Abstract
Background
Early prehospital recognition of critical conditions such as ST‐segment–elevation myocardial infarction (STEMI) has prognostic relevance. Current international electrocardiographic STEMI thresholds are predominantly based on individuals of Western European descent. However, because of ethnic electrocardiographic variability both in health and disease, there is a need to reevaluate diagnostic ST‐segment elevation thresholds for different populations. We hypothesized that fulfillment of ST‐segment elevation thresholds of STEMI criteria (STE‐ECGs) in apparently healthy individuals is ethnicity dependent.
Methods and Results
HELIUS (Healthy Life in an Urban Setting) is a multiethnic cohort study including 10 783 apparently healthy subjects of 6 different ethnicities (African Surinamese, Dutch, Ghanaian, Moroccan, South Asian Surinamese, and Turkish). Prevalence of STE‐ECGs across ethnicities, sexes, and age groups was assessed with respect to the 2 international STEMI thresholds: sex and age specific versus sex specific. Mean prevalence of STE‐ECGs was 2.8% to 3.4% (age/sex‐specific and sex‐specific thresholds, respectively), although with large ethnicity‐dependent variability. Prevalences in Western European Dutch were 2.3% to 3.0%, but excessively higher in young (<40 years) Ghanaian males (21.7%–27.5%) and lowest in older (≥40 years) Turkish females (0.0%). Ethnicity (sub‐Saharan African origin) and other variables (eg, younger age, male sex, high QRS voltages, or anterolateral early repolarization pattern) were positively associated with STE‐ECG occurrence, resulting in subgroups with >45% STE‐ECGs.
Conclusions
The accuracy of diagnostic tests partly relies on background prevalence in healthy individuals. In apparently healthy subjects, there is a highly variable ethnicity‐dependent prevalence of ECGs with ST‐segment elevations exceeding STEMI thresholds. This has potential consequences for STEMI evaluations in individuals who are not of Western European descent, putatively resulting in adverse outcomes with both over‐ and underdiagnosis of STEMI.
Keywords: ECG, ethnicity, HELIUS study, population study, STEMI
Subject Categories: Women, Epidemiology, Electrocardiology (ECG), Acute Coronary Syndromes
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- ERP
early repolarization pattern
- HELIUS
Healthy Life in an Urban Setting
- LVH
left ventricular hypertrophy
- OR
odds ratio
- QTc
QT interval corrected for heart rate
- STE‐ECG
ECG that fulfills thresholds for STEMI
- STEMI
ST‐segment–elevation myocardial infarction
Clinical Perspective
What Is New?
In a multiethnic population cohort including 10 783 diligently selected apparently healthy individuals, we show that there is a highly variable ethnicity‐dependent (and sex‐dependent) prevalence of ECGs with ST‐segment–elevations exceeding international ST‐segment–elevation myocardial infarction thresholds (ranging from 0% to 45% in certain subgroups).
What Are the Clinical Implications?
This result implicates that current international ST‐segment–elevation myocardial infarction thresholds (predominantly based on populations of Western European descent) are to be used cautiously in patients who are not of Western European descent, as clinically relevant over‐ and underdiagnosis of acute coronary syndromes eligible for acute revascularization could occur.
In addition, increased awareness of ethnic variability and sex differences in health and disease is sincerely advised in future studies, in registries, and in international threshold definitions.
The prehospital triage of patients with acute chest pain remains a clinical challenge requiring rapid and accurate determination of ischemic versus nonischemic pathology.1, 2 Since the introduction of thrombolysis, possible detrimental effects of inaccurate diagnoses have been documented.3 Thresholds in ST‐segment shifts, formerly proposed to identify eligible thrombolysis candidates, differed between precordial and extremity leads because of higher nonzero precordial J‐point amplitudes in healthy individuals.4 This concept was expanded by investigations of differences between sexes5, 6, 7 and between age groups, where young healthy males were found to have highest ST‐segment amplitudes.6, 7, 8 These findings prompted refinement of the ST‐segment–elevation myocardial infarction (STEMI) thresholds,9 with sex‐specific and later both age‐ and sex‐specific thresholds,10 respectively, adapted in the US (American College of Cardiology Foundation/American Heart Association)1 and European (European Society of Cardiology)2 guidelines.
However, normal ECG values are not only sex and age dependent, but differences between ethnicities have also been well established.6, 11, 12, 13 For instance, individuals of African descent are known to have higher preexistent J‐point/ST‐segment amplitudes compared with individuals of Western European descent (who may have migrated to North America).12, 13, 14 STEMI evaluations in individuals who are not of Western European descent may thus be less accurate. Indeed, depending on the ethnic origin of the investigated individuals, there appear to be more false‐positive or more false‐negative referrals for urgent coronary interventions.15, 16 With the increasing diversity of populations worldwide, there is thus a growing need to reevaluate thresholds for health and disease, such as STEMI, with a focus on ethnicity, as this may impact recognition, treatment, and outcome. Urgent coronary catheterization should preferably be limited to patients with a high suspicion of acute myocardial ischemia. Also, in more remote, often non‐Western areas, hazardous unnecessary prehospital thrombolysis should be prevented.3, 17 While capabilities for urgent coronary interventions in areas with populations who are not predominantly of Western European descent are increasing, many Western metropolitan areas are becoming increasingly multiethnic. This increases the chances of referrals for urgent coronary interventions of patients who are not of Western European descent, which first demands knowledge of background ethnic variability.
To determine background variability across ethnicities in ECGs exceeding ST‐segment elevation thresholds of the STEMI criteria (STE‐ECGs), we studied the performance of non–ethnicity‐specific STEMI thresholds1, 2 by investigating prevalences of STE‐ECGs in the apparently healthy multiethnic population from the HELIUS (Healthy Life in an Urban Setting) study.
Methods
The data, analytic methods, and study materials can be made available to other researchers for purposes of reproducing the results or replicating the procedure, after completion of a research proposal to the authors and the HELIUS scientific coordinator, including a data use agreement, and only after approval by the HELIUS executive board.
Study Design, Setting, and Participants
HELIUS is a multiethnic cohort study including inhabitants of the metropolitan area of Amsterdam, the Netherlands,18, 19 with an approximately equal representation of the largest migrant groups in Europe from outside the European Union next to the indigenous Western European Dutch population. HELIUS's general aim is to assess differences in disease prevalence across ethnic groups, unravel their causes, and ultimately enable improvement of health care and prevention strategies. Initial inclusion consisted of nearly 25 000 participants mainly of 6 different ethnic origins (African Surinamese, Dutch, Ghanaian, Moroccan, South Asian Surinamese, and Turkish; see Figure S1 for the migration history of these ethnicities). Baseline investigations used for this specific study were electively performed in ambulatory subjects and included questionnaires, physical examinations, an ECG, and blood sampling. The study was approved by our Medical Ethics Committee before data collection, and all participants provided written informed consent. A more detailed description of HELIUS was published previously.18, 19
Clinical Diagnoses
To identify apparently healthy subjects for the current study, medical history was retrieved from the questionnaires combined with physical examination and blood test results. Arterial disease was defined by self‐reported stroke; transient ischemic attack; myocardial infarction; (coronary) bypass surgery or percutaneous intervention; or use of antithrombotics, anticoagulation therapy, or nitrates. Subjects were labeled hypertensive when they reported a history of hypertension, used antihypertensive medication, or had current hypertension defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg (World Health Organization criteria), each based on a mean of 2 measurements. Antihypertensive agents can also be used for other conditions, which were therefore also excluded. Diabetes mellitus was based on self‐reported diagnosis, fasting glucose (≥7 mmol/L), hemoglobin A1c (≥48 mmol/mol), or the use of glucose‐lowering medication. Chronic kidney disease was defined as Chronic Kidney Disease Epidemiology Collaboration stage ≥3 (estimated glomerular filtration rate <60 mL/min per 1.73 m2) or a Kidney Disease Improving Global Outcomes albumin‐to‐creatinine ratio ≥3 mg/mmol. Possible ECG‐modifying medications were determined as self‐reported use of any antiarrhythmic Vaughan‐Williams classification medication plus digoxin or the daily use of a psychotropic medication.
ECG Processing and Analysis
General
Standard 12‐lead supine digital resting ECGs were recorded (GE MAC5500, 500 samples/sec) and processed with the Modular ECG Analysis System program,20 which determines common P‐wave, QRS, and T‐wave onsets and offsets for all 12 leads together on 1 representative averaged beat. All on‐ and offsets were manually checked and adjusted when necessary. The QRS offset/J‐point was positioned after a potential end‐QRS notch/slur. Various ECG variables were subsequently computed, including heart rate, QRS interval, QTc (Bazett), QRS complex amplitudes, and J‐point amplitudes. Additionally, the ST/J‐point vector21, 22 was computed after synthesizing vectorcardiographic leads from the 12‐lead ECGs.
Additionally, early repolarization pattern (ERP) was fully automatically assessed by the University of Glasgow ECG core laboratory and defined as follows: end‐QRS notching or slurring (irrespective of ST‐segment elevation) in at least 2 contiguous leads (lateral ERP [aVL, I], inferior ERP [II, aVF, III], anterolateral ERP [V4‐V6]) with J peak or end QRS slur onset ≥0.1 mV.23, 24 High QRS voltages were initially identified using the European Society of Cardiology hypertension guideline for electrocardiographic criteria of left ventricular hypertrophy (LVH).25 Because these criteria resulted in an excessively high prevalence in our normotensive subjects indicative of low specificity (Table S1), we defined high QRS voltages with broadly used composite LVH ECG criteria.26
Three methods were used to evaluate each ECG: Minnesota coding,27 the GE Marquette 12SL report, and assessment by a cardiologist. ECG abnormalities, used for the exclusion process (see “Exclusion Criteria”), were assessed using these 3 methods. In case of discrepancies among the 3 methods, recommendations of international expert groups10, 28 were used for final diagnoses. Using the Modular ECG Analysis System measurements, diagnoses were further verified (eg, an assigned complete right bundle branch block required a measured QRS duration of ≥120 ms). QTc was scored following the description of Viskin (very long/long/normal/short/very short).29 Low QRS voltages were defined as peak‐to‐peak QRS amplitudes of <0.5 mV in all limb leads or <1.0 mV in all precordial leads.
Criteria Used to Recognize STE‐ECGs
Since the American College of Cardiology Foundation/American Heart Association and European Society of Cardiology STEMI thresholds slightly differ, ECGs were classified twice by applying 2 sets of thresholds on the J‐point amplitudes2, 10:
Sex‐specific STEMI thresholds: 2013 American College of Cardiology Foundation/American Heart Association STEMI guidelines1 (lead V2‐V3 ≥0.2 mV [men], ≥0.15 mV [women], other leads ≥0.1 mV).
Age‐ and sex‐specific STEMI thresholds: 2017 European Society of Cardiology STEMI guidelines2 (lead V2‐V3 ≥0.25 mV [men <40 years], ≥0.20 mV [men ≥40 years], ≥0.15 mV [women], other leads ≥0.1 mV).
Exclusion Criteria
Subjects were excluded when questionnaires were incomplete or no ECG of sufficient quality was recorded. To allow statistically meaningful analyses, only ethnicities with a sufficient number of subjects were included, resulting in subgroups of African Surinamese (South American with African roots), Dutch (Western Europe), Ghanaian (Western Africa), Moroccan (Northern Africa), South Asian Surinamese (South Asia), and Turkish (Middle East) ethnic origin (Figure S1). Figure 1 depicts the exclusion process for establishing an apparently healthy population based on clinical diagnoses, medication, and ECG characteristics.
Figure 1. Inclusion and exclusion flowchart.
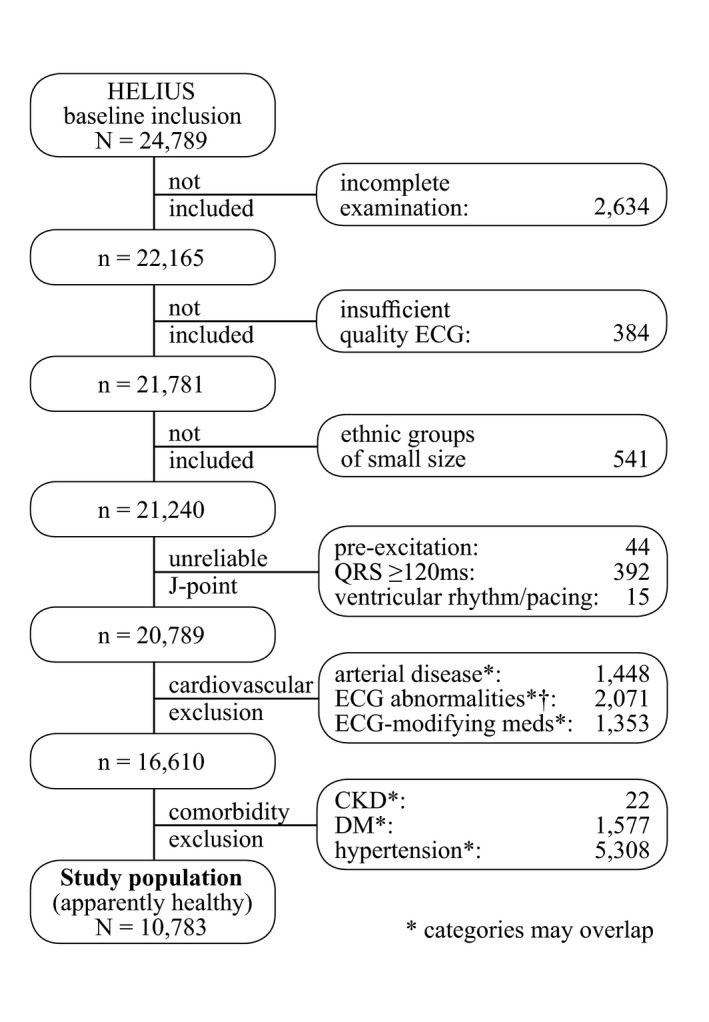
†ECG abnormalities: overt tachycardia (>110/min), supraventricular arrhythmia, second‐ or third‐degree atrioventricular block, left, right, extreme or indeterminate axis, pathological Q‐waves or high R‐waves V1/V2, low QRS voltages, T‐wave abnormalities, very long or very short QTc, suspicion of cardiomyopathy or other overt ECG abnormalities (eg, dextrocardia).
Statistical Analysis
The prevalence and corresponding Wilson score 95% CIs of STE‐ECGs were computed using sex‐specific and age‐ and sex‐specific STEMI thresholds. For initial analyses, the prevalence of STE‐ECGs was computed in the total HELIUS population still including subjects with comorbidities (presented in Table S2). For further statistical analyses, only apparently healthy subjects were investigated, using age‐ and sex‐specific thresholds.
Because differences in the magnitude of measured ST‐segment elevation on a 12‐lead ECG could possibly be attributable to different 3‐dimensional (ie, spatial) orientation of the ST vector as measured at the J‐point, we evaluated the ST vector from the synthesized vectorcardiographic ECGs. Possible differences between ethnicities in spatial orientation of the largest ST‐segment elevation were subsequently explored by plotting interquartile ranges of ST vectors on the cordiform Stab‐Werner projection.30 The distribution of ethnicity‐ and sex‐based subgroups within the STE‐ECGs was depicted after correction for the study population distribution regarding ethnicity, sex and the 2 age groups (</≥40 years).
Using logistic regression, associations between an STE‐ECG pattern as the outcome parameter (yes/no, using age‐ and sex‐specific thresholds) and predictor variables influencing ST‐segment elevation (ie, ethnicity, age, sex, high QRS voltages, ERP, QRS duration and QTc) were tested (see Table S3). All single 2‐way interactions were tested while correcting for the other variables. Finally, multivariable logistic regression including all significant variables was performed to estimate associations’ effect sizes. The Bonferroni corrected significance threshold was 0.001. Statistical analyses were performed in R software version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population Description
After exclusion, 10 783 apparently healthy subjects remained (Figure 1). Study population characteristics are detailed in Table and Figure S2. The median age was 38 (interquartile range, 20) and the male/female ratio was 4079/6704, while the 6 ethnic subgroups consisted of 870 to 2603 subjects. A description of exclusions stratified per ethnicity is provided (Table S4).
Table 1.
Characteristics of the Study Population
| Apparently Healthy Population (N=10 783) | |
|---|---|
| Age, y, median (quartile 1–3)[min‐max] | 38 (28–48) [18–71] |
| Sex, male/female | 4079/6704 |
| Ethnicity, n (%) | |
| African Surinamese | 1660 (15) |
| Dutch | 2603 (24) |
| Ghanaian | 870 (8) |
| Moroccan | 2384 (22) |
| South Asian Surinamese | 1318 (12) |
| Turkish | 1948 (18) |
STE‐ECG Prevalence
The STE‐ECG prevalence in the total apparently healthy population was 3.43% (95% CI, 3.10%–3.79%) using the sex‐specific thresholds and slightly lower (2.76%; 95% CI, 2.47%–3.09%), when using both age‐ and sex‐specific thresholds. The STE‐ECG prevalence using age‐ and sex‐specific thresholds was higher in men (6.15%; 95% CI, 5.46%–6.93%) than in women (0.70%; 95% CI, 0.53%–0.93%). Younger (<40 years) individuals had a higher STE‐ECG prevalence (3.45%; 95% CI, 3.01%–3.95%) compared with older subjects (≥40 years) (1.98%; 95% CI, 1.63%–2.40%).
Additionally, evident ethnic differences in STE‐ECG prevalence were observed (Figure 2, Table S5). While prevalences were relatively low in Dutch Western European subjects, prevalences were highest in Ghanaian and lowest in Turkish subjects. Ghanaian men aged <40 had the highest STE‐ECG prevalence (21.7%–27.5%), while none of the Turkish women aged ≥40 had an STE‐ECG. Within the STE‐ECGs, correction for study population distributions (eg, men/women) further elaborates this ethnic variability (Figure 3).
Figure 2. STE‐ECG prevalence stratified per ethnicity, sex, and age group.
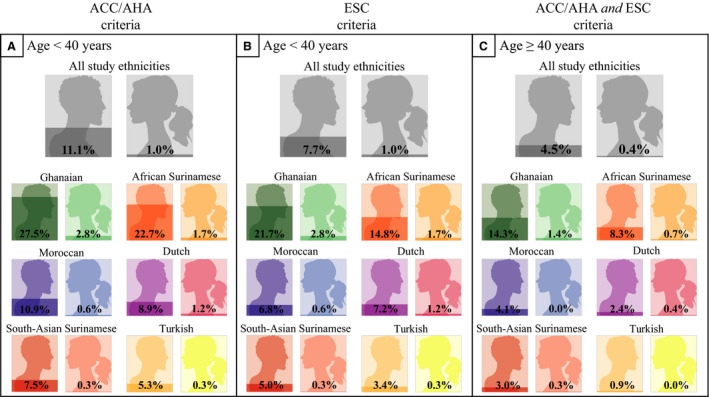
Application of the 2 STEMI thresholds for the different ethnicity, sex, and age groups. ACCF/AHA sex‐specific STEMI thresholds: lead V2 to V3 ≥0.2 mV [men], ≥0.15 mV [women], other leads ≥0.1 mV. ESC age‐ and sex‐specific STEMI thresholds: lead V2‐V3 ≥0.25 mV [men <40 y], ≥0.20 mV [women ≥40 y], ≥0.15 mV [women], other leads ≥0.1 mV. Note the increase in prevalence when using only sex‐specific thresholds. Furthermore, note the higher prevalence with younger age, male sex (despite sex‐specific thresholds), and in certain ethnicities. ACCF/AHA indicates American College of Cardiology Foundation/American Heart Association; ESC, European Society of Cardiology; STE‐ECG, ECG that fulfills thresholds for STEMI; and STEMI, ST‐segment–elevation myocardial infarction.
Figure 3. Corrected distribution of ethnicity and sex within the STE‐ECGs.
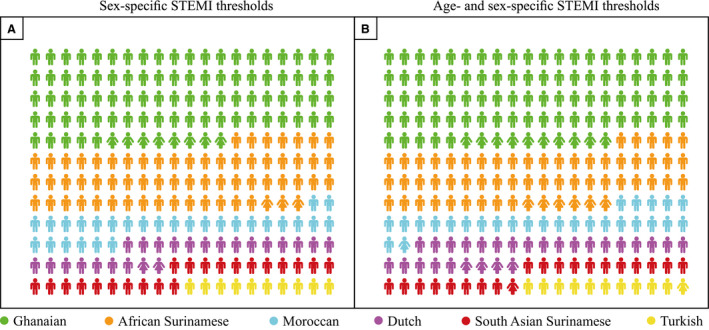
Distribution of ethnicity‐ and sex‐ based subgroups within the STE‐ECGs plotted after correction for the study population distribution regarding ethnicity, sex, and the 2 age groups (cutoff 40 years). ACC/AHA sex‐specific STEMI thresholds: lead V2‐V3 ≥0.2 mV [men], ≥0.15 mV [women], other leads ≥0.1 mV. ESC age‐ and sex‐specific STEMI thresholds: lead V2‐V3 ≥0.25 mV [men <40 y], ≥0.20 mV [men ≥40 y], ≥0.15 mV [women], other leads ≥0.1 mV. Note that subjects originating from Western Africa account for more than half (sex‐specific or thresholds) or up to two thirds (age‐ and sex‐specific thresholds) of all STE‐ECGs. ACCF/AHA indicates American College of Cardiology Foundation/American Heart Association; ESC, European Society of Cardiology; STE‐ECG, ECG that fulfills thresholds for STEMI; and STEMI, ST‐segment–elevation myocardial infarction.
Factors Contributing to STE‐ECGs
J‐Point Amplitudes and ST‐Segment Elevation Location
The J‐point amplitudes of all 12 ECG leads with corresponding STEMI thresholds are depicted in Figure 4A. The most prevalent leads exceeding STEMI thresholds were V4–V5 (Table S6). In 89% of all STE‐ECGs, an above‐threshold V4 J‐point amplitude was present. Highest V4 medians were documented in African Surinamese and Ghanaian men aged <40 years, respectively, just above (109 μV) and slightly under (95 μV) the STEMI threshold (Figure S3). To further investigate the location of the largest ST‐segment elevation per patient, the spatial orientations of the ST/J‐point vectors were 2‐fold plotted in the cordiform Stab‐Werner projection30 (Figure 4B and 4C). No clear difference in spatial ST vector distribution could be visually observed between ethnicities, pointing to the magnitude and not the location of the ST‐segment elevation as an explanation for STE‐ECG prevalence differences among ethnicities.
Figure 4. J‐point amplitudes and ST‐segment elevation location.
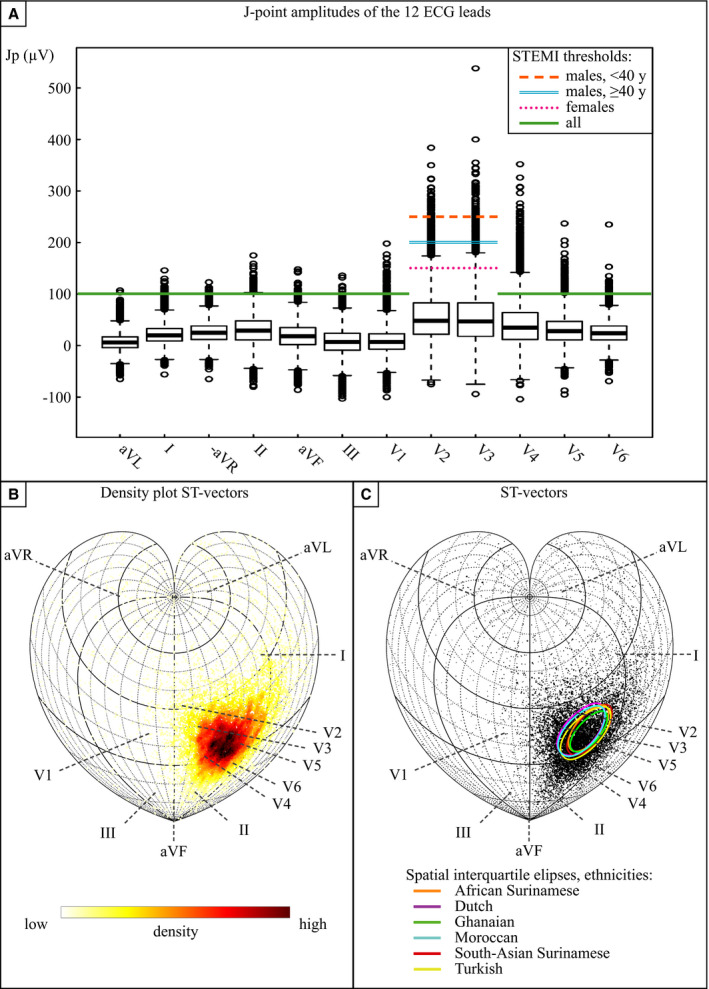
A, The colored lines represent the current age‐ and sex‐specific STEMI thresholds for each lead. Black stripes box: Q1, Q2, Q3, whiskers: Q1–1.5·interquartile range and Q3+1.5·interquartile range. Boxplots of the J‐point amplitudes in the total apparently healthy population (N=10 783). Appreciate the amount of J‐point amplitudes above the STEMI threshold in leads V2, V3, and V4. (B and C) general: The directions of the 3‐dimentional ST vectors of all subjects are shown on a sphere in the 2‐dimensional plane by cordiform Stab‐Werner projections. Lead vector projections are marked with dashed lines. B, Density plot. Note the precordial orientation of most ST vectors. C, ST vector of all subjects in which the marker size represents the size of the ST vector. Interquartile ellipses of a combination of azimuth and elevation are stratified per ethnicity. Because the direction of small ST vectors is rather unreliable, small markers with a deviant direction should, in our opinion, not be seen as actual outliers. No evident ethnic difference in spatial ST vector distribution can be appreciated. Jp indicates J‐point; STEMI, ST‐segment–elevation myocardial infarction; and y, years old.
Associated Variables
All tested variables (ethnicity, age, sex, high QRS voltages, ERP, QRS duration, and QTc) were statistically significantly associated with the occurrence of an STE‐ECG, using the age‐ and sex‐specific thresholds (Table S3). None of the 2‐way interactions was statistically significant. African Surinamese and Ghanaian ethnicity had the highest significant odds ratio (OR) for the presence of an STE‐ECG (4.49; 95% CI, 2.66–7.57; and 5.71; 95% CI, 3.25–10.02), respectively. An anterolateral ERP was significantly associated with an STE‐ECG, whether or not in combination with another ERP location, with ORs of 3.16 (95% CI, 2.11–4.72) and 4.06 (95% CI, 2.85–5.80). The OR for the occurrence of an STE‐ECG was 2.80 (95% CI, 2.08–3.76) for high QRS voltages and 4.06 (95% CI, 2.79–5.90) for male sex. Age and QTc were negatively associated with an STE‐ECG (OR, 0.97; 95% CI, 0.96–0.98; and 0.98; 95% CI, 0.97–0.99 per unit [year, millisecond]), respectively. QRS duration was positively associated with an STE‐ECG (OR, 1.06; 95% CI, 1.05–1.08 per millisecond).
Discussion
Prevalence of ECGs Exceeding STEMI Thresholds
The 12‐lead ECG still represents a cornerstone in the accurate prehospital (and also in‐hospital) emergency triage of patients with symptoms possibly or probably attributable to acute myocardial ischemia, which impacts resultant survival and morbidity.10 Diagnostic accuracy and error during these critical initial evaluations follow from balancing ratios of correct versus false‐positive and false‐negative test results in history taking, physical examinations, and ECG interpretation. Additional investigations to rule in or rule out cardiac ischemia such as echocardiography or cardiac biomarker assessment are often either unavailable (eg, prehospital) or too time consuming for initial decision making in a STEMI triage system selecting patients for direct thrombolysis or urgent coronary angiography.
It is already known that age and sex impact a STEMI classification,5, 6, 7, 8, 9 but reference values are predominantly derived from populations with a Western European descent. This has resulted in age‐ and sex‐specific STEMI thresholds in international guidelines and consensus documents. However, current US guidelines1 have not yet incorporated the age‐specific criteria suggested in the last universal definition of myocardial infarction,10 putatively resulting in more false‐positive STEMI diagnoses. Currently, ethnicity is not incorporated in the guidelines.1, 2, 10 Our findings, however, confirm that ethnicity is an important element to be considered, while there remain significant age‐ and sex‐dependent differences despite age‐ and sex‐specific thresholds. This is relevant in our era with increasing diversity of populations worldwide, especially in areas with large multiethnic populations (eg, metropolitan areas) and in parts of the world where riskful thrombolysis is administered more frequently. When current thresholds are used to evaluate health and disease, patients with acute chest pain who are not of Western European descent may thus be less accurately evaluated because of either a higher (eg, men from sub‐Saharan African descent) or lower (eg, Turkish women) incidence of preexisting ST‐segment elevation. This could putatively result in worse outcome.
Factors Contributing to STE‐ECGs
J‐Point Amplitudes and ST‐Segment Elevation Location
Since no clear differences were observed in the location of ST‐segment elevation (Figure 4), classification of STE‐ECGs across ethnicities, sex, and age is predominantly determined by the J‐point amplitude magnitude. In this respect, lead V4 appears in our study to be the most vulnerable for exceeding STEMI thresholds. In earlier studies, anterolateral ST‐segment elevation proved to cause most false‐positive catheterization laboratory activations,16 which is currently mirrored in higher V2/V3 thresholds but not V4.
Sex, Age, and Ethnicity
Male sex and younger age are well known to be associated with higher J‐point amplitudes,6, 7, 8 which is confirmed in this study. In contrast, female sex and older age indeed showed lower prevalences of nonischemic STE‐ECGs. Notably, despite different STEMI thresholds according to age and sex categories, we still noted overt differences in STE‐ECGs exceeding STEMI thresholds in our study (eg, up to 8‐fold higher prevalence in young males compared with older females while applying age‐ and sex‐specific thresholds). The observed association of STE‐ECGs and ethnic origin, especially sub‐Saharan African origin, was not unexpected.12, 14 However, the magnitude of this ethnic variability surpassed our prior understanding of this phenomenon at both extremes of the spectrum. Ethnicity, especially in combination with age and sex, jeopardizes both current STEMI thresholds for false‐positive (particularly in men from sub‐Saharan African descent) and for false‐negative (particularly in older women of Turkish origin) STEMI diagnoses when these individuals present with signs or symptoms of suggestive of acute coronary syndrome (ACS).
High QRS Voltage
LVH is a known confounder of ECG interpretation31, 32 and complicates triage,15, 16 typically manifesting with high QRS voltages combined with pronounced ST elevation in right precordial leads and ST depression in lateral leads.33 In our study, we excluded individuals with known, treated, or measured hypertension. Additionally, typical electrocardiographic LVH does usually not affect the lead V4 ST segment, while STE‐ECGs in this study are dominated by V4. Our STE‐ECGs are therefore unlikely to result from actual LVH in this nonhypertensive population. Hence, ST‐segment elevation reaching thresholds for STEMI in the absence of LVH are abundantly present in certain ethnicities, especially in younger males. This supports careful ST‐segment elevation assessment in combination with high QRS voltages, even in the absence of typical strain patterns.34
QRS Duration and QTc
Associations between the occurrence of an STE‐ECG, QRS duration prolongation and QTc shortening, might be explained by elevation of the J‐point attributable to larger overlap between depolarization and repolarization vectors, as proposed earlier.14 Noteworthy, myocardial ischemia can cause alterations in both QRS duration (peri‐ischemic conduction slowing)35 and QTc, troubling such an assessment.36
Early Repolarization Pattern
Although both the pathophysiologic mechanism37 as well as the definition23 of the pattern called “early repolarization” are debated, ERP is known to hamper ST‐segment elevation interpretation.38 As recommended by a 2015 consensus paper,23 we defined ERP as notching or slurring with or without accompanying ST‐segment elevation. Clearly, including isolated ST‐segment elevation as an ERP criterion would render statistical analysis with the occurrence of STE‐ECGs futile. Interpreting ERP ECGs of patients with symptoms suggestive of STEMI remains challenging because notches and slurs can also result from ischemia.39 Importantly, the occurrence of inferior ERP does not associate with STE‐ECGs in this study, inhibiting the possibility to use inferior ERPs to exclude ACS.
Clinical Implications and Applications
Our study identifies multiple factors associated with the occurrence of a nonischemic or preexisting STE‐ECG. The results of our logistic regression can modify the likelihood of an actual STEMI diagnosis by demonstrating the odds of a specific patient's having an STE‐ECG in nonischemic conditions. Automated ECG analysis systems have the opportunity to use additional checks to acknowledge ethnicity, high QRS voltages, or ERP, and so on, which could aid reporting. However, caution is advised since considerable overlap exists among ethnicities, sexes, and age groups. Additionally, morphological features of nonischemic and ischemic STE‐ECGs can be similar.31, 32
Although a specificity on the order of 97.5% is acceptable, our findings suggest value of ethnicity‐specific modification of the current international STEMI thresholds (based predominantly on values from apparently healthy individuals of Western European descent). Since we found a high (20%–30%) nonischemic STE‐ECG prevalence in apparently healthy male subjects originating from sub‐Saharan Africa, and even higher (>45%) when certain ECG characteristics prevail (eg, high voltage), electrocardiographic myocardial infarction diagnostics are rather complicated. An additional approach may be comparing the acute ECG to an earlier‐made nonacute ECG of the same patient, revealing whether the ST‐segment elevation is preexisting.21, 22 While biomarkers and echocardiography can assist in‐hospital ACS triage (although time consuming), the ECG is currently the only prehospital tool for ACS evaluation. Furthermore, the extremely low STE‐ECG prevalence found predominantly in females and particularly in certain ethnic subgroups, could result in an undesirable high yield of false‐negative STEMI diagnoses. Since their baseline ST value is low, they have to develop more ST‐segment elevation to exceed the thresholds. Possibly, lowering thresholds in certain female subgroups (particularly ethnicity‐dependent) could improve STEMI sensitivity and improve treatment and outcome. Whether there are additional ethinicity‐dependent differences in the amount of ST‐segment deviation during an ACS that may augment or decrease these ethnicity‐dependent background differences, is currently unknown.
Strengths and Limitations
Our study demonstrates differences in the ethnicity‐dependent prevalence of ECGs exceeding STEMI thresholds in electively recorded ECGs in apparently healthy subjects. The scale of this study and the representation of six distinct ethnicities originating from Western Africa, Northern Africa, Western Europe, the Middle East, and South Asia is not, to our knowledge, matched by earlier studies. Moreover, although ST‐segment amplitudes in different ethnicities were studied before,12, 14 a quantification of the problem of the exceeding of STEMI thresholds in these specific ethnicities and also the correlation with other ECG variables, to our knowledge, has not been evaluated earlier. Additionally, this study was performed with high precision with respect to ECG assessment and subject evaluations.
Because of the inclusion of a relatively young population (Figure S2), the prevalence of STE‐ECGs in subjects aged >70 years was not investigated. However, the median age of the subjects included in this study (38 years) mirrors the age cutoff value of the STEMI threshold (40 years), which is valuable. Although this study represents ethnicities from different areas around the world, many ethnicities remain to be investigated. For example, since Chinese do not form a substantial proportion of the Amsterdam population in HELIUS, no Chinese subjects were included. In previous studies, Chinese were found to have even higher J‐point amplitudes than subjects of sub‐Saharan African descent,6, 11, 12, 13 although that difference was not significant in our “culprit” lead V4.12
Despite our substantial efforts to exclude subjects with possible or current cardiovascular disease, possible subclinical disease may exist among our apparently healthy population, especially since this study does not include cardiac imaging results. This notwithstanding, subjects with possible or current cardiovascular disease are more likely to be evaluated for possible STEMI as compared with apparently healthy individuals. Because thresholds for health and disease are predominantly based on data from apparently healthy individuals, this could introduce bias during ECG evaluations for STEMI, although in subjects with possible or current cardiovascular disease, their previous medical history will have a larger effect size on decision making compared with apparently healthy subjects. From Table S2, it can be appreciated that the prevalence of (outpatient) STE‐ECGs actually decreases when also including patients with possible or current cardiovascular disease (which might also include an age effect).
Another important limitation is that our study does not include ACS cases. Therefore, the sensitivity of current STEMI thresholds remains unknown in these ethnicities. This is predominantly caused by the worldwide ethical issues associated with routine registration of ethnic background. The establishment of acute chest pain databases for multiethnic research would facilitate evaluations of diagnostic accuracy of STEMI criteria. Defining ethnic background is a sensible matter; the identifier Caucasian, for example, covers many different backgrounds, from Northern Europe to the Mediterranean to a part of the Middle East, while similar differentiations can be made for (sub‐Saharan) African and so on. This notwithstanding, our data are relevant only for healthcare professionals who work in an area with appreciable patient populations of individuals who are not of Western European descent. Finally, in accordance with the guidelines, we used ST‐segment amplitude criteria in isolation, but the ST‐segment morphology and other ECG features, such as reciprocal ST‐segment depression, are also reviewed in clinical practice.
Conclusion
Although accurate identification of STEMI patients impacts on prognosis, current STEMI thresholds are not ethnicity specific, while background variation in ST‐segment elevation is ethnicity dependent. We found a highly variable prevalence of ST‐segment elevation ECGs exceeding STEMI thresholds in apparently healthy individuals across ethnicities, sexes, and age groups. Putatively, when presenting with symptoms or signs possibly caused by acute myocardial ischemia, straightforward application of current international ST‐segment elevation thresholds could result in diagnostic error. Because of the high interindividual variability in preexisting J‐point amplitudes, current guidelines should be used with caution in subjects of certain age, sex, and ethnicity and with specific ECG characteristics.
Sources of Funding
This work was supported by the Dutch Heart Foundation (grant number: 2010T084), the Netherlands Organization for Health Research and Development (ZonMw) (grant number: 200 500 003), the European Union, (FP‐7) (grant number: 278 901), and the European Fund for the Integration of non‐EU immigrants (EIF) (grant number: 2013EIF013). The HELIUS study is being conducted by the Academic Medical Center Amsterdam and the Public Health Service of Amsterdam (core support for HELIUS).
Disclosures
None.
Supporting information
Tables S1–S6 Figures S1–S3
Acknowledgments
We are most grateful to the participants of the HELIUS study and the management team, research nurses, interviewers, research assistants, and other staff who have taken part in gathering the data for this study and to Prof. R.J. de Winter (Amsterdam UMC) for inspirational discussions.
(J Am Heart Assoc. 2020;9:e015477 DOI: 10.1161/JAHA.119.015477.)
For Sources of Funding and Disclosures, see Page 10.
References
- 1. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. [DOI] [PubMed] [Google Scholar]
- 2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 3. Eriksen UH, Mølgaard H, Ingerslev J, Nielsen TT. Fatal haemostatic complications due to thrombolytic therapy in patients falsely diagnosed as acute myocardial infarction. Eur Heart J. 1992;13:840–843. [DOI] [PubMed] [Google Scholar]
- 4. Nahum LH, Mauro A, Levine H, Abrahams DG. Potential field during the ST segment. J Appl Physiol. 1953;5:693–697. [DOI] [PubMed] [Google Scholar]
- 5. Dellborg M, Herlitz J, Emanuelsson H, Swedberg K. ECG changes during myocardial ischemia. Differences between men and women. J Electrocardiol. 1994;27(suppl):42–45. [DOI] [PubMed] [Google Scholar]
- 6. Macfarlane PW. Age, sex, and the ST amplitude in health and disease. J Electrocardiol. 2001;34(suppl):235–241. [DOI] [PubMed] [Google Scholar]
- 7. Rijnbeek PR, van Herpen G, Bots ML, Man S, Verweij N, Hofman A, Hillege H, Numans ME, Swenne CA, Witteman JCM, Kors JA. Normal values of the electrocardiogram for ages 16–90 years. J Electrocardiol. 2014;47:914–921. [DOI] [PubMed] [Google Scholar]
- 8. Hiss RG, Lamb LE, Allen MF. Electrocardiographic findings in 67,375 asymptomatic subjects X. Normal values. Am J Cardiol. 1960;6:200–231. [DOI] [PubMed] [Google Scholar]
- 9. Macfarlane PW, Browne D, Devine B, Clark E, Miller E, Seyal J, Hampton D. Modification of ACC/ESC criteria for acute myocardial infarction. J Electrocardiol. 2004;37(suppl):98–103. [DOI] [PubMed] [Google Scholar]
- 10. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 11. Wu J, Kors JA, Rijnbeek PR, van Herpen G, Lu Z, Xu C. Normal limits of the electrocardiogram in Chinese subjects. Int J Cardiol. 2003;87:37–51. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VK, Gapstur SM, Prineas R, Colangelo LA, Ouyang P, Kadish AH. Ethnic differences in ST height in the multiethnic study of atherosclerosis. Ann Noninvasive Electrocardiol. 2008;13:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macfarlane PW, Katibi IA, Hamde ST, Singh D, Clark E, Devine B, Francq BG, Lloyd S, Kumar V. Racial differences in the ECG–selected aspects. J Electrocardiol. 2014;47:809–814. [DOI] [PubMed] [Google Scholar]
- 14. Rautaharju PM, Zhang Z‐M, Haisty WK, Gregg RE, Warren J, Horaĉek MB, Kucharska‐Newton AM, Rosamond W, Soliman EZ. Race‐ and sex‐associated differences in rate‐adjusted QT, QTpeak, ST elevation and other regional measures of repolarization: the Atherosclerosis Risk in Communities (ARIC) Study. J Electrocardiol. 2014;47:342–350. [DOI] [PubMed] [Google Scholar]
- 15. McCabe JM, Armstrong EJ, Kulkarni A, Hoffmayer KS, Bhave PD, Garg S, Patel A, MacGregor JS, Hsue P, Stein JC, et al. Prevalence and factors associated with false‐positive ST‐segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate‐SF registry. Arch Intern Med. 2012;172:864–871. [DOI] [PubMed] [Google Scholar]
- 16. Shamim S, McCrary J, Wayne L, Gratton M, Bogart DB. Electrocardiograhic findings resulting in inappropriate cardiac catheterization laboratory activation for ST‐segment elevation myocardial infarction. Cardiovasc Diagn Ther. 2014;4:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iyengar SS, Godbole GS. Thrombolysis in the era of intervention. J Assoc Physicians India. 2011;59(suppl):26–30. [PubMed] [Google Scholar]
- 18. Stronks K, Snijder MB, Peters RJG, Prins M, Schene AH, Zwinderman AH. Unravelling the impact of ethnicity on health in Europe: the HELIUS study. BMC Public Health. 2013;13:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJG, Zwinderman AH, Stronks K. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open. 2017;7:e017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Bemmel JH, Kors JA, van Herpen G. Methodology of the modular ECG analysis system MEANS. Methods Inf Med. 1990;29:346–353. [PubMed] [Google Scholar]
- 21. ter Haar CC, Maan AC, Schalij MJ, Swenne CA. Directionality and proportionality of the ST and ventricular gradient difference vectors during acute ischemia. J Electrocardiol. 2014;47:500–504. [DOI] [PubMed] [Google Scholar]
- 22. Ter Haar CC, Man S‐C, Maan AC, Schalij MJ, Swenne CA. Subtraction electrocardiography: detection of ischemia‐induced ST displacement without the need to identify the J point. J Electrocardiol. 2016;49:316–322. [DOI] [PubMed] [Google Scholar]
- 23. Macfarlane PW, Antzelevitch C, Haissaguerre M, Huikuri HV, Potse M, Rosso R, Sacher F, Tikkanen JT, Wellens H, Yan G‐X. The early repolarization pattern: a consensus paper. J Am Coll Cardiol. 2015;66:470–477. [DOI] [PubMed] [Google Scholar]
- 24. Clark EN, Katibi I, Macfarlane PW. Automatic detection of end QRS notching or slurring. J Electrocardiol. 2014;47:151–154. [DOI] [PubMed] [Google Scholar]
- 25. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 26. Bacharova L, Chen H, Estes EH, Mateasik A, Bluemke DA, Lima JAC, Burke GL, Soliman EZ. Determinants of discrepancies in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am J Cardiol. 2015;115:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kors JA, van Herpen G, Wu J, Zhang Z, Prineas RJ, van Bemmel JH. Validation of a new computer program for Minnesota coding. J Electrocardiol. 1996;29(suppl):83–88. [DOI] [PubMed] [Google Scholar]
- 28. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982–991. [DOI] [PubMed] [Google Scholar]
- 29. Viskin S. The QT interval: too long, too short or just right. Heart Rhythm. 2009;6:711–715. [DOI] [PubMed] [Google Scholar]
- 30. Man S, Rahmattulla C, Maan AC, van der Putten NHJJ, Dijk WA, van Zwet EW, van der Wall EE, Schalij MJ, Gorgels AP, Swenne CA. Acute coronary syndrome with a totally occluded culprit artery: relation of the ST injury vector with ST‐elevation and non‐ST elevation ECGs. J Electrocardiol. 2014;47:183–190. [DOI] [PubMed] [Google Scholar]
- 31. Brady WJ, Perron AD, Chan T. Electrocardiographic ST‐segment elevation: correct identification of acute myocardial infarction (AMI) and non‐AMI syndromes by emergency physicians. Acad Emerg Med. 2001;8:349–360. [DOI] [PubMed] [Google Scholar]
- 32. Bosson N, Sanko S, Stickney RE, Niemann J, French WJ, Jollis JG, Kontos MC, Taylor TG, Macfarlane PW, Tadeo R, et al. Causes of prehospital misinterpretations of ST elevation myocardial infarction. Prehosp Emerg Care. 2017;21:283–290. [DOI] [PubMed] [Google Scholar]
- 33. Devereux RB, Reichek N. Repolarization abnormalities of left ventricular hypertrophy. Clinical, echocardiographic and hemodynamic correlates. J Electrocardiol. 1982;15:47–53. [DOI] [PubMed] [Google Scholar]
- 34. Birnbaum Y, Alam M. LVH and the diagnosis of STEMI—how should we apply the current guidelines? J Electrocardiol. 2014;47:655–660. [DOI] [PubMed] [Google Scholar]
- 35. Wagner NB, Sevilla DC, Krucoff MW, Lee KL, Pieper KS, Kent KK, Bottner RK, Selvester RH, Wagner GS. Transient alterations of the QRS complex and ST segment during percutaneous transluminal balloon angioplasty of the left anterior descending coronary artery. Am J Cardiol. 1988;62:1038–1042. [DOI] [PubMed] [Google Scholar]
- 36. Surawicz B, Orr CM, Hermiller JB, Bell KD, Pinto RP. QRS changes during percutaneous transluminal coronary angioplasty and their possible mechanisms. J Am Coll Cardiol. 1997;30:452–458. [DOI] [PubMed] [Google Scholar]
- 37. Postema PG, Wilde AAM. Do J waves constitute a syndrome? J Electrocardiol. 2013;46:461–465. [DOI] [PubMed] [Google Scholar]
- 38. Birnbaum Y. The burden of nonischemic ST‐segment elevation. J Electrocardiol. 2007;40:6–9. [DOI] [PubMed] [Google Scholar]
- 39. Demidova MM, Martín‐Yebra A, van der Pals J, Koul S, Erlinge D, Laguna P, Martínez JP, Platonov PG. Transient and rapid QRS‐widening associated with a J‐wave pattern predicts impending ventricular fibrillation in experimental myocardial infarction. Heart Rhythm. 2014;11:1195–1201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6 Figures S1–S3


