Abstract
Background:
There has been an increasing focus on late functional effects of head and neck cancer (HNC) treatment. This study was undertaken to evaluate the incidence of late proximal esophageal stricture in patients undergoing total laryngectomy (TL) and radiation therapy (RT).
Material and Methods:
An institutional retrospective review of HNC patients treated between 1995 and 2003 with TL and RT was undertaken. Thirty-three patients with stage II–IV disease were included; 25 patients had TL and postoperative RT (group 1), while 8 patients had definitive RT with salvage laryngectomy (group 2).
Results:
The median follow-up was 28 months. At the last follow-up, 25 patients (76%) were alive and disease free. Four had died and 3 developed distant metastasis. Dysphagia or stenosis developed in 40% in group 1 and 75% in group 2 patients. The median time to dysphagia was 5.5 months for all patients.
Conclusions:
The incidence of esophageal stenosis was 33% for all patients. Contributing factors for esophageal stenosis after TL and RT include continued alcohol and tobacco use, the dose-volume relationship of the RT and normal tissue damage from the tumor and the treatment.
Keywords: Head and neck cancer, Total laryngectomy, Radiation therapy, Esophageal dysfunction
Introduction
In the past, surgery has been the mainstay of head and neck cancer (HNC) treatment with postoperative radiation therapy (PORT) for high-risk patients. Many recently published studies support organ preservation in locally advanced HNC patients with radiotherapy (RT) and chemotherapy [1–5]. With the increased interest in organ preservation [6], the issue of functional preservation has become more relevant and important. The major focus of treatment in the past was survival and local control. However, there has been a growing interest in the quality of life for those patients who tolerate the treatment and survive the cancer.
In HNC, one of the main issues after treatment is the patient’s ability to swallow. Swallowing is a complex process that requires the coordination of multiple muscles and nerves. Radiation and surgery may affect one or more steps in swallowing. A swallowing disorder is defined as any disruption in bolus passage from the oral cavity to the stomach. Dysphagia typically refers to a patient’s complaint or symptom of an underlying swallowing disorder. The nature and severity of the swallowing disorder and patient complaint may be related to surgical ablation of structures known to be critical for safe and efficient bolus passage and/or to stenosis or stricture related to RT [7–9].
Further, some patients may have a preexisting swallowing difficulty related to the tumor prior to the initiation of any treatment. There is a need to thoroughly assess swallowing function at various intervals during cancer treatment because the onset and chronicity of swallowing problems greatly impact the patient’s quality of life [10].
There are retrospective reports in the literature discussing the swallowing toxicity and rate of esophageal stenosis or strictures among patients who have been treated with both RT and total laryngectomy (TL) [11–16]. However, there is no clear consensus as to the true incidence of swallowing problems in these patients. This retrospective study was undertaken to determine the incidence of dysphagia and particularly esophageal strictures for patients treated with both a TL and RT at the Medical University of South Carolina.
Patients and Materials
Patients
Between 1995 and 2003, 33 consecutive patients with nonmetastatic HNC treated with RT and TL at the Medical University of South Carolina were identified. Institution review board approval was obtained prior to the start of this project. The characteristics of the 33 evaluable patients are presented in table 1. The 28 men and 5 women in our analysis had a median age of 59 years (range 45–75). Twenty-seven patients (82%) presented with laryngeal cancer, while the remainder had primaries located in the hypopharynx (n = 6). The population included stage II (n = 2), III (n = 11) and IV (n = 20) cancers.
Table 1.
Individual patient data
| Patient | Age years | Sex | Tumor site | Stage | Chemotherapy | RT dose Gy/fx | Salvage laryngectomy | Surgery/closure | Swallowing outcome | Time to dysphagia months | Follow-up months | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | larynx | T2 N0 | no | 62/31 | yes | TL/no data | dysphagia | 4 | 22 | 3 primaries, NED |
| 2 | 58 | M | HP | T1 N3 | yes | 66/33 | yes | TL/no data | stenosis | 0 | 48 | NED |
| 3 | 55 | M | HP | T4 N1 | no | 66/33 | no | T/no | stenosis | 84 | 105 | NED, CLL, melanoma |
| 4 | 59 | F | larynx | T2 N1 | no | 66/33 | yes | T/PC fistula | normal | NA | 12 | NED after 3 primaries |
| 5 | 54 | M | larynx | T3 N0 | no | 60/30 | no | TL/no | normal | NA | 96 | NED |
| 6 | 56 | M | larynx | T4 N0 | no | 60/30 | no | TL/no | normal | NA | 90 | NED |
| 7 | 62 | F | HP | T3 N1 | no | 60/30 | no | TL/no | normal | NA | 85 | NED |
| 8 | 74 | M | larynx | T4 N0 | no | 60/30 | no | TL/no | normal | NA | 84 | NED, prostate cancer |
| 9 | 73 | M | larynx | T2 N0 | no | 64/32 | no | TL/no | normal | NA | 84 | NED |
| 10 | 53 | F | larynx | T3 N0 | no | 60/30 | no | vert./no | normal | NA | 87 | NED |
| 11 | 53 | M | larynx | T4 N2b | no | 70/35 | yes | vert./no | stenosis | 0 | 62 | died |
| 13 | 58 | M | larynx | T3 N0 | no | 60/30 | no | T/no | dysphagia | 30 | 36 | NED, lost to FU |
| 14 | 67 | M | larynx | T3 N2b | no | 60/30 | no | T/no | normal | NA | 1 | died |
| 15 | 55 | M | larynx | T4 N2c | no | 60/30 | no | vert./no data | normal | NA | 48 | NED |
| 16 | 67 | M | larynx | T3 N0 | no | 60/30 | no | TL/no data | stenosis | 18 | 54 | NED |
| 17 | 54 | M | larynx | T3 N2b | yes | 70/35 | yes | TL/PC fistula | stenosis | 6 | 42 | NED, lost to FU |
| 18 | 50 | M | HP | T2 N3 | no | 60/30 | no | TL-PMC/no | stenosis | 2 | 51 | NED |
| 19 | 54 | M | HP | T2 N2b | no | 64/32 | no | TL/no data | dysphagia | 5 | 28 | died |
| 20 | 75 | F | larynx | T2 N1 | no | 64/32 | no | T/no | stenosis | 18 | 42 | NED |
| 21 | 57 | M | larynx | T2 N1 | no | 70/35 | yes | TL/no data | dysphagia | 6 | 6 | NED |
| 22 | 45 | M | larynx | T3 N3 | no | 61.2/34 | no | TL/no data | normal | NA | 31 | NED |
| 23 | 72 | M | larynx | T4 N2c | no | 60/30 | no | TL/no | normal | NA | 0.3 | died 11 days after RT |
| 24 | 48 | M | larynx | T3 N2c | no | 66/33 | no | TL/no | normal | NA | 6 | NED, lost to FU |
| 25 | 56 | M | larynx | T4 N2b | no | 66/33 | no | TL/no | normal | NA | 26 | lung and bone metastases |
| 26 | 56 | M | larynx | T3 N0 | no | 56/28 | no | TL/no | dysphagia | 12 | 21 | lung metastasis |
| 27 | 49 | M | larynx | T3 N0 | no | 54/27 | no | TL/no | normal | 1.5 | 18 | recurrent cancer |
| 30 | 52 | M | larynx | T4 N0 | no | 66/33 | no | vert./no | normal | NA | 12 | NED |
| 31 | 59 | F | larynx | T4 N2c | no | 60/30 | no | T/no | stenosis | 3 | 15 | NED |
| 32 | 54 | M | larynx | T4 N2c | yes | 70/35 | yes | TL-PMC/no | normal | 0 | 6 | NED |
| 33 | 67 | M | larynx | T4 N2c | no | 60/30 | no | TL/no | normal | NA | 9 | NED |
fx = fractions; NED = no evidence of disease; HP = hypopharynx; T = T closure; CLL = chronic myelogenous leukemia; PC = pharyngocutaneous; NA = not assessed; vert. = vertical closure; FU = follow-up; PMC = pectoralis myocutaneous flap.
Treatment
Chemotherapy was administered in 3 patients concurrent with RT for definitive treatment. No chemotherapy was given to patients who received PORT after a TL. The majority of the patients had PORT after a TL (n = 25), while the remaining patients had a salvage laryngectomy (SL) after RT either for laryngeal dysfunction (n = 1), new cancer primary (n = 3) or salvage of persistent disease (n = 4).
All patients were treated in the supine position with the aid of immobilization devices. All patients’ treatments prior to 2001 were planned two-dimensionally with plain films obtained under fluoroscopy. Patients were treated with a three-field standard head and neck setup. The opposed lateral radiation fields were half-beam blocked inferiorly to match with a superiorly half-beam blocked anterior supraclavicular radiation field in order to avoid overlapping of the fields. Custom-made Cerrobend blocks were used to keep normal structures from being treated to reduce acute and late toxicities. However, most patients’ treatments from 2001 to 2003 were planned three-dimensionally from contiguous computed tomography slices. No tissue inhomogeneity correction was attempted in the planning process. All patients in this analysis were treated on a 6-megavoltage linear accelerator. Treatments were given on a daily basis for 5 days a week. The mean dose in the group treated with definitive RT was 68 Gy (range 66–70). In the postoperative setting, the mean RT dose was 63 Gy (range 54–66).
In this cohort of 34 patients for whom long-term follow-up is available, we found that surgical therapy typically consisted of TL with primary closure of the resultant pharyngotomy. A vertical pharyngeal closure was used in the majority of cases, with 5 T-type closures and 3 pectoralis major myocutaneous flaps in the remainder. No free flap reconstructions were used in this cohort. One postoperative pharyngocutaneous fistula was identified, in a patient who had received RT prior to surgery. A T-type closure was used in this patient. Another patient developed a wound breakdown, without a fistula identified. This was managed conservatively, with resolution. Interestingly, a T-type closure was utilized in this patient as well, but there was no prior history of RT.
Swallowing Assessment
Swallowing function was mainly obtained by physician assessment of the patient at follow-up appointments. If the patient complained of dysphagia, either modified barium swallowing examination or an esophagogram was obtained. Regular radiographic assessment of swallowing was not obtained when the patient was asymptomatic. Stenosis was operationally defined as a narrowing in the region of the cervical esophagus or neopharynx resulting in partial obstruction of bolus flow. Stricture was defined as lack of tissue separation in the region of the cervical esophagus or neopharynx with complete obstruction to bolus flow. If a stenosis or stricture was noted on the esophagogram, the patient was then brought to the operating room and dilated with a Mallory dilator either by the gastroenterologist or the otolaryngologist. Some patients underwent dilatation by the treating otolaryngologist without a previous esophagogram if clinical suspicion was strong enough. Whenever a modified barium swallowing or an esophagogram was available to document the stenosis, we attempted to define the particular region involved in order to correlate it with the anterior and lateral radiation fields’ match point.
Follow-Up
Time to follow-up was calculated from the completion of both RT and TL (in any order) until the last assessment by a physician in the radiation, otolaryngology or medical oncology department. Most patients were followed more regularly by the head and neck surgeons than by the other two departments.
Statistical Analysis
Patients were divided into two groups for statistical analysis: those treated with TL followed by PORT (n = 25; group 1) and those treated initially with RT followed by SL (n = 8; group 2). First, Kaplan-Meier survival curves for two groups were generated to make an initial exploratory comparison of time to dysphagia between patients receiving RT first and patients receiving TL first. Then, log-rank and Wilcoxon tests were used to determine whether there was a statistically significant evidence of difference in dysphagia-free survival rate between the two groups.
Results
At a median follow-up of 28 months (range 1–105), 25 patients (76%) were alive with no evidence of disease at the last follow-up (table 1). Five patients were eventually lost to follow-up. Four patients have died and 3 other patients have developed lung and bone metastases. Three patients had either a second or third primary in the head and neck region. All of these patients had surgery that included a TL to remove the new cancer. Two other patients developed another non-HNC (chronic myelogenous leukemia, melanoma and prostate cancer). A total of 8 patients (24%) required an SL for laryngeal dysfunction (n = 1), new primaries (n = 3) or for salvage of persistent (residual or recurrent) disease (n = 4).
The patients with abnormal swallowing function after both radiation and total laryngectomy are listed in table 2. A total of 16 patients (48%) had complaints or observations of mechanical swallowing problems, i.e. dysphagia, or had confirmed stenosis in the radiographic examination. Eleven patients (33%) had stenosis and all required esophageal dilatations for relief of symptoms. Three out of 5 patients with dysphagia did not require intervention because no abnormalities were detected on barium esophagograms. Ten (40%) of the 25 patients in group 1 developed dysphagia of whom 7 (28%) developed stenosis. These patients had an average of 1.6 dilatations (range 1–3) with improved swallowing after the procedure. The median time to the development of dysphagia was 8.5 months (range 1.5–84). Six of the 8 patients (75%) in group 2 developed dysphagia of which 4 (50%) developed stenosis. These patients had an average of 1.5 (range 1–2) dilatations with improvement of function. The median time to the development of dysphagia in this group was 5 months (range 0–9).
Table 2.
Patients with swallowing abnormality after TL and RT
| Patient | Swallowing dysfunction | Time to esophageal dysfunction, months | Esophageal dilatations | Gastroesophageal reflux disease | SL |
|---|---|---|---|---|---|
| 1 | stenosis | 4 | 1 | yes | yes |
| 2 | dysphagia | – | 0 | not known | yes |
| 3 | stenosis | 84 | 1 | not known | no |
| 11 | stenosis | 0 | 1 | no | yes |
| 12 | stenosis | 9 | 2 | no | yes |
| 13 | dysphagia | 30 | 1 | no | no |
| 16 | stenosis | 18 | 3 | yes | no |
| 17 | stenosis | 6 | 2 | no | yes |
| 18 | stenosis | 2 | 1 | no | no |
| 19 | dysphagia | 5 | 0 | yes | no |
| 20 | stenosis | 18 | 3 | no | no |
| 21 | dysphagia | 6 | 0 | yes | yes |
| 26 | dysphagia | 12 | 1 | no | no |
| 28 | stenosis | 3 | 2 | no | no |
| 29 | stenosis | 1.5 | 2 | no | no |
| 31 | stenosis | 3 | 2 | no | no |
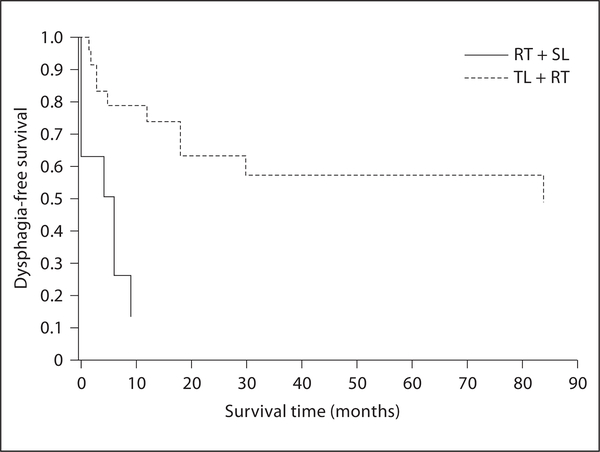
The Kaplan-Meier estimates (Kaplan-Meier curves) of the dysphagia-free survival rates were compared between both groups in figure 1. In the plot, the difference between the two groups in terms of dysphagia-free survival rates at any time point is very evident, and the difference in survival rates seems to be widening over time. Very strong statistical evidence of a better dysphagia-free survival rate for patients treated initially with TL followed by PORT (compared to the dysphagia-free survival rate of the other group) was found by both log-rank and Wilcoxon tests (p < 0.001 for both tests). Though numbers are small, it is important to note that the ratio of risk of dysphagia development between the two groups does not seem to decrease over time. This suggests that the risk of developing dysphagia for patients who received RT first followed by SL is higher at any time point comparison than for patients who received TL first followed by PORT. Using the Lifetest procedure of the statistical package SAS and under the assumption of Cox’s proportional hazards model, we found the estimated hazard ratio of dysphagia between two groups to be 0.183. This implies that the risk of dysphagia at any time point for a patient receiving TL followed by PORT is estimated to be 18.3% of the corresponding risk of a patient receiving RT followed by SL (under the assumption that the ratio of risks between two groups remains constant over time).
Fig. 1.
Kaplan-Meier curves showed that dysphagia-free survival was significantly better for patients receiving TL first followed by RT (p < 0.001 by log-rank and Wilcoxon tests). SL = Salvage laryngectomy.
Discussion
Swallowing dysfunction may result from several factors in HNC patients treated with surgery and RT. The cancer itself may have caused irreparable damage to the muscles and nerves involved in swallowing. Radiation can alter the ability to swallow by causing xerostomia, fibrosis, cranial nerve damage and restriction of laryngeal elevation [17]. Having a TL will prevent aspiration of food, but will lead to lack of coordination between the pharyngeal constrictors and upper esophageal sphincter [18, 19]. Although chemotherapy was only given to 3 patients in this study in the postoperative setting, it can potentiate the radiation and thus increase the toxicity of RT.
Weber et al. [2] reported on the outcome of salvage TL following organ preservation in the Radiation Therapy Oncology Group trial 91–11 and showed an increased incidence of pharyngocutaneous fistula among patients receiving chemotherapy along with radiation as organ preservation therapy.
Our retrospective study showed that 48% of the patients who had both a TL and RT had either subjective or objective dysphagia with 33% of the entire study population showing evidence of an esophageal stenosis on the barium esophagogram or modified barium swallowing. The relatively high rate of stenosis may be due to the inclusion of SL patients who had previously received full-dose RT. There are many published articles that address patients who require TL and RT for the treatment of HNC, but the main focus is usually on local control and survival. Few studies have discussed late esophageal toxicities from these treatments. These studies have quoted esophageal stricture rates between 1 and 13% [12–15]. It is hard to know if esophageal stenosis is included as a category of stricture since the terms have often been interchangeable. These reports of late toxicities do not specify how these patients were evaluated for their swallowing problems. Our esophageal stenosis rate is higher than that of any other reported study, and perhaps this late toxicity is underreported in the literature. There has been a greater focus on organ preservation in laryngeal and hypopharyngeal cancers with the success of several randomized studies showing that a nonsurgical approach can lead to comparable survival rates to surgery [3–5]. The emergence of organ preservation will most likely lead to increased interest in functional preservation and a greater number of publications on long-term swallowing ability after cancer treatment. This retrospective review included a small number of patients, and this may have contributed to the higher rate of stenosis reported, although other retrospective studies have included 30 patients or less in their analysis [12–15]. We would like to collaborate and pool our data with other institutions in the future in order to better assess the true rate of late esophageal strictures and stenosis.
The median time to the development of dysphagia after radiation has been reported to be 6–8 months [13, 20, 21]. Our study confirms this and found that the median time to the development of esophageal stenosis or dysphagia was 6–8.5 months after the completion of either radiation or TL (whichever one was later). Dysphagia-free survival was significantly better for patients treated with TL and PORT compared to patients treated with primary RT and SL. We do not provide data for patients treated with RT alone.
There are several possible reasons why these HNC patients develop late esophageal stenosis. Many of these patients have a history of heavy alcohol and tobacco use and often will continue these habits throughout and after the completion of treatment. These toxins can increase the risk of damage to already compromised tissue.
Acute esophagitis was once postulated to be a contributing factor in the development of late esophageal toxicities, but many authors have argued against this connection. Acute esophagitis is secondary to pseudomembranous inflammation and is not a contributing factor in the development of long-term esophagitis or fibrosis [22, 23]. Esophageal stricture is due to submucosal fibrosis and chronic arteriolitis [23].
There are 5 cranial nerves involved in swallowing and these may become damaged from surgery, RT and tumor and may contribute to the patient’s inability to swallow correctly [24].
These esophageal strictures and stenosis can be both due to a benign or malignant process. The proximal esophagus is often an innocent bystander to the radiation in the treatment of the head and neck area. Radiation exposure has been well proven to be a carcinogen [25–29] and may rarely be a very late cause of esophageal strictures or stenosis in patients treated with RT for HNC. In our study, we had only 1 patient who had cancer present at the proximal esophagus, but this cancer was an extension of a large stomal recurrence and not due to the development of a malignant proximal esophageal stenosis.
Approximately 80% of benign esophageal strictures may be due to gastroesophageal reflux disease [30]. However, they are most commonly found in the distal esophagus because this area is exposed to the largest amount of acid [30]. The esophageal stenoses that formed in our patients were proximal. Also, we did not find a correlation between gastroesophageal reflux disease and the development of dysphagia or stenosis.
One of the interesting observations in this study was the relatively small number of dilatations (mean = 1.6) required to improve the stenosis. This may indicate that the stenosis following TL and RT may differ from esophageal strictures or stenosis due to other causes including those resulting from the radiation treatment of the esophagus and lung.
A recent retrospective study by Laurell et al. [13] found a dose-volume relationship in the development of proximal esophageal strictures for patients treated definitively with RT for HNC. Patients with esophageal strictures all received at least 60 Gy in greater than 80% of the first 2 cm of the proximal esophagus. There was no radiation injury noted in patients receiving less than 60 Gy. We were not able to find a correlation between dose and esophageal stenosis because the majority of our patients were treated with two-dimensional planning. Only 6 patients had treatment planned with CT images, and numbers were too small for meaningful analysis.
We attempted to correlate the region of stenosis to the area where the lateral and anterior radiation ports were matched. Of the 16 patients who complained of dysphagia, we were able to review the studies of only 3 patients who had a swallowing study done prior to any dilatation. These 3 patients all had stenosis at the level of C3 to C5, which was higher than the radiation port matchline of the C6 /C7 interspace in most patients.
Our retrospective review showed a higher rate of stenosis in patients with definitive RT with SL (group 2). It should be clarified that these two patient groups are not directly comparable. Group 2 received a higher average RT dose (68 vs. 63 Gy). Also, chemotherapy was used as a radiosensitizer in 3 of 8 patients with SL, thereby increasing the risk of fibrosis. The possibly higher stenosis rate in group 2 patients should lead to a greater consideration of free flaps instead of primary closure in patients whose native tissues seem compromised at the time of resection. Soft-tissue complications including fibrosis are seen more commonly after SL due to preexisting radiation-induced soft-tissue fibrosis in the neck.
In this study, flaps were considered in patients with significant loss of mucosa of the hypopharynx that did not allow for an estimated esophageal lumen of at least 1 cm. Most patients had primary closure by either linear (vertical) fashion or a T closure. The method or closure was largely by surgeon preference and not due to patient or disease factors.
Although esophageal strictures have been found to be less than 15% in patients treated with both RT and TL [12, 14, 15], it can lead to a very poor quality of life for the few patients affected. Since dose was found to be correlated with the risk of developing esophageal strictures in one study [13], we believe that carefully avoiding treating the structures involved in the swallowing process with the use of intensity-modulated radiation therapy may help decrease the morbidity of HNC treatments.
Footnotes
The results of this paper were presented at the American Radium Society May 2004 annual meeting as an oral presentation (abstract No. 50).
References
- 1.Al-Sarraf M, LeBlanc M, Giri PG, et al. : Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol 1998; 16: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 2.Weber RS, Berkey BA, Forastiere A, et al. : Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91–11. Arch Otolaryngol Head Neck Surg 2003; 129:44–49. [DOI] [PubMed] [Google Scholar]
- 3.Denis F, Garaud P, Bardet E, et al. : Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004; 22: 69–76. [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre JL, Chevalier D, Luboinski B, et al. : Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst 1996; 88: 890–899. [DOI] [PubMed] [Google Scholar]
- 5.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med 1991; 324: 1685–1690. [DOI] [PubMed] [Google Scholar]
- 6.Forastiere AA, Goepfert H, Maor M, et al. : Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349: 2091–2098. [DOI] [PubMed] [Google Scholar]
- 7.Pauloski BR, Logemann JA: Impact of tongue base and posterior pharyngeal wall biomechanics on pharyngeal clearance in irradiated postsurgical oral and oropharyngeal cancer patients. Head Neck 2000; 22: 120–131. [DOI] [PubMed] [Google Scholar]
- 8.Denittis AS, Machtay M, Rosenthal DI, et al. : Advanced oropharyngeal carcinoma treated with surgery and radiotherapy: oncologic outcome and functional assessment. Am J Otolaryngol 2001; 22: 329–335. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A, Lyden T, Bradford CR, et al. : Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2002; 53:23–28. [DOI] [PubMed] [Google Scholar]
- 10.DeSanto LW, Olsen KD, Perry WC, et al. : Quality of life after surgical treatment of cancer of the larynx. Ann Otol Rhinol Laryngol 1995; 104: 763–769. [DOI] [PubMed] [Google Scholar]
- 11.Laccourreye O, Hans S, Borzog-Grayeli A, et al. : Complications of postoperative radiation therapy after partial laryngectomy in supraglottic cancer: a long-term evaluation. Otolaryngol Head Neck Surg 2000; 122: 752–757. [DOI] [PubMed] [Google Scholar]
- 12.Ampil FL, Nathan CA, Caldito G, et al. : Total laryngectomy and postoperative radiotherapy for T4 laryngeal cancer: a 14-year review. Am J Otolaryngol 2004; 25: 88–93. [DOI] [PubMed] [Google Scholar]
- 13.Laurell G, Kraepelien T, Mavroidis P, et al. : Stricture of the proximal esophagus in head and neck carcinoma patients after radiotherapy. Cancer 2003; 97: 1693–1700. [DOI] [PubMed] [Google Scholar]
- 14.Parsons JT, Mendenhall WM, Stringer SP, et al. : Salvage surgery following radiation failure in squamous cell carcinoma of the supraglottic larynx. Int J Radiat Oncol Biol Phys 1995; 32: 605–609. [DOI] [PubMed] [Google Scholar]
- 15.Zelefsky MJ, Kraus DH, Pfister DG, et al. : Combined chemotherapy and radiotherapy versus surgery and postoperative radiotherapy for advanced hypopharyngeal cancer. Head Neck 1996; 18: 405–411. [DOI] [PubMed] [Google Scholar]
- 16.Thawley SE: Complications of combined radiation therapy and surgery for carcinoma of the larynx and inferior hypopharynx. Laryngoscope 1981; 91: 677–700. [PubMed] [Google Scholar]
- 17.Lazarus CL: Effects of radiation therapy and voluntary maneuvers on swallow functioning in head and neck cancer patients. Clin Commun Disord 1993; 3: 11–20. [PubMed] [Google Scholar]
- 18.Duranceau A, Jamieson G, Hurwitz AL, et al. : Alteration in esophageal motility after laryngectomy. Am J Surg 1976; 131: 30–35. [DOI] [PubMed] [Google Scholar]
- 19.Hanks JB, Fisher SR, Meyers WC, et al. : Effect of total laryngectomy on esophageal motility. Ann Otol Rhinol Laryngol 1981; 90: 331–334. [DOI] [PubMed] [Google Scholar]
- 20.O’Rourke IC, Tiver K, Bull C, et al. : Swallowing performance after radiation therapy for carcinoma of the esophagus. Cancer 1988; 61:2022–2026. [DOI] [PubMed] [Google Scholar]
- 21.Lepke RA, Libshitz HI: Radiation-induced injury of the esophagus. Radiology 1983; 148:375–378. [DOI] [PubMed] [Google Scholar]
- 22.Silvain C, Barrioz T, Besson I, et al. : Treatment and long-term outcome of chronic radiation esophagitis after radiation therapy for head and neck tumors: a report of 13 cases. Dig Dis Sci 1993; 38: 927–931. [DOI] [PubMed] [Google Scholar]
- 23.Vanagunas A, Jacob P, Olinger E: Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol 1990; 85: 808–812. [PubMed] [Google Scholar]
- 24.Mittal BB, Pauloski BR, Haraf DJ, et al. : Swallowing dysfunction – Preventative and rehabilitation strategies in patients with headand-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. Int J Radiat Oncol Biol Phys 2003; 57:1219–1230. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, Kopecky KJ, Fujikura T, et al. : Lung cancer incidence among Japanese Abomb survivors, 1950–80. J Radiat Res (Tokyo) 1987; 28: 156–171. [DOI] [PubMed] [Google Scholar]
- 26.Upton AC: The dose-response relation in radiation-induced cancer. Cancer Res 1961; 21:717–729. [PubMed] [Google Scholar]
- 27.Travis LB, Curtis RE, Boice JD Jr: Late effects of treatment for childhood Hodgkin’s disease. N Engl J Med 1996; 335: 352–353. [PubMed] [Google Scholar]
- 28.Land CE: Studies of cancer and radiation dose among atomic bomb survivors: the example of breast cancer. JAMA 1995; 274: 402–407. [PubMed] [Google Scholar]
- 29.Modan B, Baidatz D, Mart H, et al. : Radiation-induced head and neck tumours. Lancet 1974;i:277–279. [DOI] [PubMed] [Google Scholar]
- 30.Richter JE: Peptic strictures of the esophagus. Gastroenterol Clin North Am 1999; 28:875–891. [DOI] [PubMed] [Google Scholar]