Abstract
Medicine and public health have traditionally separated the prevention and treatment of communicable and noncommunicable diseases. The coronavirus disease 2019 (COVID‐19) pandemic has challenged this paradigm, particularly in the setting of cardiovascular disease (CVD). Overall, individuals with underlying CVD who acquire severe acute respiratory syndrome coronavirus 2 experience up to a 10‐fold higher case‐fatality rate compared with the general population. Although the impact of the pandemic on cardiovascular health continues to evolve, few have defined this association from a frontline, public health perspective of populations disproportionately affected by CVD and COVID‐19. Louisiana is ranked within the bottom 5 states for cardiovascular health, and it is home to several parishes that have experienced among the highest COVID‐19 case‐fatality rates nationally. Herein, we review CVD prevention and implications of COVID‐19 in New Orleans, LA, a city holding a sobering yet resilient history with previous public health disasters. In particular, we discuss potential pandemic‐driven changes in access to health care, preventive pharmacotherapy, and lifestyle behaviors, all of which may adversely affect CVD prevention and management, while amplifying racial disparities. Through this process, we highlight proposed recommendations for how CVD prevention efforts can be improved in the midst of the current COVID‐19 pandemic and future public health crises.
Keywords: cardiovascular disease, coronavirus, COVID‐19, diet, primary prevention, quarantine, race
Subject Categories: Cardiovascular Disease, Epidemiology, Lifestyle, Primary Prevention, High Blood Pressure
Nonstandard Abbreviations and Acronyms
- COVID‐19
coronavirus disease 2019
- CVD
cardiovascular disease
The coronavirus disease 2019 (COVID‐19) pandemic poses the most significant modern‐day public health challenge since poliomyelitis. Although medicine and epidemiology have traditionally separated the prevention, treatment, and public health response between communicable and noncommunicable diseases, we are quickly beginning to appreciate and understand the intricate overlap between these 2 outcomes. The implications of COVID‐19 on cardiovascular health have not been clearly defined from the frontline perspective of populations susceptible to both cardiovascular disease (CVD) and infectious disease.
Louisiana, a state with an already disproportionately high burden of CVD‐related mortality, experienced one of the fastest growth rates of COVID‐19 cases globally during the initial outbreak.1 Notably, both the Orleans (6.4%) and St. John the Baptist Parishes (8.5%) have had among the highest COVID‐19 case‐fatality rates in the country through the end of April 2020.1 Hypertension (52.7%), type 2 diabetes mellitus (32.6%), heart disease (18.4%), and obesity (17.4%) have been implicated in a substantial proportion of COVID‐19 deaths in Louisiana, suggesting that individuals with underlying CVD experience worse health outcomes on infection with severe acute respiratory syndrome coronavirus 2 compared with the general population (Figure). Furthermore, the potential multiple epidemic waves predicted to accompany COVID‐19 may produce similar consequences on CVD prevention as those brought on by previous natural disasters in the area.
Figure 1. Characteristics of coronavirus disease 2019 (COVID‐19) deaths in Louisiana, according to underlying health condition.
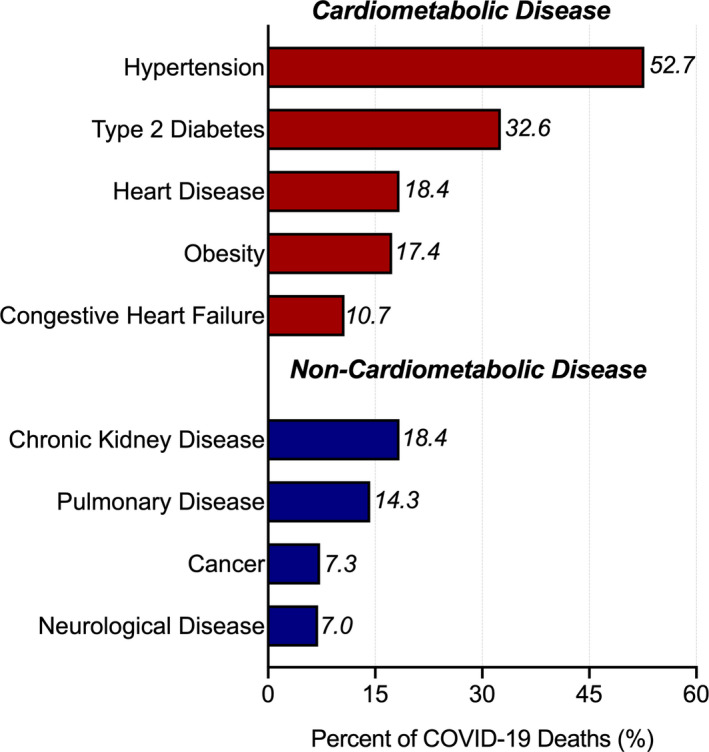
Source: Louisiana Department of Health (as of May 4, 2020).
The effects of Hurricane Katrina on the burden of CVD in the greater New Orleans, LA, community have persisted for at least one decade. Acute myocardial infarction incidence increased 4‐fold one decade after Katrina when compared with years just before the storm.2 These observations have partially been caused by poststorm increases in the cumulative burden of traditional CVD risk factors, including hypertension, hyperlipidemia, smoking, and type 2 diabetes mellitus.2 However, the chronobiological features of acute myocardial infarction have also changed since Katrina, with more events happening on nights and weekends,2 suggesting that changes in traditional risk factors alone may not fully explain the CVD implications of such public health crises. Posttraumatic stress disorder, for example, emerged as an independent predictor of incident CVD events after Hurricane Katrina.3 Thus, the crescent city's sobering yet resilient history with natural disasters serves an important role when considering the cardiovascular implications of COVID‐19 for broader populations.
Unlike Hurricane Katrina, the COVID‐19 pandemic will not lead to the physical destruction of homes and communal spaces. However, pandemic‐driven changes in access to health care and preventive pharmacotherapy and alterations in lifestyle behaviors may all adversely affect CVD prevention, detection, and management, while amplifying racial disparities. These interventions may largely stem from population‐based approaches that are highly effective at containing infectious diseases and “flattening the curve.” Nonpharmacological interventions, including quarantines and social distancing, were recently estimated to have prevented up to 95% of new severe acute respiratory syndrome coronavirus 2 infections in Wuhan, China.4 Thus, containment strategies will undoubtedly need to continue to be among our first‐line agents in the setting of COVID‐19 and future infectious disease outbreaks, yet they may also carry unintended negative consequences for CVD prevention.
Physical activity and dietary patterns may be among the first lifestyle behaviors adversely affected by the COVID‐19 pandemic. Many Americans living in urban environments, like New Orleans, rely on public gymnasiums and fitness centers as a safe means to obtain regular physical activity. However, residents are currently under a stay‐home mandate and unable to access these sites during COVID‐19 containment policies. Greater sedentary time confers increased risks for both CVD and all‐cause mortality,5 and infectious disease containment policies may inadvertently further strengthen such associations. To prevent such adverse effects, public health messaging should focus on promoting stationary aerobic exercises for those who cannot safely exercise outdoors. For example, moderate (3–6 metabolic equivalents) and vigorous (>6 metabolic equivalents) intensity physical activity may be achieved through yoga and jogging in place, respectively.6 Individuals should be encouraged to perform several bouts of aerobic activity throughout the day, and this recommendation may work particularly well given the increased flexibility in daytime work schedules. In addition, COVID‐19 study supplements that test the efficacy of novel, home‐based lifestyle interventions should be added to a large majority of longitudinal CVD cohort studies in the United States. Findings from such work will promote discovery of the most effective methods for maintaining adherence to regular physical activity amidst social distancing and containment policies. Such efforts have already begun for the BHS (Bogalusa Heart Study), a long‐term community study that began in 1972 and examines the cardiovascular health of black and white individuals residing in Bogalusa, LA.7
Likewise, COVID‐19 and future similar outbreaks will almost certainly change dietary behaviors in an unhealthy direction. The COVID‐19 pandemic has the potential to further enhance food insecurity and poor dietary quality in urban environments, impacting decision making in food purchasing behaviors among both affluent and underprivileged citizens. Individuals are likely to shift toward consuming more nonperishable foods, traditionally high in sodium, added sugars, and saturated fat, to reduce grocery store trips and repeated potential exposures to severe acute respiratory syndrome coronavirus 2. Such changes in behavior may also be attributable to a general distrust in fresh fruit and vegetable supplies, even in the absence of foodborne transmission during the pandemic. Amidst current and future pandemics, federal and American Heart Association nutrition guidelines8 should continue to promote safe consumption of fruits and vegetables, by whole or frozen means, for cardiovascular health. The CVD prevention community should also vocally encourage the consumption of healthy nonperishable foods, such as beans, lentils, and nuts. For example, although habitual legume intake confers a decreased risk of hypertension,9 type 2 diabetes mellitus,10 and hypercholesterolemia,11 <10% of adults report consumption of legumes on a given day.12 In a time where individuals may have limited access to their traditional diets, many will be more readily susceptible to dietary behavior changes. Thus, a high demand for nonperishable foods in the setting of infectious disease pandemics can also provide opportunities to increase consumption of cardiovascular health–promoting foods and broadly shift Americans toward a more healthful, plant‐based dietary pattern.
With respect to social determinants of health, Louisiana was among the first states to stratify COVID‐19 pandemic data by race/ethnicity. Recent data illustrated that an alarming 57.4% of all COVID‐19 deaths in Louisiana were among blacks (Table), a community comprising just one third of the state's population.1 These early reports make it clear that modern‐day public health disasters, including infectious disease pandemics like COVID‐19, may further reinforce racial disparities,13 perhaps specifically those related to CVD. As an example, the effects of Hurricane Katrina on different race/ethnicity groups have been previously shown, as blacks living in Louisiana parishes were more likely to be hospitalized for CVD, particularly ischemic heart disease and heart failure, compared with white New Orleans residents, during and after the storm.14 Thus, defeating both global pandemics and excess CVD burden may in part be achieved by incentivizing and implementing large‐scale primordial CVD prevention strategies that address racial inequities and disparities across the country. The recent novel cluster‐randomized trial to improve blood pressure control through black barbershops may serve as a notable example of such an approach for CVD prevention.15 Overall, these observations illustrate how the COVID‐19 pandemic underlines the interconnectedness of both communicable and noncommunicable disease prevention.
Table 1.
Characteristics of COVID‐19 Deaths in Louisiana, According to Race
| Race | Deaths, n (%) |
|---|---|
| Asian | 17 (0.8) |
| Black | 1237 (57.4) |
| Native Hawaiian/Pacific Islander | 4 (0.2) |
| Other | 17 (0.8) |
| Unknown | 9 (0.4) |
| White | 870 (40.4) |
Total deaths as of May 4, 2020 (n=2154). Source: Louisiana Department of Health. COVID‐19 indicates coronavirus disease 2019.
Last, the COVID‐19 pandemic more broadly provides a paradoxical challenge and opportunity for cardiovascular preventive care. Cancellation of nonessential visits will shift even more of the responsibility of preventive care to patients, and patients will need to be proactive in medication refills for common preventive pharmacotherapies, including statins and blood pressure–lowering medications. COVID‐19 case‐fatality rates are already markedly high among patients with CVD,16 thus it is even more prudent to continue such preventive pharmacotherapies during the pandemic among those with high risk. As severe acute respiratory syndrome coronavirus 2 uses the angiotensin‐converting enzyme 2 for host cell entry,17 robust observational studies and clinical trials are required to find the most optimal blood pressure–lowering regimen(s) for individuals with hypertension in the setting of CVD and COVID‐19 prevention. Furthermore, this situation will also be a fire drill–like test run for cardiovascular telehealth, particularly in the field of prevention. Artificial intelligence–powered wearable technologies that can accurately measure blood pressure and ECG screen for early ischemic changes and arrhythmias in high‐risk patients may hence be imperative to design and validate for remote telemonitoring in the future.
In the setting of modern‐day public health disasters, urban cities that are already disproportionately affected by CVD in the United States, such as New Orleans, hold a double burden of communicable and noncommunicable disease. Natural disasters and outbreaks, like COVID‐19, will continue to test our collective strength and resolve to discover new biological and public health interventions that mitigate both infectious disease and CVD risk. COVID‐19 will not be our last public health challenge, and we must continue to learn from our experiences to ensure that societal structures and healthcare delivery models improve, and not negate, the tremendous strides in cardiovascular health that we have made in the past several decades.
Sources of Funding
Investigators contributing to this work were supported by the National Institutes of Health under the following grants: F30HL147486 (Dr Razavi), P20GM109036 (Drs He, Krousel‐Wood, and Whelton), and R01AG041200 (Dr Bazzano).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e016997 DOI: 10.1161/JAHA.120.016997.)
For Sources of Funding and Disclosures, see page 4.
References
- 1. Louisiana Department of Health . Louisiana coronavirus COVID‐19 updated. https://ldh.la.gov/coronavirus/. Accessed May 4, 2020.
- 2. Moscona JC, Peters MN, Maini R, Katigbak P, Deere B, Gonzales H, Westley C, Baydoun H, Yadav K, Ters P, et al. The incidence, risk factors, and chronobiology of acute myocardial infarction ten years after Hurricane Katrina. Disaster Med Public Health Prep. 2019;13:217–222. [DOI] [PubMed] [Google Scholar]
- 3. Lenane Z, Peacock E, Joyce C, Frohlich ED, Re RN, Muntner P, Krousel‐Wood M. Association of post‐traumatic stress disorder symptoms following Hurricane Katrina with incident cardiovascular disease events among older adults with hypertension. Am J Geriatr Psychiatry. 2019;27:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang C, Liu L, Hao X, Guo H, Wang Q, Huang J, He N, Yu H, Lin X, Pan A, et al. Evolving epidemiology and impact of non‐pharmaceutical interventions on the outbreak of coronavirus disease 2019 in Wuhan, China. MedArxiv 2020.
- 5. Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Safford MM, Blair SN, Hooker SP. Patterns of sedentary behavior and mortality in U.S. middle‐aged and older adults a national cohort study. Ann Intern Med. 2017;167:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019;74:e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berenson GS, Wattigney WA, Bao W, Srinivasan SR, Radhakrishnamurthy B. Rationale to study the early natural history of heart disease: the Bogalusa Heart Study. Am J Med Sci. 1995;310:S22–S28. [DOI] [PubMed] [Google Scholar]
- 8. Van Horn L, Carson JAS, Appel LJ, Burke LE, Economos C, Karmally W, Lancaster K, Lichtenstein AH, Johnson RK, Thomas RJ, et al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association. Circulation. 2016;134:e505–e529. [DOI] [PubMed] [Google Scholar]
- 9. Jayalath VH, De Souza RJ, Sievenpiper JL, Ha V, Chiavaroli L, Mirrahimi A, Di Buono M, Bernstein AM, Leiter LA, Kris‐Etherton PM, et al. Effect of dietary pulses on blood pressure: a systematic review and meta‐analysis of controlled feeding trials. Am J Hypertens. 2014;27:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenkins DJA, Kendall CWC, Augustin LSA, Mitchell S, Sahye‐Pudaruth S, Blanco Mejia S, Chiavaroli L, Mirrahimi A, Ireland C, Bashyam B, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2012;172:1653–1660. [DOI] [PubMed] [Google Scholar]
- 11. Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM. Non‐soy legume consumption lowers cholesterol levels: a meta‐analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell DC, Lawrence FR, Hartman TJ, Curran JM. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J Am Diet Assoc. 2009;109:909–913. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW. COVID‐19 and African Americans. JAMA. 2020;323:1891–1892. [DOI] [PubMed] [Google Scholar]
- 14. Becquart NA, Naumova EN, Singh G, Chui KKH. Cardiovascular disease hospitalizations in Louisiana parishes’ elderly before, during and after Hurricane Katrina. Int J Environ Res Public Health. 2019;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx‐Drew D, et al. A cluster‐randomized trial of blood‐pressure reduction in black barbershops. N Engl J Med. 2018;378:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vital Surveillances . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


