Abstract
Background
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a measure of heart failure (HF) health status. Worse KCCQ scores are common in patients with chronic kidney disease (CKD), even without diagnosed heart failure (HF). Elevations in the cardiac biomarkers GDF‐15 (growth differentiation factor‐15), galectin‐3, sST2 (soluble suppression of tumorigenesis‐2), hsTnT (high‐sensitivity troponin T), and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) likely reflect subclinical HF in CKD. Whether cardiac biomarkers are associated with low KCCQ scores is not known.
Methods and Results
We studied participants with CKD without HF in the multicenter prospective CRIC (Chronic Renal Insufficiency Cohort) Study. Outcomes included (1) low KCCQ score <75 at year 1 and (2) incident decline in KCCQ score to <75. We used multivariable logistic regression and Cox regression models to evaluate the associations between baseline cardiac biomarkers and cross‐sectional and longitudinal KCCQ scores. Among 2873 participants, GDF‐15 (adjusted odds ratio 1.42 per SD; 99% CI, 1.19–1.68) and galectin‐3 (1.28; 1.12–1.48) were significantly associated with KCCQ scores <75, whereas sST2, hsTnT, and NT‐proBNP were not significantly associated with KCCQ scores <75 after multivariable adjustment. Of the 2132 participants with KCCQ ≥75 at year 1, GDF‐15 (adjusted hazard ratio, 1.36 per SD; 99% CI, 1.12–1.65), hsTnT (1.20; 1.01–1.44), and NT‐proBNP (1.30; 1.08–1.56) were associated with incident decline in KCCQ to <75 after multivariable adjustment, whereas galectin‐3 and sST2 did not have significant associations with KCCQ decline.
Conclusions
Among participants with CKD without clinical HF , GDF‐15, galectin‐3, NT‐proBNP, and hsTnT were associated with low KCCQ either at baseline or during follow‐up. Our findings show that elevations in cardiac biomarkers reflect early symptomatic changes in HF health status in CKD patients.
Keywords: cardiac biomarkers, heart failure, quality of life
Subject Categories: Quality and Outcomes, Heart Failure, Biomarkers
Nonstandard Abbreviations and Acronyms
- aHR
adjusted hazard ratio
- aOR
adjusted odds ratio
- CKD
chronic kidney disease
- CKD‐EPI
Chronic Kidney Disease Epidemiology Collaboration
- CRIC
Chronic Renal Insufficiency Cohort
- ESKD
end‐stage kidney disease
- GDF‐15
growth differentiation factor‐15
- HF
heart failure
- hsTnT
high‐sensitivity troponin T
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- sST2
soluble suppression of tumorigenesis‐2
Clinical Perspective.
What Is New?
Our study found that cardiac biomarkers GDF‐15 (growth differentiation factor‐15), galectin‐3, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), and hsTnT (high‐sensitivity troponin T) were associated with health status consistent with early heart failure, as measured by the Kansas City Cardiomyopathy Questionnaire, among participants with chronic kidney disease without baseline heart failure.
What Are The Clinical Implications?
Elevations in cardiac biomarkers may reflect early symptoms of heart failure in patients with chronic kidney disease.
Patients with chronic kidney disease (CKD) are at high risk of developing heart failure (HF).1 Cardiac biomarkers and HF health status scores are subclinical and early clinical markers of HF in CKD and other populations.2, 3 The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a health status score used in prevalent HF patients, and lower scores are associated with an increased risk of HF hospitalizations and mortality.4 The KCCQ is an accepted clinical trial outcome as a surrogate marker of HF outcomes and can also be used to track longitudinal health status.5 In our previous work, we reported that CKD patients without diagnosed HF had a high prevalence of low KCCQ scores and that lower KCCQ scores were associated with incident HF.6, 7 However, the relationships between cardiac biomarkers and heart failure health status, as measured by the KCCQ, have not been studied to our knowledge.
In studies of persons with and without CKD, elevations in cardiac biomarkers GDF‐15 (growth differentiation factor‐15), galectin‐3, sST2 (soluble suppression of tumorigenesis‐2), hsTnT (high‐sensitivity troponin T), and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) have been associated with an increased risk of incident HF.2, 3, 8, 9, 10, 11 GDF‐15 is a stress‐related cytokine upregulated in cardiac ischemia/reperfusion injury.12 Galectin‐3 is a protein involved with broad cellular functions including cell‐cell adhesion and cell‐matrix interactions and is involved in HF pathogenesis via fibrosis and inflammation.13 sST2, a member of the interleukin‐1 receptor family, binds to the cardioprotective cytokine interleukin‐33 in settings of cardiac stress, contributing to cardiac injury.14 hsTnT is a component of cardiac myocytes and specific marker of cardiac ischemia, and NT‐proBNP is released in response to myocardial stretch.15, 16 Understanding the associations of cardiac biomarkers with patient health status related to HF may improve early detection and diagnosis of HF in CKD patients and could offer pathophysiological insights into HF development in CKD.
To understand the relationships between cardiac biomarkers and health status consistent with early HF, we evaluated the association of GDF‐15, galectin‐3, sST‐2, hsTnT, and NT‐proBNP with KCCQ health status scores at baseline as well as longitudinally. We hypothesized that cardiac biomarkers would be associated with low (worse) KCCQ scores at baseline and with worsening KCCQ scores over a 4‐year period.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the CRIC (Chronic Renal Insufficiency Cohort) Study at www.cristudy.org.
Study Design and Population
We performed cross‐sectional and longitudinal analyses to evaluate associations of cardiac biomarkers with HF health status among persons with CKD in the CRIC Study. The CRIC Study is a prospective multicenter longitudinal cohort study that included 3939 adult patients with mild to moderate CKD, with an estimated glomerular filtration rate of 20 to 70 mL/min.17 Patients were recruited at 7 clinical centers (with 13 enrolling sites) across the United States from May 2003 to March 2007. Exclusion criteria were presence of New York Heart Association class III or IV HF, cirrhosis, HIV, pregnancy, previous receipt of dialysis, history of transplant, polycystic kidney disease, and others as previously described.18 All study participants provided written informed consent, and the study protocol was approved by institutional review boards at each of the participating sites.
For the present analysis, we excluded CRIC participants who did not have all 5 cardiac biomarkers measured at baseline (N=277); were missing a KCCQ score at year 1 (N=410) which was the earliest measure of the KCCQ in CRIC; or had end‐stage kidney disease (ESKD) at year 1 (N=40). We further excluded patients with HF or missing HF status at year 1 (N=339) as our objective was to evaluate CKD participants without diagnosed HF. Of the 3939 participants in the original CRIC study population, 2873 participants were included in the analytic population for our cross‐sectional analysis.
Predictors
The primary predictors were levels of the cardiac biomarkers GDF‐15, galectin‐3, sST‐2, hsTnT, and NT‐proBNP measured at baseline. GDF‐15, galectin‐3, and sST2 were measured from EDTA plasma stored at 70°C from samples at baseline in batch at the University of Pennsylvania Laboratory in 2017. GDF‐15, galectin‐3, and sST2 were measured using ELISA (R&D Systems) and had intra‐assay coefficients of variation of 2.0%, 4.0%, and 2.6%, respectively. All assays were measured in duplicate.
The biomarkers hsTnT and NT‐proBNP were measured at baseline in 2008 from EDTA plasma stored at −70°C using a chemiluminescent microparticle immunoassay (www.roche-diagnostics.us) on the ElecSys 2010 at the University of Maryland. HsTnT was measured using the highly sensitive assay with a range of values from 3 to 10 000 ng/L. Any values below the lower limit of blank were characterized as “undetectable.” The coefficient of variation was 6.0% at a level of 26 ng/L and 5.4% at 2140 ng/L. The value at the 99th percentile cutoff from a healthy reference population was 13 ng/L for hsTnT with a 10% coefficient of variation. The range of values for NT‐proBNP, was from 5 to 35 000 pg/mL and the coefficient of variation was 9.3% at a level of 126 pg/mL and 5.5% at 4319 pg/mL.
Outcomes
The primary outcomes were baseline KCCQ Overall Summary Scores and an incident decline in KCCQ Overall Summary Scores. The KCCQ Overall Summary Score includes the total symptom, physical function, social limitations and quality of life scores, and ranges from 0 to 100, with lower scores reflecting worse health status. For the present study, initial KCCQ scores were measured at year 1. We dichotomized the score at 75 to indicate health status consistent with early HF, which was considered a meaningful threshold of health status consistent with HF based on prior studies and our work in the CRIC Study.4, 6, 19 The KCCQ is a 23‐item self‐administered questionnaire measuring participants’ perception of their health status, completed by participants at annual CRIC study visits.4 It is a validated survey instrument that is highly sensitive for monitoring changes in HF health status.5, 20, 21 The KCCQ incorporates questions related to respiratory symptoms, activities of daily living, extremity swelling, fatigue, and lifestyle. It consists of 6 domains: symptoms, physical function, quality of life, social limitation, self‐efficacy, and symptom stability.
As an additional outcome, we examined the development of incident decline in KCCQ scores using annual measures of KCCQ ascertained at CRIC Study visits from years 1 to 5. This analysis was restricted to the 2132 CRIC participants included in our study who had KCCQ ≥75 at year 1. We defined incident decline as a change in KCCQ meeting 2 criteria: (1) crossing the threshold of a KCCQ score of 75 by transitioning from KCCQ≥75 to a KCCQ<75, and (2) a mean of >3 points per year decrease over the participant's follow‐up time, equivalent to a >12 point decline in KCCQ score from year 1 to year 5. We defined this threshold for a clinically significant change based on clinical trial data in other populations that have evaluated the trajectory of KCCQ over time.22, 23, 24, 25
Covariates
Covariates were measured at year 1 and included participant demographics, comorbidities, clinical variables, laboratory variables, and medications known to be associated with cardiac biomarkers and health status. At the baseline study visit, participants provided information on their demographic characteristics, including age, sex, and race/ethnicity.17 Race/ethnicity was categorized as non‐Hispanic white, non‐Hispanic black, Hispanic, or other. At the baseline study visit and each subsequent study visit, participants reported their comorbidities including cardiovascular disease, myocardial infarction/revascularization, chronic obstructive pulmonary disease, atrial fibrillation, and stroke. Diabetes mellitus was defined as a fasting glucose >126 mg/dL, a nonfasting glucose >200 mg/dL, or use of insulin or other antidiabetic medication. Blood pressure and anthropometric measurements were assessed using standard protocols.26 Body mass index was calculated as weight in kilograms divided by height in meters squared. Serum creatinine was measured using an enzymatic method on an Ortho Vitros 950 at the CRIC Central Laboratory and standardized to isotope dilution mass spectrometry‐traceable values.27 Estimated glomerular filtration rate was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation.28 Additional assays measured 24‐hour urine total protein, which was measured at the CRIC Study Central Laboratory. Participants reported their medication usage at baseline and subsequent study visits, including use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, diuretics, and beta blockers.17 Transthoracic echocardiograms were performed at 1 year and provided data on left ventricular ejection fraction and left ventricular mass index as previously described.29 Assessments were performed using 2‐dimensional images and a standard imaging protocol according to American Society of Echocardiography guidelines and quantified centrally by a highly trained Registered Diagnostic Cardiac Sonographer.30 Our analyses were adjusted using covariates at year 1 time points.
Statistical Analysis
We first described baseline characteristics of the overall study population. Levels of GDF‐15, galectin‐3, sST‐2, and NT‐proBNP were divided into quintiles (because there are no clinically meaningful or prespecified cutoffs in patients with CKD). Because of the large number of participants with undetectable hsTntT, we categorized hsTnT as undetectable (<10 ng/L) and in tertiles across the detectable range, similar to our prior published work.31, 32 Characteristics of the study population by biomarker category were reported as mean and SD or median and interquartile range for continuous variables and as number and percentage for categorical variables. Proportions of participants with KCCQ scores <75 were reported across biomarker categories.
In cross‐sectional analyses, separate multivariable logistic regression models were used to evaluate the associations between each cardiac biomarker and KCCQ scores <75. Cardiac biomarkers were modeled as both continuous variables (per SD increase) and in categories. We performed a series of nested models that were specified prior to conducting the analyses. Model 1 was adjusted for age, sex, and race/ethnicity. Model 2 was additionally adjusted for biologically relevant covariates: cardiovascular disease, myocardial infarction/revascularization, chronic obstructive pulmonary disease, atrial fibrillation, stroke, diabetes mellitus, systolic blood pressure, body mass index, current smoking, estimated glomerular filtration rate, 24‐hour urinary protein, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker use, diuretic use, and beta blocker use.
Using Cox regression, we then examined the association of baseline biomarker levels with incident decline in KCCQ scores among participants with year 1 KCCQ ≥75, which we defined as a decrease in KCCQ scores to <75 and a mean of >3 points per year decrease over the participant's follow‐up time. This analysis was limited to 2132 participants who had KCCQ ≥75 at year 1. Participants were censored at year 5, loss to follow‐up, withdrawal, death, development of ESKD, or development of incident HF. We censored participants at the development of ESKD for several reasons. First, the risk of subclinical HF is likely different in patients with ESKD compared with CKD. Further, the KCCQ has not been validated for use in the ESKD population. We chose to censor participants who developed incident HF, as we sought to evaluate the association of cardiac biomarkers with health status consistent with early HF before the development of HF. Models were adjusted for the same covariates as in the cross‐sectional analysis listed previously. We evaluated the proportional hazards assumption and found no violations for any biomarker in continuous unadjusted models (GDF‐15: P=0.66; galectin‐3: P=0.48; sST‐2: P=0.90; hsTNT: P=0.24; NT‐proBNP: P=0.90).
We performed several sensitivity analyses to evaluate the robustness of our results. We first included all 5 biomarkers in the multivariable model to evaluate if the associations with biomarkers and KCCQ scores were independent of the other biomarkers (Model 3). We also adjusted the model for left ventricular (LV) mass and ejection fraction to determine if the observed associations were independent of other established subclinical HF measures (Model 4). For the longitudinal analyses, we added adjustment for baseline KCCQ scores to evaluate the decline in KCCQ scores independent of the baseline value (Model 5). In another sensitivity analysis, we evaluated a more restrictive definition of KCCQ decline, defined as developing a KCCQ <60 and having an average decline in KCCQ score of >5 points/year among participants with a baseline KCCQ≥60.
All analyses were performed using the R 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria) software environment. We accounted for multiple comparisons using a Bonferroni correction, where a 2‐sided P value of 0.01 was considered statistically significant for all analyses.
Results
Characteristics of the Study Population
Among the 2873 participants included in the cross‐sectional analysis, mean age was 59 years and estimated glomerular filtration rate was 43 mL/min per 1.73 m2. Participants had a high prevalence of comorbidities, including diabetes mellitus (47%) and cardiovascular disease (28%), and the majority were prescribed angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers or diuretic medications (Table 1). The median and interquartile range for each biomarker were as follows: GDF‐15 median 1377, interquartile range (949–2016) pg/mL; galectin‐3 13.7 (9.9–18.7) pg/mL; sST‐2 15.1 (11.2–20.2) pg/mL; hsTnT 13.2 (8.1–22.4) ng/L; and NT‐proBNP 109 (41–285) pg/mL. Participants with higher levels of GDF‐15 were older; more likely to be male, black, or Hispanic; and had a higher prevalence of comorbidities. Participants with higher GDF‐15 were more likely to use angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, diuretics, and beta blockers (Table 1). Similar patterns were seen across the other biomarkers (Tables S1‐S4).
Table 1.
Demographic Characteristics by Quintile of Growth Differentiation Factor‐15 (N=2873)
| Overall (N=2873) | Quintile 1 ≤856 pg/mL (N=579) | Quintile 2 857 to 1200 pg/mL (N=570) | Quintile 3 1201 to 1570 pg/mL (N=575) | Quintile 4 1571 to 2220 pg/mL (N=574) | Quintile 5 >2220 pg/mL (N=575) | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 59 (10.8) | 53 (11.3) | 58 (11.0) | 60 (10.5) | 62 (9.2) | 62 (9.0) |
| Male | 1558 (54) | 287 (50) | 304 (53) | 315 (55) | 313 (55) | 339 (59) |
| Race/ethnicity | ||||||
| Non‐Hispanic white | 1314 (46) | 334 (58) | 266 (47) | 277 (48) | 237 (41) | 200 (35) |
| Non‐Hispanic black | 1111 (39) | 192 (33) | 222 (39) | 209 (36) | 242 (42) | 246 (43) |
| Hispanic | 324 (11) | 30 (5) | 48 (8) | 72 (13) | 69 (12) | 105 (18) |
| Other | 124 (4) | 23 (4) | 34 (6) | 17 (3) | 26 (5) | 24 (4) |
| Comorbidities | ||||||
| Cardiovascular disease | 806 (28) | 68 (12) | 120 (21) | 175 (30) | 218 (38) | 225 (39) |
| Myocardial infarction/prior revascularization | 530 (18) | 47 (8) | 82 (14) | 108 (19) | 143 (25) | 150 (26) |
| Chronic obstructive pulmonary disease | 121 (4) | 14 (2) | 22 (4) | 30 (5) | 23 (4) | 32 (6) |
| Atrial fibrillation | 419 (15) | 70 (12) | 65 (11) | 84 (15) | 94 (16) | 106 (18) |
| Stroke | 269 (9) | 19 (3) | 39 (7) | 59 (10) | 78 (14) | 74 (13) |
| Diabetes mellitus | 1341 (47) | 100 (17) | 207 (36) | 295 (51) | 338 (59) | 401 (70) |
| Clinical variables | ||||||
| Systolic blood pressure, mm Hg | 126.4 (21.3) | 118.5 (18.2) | 123.5 (19.5) | 125.8 (19.8) | 130.8 (21.9) | 133.8 (23.4) |
| Body mass index, kg/m2 | 32 (7.6) | 31 (7.2) | 32 (7.5) | 33 (7.6) | 32 (7.7) | 32 (7.8) |
| Current smoking | 340 (12) | 26 (4) | 45 (8) | 67 (12) | 94 (16) | 108 (19) |
| Laboratory variables | ||||||
| Estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration), mL/min per 1.73 m2 | 43.2 (15.7) | 58.1 (13.3) | 46.4 (12.2) | 41.1 (12.5) | 37.3 (13.2) | 32.6 (13.8) |
| Urinary protein to creatinine ratio from 24 h urine test | 117 (52–602) | 60 (39–107) | 92 (48–332) | 123 (54–530) | 178 (62–905) | 448 (121–1958) |
| Ejection fraction, % | 55.3 (7.3) | 55.2 (7.1) | 55.8 (7.1) | 55.4 (7.1) | 55.3 (7.4) | 54.5 (7.7) |
| Left ventricular mass index, g | 62.8 (22.3) | 54.6 (20.1) | 59.7 (19.8) | 63.2 (20.7) | 66.5 (22.2) | 71.9 (24.9) |
| Medications | ||||||
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 1972 (69) | 314 (54) | 411 (72) | 427 (74) | 423 (74) | 397 (69) |
| Diuretics | 1616 (56) | 204 (35) | 290 (51) | 353 (61) | 373 (65) | 396 (69) |
| Beta blockers | 1349 (47) | 173 (30) | 259 (45) | 276 (48) | 315 (55) | 326 (57) |
Entries are mean (SD) for continuous covariates or N (%) for categorical covariates, except as noted.
Cross‐sectional Associations of Cardiac Biomarkers with KCCQ Scores
Higher quintiles of GDF‐15, galectin‐3, and sST‐2 had higher proportions of KCCQ scores <75 and were incrementally associated with KCCQ scores of <75 in unadjusted analyses (Figure and Table 2). When adjusting for patient demographics and biologically relevant covariates, GDF‐15 (adjusted odds ratio [aOR] 1.42 per SD higher GDF‐15; 99% CI, 1.19–1.68) was significantly associated with KCCQ scores of <75, and the highest quintile of GDF‐15 compared with the lowest quintile was associated with an approximately 2‐fold odds of KCCQ <75 (Table 2). Galectin‐3 was also significantly associated with KCCQ scores <75 when modeled continuously (aOR 1.28 per SD higher galectin‐3; 99% CI, 1.12–1.48), and the highest quintile was also associated with an approximately 2‐fold odds of KCCQ <75 when compared with the lowest quintile (Table 2). In continuous analyses, sST‐2 was significantly associated with KCCQ scores <75 (aOR 1.20 per SD higher sST2; 99% CI, 1.05–1.38) when adjusted for participant demographics, but this association did not reach statistical significance after adjustment for biologically relevant covariates (Table 2).
Figure 1.
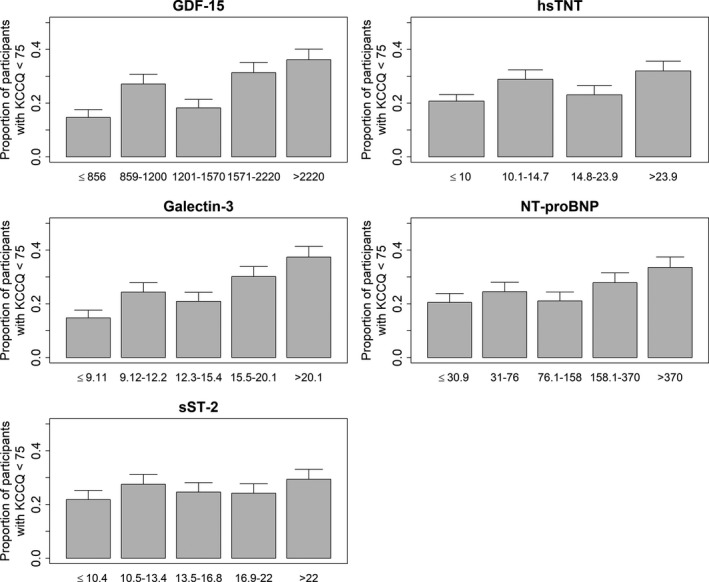
Proportion of participants with year 1 KCCQ<75 by biomarker category (N=2873). KCCQ indicates Kansas City Cardiomyopathy Questionnaire.
Table 2.
Cross‐Sectional Association of Cardiac Biomarkers With Year 1 KCCQ Score <75 in Persons With CKD Without HF in the CRIC Study (N=2873)
| KCCQ score <75 Model 0 OR (99% CI) | KCCQ score <75 Model 1 aOR (99% CI) | KCCQ Score <75 Model 2 aOR (99% CI) | |
|---|---|---|---|
| GDF‐15 continuous model | 1.56 (1.39, 1.75)a | 1.60 (1.41, 1.82)a | 1.42 (1.19, 1.68)a |
| Log(GDF‐15) per 1 SD (0.58 pg/mL) increase | |||
| GDF‐15 categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤856 pg/mL) | |||
| Quintile 2 (857–1200 pg/mL) | 1.30 (0.86, 1.96) | 1.31 (0.86, 2.01) | 1.08 (0.67, 1.73) |
| Quintile 3 (1201–1570 pg/mL) | 2.16 (1.47, 3.19)a | 2.31 (1.54, 3.47)a | 1.65 (1.03, 2.66)a |
| Quintile 4 (1571–2220 pg/mL) | 2.66 (1.81, 3.89)a | 2.81 (1.87, 4.23)a | 1.93 (1.17, 3.18)a |
| Quintile 5 (>2220 pg/mL) | 3.29 (2.26, 4.80)a | 3.52 (2.34, 5.29)a | 2.35 (1.39, 3.97)a |
| Galectin‐3 continuous model | 1.61 (1.42, 1.83)a | 1.49 (1.31, 1.69)a | 1.28 (1.12, 1.48)a |
| Log(Galectin‐3) per 1 SD (0.50 pg/mL) increase | |||
| Galectin‐3 categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤9.11 pg/mL) | |||
| Quintile 2 (9.12–12.2 pg/mL) | 1.53 (1.02, 2.29)a | 1.45 (0.96, 2.18) | 1.29 (0.84, 1.99) |
| Quintile 3 (12.3–15.4 pg/mL) | 1.86 (1.26, 2.75)a | 1.64 (1.10, 2.45)a | 1.36 (0.88, 2.08) |
| Quintile 4 (15.5–20.1 pg/mL) | 2.49 (1.70, 3.65)a | 2.15 (1.46, 3.19)a | 1.63 (1.06, 2.50)a |
| Quintile 5 (>20.1 pg/mL) | 3.45 (2.37, 5.02)a | 2.76 (1.87, 4.07)a | 1.80 (1.17, 2.78)a |
| sST‐2 continuous model | 1.12 (0.99, 1.26) | 1.20 (1.05, 1.38)a | 1.12 (0.98, 1.28) |
| Log(sST‐2) per 1 SD (0.55 pg/mL) increase | |||
| sST‐2 categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤10.4 pg/mL) | |||
| Quintile 2 (10.5–13.4 pg/mL) | 1.17 (0.81, 1.67) | 1.25 (0.86, 1.80) | 1.09 (0.73, 1.64) |
| Quintile 3 (13.5–16.8 pg/mL) | 1.36 (0.95, 1.94) | 1.51 (1.05, 2.17)a | 1.35 (0.91, 2.01) |
| Quintile 4 (16.9–22 pg/mL) | 1.14 (0.80, 1.64) | 1.37 (0.94, 2.00) | 1.06 (0.70, 1.60) |
| Quintile 5 (> 22 pg/mL) | 1.49 (1.05, 2.12)a | 1.90 (1.31, 2.75)a | 1.51 (1.00, 2.28)a |
| hsTnT continuous model | 1.30 (1.17, 1.45)a | 1.44 (1.26, 1.63)a | 1.10 (0.93, 1.31) |
| Log(hsTnT) per 1 SD (0.77 ng/L) increase | |||
| hsTnT categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| <Lower limit of detection (<10 ng/L) | |||
| Tertile 1 (10.1–14.7 ng/L) | 1.15 (0.83, 1.59) | 1.33 (0.95, 1.87) | 1.06 (0.73, 1.55) |
| Tertile 2 (14.8–23.9 ng/L) | 1.55 (1.15, 2.10)a | 1.91 (1.37, 2.65)a | 1.20 (0.82, 1.75) |
| Tertile 3 (>23.9 ng/L) | 1.80 (1.34, 2.42)a | 2.26 (1.61, 3.16)a | 1.17 (0.77, 1.78) |
| NT‐proBNP continuous model | 1.29 (1.15, 1.46)a | 1.30 (1.14, 1.47)a | 1.03 (0.88, 1.21) |
| Log(NT‐proBNP) per 1 SD (1.60 pg/mL) increase | |||
| NT‐proBNP categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤30.9 pg/mL) | |||
| Quintile 2 (31–76 pg/mL) | 1.03 (0.71, 1.50) | 1.00 (0.68, 1.46) | 0.86 (0.57, 1.30) |
| Quintile 3 (76.1–158 pg/mL) | 1.26 (0.87, 1.81) | 1.26 (0.87, 1.84) | 0.95 (0.63, 1.44) |
| Quintile 4 (158.1–370 pg/mL) | 1.50 (1.05, 2.14)a | 1.50 (1.03, 2.17)a | 0.95 (0.61, 1.47) |
| Quintile 5 (>370 pg/mL) | 1.96 (1.38, 2.78)a | 1.92 (1.32, 2.79)a | 1.03 (0.65, 1.63) |
Model 0: Unadjusted; Model 1: Age, sex, race/ethnicity; Model 2: M1 + myocardial infarction, chronic obstructive pulmonary disease, atrial fibrillation, stroke, diabetes mellitus, systolic blood pressure, body mass index, current smoking, estimated glomerular filtration rate, 24 h urinary protein, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, diuretics, and beta blocker use; aOR indicates, adjusted odds ratio; CKD, chronic kidney disease; CRIC, Chronic Renal Insufficiency Cohort; GDF‐15, growth differentiation factor‐15; HF, heart failure; hsTnT, high‐sensitivity troponin T; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OR, odds ratio; and sST‐2, soluble suppression of tumorigenesis‐2.
P<0.01.
The clinically available biomarkers hsTnT and NT‐proBNP were significantly associated with year 1 KCCQ scores of <75 in unadjusted analyses (Table 2). These associations did not attenuate when adjusting for age, sex, and race/ethnicity. In the continuous model adjusted for comorbidities and clinical variables, the association of hsTnT with KCCQ scores was not statistically significant (aOR 1.10 per SD higher hsTnT; 99% CI, 0.93–1.30). Similarly, NT‐proBNP was not associated with KCCQ scores in the adjusted model (aOR 1.03 per SD higher NT‐proBNP; 99% CI, 0.88–1.21). In categorical analyses, neither hsTnT nor NT‐proBNP was significantly associated with cross‐sectional KCCQ scores in adjusted analyses.
Cardiac Biomarkers and Incident Decline in KCCQ
Of the 2132 participants with year 1 KCCQ ≥75, 362 declined to KCCQ <75 with a >3 point per year average decline. Persons with incident decline in KCCQ were more likely to be black or Hispanic and had a higher prevalence of cardiovascular disease and diabetes mellitus, compared with those without incident decline (Table 3). GDF‐15, galectin‐3, NT‐proBNP, and hsTnT were associated with incident decline in KCCQ scores between years 1 and 5 when modeled continuously and adjusted for age, sex, and race/ethnicity (Table 4). The association of sST‐2 and incident decline in KCCQ scores did not reach statistical significance in unadjusted analyses and in analyses adjusted for participant demographics. There were 163 ESKD events (incidence rate 26.1 per 100 person‐years; 95% CI, 23.4–28.8) and 80 incident HF events (incidence rate 3.5 per 100 person‐years; 95% CI, 3.0–3.9), which were censoring events in the longitudinal analyses. When adjusted for comorbidities and clinical variables in Model 2, GDF‐15 (adjusted hazard ratio [aHR] 1.36 per SD higher GDF‐15; 99% CI, 1.12–1.65), hsTnT (aHR 1.20 per SD higher hsTnT; 99% CI, 1.01–1.44), and NT‐proBNP (aHR 1.30 per SD higher NT‐proBNP; 99% CI, 1.08–1.56) were significantly associated with incident decline in KCCQ scores. The highest quintile of GDF‐15 was associated with a greater than 3‐fold risk of incident decline in KCCQ scores, the largest association among the 5 biomarkers.
Table 3.
Characteristics of Participants Who Had Incident Decline in KCCQ Scoresa, as Defined by KCCQ <75 and An Average Decline in KCCQ Score of >3 Points/Year (N=2132)
| Incident Decline in KCCQa(N=362) | No Incident Decline in KCCQ(N=1770) | |
|---|---|---|
| Demographics | ||
| Age | 61.3 (10.2) | 58.4 (11.3) |
| Male | 182 (50) | 1049 (59) |
| Race/ethnicity | ||
| Non‐Hispanic white | 143 (40) | 931 (53) |
| Non‐Hispanic black | 150 (41) | 585 (33) |
| Hispanic | 55 (15) | 170 (10) |
| Other | 14 (4) | 84 (5) |
| Comorbidities | ||
| Cardiovascular disease | 127 (35) | 372 (21) |
| Myocardial infarction/prior revascularization | 86 (24) | 254 (14) |
| Chronic obstructive pulmonary disease | 16 (4) | 38 (2) |
| Atrial fibrillation | 57 (16) | 208 (12) |
| Stroke | 51 (14) | 108 (6) |
| Diabetes mellitus | 185 (51) | 707 (40) |
| Clinical variables | ||
| Systolic blood pressure, mm Hg | 128.6 (20.9) | 124.4 (20.5) |
| Body mass index, kg/m2 | 32.4 (7.2) | 30.2 (6.4) |
| Current smoking | 47 (13) | 158 (9) |
| Laboratory variables | ||
| Estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration), mL/min per 1.73 m2 | 41.4 (13.5) | 44.7 (15.6) |
| Urinary protein to creatinine ratio from 24 h urine test | 149.9 (58.0–635.7) | 99.9 (48.7–498.0) |
| Ejection fraction, % | 54.7 (7.2) | 55.4 (7.3) |
| Left ventricular mass index, g | 65.0 (21.1) | 59.3 (21.0) |
| Medications | ||
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 249 (69) | 1217 (69) |
| Diuretics | 219 (60) | 889 (50) |
| Beta blockers | 177 (49) | 749 (42) |
Entries are mean (SD) for continuous covariates or N (%) for categorical covariates, except as noted.
KCCQ indicates Kansas City Cardiomyopathy Questionnaire.
Incident decline in KCCQ defined as participants with KCCQ≥75 developing a KCCQ <75 and having an average decline in KCCQ score of >3 points/y.
Table 4.
Association of Cardiac Biomarkers With Incident Decline in KCCQ Scoresa, Among Participants With Year 1 KCCQ ≥75 (N=2132)
| Incident Decline in KCCQa Model 0 HR (99% CI) | Incident Decline in KCCQ Model 1 aHR (99% CI) | Incident Decline in KCCQ Model 2 aHR (99% CI) | |
|---|---|---|---|
| GDF‐15 continuous model | 1.67 (1.45, 1.92)b | 1.57 (1.35, 1.83)b | 1.36 (1.12, 1.65)b |
| Log(GDF‐15) per 1 SD (0.58 pg/mL) increase | |||
| GDF‐15 categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤856 pg/mL) | |||
| Quintile 2 (857–1200 pg/mL) | 2.06 (1.23, 3.44)b | 1.89 (1.12, 3.20)b | 1.63 (0.94, 2.83) |
| Quintile 3 (1201–1570 pg/mL) | 2.33 (1.39, 3.90)b | 2.08 (1.23, 3.53)b | 1.59 (0.89, 2.83) |
| Quintile 4 (1571–2220 pg/mL) | 3.77 (2.30, 6.18)b | 3.28 (1.94, 5.53)b | 2.34 (1.27, 4.30)b |
| Quintile 5 (>2220 pg/mL) | 5.28 (3.23, 8.63)b | 4.55 (2.70, 7.68)b | 3.15 (1.68, 5.89)b |
| Galectin‐3 continuous model | 1.36 (1.17, 1.58)b | 1.22 (1.05, 1.42)b | 1.08 (0.92, 1.26) |
| Log(Galectin‐3) per 1 SD (0.50 pg/mL) increase | |||
| Galectin‐3 categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤9.11 pg/mL) | |||
| Quintile 2 (9.12–12.2 pg/mL) | 1.53 (0.96, 2.45) | 1.42 (0.89, 2.28) | 1.33 (0.83, 2.15) |
| Quintile 3 (12.3–15.4 pg/mL) | 1.84 (1.16, 2.91)b | 1.61 (1.02, 2.56)b | 1.43 (0.90, 2.28) |
| Quintile 4 (15.5–20.1 pg/mL) | 2.26 (1.44, 3.55)b | 1.84 (1.17, 2.91)b | 1.46 (0.91, 2.33) |
| Quintile 5 (>20.1 pg/mL) | 2.79 (1.76, 4.42)b | 2.08 (1.29, 3.34)b | 1.50 (0.92, 2.46) |
| sST‐2 continuous model | 1.11 (0.96, 1.29) | 1.16 (0.99, 1.35) | 1.08 (0.92, 1.26) |
| Log(sST‐2) per 1 SD (0.55 pg/mL) increase | |||
| sST‐2 categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤10.4 pg/mL) | |||
| Quintile 2 (10.5–13.4 pg/mL) | 1.13 (0.74, 1.73) | 1.15 (0.75, 1.77) | 1.02 (0.66, 1.58) |
| Quintile 3 (13.5–16.8 pg/mL) | 1.00 (0.64, 1.56) | 1.00 (0.64, 1.57) | 0.90 (0.57, 1.41) |
| Quintile 4 (16.9–22 pg/mL) | 1.16 (0.75, 1.78) | 1.22 (0.78, 1.91) | 0.97 (0.61, 1.53) |
| Quintile 5 (> 22 pg/mL) | 1.51 (1.00, 2.29)b | 1.77 (1.15, 2.73)b | 1.46 (0.93, 2.28) |
| hsTnT continuous model | 1.42 (1.24, 1.62)b | 1.44 (1.24, 1.68)b | 1.20 (1.01, 1.44)b |
| Log(hsTnT) per 1 SD (0.78 ng/L) increase | |||
| hsTnT categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| < lower limit of detection (<10 ng/L) | |||
| Tertile 1 (10.1–14.7 ng/L) | 1.51 (1.03, 2.23)b | 1.56 (1.05, 2.32)b | 1.34 (0.89, 2.02) |
| Tertile 2 (14.8–23.9 ng/L) | 1.81 (1.25, 2.64)b | 1.86 (1.25, 2.79)b | 1.40 (0.92, 2.14) |
| Tertile 3 (>23.9 ng/L) | 2.52 (1.74, 3.65)b | 2.67 (1.76, 4.04)b | 1.75 (1.10, 2.80)b |
| NT‐proBNP continuous model | 1.59 (1.37, 1.85)b | 1.51 (1.29, 1.76)b | 1.30 (1.08, 1.56)b |
| Log(NT‐proBNP) per 1 SD (1.60 pg/mL) increase | |||
| NT‐proBNP categorical model | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| Quintile 1 (≤31.9 pg/mL) | |||
| Quintile 2 (31–76 pg/mL) | 1.64 (1.01, 2.67)b | 1.51 (0.93, 2.46) | 1.31 (0.79, 2.15) |
| Quintile 3 (76.1–158 pg/mL) | 1.57 (0.95, 2.58) | 1.42 (0.86, 2.37) | 1.12 (0.66, 1.88) |
| Quintile 4 (158.1–370 pg/mL) | 2.73 (1.72, 4.32)b | 2.47 (1.54, 3.98)b | 1.75 (1.05, 2.92)b |
| Quintile 5 (>370 pg/mL) | 3.48 (2.19, 5.55)b | 2.89 (1.78, 4.69)b | 1.88 (1.09, 3.24)b |
Model 0: Unadjusted; Model 1: Age, sex, race/ethnicity; Model 2: M1 + myocardial infarction, chronic obstructive pulmonary disease, atrial fibrillation, stroke, diabetes mellitus, systolic blood pressure, body mass index, current smoking, estimated glomerular filtration rate, 24 h urinary protein, angiotensin‐converting enzyme inhibitosr/angiotensin receptor blockers, diuretics, and beta blocker use; aHR indicates adjusted hazard ratio; GDF‐15, growth differentiation factor‐15; HR, hazard ratio; hsTnT, high‐sensitivity troponin T; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and sST‐2, soluble suppression of tumorigenesis‐2.
Incident decline in KCCQ defined as participants with KCCQ≥75 developing a KCCQ<75 and having an average decline in KCCQ score of >3 points/y.
P<0.01.
Sensitivity Analyses
When adjusting for all 5 biomarkers in our multivariable model (correlation matrix in Table S5), GDF‐15 and galectin‐3 were associated with KCCQ scores <75 in cross‐sectional analyses. In longitudinal analyses, GDF‐15 and NT‐proBNP were associated with incident KCCQ <75; however, the association of hsTnT was no longer statistically associated with a decline in KCCQ scores after adjustment for the other biomarkers (Model 3, Table S6). Additional adjustment for left ventricular mass and left ventricular ejection fraction did not meaningfully attenuate the associations between the cardiac biomarkers of interest and KCCQ scores <75 at baseline or incident decline in KCCQ scores (Model 4, Table S6). The additional adjustment for baseline KCCQ scores also did not meaningfully change the results of longitudinal analyses (Model 5, Table S6).
The cardiac biomarkers GDF‐15 and hsTnT were associated with incident decline to KCCQ <60 and having an average decline in KCCQ score of >5 points/year, among participants with a baseline KCCQ≥60, similar to the primary analysis (Table S7). Although NT‐proBNP was associated with KCCQ decline to <75, the association of NT‐proBNP and KCCQ decline to <60 did not reach statistical significance.
Discussion
Previous work has shown that persons living with CKD without diagnosed HF have low KCCQ scores, suggestive of health status consistent with early HF.6, 23, 24, 25 In this large, longitudinal study of CKD patients we found significant cross‐sectional associations of newer cardiac measures GDF‐15 and galectin‐3 with health status consistent with early HF, whereas sST2, NT‐proBNP, and hsTnT were not associated with baseline KCCQ scores <75 in models adjusted for numerous potential confounders. In longitudinal analyses, GDF‐15 had the strongest association with incident decline in KCCQ scores, followed by NT‐proBNP and hsTnT, whereas the associations of sST2 and galectin‐3 with decline in KCCQ scores were not statistically significant. These findings indicate that circulating cardiac biomarkers signal greater risk of both low and worsening health status consistent with early HF and may provide information related to the patient experience of early HF.
Newer cardiac biomarkers may contribute to a greater burden of health status consistent with early HF in CKD patients via several pathophysiologic mechanisms. GDF‐15 is upregulated in cardiac ischemia/reperfusion injury and conditions of pressure overload and cardiac hypertrophy, which commonly occur in CKD patients even in the absence of clinical HF.12, 33, 34 Among the 5 biomarkers, GDF‐15 had a strong association with baseline and longitudinal KCCQ scores over a 4‐year period, suggesting that GDF‐15, in addition to its role in HF pathogenesis, may best capture the patient experience of early HF in this population with CKD. Similarly, galectin‐3′s role in fibrosis and inflammation has been implicated in the pathogenesis of HF, including cardiac fibroblast proliferation and collagen deposition.13, 35, 36 These changes lead to cardiac hypertrophy and ventricular remodeling, which may explain the development of dyspnea that is measured by the KCCQ. Several factors may explain why galactin‐3 was significantly associated with KCCQ <75 cross‐sectionally but not longitudinally. First, the established cardiac biomarkers hsTnT and NT‐proBNP may be more sensitive to longitudinal heart failure status and changes over time, whereas galactin‐3 may be less cardiac specific, as it is also a marker of inflammation and fibrosis. We also find that sST2 was associated with baseline KCCQ scores in models adjusted for participant demographics, but this association did not reach statistical significance at a P<0.01 in models additionally adjusted for biologically relevant covariates. sST2, a member of the interleukin‐1 receptor family, is upregulated in HF, myocardial stretch, and ischemia.37 sST2 is a marker of mortality risk in patients with cardiovascular disease, but its association with quality of life has been less studied.38 Taken together, our results suggest that the newer biomarkers GDF‐15 and galectin‐3 reflect physiological processes that are important to the patient experience of early HF.
The relationships between GDF‐15 and galectin‐3 with KCCQ scores have not been previously described; however, these cardiac biomarkers have been studied in relation to other HF quality of life instruments. An analysis in patients with HF and controls found an association between GDF‐15 and the Short Form 36 physical functioning scale; however, it did not reach statistical significance after adjusting for numerous covariates (P=0.052).39 In a trial of HF patients with preserved ejection fraction, baseline galectin‐3 levels were significantly correlated with the Short Form 36 after multivariable adjustment.40 However, 2 studies in HF patients showed no association between galectin‐3 levels and the Minnesota Living with Heart Failure Questionnaire, an alternative measure of HF health status. These findings may have differed from our results because of differences in patient population as they enrolled participants with established HF, limited power due to fewer participants, and use of instruments with different test characteristics than the KCCQ.41, 42 Our results in this study, which found significant cross‐sectional associations of GDF‐15 and galectin‐3 and health status consistent with early HF support the growing evidence of the association between newer cardiac biomarkers and patient‐reported outcomes.
Contrary to our hypotheses, we did not find a significant association between hsTnT or NT‐proBNP with cross‐sectional KCCQ scores after adjustment for potential confounders. Similarly, studies in HF outpatients found bivariate correlations of NT‐proBNP with health status scores, as measured by the Short Form 36 and KCCQ, but did not demonstrate an association after adjusting for covariates.43, 44 The authors concluded that “elevated BNP seems not to be sensed by the individual,” suggesting differing roles of NT‐proBNP over time in pathogenesis of HF versus the patient experience. Other studies in 342 and 163 HF outpatients, respectively, found that BNP levels were not correlated with baseline KCCQ scores, but they may have been underpowered.45, 46 In our study, it is possible that some of the variables in the models, such as cardiovascular medication use, may have been on the causal pathways of the associations.
In our study, we found significant associations of baseline GDF‐15, hsTnT, and NT‐proBNP levels with longitudinal decline in health status. The KCCQ measured longitudinally is an accepted clinical trial outcome and is informative of changes in clinical status.23, 24, 25 Previous studies have not examined GDF‐15 with KCCQ scores longitudinally. A secondary analysis of the PARADIGM‐HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial found that NT‐proBNP levels were associated with changes in KCCQ scores over 8 months.47 The associations of GDF‐15, hsTnT, and NT‐proBNP with longitudinal KCCQ scores have several implications. Elevations in cardiac biomarkers may signal early pathophysiology and serve to identify patients at greatest risk for a decline in health status. Predicting health status as measured by KCCQ scores could be potentially useful because abnormal HF health status may be modifiable. Educational, exercise, and medical therapies have proven effective in improving KCCQ scores.23, 48, 49 Interventions such as cardiac rehabilitation, which is known to improve health status in HF patients, have also been found to modify levels of galectin‐3 and sST‐2 over time, further underscoring the relationships between cardiac biomarkers and health status.50
Our study has several strengths. We had a large sample size that was well powered to detect an association of cardiac biomarkers with KCCQ scores. We investigated longitudinal KCCQ scores over a 4‐year time frame, evaluating the role of cardiac biomarkers in prognosticating the development of low KCCQ scores, as a marker of health status consistent with early HF. We also recognize limitations to our results. Our study is observational, so we are unable to determine causality and our analyses may be subject to residual confounding. Study participants were volunteers, which may limit generalizability to the broader CKD population. We were not able to study the effects of interventions in this study on improving HF health status or their effects on cardiac biomarkers. It is possible that the biomarkers may be associated with lower KCCQ scores via additional noncardiac mechanisms, such as inflammation, malignancy, and pulmonary disease.51, 52
In summary, in a large, longitudinal cohort of CKD patients without HF, we found a significant association between GDF‐15 and galectin‐3 and health status consistent with early HF as measured by the KCCQ. We also found associations of GDF‐15, hsTnT, and NT‐proBNP with incident decline in KCCQ scores over time. Our findings provide further insights toward a better pathophysiologic understanding of the development of HF in CKD patients. Cardiac biomarkers, particularly GDF‐15, could be useful for assessing and predicting health status consistent with early HF in patients with CKD.
Appendix
CRIC Study Investigators
Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, James P. Lash, MD, Panduranga S. Rao, MD, Mahboob Rahman, MD, Raymond R. Townsend, MD.
Sources of Funding
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR‐000424, University of Maryland GCRC M01 RR‐16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane Center of Biomedical Research Excellence (COBRE) for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/National Center for Research Resources (NCRR) UCSF‐Clinical & Translational Science Institute (CTSI) UL1 RR‐024131. This study was also supported by R01 DK103612 (Bansal) and R01 01DK104730 (Anderson). Roche Diagnostics provided partial funding for the NT‐proBNP and hsTnT assays. The authors would also like to acknowledge an unrestricted fund from the Northwest Kidney Centers.
Disclosures
Dr. Tummalapalli reports consulting fees from Bayer AG outside of the submitted work. The remaining authors have nothing to disclose.
Supporting information
Tables S1–S7
(J Am Heart Assoc. 2020;9:e014385 DOI: 10.1161/JAHA.119.014385.)
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Sri Lekha Tummalapalli, Email: srilekha.tummalapalli@ucsf.edu.
the CRIC Study Investigators:
Lawrence J. Appel, Harold I Feldman, Alan S. Go, Panduranga S. Rao, Mahboob Rahman, and Raymond R. Townsend
References
- 1. Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: A collaborative meta‐analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bansal N, Hyre Anderson A, Yang W, Christenson RH, deFilippi CR, Deo R, Dries DL, Go AS, He J, Kusek JW, et al. High‐sensitivity troponin t and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and risk of incident heart failure in patients with CKD: The Chronic Renal Insufficiency Cohort (CRIC) study. J Am Soc Nephrol. 2015;26:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushita K, Sang Y, Ballew SH, Astor BC, Hoogeveen RC, Solomon SD, Ballantyne CM, Woodward M, Coresh J. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: The atherosclerosis risk in communities study. Arterioscler Thromb Vasc Biol. 2014;34:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–756. [DOI] [PubMed] [Google Scholar]
- 5. Spertus J. Medical device development tool qualification decision summary for Kansas City Cardiomyopathy Questionnaire (KCCQ) 2016.
- 6. Shlipak MG, Lash JP, Yang W, Teal V, Keane M, Cappola T, Keller C, Jamerson K, Kusek J, Delafontaine P, et al. Symptoms characteristic of heart failure among CKD patients without diagnosed heart failure. J Card Fail. 2011;17:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mishra RK, Yang W, Roy J, Anderson AH, Bansal N, Chen J, DeFilippi C, Delafontaine P, Feldman HI, Kallem R, et al. Kansas City Cardiomyopathy Questionnaire score is associated with incident heart failure hospitalization in patients with chronic kidney disease without previously diagnosed heart failure: Chronic renal insufficiency cohort study. Circulation. Heart failure. 2015;8:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuegel C, Katz R, Alam M, Bhat Z, Bellovich K, de Boer I, Brosius F, Gadegbeku C, Gipson D, Hawkins J, et al. GDF‐15, galectin 3, soluble ST2, and risk of mortality and cardiovascular events in CKD. Am J Kidney Dis. 2018;72:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fluschnik N, Ojeda F, Zeller T, Jørgensen T, Kuulasmaa K, Becher PM, Sinning C, Blankenberg S, Westermann D. Predictive value of long‐term changes of growth differentiation factor‐15 over a 27‐year‐period for heart failure and death due to coronary heart disease. PLoS ONE. 2018;13:e0197497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho JE, Lyass A, Courchesne P, Chen G, Liu C, Yin X, Hwang SJ, Massaro JM, Larson MG, Levy D. Protein biomarkers of cardiovascular disease and mortality in the community. JAHA. 2018;7. doi: 10.1161/JAHA.117.008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stenemo M, Nowak C, Byberg L, Sundström J, Giedraitis V, Lind L, Ingelsson E, Fall T, Ärnlöv J. Circulating proteins as predictors of incident heart failure in the elderly. Eur J Heart Fail. 2018;20:55–62. [DOI] [PubMed] [Google Scholar]
- 12. Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin J. The transforming growth factor‐β superfamily member growth‐differentiation factor‐15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. [DOI] [PubMed] [Google Scholar]
- 13. Lin YH, Lin LY, Wu YW, Chien KL, Lee CM, Hsu RB, Chao CL, Wang SS, Hsein YC, Liao LC, et al. The relationship between serum galectin‐3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009;409:96–99. [DOI] [PubMed] [Google Scholar]
- 14. Lupu S, Agoston‐Coldea L. Soluble ST2 in ventricular dysfunction. Adv Clin Chem. 2015;69:139–159. [DOI] [PubMed] [Google Scholar]
- 15. Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. New Eng J Med. 2009;361:2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall C. NT‐proBNP: The mechanism behind the marker. J Cardiac Fail. 2005;11:S81–83. [DOI] [PubMed] [Google Scholar]
- 17. Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin‐Becker ED, Go AS, Hamm LL, et al. The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol: JASN. 2003;14:S148–153. [DOI] [PubMed] [Google Scholar]
- 18. Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, et al. Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khariton Y, Hernandez AF, Fonarow GC, Sharma PP, Duffy CI, Thomas L, Mi X, Albert NM, Butler J, McCague K, et al. Health status variation across practices in outpatients with heart failure: Insights from the CHAMP‐HF (change the management of patients with heart failure) registry. Circulation. Cardiovascular Quality and Outcomes. 2018;11:e004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L, Valderas JM, Guillemin F, Revicki D, Alonso J. Assessing health‐related quality of life in patients with heart failure: A systematic, standardized comparison of available measures. Heart Fail Rev. 2014;19:359–367. [DOI] [PubMed] [Google Scholar]
- 21. Ortega T, Diaz‐Molina B, Montoliu MA, Ortega F, Valdes C, Rebollo P, Almenar M, Iscar M. The utility of a specific measure for heart transplant patients: Reliability and validity of the Kansas City Cardiomyopathy Questionnaire. Transplantation. 2008;86:804–810. [DOI] [PubMed] [Google Scholar]
- 22. Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Pina IL, Weinfurt KP. Relationships between changes in patient‐reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012;163(88–94):e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis EF, Kim HY, Claggett B, Spertus J, Heitner JF, Assmann SF, Kenwood CT, Solomon SD, Desai AS, Fang JC, et al. Impact of spironolactone on longitudinal changes in health‐related quality of life in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circulation. Heart Failure. 2016;9:e001937. [DOI] [PubMed] [Google Scholar]
- 24. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: A secondary analysis of 2 randomized clinical trials. JAMA cardiol. 2017;2:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: A comparison of methods. Am Heart J. 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 26. National Center for Health Statistics (NCHS): National Health and Nutrition Examination Survey Anthropometry Procedures Manual A, GA, Centers for Disease Control and Prevention, 2000.
- 27. Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL. Variability of creatinine measurements in clinical laboratories: Results from the CRIC study. Am J Nephrol. 2010;31:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2012;60:250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bansal N, Keane M, Delafontaine P, Dries D, Foster E, Gadegbeku CA, Go AS, Hamm LL, Kusek JW, Ojo AO, et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin J Am Soc Nephrol: CJASN. 2013;8:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's guidelines and standards committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 31. Bansal N, Zelnick L, Go A, Anderson A, Christenson R, Deo R, Defilippi C, Lash J, He J, Ky B, et al. Cardiac biomarkers and risk of incident heart failure in chronic kidney disease: The CRIC (Chronic Renal Insufficiency Cohort) study. J Am Heart Assoc. 2019;8.;. doi: 10.1161/JAHA.119.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamprea‐Montealegre JA, Zelnick LR, Shlipak MG, Floyd JS, Anderson AH, He J, Christenson R, Seliger SL, Soliman EZ, Deo R, et al. Cardiac biomarkers and risk of atrial fibrillation in chronic kidney disease: The CRIC study. J Am Heart Assoc. 2019;8.;. doi: 10.1161/JAHA.119.012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/mic‐1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342–350. [DOI] [PubMed] [Google Scholar]
- 34. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–151. [DOI] [PubMed] [Google Scholar]
- 35. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, André S, Crijns HJ, Gabius H‐J, Maessen JJC. Galectin‐3 marks activated macrophages in failure‐prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. [DOI] [PubMed] [Google Scholar]
- 36. Yan Y‐P, Lang BT, Vemuganti R, Dempsey RJ. Galectin‐3 mediates post‐ischemic tissue remodeling. Brain Res. 2009;1288:116–124. [DOI] [PubMed] [Google Scholar]
- 37. Shah RV, Januzzi JL. ST2: A novel remodeling biomarker in acute and chronic heart failure. Curr Heart Failure Rep. 2010;7:9–14. [DOI] [PubMed] [Google Scholar]
- 38. Chen LQ, de Lemos JA, Das SR, Ayers CR, Rohatgi A. Soluble ST2 is associated with all‐cause and cardiovascular mortality in a population‐based cohort: The Dallas Heart Study. Clin Chem. 2013;59:536–546. [DOI] [PubMed] [Google Scholar]
- 39. Stahrenberg R, Edelmann F, Mende M, Kockskamper A, Dungen HD, Luers C, Binder L, Herrmann‐Lingen C, Gelbrich G, Hasenfuss G, et al. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail. 2010;12:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher‐Krainer E, Duvinage A, Unkelbach I, Dungen HD, Tschope C, et al. Galectin‐3 in patients with heart failure with preserved ejection fraction: Results from the ALDO‐DHF trial. Eur J Heart Fail. 2015;17:214–223. [DOI] [PubMed] [Google Scholar]
- 41. AbouEzzeddine OF, Haines P, Stevens S, Nativi‐Nicolau J, Felker GM, Borlaug BA, Chen HH, Tracy RP, Braunwald E, Redfield MM. Galectin‐3 in heart failure with preserved ejection fraction. A RELAX trial substudy (Phosphodiesterase‐5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure). JACC Heart failure. 2015;3:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gandhi PU, Motiwala SR, Belcher AM, Gaggin HK, Weiner RB, Baggish AL, Fiuzat M, Brunner‐La Rocca HP, Januzzi JL Jr. Galectin‐3 and mineralocorticoid receptor antagonist use in patients with chronic heart failure due to left ventricular systolic dysfunction. Am Heart J. 2015;169(404–411):e403. [DOI] [PubMed] [Google Scholar]
- 43. Peters‐Klimm F, Kunz CU, Laux G, Szecsenyi J, Muller‐Tasch T. Patient‐ and provider‐related determinants of generic and specific health‐related quality of life of patients with chronic systolic heart failure in primary care: A cross‐sectional study. Health Qual Life Outcomes. 2010;8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parissis JT, Nikolaou M, Farmakis D, Paraskevaidis IA, Bistola V, Venetsanou K, Katsaras D, Filippatos G, Kremastinos DT. Self‐assessment of health status is associated with inflammatory activation and predicts long‐term outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luther SA, McCullough PA, Havranek EP, Rumsfeld JS, Jones PG, Heidenreich PA, Peterson ED, Rathore SS, Krumholz HM, Weintraub WS, et al. The relationship between B‐type natriuretic peptide and health status in patients with heart failure. J Cardiac Fail. 2005;11:414–421. [DOI] [PubMed] [Google Scholar]
- 46. Gallagher AM, Lucas R, Cowie MR. Assessing health‐related quality of life in heart failure patients attending an outpatient clinic: A pragmatic approach. ESC Heart Failure. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, et al. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: A secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol. 2018;3:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sherwood A, Blumenthal JA, Koch GG, Hoffman BM, Watkins LL, Smith PJ, O'Connor CM, Adams KF Jr., Rogers JG, Sueta C, et al. Effects of coping skills training on quality of life, disease biomarkers, and clinical outcomes in patients with heart failure: a randomized clinical trial. Circulation: Heart Failure. 2017;10:e003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, et al. Effects of exercise training on health status in patients with chronic heart failure: HF‐action randomized controlled trial. JAMA. 2009;301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Billebeau G, Vodovar N, Sadoune M, Launay JM, Beauvais F, Cohen‐Solal A. Effects of a cardiac rehabilitation programme on plasma cardiac biomarkers in patients with chronic heart failure. Eur J Prev Cardiol. 2017;24:1127–1135. [DOI] [PubMed] [Google Scholar]
- 51. Husebo GR, Gronseth R, Lerner L, Gyuris J, Hardie JA, Bakke PS, Eagan TM. Growth differentiation factor‐15 is a predictor of important disease outcomes in patients with COPD. Europ Resp J. 2017;49.. [DOI] [PubMed] [Google Scholar]
- 52. Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble ST2, galectin‐3 and growth‐differentiation factor‐15 with heart failure and other non‐cardiac diseases. Clin Chim Acta. 2015;445:155–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


