Abstract
Background
Although ischemic heart disease has a complex and multilevel origin, the diagnostic approach is mainly focused on focal obstructive disease as assessed by pressure‐derived indexes. The prognostic relevance of coronary flow over coronary pressure has been suggested and implies that identification of relevant perfusion abnormalities by invasive physiology techniques is critical for the correct identification of patients who benefit from coronary revascularization. The purpose of this study was to evaluate the diagnostic potential of a sequential approach using pressure‐derived indexes instantaneous wave‐free ratio (iFR), fractional flow reserve (FFR), and coronary flow reserve (CFR) measurements to determine the number of intermediate lesions associated with flow abnormalities after initial pressure measurements.
Methods and Results
A total of 366 intermediate lesions were assessed with simultaneous intracoronary pressure and flow velocity measurements. Contemporary clinical iFR, FFR, and CFR cut points for myocardial ischemia were applied. A total of 118 (32%) lesions were FFR+ and 136 (37%) lesions were iFR+. Subsequent CFR assessment resulted for FFR in a total of 91 (25%) FFR+/CFR+ and for iFR a total of 111 (30%) iFR+/CFR+ lesions. An iFR, FFR, and invasive flow velocity assessment approach would have yielded 20% of lesions (74 of 366) as ischemic.
Conclusions
Ultimately, 20% of intermediate lesions are associated with flow abnormalities after applying a pressure and flow velocity sequential approach. If iFR is borderline, FFR has limited additional value, in contrast with CFR. These results emphasize the use of coronary physiology in assessing stenosis severity but may also further question the contemporary reputation of a pressure‐based approach as a gold standard for the detection of myocardial ischemia in ischemic heart disease.
Keywords: coronary flow capacity, coronary flow reserve, fractional flow reserve, instantaneous wave‐free ratio, myocardial ischemia
Subject Categories: Diagnostic Testing, Angiography, Physiology
Nonstandard Abbreviations and Acronyms
- CAG
coronary angiography
- CFC
coronary flow capacity
- CFR
coronary flow reserve
- FFR
fractional flow reserve
- hAPV
hyperemic average peak velocity
- iFR
instantaneous wave‐free ratio
- IHD
ischemic heart disease
- MACE
major adverse cardiac event
- PCI
percutaneous coronary intervention
Clinical Perspective
What Is New?
Ischemic heart disease has an origin of focal, diffuse, and microvascular diseases, and the improved selection of which lesions are truly ischemia inducing and will benefit from revascularization is warranted.
We show that after a sequential approach of invasive pressure and flow measurements, a small number of all intermediate lesions are associated with inducible myocardial ischemia.
What Are the Clinical Implications?
Contemporary clinical decision making in the catheterization laboratory is mainly driven by pressure‐derived physiology parameters such as instantaneous wave‐free ratio and fractional flow reserve in addition to coronary angiography.
In this era, where instantaneous wave‐free ratio and fractional flow reserve are used interchangeably, flow assessment provides valuable information regarding which lesions will benefit from revascularization and in the future may be included in clinical revascularization guidelines.
In contrast with a historical stenosis‐centered approach to angina pectoris, ischemic heart disease (IHD) is now recognized to be a complex disease involving multiple levels of the coronary circulation. As a result, coronary angiography (CAG) alone fails to properly select patients who will benefit from revascularization. The coronary pressure‐derived fractional flow reserve (FFR) has emerged as an important addition to CAG in clinical decision making regarding IHD,1 where its routine use to guide coronary intervention leads to the swift alleviation of angina2 while reducing the number of revascularizations.3, 4, 5, 6, 7 The instantaneous wave‐free ratio (iFR), a pressure‐derived alternative to FFR obtained during resting conditions, was documented to be noninferior to FFR in terms of the 1‐year occurrence of major adverse cardiac events (MACEs).8, 9 However, ultimately the purpose of invasive physiological assessment is the identification of perfusion abnormalities that are associated with the occurrence of myocardial ischemia and its clinical sequelae. Thus, it is important to realize that pressure‐derived estimates of perfusion impairment, such as FFR and iFR, are not the same as the direct assessment of coronary flow or flow reserve that represent the critical determinants of myocardial ischemia. This is illustrated by low FFR values without the occurrence of signs of ischemia as long as coronary flow remains above ischemic levels.10, 11 The prognostic relevance of coronary flow over coronary pressure has now been suggested in several clinical studies and implies that the identification of relevant perfusion abnormalities by invasive physiology techniques is critical for the correct identification of patients who benefit from coronary revascularization. Coronary flow reserve (CFR) is a well‐studied coronary flow‐based index that represents the available vasodilator reserve of the coronary circulation and has strong prognostic value. Moreover, its accuracy for clinically relevant perfusion abnormalities has recently been enhanced by the incorporation of CFR and hyperemic flow in the concept of coronary flow capacity (CFC).
Accordingly, using the international multicenter IDEAL (Iberian‐Dutch‐English) registry, we sought to determine the association of contemporary coronary pressure indexes with coronary perfusion abnormalities identified by the direct invasive measurement of coronary flow expressed by CFR and CFC. Moreover, we sought to identify a sequential approach of coronary pressure and flow measurements to improve the identification of patients with clinically pertinent perfusion impairment.
Methods
We used data from the IDEAL study, where combined pressure and Doppler flow velocity measurements were performed in 567 vessels (of which 366 vessels with an angiographically visible stenosis between 40% and 70% and 201 angiographically unobstructed vessels with a diameter stenosis on visual estimation <30%) of 301 consecutive enrolled patients who were scheduled for elective angiography in 4 international hospitals.10 The study was approved by the institutional review board, and written informed consent was obtained before the intervention. The data that support the findings of this study are available from the corresponding author upon reasonable request. Recruitment and inclusion criteria were different for each enrolling site, and the exclusion criteria were similar: severe valvular heart disease, body weight >200 kg, previous coronary artery bypass surgery, and vessels with evidence of myocardial bridging during angiography or collateral arteries and vessels with prior myocardial infarction or myocardial infarction within 48 hours before inclusion. For the current analyses, we only included the 366 stenosed vessels.
Cardiac Catheterization and Coronary Physiology Assessment
As described previously,10 CAG was performed according to American Heart Association guidelines.11 Prior to physiology assessment, 200 to 300 μg. intracoronary nitrates were administered. Combined pressure and Doppler flow velocity wires (ComboWire XT, Volcano/Philips Corporation, San Diego, CA) were used for physiology assessments of coronary lesions. The ComboWire, with the pressure and Doppler flow sensor at 1.5 cm offset, was equalized and normalized with aortic pressure before measurements were performed. Hyperemia was induced by intracoronary (bolus injection of 60–150 μg) or intravenous infusion (140 μg/kg per minute) of adenosine in, respectively, 196 (53.6%) and 170 (46.4%) measurements.
FFR was calculated as the ratio of the mean coronary artery pressure distal of the stenosis divided by the mean aortic pressure during hyperemia. Lesions with FFR ≤0.80 are presented as FFR+, and lesions with FFR >0.80 as FFR−. iFR was calculated as the mean pressure distal of the stenosis divided by the mean aortic pressure during the wave‐free period of the diastole. Lesions with iFR ≤0.89 are presented as iFR+, and lesions with iFR >0.89 as iFR−. CFR was calculated as the ratio of hyperemic average peak velocity (hAPV) and basal average peak flow velocity. Lesions with CFR <2.0 are presented as CFR+, and lesions with CFR ≥2.0 as CFR−. The definition of CFC categories is determined as previously described by van de Hoef et al12 and Kern et al13 and are shown in Table 1. Normal CFC was defined as a CFR ≥2.8, as encountered in patients with risk factors for IHD without epicardial narrowing,13 with its corresponding hAPV of ≥49.0 cm/s. Mildly reduced CFC was defined as a CFVR <2.8 but >2.1, which reflects the upper limit of reported CFR cut‐off values for inducible ischemia,14 and the corresponding hAPV of <49.0 and >33.0 cm/s, respectively. Moderately reduced CFC was defined as CFR ≤2.1 and >1.7, analogous to the reported range of CFR cut‐off values for inducible myocardial ischemia,14 and the corresponding hAPV of ≤33.0 and >26.0 cm/s, respectively. Finally, severely reduced CFC was defined as a CFR ≤1.7, which is the lower limit of CFR cut‐off values reported for inducible myocardial ischemia and analogous to the ischemic CFR threshold in noninvasive imaging,14, 15 and the corresponding hAPV of ≤26.0 cm/s. Lesions with a moderately to severely reduced CFC are with great certainty associated with inducible myocardial ischemia12 and therefore will be reported combined as “reduced CFC” in the sequential approach.
Table 1.
Patient (n=222) and Stenosis (n=366) Characteristics
| No. (%) or Mean±SD | |
|---|---|
| Age, y | 61.8±9.7 |
| Male | 281 (76.8) |
| Hypertension | 196 (53.6) |
| Hyperlipidemia | 236 (64.6) |
| Current or ex‐smoker | 148 (40.4) |
| Diabetes mellitus | 92 (25) |
| Chronic renal impairment | 13 (3.5) |
| Family history of CAD | 147 (40) |
| Previous myocardial infarction | 47 (12.8) |
| Impaired left ventricle function (LVEF <30%) | 3 (0.8) |
| Stable angina | 346 (94.5) |
| Unstable angina | 20 (5.5) |
| Multivessel disease | 115 (31.4) |
| Coronary artery physiology assessment | |
| Left anterior descending | 207 (56.6) |
| Left circumflex coronary artery | 90 (24.6) |
| Right coronary artery | 69 (18.8) |
| Adenosine administration | |
| Intravenous infusion | 196 (53.6) |
| Intracoronary bolus | 170 (46.4) |
CAD indicates coronary artery disease; and LVEF, left ventricle ejection fraction.
Statistical Analysis
Data were analyzed on a per‐vessel basis for all calculations. Normality of the variance was tested using the Shapiro–Wilk test. Continuous variables are presented as mean± SD. Categorical variables are presented as counts and percentages, and mean±SE (95% CI). Categorical variables were compared using the Fisher exact test. Sensitivity, specificity, positive predictive value, and negative predictive value of iFR, FFR, and CFR with contemporary cut‐off values were calculated against moderately to severely reduced CFC as the reference. The Student t test was used to compare means of continuous variables. A P value below 0.05 was considered statistically significant. All data were analyzed by using Stata 14.2 (StataCorp LP, College Station, TX).
Results
Patient and vessel characteristics are shown in Table 1. iFR, FFR, and CFR ranges and means can be found in Figure 1. The distribution of iFR, FFR, and CFR according to CFC category is shown in Figure 2 and Table 2, with a subdivision for iFR, FFR, and CFR cut‐off values in Table 3. The distribution of physiology measurements was 207 vessels for the left anterior descending artery (LAD) (56.6%), 90 vessels for the left circumflex artery (LCx) (24.6%), and 69 vessels for the right coronary artery (RCA) (18.8%).
Figure 1. The iFR, FFR, and CFR ranges and means.
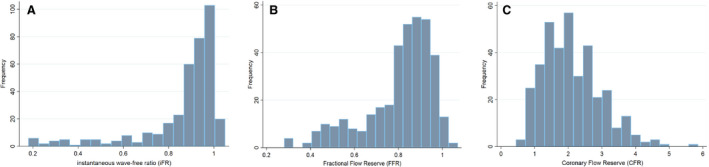
A, iFR distribution, mean iFR 0.86±0.18. B, FFR distribution, mean FFR 0.81±0.16. C, CFR distribution, mean CFR 2.1±0.86. Values are displayed as mean±standard deviation (SD). CFR indicates coronary flow reserve; FFR, fractional flow reserve; and iFR, instantaneous wave‐free ratio.
Figure 2. Distribution of iFR (A), FFR (B), and CFR (C) with normal (blue), mildly reduced (green), moderately reduced (orange), and severely reduced CFC (red).
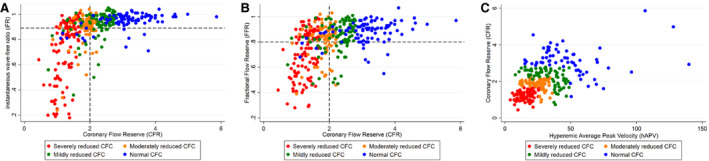
Horizontal and vertical dashed lines indicate contemporary cut‐off values for iFR (≤0.89), FFR (≤0.80), and CFR (<2.0). CFC indicates coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; hAPV, hyperemic average peak velocity; and iFR, instantaneous wave‐free ratio.
Table 2.
iFR, FFR, and CFR Measurement Distributions in the Different CFC Categories
| Normal CFC Mean±SD (n=88) | Mildly Reduced CFC Mean±SD (n=94) | Moderately Reduced CFC Mean±SD (n=80) | Severely Reduced CFC Mean±SD (n=104) | |
|---|---|---|---|---|
| iFR | 0.95±0.06 | 0.92±0.11 | 0.89±0.13 | 0.72±0.24 |
| FFR | 0.88±0.09 | 0.85±0.13 | 0.81±0.13 | 0.70±0.19 |
| CFR | 3.17±0.80 | 2.27±0.44 | 1.85±0.28 | 1.27±0.27 |
CFC indicates coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; and iFR, instantaneous wave‐free ratio.
Table 3.
iFR, FFR, and CFR Measurement Distributions With Contemporary Cut‐Off Values in the Different CFC Categories
| Normal CFC Mean±SD (n) | Mildly Reduced CFC Mean±SD (n) | Moderately Reduced CFC Mean±SD (n) | Severely Reduced CFC Mean±SD (n) | |
|---|---|---|---|---|
| iFR ≤0.89 (n=136) | 0.82±0.05 (13) | 0.76±0.14 (21) | 0.77±0.17 (29) | 0.63±0.24 (73) |
| FFR ≤0.80 (n=68) | 0.74±0.11 (3) | 0.59±0.13 (9) | 0.55±0.08 (10) | 0.54±0.14 (46) |
| CFR <2.0 (n=185) | 1.55±0.31 (8) | 1.57±0.35 (20) | 1.73±0.27 (53) | 1.27±0.27 (104) |
CFC indicates coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; and iFR, instantaneous wave‐free ratio.
Sequential Approach of iFR and Invasive Flow Measurements
iFR and the subsequent CFR assessment is shown in Figure 3A. After initial iFR assessments with iFR ≤0.89, the majority of all lesions (63%) were iFR−, of which also the majority were CFR− (n=156, 68%). Direct iFR assessment yielded 136 of 366 lesions as iFR+ (37%). Subsequent CFR assessments in iFR+ lesions resulted in a total of 111 of 136 iFR+/CFR+ lesions (82%), of which 75 lesions (68%) had reduced CFC. CFC distribution after an iFR and CFR approach is shown in Figure 4A. iFR ≤0.89 had a sensitivity of 84.6%, specificity of 60.4%, positive predictive value of 55.8%, and negative predictive value of 86.9% for the identification of moderately to severely reduced CFC (Table S1).
Figure 3. Sequential approach based on contemporary clinical cut‐off values to evince myocardial ischemia.
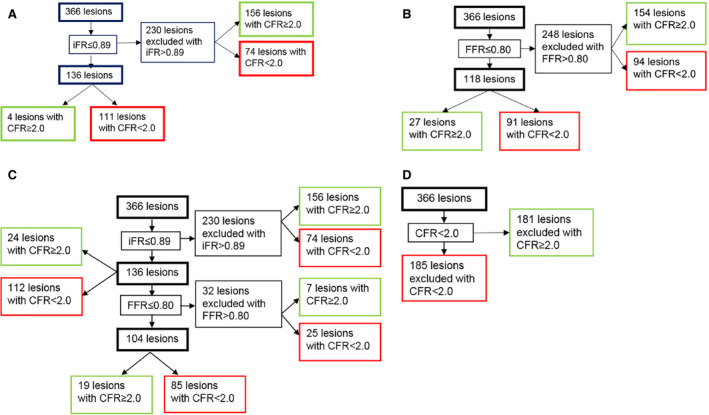
A, iFR and CFR approach. Of the 366 lesions, 111 (30.3%) were iFR+ and CFR+. B, FFR and CFR approach. Of the 366 lesions, 91 (24.9%) were FFR+ and CFR+. C, iFR, FFR, and CFR approach. Of the 366 lesions, 85 (23%) were iFR+, FFR+, and CFR+. D, CFR approach. After a sequential approach, 185 of the 366 lesions (50.5%) were CFR+. CFR indicates coronary flow reserve; FFR, fractional flow reserve; and iFR, instantaneous wave‐free ratio.
Figure 4. CFC distribution after different sequential approaches.
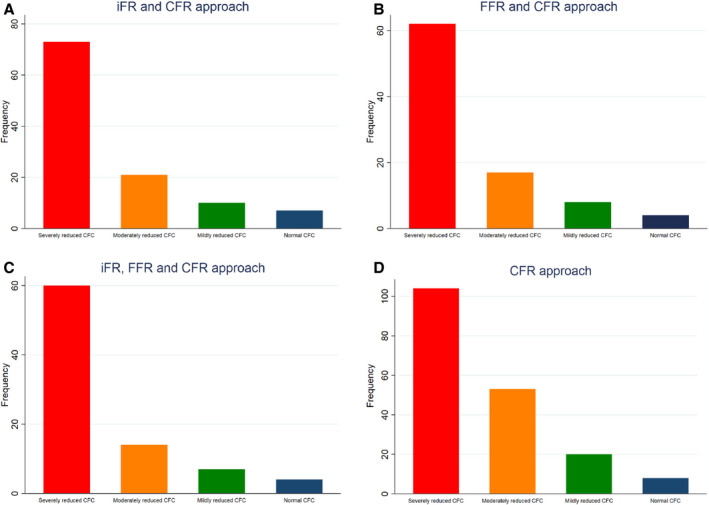
A, iFR and CFR approach. A total of 111 lesions were iFR+/CFR+. Of these lesions, n=73 (66%) had a severely reduced CFC, n=21 (19%) a moderately reduced CFC, n=10 (9%) a mildly reduced CFC, and n=7 (6%) a normal CFC. B, FFR and CFR approach. A total of 91 lesions were FFR+/CFR+. Of these lesions, n=62 (68%) had a severely reduced CFC, n=17 (19%) a moderately reduced CFC, n=8 (9%) a mildly reduced CFC, and n=4 (4%) a normal CFC. C, iFR, FFR, and CFR approach. A total of 85 lesions were iFR+/FFR+/CFR+. Of these lesions, n=60 (71%) had a severely reduced CFC, n=14 (16%) a moderately reduced CFC, n=7 (8%) a mildly reduced CFC, and n=4 (5%) a normal CFC. D, CFR approach. A total of 185 lesions were CFR+. Of these lesions, n=104 (56%) had a severely reduced CFC, n=53 (29%) a moderately reduced CFC, n=20 (11%) a mildly reduced CFC, and n=8 (4%) a normal CFC. CFC indicates coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; and iFR, instantaneous wave‐free ratio.
Sequential Approach of FFR and Invasive Flow Measurements
FFR and subsequent CFR assessment is shown in Figure 3B. Direct assessment of FFR with a cut‐off value ≤0.80 would have identified 118 of 366 lesions (32%) as FFR+, and subsequent assessment of CFR with CFR <2.0 would have yielded a total of 91 lesions (77%) as FFR+/CFR+ (25%) (Figure 2B). Of these 91 lesions, the majority (n=65, 71%) had a reduced CFC. CFC distribution after the FFR and CFR approach is shown in Figure 4B. FFR ≤0.80 had a sensitivity of 46.6%, specificity of 86.3%, positive predictive value of 81.4%, and negative predictive value of 55.7% for the identification of moderately to severely reduced CFC (Table S2).
Value of FFR After Initial iFR Assessment
After selecting iFR ≤0.89, FFR ≤0.80, and CFR <2.0 lesions, 85 of 366 lesions (23%) remained (Figure 3C). Of these iFR+, FFR+, and CFR+ lesions, 60 had a reduced CFC (71%). The direct selection of FFR+ or iFR+ lesions would have resulted in the revascularization of 25% and 30% of CFR+ lesions. FFR assessment with FFR ≤0.80 after initial iFR assessment with iFR ≤0.89 identified 32 extra iFR+ and FFR+ lesions, of which 25 lesions were CFR+ with a CFR <2.0. Of 136 iFR+ and CFR− lesions, 25 (18%) would be revascularized based on iFR alone (Figure 3A). For FFR+ lesions, 27 of 118 FFR+ and CFR− lesions (23%) would be revascularized based on FFR assessment alone (Figure 3B). Of 366 lesions, 50 (14%) were iFR/FFR discordant, either iFR+/FFR− (n=28) or iFR−/FFR+ (n=22) (Figure 5A and 5B). Eventually, 24 lesions were iFR+/FFR−/CFR+, 6 lesions were iFR−/FFR+/CFR+ (P<0.001). Both iFR+ and FFR+ lesions had a significant lower hAPV (24±14.8 cm/s, P<0.001 and 24±13.8 cm/s, P<0.001 [mean±SD]) compared with iFR− and FFR− lesions (33±18.7 cm/s and 33±18.8 cm/s). Baseline average peak velocity did not significantly differ between iFR (15±7.1 cm/s versus 15±8.2 cm/s for iFR− and iFR+, P=0.807) and FFR (15±7.6 cm/s versus 15±7.4 cm/s for FFR− and FFR+, P=0.298) lesions. CFC distributions after the iFR, FFR, and CFR approach are shown in Figure 4C.
Figure 5. Distribution of iFR ‐ FFR discordance.
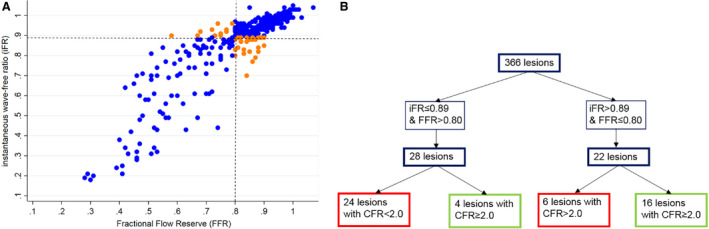
A, Scatterplot showing iFR—FFR discordance. The dashed lines represent the cut‐off values for FFR (≤0.80) and iFR (≤0.89). Concordant cases are colored blue, and discordant cases are colored orange. B, iFR+/FFR− and iFR−/FFR+ discordance and distribution of CFR. A total of 24 iFR+/FFR− lesions were CFR+, and 6 iFR−/FFR+ lesions were CFR+. CFR indicates coronary flow reserve; FFR, fractional flow reserve; and iFR, instantaneous wave‐free ratio.
Direct CFR Assessment
Direct CFR assessment with CFR <2.0 results in 185 of 366 CFR+ (51%), of which 124 lesions had a reduced CFC (67%) (see Figure 3D). After CFR assessment, 181 lesions (49%) were CFR−. CFC distribution after a CFR approach is shown in Figure 4D. CFR <2.0 had a sensitivity of 89.8%, specificity of 100%, positive predictive value of 55.8%, and negative predictive value of 88% for the identification of moderately to severely reduced CFC (Table S3).
Discussion
We evaluated a sequential approach of pressure and flow indexes in intermediate lesions in patients with stable IHD to determine what number of lesions would be significant according to invasive pressure and flow assessment. We found that (1) solely 20% of all lesions were iFR+, FFR+, CFR+, and CFC+; (2) 37% of all stenoses were iFR+, and the added diagnostic value of FFR after initial iFR approach is limited; and (3) iFR and FFR were discordant in 14% of all lesions.
Pressure‐Based Approach in Stenosis Severity: Why the Shift to Flow Is Important
Coronary physiology assessment in addition to CAG has proven its value over CAG alone in clinical decision making in the cardiac catheterization laboratory.16, 17, 18 Currently, several physiology indexes are available to guide the revascularization of coronary lesions, of which the pressure‐derived iFR and FFR indexes are most renowned. It is commonly neglected that pressure‐derived indexes only estimate the number of coronary flow impairment attributed to a coronary lesion, which is not the same as direct flow assessment. In addition, several studies have confirmed that coronary flow is more important than coronary pressure in maintaining myocardial function.5, 19 Coronary flow remains stable because of coronary autoregulation even up to 80% epicardial narrowing; hereafter, coronary flow starts to decrease.10, 20 Vessels with severe myocardial blood flow impairment and thus a low CFR may show signs of inducible ischemia, whereas this is less likely in those areas of the myocardium that are perfused by vessels with high myocardial flow as represented by high CFR.15, 21 In our analysis, 27 of 118 (23%) FFR+/CFR− lesions, or non‐flow‐limiting lesions, would be revascularized while coronary flow is not reduced, and 94 of 248 FFR− lesions (38%), which are CFR+ or flow limiting, would not be revascularized. Hence, a low FFR is not necessarily associated with myocardial flow impairment and thus a low CFR; moreover, lesions with an abnormal FFR but normal CFR are less at risk for MACEs than high FFR but low CFR lesions.5 Therefore, the current pressure‐derived approach in assessing stenosis severity seems to fail in accurately selecting lesions that benefit most by revascularization. The currently available and most widely used pressure‐derived indexes are FFR and iFR, which have similar diagnostic accuracy to assess stenosis severity.8, 9 In our study, the diagnostic accuracy for iFR and FFR was similar, where eventually ≈7% of all lesions would be revascularized while these are not flow limiting, that is, with a CFR ≥2.0. In addition, the concept of non‐flow‐limiting lesions is confirmed by the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) II study, which compared FFR‐guided percutaneous coronary intervention (PCI) with angiography‐guided PCI. The FAME II trial showed that 50% of patients with a FFR ≤0.80 treated with optimal medical therapy did not require revascularization nor did 70% suffer from MACEs during the 5 years of follow‐up.18 FFR seems to fail to properly distinguish which lesions are associated with flow abnormalities—as FFR is based on pressure‐derived estimates of coronary flow impairment, and not on direct flow measurement itself as assessed by CFR. In addition, adding CFC in the sequential approach results in an improved selection of lesions that will benefit from revascularization and subsequently more refined risk stratification in terms of MACEs.12
Dealing With Discordance Between Pressure and Flow: Putting Flow First
Of 366 lesions, 50 (14%) showed iFR–FFR discordance in our study. This number is similar to other studies that reported 80% to 90% agreement between iFR and FFR in >2000 lesions.22, 23, 24, 25 iFR and FFR are currently applied concomitantly and assumed to have equal diagnostic value. Nonetheless, no clear approach on the management of discordance between iFR and FFR exists. Discordance between iFR and FFR is thought to originate from a lower hAPV because iFR positive lesions frequently have a lower CFR that is driven by a reduced hAPV,26 which suggests that a reduced hyperemic response is likely the explanation for discordance between pressure and flow.27 In our study, both iFR+ and FFR+ lesions had a significantly reduced hAPV compared with iFR− and FFR− lesions. Aside from hyperemic flow, several other factors play an important role in iFR/FFR discordance. Focal disease is frequently present in iFR−/FFR+ lesions, whereas diffuse disease is frequently present in iFR+/FFR− lesions.28 In addition, stenosis‐specific characteristics (location, severity) heart rate, age, and beta blocker use affect FFR and should be anticipated when assessing both FFR and iFR.29
Discordance between FFR and CFR occurs more frequently in 30% to 40% of all cases.3 A stenosis with an abnormal pressure measurement but normal CFR suggests the presence of a non‐flow‐limiting stenosis and vice versa. Our findings further address the importance of coronary flow rather than coronary pressure as >75% of all lesions had a normal to mildly impaired CFC—and among those lesions, the majority had a normal to mildly reduced CFC and were thus not associated with ischemia based on invasive flow velocity assessment. Moreover, we have shown that FFR assessment after iFR has only limited value, as this resulted solely in 9% more lesions that were ischemia inducing, of which 22% had a CFR ≥2.0. In addition, contemporary clinical cut‐off values of FFR and to a lesser extent iFR showed relatively low diagnostic accuracy against moderately to severely reduced CFC, which could potentially lead to inappropriate revascularization of vessels.30 Interestingly, the ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) trial, comparing pressure‐guided versus sham PCI in 200 patients with stable CAD and at least 1 vessel with ≥70% diameter stenosis, did not lead to a significant alleviation of angina driven by iFR nor FFR after 6 weeks of follow‐up, whereas the mean iFR was 0.76 and mean FFR was 0.69.31 Hence, these findings give rise to the need to revise contemporary iFR and FFR cut‐off values or revascularization guidelines.
Clinical and Future Implications
The aim of this study was to determine which number of intermediate lesions are associated with flow abnormalities and are assumed to be ischemia inducing by applying coronary pressure and flow indexes through a sequential approach. After pressure and flow assessment, a small number of lesions were positive according to all assessed indexes. If initial iFR assessment is (borderline) significant, subsequent CFR assessment provides diagnostic value and guidance regarding revascularization than another pressure‐derived assessments such as FFR, especially because iFR correlates better with CFR.27 If this results in CFR <2.0, it is probably better to perform PCI because lesions with impaired CFR are highly associated with MACEs after up to 10 years of follow‐up.5, 32, 33 Because myocardial flow is important in maintaining myocardial pressure, our study implicates that the current pressure‐based clinical guidelines for revascularization may be reconsidered to a complemental pressure and flow‐based approach in the future to further improve the selection of patients who may truly benefit from PCI. Therefore, the follow‐up of the DEFINE FLOW (Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect ‐ Combined Pressure and Doppler FLOW Velocity Measurements) study (NCT02328820), a large clinical trial that assessed combined pressure and flow measurements and deferred patients from treatment if FFR was ≤0.80 and CFR ≥2.0 and vice versa, is eagerly awaited as this study may further endorse the prognostic value of coronary flow rather than coronary pressure in IHD.
Limitations
This study has several limitations. First, this study assesses the abstract decision tree of assumed ischemia based on the contemporary clinical cut‐off values for iFR, FFR, and CFR. This results in second quantities where inducible myocardial ischemia is assumed, but not confirmed in terms of MACEs, as follow‐up in this particular article is lacking. The decision to perform PCI was left at the operator's discretion. Moreover, assessing coronary flow can be challenging as it requires operator‐specific experience. Nonetheless, all operators contributing to this study have comprehensive experience in measuring coronary flow at the beginning of the study. Next, wedge pressure was not measured in a majority of the lesions, leaving the attribution of collateral flow to the physiology indexes uncertain. Individual differences in diffuse coronary artery disease and microcirculatory involvement are not deemed impossible based on physiological assessment alone and thus have not been accounted for in the present study.
Conclusions
Ultimately, 20% of intermediate lesions are associated with flow abnormalities according to a iFR+, FFR+, CFR+, and reduced CFC approach based on contemporary cut‐off values. iFR already identifies ischemia in 37% of lesions, and the added diagnostic value of FFR in this approach is limited. In addition, if initial iFR assessment is (borderline) significant, subsequent FFR assessment has no added diagnostic value in contrast with coronary flow assessment by CFR. These results emphasize the use of coronary physiology assessment in assessing stenosis severity over CAG alone, but may also further question the contemporary reputation of a pressure‐based approach as a gold standard for the detection of myocardial ischemia in the cardiac catheterization laboratory.
Sources of Funding
This work was supported by the Institute for Cardiovascular Research of the Amsterdam UMC–location VUmc (to Drs de Waard and van Royen) and the Medical Research Council, British Heart Foundation, and the National Institute for Health Research Imperial Biomedical Research Centre (to Drs Nijjer, Sen, and Petraco). Dr de Waard is supported by a fellowship grant provided by the Netherlands Heart Institute. Funding to pay the open access publication charges for this article was provided by the Amsterdam UMC ‐ location AMC.
Disclosures
Drs Davies and Piek report consultancies work for Volcano Corporation. Dr Davies reports consultancies for Philips‐Volcano Corporation and St Jude Medical. Dr Davies holds intellectual property that is under license. Dr Wijntjens is partially supported by a research grant from Philips‐Volcano Corporation. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
(J Am Heart Assoc. 2020;9:e015559 DOI: 10.1161/JAHA.119.015559.)
For Sources of Funding and Disclosures, see pages 8 and 9.
References
- 1. van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrom T, Klauss V, MacCarthy PA, Manoharan G, Oldroyd KG, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5‐year follow‐up of a randomised controlled trial. Lancet. 2015;386:1853–1860. [DOI] [PubMed] [Google Scholar]
- 2. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, et al. Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 3. Meuwissen M, Chamuleau SA, Siebes M, Schotborgh CE, Koch KT, de Winter RJ, Bax M, de Jong A, Spaan JA, Piek JJ. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation. 2001;103:184–187. [DOI] [PubMed] [Google Scholar]
- 4. Petraco R, Al‐Lamee R, Gotberg M, Sharp A, Hellig F, Nijjer SS, Echavarria‐Pinto M, van de Hoef TP, Sen S, Tanaka N, et al. Real‐time use of instantaneous wave‐free ratio: results of the ADVISE in‐practice: an international, multicenter evaluation of instantaneous wave‐free ratio in clinical practice. Am Heart J. 2014;168:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, et al. Physiological basis and long‐term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. [DOI] [PubMed] [Google Scholar]
- 6. Sen S, Asrress KN, Nijjer S, Petraco R, Malik IS, Foale RA, Mikhail GW, Foin N, Broyd C, Hadjiloizou N, et al. Diagnostic classification of the instantaneous wave‐free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (classification accuracy of pressure‐only ratios against indices using flow study). J Am Coll Cardiol. 2013;61:1409–1420. [DOI] [PubMed] [Google Scholar]
- 7. Echavarria‐Pinto M, van de Hoef TP, van Lavieren MA, Nijjer S, Ibanez B, Pocock S, Quiros A, Davies J, Meuwissen M, Serruys PW, et al. Combining baseline distal‐to‐aortic pressure ratio and fractional flow reserve in the assessment of coronary stenosis severity. JACC Cardiovasc Interv. 2015;8:1681–1691. [DOI] [PubMed] [Google Scholar]
- 8. Davies JE, Sen S, Dehbi HM, Al‐Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, et al. Use of the instantaneous wave‐free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824–1834. [DOI] [PubMed] [Google Scholar]
- 9. Gotberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Ohagen P, Olsson H, Omerovic E, et al. Instantaneous wave‐free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813–1823. [DOI] [PubMed] [Google Scholar]
- 10. Nijjer SS, de Waard GA, Sen S, van de Hoef TP, Petraco R, Echavarrıa‐Pinto M, van Lavieren MA, Meuwissen M, Danad I, Knaapen P, et al. Coronary pressure and flow relationships in humans: phasic analysis of normal and pathological vessels and the implications for stenosis assessment: a report from the Iberian–Dutch–English (IDEAL) collaborators. Eur Heart J. 2016;37:2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB III, Morrison DA, O'Neil WW, Schaff HV, Whitlow PL, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI writing committee to update 2001 guidelines for percutaneous coronary intervention). Circulation. 2006;113:e166–e286. [DOI] [PubMed] [Google Scholar]
- 12. van de Hoef TP, Echavarria‐Pinto M, van Lavieren MA, Meuwissen M, Serruys PW, Tijssen JG, Pocock SJ, Escaned J, Piek JJ. Diagnostic and prognostic implications of coronary flow capacity: a comprehensive cross‐modality physiological concept in ischemic heart disease. JACC Cardiovasc Interv. 2015;8:1670–1680. [DOI] [PubMed] [Google Scholar]
- 13. Kern MJ, Bach RG, Mechem CJ, Caracciolo EA, Aguirre FV, Miller LW, Donohue TJ. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28:1154–1160. [DOI] [PubMed] [Google Scholar]
- 14. Meuwissen M. Role of fractional and coronary flow reserve in clinical decision making in intermediate coronary lesions. Interv Cardiol. 2009;1:237–255. [Google Scholar]
- 15. Johnson NP, Gould KL. Physiological basis for angina and ST‐segment change PET‐verified thresholds of quantitative stress myocardial perfusion and coronary flow reserve. JACC Cardiovasc Imaging. 2011;4:990–998. [DOI] [PubMed] [Google Scholar]
- 16. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary‐artery stenoses. N Engl J Med. 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 17. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrom T, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2‐year follow‐up of the FAME (fractional flow reserve versus angiography for multivessel evaluation) study. J Am Coll Cardiol. 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 18. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrom T, Kaab S, Dambrink JH, Rioufol G, et al. Five‐year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 19. Smalling RW, Kelley K, Kirkeeide RL, Fisher DJ. Regional myocardial function is not affected by severe coronary depressurization provided coronary blood flow is maintained. J Am Coll Cardiol. 1985;5:948–955. [DOI] [PubMed] [Google Scholar]
- 20. Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87–94. [DOI] [PubMed] [Google Scholar]
- 21. Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5:430–440. [DOI] [PubMed] [Google Scholar]
- 22. Chamuleau SA, Meuwissen M, van Eck‐Smit BL, Koch KT, de Jong A, de Winter RJ, Schotborgh CE, Bax M, Verberne HJ, Tijssen JG, et al. Fractional flow reserve, absolute and relative coronary blood flow velocity reserve in relation to the results of technetium‐99 m sestamibi single‐photon emission computed tomography in patients with two‐vessel coronary artery disease. J Am Coll Cardiol. 2001;37:1316–1322. [DOI] [PubMed] [Google Scholar]
- 23. Christou MA, Siontis GC, Katritsis DG, Ioannidis JP. Meta‐analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol. 2007;99:450–456. [DOI] [PubMed] [Google Scholar]
- 24. Miller DD, Donohue TJ, Younis LT, Bach RG, Aguirre FV, Wittry MD, Goodgold HM, Chaitman BR, Kern MJ. Correlation of pharmacological 99mTc‐sestamibi myocardial perfusion imaging with poststenotic coronary flow reserve in patients with angiographically intermediate coronary artery stenoses. Circulation. 1994;89:2150–2160. [DOI] [PubMed] [Google Scholar]
- 25. van de Hoef TP, Nolte F, Damman P, Delewi R, Bax M, Chamuleau SA, Voskuil M, Siebes M, Tijssen JG, Spaan JA, et al. Diagnostic accuracy of combined intracoronary pressure and flow velocity information during baseline conditions: adenosine‐free assessment of functional coronary lesion severity. Circ Cardiovasc Interv. 2012;5:508–514. [DOI] [PubMed] [Google Scholar]
- 26. Cook CM, Jeremias A, Petraco R, Sen S, Nijjer S, Shun‐Shin MJ, Ahmad Y, de Waard G, van de Hoef T, Echavarria‐Pinto M, et al. Fractional flow reserve/instantaneous wave‐free ratio discordance in angiographically intermediate coronary stenoses: an analysis using Doppler‐derived coronary flow measurements. JACC Cardiovasc Interv. 2017;10:2514–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petraco R, van de Hoef TP, Nijjer S, Sen S, van Lavieren MA, Foale RA, Meuwissen M, Broyd C, Echavarria‐Pinto M, Foin N, et al. Baseline instantaneous wave‐free ratio as a pressure‐only estimation of underlying coronary flow reserve: results of the JUSTIFY‐CFR Study (joined coronary pressure and flow analysis to determine diagnostic characteristics of basal and hyperemic indices of functional lesion severity‐coronary flow reserve). Circ Cardiovasc Interv. 2014;7:492–502. [DOI] [PubMed] [Google Scholar]
- 28. Warisawa T, Cook CM, Howard JP, Ahmad Y, Doi S, Nakayama M, Goto S, Yakuta Y, Karube K, Shun‐Shin MJ, et al. Physiological pattern of disease assessed by pressure‐wire pullback has an influence on fractional flow reserve/instantaneous wave‐free ratio discordance. Circ Cardiovasc Interv. 2019;12:e007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Derimay F, Johnson NP, Zimmermann FM, Adjedj J, Witt N, Hennigan B, Koo BK, Barbato E, Esposito G, Trimarco B, et al. Predictive factors of discordance between the instantaneous wave‐free ratio and fractional flow reserve. Catheter Cardiovasc Interv. 2019;94:356–363. [DOI] [PubMed] [Google Scholar]
- 30. Modi BN, Rahman H, Kaier T, Ryan M, Williams R, Briceno N, Ellis H, Pavlidis A, Redwood S, Clapp B, et al. Revisiting the optimal fractional flow reserve and instantaneous wave‐free ratio thresholds for predicting the physiological significance of coronary artery disease. Circ Cardiovasc Interv. 2018;11:e007041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al‐Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double‐blind, randomised controlled trial. Lancet. 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 32. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long‐term prognostic value of 13N‐ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. [DOI] [PubMed] [Google Scholar]
- 33. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


