Abstract
Background:
Teens with type 1 diabetes (T1D) experience increased sleep disturbances, which have been linked to problems with adherence and glycemic control. As such, sleep represents a novel target to improve outcomes in teens.
Objective:
To evaluate the feasibility, acceptability, and preliminary efficacy of a sleep-promoting intervention in teens with T1D.
Research Design and Methods:
Teens aged 13 to 17 with T1D (n = 39) completed measures of sleep quality and diabetes management and wore actigraphs to obtain an objective measure of sleep. Hemoglobin A1C (HbA1c) was collected from medical records. Teens were randomized to Usual Care (n = 19) or the Sleep Coach intervention (n = 20). Teens in the Sleep Coach group received educational materials on healthy sleep habits and completed three individual telephone sessions. Follow-up data were collected at 3 months, including exit interviews with teens and parents.
Results:
Feasibility of the study was excellent; 80% of teens in the Sleep Coach group completed all three sessions, and retention was high (90%). Based on actigraphy data, a significant improvement in sleep efficiency and sleep duration was observed (48-minute increase) among teens randomized to the Sleep Coach intervention, and teens in the control group were 7.5 times more likely to report poor sleep quality after 3 months than intervention participants. No change in HbA1c was observed.
Conclusions:
The Sleep Coach intervention for teens with T1D is a feasible and acceptable program that increased sleep duration and improved sleep quality for this high-risk population.
Keywords: adolescents, clinical trial, sleep, type 1 diabetes
1 |. INTRODUCTION
Adolescents with type 1 diabetes (T1D) obtain shorter sleep duration and experience increased sleep disturbances compared to adolescents without T1D.1,2 While adolescents with T1D experience many of the same barriers to obtaining sufficient sleep as the general population (eg, academic and extracurricular commitments, electronics use, early school start times), they also experience diabetes-related sleep disturbances, such as treating episodes of nocturnal hypoglycemia and awakenings related to device alarms.3,4
Accumulating evidence indicates that sleep duration, quality, and timing influence glycemic control and self-management in adolescents with T1D.5,6 For example, one study found that adherence to blood glucose checks and insulin boluses increased with every additional 15 to 20 minutes adolescents slept.7 Furthermore, a recent study found that variability in sleep duration was significantly related to glycemic control and adherence among adolescents with T1D.4 Given that most adolescents struggle to reach glycemic targets, despite increased use of new diabetes technology,8 innovative approaches are needed to improve diabetes-related outcomes in this population. Sleep habits may represent a novel target for improving T1D management in adolescents.
The current study pilot tested a sleep-promoting intervention to assess feasibility and acceptability among adolescents with T1D. In addition, we explored intervention effects on sleep (sleep duration, quality, and efficiency), daytime functioning, and diabetes indicators (diabetes management, glycemic control).
2 |. RESEARCH DESIGN AND METHODS
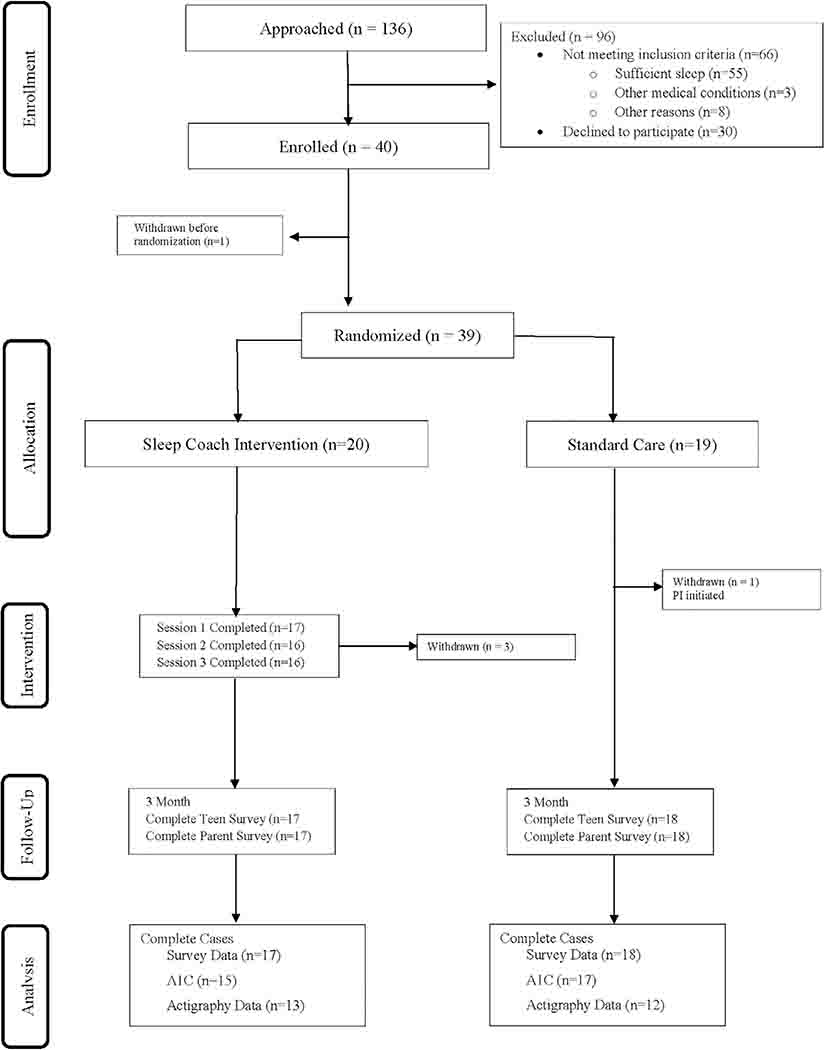
We conducted a randomized pilot of a sleep-promoting intervention for teens with T1D (NCT02786953). Adolescents were eligible if they were between the ages of 13 and 17 years, had been diagnosed with T1D for at least 12 months, and self-reported insufficient sleep (<8 hours on most school nights). Enrollment was timed so that all data were collected during the school year. Of the 136 adolescents approached during regularly-scheduled diabetes clinic visits, 49% (n = 66) were ineligible (most common reason was they were obtaining sufficient sleep), and 43% of eligible adolescents (n = 30) declined to participate, with no significant differences in teen age, sex, race/ethnicity, or A1C between those who declined and those who enrolled. Among the 40 enrolled participants, five withdrew from the study (one withdrew after enrollment but before randomization, one was withdrawn by the PI after randomization due to a new diagnosis, and three withdrew from the Sleep Coach group due to the time commitment), see Figure 1. Adolescents and their caregivers provided informed consent/assent in line with the protocol approved by the University Institutional Review Board. After completing baseline data collection, teens were randomized to the Sleep Coach Intervention (n = 20) or Usual Care (n = 19). Randomization was determined by a computerized program created by the study biostatistician, and teens were stratified by treatment type (insulin pump vs injections) to avoid confounding by differences related to insulin regimen. Adolescents and parents were invited to take part in optional exit interviews at the 3-month data collection to provide more detailed information regarding feasibility and acceptability.
FIGURE 1.
CONSORT diagram
3 |. MEASURES
Adolescents and their caregivers completed several validated measures at baseline and 3 months. Follow-up data collection was timed to coincide with regularly scheduled diabetes clinic visits.
3.1 |. Sleep measures
Adolescents completed the Pittsburgh Sleep Quality Index (PSQI,9), a self-report measure of sleep quality. A global score > 5 on the PSQI suggests clinically significant sleep disturbances. Teens also completed the Epworth Sleepiness Scale (ESS,10), which assesses daytime functioning. Cronbach’s alpha was .76 for adolescents’ self-report.
To obtain a more objective measure of sleep, adolescents wore an actigraph (Philips Respironics Spectrum Plus) at night and completed a sleep diary for seven nights prior to randomization and again at 3 months. The sleep diary included spaces for adolescents to indicate bedtime and waketime, whether it was a school night, naps, nighttime BG checks, and other factors that may influence sleep (eg, activity level, caffeine intake, use of electronics). Based on earlier studies (23), we configured actigraph watches using a 1-minute epoch, with a sleep interval of 10 epochs for onset of sleep, and an awake threshold setting of 40 (medium). Data from the watches were analyzed using Philips Actiware software to calculate sleep efficiency (ratio of total sleep time to time in bed) and sleep duration (total sleep time). All participants were mailed a summary of their sleep characteristics (mean total sleep time and sleep efficiency) after completing baseline data collection.
3.2 |. Diabetes indicators
Adolescents and their parents completed the Self-Care Inventory (SCI,11) as a measure of diabetes management. The SCI consists of 14 items, and higher scores indicate better management. Cronbach’s alpha was .78 for adolescent’ self-report and .70 for parent report.
Hemoglobin A1C (HbA1c), a measure of glycemic control over the prior 8 to 12 weeks, was extracted from participants’ medical records from the clinic visit on the day of enrollment and regular clinic visits 3 and 6 months later.
4 |. INTERVENTIONS
Teens in the Sleep Coach group received a binder with psychoeducational materials on healthy sleep habits,12 supplemented with diabetes-specific examples from previous qualitative interviews with teens and their caregivers.3 A trained member of the research team conducted three individual phone calls with teens in the Sleep Coach group (see Table 1). The first call focused on healthy sleep habits and lasted approximately 20 to 30 minutes. The two booster calls occurred approximately 1 week and 1 month after the initial call, and each call lasted about 10 minutes. The sessions were designed to be interactive and individualized; participants were asked to describe current behaviors and choose specific goals (eg, fewer caffeinated drinks after 5 PM). Participants were also asked to choose one of three areas to focus on: relaxation and mindfulness (chosen by 11 teens), sleep timing (chosen by 6 teens), or coping thoughts (chosen by 1 teen). Parents were given the psychoeducational materials with the following instructions: “Your teen will be working with a sleep coach, and your role is to support your teen’s efforts to change.” All calls were recorded and 20% were randomly selected for a fidelity check by an objective rater. Fidelity scores ranged from 88.5% to 100%, with an average of 99%.
TABLE 1.
Intervention content
| Content | Purpose/concept | Example |
|---|---|---|
| Session 1 (20–30 minutes) | ||
| Stimulus control (sleep hygiene) | Identify daytime and evening habits that can affect sleep and sleep environment. Note the relationship between diabetes management and sleep. |
Remove electronic devices from bedroom, set a communication curfew, limit caffeine use, create a bedtime routine. |
| Modules (Chosen by individual participants to tailor the intervention to their needs) | ||
| Sleep timing | Identify sleep amount/timing/regularity, noting differences between weekday and weekend sleep schedules. | Set goals for more consistent bedtime and wake time, use sleep diaries to monitor progress toward goals. |
| Relaxation training | Reduce tension at bedtime or during night wakings. | Use progressive muscle relaxation and/or imagery at bedtime/wakings. |
| Coping thoughts | Address worried thoughts related to difficulty falling asleep or staying asleep. | Develop coping thoughts to replace negative thoughts at bedtime/wakings. |
| Session 2 (1 week later, 10 minutes) | ||
| To Sleep or Not to Sleep? | Validate difficulty with changing habits, highlight benefits of new habits. | Removing phone from room may mean missed call/texts from friends, but teen may feel more rested the next day. |
| Session 3 (1 month later, 10 minutes) | ||
| Planning for setbacks | Identifying potential events that may disrupt sleep. | Plan to take a nap the day after a sleepover. |
5 |. USUAL CARE
Participants in the Usual Care continued with regular outpatient clinic visits with a physician or nurse practitioner (quarterly) and telephone access to a health care provider 24 hours a day, 7 days a week.
6 |. DATA ANALYSIS
We conducted intention-to-treat analyses to determine the effects of the intervention on outcomes using complete case analysis. The association of continuous outcomes at 3 months with treatment group was estimated using linear regression controlling for baseline. For sleep duration and efficiency measured by actigraphy, we also controlled for age and having school the next day. Odds ratios were calculated for binary outcomes. Exit interviews were transcribed and coded to identify text about participants’ experiences in and perceptions about the study. We used a thematic analytic approach to organize patterns (themes) across the data.13
7 |. RESULTS
Mean age of the adolescents was 15.3 years; 54% were female; 74% were White, non-Hispanic, 8% were Hispanic, 10% were Black, and 8% were biracial (see Table 2). Mean duration of diabetes was 8.4 years; 59% used insulin pumps, and 5% used continuous glucose monitors. Mean HbA1c was 9.2% (77.0 mmol/mol). The only significant difference between groups observed at baseline was age; adolescents randomized to the sleep coach group were significantly younger than adolescents randomized to the usual care group.
TABLE 2.
Demographics and baseline clinical characteristics by treatment group
| Characteristic | SC (n = 20) | UC (n = 18) | Total sample (n = 38) |
|---|---|---|---|
| Adolescent age, M (SD) | 14.85 (1.27) | 15.72 (1.32)* | 15.26 (1.35) |
| Duration of diabetes, M (SD) | 6.55 (3.27) | 7.33 (3.91) | 6.66 (3.54) |
| A1C, M (SD) | 9.29 (2.17) | 9.06 (1.45) | 9.18 (1.84) |
| Sex | |||
| Male, n (%) | 10 (50) | 8 (44) | 18 (47) |
| Female, n (%) | 10 (50) | 10 (56) | 20 (53) |
| Race/ethnicity | |||
| White, non-Hispanic, n (%) | 15 (75) | 14 (78) | 29 (76) |
| Non-White, n (%) | 5 (25) | 4 (22) | 9 (24) |
| Annual income (USD) | |||
| <39 000, n (%) | 7 (37) | 2 (12) | 9 (24) |
| 40 000–79 000, n (%) | 7 (37) | 5 (29) | 12 (33) |
| >80 000, n (%) | 5 (26) | 10 (59) | 15 (42) |
| Treatment type | |||
| Insulin Pump, n (%) | 12 (60) | 11 (61) | 23 (61) |
| Injection, n (%) | 8 (40) | 7 (39) | 15 (39) |
| CGM Use, n (%) | 2 (10) | 0 (0) | 2 (5) |
Abbreviations: CGM, continuous glucose monitor; SC, sleep coach intervention; UC, usual care.
Significant difference between groups (P < .05).
At baseline, 27% of teens reported poor sleep quality (score > 5 on the PSQI). Based on actigraphy data (90% usable data), the mean sleep duration was 6.9 hours (well below the recommended duration of 8–10 hours for teens), and mean sleep efficiency was 83% (recommended efficiency is ≥85%).
7.1 |. Feasibility
Of the 20 participants randomized to the Sleep Coach intervention, 17 (85%) completed the first session, and 80% completed all three individual sessions. In addition, excluding the participants who withdrew from the study (n = 5), we obtained follow-up survey data from 100% of the teens and caregivers at 3 months and usable actigraphy data from 71% of adolescents. There were no differences in demographic or clinical variables between teens who withdrew and those who completed the study.
7.2 |. Acceptability
Adolescents reported that the study was generally helpful (mean score was 3.5 on a 1–5 scale, where 1 = not helpful, 2 = a little helpful, 3 = somewhat helpful, 4 = pretty helpful, and 5 = very helpful), and enjoyable (mean score = 3.4), and 89% reported that they would recommend the study to others. Groups did not differ significantly in satisfaction ratings.
Exit interviews conducted with teens (n = 29, 13 Sleep Coach participants, 16 Usual Care participants) revealed an overall favorable experience and few barriers to participation. Teens described challenges to wearing or using the actigraphy watch, such as remembering to put it on or to press the button when going to sleep or upon waking (21% of teens interviewed). Some teens also experienced mild discomfort wearing the watch (28%). For example, they reported that the watches were “irritating” (M, 15 years), “clunky” (M, 15 years), and “bulky and hard to sleep with” (M, 14 years). In addition, eight teens reported a challenge of remembering to write in the sleep diary (28%).
Despite these barriers, approximately one-third of teens (31%) reported that they enjoyed learning about their own sleep patterns. Many teens (28%) reported that they liked tracking their sleep and recording their blood glucose checks as part of the sleep diary (completed by the Sleep Coach and Usual Care participants), particularly for helping hold them accountable to a schedule. “I could get a sense of how I slept and having the journal helped me see…different things about my sleep schedule and different blood sugar checks. Just having it written down and everything…kind of fascinated me” (M, 17 years). Similarly, another teen (M, 14 years) liked “track[ing] how many hours I’ve slept and to know what my blood sugar was in the morning and how it changed in the afternoon.” More than two-thirds of teens interviewed (69%) found the Sleep Coach intervention binder useful, and almost all of the teens (92%) reported sustained use of new sleep strategies (“After a while, it just became a habit” (M, 17 years). For example, one teen (F, 13 years) noted, “I don’t drink Cokes late at night like I was doing before.”
7.3 |. Efficacy
As seen in Table 3, we observed medium effect sizes of the intervention on mean total sleep time (using actigraphy data) and sleep quality (based on adolescents’ self-report), and small intervention effects on sleep efficiency (using actigraphy data), and daytime functioning (based on adolescents’ self-report). Furthermore, in regression analyses controlling for age at baseline and school night vs weekend night, at 3 months, Sleep Coach participants slept, on average, 48 minutes longer (95% CI = 6–91 minutes, P = .025). Similarly, Sleep Coach participants exhibited higher sleep efficiency at 3 months (4.4% higher, 95% CI = 0.6–8.3, P = .024). In terms of self-reported measures, teens in the Usual Care group were 7.5 times more likely to report poor sleep quality at 3 months (95% CI = 0.7–76.6, P = .04) compared to intervention participants. However, controlling for baseline scores, there was insufficient evidence to demonstrate that being in the intervention group was associated with improved daytime functioning (lower ESS scores) at 3 months (P = .07). There was not a significant effect of intervention on glycemic control; on average, HbA1c for teens in the Sleep Coach was 0.06% lower at 3 months as compared to teens in the Usual Care group, after adjusting for baseline levels (P = .89). There was no significant difference in diabetes management (parent and self-reported SCI scores did not change).
TABLE 3.
Summary of Sleep and Diabetes Outcomes at Each Time Point by Intervention Group
| Variable | Baseline (M ± SD) | 3 months (M ± SD) | Effect size (d) |
|---|---|---|---|
| Mean TST | |||
| SC | 6.85 ± 0.82 | 7.02 ± 0.78* | .76 |
| UC | 6.69 ± 1.00 | 6.37 ± 0.92 | |
| Mean efficiency | |||
| SC | 84.09 ± 3.75 | 83.01 ± 4.04* | .38 |
| UC | 81.24 ± 6.52 | 80.27 ± 9.30 | |
| PSQI | |||
| SC | 4.32 ± 1.67 | 3.86 ± 1.46 | −.63 |
| UC | 4.47 ± 1.46 | 4.81 ± 1.56 | |
| ESS | |||
| SC | 7.45 ± 4.86 | 6.06 ± 4.67 | −.35 |
| UC | 7.22 ± 3.15 | 7.61 ± 4.12 | |
| HbA1c | |||
| SC | 9.38 ± 2.17 | 9.29 ± 2.31 | .07 |
| UC | 9.06 ± 1.45 | 9.15 ± 1.38 | |
| SCI (teen) | |||
| SC | 27.60 ± 5.31 | 26.94 ± 3.21 | −.02 |
| UC | 26.11 ± 5.48 | 27.00 ± 4.12 | |
| SCI (parent) | |||
| SC | 26.25 ± 4.59 | 26.81 ± 5.88 | .14 |
| UC | 26.28 ± 4.82 | 26.06 ± 5.08 | |
| PSQI >5 | n (%) | n (%) | |
| SC | 2 (10) | 1 (6) | |
| UC | 3 (17) | 7 (39) |
Abbreviations: d, Cohen’s d; ESS, Epworth Sleepiness Scale; PSQI, Pittsburgh Sleep Quality Index; SC, sleep coach intervention; TST, total sleep time; SCI, Self-Care Inventory; UC, usual care.
P < .05.
8 |. DISCUSSION
The current study was one of the first to target sleep in teens with T1D. Our results indicate that the Sleep Coach program was feasible and acceptable, and that the program increased sleep duration and efficiency among teens with T1D. The mean increase of 48 minutes among teens in the intervention group is clinically meaningful, as a 15 to 20-minute increase in sleep time has been associated with greater adherence to diabetes treatment in teens with T1D.7 The sleep quality result is driven by subjects in the Usual Care group having worse sleep quality over time, suggesting that the Sleep Coach program may attenuate the decrease in sleep quality and duration typically observed during adolescence.14
Although we did not observe significant intervention effects on diabetes management or HbA1c, we may expect those differences to occur over a longer follow-up period. Based on the research in sleep, we would expect improvements in sleep to precede improvements in glycemic control, and other measures of glycemic control, such as time in range, may be more sensitive to changes in sleep.15 As the use of CGM increases among adolescents with T1D, there will be more opportunities to examine sleep in relation to this outcome. The only other sleep-promoting intervention for adolescents with T1D, focused on sleep extension, did not report effects on glycemic control.16
As a pilot randomized trial, the current study was limited by a small sample size. Furthermore, while results may be limited by the fact that the teens agreed to take part in a sleep study, the sleep duration and glycemic control observed in the current sample was similar to other recent studies in adolescents with T1D.6 A larger sample size and longer follow-up period is needed to assess the efficacy of the Sleep Coach program on diabetes outcomes. In addition, future studies should compare the occurrence of nocturnal hypoglycemia in participants. Finally, we did not ask parents to play an active role in the Sleep Coach intervention, and it would be important to measure parental involvement in future studies.
Despite these limitations, teens in the Sleep Coach intervention group displayed a high level of participation, indicating that this is a feasible intervention program for this high-risk population. Furthermore, satisfaction ratings and exit interview comments support the acceptability of the intervention, but more support may be needed to obtain a higher percentage of usable actigraphy data. By educating adolescents regarding healthy sleep habits with content tailored to their individual needs, we created a program that was engaging and relevant for teens with T1D who obtain insufficient sleep.
ACKNOWLEDGEMENTS
We would like to thank the families who participated in the study. This research was funded by the National Institutes of Health grant R21-DK110657.
Footnotes
CONFLICT OF INTEREST
Dr. B.A.M. has received funding from Rockmelon for consulting on behavioral sleep modules for children with autism. The other authors have no conflicts of interest to report.
REFERENCES
- 1.Perez KM, Hamburger ER, Lyttle M, et al. Sleep in type 1 diabetes: implications for glycemic control and diabetes management. Curr Diabetes Rep. 2018;18(2):5 10.1007/s11892-018-0974-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reutrakul S, Thakkinstian A, Anothaisintawee T, et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med. 2016;23:26–45. 10.1016/j.sleep.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergner EM, Williams R, Hamburger ER, et al. Sleep in teens with type 1 diabetes: perspectives from adolescents and their caregivers. Diabetes Educ. 2018;44(6):541–548. 10.1177/0145721718799086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel NJ, Savin KL, Kahanda SN, et al. Sleep habits in adolescents with type 1 diabetes: variability in sleep duration linked with glycemic control. Pediatr Diabetes. 2018;19(6):1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perfect MM, Patel PG, Scott RE, et al. Sleep, glucose, and functioning in youth with type 1 diabetes. Sleep. 2012;35:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frye SS, Perfect MM, Silva GE. Diabetes management mediates the association between sleep duration and glycemic control in youth with type 1 diabetes mellitus. Sleep Med. 2019;60:132–138. 10.1016/j.sleep.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 7.McDonough RJ, Clements MA, DeLurgio SA, Patton SR. Sleep duration and its impact on adherence in adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18(4):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the US: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 10.Johns MW. Reliability and factor analysis of the Epworth sleepiness scale. Sleep. 1992;15(4):376–381. [DOI] [PubMed] [Google Scholar]
- 11.La Greca A Manual for the Self Care Inventory. Miami, FL: University of Miami; 2004. [Google Scholar]
- 12.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 13.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 14.Owens J Adolescent sleep working group committee on adolescence. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;13:e921–e932. 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perfect MM, Beebe D, Levine-Donnerstein D, Frye SS, Bluez GP, Quan SF. The development of a clinically relevant sleep modification protocol for youth with type 1 diabetes. Clin Prac Pediatr Psychol. 2016;4:227–240. 10.1037/cpp0000145. [DOI] [PMC free article] [PubMed] [Google Scholar]