Abstract
Background
The use of extracorporeal life support (ECLS) has expanded to include unique populations such as peripartum women. This systematic review aims to (1) quantify the number of cases and indications for ECLS in women during the peripartum period reported in the literature and (2) report maternal and fetal complications and outcomes associated with peripartum ECLS.
Methods and Results
This review was registered in PROSPERO (CRD42018108142). MEDLINE, Embase, and CINAHL were searched for case reports, case series, and studies reporting cases of ECLS during the peripartum period that reported one or more of the following outcomes: maternal survival, maternal complications, fetal survival, and/or fetal complications. Qualitative assessment of 221 publications evaluated the number of cases, clinical details, and maternal and fetal outcomes of ECLS during the peripartum period. There were 358 women included and 68 reported fetal outcomes in cases where the mother was pregnant at the time of cannulation. The aggregate maternal survival at 30 days was 270 (75.4%) and at 1 year was 266 (74.3%); fetal survival was 44 (64.7%). The most common indications for ECLS overall in pregnancy included acute respiratory distress syndrome 177 (49.4%), cardiac failure 67 (18.7%), and cardiac arrest 57 (15.9%). The most common maternal complications included mild to moderate bleeding 66 (18.4%), severe bleeding requiring surgical intervention 48 (13.4%), and intracranial neurologic morbidity 19 (5.3%). The most commonly reported fetal complications included preterm delivery 33 (48.5%) and neonatal intensive care unit admission 19 (27.9%).
Conclusions
Reported rates of survival in ECLS in pregnant and postpartum women are high and major complications relatively low.
Keywords: extracorporeal circulation, extracorporeal membrane oxygenation, pregnancy and postpartum
Subject Categories: Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- ARDS
acute respiratory distress syndrome
- ECLS
extracorporeal life support
- ECMO
extracorporeal membrane oxygenation
Clinical Perspective
What Is New?
With the increasing medical complexity of women of childbearing age, the potential for catastrophic complications of pregnancy may call for advanced therapies including extracorporeal life support.
What Are the Clinical Implications?
Venovenous and venoarterial extracorporeal support may be implemented for a variety of severe cardiopulmonary conditions in pregnant patients with reasonable success and safety for mother and fetus.
Cardiovascular disease is currently the leading cause of pregnancy‐related mortality in the United States, contributing to more than a quarter (26%) of maternal deaths and as well as severe maternal morbidity.1, 2 Pregnant women are becoming more medically complex with chronic health conditions that may predispose them to cardiopulmonary complications during pregnancy.3, 4, 5, 6 Given the rise in conditions for which extracorporeal life support (ECLS) including extracorporeal membrane oxygenation (ECMO) may be indicated, providers must understand the uses and limitations of advanced medical therapies such as ECLS. The relative rarity of catastrophic cardiopulmonary disease in the peripartum period requiring ECLS makes it difficult to study. Existing systematic reviews are narrow in scope (for example, evaluating only certain causative conditions such as acute respiratory distress syndrome [ARDS]) and small in size (the largest studies report <70 patients).7, 8, 9, 10 Combining past studies for all conditions in a systematic review will enhance the understanding of how to identify patients who will benefit from ECLS, develop appropriate use, and provide data to counsel patients and their families. The aim of this systematic review is to perform a comprehensive search of ECLS in the pregnant and postpartum periods, to define the reported indications as well as maternal and fetal survival, and to identify associated complications.
Methods
This systematic review was registered in PROSPERO (CRD42018108142) and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines were reviewed and maintained when performing this systematic review.11 This study was exempt from institutional review board approval as a systematic review of published literature. The authors declare that all supporting data are available within the article and its online supplementary files.
Literature Search
A comprehensive literature search was performed on September 11, 2018 for this systematic review using OVID MEDLINE (1946‐), Elsevier Embase (1947‐), and EBSCOhost CINAHL (1937‐) databases from time of inception to capture studies regarding ECLS in the pregnant and postpartum periods with no limit placed on language or date of publication, but studies that describe the use of ECMO in children were excluded. A selection of sentinel articles was used to generate search terms and test retrieval in all of the databases. The complete searches can be found in Data S1.
Study Selection
After the search was completed, 2 authors (E.E.N., A.N.C.) reviewed each abstract independently for consideration of full text review. The same 2 authors independently reviewed the full text articles for inclusion in the systematic review. A search using the Web of Science from February 28 through March 1, 2019 identified the articles in the bibliographies as well as citations of the selected articles and were also screened for inclusion. An additional study was found through preexisting subject knowledge and included. Once final articles were selected, data were extracted by one author (E.E.N.) and validated by another (A.N.C.). Google Translate was used for non‐English texts and information was collected from adequately translated articles for those that met inclusion criteria. Any discrepancies were resolved by discussion. Figure 1 depicts a flowchart of study selection.
Figure 1. Flowsheet for study inclusion/exclusion.
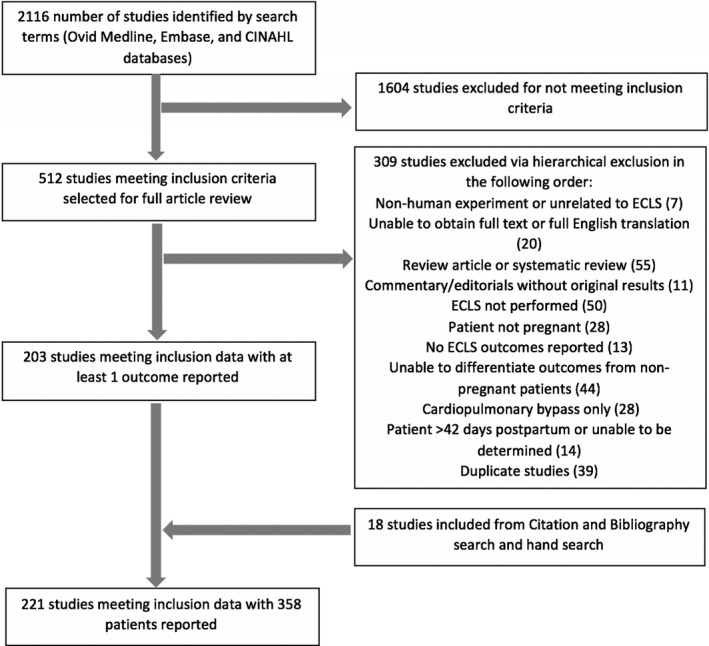
Flow diagram of search results and study selection.
Case reports, case series, meeting abstracts, correspondences, and any other type of study that reported one or more of the following were included: maternal survival, fetal survival, maternal complications, and/or fetal complications with the use of ECLS during pregnancy or within the postpartum period (the first 42 days after delivery). Studies in which the patients did not clearly undergo ECLS, underwent cardiopulmonary bypass for cardiac surgery only, or underwent immediate cardiac mechanical support (eg, left ventricular assist device, biventricular assist device) were excluded. Cases in which initiation of extracorporeal support was unclear were reviewed by 2 authors (E.E.N., M.E.B.) for inclusion and those where initiation was more than 42 days postpartum were excluded.
The following criteria were used for exclusion of studies: patients who were not pregnant or more than 42 days postpartum, studies not reporting either maternal or fetal outcomes, nonhuman studies, non‐English studies without adequate translation, editorials, narrative review articles, and systematic review articles. Articles that were excluded after full text review were recorded with reasons for exclusion. If more than one reason for exclusion was identified, articles were excluded based on the following hierarchical order: nonhuman experiment or unrelated to ECLS; unable to obtain full text or full English translation; review article or systematic review; commentary/editorials without original results; ECLS not performed; patient not pregnant; no ECLS outcomes reported; unable to differentiate outcomes from nonpregnant patients; patient >42 days postpartum; duplicate studies or overlapping cohorts.
Interpretation of Data
Data were collected for indication; maternal demographics; gestational age; timing, duration, and type of ECLS; and maternal and fetal morbidity and mortality. Maternal information collected included age, gravidity/parity, disease process, ECLS duration, complications, and mortality. Obstetrical information was collected for gestational age and delivery type. Fetal information was collected for gestational age at delivery, preterm delivery, neonatal intensive care unit admission, complications, and mortality. ECLS information was collected for support type (venoarterial, venovenous, or both [venoarterial+venovenous]), duration, and indication. For cohort studies, the mean age, gravidity and parity, gestational age, and duration on ECLS were recorded. One author collected the data (E.E.N.) and the second author (A.N.C.) reviewed each study and confirmed the data. Discrepancies in the data were resolved by discussion, and if still unresolved, a third author (M.E.B.) reviewed for resolution. Authors of published articles with incomplete information were contacted by e‐mail and/or telephone for further information.
Gravidity and parity was defined as described in reports on hospital admission. Vaginal deliveries included spontaneous vaginal deliveries and operative vaginal deliveries. Deliveries prior to 37 weeks were considered preterm deliveries. Any delivery prior to 32 weeks was considered to have been admitted to the neonatal intensive care unit whether or not it was explicitly stated, in accordance with the American Academy of Pediatrics guidelines on levels of neonatal care.12
Fetal outcomes were reported for patients who underwent ECLS while pregnant; fetal outcomes from patients who underwent ECLS immediately postpartum, postpartum, or unknown timing relative to delivery were not included. Ectopic pregnancies were not counted toward fetal mortality. Maternal cases <24 weeks with a fetal loss were reported as a spontaneous abortion and were not included in the preterm delivery count. Spontaneous abortions that occurred prior to ECLS were not included in fetal mortality. Therapeutic abortions were included in fetal mortality if the termination occurred on ELCS for maternal indications or if abortion was performed because of catastrophic fetal injury incurred due to ECLS. Fetal survival was reported within the outcome only if it was explicitly stated in the study.
Patients were classified as being antepartum, immediately postpartum, or postpartum. Antepartum patients were those cannulated and initiated on ECLS prior to delivery. Immediately postpartum cases were identified as those patients who were cannulated and initiated on ECLS within 24 hours of delivery. Postpartum cases included patients who were cannulated and initiated on ECLS >24 hours after delivery but within 42 days of parturition. Cases that were identified as postpartum but did not have a clear time frame of <24 hours of delivery were included in the postpartum category. Indications for ECLS were noted based on case reports; some patients had multiple indications for ECLS. Patients with underlying ischemic, structural, or valvular heart disease were defined as having heart disease as well as cardiac failure as the indication for ECLS.
Mechanical support was identified as venoarterial, venovenous, or both as described in the study. Extracorporeal carbon dioxide removal, extracorporeal arteriovenous carbon dioxide removal, interventional lung assist membrane, and percutaneous extracorporeal lung assist supports were included with venovenous therapies. Cases in which the patient underwent cardiopulmonary bypass and had been previously initiated on ECLS or underwent post‐cardiopulmonary bypass ECLS were included. Duration of ECLS was reported for all patients regardless of survival. Survival was reported within 30 days of discontinuation of ECLS therapy as the aim of this study is to evaluate the rescue survivability of ECLS; additional deaths within 1 year were also reported separately. Complications included mild to moderate bleeding (qualitatively described as mild to moderate and/or the need for transfusion without invasive intervention), severe bleeding (requiring surgical or endoscopic interventions), vascular complications (limb ischemia, pseudoaneurysm, wound infection), intracranial morbidity (hypoxic brain injury, intracranial hemorrhage, hemiplegia, cerebral infarct), deep vein thrombosis, and other neurologic morbidity (peripheral neuropathy, cognitive dysfunction, need for rehabilitation after discharge, myoclonic epilepsy, amnestic disorder). Some patients had multiple complications.
Quality Assessment
Because of the low level of evidence and high risk of bias, case reports and case series were not assessed for the level of quality. Given the limited information presented, meeting abstracts and correspondence were also not assessed for level of quality.
Results
Overall, 2116 studies were identified and reviewed and 221 studies met inclusion criteria. Table S1 contains the complete list of articles included and description of the studies and patients. These papers were published between 1974 and 2019. Figure 2 depicts the increasing rate of reporting of cases. Table 1 reports the patient characteristics. There were a total of 358 cases of ECLS during the peripartum period reported. The maternal survival at 30 days was 270 (75.4%) patients; maternal survival at 1 year was 266 (74.3%). Of the 358 cases, there were 210 (58.7%) cases that described the fetal outcome. There were 81 cases where the mother was on ECLS during pregnancy; of these cases, 68 (84.0%) had fetal outcomes reported. Fetal survival reported for patients who underwent ECLS during the antepartum period was 44 (64.7%) patients.
Figure 2. Case reports by year of publication.
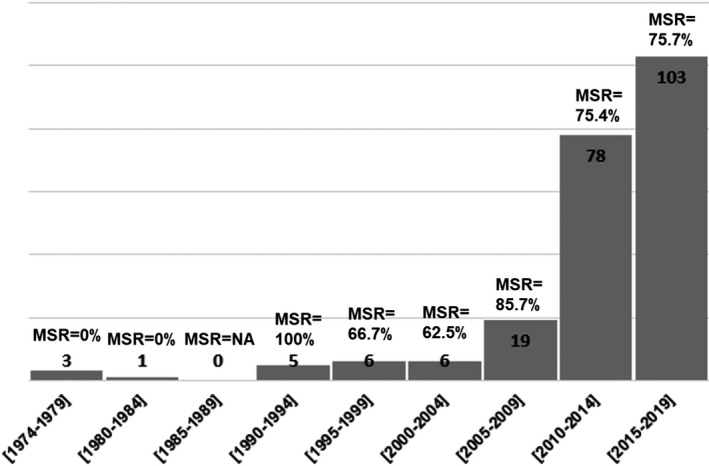
Results of the case reports by year of publication. MSR indicates the maternal survival rate for each time period described.
Table 1.
Patient Characteristics
| Total Patients, n (%) | |
|---|---|
| Maternal count | 358 (100) |
| Antepartum cases with fetal outcome | 68 (84.0) |
| Average maternal age, y (SD) | 29.5 (6.1) |
| Median gravity/parity (range) | 2/1 (G1P0–G9P8) |
| Median gestational age (SD) | 24 wk (6.2) |
| Interquartile range | 6.14 wk |
| Stage of pregnancy, n (%) | |
| Antepartum | 81 (22.6) |
| Immediate postpartum | 69 (19.3) |
| Postpartum | 126 (35.2) |
| Uncategorized | 82 (22.9) |
| Delivery type, n (%) | |
| Vaginal | 67 (18.7) |
| Cesarean | 158 (44.1) |
| Dilation and extraction | 5 (1.4) |
| Not reported | 128 (35.8) |
| Deliveries on extracorporeal membrane oxygenation, n (%) | 35 (9.8) |
| Extracorporeal life support indications, n (%)a | |
| Acute respiratory distress syndrome | 177 (49.4) |
| Cardiac failure | 67 (18.7) |
| Cardiac arrest | 57 (15.9) |
| Peripartum cardiomyopathy | 45 (12.6) |
| Pulmonary arterial hypertension | 28 (7.8) |
| Amniotic fluid embolism | 27 (7.5) |
| Pulmonary embolism | 17 (4.7) |
| Heart disease | 14 (3.9) |
| Septic shock | 11 (3.1) |
| Asthma | 7 (2.0) |
| Malignancy | 7 (2.0) |
| Takotsubo cardiomyopathy | 6 (1.7) |
| Pheochromocytoma | 5 (1.4) |
| Spontaneous coronary artery dissection | 3 (0.8) |
| Aspiration pneumonitis | 2 (0.6) |
| Trauma | 2 (0.6) |
| Distributive shock | 1 (0.3) |
| Propofol infusion syndrome | 1 (0.3) |
| Hemorrhagic shock | 1 (0.3) |
| Diffuse alveolar hemorrhage | 1 (0.3) |
| Pulmonary alveolar proteinosis | 1 (0.3) |
| Interstitial lung disease | 1 (0.3) |
| Sickle cell crisis | 1 (0.3) |
| Aortic dissection | 1 (0.3) |
| Transfusion related acute lung injury | 1 (0.3) |
| Cerebral venous thrombosis | 1 (0.3) |
| Pulmonary hemorrhage | 1 (0.3) |
| Cystic fibrosis | 1 (0.3) |
| Dengue fever | 1 (0.3) |
| Extracorporeal membrane oxygenation cannulation mode, n (%) | |
| Venoarterial | 145 (40.5) |
| Venovenous | 96 (26.8) |
| Both venoarterial and venovenous | 19 (5.3) |
| Not reported | 98 (27.4) |
| Maternal overall survival at 30 d, n (%) | 270 (75.4) |
| Antepartum | 65 (81.2) |
| Immediate postpartum | 58 (84.1) |
| Postpartum | 85 (67.5) |
| Timeframe unspecified | 62 (75.6) |
| Maternal overall survival at 1 y, n (%) | 266 (74.3) |
| Maternal complications, n (%)b | |
| Mild to moderate bleeding | 66 (18.4) |
| Severe bleeding requiring surgical intervention (laparotomy, hysterectomy) | 48 (13.4) |
| Intracranial neurologic morbidity | 19 (5.3) |
| Vascular complications (extremity ischemia, infection) | 14 (3.9) |
| Deep vein thrombosis | 10 (2.8) |
| Other (peripheral neuropathy, need for rehabilitation on discharge, hyperbilirubinemia, reperfusion injury, bradycardia with cannulation) | 22 (6.1) |
| Fetal survival, n (%) | 44 (64.7) |
| Fetal complications, n (%)b | |
| Preterm delivery | 33 (48.5) |
| Neonatal intensive care unit admission | 19 (27.9) |
Some cases had multiple indications for ECLS.
Some cases had multiple complications.
The most common indications for ECLS overall in pregnancy included ARDS 177 (49.4%), cardiac failure 67 (18.7%), and cardiac arrest 57 (15.9%). Table 2 reports the survival of patients based on indication for ECLS. The most common maternal complications included mild to moderate bleeding 66 (18.4%), severe bleeding requiring surgical intervention 48 (13.4%), and intracranial neurologic morbidity 19 (5.3%). There were 18 (5.0%) patients who had peripheral neurologic deficits or required rehabilitation resulting in an intact neurologic maternal survival of 245 (68.4%).
Table 2.
Extracorporeal Life Support Indications and Outcomes
| Total, n (%) | Survival, n (%) | |
|---|---|---|
| Indications | ||
| Acute respiratory distress syndrome | 177 (49.3) | 141 (79.7) |
| Cardiac failure | 67 (18.7) | 52 (77.6) |
| Cardiac arrest | 57 (15.9) | 50 (87.7) |
| Peripartum cardiomyopathy | 45 (12.5) | 36 (78.3) |
| Pulmonary arterial hypertension | 28 (7.8) | 14 (50) |
| Amniotic fluid embolism | 27 (7.5) | 14 (51.9) |
| Pulmonary embolism | 17 (4.7) | 11 (64.7) |
| Heart disease | 14 (3.9) | 11 (78.6) |
| Septic shock | 11 (3.1) | 10 (90.9) |
Outcomes separated by indication for extracorporeal support.
There were 81 (22.6%) cases in the antepartum period, 69 (19.3%) cases in the immediately postpartum period (<1 day), and 126 (35.2%) cases in the postpartum period (1–42 days). There were 82 (22.9%) cases that could not be classified into a defined time period. The most common antepartum indications were ARDS 53 (65.4%), cardiac failure 8 (9.9%), pulmonary arterial hypertension 7 (8.6%), and cardiac arrest 7 (8.6%). Complications included mild to moderate bleeding 27 (33.3%) and severe bleeding 7 (8.6%). Cannulation was venovenous in 46 (56.8%), venoarterial in 21 (25.9%), venoarterial+venovenous in 5 (6.2%), and unknown in 9 (11.1%). The average duration of ECLS was 10.8 days. Maternal survival was 65 (80.2%), fetal survival was 44 (64.7%). The most commonly reported fetal complications include preterm delivery 33 (48.5%) and neonatal intensive care unit admission 19 (27.9%). Fetal neurologic complications included intraventricular hemorrhage 2 (2.9%), ventriculomegaly 1 (1.5%), ventriculomegaly/cerebral ischemia 1 (1.5%), and asphyxia with therapeutic hypothermia 1 (1.5%).
The most common immediately postpartum indications included cardiac arrest 39 (56.6%), cardiac failure 16 (23.2%), and amniotic fluid embolism 15 (21.7%). Complications included mild to moderate bleeding 17 (24.6%) and severe bleeding 18 (26.1%). Cannulation was venoarterial in 53 (76.8%), venovenous in 6 (8.7%), venoarterial+venovenous in 6 (8.7%), and unknown in 4 (5.8%). The average duration of ECLS was 5.5 days and maternal survival was 58 (84.1%).
The most common postpartum indications were ARDS 50 (39.7%), peripartum cardiomyopathy 32 (25.4%), and cardiac failure 24 (19.0%). Complications included mild to moderate bleeding 17 (13.5%) and severe bleeding 18 (14.3%). Cannulation was venoarterial in 63 (50%), venovenous in 37 (29.4%), venovenous+venoarterial in 7 cases (5.6%), and unknown in 19 (15.1%). The average duration of ECLS was 17.9 days and maternal survival was 85 (67.5%).
There were 35 deliveries on ECLS at an average gestational age of 26.3 weeks. Women who had deliveries on ECLS had a survival of 27 (79.4%) and of the reported fetal outcomes, the fetal survival was 18 (56.3%). There were 14 (41.2%) vaginal deliveries, 1 (2.9%) vacuum‐assisted vaginal delivery, and 20 (57.1%) cesarean deliveries. Of the deliveries on ECLS, 16 (45.7%) were performed with the patient on anticoagulation including 10 (63%) cesarean deliveries and 6 vaginal deliveries (37%). Anticoagulation was held for the delivery in 3 (8.6%) cases and 16 (45.7%) did not explicitly outline anticoagulation management. Additional details on these cases can be found in Table S2. There were 11 cases including cardiopulmonary bypass pre‐ or post‐ECLS. Additional details on these cases can be found in Table S3.
We also analyzed cases based on the cannulation mode as seen in Table 3. Extracorporeal carbon dioxide removal removal 4 (1.1%), extracorporeal arteriovenous carbon dioxide removal removal 1 (0.28%), interventional lung assist membrane 2 (0.56%), and percutaneous extracorporeal lung assist 1 (0.28%) were included within the venovenous cases. Venoarterial cases were more likely to be of shorter duration (average 6.9 days) and for cases of cardiac arrest (28.3%), cardiac failure (26.2%) or peripartum cardiomyopathy (25.5%). Venovenous cases were on average longer in duration (average 18.8 days) and the vast majority were indicated for ARDS 74 (77.1%) followed by asthma 6 (6.25%) and cardiac failure 4 (4.2%). A smaller percentage of patients (5.3%) had both venoarterial or venovenous at different times during the peripartum period and this was most frequently used for cardiac arrest 6 (31.6%), ARDS 6 (31.6%), or cardiac failure 4 (21.1%). The survival between these groups as well as reports where the cannulation mode was unknown was comparable.
Table 3.
Cannulation Outcomes
| Venoarterial | Total, n (%) | Venoarterial+Venovenous | Total, n (%) |
|---|---|---|---|
| 145 (40.5) | 19 (5.3) | ||
| Indications | Indications | ||
| Cardiac arrest | 41 (28.3) | Cardiac arrest | 6 (31.6) |
| Cardiac failure | 39 (26.9) | ARDS | 6 (31.6) |
| Peripartum cardiomyopathy | 37 (25.5) | Cardiac failure | 4 (21.1) |
| Duration | 6.9 d | Duration | 13.9 d |
| Maternal survival | 105 (72.4) | Maternal survival | 14 (73.7) |
| Maternal complications | Maternal complications | ||
| Mild to moderate bleeding | 29 (20.0) | Mild to moderate bleeding | 6 (31.6) |
| Severe bleeding | 23 (15.9) | Severe bleeding | 5 (26.3) |
| Intracranial complications | 10 (6.9) | Vascular complications | 3 (15.8) |
| Venovenous | 96 (26.8) | Unknown Cannulation | 98 (27.4) |
|---|---|---|---|
| Indications | Indications | ||
| ARDS | 74 (77.1) | ARDS | 83 (84.7) |
| Asthma | 7 (7.3) | Cardiac failure | 20 (20.4) |
| Cardiac failure | 4 (4.2) | Cardiac arrest | 7 (7.1) |
| Duration | 18.8 d | Duration | 12.7 d |
| Maternal survival | 77 (80.2) | Maternal survival | 74 (76.3) |
| Maternal complications | Maternal complications | ||
| Mild to moderate bleeding | 29 (30.2) | Mild to moderate bleeding | 4 (4.1) |
| Severe bleeding | 12 (12.5) | Severe bleeding | 8 (8.2) |
| Intracranial complications | 6 (6.3) | Deep vein thrombosis | 5 (5.1) |
Indications and outcomes separated by cannulation type. ARDS indicates acute respiratory distress syndrome.
Discussion
In this study, we found an overall 30‐day survival rate of 75.4% (n=270) for mothers requiring ECLS and a survival rate of 64.7% (n=44) for fetuses exposed to ECLS. However, our fetal survival rate is limited by missing fetal outcomes and lack of long‐term follow‐up. Complications associated with ECLS in pregnant patients were consistent with the general population including bleeding, deep vein thrombosis, and vascular complications.13, 14, 15 The indications for ECLS and cannulation methods varied based on the timing relative to delivery. From the few reviews published to date, the survival rate for pregnant patients undergoing ECLS has been significantly higher than the overall survival with adult ECLS for pulmonary (59%) or cardiac (43%) causes16 with reported maternal survival rates ranging from 70% to 80%7, 8, 9, 10 and reported fetal survival rates 65% to 72%.7, 8, 9, 10
Despite concerns that pregnant or immediately postpartum women may be at risk of bleeding complications and/or at risk for thromboembolic phenomenon in the immediate postpartum period, our findings do not reflect this concern and suggest that the conditions leading these women to require ECLS may be reversible and in fact, more amenable to mechanical support than the standard adult ECLS. Mild to moderate bleeding complications were noted in 18.4% (n=66) of cases and severe bleeding requiring operation was present in 13.4% (n=48) of cases; this was comparable to the reported range in other adults studies ranging from 28% to 32%.13, 14, 15 Deep vein thromboses and vascular complications were uncommon at 2.8% (n=10) and 3.9% (n=14) respectively and comparable to the ranges reported for limb ischemia in other adult populations, 2% to 14%.13, 14, 15, 17 Collectively, our findings demonstrate that pregnant patients had more favorable survival than prior reported rates for the general population with similar rates of complications.13, 14, 15, 17 These patients represent an overall younger, healthier group who are more likely to have ECLS for acute, reversible indications than the general population of ECLS cases and our findings reflect this underlying favorability for better outcomes.
Survival varied depending on the indication for ECLS. Women who were cannulated for cardiac arrest had a survival rate of 87.7%; comparing this with a population‐based study on maternal cardiac arrest that reported a 58.9% survival suggests that ECLS may have a role in improving clinical outcomes in this context.18 Neurologically intact survival for patients who undergo extracorporeal cardiopulmonary resuscitation has been reported at 28.5%, in this study it was found to be 78.9% with neurologic deficits including hemiparesis 1 (1.8%), limb motor deficits 2 (3.5%), hand weakness 1 (1.8%), and need for rehabilitation on discharge 2 (3.5%).19 The current literature regarding amniotic fluid embolism reports a wide range of survival from 39% to 89%.20, 21, 22 This review found an overall survival of 51.9% (n=14), consistent with the current literature; however, the rate of neurologically intact survival was 74% (n=20) compared with 15% in previously reported literature.20 Pulmonary arterial hypertension has a very poor prognosis in pregnant women and prior case series have reported very poor survival rates with ECLS (16.7%); however, our study reported a survival of 50% (n=14).23 Several reports of antepartum placement of venous and arterial sheaths for potential rapid initiation of ECLS in patients with severe pulmonary arterial hypertension suggest that these anticipatory interventions may improve outcomes in these high‐risk patients.24, 25
We separated peripartum patients who underwent ECLS into 3 time periods and observed that there were different indications and survival rates for advanced mechanical support at different stages of pregnancy. The majority of cases during the antepartum period were for cases of ARDS and roughly one‐third of those patients delivered while on ECLS. The decision to deliver the neonate versus continue ECLS in these patients is one that requires multidisciplinary discussion and is made on an individual basis depending on the disease process, evolution of illness, institutional experience, and expert opinion. Notably, survival in the immediately postpartum group was highest at 84.1% (n=58). The higher risk of bleeding in the immediately postpartum group (mild to moderate bleeding 17 [24.6%] and severe bleeding 18 [26.1%]) may not be surprising given the number of cases associated with amniotic fluid embolism and postpartum hemorrhage, 2 pathologies known to induce coagulopathy and hyperfibrinolysis. Of the 3 groups, postpartum ECLS had the longest average duration of support and lowest survival rates.
Cardiac arrest was the most common indication in the immediately postpartum period and many of these reports are cases of extracorporeal cardiopulmonary resuscitation, defined as application of venoarterial ECMO during cardiopulmonary resuscitation. The 57 cases of cardiac arrest in this review had very favorable survival (87.7%) compared with the general adult population survival with extracorporeal cardiopulmonary resuscitation (29%).16 These findings support consideration and use of extracorporeal cardiopulmonary resuscitation in pregnant and immediately postpartum patients as an advantageous group.
Our study has several limitations. These results may be confounded by publication bias as authors may be more likely to report favorable outcomes than poor ones. It may be that the current literature is unduly optimistic with successful cases being overrepresented but could also be underreporting of uneventful but successful cases. Another limitation is that the current data come from case reports and case series. We considered estimating our outcomes using 95% CIs; however, we did not have an unexposed population without ECLS in order to compare our outcomes. Additionally, when calculating CIs to estimate a risk/proportion, there is also the underlying assumption that the observations come from the same population with the same “true” risk and that these are randomly drawn from the population. We rejected this assumption as there is likely to be publication bias when synthesizing results from case reports and case series. Sample size limitations were also a major concern in the consideration of fitting a random effects model. Therefore, we included only the proportion of what is reported in the literature in our sample. There were 4 (1.1%) cases in which the authors noted maternal deaths more than 30 days postpartum that were included to estimate a 1‐year maternal survival, however, outcomes at 1 year were not uniformly followed nor reported and this may be an underestimation. We are unable to draw any conclusions about maternal survival over time because of the limited reports prior to 2009. However, the contemporary reports from 2009 to 2019 have a maternal survival rate of 75%. Prospective and detailed reporting with multicenter collaboration may help to better evaluate the use of ECLS in pregnancy including indications, complications, outcomes, and best management strategies for this unique population.
The findings of this study are encouraging but one also has to consider the long‐term functional outcomes in these patients. Although studies suggest that patients who have undergone ECMO are able to achieve reasonable physical and neuropsychologic recovery, functional deficits may persist.26, 27, 28, 29, 30 This is an important consideration in this young population as they may benefit from targeted medical or psychosocial rehabilitation.31 Intracranial neurologic morbidity occurred in 5.3% (n=19) of patients and overall neurologically intact survival in this study was 68.4% (n=245). These findings suggest a lower incidence of poor neurologic outcome and mortality compared with other ECMO studies that cite neurologic morbidity in up to 16.0% of patients.17, 19, 32, 33 Another limitation to this study is the lack of information on fetal outcomes as they were only reported in 84.0% (n=68) of antepartum cases. With these data alone, it is challenging to accurately predict the fetal risk portended with maternal ECLS. Prospective studies may better elucidate the association of maternal and fetal outcomes with ECLS use during pregnancy.
These limitations notwithstanding, our findings prompt providers to consider the use of ECLS during the peripartum period as a potentially lifesaving intervention for patients with cardiac and/or respiratory failure. Centers that manage high‐risk pregnancies, particularly those that include women with congenital heart disease, cardiovascular disease, and/or severe respiratory illness, should be prepared to initiate and manage patients with ECLS in the event of a devastating cardiopulmonary event during the peripartum period.
Conclusions
ECLS in the peripartum period has been successfully utilized with a maternal survival rate of 75.4% and should be considered in catastrophic cardiopulmonary conditions. Pregnancy brings a set of challenges and unique considerations for ECLS. At present, there are no formal guidelines to lead physicians to best manage these patients and future directions of research include optimal anticoagulation strategies, choice of cannulation sites, fetal monitoring, and/or method and timing of delivery. Future studies may want to assess long‐term outcomes of neonates born to women who underwent ECLS during the antepartum period. Moving forward, to more clearly assess outcomes, an emphasis should be made on reporting all cases of ECLS in pregnancy in a prospective manner with more granular case details to establish an inclusive assessment of outcomes and complications in this unique population.
Sources of Funding
This work was supported by the University of Michigan Department of Anesthesiology.
Disclosures
None.
Supporting information
Data S1 Tables S1–S3 References 7, 8, 9, 23, and 34–250
Acknowledgments
The authors would like to thank Dana Rector, Madeline McCabe, Dana Labuda, and Jasmine Purtell (Research Assistants, University of Michigan, funded by the Department of Anesthesiology) for their administrative assistance with this systematic review and Ruth Cassidy (Senior Statistician, University of Michigan, funded by the Department of Anesthesiology) for her assistance with statistical consultation with this review.
(J Am Heart Assoc. 2020;9:e016072 DOI: 10.1161/JAHA.119.016072.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy‐related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Severe maternal morbidity in the United States. 2019.
- 3. Joseph KS, Lisonkova S, Muraca GM, Razaz N, Sabr Y, Mehrabadi A, Schisterman EF. Factors underlying the temporal increase in maternal mortality in the United States. Obstet Gynecol. 2017;129:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agrawal P. Maternal mortality and morbidity in the United States of America. Bull World Health Organ. 2015;93:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth. 2019;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bateman BT, Bansil P, Hernandez‐Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore SA, Dietl CA, Coleman DM. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016;151:1154–1160. [DOI] [PubMed] [Google Scholar]
- 8. Sharma NS, Wille KM, Bellot SC, Diaz‐Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J. 2015;61:110–114. [DOI] [PubMed] [Google Scholar]
- 9. Anselmi A, Ruggieri VG, Letheulle J, Robert AL, Tomasi J, Le Tulzo Y, Verhoye JP, Flecher E. Extracorporeal membrane oxygenation in pregnancy. J Card Surg. 2015;30:781–786. [DOI] [PubMed] [Google Scholar]
- 10. Saad AF, Rahman M, Maybauer DM, Fraser JF, Costantine MM, Pacheco LD, Maybauer MO. Extracorporeal membrane oxygenation in pregnant and postpartum women with H1N1‐related acute respiratory distress syndrome: a systematic review and meta‐analysis. Obstet Gynecol. 2016;127:241–247. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 12. Levels of neonatal care. Pediatrics. 2012;130:587–597. [DOI] [PubMed] [Google Scholar]
- 13. Swol J, Brodie D, Napolitano L, Park PK, Thiagarajan R, Barbaro RP, Lorusso R, McMullan D, Cavarocchi N, Hssain AA, et al. Indications and outcomes of extracorporeal life support in trauma patients. J Trauma Acute Care Surg. 2018;84:831–837. [DOI] [PubMed] [Google Scholar]
- 14. Haas NL, Coute RA, Hsu CH, Cranford JA, Neumar RW. Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out‐of‐hospital cardiac arrest—an ELSO registry study. Resuscitation. 2017;119:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yeo HJ, Kim D, Jeon D, Kim YS, Rycus P, Cho WH. Extracorporeal membrane oxygenation for life‐threatening asthma refractory to mechanical ventilation: analysis of the Extracorporeal Life Support Organization registry. Crit Care. 2017;21:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ELSO registry report. July 2019.
- 17. Wang L, Wang H, Hou X. Clinical outcomes of adult patients who receive extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock: a systematic review and meta‐analysis. J Cardiothorac Vasc Anesth. 2018;32:2087–2093. [DOI] [PubMed] [Google Scholar]
- 18. Mhyre JM, Tsen LC, Einav S, Kuklina EV, Leffert LR, Bateman BT. Cardiac arrest during hospitalization for delivery in the United States, 1998–2011. Anesthesiology. 2014;120:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryu JA, Chung CR, Cho YH, Sung K, Jeon K, Suh GY, Park TK, Lee JM, Song YB, Hahn JY, et al. Neurologic outcomes in patients who undergo extracorporeal cardiopulmonary resuscitation. Ann Thorac Surg. 2019;108:749–755. [DOI] [PubMed] [Google Scholar]
- 20. Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158–1167; discussion 1167–9. [DOI] [PubMed] [Google Scholar]
- 21. Benson MD. Amniotic fluid embolism mortality rate. J Obstet Gynaecol Res. 2017;43:1714–1718. [DOI] [PubMed] [Google Scholar]
- 22. Knight M, Berg C, Brocklehurst P, Kramer M, Lewis G, Oats J, Roberts CL, Spong C, Sullivan E, van Roosmalen J, et al. Amniotic fluid embolism incidence, risk factors and outcomes: a review and recommendations. BMC Pregnancy Childbirth. 2012;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meng ML, Landau R, Viktorsdottir O, Banayan J, Grant T, Bateman B, Smiley R, Reitman E. Pulmonary hypertension in pregnancy: a report of 49 cases at four tertiary north American sites. Obstet Gynecol. 2017;129:511–520. [DOI] [PubMed] [Google Scholar]
- 24. Minicucci S, Segala V, Verdecchia C, Sismondi P, Casabona R, Sansone F. Safe management of cesarean section in a patient of Eisenmenger syndrome. Ann Card Anaesth. 2012;15:296–298. [DOI] [PubMed] [Google Scholar]
- 25. Rosenzweig EB, Biscotti M, Cleary K, Smiley R, Bacchetta MD. Chronic thromboembolic pulmonary hypertension, pregnancy, and a pulmonary endarterectomy: a rare challenge. Pulm Circ. 2016;6:384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Norkiene I, Jovaisa T, Scupakova N, Janusauskas V, Rucinskas K, Serpytis P, Laurusonis K, Samalavicius R. Long‐term quality of life in patients treated with extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock. Perfusion. 2019;34:285–289. [DOI] [PubMed] [Google Scholar]
- 27. Sylvestre A, Adda M, Maltese F, Lannelongue A, Daviet F, Parzy G, Coiffard B, Roch A, Loundou A, Baumstarck K, et al. Long‐term neurocognitive outcome is not worsened by of the use of venovenous ECMO in severe ARDS patients. Ann Intensive Care. 2019;9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grasselli G, Scaravilli V, Tubiolo D, Russo R, Crimella F, Bichi F, Corinna Morlacchi L, Scotti E, Patrini L, Gattinoni L, et al. Quality of life and lung function in survivors of extracorporeal membrane oxygenation for acute respiratory distress syndrome. Anesthesiology. 2019;130:572–580. [DOI] [PubMed] [Google Scholar]
- 29. von Bahr V, Kalzen H, Hultman J, Frenckner B, Andersson C, Mosskin M, Eksborg S, Holzgraefe B. Long‐term cognitive outcome and brain imaging in adults after extracorporeal membrane oxygenation. Crit Care Med. 2018;46:e351–e358. [DOI] [PubMed] [Google Scholar]
- 30. Roll MA, Kuys S, Walsh JR, Tronstad O, Ziegenfuss MD, Mullany DV. Long‐term survival and health‐related quality of life in adults after extra corporeal membrane oxygenation. Heart Lung Circ. 2019;28:1090–1098. [DOI] [PubMed] [Google Scholar]
- 31. Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Leger P, Pavie A, Chastre J. Outcomes and long‐term quality‐of‐life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36:1404–1411. [DOI] [PubMed] [Google Scholar]
- 32. Klinzing S, Wenger U, Stretti F, Steiger P, Rushing EJ, Schwarz U, Maggiorini M. Neurologic injury with severe adult respiratory distress syndrome in patients undergoing extracorporeal membrane oxygenation: a single‐center retrospective analysis. Anesth Analg. 2017;125:1544–1548. [DOI] [PubMed] [Google Scholar]
- 33. Lorusso R, Barili F, Mauro MD, Gelsomino S, Parise O, Rycus PT, Maessen J, Mueller T, Muellenbach R, Belohlavek J, et al. In‐hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. 2016;44:e964–e972. [DOI] [PubMed] [Google Scholar]
- 34. Abbal B, Perbet S, Jabaudon M, Legault B, Gallot D, Constantin JM. [Extracorporeal membrane oxygenation for refractory hypoxia secondary to a severe viral pneumonia due to influenza A (H1N1) in a pregnant woman: continuation or termination of pregnancy?]. Ann Fr Anesth Reanim. 2014;33:55–57. [DOI] [PubMed] [Google Scholar]
- 35. Abid Memon H, Safdar Z, Goodarzi A. Use of extracorporeal membrane oxygenation in postpartum management of a patient with pulmonary arterial hypertension. Case Rep Pulmonol. 2018;2018:7031731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agerstrand C, Abrams D, Biscotti M, Moroz L, Rosenzweig EB, D'Alton M, Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for cardiopulmonary failure during pregnancy and postpartum. Ann Thorac Surg. 2016;102:774–779. [DOI] [PubMed] [Google Scholar]
- 37. Alamo M, Castro J, Alvarez E, Brunet L. [Extracorporeal membrane oxygenation in adult respiratory distress syndrome. Experience in 2 patients]. Rev Med Chil. 1995;123:1275–1283. [PubMed] [Google Scholar]
- 38. Alyamani O, Mazzeffi MA, Bharadwaj S, Galey JH, Yao R, Shah NG, Malinow AM. Venovenous extracorporeal membrane oxygenation to prolong pregnancy: a case report. A A Pract. 2018;10:229–231. [DOI] [PubMed] [Google Scholar]
- 39. Amancio RT, Acra CM, Souza Dantas VC. Extra‐corporeal membrane oxygenation as an indispensable tool for a successful treatment of a pregnant woman with H1N1 infection in Brazil. Respir Med Case Rep. 2017;20:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arlt M, Philipp A, Iesalnieks I, Kobuch R, Graf BM. Successful use of a new hand‐held ECMO system in cardiopulmonary failure and bleeding shock after thrombolysis in massive post‐partal pulmonary embolism. Perfusion. 2009;24:49–50. [DOI] [PubMed] [Google Scholar]
- 41. Barbalic B, Tokmadzic VS, Petrovic O, Kricka IB, Sustic A. Life saving use of ECMO in an obstetric patient with massive hemorrhage following uterine atony: a case report. Signa Vitae. 2016;12:121–124. [Google Scholar]
- 42. Barnes L, Passarelli PJ, Hsiao R, Sell RE. Surfactant protein C deficiency and rheumatoid arthritis interstitial lung disease in pregnancy—a fatal combination. Am J Respir Crit Care Med. 2018;197: A1541. MeetingAbstracts. [Google Scholar]
- 43. Bartlett RH, Gazzaniga AB, Fong SW, Burns NE. Prolonged extracorporeal cardiopulmonary support in man. J Thorac Cardiovasc Surg. 1974;68:918–932. [PubMed] [Google Scholar]
- 44. Bataillard A, Hebrard A, Gaide‐Chevronnay L, Casez M, Dessertaine G, Durand M, Chavanon O, Albaladejo P. Extracorporeal life support for massive pulmonary embolism during pregnancy. Perfusion. 2016;31:169–171. [DOI] [PubMed] [Google Scholar]
- 45. Ortiz‐Bautista C, Lopez‐Gude MJ, Grande Garcia J, Perez‐Vela JL, Perez‐Gonzalez V, Escribano‐Subias P. Extracorporeal membrane oxygenation support during pregnancy in pulmonary veno‐occlusive disease. Rev Esp Cardiol. 2018;07:07. [DOI] [PubMed] [Google Scholar]
- 46. Bellissima P, Bellissima G. [Pulmonary complications from pandemic AH1N1 influenza: clinical‐radiological features]. Infez Med. 2011;19:20–27. [PubMed] [Google Scholar]
- 47. Benetis R, Nadisauskiene R, Sirvinskas E, Lenkutis T, Siudikas A, Kadusauskaite V, Railaite D, Sukovas A, Abraitis V. Successfully treated severe obstetric sepsis and acute respiratory distress syndrome with extracorporeal membrane oxygenation. Perfusion. 2016;31:343–346. [DOI] [PubMed] [Google Scholar]
- 48. Besnier E, Hubscher C, Doguet F, Bessou JP, Dureuil B. [Extracorporeal membrane oxygenation support for tako‐tsubo syndrome after urgent caesarean section]. Ann Fr Anesth Reanim. 2013;32:704–706. [DOI] [PubMed] [Google Scholar]
- 49. Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Leger P, Pavie A, Combes A, Leprince P. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac‐RESCUE program). Eur Heart J. 2013;34:112–120. [DOI] [PubMed] [Google Scholar]
- 50. Biderman P, Carmi U, Setton E, Fainblut M, Bachar O, Einav S. Maternal salvage with extracorporeal life support: lessons learned in a single center. Anesth Analg. 2017;125:1275–1280. [DOI] [PubMed] [Google Scholar]
- 51. Bok JS, Jun JH, Lee HJ, Park IK, Kang CH, Yang J, Kim YT. A successful bilateral lung transplantation in a patient with high panel reactive antibody and positive cross matching. Korean J Thorac Cardiovasc Surg. 2014;47:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bouabdallaoui N, Demondion P, Leprince P, Lebreton G. Short‐term mechanical circulatory support for cardiogenic shock in severe peripartum cardiomyopathy: La Pitie‐Salpetriere experience. Interact Cardiovasc Thorac Surg. 2017;25:52–56. [DOI] [PubMed] [Google Scholar]
- 53. Bowkalow S, Brauer M, Gross W, Schleussner E. Severe H1N1‐infection during pregnancy. Arch Gynecol Obstet. 2011;284:1133–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bruch C, Paraforos A, Kunitz O. [Case report: extracorporeal life support for treatment of fulminant pulmonary embolism in a young woman during pregnancy]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2013;48:682–684. [DOI] [PubMed] [Google Scholar]
- 55. Cerene A, Barthelemy R, Vives M. Respiratory support by long term extracorporeal circulation (with a report of a successfully treated case of Mendelson's syndrome). Ann Chir. 1977;31:35–37. [PubMed] [Google Scholar]
- 56. Chambers J, Smith N, Sehring M, Chittivelu S. Acute chest syndrome progressing to ARDS in a patient of 25‐week gestation. Case Rep Crit Care. 2018;2018:4243569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Champion S, Belcour D, Gaüzère BA. Thrombotic thrombocytopenic purpura presenting as a severe peripartum cardiogenic shock: role of myocardial biopsy and assist device for diagnosis and resuscitation. Eur Heart J Acute Cardiovasc Care. 2017;2048872617740829. [DOI] [PubMed] [Google Scholar]
- 58. Chao AS, Chang YL, Lien R, Tsai FC, Wu TS, Chiu TF, Chao A. Successful childbirth after extracorporeal membrane oxygenation in previous pregnancy: two case reports. Eur J Obstet Gynecol Reprod Biol. 2016;198:168–169. [DOI] [PubMed] [Google Scholar]
- 59. Chehab O, Simon A. Rapid onset cardiogenic shock secondary to a case of peripartum cardiomyopathy. Eur J Heart Fail. 2015;17(suppl 1):22–23. [Google Scholar]
- 60. Chillcott S, Sheridan PS. ECCO2R: an experimental approach to treating ARDS. Crit Care Nurse. 1995;15:50–56. [PubMed] [Google Scholar]
- 61. Cho FN, Chen SN. Use of extracorporeal membrane oxygenation to manage post‐partum pulmonary haemorrhage associated with pulmonary hypertension and aberrant right pulmonary artery. Aust N Z J Obstet Gynaecol. 2006;46:556–558. [DOI] [PubMed] [Google Scholar]
- 62. Choi CW, Lim JW, Kim TH. Re: microcirculation in women with severe pre‐eclampsia and HELLP syndrome: a case control study: effect of timing of extracorporeal membrane oxygenation during HELLP syndrome. BJOG. 2016;123:1710. [DOI] [PubMed] [Google Scholar]
- 63. Chuang CJ, Hsu CS. Successful application of extracorporeal membrane oxygenation and pulmonary thromboembolectomy in a patient with a life‐threatening pulmonary embolism. Taiwan J Obstet Gynecol. 2015;54:467–468. [DOI] [PubMed] [Google Scholar]
- 64. Clark GP, Dobson PM, Thickett A, Turner NM. Chickenpox pneumonia, its complications and management. A report of three cases, including the use of extracorporeal membrane oxygenation. Anaesthesia. 1991;46:376–380. [DOI] [PubMed] [Google Scholar]
- 65. Clifford C, Mhatre M, Craigo S. Successful use of extracorporeal membrane oxygenation for status asthmaticus in a woman with a periviable pregnancy. Obstet Gynecol. 2018;17:17. [DOI] [PubMed] [Google Scholar]
- 66. Coscia AP, Cunha HF, Longo AG, Martins EG, Saddy F, Japiassu AM. Report of two cases of ARDS patients treated with pumpless extracorporeal interventional lung assist. J Bras Pneumol. 2012;38:408–411. [DOI] [PubMed] [Google Scholar]
- 67. Courouble P, Geukens P, Laarbaui F, Beauloye C, Van Caenegem O, Jacquet LM. Adult respiratory distress syndrome caused by 2009 H1N1 influenza during pregnancy: success of ECMO for both the mother and the child. J Extra Corpor Technol. 2011;43:75–78. [PMC free article] [PubMed] [Google Scholar]
- 68. Crawford TC, Grimm JC, Magruder JT, Stephens RS, Sciortino CM, Vaught AJ, Althaus J, Shah AS, Kim BS. A curious case of acute respiratory distress syndrome. J Surg Case Rep. 2015;11:09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al‐Samarrai T, Schwarz AG, Chu SY, Sackoff JE, Jamieson DJ, Fine AD, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–726. [DOI] [PubMed] [Google Scholar]
- 70. Cunningham JA, Devine PC, Jelic S. Extracorporeal membrane oxygenation in pregnancy. Obstet Gynecol. 2006;108:792–795. [DOI] [PubMed] [Google Scholar]
- 71. Dabas N, MacOn C, Loebe M, Brozzi N, Ghodsizad A, Badiye A. Transposition of the great arteries, pregnancy, cardiac arrest and ECMO: a challenging case. J Am Coll Cardiol. 2018;71(11 supplement 1):A2476. [Google Scholar]
- 72. Di Lorenzo G, Martucci G, Sciacca S, Longo R, Pilato M, Arcadipane A. Dysfunction of mechanical mitral prosthesis at 33rd week of pregnancy: ECMO support as a complex strategy for the mother and the fetus. Perfusion. 2016;31:611–613. [DOI] [PubMed] [Google Scholar]
- 73. Ecker JL, Solt K, Fitzsimons MG, MacGillivray TE. Case records of the Massachusetts General Hospital. Case 40‐2012. A 43‐year‐old woman with cardiorespiratory arrest after a cesarean section. N Engl J Med. 2012;367:2528–2536. [DOI] [PubMed] [Google Scholar]
- 74. Ellington SR, Hartman LK, Acosta M, Martinez‐Romo M, Rubinson L, Jamieson DJ, Louie J. Pandemic 2009 influenza A (H1N1) in 71 critically ill pregnant women in California. Am J Obstet Gynecol. 2011;204:S21–S30. [DOI] [PubMed] [Google Scholar]
- 75. Erb CT, Black JD. Successful treatment of severe peripartum cardiomyopathy with intra‐aortic balloon pump (IABP), biventricular assist device (BiVAD), and extra‐corporeal membrane oxygenation (ECMO). Am J Respir Crit Care Med. 2013;187:A4430.MeetingAbstracts. [Google Scholar]
- 76. Evans R. Post‐partum spontaneous coronary artery dissection and the use of veno‐arterial extra‐corporeal membrane oxygenation. Nurs Crit Care. 2014;19:304–309. [DOI] [PubMed] [Google Scholar]
- 77. Ezri T, Golan A, Sasson L, Rozenman Y. Pheochromocytoma induced fulminant cardiogenic shock following laparoscopic salpingectomy, successfully managed with extracorporeal membrane oxygenation. Jurnalul Roman de Anestezie Terapie Intensiva. 2009;16:154–158. [Google Scholar]
- 78. Fabricius AM, Autschbach R, Doll N, Mohr W. Acute aortic dissection during pregnancy. Thorac Cardiovasc Surg. 2001;49:56–57. [DOI] [PubMed] [Google Scholar]
- 79. Faerber G, Kirov H, Sandhaus T, Doenst T. Successful weaning and explantation of the HeartMate3 left ventricular assist device using a pre‐designed metal plug. J Heart Lung Transplant. 2018;37:S288. [Google Scholar]
- 80. Fang ZA, Van Diepen S; Royal Alexandra H and University of Alberta Hospital Cardiac Arrest T . Successful inter‐hospital transfer for extracorporeal membrane oxygenation after an amniotic fluid embolism induced cardiac arrest. Can J Anaesth. 2016;63:507–508. [DOI] [PubMed] [Google Scholar]
- 81. Fayad G, Larrue B, Modine T, Azzaoui R, Regnault A, Koussa M, Gourlay T, Fourrier F, Decoene C, Warembourg H. Extracorporeal membrane oxygenation in severe acute respiratory failure in postpartum woman with rheumatic mitral valve disease: benefit, factors furthering the success of this procedure, and review of the literature. J Extra Corpor Technol. 2007;39:112–116. [PMC free article] [PubMed] [Google Scholar]
- 82. Fernandes P, Allen P, Valdis M, Guo L. Successful use of extracorporeal membrane oxygenation for pulmonary embolism, prolonged cardiac arrest, post‐partum: a cannulation dilemma. Perfusion. 2015;30:106–110. [DOI] [PubMed] [Google Scholar]
- 83. Fernandez L, Sua LF, Garcia L, Velasquez M, Garcia C. A fatal case of giant cell pulmonary sarcomatoid carcinoma diagnosed in a pregnant woman: case report. Am J Respir Crit Care Med. 2015;191:A3621. MeetingAbstracts. [Google Scholar]
- 84. Firstenberg MS, Abel E, Blais D, Louis LB, Steinberg S, Sai‐Sudhakar C, Martin S, Sun B. The use of extracorporeal membrane oxygenation in severe necrotizing soft tissue infections complicated by septic shock. Am Surg. 2010;76:1287–1289. [DOI] [PubMed] [Google Scholar]
- 85. Fuchs A, McLaren R, Saunders P, Karakash S, Minkoff H. Human metapneumovirus infection and acute respiratory distress syndrome during pregnancy. Obstet Gynecol. 2017;130:630–632. [DOI] [PubMed] [Google Scholar]
- 86. Futoran JM, Hill JD. Pulmonary insufficiency associated with pregnancy. Am J Obstet Gynecol. 1975;121:637–640. [DOI] [PubMed] [Google Scholar]
- 87. Garcia‐Aranda Dominguez B, Diaz‐Anton B, Villar OP, Garcia‐Burguillo A, Hernandez‐Gonzalez I, Quezada A, Real MI, Martin‐Asenjo R, Lopez‐Gude MJ, Escribano‐Subias P. Outcome of pregnancy in patients with cardiovascular disease, experience in a centre. Eur Heart J. 2017;38(supplement 1):351. [Google Scholar]
- 88. Gattinoni L, Agostoni A, Pesenti A, Pelizzola A, Rossi GP, Langer M, Vesconi S, Uziel L, Fox U, Longoni F, et al. Treatment of acute respiratory failure with low‐frequency positive‐pressure ventilation and extracorporeal removal of CO2 . Lancet. 1980;2:292–294. [DOI] [PubMed] [Google Scholar]
- 89. Gauzere BA, Bussienne F, Bouchet B, Jabot J, Roussiaux A, Drouet D, Djourhi S, Leaute B, Belcour D, Bossard G, et al. [Severe cases of A (H1N1) v2009 infection in Reunion Island in 2009 and 2010]. Bull Soc Pathol Exot. 2011;104:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gevaert S, Van Belleghem Y, Bouchez S, Herck I, De Somer F, De Block Y, Tromp F, Vandecasteele E, Martens F, De Pauw M. Acute and critically ill peripartum cardiomyopathy and ‘bridge to’ therapeutic options: a single center experience with intra‐aortic balloon pump, extra corporeal membrane oxygenation and continuous‐flow left ventricular assist devices. Crit Care. 2011;15:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gijs J, Lambert J, Meyfroidt G, Demeestere J. Cerebral microbleeds and intracerebral hemorrhage associated with veno‐venous extracorporeal membrane oxygenation. Acta Neurol Belg. 2018;118:513–515. [DOI] [PubMed] [Google Scholar]
- 92. Goto M, Watanabe H, Ogita K, Matsuoka T. Perimortem cesarean delivery and subsequent emergency hysterectomy: new strategy for maternal cardiac arrest. Acute Med. 2017;4:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Grasselli G, Bombino M, Patroniti N, Giuffrida A, Marcolin R, Vergani P, Pesenti A. Use of extracorporeal respiratory support during pregnancy: a case report and literature review. ASAIO J. 2012;58:281–284. [DOI] [PubMed] [Google Scholar]
- 94. Greenberg LR, Moore TR. Staphylococcal septicemia and adult respiratory distress syndrome in pregnancy treated with extracorporeal carbon dioxide removal. Obstet Gynecol. 1995;86:657–660. [DOI] [PubMed] [Google Scholar]
- 95. Grimme I, Winter R, Kluge S, Petzoldt M. Hypoxic cardiac arrest in pregnancy due to pulmonary haemorrhage. BMJ Case Rep. 2012;24:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guenther SPW, Reichelt A, Buchholz S, Born F, Fischer M, Schramm R, Pichlmaier AM, Hagl C, Khaladj N. Fulminant amniotic fluid embolism. Mobile ECLS team in the delivery ward. Z Herz Thorax Gefasschir. 2015;29:312–316. [Google Scholar]
- 97. Guillaume A, Sananes N, Akladios CY, Boudier E, Diemunsch P, Averous G, Nisand I, Langer B. Amniotic fluid embolism: 10‐year retrospective study in a level III maternity hospital. Eur J Obstet Gynecol Reprod Biol. 2013;169:189–192. [DOI] [PubMed] [Google Scholar]
- 98. Halldorsdottir H, Nordstrom J, Brattstrom O, Sennstrom MM, Sartipy U, Mattsson E. Early postpartum mitral valve thrombosis requiring extra corporeal membrane oxygenation before successful valve replacement. Int J Obstet Anesth. 2016;26:75–78. [DOI] [PubMed] [Google Scholar]
- 99. Hamdan R, Nassar P, Zein A, Issa M, Mansour H, Saab M. Peripartum cardiomyopathy, place of drug therapy, assist devices, and outcome after left ventricular assistance. J Crit Care. 2017;37:185–188. [DOI] [PubMed] [Google Scholar]
- 100. Hansen AJ, Sorrell VL, Cooper AD, Moulton MJ. Postpartum rupture of the posteromedial papillary muscle. J Card Surg. 2012;27:313–316. [DOI] [PubMed] [Google Scholar]
- 101. Hara R, Hara S, Ong CS, Schwartz G, Sciortino C, Hibino N. Cesarean section in the setting of severe pulmonary hypertension requiring extracorporeal life support. Gen Thorac Cardiovasc Surg. 2017;65:532–534. [DOI] [PubMed] [Google Scholar]
- 102. Herrero T, Martin E, Poch DS, Roeder HA. Anti‐coagulation complications in pregnancies with severe pulmonary arterial hypertension. J Matern Fetal Neonatal Med. 2018;31:1209–1213. [DOI] [PubMed] [Google Scholar]
- 103. Hill JD, Ratliff JL, Fallat RJ, Tucker HJ, Lamy M, Dietrich HP, Gerbode F. Prognostic factors in the treatment of acute respiratory insufficiency with long‐term extracorporeal oxygenation. J Thorac Cardiovasc Surg. 1974;68:905–917. [PubMed] [Google Scholar]
- 104. Ho CH, Chen KB, Liu SK, Liu YF, Cheng HC, Wu RS. Early application of extracorporeal membrane oxygenation in a patient with amniotic fluid embolism. Acta Anaesthesiol Taiwan. 2009;47:99–102. [DOI] [PubMed] [Google Scholar]
- 105. Ho YK, Wang CP, Wu YL, Lee TH, Ying TH, Lee MS. Pulmonary embolism after cesarean section and successful treatment with early application of extracorporeal membrane oxygenation system and anticoagulant agents. Taiwan J Obstet Gynecol. 2014;53:273–275. [DOI] [PubMed] [Google Scholar]
- 106. Holzgraefe B, Broome M, Kalzen H, Konrad D, Palmer K, Frenckner B. Extracorporeal membrane oxygenation for pandemic H1N1 2009 respiratory failure. Minerva Anestesiol. 2010;76:1043–1051. [PubMed] [Google Scholar]
- 107. Hou X, Guo L, Zhan Q, Jia X, Mi Y, Li B, Sun B, Hao X, Li H. Extracorporeal membrane oxygenation for critically ill patients with 2009 influenza A (H1N1)‐related acute respiratory distress syndrome: preliminary experience from a single center. Artif Organs. 2012;36:780–786. [DOI] [PubMed] [Google Scholar]
- 108. Hsieh YY, Chang CC, Li PC, Tsai HD, Tsai CH. Successful application of extracorporeal membrane oxygenation and intra‐aortic balloon counterpulsation as lifesaving therapy for a patient with amniotic fluid embolism. Am J Obstet Gynecol. 2000;183:496–497. [DOI] [PubMed] [Google Scholar]
- 109. Huang KY, Li YP, Lin SY, Shih JC, Chen YS, Lee CN. Extracorporeal membrane oxygenation application in post‐partum hemorrhage patients: is post‐partum hemorrhage contraindicated? J Obstet Gynaecol Res. 2017;43:1649–1654. [DOI] [PubMed] [Google Scholar]
- 110. Huang HC, Chang SC, Huang CS. Airway exchange catheter used in a lymphoma patient with acute respiratory failure who failed to be mechanically ventilated. Am J Respir Crit Care Med. 2017;195:A3621. [Google Scholar]
- 111. Hur JA. A fatal case in pregnant woman infected by H1N1 2009 in Korea. Infect Chemother. 2011;43:225–228. [Google Scholar]
- 112. Imaeda T, Nakada TA, Abe R, Tateishi Y, Oda S. Veno‐arterial extracorporeal membrane oxygenation for Streptococcus pyogenes toxic shock syndrome in pregnancy. J Artif Organs. 2016;19:200–203. [DOI] [PubMed] [Google Scholar]
- 113. Isbir S, Ak K, Aslantas M, Kepez A, Cinel I, Arsan S. Peripartum cardiomyopathy mimicking acute aortic dissection: successful salvage with extracorporeal membrane oxygenation support. Turk Gogus Kalp Damar Cerrahisi Derg. 2014;22:843–846. [Google Scholar]
- 114. Itagaki T, Onodera M, Okuda N, Nakataki E, Imanaka H, Nishimura M. Successful use of extracorporeal membrane oxygenation in the reversal of cardiorespiratory failure induced by atonic uterine bleeding: a case report. J Med Case Rep. 2014;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ius F, Sommer W, Tudorache I, Avsar M, Siemeni T, Salman J, Puntigam J, Optenhoefel J, Greer M, Welte T, et al. Veno‐veno‐arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: technique and early outcomes. Interact Cardiovasc Thorac Surg. 2015;20:761–767. [DOI] [PubMed] [Google Scholar]
- 116. Jacquens A, Kerever S, Guidet B, Aegerter P, Das V, Cariou A, Fartoukh M, Hayon J, Combes A, De Montmollin E, et al. Pneumonia during pregnancy and the post partum period: a reappraisal of etiologies requiring intermediate or intensive care‐a retrospective study through the CUBREA network. Ann Intensive Care. 2017;7:177–178. [Google Scholar]
- 117. Jais X, Olsson KM, Barbera JA, Blanco I, Torbicki A, Peacock A, Vizza CD, Macdonald P, Humbert M, Hoeper MM. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J. 2012;40:881–885. [DOI] [PubMed] [Google Scholar]
- 118. Jandhyala R, Haydon P, Czaplicka C, Claprood C, Casey K. Successful vaginal delivery of a male infant during extracorporeal carbon dioxide removal: a case report. J Extra Corpor Technol. 1994;26:87–90. [Google Scholar]
- 119. Janssen MJ, van de Wetering K, Arabin B. Sepsis due to gestational psittacosis: a multidisciplinary approach within a perinatological center–review of reported cases. Int J Fertil Womens Med. 2006;51:17–20. [PubMed] [Google Scholar]
- 120. Jo YY, Park S, Choi YS. Extracorporeal membrane oxygenation in a patient with stress‐induced cardiomyopathy after caesarean section. Anaesth Intensive Care. 2011;39:954–957. [DOI] [PubMed] [Google Scholar]
- 121. John SG, Carr G, Raz Y. Independent ventilation and ECMO for severe refractory pulmonary edema in a pregnant patient with rheumatic mitral stenosis. Crit Care Med. 2012;40:U336–U337. [Google Scholar]
- 122. Ju YH, Hyun CW, Dong‐Hyung L, Gon KH, Jung SY, Sup BK, Seok KH, Hoon YS, Eun LS, Do Hyung K, et al. The role of extracorporeal membrane oxygenation in obstetric emergencies with unexpected refractory shock. Clin Exp Obstet Gynecol. 2018;45:466–469. [Google Scholar]
- 123. Kapoor A. Pulmonary hypertension (PH) and pregnancy: watch for catastrophe. Am J Respir Crit Care Med. 2012;185:A4625. MeetingAbstracts. [Google Scholar]
- 124. Kaliyev R, Kapyshev T, Goncharov A, Lesbekov T, Pya Y. Maternal and fetal recovery after severe respiratory failure: a case report of air transportation of a pregnant woman on ECMO using the CentriMag transporter system. ASAIO J. 2015;61:e33–e35. [DOI] [PubMed] [Google Scholar]
- 125. King PT, Rosalion A, McMillan J, Buist M, Holmes PW. Extracorporeal membrane oxygenation its pregnancy. Lancet. 2000;356:45–46. [DOI] [PubMed] [Google Scholar]
- 126. Kinni H, Hegab S. Uncommon etiology of diffuse alveolar hemorrhage: hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med. 2018;197:A3714. MeetingAbstracts. [Google Scholar]
- 127. Knapp KE, Weis RA, Cubillo EI, Chapital AB, Ramakrishna H. Spontaneous, postpartum coronary artery dissection and cardiogenic shock with extracorporeal membrane oxygenation assisted recovery in a 30‐year‐old patient. Case Rep Cardiol. 2016;2016:1048708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ko WJ, Ho HN, Chu SH. Postpartum myocardial infarction rescued with an intraaortic balloon pump and extracorporeal membrane oxygenator. Int J Cardiol. 1998;63:81–84. [DOI] [PubMed] [Google Scholar]
- 129. Konstantinidis K, Schulman S. Peripartum management of severe idiopathic pulmonary arterial hypertension and the use of extracorporeal membrane oxygenation during delivery. J Am Coll Cardiol. 2015;65:A646. [Google Scholar]
- 130. International Session Workshop (IS‐WS) . 69th Annual Congress of the Japan Society of Obstetrics and Gynecology. Hiroshima, Japan, 13–16 April, 2017. J Obstet Gynaecol Res. 2017;43:1897–1922. [Google Scholar]
- 131. Kumar S, Shumaster V, Jacoby DA, Mangi A. BiVAD and ECMO for bridge to recovery in peripartum cardiomyopathy. Heart Surg Forum. 2012;15(suppl 1):S109–S110. [Google Scholar]
- 132. Krumnikl JJ, Toller WG, Prenner G, Metzler H. Beneficial outcome after prostaglandin‐induced post‐partum cardiac arrest using levosimendan and extracorporeal membrane oxygenation. Acta Anaesthesiol Scand. 2006;50:768–770. [DOI] [PubMed] [Google Scholar]
- 133. Kunstyr J, Lips M, Belohlavek J, Prskavec T, Mlejnsky F, Koucky M, Sebron V, Balik M. Spontaneous delivery during veno‐venous extracorporeal membrane oxygenation in swine influenza‐related acute respiratory failure. Acta Anaesthesiol Scand. 2010;54:1154–1155. [DOI] [PubMed] [Google Scholar]
- 134. Kutlesa M, Santini M, Krajinovic V, Raffanelli D, Barsic B. Novel observations during extracorporeal membrane oxygenation in patients with ARDS due to the H1N1 pandemic influenza. Wien Klin Wochenschr. 2011;123:117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Law C, Khaliq A, Guglin M. Reversible cardiogenic shock due to catecholamine‐induced cardiomyopathy: a variant of takotsubo? Am J Emerg Med. 2013;31:1621.e1–1621.e3. [DOI] [PubMed] [Google Scholar]
- 136. Lee WA, Kolla S, Schreiner RJ Jr, Hirschl RB, Bartlett RH. Prolonged extracorporeal life support (ECLS) for varicella pneumonia. Crit Care Med. 1997;25:977–982. [DOI] [PubMed] [Google Scholar]
- 137. Lee AJ, Koyyalamudi PL, Martinez‐Ruiz R. Severe transfusion‐related acute lung injury managed with extracorporeal membrane oxygenation (ECMO) in an obstetric patient. J Clin Anesth. 2008;20:549–552. [DOI] [PubMed] [Google Scholar]
- 138. Legrand M, Rossignol M, Muller F, Payen D. [Amniotic fluid embolism: an update]. Ann Fr Anesth Reanim. 2013;32:189–197. [DOI] [PubMed] [Google Scholar]
- 139. Lemaire A, Bonnin M, Storme B, Fournet‐Fayard A, Rosano G, Vernis L, Cayot S, Accocebery M, Dechelotte P, Boyer L, et al. Hemoperitoneum in peripartum: a case‐series. J Neonatal Perinatal Med. 2017;10:451–454. [DOI] [PubMed] [Google Scholar]
- 140. Lembrikov I, Rudis E, Weiniger CF. Postpartum cardiogenic shock diagnosed by focused cardiac ultrasound and treated with venoarterial extracorporeal membrane oxygenation: a case report. A A Pract. 2019;12:277–280. [DOI] [PubMed] [Google Scholar]
- 141. Li HL, Du HY, Jiang YH, Zhao YY, Zhu X, Yao GQ. Nine‐month follow‐up of an acute respiratory distress syndrome caused by 2009 H1N1 influenza during pregnancy. Chin Med J. 2013;126:1999–2000. [PubMed] [Google Scholar]
- 142. Liu C, Sun W, Wang C, Liu F, Zhou M. Delivery during extracorporeal membrane oxygenation (ECMO) support of pregnant woman with severe respiratory distress syndrome caused by influenza: a case report and review of the literature. J Matern Fetal Neonatal Med. 2019;32:2570–2574. [DOI] [PubMed] [Google Scholar]
- 143. Loizos S, Cirillo C, Trimlett R, Price S. A pheochromocytoma‐induced takotsubo syndrome case treated by venoarterial extracorporeal membrane oxygenation. Eur Heart J. 2017;38(supplement 1):1431. [Google Scholar]
- 144. Lueck S, Sindermann J, Martens S, Scherer M. Mechanical circulatory support for patients with peripartum cardiomyopathy. J Artif Organs. 2016;19:305–309. [DOI] [PubMed] [Google Scholar]
- 145. Lund LH, Grinnemo K‐H, Svenarud P, van der Linden J, Eriksson MJ. Myocardial recovery in peri‐partum cardiomyopathy after continuous flow left ventricular assist device. J Cardiothorac Surg. 2011;6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lysenko L, Zaleska‐Dorobisz U, Blok R, Dumanski A, Zielinska M, Kustrzycki W, Durek G. A successful cesarean section in a pregnant woman with A (H1N1) influenza requiring ECMO support. Kardiochir Torakochirurgia Pol. 2014;11:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Eiras Mariño MDM, Taboada Muñiz M, Otero Castro P, Adrio Nazar B, Reija López L, Agra Bermejo R. Venoarterial extracorporeal membrane oxygenation and ventricular assistance with impella CP in an amniotic fluid embolism. Rev Esp Cardiol (Engl Ed). 2019;72:679–680. [DOI] [PubMed] [Google Scholar]
- 148. McDonald C, Laurie J, Janssens S, Zazulak C, Kotze P, Shekar K. Successful provision of inter‐hospital extracorporeal cardiopulmonary resuscitation for acute post‐partum pulmonary embolism. Int J Obstet Anesth. 2017;30:65–68. [DOI] [PubMed] [Google Scholar]
- 149. McKechnie RS, Patel D, Eitzman DT, Rajagopalan S, Murthy TH. Spontaneous coronary artery dissection in a pregnant woman. Obstet Gynecol. 2001;98:899–902. [DOI] [PubMed] [Google Scholar]
- 150. McNamee K, Dawood F. Severe H1N1 virus in pregnancy requiring extracorporeal membrane oxygenation and lobectomy. Obstet Med. 2010;3:156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mendes PV, Moura E, Barbosa EVS, Hirota AS, Scordamaglio PR, Ajjar FM, Costa ELV, Azevedo LCP, Park M. Challenges in patients supported with extracorporeal membrane oxygenation in Brazil. Clinics (Sao Paulo). 2012;67:1511–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Meng ML, Fu A, Westhoff C, Bacchetta M, Rosenzweig EB, Landau R, Smiley R. Eisenmenger syndrome in pregnancy: when is it time for ECMO?: a case report. A A Pract. 2018;11:11. [DOI] [PubMed] [Google Scholar]
- 153. Miessau J, Yang Q, Unai S, Entwistle JW, Cavarocchi NC, Hirose H. Veno‐venous extracorporeal membrane oxygenation using a double‐lumen bi‐caval cannula for severe respiratory failure post total artificial heart implantation. Perfusion. 2015;30:410–414. [DOI] [PubMed] [Google Scholar]
- 154. Mikami T, Kamiunten H. Emergent caesarean section under mechanical circulatory support for acute severe peripartum cardiomyopathy. J Cardiol Cases. 2018;17:200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mita K, Tsugita K, Yasuda Y, Matsuki Y, Obata Y, Matsuki Y, Kamisawa S, Shigemi K. A successfully treated case of cardiac arrest after caesarean section complicated by pheochromocytoma crisis and amniotic fluid embolism. J Anesth. 2017;31:140–143. [DOI] [PubMed] [Google Scholar]
- 156. Morsolini M, Sciacca S, Panarello G, Martucci G, Bertani A, Longo R, Vitulo P, Arcadipane A, Pilato M. Delivery during ECMO: a single‐center case series. J Heart Lung Transplant. 2017;36:S333–S334. [Google Scholar]
- 157. Nair P, Davies AR, Beca J, Bellomo R, Ellwood D, Forrest P, Jackson A, Pye R, Seppelt I, Sullivan E, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med. 2011;37:648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nair L, Oates M, Russell L, Smith I, Thomson B, Wall D, Fiene A. Critical pulmonary arterial hypertension necessitating bilateral lung transplantation. Pulm Circ. 2017;7:272. [Google Scholar]
- 159. Naqvi SY, Yoruk A, Pressman E, Olson‐Chen C, Prasad S, Barrus B, Gosev I, Alexis J, Thomas S. Cardiomyopathy bridged to heart transplant with ambulatory extracorporeal membrane oxygenation in a peripartum patient. J Am Coll Cardiol. 2018;71:2418. [Google Scholar]
- 160. Neurath M, Benzing A, Knolle P, Grundmann H, Dippold W, Zumbuschenfelde KHM. Acute respiratory‐failure in falciparum‐malaria during pregnancy—successful treatment using extracorporeal CO2 elimination. Dtsch Med Wochenschr. 1993;118:1060–1066. [DOI] [PubMed] [Google Scholar]
- 161. Ngatchou W, Ramadan AS, Van Nooten G, Antoine M. Left tilt position for easy extracorporeal membrane oxygenation cannula insertion in late pregnancy patients. Interact Cardiovasc Thorac Surg. 2012;15:285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Noah MA, Dawrant M, Faulkner GM, Hill AM, Harvey C, Hussain A, Jenkins DR, Nichani S, Peek GJ, Sosnowski AW, et al. Panton‐Valentine leukocidin expressing Staphylococcus aureus pneumonia managed with extracorporeal membrane oxygenation: experience and outcome. Crit Care Med. 2010;38:2250–2253. [DOI] [PubMed] [Google Scholar]
- 163. O'Gara PT, Shepard JAO, Yared K, Sohani AR. Case 39‐2009: a 28‐year‐old pregnant woman with acute cardiac failure. N Engl J Med. 2009;361:2462–2473+2404. [DOI] [PubMed] [Google Scholar]
- 164. Pagel PS, Lilly RE, Nicolosi AC. Use of ECMO to temporize circulatory instability during severe Brugada electrical storm. Ann Thorac Surg. 2009;88:982–983. [DOI] [PubMed] [Google Scholar]
- 165. Panarello G, Occhipinti G, Capitanio G, Ferrazza V, Vitulo P, Pilato M, Di Lorenzo G, Arcadipane AF. Treatment of A/H1N1 related severe ARDS by extracorporeal membrane oxygenation in pregnant and postpartum women, during the 2009 flu pandemic. Single center experience. Intensive Care Med. 2010;36(suppl 2):S235. [Google Scholar]
- 166. Panarello G, D'Ancona G, Capitanio G, Occhipinti G, Attardo G, Bertani A, Arcadipane A. Cesarean section during ECMO support. Minerva Anestesiol. 2011;77:654–657. [PubMed] [Google Scholar]
- 167. Park SH, Chin JY, Choi MS, Choi JH, Choi YJ, Jung KT. Extracorporeal membrane oxygenation saved a mother and her son from fulminant peripartum cardiomyopathy. J Obstet Gynaecol Res. 2014;40:1940–1943. [DOI] [PubMed] [Google Scholar]
- 168. Parkins MD, Fonseca K, Peets AD, Laupland KB, Shamseddin K, Gill MJ. A potentially preventable case of serious influenza infection in a pregnant patient. Can Med Assoc J. 2007;177:851–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Paruchuri Y, Hauspurg A, Donadee C, Sakamoto S, Murugan R. Can we achieve low tidal volume ventilation in pregnant women with acute respiratory distress syndrome? Am J Obstet Gynecol. 2018;218:S470–S471. [Google Scholar]
- 170. Patel S, Loveridge R, Willars C, Best T, Gelandt E, Morgan L, Butt S, Auzinger G. First for a child, now for a new mother: extending the reach of emergency liver transplantation by bridging with ECMO—a case report. Eur J Heart Fail. 2017;19:41–42. [Google Scholar]
- 171. Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, et al. The Italian ECMO network experience during the 2009 influenza A (H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Patrut GV, Neamtu C, Ionac M. Leg for life? The use of sartorius muscle flap for the treatment of an infected vascular reconstructions after VA‐ECMO use. A case report. Int J Surg Case Rep. 2015;16:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pechulis R, Miller C, Wu J, Pechulis M, Lindauer L. Extreme physical therapy‐progression of functional mobility of 25 year old on ECMO 72 days. Crit Care Med. 2014;42:A1653. [Google Scholar]
- 174. Pekka H, Markku S, Kalervo W. Case report: ECMO as comprehensive treatment for high risk pulmonary embolism (PE). Int J Artif Organs. 2013;36:279. [Google Scholar]
- 175. Perdue SM, Poore BJ, Babu AN, Stribling WK. Successful use of extracorporeal membrane oxygenation support in severe septic shock with associated acute cardiomyopathy. J Card Surg. 2018;33:50–52. [DOI] [PubMed] [Google Scholar]
- 176. Pereira GB, Barney J. Non‐familial pulmonary alveolar proteinosis in a pregnancy complicated by stenotrophomonas maltophilia. Am J Respir Crit Care Med. 2018;197:A4790. MeetingAbstracts. [Google Scholar]
- 177. Pham T, Combes A, Roze H, Chevret S, Mercat M, Roch A, Mourvillier B, Ara‐Somohano C, Bastien O, Zogheib E, et al. Extracorporeal membrane oxygenation for pandemic influenza A (H1N1)‐induced acute respiratory distress syndrome a cohort study and propensity‐matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. [DOI] [PubMed] [Google Scholar]
- 178. Phillips MR, Klein M, Shah M, Charles AG. Veno‐venous extracorporeal membrane oxygenation in pregnancy: does foetal viability matter? Anaesth Intensive Care. 2017;45:524–525. [PubMed] [Google Scholar]
- 179. Piatrovich NS, Shestakova LG, Ostrovsky YP. Successful application of extracorporeal membrane oxygenation in woman after childbirth. Eur J Heart Fail. 2018;20(supplement 1):174. [Google Scholar]
- 180. Pirsaheli R, Wijman C, Hobbs K, Snider R. Survival of a patient with propofol infusion syndrome and prolonged asystole using ECMO. Neurocrit Care. 2012;17(suppl 2):S185. [Google Scholar]
- 181. Plotkin JS, Shah JB, Lofland GK, DeWolf AM. Extracorporeal membrane oxygenation in the successful treatment of traumatic adult respiratory distress syndrome: case report and review. J Trauma. 1994;37:127–130. [DOI] [PubMed] [Google Scholar]
- 182. Power CA, Van Heerden PV, Moxon D, Martindale G, Roberts B. Extracorporeal membrane oxygenation for critically ill patients with influenza A (H1N1) 2009: a case series. Crit Care Resusc. 2011;13:38–43. [PubMed] [Google Scholar]
- 183. Prakash S, Bihari S, Wiersema U. A rare case of rapidly enlarging tracheal lobular capillary hemangioma presenting as difficult to ventilate acute asthma during pregnancy. BMC Pulm Med. 2014;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Rabin J, Kon Z, Garcia J, Griffith B, Herr D. Successful bridge to transplant after 155 days of V‐V ECMO. Crit Care Med. 2012;40:292. [Google Scholar]
- 185. Rademacher W, Lauten A, Lauten A, Ragoschke‐Schumm A, Figulla HR. Postpartum unmasking of a severe triple‐vessel‐disease with acute myocardial infarction. Clin Res Cardiol. 2010;99:463–466. [DOI] [PubMed] [Google Scholar]
- 186. Radsel P, Gorjup V, Jazbec A, Knafelj R, Lucovnik M, Kavsek G, Kornhauser Cerar L, Noc M. Pregnancy complicated by influenza A ARDS requiring consecutive VV‐ECMO treatment with successful vaginal delivery. J Artif Organs. 2018;17:17. [DOI] [PubMed] [Google Scholar]
- 187. Raley M, Gill B, Grier L, Conrad S. Arteriovenous CO2 removal (AVCO2R) in a postpartum patient with refractory status asthmaticus. Crit Care Med. 2015;43:316. [Google Scholar]
- 188. Ramirez RL, Zarafshar S. A heartbreaking tale: a young pregnant woman presents with acute cardiomyopathy and a surprising cause. J Gen Intern Med. 2018;33:426. [Google Scholar]
- 189. Rein JL, Etra AM, Patel JJ, Stein JL, Rivers AL, Gershengorn HB, Awerbuch E, Kreiswirth BN, Koshy SC. Death of woman with peripartum influenza B virus infection and necrotizing pneumonia. Emerg Infect Dis. 2014;20:1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Reyftmann L, Morau E, Dechaud H, Frapier JM, Hedon B. Extracorporeal membrane oxygenation therapy for circulatory arrest due to postpartum hemorrhage. Obstet Gynecol. 2006;107:511–514. [DOI] [PubMed] [Google Scholar]
- 191. Riddell J IV, Chenoweth CE, Kauffman CA. Disseminated Scedosporium apiospermum infection in a previously healthy woman with HELLP syndrome. Mycoses. 2004;47:442–446. [DOI] [PubMed] [Google Scholar]
- 192. Riester A, Weismann D, Quinkler M, Lichtenauer UD, Sommerey S, Halbritter R, Penning R, Spitzweg C, Schopohl J, Beuschlein F, et al. Life‐threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173:757–764. [DOI] [PubMed] [Google Scholar]
- 193. Robbins T, Kanu C, Lam SJ, Zammit‐Mangion M. Extracorporeal membrane oxygenation in the treatment of an infective exacerbation of asthma in pregnancy. BJOG. 2015;122:42. [Google Scholar]
- 194. Robertson LC, Allen SH, Konamme SP, Chestnut J, Wilson P. The successful use of extra‐corporeal membrane oxygenation in the management of a pregnant woman with severe H1N1 2009 influenza complicated by pneumonitis and adult respiratory distress syndrome. Int J Obstet Anesth. 2010;19:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rodriguez Chaverri A, Ponz De Antonio I, Garcia‐Cosio MD, Moran Fernandez L, De Juan Baguda J, Arribas Ynsaurraga F, Delgado Jimenez JF. A case of advanced heart failure: when not everything is as it seems. Eur J Heart Fail. 2018;20(supplement 1):21. [Google Scholar]
- 196. Roncon‐Albuquerque R, Basilio C, Figueiredo P, Silva S, Mergulhao P, Alves C, Veiga R, Castelo‐Branco S, Paiva L, Santos L, et al. Portable miniaturized extracorporeal membrane oxygenation systems for H1N1‐related severe acute respiratory distress syndrome: a case series. J Crit Care. 2012;27:454–463. [DOI] [PubMed] [Google Scholar]
- 197. Rubin E, Minkin R, Khouli H. Successful use of extracorporeal membrane oxygenation (ECMO) to treat pulmonary thromboembolism and amniotic fluid embolism in a postpartum patient. Am J Respir Crit Care Med. 2013;187:A4431. [Google Scholar]
- 198. Sakamoto C, Miyashita S, Ochiai S, Motegi E, Kuno T, Tada K, Fukasawa I. Perimortem cesarean section (PMCS)‐successful maternal resuscitation of out‐hospital onset cardiopulmonary arrest, a case report. J Obstet Gynaecol Res. 2018;44:1568. [Google Scholar]
- 199. Sakanashi Y, Terasaki H, Tajiri A, Mizoguchi S, Chung IS, Okamoto K, Morioka T. A case of Eisenmenger's syndrome treated with extracorporeal lung and heart assist in the postpartum period. J Anesth. 1994;8:107–109. [DOI] [PubMed] [Google Scholar]
- 200. Salazar LA, Schreuder CM, Eslava JA, Murcia AS, Forero MJ, Orozco‐Levi MA, Echeverria LE, Figueredo AI. Extracorporeal membrane oxygenation in dengue, malaria, and acute Chagas disease. ASAIO J. 2017;63:e71–e76. [DOI] [PubMed] [Google Scholar]
- 201. Satoh H, Masuda Y, Izuta S, Yaku H, Obara H. Pregnant patient with primary pulmonary hypertension: general anesthesia and extracorporeal membrane oxygenation support for termination of pregnancy. Anesthesiology. 2002;97:1638–1640. [DOI] [PubMed] [Google Scholar]
- 202. Scherrer V, Lasgi C, Hariri S, Dureuil B, Savoure A, Tamion F, Doguet F. Radiofrequency ablation under extracorporeal membrane oxygenation for atrial tachycardia in postpartum. J Card Surg. 2012;27:647–649. [DOI] [PubMed] [Google Scholar]
- 203. Scriven J, McEwen R, Mistry S, Green C, Osman H, Bailey M, Ellis C. Swine flu: a Birmingham experience. Clin Med. 2009;9:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Seczynska B, Jankowski M, Nowak I, Szczeklik W, Szuldrzynski K, Krolikowski W. Pregnancy‐related H1N1 influenza and severe acute respiratory distress syndrome successfully treated with extracorporeal membrane oxygenation despite difficult vascular access. Pol Arch Med Wewn. 2014;124:136–137. [DOI] [PubMed] [Google Scholar]
- 205. Seidler D, Franklin M. Metastatic choriocarcinoma to the lung: changing dogma. Chest. 2016;150:765A. [Google Scholar]
- 206. Sellami W, Hajjej Z, Ferjani M. Fulminant acute myocarditis in a pregnant woman treated with circulatory assistance. Reanimation. 2017;26:523–527. [Google Scholar]
- 207. Seong GM, Kim SW, Kang HS, Kang HW. Successful extracorporeal cardiopulmonary resuscitation in a postpartum patient with amniotic fluid embolism. J Thorac Dis. 2018;10:E189–E193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Seth B, Gonzalez M, Schembri F. Status asthmaticus in pregnancy requiring sevoflurane and ECMO. Am J Respir Crit Care Med. 2018;197:A5242. MeetingAbstracts. [Google Scholar]
- 209. Shah N, Perrin F. Extracorporeal membrane oxygenation (ECMO) in cystic fibrosis. Paediatr Respir Rev. 2014;15(suppl 1):26–28. [DOI] [PubMed] [Google Scholar]
- 210. Shamsah M, Alfoudri H. Extracorporeal membrane oxygenation for cardiac arrest in pregnancy secondary to severe pulmonary hypertension. International Journal of Obstetric Anesthesia. 2017;31(supplement 1):S59. [Google Scholar]
- 211. Shen HP, Chang WC, Yeh LS, Ho M. Amniotic fluid embolism treated with emergency extracorporeal membrane oxygenation: a case report. J Reprod Med. 2009;54:706–708. [PubMed] [Google Scholar]
- 212. Shuang L, Yang C, Li Z, Lin S, Li Z, Zhou J, Mai Y. Clinical analysis of the first maternal patient infected with novel avian influenza A (H5N6) virus in the world. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016;28:988–993. [Google Scholar]
- 213. Sim SS, Chou HC, Chen JW, Ma MH. Extracorporeal membrane oxygenation in maternal arrhythmic cardiogenic shock. Am J Emerg Med. 2012;30:1012.e3–5. [DOI] [PubMed] [Google Scholar]
- 214. Skolnik S, Ioscovich A, Eidelman LA, Davis A, Shmueli A, Aviram A, Orbach‐Zinger S. Anesthetic management of amniotic fluid embolism—a multi‐center, retrospective, cohort study. J Matern Fetal Neonatal Med. 2019;32:1262–1266. [DOI] [PubMed] [Google Scholar]
- 215. Smiechowicz J, Barteczko B, Grotowska M, Kaiser T, Zielinski S, Kubler A. [Severe acute respiratory distress syndrome complicating type A (H1N1) influenza treated with extracorporeal CO2 removal]. Anestezjol Intens Ter. 2011;43:98–103. [PubMed] [Google Scholar]
- 216. Smith IJ, Gillham MJ. Fulminant peripartum cardiomyopathy rescue with extracorporeal membranous oxygenation. Int J Obstet Anesth. 2009;18:186–188. [DOI] [PubMed] [Google Scholar]
- 217. Stankiewicz A, Frank M, Dmitruk I, Czudzinowicz K, Teodorowski W, Walicka‐Pylko A, Matulewicz‐Gilewicz J, Bernacki A, Juszczyk G, Matlak K, et al. Effective therapy of influenza A/H1N1 acute respiratory distress syndrome using extracorporeal membrane oxygenation. Kardiochir Torakochirurgia Pol. 2011;8:462–464. [Google Scholar]
- 218. Steinack C, Lenherr R, Hendra H, Franzen D. The use of life‐saving extracorporeal membrane oxygenation (ECMO) for pregnant woman with status asthmaticus. J Asthma. 2017;54:84–88. [DOI] [PubMed] [Google Scholar]
- 219. Strecker T, Munch F, Weyand M. One hundred ten days of extracorporeal membrane oxygenation in a young woman with postpartum cerebral venous thrombosis and acute respiratory distress syndrome. Heart Surg Forum. 2012;15:180–E181. [DOI] [PubMed] [Google Scholar]
- 220. Su TW, Tseng YH, Wu TI, Lin PJ, Wu MY. Extracorporeal life support in adults with hemodynamic collapse from fulminant cardiomyopathies: the chance of bridging to recovery. ASAIO J. 2014;60:664–669. [DOI] [PubMed] [Google Scholar]
- 221. Takacs ME, Damisch KE. Extracorporeal life support as salvage therapy for massive pulmonary embolus and cardiac arrest in pregnancy. J Emerg Med. 2018;55:121–124. [DOI] [PubMed] [Google Scholar]
- 222. Takeda S, Kotani T, Nakagawa S, Ichiba S, Aokage T, Ochiai R, Taenaka N, Kawamae K, Nishimura M, Ujike Y, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) severe respiratory failure in Japan. J Anesth. 2012;26:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Tampo A, Yamaki M, Kawata D, Suzuki A, Akasaka N, Fujita S. A severe case of postpartum cardiomyopathy required veno‐atrial extracorporeal membrane oxygenation. Eur J Anaesthesiol. 2014;31(suppl 52):178.24448382 [Google Scholar]
- 224. Theodorou M, Gilbert E. Extracorporeal membrane oxygenation in a case of refractory status asthmaticus in pregnancy. Chest. 2015;148:(4_MeetingAbstracts):279A.26149547 [Google Scholar]
- 225. Tincrès F, Conil JM, Crognier L, Rouget A, Georges B, Ruiz S. Veno‐arterial extracorporeal membrane oxygenation in a case of amniotic fluid embolism with coexisting hemorrhagic shock: lessons learned. Int J Obstet Anesth. 2018;33:99–100. [DOI] [PubMed] [Google Scholar]
- 226. Ull C, Schildhauer TA, Strauch JT, Mugge A, Swol J. Veno‐venous ECMO as a safe bridge to recovery in a patient with severe peripartum cardiomyopathy—learning from errors. Perfusion. 2017;32:328–332. [DOI] [PubMed] [Google Scholar]
- 227. Unterberg M, Nowak H, Gottschalk A. [Hypercapnic raised intracerebral pressure—ECMO therapy despite major intracerebral haemorrhage]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2017;52:376–381. [DOI] [PubMed] [Google Scholar]
- 228. Uribarri A, Cruz‐Gonzalez I, Dalmau MJ, Rubia‐Martin MC, Ochoa M, Sanchez PL. Interhospital transfer in patients on ECMO support. An essential tool for a critical care network. Rev Esp Cardiol. 2017;70:1147–1149. [DOI] [PubMed] [Google Scholar]
- 229. van Zwet CJ, Rist A, Haeussler A, Graves K, Zollinger A, Blumenthal S. Extracorporeal membrane oxygenation for treatment of acute inverted takotsubo‐like cardiomyopathy from hemorrhagic pheochromocytoma in late pregnancy. A A Case Rep. 2016;7:196–199. [DOI] [PubMed] [Google Scholar]
- 230. Veld Van Wingerden AWN, Maas J, Harinck HIJ, Mauritz R. Long‐term extracorporeal membrane oxygenation in acute respiratory distress syndrome. Neth J Crit Care. 2015;19:20–24. [Google Scholar]
- 231. Verroust N, Zegdi R, Ciobotaru V, Tsatsaris V, Goffinet F, Fabiani J‐N, Mignon A. Ventricular fibrillation during termination of pregnancy. Lancet. 2007;369:1900. [DOI] [PubMed] [Google Scholar]
- 232. Visveswaran GK, Gidea C, Baran D, Cohen M, Zucker MJ. Acute left ventricular dysfunction complicating pregnancy on ECMO: tri‐iodothyronine to the rescue with real time transesophageal echocardiography. J Cardiol Cases. 2016;13:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Vitulo P, Beretta M, Martucci G, Hernandez Baravoglia CM, Romano G, Bertani A, Martino L, Callari A, Panarello G, Pilato M, et al. Challenge of pregnancy in patients with pre‐capillary pulmonary hypertension: veno‐arterial extracorporeal membrane oxygenation as an innovative support for delivery. J Cardiothorac Vasc Anesth. 2017;31:2152–2155. [DOI] [PubMed] [Google Scholar]
- 234. Wall CJ, Santamaria J. Extracorporeal membrane oxygenation: an unusual cause of acute limb compartment syndrome. Anaesth Intensive Care. 2010;38:560–562. [DOI] [PubMed] [Google Scholar]
- 235. Wang G, Zhou Y, Gong S, Dong H, Wu G, Xiang X, Tang J. A pregnant woman with avian influenza A (H7N9) virus pneumonia and ARDS managed with extracorporeal membrane oxygenation. Southeast Asian J Trop Med Public Health. 2015;46:444–448. [PubMed] [Google Scholar]
- 236. Wei J, Yang HS, Tsai SK, Hsiung MC, Chang CY, Ou CH, Chang YC, Lee KC, Sue SH, Chou YP. Emergent bedside real‐time three‐dimensional transesophageal echocardiography in a patient with cardiac arrest following a caesarean section. Eur J Echocardiogr. 2011;12:E16. [DOI] [PubMed] [Google Scholar]
- 237. Weinberg L, Kay C, Liskaser F, Jones D, Tay S, Jaffe S, Seevanayagam S, Doolan L. Successful treatment of peripartum massive pulmonary embolism with extracorporeal membrane oxygenation and catheter‐directed pulmonary thrombolytic therapy. Anaesth Intensive Care. 2011;39:486–491. [DOI] [PubMed] [Google Scholar]
- 238. Welch SA, Snowden LN, Buscher H. Pandemic (H1N1) 2009 influenza, pregnancy and extracorporeal membrane oxygenation. Med J Aust. 2010;192:668. [DOI] [PubMed] [Google Scholar]
- 239. Wertaschnigg D, Lucovnik M, Moertl MG. Differences between venovenous and venoarterial extracorporeal membrane oxygenation in pregnancies. Ann Thorac Surg. 2017;103:361. [DOI] [PubMed] [Google Scholar]
- 240. Weyrich J, Bogdanski R, Ortiz JU, Kuschel B, Schneider KT, Lobmaier SM. Interdisciplinary peripartum management of acute respiratory distress syndrome with extracorporeal membrane oxygenation—a case report and literature review. Geburtshilfe Frauenheilkd. 2016;76:273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Williams J, Kim A, Bartlett R, Mychaliska G, Chames M. Life‐threatening cardiopulmonary failure in pregnancy and the peripartum period: is extracorporeal life support effective? Am J Obstet Gynecol. 2008;199:S152. [Google Scholar]
- 242. Wise EM, Harika R, Zahir F. Successful recovery after amniotic fluid embolism in a patient undergoing vacuum‐assisted vaginal delivery. J Clin Anesth. 2016;34:557–561. [DOI] [PubMed] [Google Scholar]
- 243. Yang HS, Hong YS, Rim SJ, Yu SH. Extracorporeal membrane oxygenation in a patient with peripartum cardiomyopathy. Ann Thorac Surg. 2007;84:262–264. [DOI] [PubMed] [Google Scholar]
- 244. Yang YP, Lin LS. A pregnant woman with acute massive pulmonary embolism. Acta Cardiol. 2014;30:82–85. [PMC free article] [PubMed] [Google Scholar]
- 245. Yeh TC, Liu CP, Tseng CJ, Liou JC. Postpartum patient with congenital patent ductus arteriosus mimicking acute pulmonary embolism. BMJ Case Rep. 2013;22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Young S, Pritzker M, Rosenberg M. Vacuum‐assisted thrombectomy of massive pulmonary embolism. J Vasc Interv Radiol. 2016;27:1094–1096. [DOI] [PubMed] [Google Scholar]
- 247. Zhang J, Lu J, Zhou X, Xu X, Ye Q, Ou Q, Li Y, Huang J. Perioperative management of pregnant women with idiopathic pulmonary arterial hypertension: an observational case series study from China. J Cardiothorac Vasc Anesth. 2018;07:07. [DOI] [PubMed] [Google Scholar]
- 248. Zhang JL, Lu JK, Zhou XR, Xu XF, Ye Q, Ou QT, Li YN, Huang JP. Perioperative management of pregnant women with idiopathic pulmonary arterial hypertension: an observational case series study from China. J Cardiothorac Vasc Anesth. 2018;32:2547–2559. [DOI] [PubMed] [Google Scholar]
- 249. Zingel D, Avsar M, Schmitto JD, Malehsa D, Haverich A, Strueber M. Left ventricular assist device implantation via mini‐sternotomy in a case of peripartum cardiomyopathy. Artif Organs. 2012;36:A43. [Google Scholar]
- 250. Zykova I, Švancar P, Prattingerová J, Sedlák P, Krejzar Z, Remeta P, Škantárová J, Žihlová L, Morman D. Analysis of the impact of pandemic influenza 2009 a H1N1 outbreak on intensive care in a tertiary care hospital. Intensive Care Med. 2010;36(suppl 2):S284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Tables S1–S3 References 7, 8, 9, 23, and 34–250


