existing valve using a catheter (a long, flexible tube), most commonly through an artery in the leg. There is no need for open-heart surgery. At present in Ontario, TAVI is not publicly funded for people at low surgical risk.
This health technology assessment looked at how safe and effective TAVI is for people with severe aortic valve stenosis who are at low surgical risk. It looked at whether TAVI is cost-effective and at the budget impact of publicly funding TAVI in people at low surgical risk. It also looked at the experiences, preferences, and values of people with aortic valve stenosis and their families and caregivers.
What Did This Health Technology Assessment Find?
The TAVI procedure is less invasive than SAVR and, in the short term (30 days after the procedure), results in greater symptom improvement and quality of life, and has a slightly lower risk of mortality and disabling stroke. At 1 year, the risk of mortality is similar between TAVI and SAVR, but TAVI may have a slightly lower risk of disabling stroke than SAVR. We also found that the two treatments have different patterns of complications. Researchers say that longer-term follow-up is needed both to determine how long the TAVI valve will last and to draw definitive conclusions on long-term (beyond 1 year) outcomes of TAVI compared with SAVR.
Device costs are much higher for TAVI than for SAVR, but they are partially offset by lower costs for hospitalization and complications. Compared with SAVR, TAVI could be cost-effective. We estimate that publicly funding TAVI for people with severe aortic valve stenosis at low surgical risk in Ontario over the next 5 years would cost an additional $5 million to $8 million annually.
We did not find any quantitative or qualitative evidence on patient preferences and values specific to the low-risk surgical group. From qualitative literature and in direct engagement among a mixed or generally high-risk and elderly population, people typically preferred the less invasive nature and the faster recovery time of TAVI compared with SAVR.
Contributor Information
Ontario Health (Quality):
Vania Costa, Myra Wang, Lindsey Falk, Sean Tiggelaar, David Wells, Melissa Walter, and Corinne Holubowich
About Us
This health technology assessment was produced by Ontario Health, the government agency that when fully established will be responsible for ensuring all Ontarians receive high-quality health care where and when they need it.
For more information about Ontario Health, visit ontariohealth.ca
REFERENCES
- (1).Smith A, Argaez C. Transcatheter aortic valve implantation for aortic stenosis: a rapid qualitative review [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019. [cited 2020 Jan 5]. Available from: https://cadth.ca/sites/default/files/pdf/htis/2019/RC1180%20TAVI%20Final.pdf [PubMed] [Google Scholar]
- (2).Health Quality Ontario. Transcatheter aortic valve implantation for treatment of aortic valve stenosis: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2016;16(19):1–94. [PMC free article] [PubMed] [Google Scholar]
- (3).Liu Z, Duarte R, Kidney E, Bem D, Bramley G, Rooney S, et al. Clinical effectiveness and safety of transcatheter aortic valve implantation (TAVI) for aortic stenosis: a systematic review and meta-analysis [Internet]. Birmingham (UK): University of Birmingham; 2016. [cited 2019 Oct 17]. Available from: https://www.nice.org.uk/guidance/ipg586/documents/supporting-documentation [Google Scholar]
- (4).Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. 2014;56(6):565–71. [DOI] [PubMed] [Google Scholar]
- (5).Indolfi C, Bartorelli AL, Berti S, Golino P, Esposito G, Musumeci G, et al. Updated clinical indications for transcatheter aortic valve implantation in patients with severe aortic stenosis: expert opinion of the Italian Society of Cardiology and GISE. J Cardiovasc Med (Hagerstown). 2018;19(5):197–210. [DOI] [PubMed] [Google Scholar]
- (6).Zakkar M, Bryan AJ, Angelini GD. Aortic stenosis: diagnosis and management. BMJ (Clin Res Ed). 2016;355:i5425. [DOI] [PubMed] [Google Scholar]
- (7).Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–12. [DOI] [PubMed] [Google Scholar]
- (8).Thourani VH, Suri RM, Gunter RL, Sheng S, O'Brien SM, Ailawadi G, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg. 2015;99(1):55–61. [DOI] [PubMed] [Google Scholar]
- (9).Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Fleisher LA, et al. 2017. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135(25):e1159–e95. [DOI] [PubMed] [Google Scholar]
- (10).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. The patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (11).Webb J, Rodes-Cabau J, Fremes S, Pibarot P, Ruel M, Ibrahim R, et al. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol. 2012;28(5):520–8. [DOI] [PubMed] [Google Scholar]
- (12).Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–20. [DOI] [PubMed] [Google Scholar]
- (13).Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;60(6):483–92. [DOI] [PubMed] [Google Scholar]
- (14).Vahanian A, Himbert D, Brochet E. Transcatheter valve implantation for patients with aortic stenosis. Heart (British Cardiac Society). 2010;96(22):1849–56. [DOI] [PubMed] [Google Scholar]
- (15).Health Quality Ontario. Transcatheter aortic valve implantation for treatment of aortic valve stenosis: OHTAC recommendation [Internet]. Toronto (ON): Queen's Printer for Ontario; 2016. November [cited 2019 Oct 17]. Available from: http://www.hqontario.ca/evidence-to-improvecare/recommendations-and-reports/OHTAC/aortic-valve-stenosis [Google Scholar]
- (16).Ontario Health (Quality). Transcatheter aortic valve implantation in patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk: recommendation. [Internet]. Toronto (ON): Queen's Printer for Ontario; 2020. Mar [cited 2020 Mar]. Available from: https://www.hqontario.ca/evidence-to-improve-care/health-technology-assessment/reviews-and-recommendations/transcatheter-aortic-valve-implantation-in-patients-with-severe-symptomatic-aortic-valve-stenosis-at-intermediate-surgical-risk [PMC free article] [PubMed] [Google Scholar]
- (17).Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–15. [DOI] [PubMed] [Google Scholar]
- (18).Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–705. [DOI] [PubMed] [Google Scholar]
- (19).Health Canada. Health Canada Medical Devices Active Licence Listing (MDALL). [Internet]. Ottawa (ON): Queen's Printer for Canada; 2019. [cited 2019 Oct 17]. Available from: https://health-products.canada.ca/mdall-limh/ [Google Scholar]
- (20).Health Technology Assessment International. FAQ for HTA agencies and policy makers [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/interest-groups/patient-and-citizen-involvement/resources/for-hta-agencies-and-policy-makers/faq-for-hta-agencies-and-policy-makers.html#c3229 [Google Scholar]
- (21).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (22).Covidence systematic review software. Veritas Health Innovation. Melbourne (Australia). Available at: https://www.covidence.org/home.
- (23).O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- (24).Tian L, Cai T, Pfeffer MA, Piankov N, Cremieux PY, Wei LJ. Exact and efficient inference procedure for meta-analysis and its application to the analysis of independent 2 × 2 tables with all available data but without artificial continuity correction. Biostatistics (Oxford, England). 2009;10(2):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- (26).R Foundation for Statistical Computing. R statistical computing software. [Internet]. Vienna: R Foundation; 2019. [cited 2019 Oct 17]. Available from: http://www.Rproject.org [Google Scholar]
- (27).Higgins JP, Altman DG, Sterne JA. Assessing risk of bias in included studies. In: Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Internet]. The Cochrane Collaboration, 2011. [updated 2011 Mar; cited 2019 Oct 17]. Available from www.handbook.cochrane.org. [Google Scholar]
- (28).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- (29).Baron SJ, Magnuson EA, Lu M, Wang K, Chinnakondepalli K, Mack M, et al. Health status after transcatheter vs. surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. In press. [DOI] [PubMed]
- (30).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65(20):2184–94. [DOI] [PubMed] [Google Scholar]
- (32).Nielsen HH, Klaaborg KE, Nissen H, Terp K, Mortensen PE, Kjeldsen BJ, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention. 2012;8(3):383–9. [DOI] [PubMed] [Google Scholar]
- (33).Siontis GCM, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J. In press. [DOI] [PubMed]
- (34).Ando T, Ashraf S, Villablanca P, Kuno T, Pahuja M, Shokr M, et al. Meta-analysis of effectiveness and safety of transcatheter aortic valve implantation versus surgical aortic valve replacement in low-to-intermediate surgical risk cohort. Am J Cardiol. 2019;124(4):580–5. [DOI] [PubMed] [Google Scholar]
- (35).US Food and Drug Administration. Summary of safety and effectiveness data: Edwards SAPIEN 3 Transcatheter Heart Valve System; Edwards SAPIEN 3 Ultra Transcatheter Heart Valve System P140031/S085 [Internet]. 2019. [cited 2019 Oct 17]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140031S085b.pdf
- (36).US Food and Drug Administration. Summary of safety and effectiveness data: Medtronic CoreValve Evolut R System, Medtronic CoreValve Evolut PRO System P130021/S058 [Internet]. 2019. [cited 2019 Oct 17]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130021S058B.pdf
- (37).Mack MJ, Leon MB, Thourani VH. PARTNER 3 trial protocol. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients [Internet] 2019. [cited 2019 Oct 17]. Available from: https://www.nejm.org/doi/suppl/10.1056/NEJMoa1814052/suppl_file/nejmoa1814052_protocol.pdf
- (38).American Heart Association. Classes of heart failure [Internet]. Dallas (TX): American Heart Association; 2017. [cited 2019 Aug 1]. Available from: http://www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/ClassesofHeart-Failure_UCM_306328_Article.jsp#.WiAvz0qnGUk. [Google Scholar]
- (39).Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61–7. [DOI] [PubMed] [Google Scholar]
- (40).Baron SJ, Arnold SV, Wang K, Magnuson EA, Chinnakondepali K, Makkar R, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2(8):837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. [DOI] [PubMed] [Google Scholar]
- (42).S⊘ndergaard L, Olsen PS. Comparison of transcatheter versus surgical aortic valve replacement in younger low surgical risk patients with severe aortic stenosis (NOTION-2) [Internet]. Last update October 18, 2018. [cited 2019 Oct 17]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02825134?term=notion+2&rank=1
- (43).Blankenberg S, Cremer J. Randomized trial of TAVI vs. SAVR in patients with severe aortic valve stenosis at intermediate risk of mortality (DEDICATE) [Internet]. Last updated June 27, 2019. [cited 2019 Oct 17]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03112980?term=dedicate&rank=1
- (44).Bourantas CV, Serruys PW. Evolution of transcatheter aortic valve replacement. Circ Res. 2014;114(6):1037–51. [DOI] [PubMed] [Google Scholar]
- (45).Mack MJ, Leon MB. Transcatheter aortic-valve replacement in low-risk patients. reply. N Engl J Med. 2019;381(7):684–5. [DOI] [PubMed] [Google Scholar]
- (46).Ontario Health (Quality). Transcatheter aortic valve implantation in patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2020. Mar [cited 2020 Mar]; 20(2):1–121. Available from: https://www.hqontario.ca/evidence-to-improve-care/health-technology-assessment/reviews-and-recommendations/transcatheter-aortic-valve-implantation-in-patients-with-severe-symptomatic-aortic-valve-stenosis-at-intermediate-surgical-risk [PMC free article] [PubMed] [Google Scholar]
- (47).Otto CM. Informed shared decisions for patients with aortic stenosis. N Engl J Med. 2019;380(18):1769–70. [DOI] [PubMed] [Google Scholar]
- (48).National Institute for Health and Care Excellence. Process and methods guides. Appendix I: Quality appraisal checklist-economic evaluations [Internet]. London: The Institute; 2012. [cited 2016 Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [Google Scholar]
- (49).Tam DY, Azizi PM, Fremes SE, Chikwe J, Gaudino M, Wijeysundera HC. The cost-effectiveness of transcatheter aortic valve replacement in low surgical risk patients with severe aortic stenosis. Eur Heart J Qual Care Clin Outcomes [Internet]. 2020. Jul 9 [cited 2020 Aug 20]; doi: 10.1093/ehjqcco/qcaa058. Available from: https://academic.oup.com/ehjqcco/advance-article-abstract/doi/10.1093/ehjqcco/qcaa058/5869438?redirectedFrom=fulltext [DOI] [PubMed]
- (50).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada [Internet]. Ottawa (ON): CADTH; 2017. [cited 2018 Jan]. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [Google Scholar]
- (51).Geisler BP, Jorgensen TH, Thyregod HGH, Pietzsch JB, Sondergaard L. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients at lower surgical risk: results from the NOTION trial. EuroIntervention. 2019;15(11):e959–e67. [DOI] [PubMed] [Google Scholar]
- (52).Eggebrecht H, Mehta RH. Transcatheter Aortic Valve Implantation (TAVI) in Germany: more than 100,000 procedures and now the standard of care for the elderly. EuroIntervention. 2019;14(15):e1549–e52. [DOI] [PubMed] [Google Scholar]
- (53).CorHealth Ontario. Report on transcatheter aortic valve implantation (TAVI) in Ontario, November 2013 to March 2016. Toronto (ON): CorHealth Ontario; 2018. [Google Scholar]
- (54).Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017;377(19):1847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act. Toronto (ON): Queen's Printer for Ontario; 2015. p. 746. [Google Scholar]
- (56).Canadian Institute for Health Information,. CIHI patient cost estimator website. Available at: https://www.cihi.ca/en/spending-and-health-workforce/spending/patient-cost-estimator.
- (57).Phan K, Xie A, Di Eusanio M, Yan TD. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg. 2014;98(4):1499–511. [DOI] [PubMed] [Google Scholar]
- (58).Brown ML, McKellar SH, Sundt TM, Schaff HV. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2009;137(3):670–9.e5. [DOI] [PubMed] [Google Scholar]
- (59).Ghanta RK, Lapar DJ, Kern JA, Kron IL, Speir AM, Fonner E, Jr, et al. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: a real-world multi-institutional analysis. J Thorac Cardiovasc Surg. 2015;149(4):1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Medtronic Evolut Transcatheter Aortic Valve Replacement in low risk patients [Internet]. Last updated Oct 30, 2019. [cited 2019 Dec 17]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02701283?term=Evolut+Low+Risk&rank=1
- (61).Attizzani GF, Alkhalil A, Padaliya B, Tam CC, Lopes JP, Fares A, et al. Comparison of outcomes of transfemoral transcatheter aortic valve implantation using a minimally invasive versus conventional strategy. Am J Cardiol. 2015;116(11):1731–6. [DOI] [PubMed] [Google Scholar]
- (62).Babaliaros V, Devireddy C, Lerakis S, Leonardi R, Iturra SA, Mavromatis K, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovascular interventions. 2014;7(8):898–904. [DOI] [PubMed] [Google Scholar]
- (63).Barbanti M, Capranzano P, Ohno Y, Attizzani GF, Gulino S, Imme S, et al. Early discharge after transfemoral transcatheter aortic valve implantation. Heart (British Cardiac Society). 2015;101(18):1485–90. [DOI] [PubMed] [Google Scholar]
- (64).Kotronias RA, Teitelbaum M, Webb JG, Mylotte D, Barbanti M, Wood DA, et al. Early versus standard discharge after transcatheter aortic valve replacement: a systematic review and meta-analysis. JACC Cardiovascular interventions. 2018;11(17):1759–71. [DOI] [PubMed] [Google Scholar]
- (65).Glauber M, Ferrarini M, Miceli A. Minimally invasive aortic valve surgery: state of the art and future directions. Ann Cardiothorac Surg. 2015;4(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (67).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (68).Selva A, Sola I, Zhang Y, Pardo-Hernandez H, Haynes RB, Martinez Garcia L, et al. Development and use of a content search strategy for retrieving studies on patients' views and preferences. Health Qual Life Outcomes. 2017;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Amonn K, Stortecky S, Brinks H, Gahl B, Windecker S, Wenaweser P, et al. Quality of life in high-risk patients: comparison of transcatheter aortic valve implantation with surgical aortic valve replacement. Eur J Cardiothorac Surg. 2013;43(1):34–42. [DOI] [PubMed] [Google Scholar]
- (70).Dharmarajan K, Foster J, Coylewright M, Green P, Vavalle JP, Faheem O, et al. The medically managed patient with severe symptomatic aortic stenosis in the TAVR era: patient characteristics, reasons for medical management, and quality of shared decision making at heart valve treatment centers. PloS one. 2017;12(4):e0175926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Marsh K, Hawken N, Brookes E, Kuehn C, Liden B. Patient-centered benefit-risk analysis of transcatheter aortic valve replacement. F1000Res. 2019;8:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Rosseel L, Bieliauskas G, Brodersen BB, Olsen PS, Sondergaard L, De Backer O. Patients and informal caregivers' experience of surgical and transcatheter aortic valve replacement: real-world data contributing to establish value-based medicine in Denmark. Clin Cardiol. 2019;42(4):444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).van Mourik MS, Vendrik J, Abdelghani M, van Kesteren F, Henriques JPS, Driessen AHG, et al. Guideline-defined futility or patient-reported outcomes to assess treatment success after TAVI: what to use? Results from a prospective cohort study with long-term follow-up. Open Heart. 2018;5(2):e000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Wong S, Montoya L, Quinlan B. Transitional care post TAVI: a pilot initiative focused on bridging gaps and improving outcomes. Geriatr Nurs. 2018;39(5):548–53. [DOI] [PubMed] [Google Scholar]
- (75).Lytvyn L, Guyatt GH, Manja V, Siemieniuk RA, Zhang Y, Agoritsas T, et al. Patient values and preferences on transcatheter or surgical aortic valve replacement therapy for aortic stenosis: a systematic review. BMJ Open. 2016;6(9):e014327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Foley KM, Gelband H. Improving care [Internet]. Washington (DC): Press; 2001. [cited 2019 Mar 12]. Available from: www. [Google Scholar]
- (77).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (78).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (79).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (80).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (81).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2016 Jan]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (82).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (83).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]