Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) is an increasingly prevalent form of heart failure, representing half of the total burden of heart failure. We hypothesised that modulation of the phosphodiesterase type 3/cyclic AMP using a novel oral formulation of milrinone might exert favorable effects HFpEF via pulmonary and systemic vasodilation and enhancement of ventricular relaxation. We assessed the safety and efficacy of oral milrinone on quality of life and functional outcomes in patients with HFpEF.
Methods and Results
The MilHFPEF (Extended Release Oral Milrinone for the Treatment of Heart Failure With Preserved Ejection Fraction) study was a randomized, double‐blind, placebo‐controlled pilot study in 23 patients with symptomatic HFpEF. Efficacy end points included changes from baseline in Kansas City Cardiomyopathy Questionnaire summary score and 6‐minute walk distance. The primary safety end point was the development of clinically significant arrhythmia. The Kansas City Cardiomyopathy Questionnaire score improved significantly in milrinone‐treated patients compared with placebo (+10±13 versus −3±15; P=0.046). Six‐minute walk distance also tended to improve in the treatment group compared with placebo (+22 [−8 to 49] versus −47 [−97 to 12]; P=0.092). Heart rate (−1±5 versus −2±9 bpm; P=0.9) and systolic blood pressure (−3±18 versus +1±12 mm Hg; P=0.57) were unchanged. Early filling velocity/early mitral annular velocity (−0.3±3.0 versus −1.9±4.8; P=0.38) was unchanged. One patient in the placebo arm was hospitalized for heart failure. Holter monitoring did not demonstrate evidence of a proarrhythmic effect of milrinone.
Conclusions
In this novel pilot study, extended release oral milrinone was well tolerated and associated with improved quality of life in patients with HFpEF. Further longer‐term studies are warranted to establish the role of this therapeutic approach in HFpEF.
Registration
URL: https://www.anzctr.org.au/; Unique identifier: ACTRN12616000619448.
Keywords: diastolic heart failure, inotropes, lusitropy, pharmacotherapy, phosphodiesterase type 3
Subject Categories: Heart Failure, Translational Studies, Physiology, Echocardiography
Nonstandard Abbreviations and Acronyms
- BNP
B‐type natriuretic peptide
- BP
blood pressure
- E/e′
early filling velocity/early mitral annular velocity
- EF
ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- KCCQ
Kansas City Cardiomyopathy Questionnaire
Clinical Perspective
What Is New?
This pilot randomized controlled trial demonstrates an acceptable safety profile and signals of efficacy of a novel oral formulation of milrinone.
What Are the Clinical Implications?
Quality of life was improved with a trend toward improved 6‐minute walk distance; however, larger long‐term studies are required to demonstrated sustained efficacy, particularly on end points such as heart failure hospitalization.
Despite advances in the prevention and treatment of cardiovascular disease in general, numerous epidemiologic studies indicate that the overall burden of heart failure (HF) has increased over the past decade.1 In particular, increasing age and ongoing challenges in the management of comorbidities including hypertension, atrial fibrillation, and diabetes mellitus have contributed to the increase in prevalence and to a change in the phenotypic profile of HF patients. Presently, approximately half of those living with HF are now recognized to have heart failure with preserved ejection fraction (HFpEF), and this is rapidly becoming one of the most challenging issues in contemporary cardiovascular medicine.
In contrast to the substantial progress made in relation to the demonstration of effective treatments for Heart failure with reduced ejection fraction (HFrEF), little progress has been made for HFpEF. To some extent, the lack of success in previous trials can be attributed to the heterogeneous pathophysiology of HFpEF. The cardiovascular physiology of HFpEF is complex. While the central paradigm is that of left ventricular diastolic dysfunction leading to rapid exertion‐related rise in left atrial pressure, other elements such as subclinical systolic dysfunction,2 left atrial dysfunction,3 impaired right ventricular–pulmonary arterial coupling,4 and reduced peripheral oxygen delivery have all been implicated as potential pathophysiologic determinants of exercise intolerance.
We recently provided the first evidence for positive hemodynamic effects of milrinone in patients with HFpEF following an acute intravenous dose in a placebo‐controlled trial.5 Milrinone, a phosphodiesterase type III inhibitor, exhibits several pharmacologic actions that may be useful in HFpEF, including beneficial effects on left ventricular diastolic function, pulmonary vasodilation, and systemic vasodilation. In the present study, we evaluated the safety and efficacy of chronic, orally administered milrinone in patients with HFpEF, using a novel extended‐release formulation of the drug.
Methods
Study Design and Participants
We conducted a prospective, randomized, double‐blind, placebo‐controlled trial of novel extended‐release oral formulation of milrinone. The primary objective was to assess the safety of this novel therapeutic approach in HFpEF patients. Key inclusion criteria were age ≥18 years, New York Heart Association class III symptoms of HF, left ventricular ejection fraction ≥50%, echocardiographic features of structural or functional alteration in keeping with the European Society of Cardiology guidelines for the diagnosis of HFpEF (≥1 of left atrial volume index >34 mL/m2, left ventricular mass index ≥115 g/m2 for men or 95 g/m2 for women, and/or septal early filling velocity/early mitral annular velocity (E/e′) ≥13 and mean e′ of the septal and lateral walls <9 cm/s). Patients were also required to either have had a hospitalization for HF within 12 months, or elevated natriuretic peptides (BNP [B‐type natriuretic peptide] >35 pg/mL or NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) >125 pg/mL if sinus rhythm, and BNP >100 pg/mL or NT‐proBNP >300 pg/mL if atrial fibrillation). Patients were included only if they were on stable heart failure therapy for the 2 weeks before screening, excluding diuretic dose changes <50% of the total diuretic dose. Key exclusion criteria were myocardial infarction within 90 days before screening, systolic blood pressure <90 mm Hg, cardiac surgery within 60 days before screening, moderate or greater degree of cardiac valvular stenosis or regurgitation, and significant comorbid disease, including hepatic, renal (glomerular filtration rate <30 mL/min), or respiratory disease. Patients with a 6‐minute walk distance <150 m and poorly controlled atrial fibrillation (resting rate >100 bpm) and patients on flecainide, encainide, propafenone, dofetilide, and disopyramide were excluded. Potassium was required to be between 4 and 5.5 mEq/L and magnesium >1.0 mEq/L.
The study protocol was approved by the Alfred Hospital Human Research and Ethics Committee. All patients gave written informed consent. The study was registered with the Australian New Zealand Clinical Trials Registry, registration number ACTRN12616000619448. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Procedures
Following enrollment, patients underwent baseline evaluation including a 6‐minute walk test, quality‐of‐life scores, and single laboratory assessment of natriuretic peptides, in addition to a transthoracic echocardiogram. The estimated glomerular filtration rate was calculated with the MDRD (Modification of Diet in Renal Disease) study equation. A 24‐hour Holter monitor was performed to screen for presence of arrhythmia and control of atrial fibrillation. Echocardiography was performed as per standard American Society of Echocardiography guidelines. Patients were supplied with thigh‐mounted activity monitors (ActivPal 3, PAL Technologies Ltd., Glasgow, UK). Devices were worn for the first 2 weeks (during the placebo run‐in) and during the final 2 weeks of the trial. Actigraphic measures included step count, active hours, and up‐down transitions during each period. Each accelerometer was activated at the time it was dispensed, providing time‐ and date‐stamped data synchronized to the study protocol.
Quality of life was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ). The KCCQ is a well‐validated 23‐item questionnaire that quantifies multiple domains of quality of life and has been validated previously in HFpEF,6 with a change of ≥5 points in the overall summary score being considered clinically significant. Six‐minute walk testing and KCCQ questionnaires were repeated at completion in addition to a transthoracic echocardiogram, and a daily diary was maintained to record a daily Likert scale of their dyspnea and for nonserious adverse events. Patients were contacted by telephone 1 week after the completion of the study.
Permuted block randomization allocated patients in a 1:1 ratio between active treatment and placebo in blocks of 2 or 4 with random variation of the blocking number. Before randomization, patients entered a single‐blind placebo run‐in period to determine compliance and assess for non–drug‐related adverse events. The study drugs were administered in a gelatin capsule twice daily. Each capsule contained extended‐release milrinone minitabs (14 mg total dose) or matching placebo minitabs. Dose selection was based on previous studies of pharmacokinetics and hemodynamics in healthy volunteers. Patients were clinically reviewed at 2 weekly intervals, with the randomization occurring at day 15 and with study completion at day 43.
Outcome Measures
The primary objective was to investigate the safety of this novel therapeutic approach in HFpEF. Efficacy measures included echocardiographic measures reflecting diastolic function (change in the E/e′ ratio, right ventricular systolic pressure, and left atrial volume index); quality of life (KCCQ and self‐reported dyspnea scale rated by patients on each day of the study); functional capacity (6‐minute walk distance) and biochemical measures (natriuretic peptide levels and renal function). The study was powered on the basis of a hypothesized 20% reduction in the E/e′ from a baseline of 14.5±2, with 12 patients per group for 80% power.
Statistical Analysis
Analysis was performed as intention to treat. Baseline characteristics are presented according to assignment at randomization. Descriptive statistics for continuous variables are presented as mean±SD for parametric data, and median and interquartile range for nonparametric data. Categorical data are presented as a percentage of the group. Comparisons used the t‐test or chi‐square as appropriate for the data type, with a P value of <0.05 considered statistically significant. Nonparametric values were analyzed using the Mann–Whitney U test. Linear regression was performed and adjusted as specified in the text. Statistical analyses were performed using R (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria).
Role of the Funding Source
The study was designed by the members of the executive committee in collaboration with the sponsor of the study (Cardiora). Data collection and analysis was performed at the Alfred Hospital by the investigators with supervision by author SN. The report was drafted by the first author and revised by all authors, who have read and agreed to the report as written and the decision to submit for publication.
Results
From June 2016 to September 2018, a total of 49 patients were screened, and 23 patients were enrolled into the study. The primary reasons for screen failure were a BNP below the specified cutoff or the presence of concomitant moderate tricuspid regurgitation. Twelve patients were assigned to receive milrinone and 11 patients to placebo. The groups were well matched at baseline (Table 1), with no significant differences in key characteristics. The mean age of the patient group was 77±6 years of age, and 74% (17/23) were women. The majority of patients had a body mass index within the obese range (65%), with an overall study group median body mass index of 32 (29–34). All patients demonstrated structural and functional changes consistent with HFpEF on echocardiography, in keeping with the current guidelines established by the European Society of Cardiology for the diagnosis of HFpEF. All patients had a preserved ejection fraction (mean, 61±6%), as well as elevated septal (mean 18.2±5.7) and lateral (mean 13.8±4.0) E/e′ ratios and left atrial enlargement (left atrial volume index, 45 [38–50]). Resting estimated pulmonary artery systolic pressure was <35 mm Hg in the majority of patients (75%).
Table 1.
Baseline Characteristics
| Milrinone (n=12) | Placebo (n=11) | |
|---|---|---|
| Age, y | 77±6 | 77±5 |
| Sex (% female) | 67 | 82 |
| Body mass index, kg/m2 | 30 [28–33] | 34 [31–35] |
| Atrial fibrillation | 2 (17) | 3 (27) |
| Hypertension | 11 (92) | 9 (82) |
| Diabetes mellitus | 6 (50) | 2 (18) |
| Ischemic heart disease | 4 (33) | 2 (18) |
| Atrial fibrillation | 4 (33) | 5 (45) |
| Bloods | ||
| Creatinine, μmol/L | 78 [71–101] | 90 [81–105] |
| eGFR | 68 [61–74] | 56 [47–69] |
| N‐terminal pro‐BNP, pg/mL | 276 [209–444] | 666 [257–1203] |
| Clinical | ||
| Blood pressure, mm Hg | ||
| Systolic | 151 [135–156] | 149 [126–151] |
| Diastolic | 73 [68–81] | 74 [58–81] |
| Heart rate, bpm | 69±9 | 71±13 |
| Medications | ||
| ACE inhibitor or ARB | 10 (83) | 10 (91) |
| β‐Blocker | 3 (25) | 5 (45) |
| Loop diuretic/furosemide | 5 (42) | 7 (63) |
| Nondihydropyridine calcium channel blocker | 1 (8) | 1 (9) |
| Spironolactone | 4 (33) | 6 (63) |
| Metformin | 3 (25) | 1 (9) |
| Insulin | 1 (8) | 1 (9) |
| Statin | 7 (58) | 6 (54) |
| Dihydropyridine calcium channel blocker | 2 (17) | 2 (18) |
| Echocardiographic | ||
| Ejection fraction | 62±4 | 60±7 |
| LVEDD, mm | 46±4 | 47±5 |
| LVESD, mm | 29±6 | 31±5 |
| TAPSE, cm | 2.4±0.4 | 2.3±0.4 |
| LAVI, mL/m2 | 45 [41–47] | 46 [38–62] |
| Septal E/e′ | 17.5±3.5 | 19.8±6.6 |
| Lateral E/e′ | 13.3±3.4 | 14.6±4.2 |
| Mean E/e′ | 15.4±3.2 | 17.2±5.4 |
| RVSP, mm Hg | 30±6 | 28±8 |
| Functional | ||
| 6‐min walking distance | 394 [252–451] | 320 [236–366] |
| Baseline KCCQ Overall Summary Score | 55 [40–65] | 52 [39–63] |
Parametric variables are reported as mean±SD, and nonparametric variables presented as median [interquartile range]. Values presented are those taken at screening. There were no statistically significant differences noted in baseline variables. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; E/e′, early filling velocity/early mitral annular velocity; eGFR, estimated glomerular filtration rate; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAVI, left atrial volume index; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; RVSP, right ventricular systolic pressure; and TAPSE, tricuspid annular plane systolic excursion.
Safety
In this pilot study, the extended‐release formulation of milrinone was safe and was well tolerated. The heart rate in milrinone‐treated patients at baseline was similar to the placebo group (69±9 versus 71±13 bpm; P=0.64) and did not differ after 1 month of treatment (68±9 versus 72±11; P=0.32). There was no difference in the change in heart rate between groups (delta heart rate, −1±7 versus −1±8 bpm, milrinone versus placebo; P=0.94). Changes in systolic (−3±18 versus +1±12 mm Hg; P=0.57) and diastolic (−3±12 versus 2±8 mm Hg, P=0.29) blood pressure were also similar across both groups. Two patients discontinued the trial; the former for adverse events beginning during the placebo run‐in phase, and the latter ceased after hospitalization for an unrelated medical condition. There was 1 serious adverse event (HF hospitalization) in a placebo‐treated patient. No serious adverse events were reported in the milrinone arm. There were no atrial or ventricular arrhythmias reported in either arm.
Biochemical
NT‐proBNP was numerically higher at baseline in the placebo group but not statistically different (666 [257–1203] versus 276 [209–444]; P=0.11), with no significant differences over the course of the trial (change in NT‐proBNP; milrinone +9 [−58 to 52], placebo −2 [−169 to 68]; P=0.66). There was a small improvement in estimated glomerular filtration rate in the milrinone arm compared with a small fall in the placebo group (change in estimated glomerular filtration rate; milrinone +2±6 versus −3±7; P=0.08).
Quality of Life
There was no significant between‐group difference in KCCQ score (milrinone: 55 [40–65]; placebo: 52 [39–63]; P=0.85) or 6‐minute walk distance (394 [252–451] versus 320 [236–366]; P=0.23) at baseline. Following treatment, patients randomized to milrinone had a significantly greater improvement in KCCQ score than patients allocated to placebo (+10±13 versus −3±15; P=0.04; Figure 1), with a significant change in the quality‐of‐life subdomain (22±21 versus −7±20; P=0.004). There was a trend toward improvement in the 6‐minute walk distance (10±62 versus −42±77; P=0.092; Figure 2). There were no differences in step count adjusted for time monitored (−13 [−53 to 17] versus −18 [−29 to −4] steps/h; P=0.46).
Figure 1. Changes in KCCQ overall summary score and quality‐of‐life subdomain between groups.
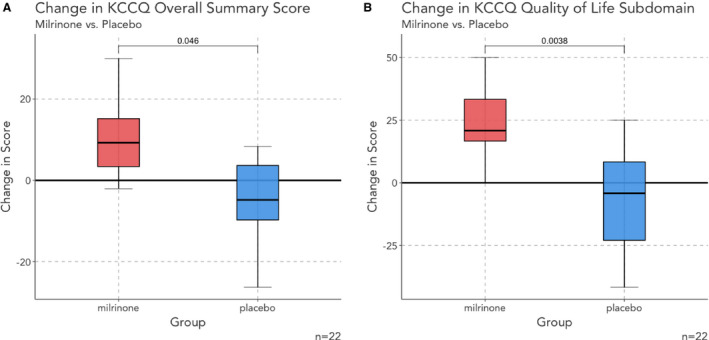
Patients treated with milrinone are indicated in red, with placebo‐treated patients indicated with blue. There was a significantly greater improvement in the overall KCCQ score (A) and quality‐of‐life subdomain score (B) in patients treated with milrinone. KCCQ indicates Kansas City Cardiomyopathy Questionnaire.
Figure 2. Change in 6‐minute walk distance between groups. Patients treated with milrinone are indicated in red, with placebo‐treated patients indicated with blue.
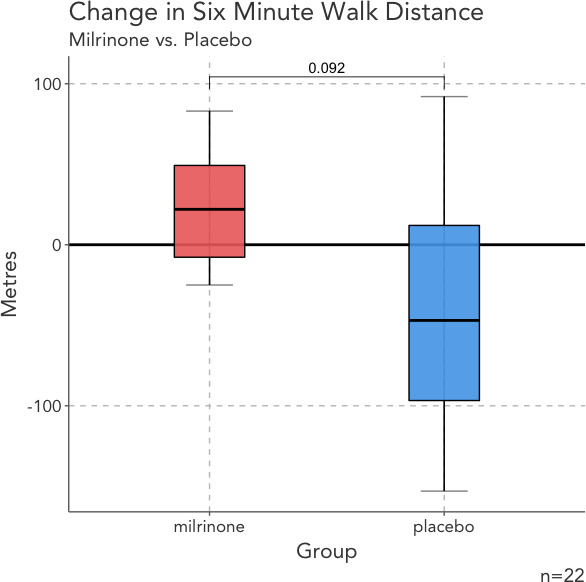
There was no statistically significant difference in 6‐minute walk distance between groups.
Echocardiography
The change in mean E/e′ was not significantly different between patients receiving milrinone and placebo (0±2.2 versus −2.2±4.3; P=0.15; Figure 3), nor were there differences in septal (−0.3±3 versus −1.1±5.2; P=0.66) or lateral E/e′ (0.2±2.3 versus −1.9±3.9; P=0.14). As shown in Table 2, within‐group changes in E/e′ were also not significant. Similarly, changes in tricuspid annular plane systolic excursion with milrinone (0.1±0.4 versus 0.1±0.3 cm; P=0.87) and estimated right ventricular systolic pressure (−1±5 versus −2±5 mm Hg; P=0.60) were no different compared with placebo. There were no differences in left ventricular size, left ventricular systolic function, and left atrial volume index, as shown in Table 1.
Figure 3. Changes in clinical, biochemical, and echocardiographic parameters.
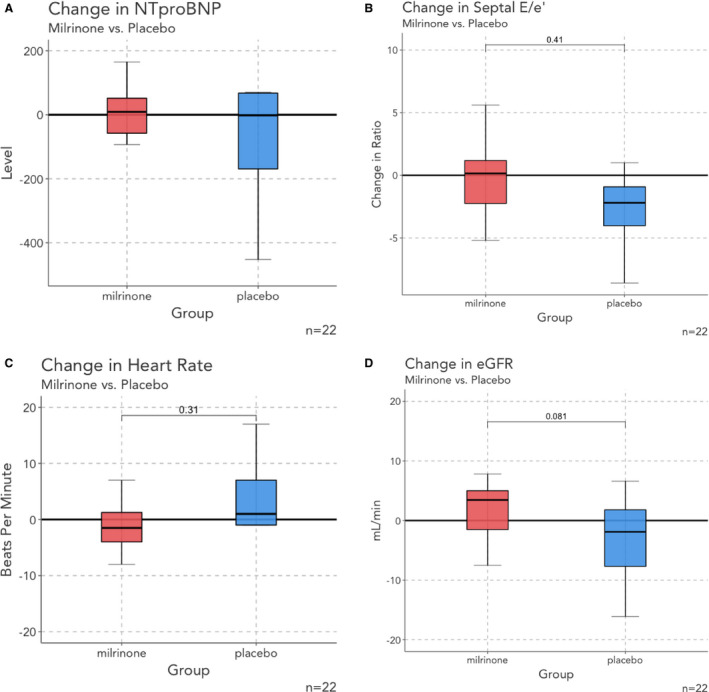
A, N‐terminal pro‐B‐type natriuretic peptide; B, septal E/e′; C, heart rate; D, change in estimated glomerular filtration rate. Patients treated with milrinone are indicated in red, with placebo‐treated patients indicated with blue. Overall, there were no significant between‐group differences in any of these parameters. E/e′ indicates early filling velocity/early mitral annular velocity; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Table 2.
Efficacy End Points
| End Points | Milrinone (n=12) | Placebo (n=11) | P Value | |
|---|---|---|---|---|
| Change in septal E/e′ ratio | Pre | 18±3 | 20±7 | |
| Delta | −0.3±3.0 | −1.9±4.8 | 0.38 | |
| Post | 17±4 | 18±6 | ||
| Change in KCCQ overall summary score | Pre | 53±19 | 51±21 | |
| Delta | 10±13 | −3±15 | 0.046 | |
| Post | 63±24 | 48±21 | ||
| Change in KCCQ quality‐of‐life subdomain | Pre | 39±25 | 49±23 | |
| Delta | 22±21 | −7±20 | 0.004 | |
| Post | 61±30 | 40±24 | ||
| Change in 6‐min walking distance | Pre | 394 [252–452] | 322 [263–371] | |
| Delta | 22 [−8 to 49] | −47 [−97 to 12] | 0.092 | |
| Post | 430 [244–467] | 308 [239–333] | ||
| Change in N‐terminal pro‐BNP | Pre | 276 [209–444] | 639 [219–814] | |
| Delta | 9 [−58 to 52] | −2 [169–68] | 0.66 | |
| Post | 234 [175–369] | 668 [347–965] | ||
| Change in eGFR | Pre | 68 [61–74] | 56 [47–69] | |
| Delta | 2±6 | −3±7 | 0.08 | |
| Post | 73 [62–77] | 48 [43–70] |
Parametric variables are reported as mean±SD, and nonparametric variables presented as median [interquartile range]. An unpaired t‐test or Wilcoxon test was used as appropriate. BNP, B‐type natriuretic peptide; E/e′, early filling velocity/early mitral annular velocity; eGFR, estimated glomerular filtration rate; and KCCQ, Kansas City Cardiomyopathy Questionnaire.
Discussion
The prevalence of HFpEF has risen significantly over the past 2 decades, and it is projected to become the most prevalent form of HF over the coming decade.7 A significant area of unmet need exists in regard to the availability of oral therapy for HFpEF. In this phase Ib study, extended‐release oral milrinone improved quality of life without a significant increase in adverse events.
In the current study, we demonstrated a clinically meaningful and significant effect of milrinone on quality of life as reflected by the KCCQ. In milrinone‐treated patients, there was an improvement in the KCCQ score of +10±13 compared with a fall of −3±15 (P=0.046) in placebo‐treated patients. Consistent with this, the quality‐of‐life subdomain improved substantially with milrinone (+22±19 versus −7±21; P=0.004). The KCCQ is one of the most widely used instruments to assess quality of life in HF, and has demonstrated to be reliable and valid in both HFrEF8 and HFpEF.6 Although reduction of hospitalization burden and mortality are important end points in HF. Quality of life remains a primary goal in HFpEF, particularly when the competing risk of death from comorbid conditions is significant in the context of a different demographic compared with HFrEF.9 In a secondary analysis of patients from the NEAT‐HFpEF (Nitrate's Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction) trial, KCCQ scores were strongly correlated with baseline functional status parameters, including 6‐minute walk distance and accelerometery. Importantly, serial changes are associated with clinical outcomes,10 with changes >5 points considered clinically significant. A large prospective HF registry confirmed the association of the KCCQ overall summary score with long‐term outcomes, with a 10‐point increase associated with a 12% decrease in the hazard of death or hospitalization.11 In this study, patients displayed a similar baseline KCCQ score to other HFpEF trials (MilHFPEF [Extended Release Oral Milrinone for the Treatment of Heart Failure With Preserved Ejection Fraction], 52±19; TOPCAT [Treatment of Preserved Ejection Fraction Heart Failure With an Aldosterone Antagonist Trial], 55±2112; NEAT‐HFpEF, 56±24).
Noninvasive estimation of filling pressure is often assessed using the ratio of the peak E/e′ and is recommended by the American Society of Echocardiography and European Society of Cardiology in the evaluation of HFpEF. In this study, baseline E/e′ was elevated across the cohort; however, there was no significant between‐group difference in the change in E/e′ over the course study. Despite its validity in HFrEF, multiple studies have demonstrated the limited accuracy of septal and lateral E/e′ in HFpEF, with poor correlations with direct measures of filling pressure.13 Furthermore, there is insufficient evidence for its use as a measure of filling pressure following intervention in HFpEF.14 There were no other changes in cardiac structure or function noted; however, the relatively short duration of the trial may not have allowed adequate exposure to observe a favorable effect.
The lack of success with previous trials of pharmacologic therapy is in part attributable to the diverse range of mechanisms and phenotypic heterogeneity. Primarily, an elevated filling pressure at rest or exercise promotes dyspnea, principally attributable to diastolic dysfunction; however, multiple other mechanisms have been implicated. Coexistent systolic dysfunction left atrial dysfunction, ventricular‐vascular stiffening, chronotropic incompetence, and impaired peripheral oxygen handling have all been implicated. Arguably, HFpEF represents a multisystem disorder with varying physiologic pathways, with single‐pathway therapies unlikely to succeed without careful phenotype selection.9 A proportion of patients with HFpEF develop significant pulmonary vascular remodeling, potentially irreversibly so, and consequently agents targeting the nitric oxide pathway alone may have diminished impact15, 16; similarly, protein kinase G modification of titin, a large protein implicated in the development of passive ventricular stiffness, is relevant in patients with more significant pulmonary and peripheral abnormalities. Renin‐angiotensin‐aldosterone blockade has been evaluated in large‐scale trials previously with negative results,17, 18, 19 perhaps again attributable to patient selection or potentially attributable to trial duration.
In the absence of a clear influence of milrinone on resting measures of left ventricular (LV) filling pressures, it is relevant to consider other means by which phosphodiesterase 3 inhibition may have improved quality of life. First, assessments of LV diastolic performance were conducted only at rest. It is possible that the actions of low‐dose oral milrinone on LV filling may be evident only during physical activity. Second, milrinone exerts several other potentially favorable actions on key pathophysiologic targets, including left and right ventricular contractility and pulmonary and systemic vascular impedance. For example, in previous work from our group, we randomized HFpEF patients to intravenous milrinone or saline in patients undergoing exercise hemodynamic evaluation.5 Milrinone administration was associated with a significant reduction in right atrial, pulmonary arterial, and pulmonary capillary wedge pressures at rest and during exercise. Furthermore, the positive inotropic effects of milrinone may potentiate reductions in filling pressure. Previous work has demonstrated coexistent systolic impairment in HFpEF2 despite a preserved ejection fraction, with reductions in LV global longitudinal strain and exercise LV stroke work index. Further work is required to define the pharmacodynamic relationship between milrinone levels and key physiologic parameters.
Oral formulations of milrinone have previously been trialed in HFrEF with disappointing outcomes. The PROMISE (Prospective Multicenter Imaging Study for Chest Pain Evaluation) trial,20 published in 1991, tested the hypothesis that long‐term milrinone therapy may improve survival in HFrEF. The study was stopped prematurely because of an increase in adverse events, primarily arrhythmic deaths. Importantly, PROMISE used a higher dose (mean dose, 40 mg/day) and an immediate‐release formulation administered 4 times per day, and was conducted before widespread use of β‐blockers and implantable cardiac defibrillators in HF management. We hypothesized that the extended‐release formulation of milrinone, providing zero‐order release and a more stable plasma profile, would result in fewer occurrences of potentially toxic plasma levels. A previous study of extended‐release milrinone in HFrEF did not result in increased arrhythmia.21 Unlike HFrEF where ventricular arrhythmia accounts for the majority of sudden death, the rates of sudden cardiac death are low in HFpEF,22 particularly when defined as an ejection fraction ≥50%.23
Limitations
This study has several limitations. The study was small and primarily designed principally to demonstrate safety. There was a high rate of screen failures; however, it is important to recognize that many patients with hemodynamically confirmed HFpEF do not have elevated natriuretic peptide levels, particularly at rest.24 In this study, there were no significant changes in E/e′ or natriuretic peptide levels. E/e′ has been shown to have limited accuracy in the estimation of pulmonary capillary wedge pressure in patients with HFpEF,25, 26 and more accurate invasive measures of filling pressure may have shown differences. Furthermore, the trial assessments were conducted at rest, and it is possible that dynamic changes in E/e′ during physical activity might be modified by phosphodiesterase 3 inhibition, given the positive effect on KCCQ. There was no change in step count and the change in 6‐minute walk distance only trended toward improvement. These parameters may have been limited by the small sample size. In addition, actigraphy is inherently reliant on volition and improvements in symptoms (as may be reflected indirectly with the KCCQ) and does not always track ambulatory activity.16 In the current study, detailed pharmacokinetics were not performed, and further studies need to be performed to relate plasma milrinone levels to treatment effects. The study was of short duration, and it is possible that reverse remodeling events might take months to appear. For example, a reduction in LV filling pressures could have an impact of left atrial and pulmonary vascular structure; however, this may take time to become evident. Finally, larger, longer‐term studies would be required to determine the impact on hard clinical end points such as HF hospitalization and mortality and to further define the safety profile.
Conclusions
In a pilot study of patients with HFpEF, a short duration of treatment with extended‐release oral milrinone appeared to be safe and resulted in improved quality of life and functional capacity without an increase in adverse events. Further longer‐term studies are warranted to establish the potential role of this therapeutic approach in HFpEF.
Sources of Funding
This work was supported by Cardiora and the National Health and Medical Research Council of Australia. Dr Nanayakkara is supported by a scholarship from the National Heart Foundation of Australia and the Baker Bright Sparks Program. Dr Kaye is supported by a Fellowship from the National Health and Medical Research Council of Australia. The Baker Institute is supported in part by the Victorian Government's OIS Program.
Disclosures
Dr Kaye is the cofounder of Cardiora Pty Ltd and coinventor of CRD‐102. Dr Byrne is a part‐time employee of Cardiora Pty Ltd. The remaining authors have no disclosures to report.
Acknowledgments
This work was performed at Department of Cardiology, Alfred, Melbourne, Australia.
(J Am Heart Assoc. 2020;9:e015026 DOI: 10.1161/JAHA.119.015026.)
For Sources of Funding and Disclosures, see page 9.
See Editorial by Silverman et al.
References
- 1. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, et al. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet. 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, et al.; Investigators P . Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santos ABS, Kraigher‐Krainer E, Gupta DK, Claggett B, Zile MR, Pieske BM, Voors AA, Lefkowitz M, Bransford T, Shi V, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hussain I, Mohammed SF, Forfia PR, Lewis GD, Borlaug BA, Gallup DS, Redfield MM. Impaired right ventricular‐pulmonary arterial coupling and effect of Sildenafil in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e002729 DOI: 10.1161/CIRCHEARTFAILURE.115.002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaye DM, Nanayakkara S, Vizi D, Byrne MJ, Mariani JA. Effects of milrinone on rest and exercise hemodynamics in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;67:2554–2556. [DOI] [PubMed] [Google Scholar]
- 6. Napier R, McNulty SE, Eton DT, Redfield MM, AbouEzzeddine O, Dunlay SM. Comparing measures to assess health‐related quality of life in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah RV, Desai AS, Givertz MM. The effect of renin‐angiotensin system inhibitors on mortality and heart failure hospitalization in patients with heart failure and preserved ejection fraction: a systematic review and meta‐analysis. J Card Fail. 2010;16:260–267. [DOI] [PubMed] [Google Scholar]
- 8. Green P, Kodali S, Leon MB, Maurer MS. Echocardiographic assessment of pressure volume relations in heart failure and valvular heart disease: using imaging to understand physiology. Minerva Cardioangiol. 2011;59:375–389. [PMC free article] [PubMed] [Google Scholar]
- 9. Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, Depre C, Desvigne‐Nickens P, Nkulikiyinka R, Lewis GD, et al. Heart failure with preserved ejection fraction expert panel report: current controversies and implications for clinical trials. JACC Heart Fail. 2018;6:619–632. [DOI] [PubMed] [Google Scholar]
- 10. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2017;2:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, Davila‐Roman VG, Mann DL, Spertus JA. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharifov OF, Schiros CG, Aban IB, Denney TS, Gupta H. Diagnostic accuracy of tissue Doppler index E/e’ for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta‐analysis. J Am Heart Assoc. 2016;5:e002530 DOI: 10.1161/JAHA.115.002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharifov OF, Gupta H. What is the evidence that the tissue Doppler index E/e′ reflects left ventricular filling pressure changes after exercise or pharmacological intervention for evaluating diastolic function? A systematic review J Am Heart Assoc. 2017;6:e004766 DOI: 10.1161/JAHA.116.004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Michael Felker G, LeWinter MM, Mann DL, et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE‐HFpEF randomized clinical trial. JAMA. 2018;320:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cleland JGF, Tendera M, Adamus J, Fremantle N, Polonski L, Taylor J; PEP‐CHF Investigators . The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J. 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 18. Pfeffer MA, Yusuf S, McMurray JJV, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 20. Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, et al. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325:1468–1475. [DOI] [PubMed] [Google Scholar]
- 21. Nanayakkara S, Mak V, Crannitch K, Byrne M, Kaye DM. Extended release oral milrinone, CRD‐102, for advanced heart failure. Am J Cardiol. 2018;122:1017–1020. [DOI] [PubMed] [Google Scholar]
- 22. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69:556–569. [DOI] [PubMed] [Google Scholar]
- 23. Bajaj NS, Claggett B, Lewis EF, Desai AS, Fang JC, O'Meara E, Shah SJ, Sweitzer NK, Fleg JL, Pitt B, et al. Influence of ejection fraction on cause‐specific mortality in heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;2:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B‐type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maeder MT, Thompson BR, Rocca HPBL, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. [DOI] [PubMed] [Google Scholar]
- 26. Sharifov OF, Schiros CG, Aban I, Denney TS, Gupta H. Diagnostic accuracy of tissue Doppler index E/è for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta‐analysis. J Am Heart Assoc. 2016;5:e002530 DOI: 10.1161/JAHA.115.002530. [DOI] [PMC free article] [PubMed] [Google Scholar]


